Abstract
Wnt proteins play major roles in development and differentiation, and abnormalities in their regulation are believed to contribute to the formation of many cancers, including colorectal malignancies. As a result, there has been an interest in identifying small molecule inhibitors of Wnt signaling as tool compounds for research or as precursors to new generations of anticancer drugs. Advancements in robotic technology along with reductions in the costs of equipment, chemical libraries, and information handling have made high-throughput drug discovery programs possible in an academic setting. In this minireview we discuss the most plausible protein targets for inhibiting Wnt signaling in colon cancer therapy, list small molecule Wnt inhibitors that have been identified through recent drug discovery efforts, and provide our laboratory's strategy for identifying novel Wnt signaling antagonists using high-throughput screening. In particular, we summarize the results of a screen of over 1,200 drug and druglike compounds we recently completed in which niclosamide was identified as a Wnt pathway antagonist.
Keywords: Wnt, Frizzled, β-catenin, receptor, dishevelled, niclosamide, screening, stem cells
systematic programs of drug discovery, once confined primarily to the pharmaceutical industry, are now becoming commonplace in university research programs. The transfer of moderate- to large-scale drug screening capabilities to academic laboratories has been facilitated by recent reductions in the cost of automated screening equipment, compound libraries, and information technology. The identification of small molecule regulators of stem cell signaling associated with cancer development is an area that can benefit greatly as a result of academic screening programs. Presently, there are no small molecules approved by the FDA for the targeted therapy of colon cancer despite its prevalence in the adult population. Wnt signaling appears to be one of the important molecular mechanisms underlying colon cancers, and this has spurred a search for small molecule inhibitors of Wnt pathway proteins. In this minireview we will present a high-throughput screening (HTS) strategy for identifying Wnt pathway signaling antagonists for use in colorectal cancer research and the treatment of colon cancers.
Colon Cancer and Targeted Therapies
Colorectal cancers are the third most common forms of cancer and the third leading cause of cancer deaths in the United States, and their incidence has remained relatively unchanged (25). In 2008 there were an estimated 149,000 new cases of colorectal cancer diagnosed and 49,960 deaths in the US (22). Small molecule drugs for treating noncancerous diseases of the gastrointestinal tract form one of the largest targeted drug therapy markets; in 2003 $13.5 billion were spent on proton pump inhibitors alone (1). In contrast, small molecule targeted treatments for colon cancers are lacking and the nontargeted drug therapies for later stage disease are relatively ineffective. In a recent study comparing two standard treatment regimens (combined 5FU, leucovorin and oxaliplatin, or irinotecan), response rates were only slightly greater than 50% and median survival was only 20–21 mo for metastatic disease (50). Targeted antibodies against VEGF and the EGFR have been added to chemotherapy regimens and have produced incremental improvements in survival time and response rates in metastatic disease (20). However, relapse eventually occurs in the majority of patients and the survival of patients with metastatic disease remains approximately two years, indicating the need for new therapies that may produce dramatic improvements.
Wnt Signaling Pathway and Tissue Development
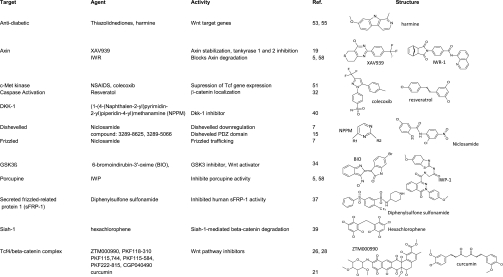
The observation that the progression of aberrant cells from occult tumors to full blown malignancies involves abnormalities in the regulation of the Wnt/Frizzled/LRP5/6 signal transduction pathway has prompted a search for anti-Wnt pathway compounds (46), and results from some of the more recent efforts are shown in the table in Fig. 1. The Wnts form a family of cysteine-rich glycoproteins that locally act to regulate receptor-mediated signaling cascades responsible for tissue development and homeostasis (4, 13, 30). Wnts have been observed to activate at least three different signaling pathways: a canonical pathway that requires β-catenin activation to modulate T-cell factor/lymphoid enhancer factor (TCF/LEF)-dependent gene transcription, and two noncanonical pathways that are independent of β-catenin and regulate cell movement and organization through Jnk or calcium signaling (29, 47). Wnt signals are associated with the homeostatic regulation of the stem cell populations of many tissues including colon, intestine, blood, bone, muscle, fat, and hair (23, 24, 38). Therefore, dysregulation of signaling by individual elements of the Wnt pathway is associated with a broad spectrum of illnesses, both nonmalignant and oncogenic. The following cancers are associated with loss of function oncogenic mutations in Wnt signaling proteins: colorectal cancer, hepatocellular carcinoma, melanoma, prostate cancer, endometrial cancer, and bone malignancies (4, 10, 30, 36).
Fig. 1.
Wnt signaling active compounds.
Canonical Wnt Signaling
In canonical Wnt signaling, Wnts bind and activate two structurally unrelated classes of coreceptors on the surface of target cells, the single transmembrane, low-density lipoprotein-related protein receptors 5 and 6 (LRP5 and LRP6) and members of the seven transmembrane (7TM) receptor Frizzled family (41, 49, 52, 56). An immediate consequence of Frizzled stimulation by Wnt is the recruitment to Frizzled of cytosolic dishevelled protein (Dvl1, 2, or 3) that occurs through a weak interaction of the dishevelled PDZ binding domain with the Frizzled COOH terminus (57). The interaction of Frizzled with dishevelled may be a key regulatory step in coordinating Wnt signaling through the LRP5/6 and Frizzled coreceptor pair. LRP5/6 and Frizzled receptors cooperatively function upon Wnt stimulation to regulate the intracellular concentration of the protein β-catenin by modulating the activity of a β-catenin destruction complex containing axin, adenomatous polyposis coli (APC), glycogen synthase kinase 3β (GSK3β), and casein kinase (13, 30). Axin contains an APC binding site and APC contains a β-catenin binding site (4). β-Catenin is a plasma membrane protein associated with cadherins for the regulation of cell adhesion and mitotic spindles presumably for the regulation of cell division (45), but β-catenin also signals by derepressing nuclear TCF/LEF transcription factors. Cytosolic β-catenin is degraded in proteasomes upon phosphorylation by a destruction complex containing GSK3β, casein kinase 1, and the ubiquitin ligase β-TrCP. Inhibition of GSK3β activity leads to stabilization of cytosolic β-catenin, redistribution of β-catenin to the nucleus, and increased nuclear activity of TCF/LEF transcription factors (4, 17, 36) (Fig. 2).
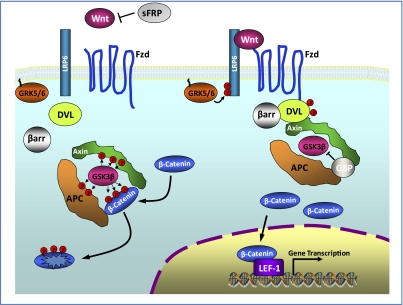
Fig. 2.
Wnt signaling pathway. In the absence of Wnt, cytosolic β-catenin is phosphorylated by GSK3β. Adenomatous polyposis coli (APC) and axin complex with GSK3β and β-catenin to enhance this destruction process. Phosphorylated β-catenin is recognized by the ubiquitin ligase βTrCP, ubiquinated, and degraded. Hence the Wnt signaling is in an “off” state (left). Wnts bind both Frizzled and LRP5/6 receptors to initiate GRK5/6-mediated LRP phosphorylation as well as dishevelled/β-arrestin-mediated Frizzled internalization. Dishevelled membrane translocation and phosphorylation leads to dissociation of the axin/APC/GSK3β destruction complex. Hence β-catenin phosphorylation is inhibited and it accumulates in the cytosol. The accumulated cytosolic β-catenin translocates into the nucleus to bind to LEF/TCFs cotranscription factors, which results in the Wnt-responsive gene transcription (right). Fzd, Frizzled; GBP, GSK3β binding proteins; DVL, Dishevelled; βarr, β-arrestin; GRK, G protein-coupled receptor kinase.
Wnt Signaling Regulation by GRKs and Arrestins
Most ligand-activated 7TM G protein coupled receptors (GPCRs) throughout the body are regulated by the widely expressed serine/threonine kinases GRKs 2, 3, 5, and 6 (42, 43), that also directly regulate a variety of non-GPCR proteins, including α-actinin, Akt, GIT1/2, PI3K, and Smad2/3 (44). GRK phosphorylated GPCRs bind intracellular β-arrestins to traffic and signal. The β-arrestins present GPCRs to components of the clathrin and AP2 endocytic machinery, and form scaffolds that bind intracellular signaling molecules such as Src and Jnk3 (14, 27, 33). Studies now indicate that Wnt signaling may also be modulated as a direct consequence of GRK and β-arrestin activation by Frizzleds and LRP5/6 (6, 8, 9). It was first observed that Frizzle4 receptors recruit β-arrestins in the presence of dishevelled proteins and internalize as a result (9). Additional support for the notion that GPCR regulatory proteins have a role in Wnt regulation stems from findings that GRK5/6 homolog activity in zebrafish appears to be necessary for Wnt/Frizzled signaling to produce normal embryonic patterning (6), the observation that GRKs5/6 phosphorylate LRP6 in a Wnt3A-dependent manner and enhance the activity of TCF/LEF transcription factors in a TopFlash luciferase reporter assay (6), and the result that GRK2 negatively regulates canonical Wnt signaling and this requires a protein-protein interaction between the regulator of G protein signaling (RGS) domain in GRK2 and APC (54). Characterizing how GRKs fit into the observed patterns of Wnt signaling and progression to cancers is a major unexplored area.
Wnt Signaling, Polyp Formation, and Colon Cancer
Polyp formation in the intestine is a premalignant condition and is a reflection of abnormal migration of undifferentiated crypt stem cells to the villus appendages that form the active lining of the gut. In humans one hundred billion cells of intestinal lining are replaced every 2–3 days as a result of the loss of the terminally differentiated cells at the apex of the villus epithelium. In APC mutant mice, the crypt cells no longer migrate appropriately, which disturbs the underlying architecture of the surrounding villus and subsequently results in polyp formation (3). Over 85% of colon cancers contain a mutation in APC genes, but other activating mutations of Wnt pathway elements have also been observed, namely in axin and β-catenin (30). What these tumor-inducing mutations have in common is an ability to lessen the destruction of β-catenin, and as a result nuclear β-catenin activity increases, as does the associated TCF-mediated gene transcription. The upregulation of β-catenin activity is sufficient to take normal intestinal cells on a path to malignancy. Evidence for this conclusion comes from genetic studies, animal knockout experiments, and studies in cell systems (13, 30). Although an outcome of β-catenin activating mutations is polyp formation, in xenograft models inhibiting the Wnt pathway using antisense RNA directed against β-catenin results in decreased tumor growth (16).
Targeting Wnt Signaling
The Wnt pathway contains multiple protein targets, each potentially capable of inhibiting β-catenin activity. The most desirable on the basis of accessibility and practicality are the membrane coreceptors Frizzled and LRP5/6. Although many of the key Wnt intracellular proteins may be equally valid as choices, β-catenin activation is the last signaling step preceding transcription, and on that basis as a signaling choke point β-catenin is a desirable target. In addition to target selection, the makeup of the screening library is critical to the search for druggable compounds; lacking compound structure activity information on the target, druglike libraries provide an abundance of proven scaffolds and a test of proof of principle.
Screens for Wnt pathway antagonists have utilized luciferase-based TCF reporter elements (5, 19, 39, 40), alkaline phosphatase-conjugated antibodies that recognize TCF/β-catenin complexes (28), and differentiation-dependent promoters as readouts for hit compounds (53). Each of these methods assesses changes in downstream Wnt signaling. We developed high-content Wnt signaling assays by modifying Frizzled and β-catenin with green fluorescent protein (GFP) tags, forming their respective GFP chimeras. Although β-catenin-GFP behavior also assesses downstream signaling, changes in Frizzled-GFP compartmentalization will assess changes in Wnt activity at the level of the plasma membrane and bias the search for Wnt inhibitors accordingly. Hit compounds in the assays that change the compartmentalization or concentration of the target chimeras are assessed in secondary assays that directly measure changes in behavior of Wnt signaling proteins, transcriptional activity, and cell growth. We will summarize many of these studies below and additionally present results in greater detail of our screen of Frizzled 1-GFP that identified the FDA-approved antihelminthic niclosamide as a β-catenin signaling inhibitor and a potential therapy for colon cancers as recently described (7).
Identification of Small Molecule Wnt Pathway Regulators
There has been a recent increase in the number of published reports of Wnt pathway modulators (5, 15, 19, 28, 32, 34, 37, 39, 40, 51, 58). Many of the studies reflect the efforts of pharmaceutical companies in identifying Wnt active compounds using high-throughput screening approaches. More focused studies from academic groups have used biochemical insights to identify Wnt active compounds.
Pharmaceutical Studies
Novartis.
Huang et al. (19) in work conducted at Novartis identified XAV939 as a small molecule inhibitor of the Wnt/β-catenin pathway from a high-throughput screen of HEK293 cells containing a Super-TopFlash luciferase reporter. XAV939 treatment decreased β-catenin expression and increased β-catenin phosphorylation at amino acids S33/S37/T41, phosphor-residues that enhance degradation. Protein, but not messenger RNA, levels of axin 1 and axin 2 were strongly increased after XAV939 treatment as was axin-GSK3β complex formation. These results suggest that XAV939 promotes the phosphorylation-dependent degradation of β-catenin by increasing the activity of the axin destruction complex.
Huang et al. (19) utilized a strategy based on the immobilization of a bioactive analog of XAV939 to isolate the XAV939 target from the affinity capture of HEK293 cell lysate proteins. Mass spectrometry identified tankyrase as one of 18 proteins competitively displaced from the affinity matrix by XAV939. The tankyrase enzymes TNKS1 and TNKS2 add ADP-ribose units to substrates (poly-ADP-ribosylation or PARsylation). Codepletion but not individual depletion of TNKS1 and TNKS2 mirrored treatment by XAV939, increased protein levels of axin 1 and 2, increased β-catenin phosphorylation, decreased β-catenin expression, and inhibited the transcription of β-catenin target genes. The authors hypothesized that tankyrase promoted the ubiquitination and degradation of axin, potentially through the direct PARsylation of axin.
In another Novartis-related study, Lepourcelet et al. (28) screened a limited library of 700 natural compounds and a synthetic library of 45,000 compounds against a complex consisting of TCF4 and β-catenin (26). Purified β-catenin fragments (amino acids 134–668) coated onto microtiter plates bound the TCF4 fragments (residues 8–54) that were fused to glutathione-S-transferase (GST), anti-GST antibody, and alkaline phosphatase-conjugated secondary antibody. Compounds tested at 10 μM that disrupted the β-catenin/TCF complex resulted in a reduction of the alkaline phosphatase signal. Secondary screens to verify hits included assessment of pathway specific genes, proliferation and TopFlash reporter assays in HCT116 colon cancer cells, and developmental studies in Xenopus. Interestingly, the smaller natural compound library produced eight hits including PKF115-584, CGP049090, PKF222-815, and ZTM000990, whereas no active compounds were isolated from the larger synthetic library. The molecular mechanisms by which the hit compounds blocked the targeted interaction were not determined. These results indicate how critical the selection of library is and that the quality of bioactive scaffolds present in a natural products library may more than compensate for the smaller number of compounds present. The success of synthetic libraries in producing hit compounds can be immeasurably improved from consideration of structure activity relationships that may be known for the proteins. Unfortunately, this type of information is not always available to enable the rational selection of a directed library containing the appropriate scaffolds.
Wyeth.
These studies are not cancer related but were undertaken to treat bone loss. In a continuing structure-activity relationship (SAR) effort at Wyeth to expand the Wnt-stimulating activities of piperidinyl diphenylsulfonyl sulfonamide scaffolds, Moore et al. (37) describe the effects of these sulfonamides on the Frizzled-related protein-1 family. (sFRP-1 or SARP-2). sFRPs form a large group of Wnt antagonists and are homologous to the membrane-bound Frizzled receptor. The 35-kDa SFRP-1 competes directly with Frizzled for Wnt binding, and compounds that inhibit sFRP-1 are Wnt signaling agonists. A goal of the internal Wyeth Wnt agonist discovery effort is to develop orally bioavailable small molecule inhibitors of sFRP-1 for the treatment of bone loss, particularly for diseases like osteoporosis. The compounds they investigated were identified in an ex vivo calvaria tissue assay, and the more potent ones apparently modulate Wnt signaling at 50 nM by perturbing protein-protein interaction between Wnt and sFRP-1. A review of the scientific and patent literature shows that parent studies were previously carried out in U2OS cell-based assays by using a TCF-tk-Luciferase reporter (USPTO number 7,563,813).
A second study out of Wyeth by Pelletier et al. (40) highlighting 2-aminopyrimidines also is concerned with bone formation. The soluble extracellular protein, Dkk-1, antagonizes an increase in bone development by binding to the cell surface receptor LRP5. To identify small molecules that inhibit the action of Dkk-1, a high-throughput assay was conducted with the Wyeth corporate compound library using an osteosarcoma cell line transfected with Wnt-3a and Dkk-1. A TCF-luciferase response element and a Renilla standard were transfected for luminescence readouts of active compounds and for controls. Lead optimization using SAR produced compounds with enhanced in vitro activity as specific Dkk-1 inhibitors that reduce GSK-3β activity. One of the leads (compound 5 in Fig. 1; Ref. 40) was shown to possess excellent pharmaceutical and pharmacokinetic (PK) properties and was able to enhance the bone formation rate in ovariectomized rats following oral administration.
Academic Studies
Small-molecule Wnt pathway antagonists.
Chen et al. (5) at Texas Southwestern Medical Center used a Super-TopFlash reporter assay of β-catenin activity to identify small-molecule Wnt pathway antagonists from a 200,000-compound synthetic chemical library (58). They recognized two classes of inhibitors. One class, the inhibitor of Wnt protein (IWP) compounds, inhibited the activity of Porcupine, a membrane-bound acyltransferase that is essential for the production of Wnt proteins. The other class, inhibitor of Wnt response (IWR) compounds, prevented destruction of axin proteins by direct binding and stabilization. The effectiveness of IWR compounds in inhibiting the Wnt/β-catenin pathway may result from the rate-limiting role that axin proteins occupy within the Wnt pathway. Elevated axin protein levels resulting from exposure to IWR compounds can potentially compensate for the loss of APC tumor suppressor function. As a test of efficacy, IWR compounds were able to inhibit zebrafish tail regeneration; in contrast, IWP compounds had no effect in this Wnt signaling model.
Hexachlorophene.
The study by Park et al. (39) at Inje University identifying hexachlorophene as a Wnt/β-catenin pathway inhibitor is a further example of using a TopFlash reporter to identify candidate ligands. For this screening effort they used a small high-quality 960-member library of bioactive compounds. Hexachlorophene is an antimicrobial that inhibits enoyl-acyl carrier protein reductase, the last enzyme in the fatty acid elongation cycle. The report indicated that hexachlorophene induced the degradation of β-catenin through the Siah/APC pathway rather than through GSK-3β promoted degradation. Siah-1 interacts with the carboxy terminus of APC, promoting β-catenin ubiquitination and leading to a decreased expression of cyclin D1.
Wnt modulators from observation.
Investigators have identified Wnt pathway ligands by extending observations developed for other signaling pathways. Meijer et al. (34) observed that cyclin-dependent kinase inhibitors were also active against GSK-3β, including the indirubins and bis-indoles that are found in indigo-producing plants, bacteria, and mollusks. These compounds form purple dyes and are also used in Chinese medicine to treat leukemia. The substituted 6-bromoindirubin from the mollusk Hexaplex trunculus was as potent a GSK-3 inhibitor as its analog Bio, 6-bromoindirubin-3-oxime that was synthesized for the study. These compounds cocrystallize with GSK-3 and reduce its phosphorylation on Tyr276/216, thus reducing enzyme activity. Tuynman et al. (51) based their work on observations that there exists a functional interaction between COX-2 enzymatic activity, receptor tyrosine kinase signaling, and Wnt activity. Studies have shown that high concentrations of nonsteroidal anti-inflammatories are able to downregulate the Wnt signaling cascade in colon cancer cells. COX-2 activity enhances the cross talk between the membrane tyrosine kinases c-Met and EGFR, resulting in nuclear accumulation of β-catenin; however, the precise mechanisms are unclear. Other known compounds that also may possess druglike activity against cancer through regulation of β-catenin include the antidiabetic harmine, the wine antioxidant resveratrol, and the spice curcumin (18, 21, 53, 55).
A high-throughput Frizzled-green fluorescent protein screen for Wnt antagonists.
This screen performed by our group, Chen et al. (7), is fundamentally different from the TopFlash-based ones discussed above in that the readout, inhibiting signaling secondary to receptor desensitization and internalization, occurs far upstream at the ligand action site rather than as a reflection of downstream responsiveness. In membrane-based assays such as this, screening reliability is typically better when the target proteins are uniformly expressed, as occurs in permanent cell vs. transient systems. For the Frizzled1-GFP chimera, a stable U2OS cell line was made by transfecting pCS2ratFrizzled1-GFP together with the puromycin resistance plasmid pLKO.1 (10:1 ratio) and selecting for puromycin-resistant green fluorescent clones. The choice of U2OS cells was based predominantly on two desirable properties, an adherence to glass and a relative flatness, properties that reduce background fluorescence and facilitate identification of cytosolic fluorescent structures. To set up the primary screening assay, ∼6,000 Frizzled-GFP U2OS cells were deposited into each well of glass-bottom 384-well plates (MGB101-1-2-LG, Matrical) by using a Multidrop 384 dispenser (Titertek Instruments). The plates were incubated overnight at 37°C in 5% CO2. The following day, compounds (12.5 μM, final concentration) from the Prestwick Chemical Library were added to the wells and left for 6 h at 37°C. After treatment, the cells were fixed in phosphate-buffered saline containing 0.5% paraformaldehyde and 0.002% DRAQ5. The plates were stored at 4°C until analysis on an ImageXpress Ultra (Molecular Devices) equipped with a 488-nm argon laser for exciting GFP and a 568-nm krypton laser for imaging DRAQ5 nuclear staining (7).
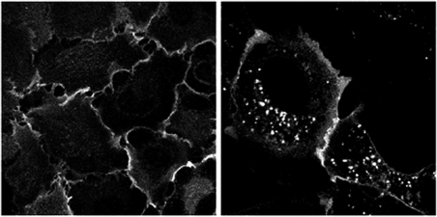
Frizzled1-GFP localized predominantly to the plasma membrane with almost no internalized vesicles apparent when the cells were not prestimulated with Wnt ligands. When treated with an agonist or exposed to a hit compound, the cells showed increased intracellular fluorescence (Fig. 3). Just how robust an assay is can be measured by testing multiple plates of test cells against only known positive and negative control compounds. The accuracy of a test assay in correctly identifying the positive and negative controls is reported as a Z factor. Larger Z values (0.4–1) are desirable in high-throughput assays and should result in fewer false positive hits, a major consideration when screening tens of thousands of compounds (59). We initially evaluated 1,200 FDA-approved drug and druglike compounds from the Prestwick Chemical Library. This Frizzled1 internalization screen produced 24 hits, only one of which, niclosamide (Prestwick 01D11), stood up to verification by secondary assays. Additionally, to substantiate these results, the Frizzled1-GFP expressing U2OS cells were also treated with niclosamide obtained from an alternate supplier.
Fig. 3.
Niclosamide-induced Frizzled1-green fluorescent protein (GFP) internalization. Frizzled1-GFP stable U2OS cells were treated with DMSO (left) or 12.5 μM niclosamide from the Prestwick Chemical Library (right) for 6 h at 37°C. Images were acquired on an ImageXpress Ultra high-throughput confocal imaging system by using a ×40 objective and 488 nm excitation.
Validation Assays
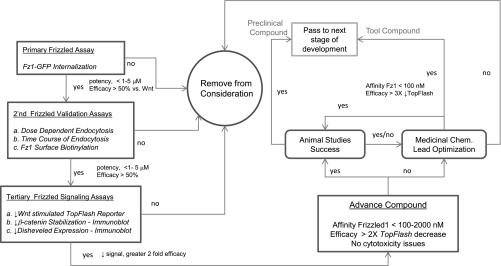
Secondary and tertiary validation assays form a significant part of any compound development strategy (Fig. 4); the ones we employed for verification of niclosamide activity assessed Frizzled receptor internalization, degradation of Wnt signaling molecules, and TCF/LEF transcription.
Fig. 4.
Compound development flowchart for Wnt pathway antagonists. The diagram depicts the decision tree for advancing Wnt antagonists toward clinical compounds. FDA-approved compounds like niclosamide have the potential to quickly pass out of the program to the next stage of development without going through any further lead optimization.
Internalization.
β2-Adrenergic receptors are prototypical for clathrin-dependent internalization of G protein-coupled receptors (2, 14), and transferrin is a well-documented standard for clathrin-mediated internalization in general (35). In cells expressing β2AR and Frizzled1 receptors prior to activation, the two receptors are not colocalized inside the cell. Exposure of cells for 2 or 6 h to the adrenergic agonist isoproterenol and to niclosamide resulted in multiple overlapping intracellular distributions of each receptor; and internalized transferrin at 2 h had significant colocalization with internalized Frizzled1. These data suggest that niclosamide-induced Frizzled1 internalization occurs through clathrin-coated pits.
Degradation of Wnt signaling molecules.
U2OS cells stimulated with either control or Wnt3A-conditioned medium and with niclosamide for 6 h resulted in a dramatic reduction of cytosolic Dvl2 protein. The half-maximal reduction of cytosolic Dvl2 occurred at a niclosamide concentration of ∼1 μM. No endogenous Dvl2 was detected in the membrane fraction, and endogenous Dvl1 and 3 were not detectable by use of commercial antibodies.
Transcription.
We generated an HEK293 cell line that stably expressed a TCF/LEF transcription factor TopFlash reporter. Niclosamide alone did not produce a statistically significant increase in the TopFlash signal, and Wnt3A stimulation produced a 140-fold induction. The addition of niclosamide to the Wnt3A conditioned medium blocked the increase of the reporter signal observed with Wnt3A alone, indicating that niclosamide inhibited Wnt/Frizzled signaling with an IC50 of 0.5 ± 0.05 μM (7).
Summary
Identifying small molecules for the targeted therapy of colon cancers will provide synergies to current treatment protocols. The different readouts utilized in screening for Wnt active compounds have provided multiple scaffolds active against Wnt pathway targets. The majority of Wnt-driven tumors associated with colon cancer, however, are related to the development of inactivating mutations of the tumor suppressor APC, implying that compounds not restoring APC function would be ineffective agents in the majority of clinical cases. Our data with niclosamide suggest that compounds that do not directly restore APC repressor activity may still be effective antitumor agents. For example, the ability of niclosamide to reduce dishevelled expression may counter the effects of APC mutations by reducing the interaction between dishevelled and axin, but this and similar scenarios remain to be expressly tested in APC-driven tumor models (31).
Even though the contribution of the Wnt pathway to tumorigenesis is not fully characterized, there is compelling evidence that Wnt signaling is critical to the formation of many cancers and Wnt proteins are high-value targets for drug discovery. The list of GPCRs associated with the growth and metastasis of colon and other gastrointestinal cancers is also extensive: EP2, EP4, LPA1, ET, PAR1, CCK-A, CCK-B, neurotensin, GRPR, CCR6, and CXCR4 (11, 12, 48). By exploiting high-throughput, high-content strategies similar to that for Frizzled1, biological assays for identifying inhibitors other than simple classical receptor binding antagonists can be developed. Our recent findings with a relatively small library of FDA-approved drugs indicate that many novel scaffolds remain to be discovered from much larger libraries. The widespread availability of automated screening platforms, their record of success with plasma membrane receptors, and the exploitation of recent advances in signaling biology suggest that novel small molecules will soon be available to modulate important intracellular targets such as β-catenin.
GRANTS
This work was supported in part by National Cancer Institute Grant 5RO1 CA113656-03 (W. Chen), Pediatric Brain Tumor Foundation (W. Chen), Susan G. Komen for the Cure (W. Chen), Alexander and Margaret Stewart Trust Fund (W. Chen and L. S. Barak). W. Chen is a V Foundation and American Cancer Society scholar.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the Duke Comprehensive Cancer Center for help in supporting our programs in stem cell-based high-throughput drug discovery.
REFERENCES
- 1.Ashburn TT, Gupta MS. The GERD market. Nat Rev Drug Discov 5: 277–278, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Barak LS, Ferguson SS, Zhang J, Martenson C, Meyer T, Caron MG. Internal trafficking and surface mobility of a functionally intact beta2-adrenergic receptor-green fluorescent protein conjugate. Mol Pharmacol 51: 177–184, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell 103: 311–320, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Cadigan KM, Peifer M. Wnt signaling from development to disease: insights from model systems (Abstract). Cold Spring Harb Perspect Biol 1: a002881, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 5: 100–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen M, Philipp M, Wang J, Premont RT, Garrison TG, Caron MG, Lefkowitz RJ, Chen W. G protein-coupled receptor kinases phosphorylate LRP6 in the Wnt pathway. J Biol Chem 284: 35040–35048, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Wang J, Lu J, Bond MC, Ren XR, Lyerly HK, Barak LS, Chen W. The anti-helminthic niclosamide inhibits Wnt/Frizzled1 signaling. Biochemistry 48: 10267–10274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Hu LA, Semenov MV, Yanagawa S, Kikuchi A, Lefkowitz RJ, Miller WE. β-Arrestin1 modulates lymphoid enhancer factor transcriptional activity through interaction with phosphorylated dishevelled proteins. Proc Natl Acad Sci USA 98: 14889–14894, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science 301: 1391–1394, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol 129: 1614–1627, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer 7: 79–94, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Evers BM. Gastrointestinal growth factors and neoplasia. Am J Surg 190: 279–284, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Fuerer C, Nusse R, Ten Berge D. Wnt signalling in development and disease. Max Delbruck Center for Molecular Medicine meeting on Wnt signaling in Development and Disease. EMBO Rep 9: 134–138, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature 383: 447–450, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Grandy D, Shan J, Zhang X, Rao S, Akunuru S, Li H, Zhang Y, Alpatov I, Zhang XA, Lang RA, Shi DL, Zheng JJ. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J Biol Chem 284: 16256–16263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green DW, Roh H, Pippin JA, Drebin JA. Beta-catenin antisense treatment decreases beta-catenin expression and tumor growth rate in colon carcinoma xenografts. J Surg Res 101: 16–20, 2001 [DOI] [PubMed] [Google Scholar]
- 17.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development 131: 1663–1677, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Hope C, Planutis K, Planutiene M, Moyer MP, Johal KS, Woo J, Santoso C, Hanson JA, Holcombe RF. Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells: implications for colon cancer prevention. Mol Nutr Food Res 52, Suppl 1: S52–S61, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461: 614–620, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Hurwitz H. Integrating the anti-VEGF-A humanized monoclonal antibody bevacizumab with chemotherapy in advanced colorectal cancer. Clin Colorectal Cancer 4, Suppl 2: S62–S68, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 21: 8414–8427, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin 59: 225–249, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Katoh M. WNT signaling in stem cell biology and regenerative medicine. Curr Drug Targets 9: 565–570, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res 13: 4042–4045, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kaz AM, Brentnall TA. Genetic testing for colon cancer. Nat Clin Pract Gastroenterol Hepatol 3: 670–679, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kiselyov AS, Tkachenko SE, Balakin KV, Ivachtchenko AV. Small-molecule modulators of Hh and Wnt signaling pathways. Expert Opin Ther Targets 11: 1087–1101, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Laporte SA, Oakley RH, Holt JA, Barak LS, Caron MG. The interaction of beta-arrestin with the AP-2 adaptor is required for the clustering of beta 2-adrenergic receptor into clathrin-coated pits. J Biol Chem 275: 23120–23126, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell 5: 91–102, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Macdonald BT, Semenov MV, He X. SnapShot: Wnt/beta-catenin signaling. Cell 131: 1204, 2007 [DOI] [PubMed] [Google Scholar]
- 30.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacDonald ML, Lamerdin J, Owens S, Keon BH, Bilter GK, Shang Z, Huang Z, Yu H, Dias J, Minami T, Michnick SW, Westwick JK. Identifying off-target effects and hidden phenotypes of drugs in human cells. Nat Chem Biol 2: 329–337, 2006 [DOI] [PubMed] [Google Scholar]
- 32.MacLachlan TK. In vino, curationis? Cancer Biol Ther 7: 1313–1314, 2008 [DOI] [PubMed] [Google Scholar]
- 33.McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 290: 1574–1577, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, Crovace C, Tarricone C, Musacchio A, Roe SM, Pearl L, Greengard P. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol 10: 1255–1266, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol 12: 575–625, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet 5: 691–701, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Moore WJ, Kern JC, Bhat R, Bodine PV, Fukyama S, Krishnamurthy G, Magolda RL, Pitts K, Stauffer B, Trybulski EJ. Modulation of Wnt signaling through inhibition of secreted frizzled-related protein I (sFRP-1) with N-substituted piperidinyl diphenylsulfonyl sulfonamides: part II. Bioorg Med Chem 18: 190–201, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Neth P, Ries C, Karow M, Egea V, Ilmer M, Jochum M. The Wnt signal transduction pathway in stem cells and cancer cells: influence on cellular invasion. Stem Cell Rev 3: 18–29, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Park S, Gwak J, Cho M, Song T, Won J, Kim DE, Shin JG, Oh S. Hexachlorophene inhibits Wnt/beta-catenin pathway by promoting Siah-mediated beta-catenin degradation. Mol Pharmacol 70: 960–966, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Pelletier JC, Lundquist JT, 4th, Gilbert AM, Alon N, Bex FJ, Bhat BM, Bursavich MG, Coleburn VE, Felix LA, Green DM, Green P, Hauze DB, Kharode YP, Lam HS, Lockhead SR, Magolda RL, Matteo JJ, Mehlmann JF, Milligan C, Murrills RJ, Pirrello J, Selim S, Sharp MC, Unwalla RJ, Vera MD, Wrobel JE, Yaworsky P, Bodine PV. (1-(4-(Naphthalen-2-yl)pyrimidin-2-yl)piperidin-4-yl)methanamine: a wingless beta-catenin agonist that increases bone formation rate. J Med Chem 52: 6962–6965, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407: 535–538, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem 67: 653–692, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol 69: 511–534, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F., Jr The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta 1768: 913–922, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Salinas PC. Modulation of the microtubule cytoskeleton: a role for a divergent canonical Wnt pathway. Trends Cell Biol 17: 333–342, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene 25: 7531–7537, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Semenov MV, Habas R, Macdonald BT, He X. SnapShot: noncanonical Wnt signaling pathways. Cell 131: 1378.01–1378.02, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Rodrigues TA, Casimiro S, Gasparinho G, Crujo C, Matos M, Ruivo J, Fernandes A, Quintela A, Costa L. Involvement of chemokine receptors CXR4, CCR7, CCR6, and CXCR5 in colorectal cancer metastasis. Am Soc Clin Oncol Abstract 374: 2009 [Google Scholar]
- 49.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature 407: 530–535, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Tournigand C, Cervantes A, Figer A, Lledo G, Flesch M, Buyse M, Mineur L, Carola E, Etienne PL, Rivera F, Chirivella I, Perez-Staub N, Louvet C, Andre T, Tabah-Fisch I, de Gramont A. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer–a GERCOR study. J Clin Oncol 24: 394–400, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Tuynman JB, Vermeulen L, Boon EM, Kemper K, Zwinderman AH, Peppelenbosch MP, Richel DJ. Cyclooxygenase-2 inhibition inhibits c-Met kinase activity and Wnt activity in colon cancer. Cancer Res 68: 1213–1220, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal 1: re9, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Waki H, Park KW, Mitro N, Pei L, Damoiseaux R, Wilpitz DC, Reue K, Saez E, Tontonoz P. The small molecule harmine is an antidiabetic cell-type-specific regulator of PPARgamma expression. Cell Metab 5: 357–370, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Gesty-Palmer D, Fields TA, Spurney RF. Inhibition of WNT signaling by G protein-coupled receptor (GPCR) kinase 2 (GRK2). Mol Endocrinol 23: 1455–1465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang PS, Chou FS, Bloomston M, Vonau MS, Saji M, Espinosa A, Pinzone JJ. Thiazolidinediones downregulate Wnt/beta-catenin signaling via multiple mechanisms in breast cancer cells. J Surg Res 153: 210–216, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Wehrli M, Dougan ST, Caldwell K, O'Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407: 527–530, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell 12: 1251–1260, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeh JR, Peterson RT. Novel Wnt antagonists target porcupine and Axin. Nat Chem Biol 5: 74–75, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4: 67–73, 1999. [DOI] [PubMed] [Google Scholar]