Abstract
Our aim was to elucidate the mechanism by which HIV transmission is increased following obstetrical hemorrhage. We investigated whether fetal allostimulation of maternal cells, which could occur following fetal-to-maternal hemorrhage, increases proliferation, HIV replication, and cellular activation. Peripheral blood mononuclear cells (PBMCs) were collected from HIV-infected mothers and their infants to assess maternal-fetal allostimulation. Responses were compared to allostimulation with unrelated donors. Maternal and fetal cells were cocultured to assess allogeneic stimulation. Cell proliferation was measured by [3H]thymidine incorporation and cell activation was assessed via fluorochrome-labeled antibody staining and flow cytometric analysis. Virus production from HIV-infected maternal cells was quantitated by p24 enzyme-linked immunosorbent assay or by branched chain DNA assay. Allostimulation with fetal cells led to maternal cell proliferation. In women with unsuppressed viral loads, virus release was also enhanced following allostimulation of maternal cells with fetal cells. Fetal cells are capable of allogeneically stimulating maternal cells, with responses comparable to those seen following allostimulation with unrelated donors. Allostimulation of maternal cells by fetal cells results in statistically significant increases in proliferation and enhanced HIV replication, suggesting a possible physiological mechanism for mother-to-child transmission of HIV in women with obstetrical hemorrhage.
Introduction
Mother-to-child transmission (MTCT) of HIV-1 may occur during the antepartum, intrapartum, or postpartum periods. Vertical HIV transmission correlates with plasma viral load and can be decreased substantially with the use of antiretroviral therapy.1–4 Obstetric complications such as preterm labor, prolonged rupture of membranes, infection, and obstetrical hemorrhage increase the vertical transmission rate of HIV.5–7 Chorioamnionitis is thought to enhance HIV transmission via the production and release of inflammatory cytokines.8 In support of this concept, our laboratory has shown that lymphocytes from infants born following preterm labor or chorioamnionitis are more activated and tend to be more susceptible to HIV infection.9 Maternal immune status has also been associated with perinatal HIV transmission,10–14 thus the immune system is likely to be an important determinant in mother-to-child transmission of HIV.
Pregnancy is characterized by major histocompatibility complex antigen (MHC) disparity between the mother and the fetus, secondary to paternally derived antigens. MHC mismatch normally results in an anti-allo-MHC immune response, involving cellular activation and proliferation, as is seen following organ transplantation and/or blood transfusion. However, within a normal pregnancy the maternal immune system does not react against the fetus, secondary to either a lack of fetal antigenic exposure, immune modulation, or a combination of factors.15 This lack of allostimulation likely contributes to the tolerance of the fetal allograft during pregnancy.15,16
It is well established that allogeneic stimulation leads to enhanced HIV replication and infection, including the reactivation of latently infected cells, using in vitro systems.17 Thus obstetrical complications that are associated with fetal-to-maternal hemorrhage, including placental abruption, could enhance maternal HIV replication via an alloimmune response. As viral load correlates with vertical HIV transmission,1–4 enhanced maternal HIV replication secondary to allostimulation could transiently increase viral load and mother-to-child transmission of HIV. Epidemiological studies have associated obstetrical hemorrhage with vertical HIV transmission,5–7 supporting this concept. These findings lead us to hypothesize that fetal cells can serve as allogeneic stimuli for maternal cells, leading to an increase in HIV replication. However, several investigators have shown that there is not a normal allostimulatory reaction when maternal cells are challenged with fetal cells,16,18–21 although work by other laboratories suggests that maternal cells can appropriately recognize and react to allogeneic fetal cells.22,23 Unfortunately these studies had limited sample sizes and utilized different methods to assess allostimulation, making cross-study comparison unfeasible. Therefore it is not certain whether fetal cells can allostimulate maternal cells and enhance HIV replication in the setting of maternal HIV infection, prompting our laboratory to investigate this phenomenon.
Our studies demonstrate that fetal cells are capable of activating HIV-infected maternal cells via allogeneic stimulation at levels comparable to unrelated cells. Cellular activation is accompanied by increased proliferation. Furthermore, HIV replication in maternal cells was enhanced in patients in whom maternal viral load was not well suppressed in vivo. Additionally, we found that HIV-exposed fetal cells were activated by allostimulation with maternal cells, although our small sample size precluded statistical analysis. These findings indicate that fetal–maternal hemorrhage could increase maternal viral load and vertical HIV transmission following obstetrical hemorrhage, representing a physiological mechanism by which allostimulation could occur in vivo.
Materials and Methods
Patient population and cell isolation
The Institutional Review Boards of each participating site (Johns Hopkins University and UCLA) approved the study and all patients gave written informed consent. Demographics and information regarding study participants are shown in Table 1. Patient viral loads were determined using Roche Diagnostics Corporation HIV-1 RNA assay kits. Cord blood was obtained by venipuncture of the umbilical cord following cleansing to eliminate maternal cell contamination and blood was collected into a sterile collection bag containing acid citrate dextrose (ACD). Maternal blood was collected using peripheral venipuncture. HIV-infected maternal peripheral blood mononuclear cells (PBMCs), HIV-exposed cord blood mononuclear cells (CBMCs), and unrelated healthy PBMCs were isolated by Ficoll–Hypaque centrifugation, and seeded at different cell concentrations, as described below, in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 6 mM l-glutamine, 100 U/ml penicillin, and 50 μg/ml streptomycin. For some experiments, PBMCs from HIV-infected mothers were depleted of CD8+ T cells using immunomagnetic beads specific for CD8 (Dynal, Lake Success, NY) according to the manufacturer's instructions. Some cells were irradiated at 5000 rads using a Mark-I irradiator, as indicated below.
Table 1.
Patient Clinical Informationa
| Patient | Age | Parity | Years of HIV+ | Antiretrovirals | Duration of suppression | Complications | Mode of delivery | VL | CD4 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | G3P0110 | 2 | AZT, 3TC, NFV | 5 months | Depression, drug use | NSVD | ND | 382 |
| 2 | 24 | G1P0 | 6 | AZT, 3TC, NFV | 6 months | NSVD | ND | 410 | |
| 3 | 25 | G5P4004 | 9 | AZT, 3TC | NA | NSVD | 102136 | 36 | |
| 4 | 27 | G4P2012 | 4 | ABC, AZT, 3TC, NVP | NA | eCS | 518 | 357 | |
| 5 | 34 | G12P3083 | 6 | AZT, 3TC, IDV, RTV | NA | GDMA2 | eCS | 66000 | 11 |
| 6 | 27 | G2P1001 | 3 | AZT, 3TC, NFV | NA | eCS | 562 | 379 | |
| 7 | 31 | G2P1001 | 2 | ABC, AZT, 3TC, NVP | 18 months | NSVD | ND | 350 | |
| 8 | 35 | G6P4004 | 8 | DDI, RTV, IDV, NVP | 1 month | GDMA2, nonreassuring fetal status | CS | ND | 196 |
| 9 | 39 | G2P1001 | 18 | DDI, TDF, NFV | >5 years | eCS | ND | 222 | |
| 10 | 31 | G2P0010 | 13 | TDF, LPV/r, SQV | >5 years | NSVD | ND | 795 |
AZT, zidovudine; 3TC, lamivudine; NFV, nelfinavir; ABC, abacavir sulfate; NVP, nevirapine; IDV, indinavir sulfate; RTV, ritonavir; DDI, didanosine; TDF, tenofovir disoproxil fumarate; LPV/r, lopinavir/ritonavir; SQV, saquinavir; GDMA2, gestational diabetes type 2; NSVD, normal spontaneous vaginal delivery; eCS, elective cesarean section; ND, nondetectable.
Establishment of allogeneically stimulated PBMC cultures and [3H]thymidine incorporation
To determine whether fetal cells stimulate maternal cell proliferation and/or activation, equal numbers of cells from a mother/child pair were mixed and cultured at a cell concentration of 2 × 106 cells/ml. CBMCs were irradiated to enable assessment of maternal cell proliferation and/or activation. As a negative control maternal cells were cultured with an equivalent number of autologous, irradiated maternal cells, and maternal cells were cocultured with unrelated, allogeneic irradiated PBMCs as a positive control. Maternal cell proliferation was quantitated on day 5 by incubating cells with [3H]thymidine at a final concentration of 1 μCi/well overnight. Cells were then harvested and [3H]thymidine incorporation was assessed using a microbeta plate reader (J 450 Microbeta).
Flow cytometric analysis
Cell activation was assessed via fluorochrome-labeled antibody staining. Cell surface expression of CD4, HIV coreceptors (CCR5 and CXCR4), and cellular activation markers (CD69, CD25, and HLA-DR) were analyzed in unstimulated and allostimulated maternal PBMCs using flow cytometry (FACScalibur, Becton Dickinson Immunocytometry Systems, San Jose, CA) following 4 days of coculture. PE-conjugated monoclonal antibodies (mAbs) to CCR5 and CXCR4 were purchased from PharMingen (San Diego, CA). PE-conjugated mAbs to CD69, CD25, and HLA-DR, as well as CD8-FITC, CD3-PerCP, CD4-APC, and isotype controls were obtained from Becton Dickinson Immunocytometry Systems. Neonatal cell activation was assessed similarly, following co-culture with lethally irradiated maternal PBMCs or unrelated, irradiated PBMCs as described above.
Virus replication assays
To determine whether fetal cells stimulate maternal virus production, CD8-depleted maternal PBMCs were cocultured with an equal number (2 × 106 cells/ml) of lethally irradiated CBMCs. Coculture with an equal number of irradiated, autologous maternal PBMCs was used as a negative control, and coculture with lethally irradiated, unrelated PBMCs was used as a positive control. Virus production was measured by release of p24 into the cell culture supernatant via enzyme-linked immunosorbent assay (ELISA) (Coulter, Miami, FL). The culture supernatants were collected for p24 antigens on days 3, 6, 9, and 12 after allostimulation. In some cases virus release was assessed by branched chain DNA quantitation of HIV for enhanced sensitivity as described.24 In some patients (sample size permitting), virus production was compared between PBMCs and CD8-depleted PBMCs. Although no differences were appreciated in these samples, virus production was assessed in CD8-depleted PBMCs to ensure maximum sensitivity.
Statistical analysis
Statistical analysis was performed using the Wilcoxon signed rank test. p values below 0.05 were considered significant.
Results
Allostimulation results in increased maternal cell proliferation
To determine whether allostimulation with fetal cells stimulates maternal cell proliferation, we cocultured maternal PBMCs with an equal number of lethally irradiated, autologous(maternal), fetal or unrelated mononuclear cells. Proliferation was assessed by thymidine incorporation on day 5 (Fig. 1). We found that fetal cells were capable of stimulating maternal cell proliferation, with a stimulation index ranging from 15 to 25. Coculture with irradiated fetal cells led to greater levels of thymidine incorporation than did coculture with irradiated, autologous cells via the Wilcoxon signed rank test (n = 5, p = 0.0313). Proliferation appeared slightly more robust when maternal cells were challenged with unrelated allogeneic cells; however, this likely reflects the presence of shared HLA alleles between infants and their mothers. These findings support the notion that fetal cells can serve as effective allostimuli for maternal cells. As proliferating cells produce more virus and are more susceptible to HIV infection,25 in vivo allostimulation could result in enhanced perinatal HIV transmission.
FIG. 1.
Allostimulation results in increased maternal cell proliferation. Proliferation of maternal PBMCs was quantitated by 3H incorporation on day 5 of coculture with irradiated cells. Maternal cell proliferation was enhanced following coculture with fetal (p = 0.0313) or unrelated mononuclear cells. Results are representative of six independent experiments.
Allostimulation results in both maternal and neonatal T cell activation
To determine whether fetal cells activate maternal cells, phenotyping was performed in a limited number of allostimulated samples. Maternal PBMCs were cocultured with an equal number of lethally irradiated maternal, fetal, or unrelated mononuclear cells. On day 4 cell activation was assessed using fluorochrome-labeled monoclonal antibodies recognizing CD25, CD69, and HLA-DR followed by flow cytometric analysis (Fig. 2). Coculture with fetal cells resulted in a trend toward increased CD69 and HLA-DR expression in maternal cells; however, statistical significance was not achieved secondary to our limited sample size. The percentage of maternal cells expressing CD25 was not increased following allostimulation; however, the mean fluorescent intensity (MFI) of positive cells was qualitatively higher. Our results suggest that allostimulation with fetal cells results in cellular activation that is comparable to that seen with unrelated donors. Similar studies were conducted using CBMCs from HIV-exposed neonates to determine whether maternal cells could activate neonatal cells via allostimulation (Fig. 3). Coculture with maternal cells increased the percentage of CD4+ T cells expressing CD69 and HLA-DR, and there was an increase in the MFI of CD25 expression. Our results suggest that maternal–fetal allostimulation leads to both maternal and neonatal CD4+ T cell activation, as is seen following allostimulation with unrelated donors.17 These qualitative findings, coupled with the increased cell proliferation observed following allostimulation, suggests that in vivo allostimulation could result in enhanced perinatal HIV transmission, as activated, proliferating cells produce more virus and are more susceptible to HIV infection.25
FIG. 2.

Maternal CD4+ T cell activation following allostimulation. Cocultured maternal PBMCs (day 4) were stained with fluorochrome-labeled monoclonal antibodies: CD4-APC, CD69-PE, CD25-PE, HLA-DR-PE, CD8-FITC, and CD3-PerCP. Flow cytometric analysis was performed, settings are based on isotype antibody staining, and samples were compensated electronically for overlap in fluorescent emission. Dot plots show lymphocytes gated for CD3 expression; the percentage of CD4+ cells expressing the indicated marker is shown in the top right. The mean fluorescence intensity (MFI) of CD25+/CD4+ staining is shown in parentheses. Results are representative of five independent experiments.
FIG. 3.

Neonatal CD4+ T cell activation following allostimulation. Unstimulated (fetal cells) and allostimulated fetal CM-BCs were cultured for 4 days and expression of cellular activation markers (CD25, CD69, HLA-DR) and HIV coreceptors (CXCR4, CCR5) was determined for CD4+ T cells by FACS. Dot plots show lymphocytes gated for CD3 expression; the percentage of CD4+ cells expressing the indicated marker is shown in the top right. The mean fluorescence intensity (MFI) of CD25+/CD4+ staining is shown in parentheses. Results are representative of three independent experiments.
Allostimulation can lead to increased virus production
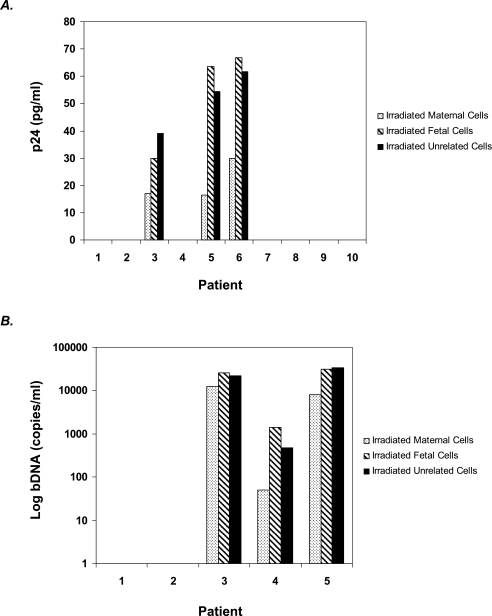
We next examined whether allostimulation with fetal cells can stimulate maternal virus production. Maternal PBMCs from HIV-infected pregnant women were cocultured with equal numbers of lethally irradiated autologous maternal, fetal, or unrelated mononuclear cells. Virus production was quantitated by release of p24 into cell culture supernatant via ELISA (Fig. 4A). Virus production in some samples was assessed by branched chain DNA quantitation of HIV for enhanced sensitivity (Fig. 4B). We were able to detect virus release in women who had detectable viral loads at delivery; moreover, allostimulation with either fetal or unrelated mononuclear cells increased virus release as quantitated by p24 (p < 0.05). In general, we were not able to detect virus release in women with undetectable viral loads even following CD8 depletion. We were able to detect a small amount of released virus following fetal allostimulation via bDNA assay in patient 4. This patient was unique in that she did not start antiretroviral therapy until 34 weeks of gestation. These findings suggest that allostimulation could increase virus production in viremic women.
FIG. 4.
Allostimulation increases virus production. (A) Cell culture supernatants were collected to measure p24 released from unstimulated and allostimulated maternal cell cocultures. Peak p24 titers are shown. Virus release was not measurable from women with undetectable viral loads. (B) Using a more sensitive assay, bDNA technology, patient 4 had low levels of virus released. Women with detectable viral loads clearly show increased viral release following stimulation with fetal cells or unrelated allogeneic cells (p < 0.05).
Discussion
Multiple factors impact mother-to-child transmission of HIV. Viral load at the time of delivery strongly correlates with HIV transmission,1–4 as does low maternal CD4 count, low birth weight, and drug use during pregnancy. Obstetric complications such as preterm labor, prolonged rupture of membranes, infection, and obstetrical hemorrhage also increase the vertical transmission rate of HIV.5–7 Mothers transmitting HIV to infants were more likely than nontransmitting mothers to have had fetal exposure to maternal blood during labor via placental abruption, use of fetal scalp electrodes, or severe lacerations.7,26,27 Furthermore, obstetric hemorrhage was found to be independently associated with perinatal HIV transmission; when procedures such as fetal scalp electrode placement and episiotomy were excluded.7,26,27 Thus, fetal exposure to maternal blood may be a critical intrapartum event accounting for MTCT of HIV.
Pregnancy is characterized by MHC antigen mismatch between the mother and the fetus. MHC discordance normally results in an anti-allo-MHC immune response. However, within a normal pregnancy the maternal immune system does not react against the fetus, secondary to either a lack of fetal antigenic exposure, immune modulation, or a combination of factors. Nevertheless, as demonstrated by rhesus (Rh) isoimmunization, there are pathological conditions in which the maternal immune system is exposed to fetal antigens, leading to an immune response. Thus obstetrical complications associated with fetal-to-maternal hemorrhage, including placental abruption, could result in an alloimmune response. This could result in enhanced perinatal HIV transmission as allostimulation has been shown to increase HIV replication,17 and viral load correlates with vertical HIV transmission.1–4 Epidemiological studies demonstrating an association between obstetrical hemorrhage and vertical HIV transmission support this concept.7,26,27
Other investigators suggest that uterine contractions routinely lead to maternal–fetal transfusions.28 Early work utilizing radiolabeled maternal cells established that there is some maternal-to-fetal transfusion during delivery29; moreover, up to 25% of infants with severe combined immunodeficiency develop graft-versus-host disease from maternal lymphocytes.30,31 More recent studies assessing HBsAg and placental alkaline phosphatase levels in infants suggest that approximately 3 ml of maternal blood is present within the fetal circulation following uncomplicated vaginal delivery.32,33 The amount of maternal blood within the fetal circulation is substantially lower following elective, scheduled C-section, but is not decreased following C-section in labor. Thus, maternal-to-fetal microtransfusion could result in some HIV-infected maternal cells within the fetal circulation potentially leading to allostimulation-induced virus production.
Given the data indicating that microtransfusion occurs late in pregnancy and that obstetrical hemorrhage is associated with increased HIV transmission, we sought to determine whether fetal cells can serve as allogeneic stimuli for maternal cells, leading to an increase in HIV replication. A secondary hypothesis was that maternal cells can, in turn, serve to allostimulate fetal cells. Our results demonstrate that fetal cells are capable of activating HIV-infected maternal cells. Allostimulation with fetal cells led to maternal CD4+ T cell proliferation (p = 0.0313) comparable to that seen following allostimulation with unrelated donor cells. Activation marker expression was determined on a subset of maternal and fetal PBMCs following allostimulation. Allostimulated samples tended to have increased activation marker expression. As activated, proliferating cells produce more virus and are highly susceptible to infection with HIV, allostimulation in the setting of pregnancy could result in increased HIV replication and transmission.
We next sought to assess the impact of neonatal cell-mediated allostimulation on maternal virus production. Our results show that neonatal cells were capable of increasing virus production in CD8-depleted PBMCs from viremic patients (p < 0.05). However, we were not able to detect virus production following allostimulation in samples from aviremic women. These results suggest that placental abruption, or other pathological conditions associated with fetal-to-maternal hemorrhage, could result in allostimulation-enhanced virus production in vivo in a subset of patients (i.e., those with unsuppressed viral loads).
Other investigators have suggested an alternative hypothesis regarding alloantigen recognition in the setting of HIV infection.35 Citing epidemiological studies linking HLA concordance with increased perinatal HIV transmission,36 they suggest that allogeneic stimulation within pregnancy might result in inhibition of HIV replication and transmission via the production of soluble factors including human chorionic gonadotropin, stromal cell-derived factor-1, leukemia inhibitory factor, and ribonucleases (RNases).35 Allostimulation also results in increased CCL chemokine production, which can result in coreceptor downregulation, potentially limiting HIV transmission.17,37 However, allostimulation clearly results in cellular activation and proliferation, which are known to be critical for HIV infection and replication.38,39 Thus, alloantigen-induced stimulation has a dichotomous effect on HIV replication, enhancing viral replication via cellular activation, while inducing factors that potentially suppress HIV replication. Our findings demonstrate that cells from HIV-infected pregnant women become activated and have increased HIV replication following allostimulation with fetal cells. Fetal allostimulation of maternal cells might explain the mechanism by which there is an increased rate of perinatal HIV transmission in women whose pregnancy is complicated by obstetrical hemorrhage.
Acknowledgments
We thank Dr. Jerome A. Zack and Audrey Kinter for helpful discussion and critical reading of this manuscript. H.B. was a Building Interdisciplinary Research Careers in Women's Health Center Scholar (5 K12 HD01400). This work was also supported in part by the Reproductive Scientist Development Program through the National Institutes of Health (5K12HD00849) and the Society for Gynecologic Investigation.
Disclosure Statement
No competing financial interests exist.
References
- 1.Connor EM. Sperling RS. Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 2.Lallemant M. Jourdain G. Le Coeur S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351:217–228. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- 3.Mofenson LM. Lambert JS. Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341:385–393. doi: 10.1056/NEJM199908053410601. [DOI] [PubMed] [Google Scholar]
- 4.Peckham C. Gibb D. Mother-to-child transmission of the human immunodeficiency virus. N Engl J Med. 1995;333:298–302. doi: 10.1056/NEJM199508033330507. [DOI] [PubMed] [Google Scholar]
- 5.Landesman SH. Kalish LA. Burns DN, et al. Obstetrical factors and the transmission of human immunodeficiency virus type 1 from mother to child. The Women and Infants Transmission Study. N Engl J Med. 1996;334:1617–1623. doi: 10.1056/NEJM199606203342501. [DOI] [PubMed] [Google Scholar]
- 6.Mandelbrot L. Landreau-Mascaro A. Rekacewicz C, et al. Lamivudine-zidovudine combination for prevention of maternal-infant transmission of HIV-1. JAMA. 2001;285:2083–2093. doi: 10.1001/jama.285.16.2083. [DOI] [PubMed] [Google Scholar]
- 7.Boyer PJ. Dillon M. Navaie M, et al. Factors predictive of maternal-fetal transmission of HIV-1. Preliminary analysis of zidovudine given during pregnancy and/or delivery. JAMA. 1994;271:1925–1930. [PubMed] [Google Scholar]
- 8.Shearer WT. Reuben J. Lee BN, et al. Role of placental cytokines and inflammation in vertical transmission of HIV infection. Acta Paediatr Suppl. 1997;421:33–38. doi: 10.1111/j.1651-2227.1997.tb18317.x. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein HB. Jackson RW. Anderson J. Kinter AL. The effect of elective cesarean delivery and intrapartum infection on fetal lymphocyte activation and susceptibility to HIV infection. Am J Obstet Gynecol. 2002;187:1283–1289. doi: 10.1067/mob.2002.126978. [DOI] [PubMed] [Google Scholar]
- 10.Jin X. Roberts CG. Nixon DF, et al. Longitudinal and cross-sectional analysis of cytotoxic T lymphocyte responses and their relationship to vertical human immunodeficiency virus transmission. ARIEL Project Investigators. J Infect Dis. 1998;178:1317–1326. doi: 10.1086/314455. [DOI] [PubMed] [Google Scholar]
- 11.Lathey JL. Tsou J. Brinker K. Hsia K. Meyer WA., 3rd Spector SA. Lack of autologous neutralizing antibody to human immunodeficiency virus type 1 (HIV-1) and macrophage tropism are associated with mother-to-infant transmission. J Infect Dis. 1999;180:344–350. doi: 10.1086/314886. [DOI] [PubMed] [Google Scholar]
- 12.Plaeger S. Bermudez S. Mikyas Y, et al. Decreased CD8 cell-mediated viral suppression and other immunologic characteristics of women who transmit human immunodeficiency virus to their infants. J Infect Dis. 1999;179:1388–1394. doi: 10.1086/314746. [DOI] [PubMed] [Google Scholar]
- 13.Tranchat C. Van de Perre P. Simonon-Sorel A, et al. Maternal humoral factors associated with perinatal human immunodeficiency virus type-1 transmission in a cohort from Kigali, Rwanda, 1988–1994. J Infect. 1999;39:213–220. doi: 10.1016/s0163-4453(99)90052-x. [DOI] [PubMed] [Google Scholar]
- 14.Wilson CC. Brown RC. Korber BT, et al. Frequent detection of escape from cytotoxic T-lymphocyte recognition in perinatal human immunodeficiency virus (HIV) type 1 transmission: The ariel project for the prevention of transmission of HIV from mother to infant. J Virol. 1999;73:3975–3985. doi: 10.1128/jvi.73.5.3975-3985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trowsdale J. Betz AG. Mother's little helpers: Mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 16.Finn R. St Hill CA. Davis JC. Hipkin LJ. Harvey M. Fetomaternal bidirectional mixed lymphocyte reaction and survival of fetal allograft. Lancet. 1977;2:1200–1202. doi: 10.1016/s0140-6736(77)90439-1. [DOI] [PubMed] [Google Scholar]
- 17.Moriuchi H. Moriuchi M. Fauci AS. Induction of HIV-1 replication by allogeneic stimulation. J Immunol. 1999;162:7543–7548. [PubMed] [Google Scholar]
- 18.Birkeland SA. Kristoffersen K. The fetus as an allograft: A longitudinal study of normal human pregnancies studied with mixed lymphocyte cultures between mother-father and mother-child. Scand J Immunol. 1980;11:311–319. doi: 10.1111/j.1365-3083.1980.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 19.Brune T. Masjoshusmann K. Reinhold D, et al. The imbalanced mixed lymphocyte reaction between maternal and fetal lymphocytes as well as between the lymphocytes of adult children and their parents is mediated by activated CD8+ T cells. Am J Reprod Immunol. 2004;52:284–289. doi: 10.1111/j.1600-0897.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- 20.Lawler SD. Ukaejiofo EO. Reeves BR. Interaction of maternal and neonatal cells in mixed-lymphocyte cultures. Lancet. 1975;2:1185–1187. doi: 10.1016/s0140-6736(75)92663-x. [DOI] [PubMed] [Google Scholar]
- 21.Saji F. Tanaka F. Fumita Y. Nakamuro K. Tanizawa O. Immunoregulatory effects of human cord blood T lymphocytes on mixed lymphocyte reaction. Nippon Sanka Fujinka Gakkai Zasshi. 1986;38:1115–1119. [PubMed] [Google Scholar]
- 22.Carr MC. Stites DP. Fudenberg HH. Cellular immune aspects of the human fetal-maternal relationship. III. Mixed lymphocyte reactivity between related maternal and cord blood lymphocytes. Cell Immunol. 1974;11:332–341. doi: 10.1016/0008-8749(74)90032-x. [DOI] [PubMed] [Google Scholar]
- 23.Moen T. Moen M. Palbo V. Thorsby E. In vitro foeto-maternal lymphocyte responses at delivery: No gross changes in MLC and PLT responsiveness. J Reprod Immunol. 1980;2:213–224. doi: 10.1016/0165-0378(80)90027-3. [DOI] [PubMed] [Google Scholar]
- 24.Chun TW. Davey RT., Jr Ostrowski M, et al. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active antiretroviral therapy. Nat Med. 2000;6:757–761. doi: 10.1038/77481. [DOI] [PubMed] [Google Scholar]
- 25.Pantaleo G. Fauci AS. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 26.Fawzi W. Msamanga G. Renjifo B, et al. Predictors of intrauterine and intrapartum transmission of HIV-1 among Tanzanian women. AIDS. 2001;15:1157–1165. doi: 10.1097/00002030-200106150-00011. [DOI] [PubMed] [Google Scholar]
- 27.Mandelbrot L. Mayaux MJ. Bongain A, et al. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: The French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am J Obstet Gynecol. 1996;175:661–667. doi: 10.1053/ob.1996.v175.a75478. [DOI] [PubMed] [Google Scholar]
- 28.Kourtis AP. Bulterys M. Nesheim SR. Lee FK. Understanding the timing of HIV transmission from mother to infant. JAMA. 2001;285:709–712. doi: 10.1001/jama.285.6.709. [DOI] [PubMed] [Google Scholar]
- 29.Zarou DM. Lichtman HC. Hellman LM. The transmission of chromium-51 tagged maternal erythrocytes from mother to fetus. Am J Obstet Gynecol. 1964;88:565–571. doi: 10.1016/0002-9378(64)90881-6. [DOI] [PubMed] [Google Scholar]
- 30.Thompson LF. O'Connor RD. Bastian JF. Phenotype and function of engrafted maternal T cells in patients with severe combined immunodeficiency. J Immunol. 1984;133:2513–2517. [PubMed] [Google Scholar]
- 31.Bastian JF. Williams RA. Ornelas W. Tani P. Thompson LF. Maternal isoimmunisation resulting in combined immunodeficiency and fatal graft-versus-host disease in an infant. Lancet. 1984;1:1435–1437. doi: 10.1016/s0140-6736(84)91932-9. [DOI] [PubMed] [Google Scholar]
- 32.Kaneda T. Shiraki K. Hirano K. Nagata I. Detection of maternofetal transfusion by placental alkaline phosphatase levels. J Pediatr. 1997;130:730–735. doi: 10.1016/s0022-3476(97)80014-5. [DOI] [PubMed] [Google Scholar]
- 33.Lin HH. Kao JH. Hsu HY. Mizokami M. Hirano K. Chen DS. Least microtransfusion from mother to fetus in elective cesarean delivery. Obstet Gynecol. 1996;87:244–248. doi: 10.1016/0029-7844(95)00385-1. [DOI] [PubMed] [Google Scholar]
- 34.Herva E. Jouppila P. Mixed lymphocyte culture reactions between parental cells in pregnancy and puerperium. Acta Pathol Microbiol Scand [C] 1977;85:99–106. doi: 10.1111/j.1699-0463.1977.tb03618.x. [DOI] [PubMed] [Google Scholar]
- 35.Rugeles MT. Shearer GM. Alloantigen recognition in utero: Dual advantage for the fetus? Trends Immunol. 2004;25:348–352. doi: 10.1016/j.it.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 36.MacDonald KS. Embree J. Njenga S, et al. Mother-child class I HLA concordance increases perinatal human immunodeficiency virus type 1 transmission. J Infect Dis. 1998;177:551–556. doi: 10.1086/514243. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y. Tao L. Mitchell E, et al. Allo-immunization elicits CD8+ T cell-derived chemokines, HIV suppressor factors and resistance to HIV infection in women. Nat Med. 1999;5:1004–1009. doi: 10.1038/12440. [DOI] [PubMed] [Google Scholar]
- 38.Fang G. Burger H. Grimson R, et al. Maternal plasma human immunodeficiency virus type 1 RNA level: A determinant and projected threshold for mother-to-child transmission. Proc Natl Acad Sci USA. 1995;92:12100–12104. doi: 10.1073/pnas.92.26.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fauci AS. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]