Abstract
We assessed the utility of the modified Kigali combined (MKC) staging system for predicting survival in HIV-infected Zambian adults in a prospective, longitudinal, open cohort. From 1995 to 2004, HIV-discordant couples (one HIV-infected partner and one HIV-negative partner) were recruited from couples' voluntary counseling and testing centers in Lusaka, Zambia and followed at 3-month intervals. MKC stage, which incorporates clinical stage with erythrocyte sedimentation rate (ESR), hematocrit, and body mass index (BMI), was determined at enrollment. Kaplan–Meier survival and Cox proportional hazard methods were used to calculate median survival and relative hazards. We enrolled 1479 HIV-discordant couples with a combined 7305 person-years of follow-up. Among HIV-infected participants over the 9-year study period, there were 333 confirmed deaths. The time to 50% mortality was 8.5 years with MKC stage 1 and 2 disease compared to 3.7 years with MKC stage 4 disease at enrollment. Survival rates at 3 years were 85% with MKC stage 1 and 2 disease, 74% with MKC stage 3 disease, and 51% with MKC stage 4 disease. A total of 275 HIV-negative partners seroconverted during follow-up. In comparison, survival rates at 3 years were 94% for HIV-negative participants and 92% for participants who seroconverted during follow-up. In multivariate analysis, MKC stage 4 disease (HR = 3.7, 95% CI = 2.7–5.0) remained a strong predictor of mortality. Incorporating ESR, hematocrit, and BMI with clinical staging is a powerful, low-cost tool to identify HIV-infected adults at high risk for mortality.
Introduction
Zambia has a severe HIV-1 epidemic, like much of Southern Africa, with an estimated HIV-1 prevalence rate of 16.5% in the general population.1 Large seroconverter cohort studies in Europe, North America, and Australia have proven essential in understanding the natural history of HIV-1 disease in high-income countries and planning healthcare resources.2,3 Despite the devastating impact of HIV in Southern Africa, few studies have evaluated the natural history of disease progression4–6 and cofactors7,8 influencing survival in HIV-infected adults in this setting. WHO clinical staging, however, has been demonstrated to be an important, independent predictor of mortality,9–13 even in the era of highly active antiretroviral therapy (HAART).14,15 The modified Kigali combined (MKC) staging system was developed as a stronger predictor of mortality than WHO clinical staging alone in Rwandan women.16 MKC staging combines a clinical staging system that is similar to WHO clinical staging but incorporates additional data, including body mass index (BMI), hematocrit, and erythrocyte sedimentation rate (ESR; a marker of systemic inflammation) (see Appendix 1 for full details). Although MKC staging has not been validated in other settings, BMI,14,15 hematocrit,15 and C-reactive protein (another marker of systemic inflammation)17,18 have all been demonstrated to be strong predictors of mortality in the era of HAART and improved access to CD4 cell count testing. To evaluate whether MKC staging remains a strong predictor of mortality in other settings in Africa, we performed a mortality analysis in a large prospective, community-based cohort of HIV-discordant couples in Zambia.
Materials and Methods
The Zambia Emory HIV Research Project (ZEHRP), in collaboration with the Zambian Ministry of Health, established couples voluntary counseling and testing (CVCT) centers in urban districts of Lusaka, a capital city with a population of 1.7 million, beginning in 1995. Details of CVCT methods and participant recruitment have been previously described.19–21 In brief, after providing informed consent HIV serostatus was determined with the rapid Dipstick HIV 1 + 2 antibody screening assay (MacDonald Scientific Ltd, Harare, Zimbabwe) and confirmed with the Capillus HIV-1/2 latex agglutination test (Cambridge Biotech Ltd, Galway, Ireland). From 1995 to 2004 over 18,000 couples received HIV testing at CVCT centers. Approximately 33% of all HIV test results were positive and 20% of all couples had discordant HIV test results (one partner was HIV infected and the other partner was HIV negative). Couples with discordant HIV test results were invited to join a prospective cohort study on methods to reduce rates of heterosexual HIV transmission. All discordant couples were extensively educated about their high risk of HIV transmission and interventions to minimize this risk. Couples were excluded from the cohort if they did not primarily reside in Lusaka, were judged to be mentally incompetent to provide informed consent, or if the female partner was greater than 48 years old and/or the male partner was greater than 65 years old.
All couples who enrolled in the prospective cohort study completed a baseline demographic and past medical history evaluation, physical examination, and laboratory testing for ESR, hematocrit, and total lymphocyte count. Couples returned at 3 month intervals to complete an interval medical history, physical examination, and laboratory testing for ESR and hematocrit. Seronegative partners had HIV testing repeated at each visit. If a couple did not return for their 3-month study visit, a community worker was sent to their home to determine the reason. The clinic provided free out-patient medical care to all participants and the study pharmacy maintained medications recommended by the WHO essential drug list. An insurance policy was provided for hospitalization and specialty outpatient care at Lusaka's major hospital, the University Teaching Hospital. Antiretroviral therapy became available at government clinics in 2004, at which point a referral system was implemented to ensure all eligible patients received an evaluation for antiretroviral therapy.
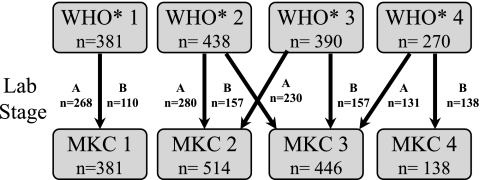
MKC staging combines WHO clinical staging (with modifications such as utilizing the BMI instead of weight loss and classifying all cases of tuberculosis as clinical stage 4 disease; see Appendix 1) with hematocrit and ESR results. Laboratory results are stratified as either stage A (normal) or stage B (an abnormal hematocrit or ESR result). For HIV-infected Zambians, an abnormal hematocrit was defined as a result less than or equal to the sex-specific 25th percentile (male: ≤38%, female: ≤34%) and an abnormal ESR was defined as a result greater than or equal to the sex-specific 75th percentile (male: ≥82 mm/h, female: ≥100 mm/h) in our cohort. An individual's final MKC stage is scored from 1 to 4 based on the combined results of their modified WHO clinical stage and laboratory stage as demonstrated in Fig. 1. As part of a nested case control study to evaluate biological factors associated with an increased risk for HIV transmission, 213 (14%) HIV-infected participants had a CD4+ cell count determined with the TRAx CD4 assay (T Cell Diagnostics, Inc., Cambridge, MA).22
FIG. 1.
Algorithm for modified Kigali combined (MKC) staging with number of HIV-infected participants from this cohort in each stage (WHO, Lab, and MKC) at enrollment. To demonstrate the flow of this algorithm, 390 participants were staged as clinical (WHO*) stage 3 at enrollment. Of these 390 participants, 230 participants were laboratory stage A (normal hematocrit and ESR results) and 157 participants were laboratory stage B (abnormal hematocrit or ESR results). The 230 participants with clinical stage 3 and laboratory stage A were classified as MKC stage 2 and the 157 participants with clinical stage 3 and laboratory stage B were classified as MKC stage 3. WHO*: clinical stage based on WHO staging with modifications described in Appendix 1.
The primary endpoint was mortality. Kaplan-Meier survival methods were used to evaluate median survival. For Kaplan-Meier analysis, survival time was censored on December 31, 2004 for all surviving active participants since HAART became available after this date.15 Survival times were stratified by MKC stage and compared by log-rank tests. Cox proportional hazards regression was employed (SAS version 9.1, SAS Institute, Cary, NC) to identify variables associated with mortality. Variables associated with mortality in univariate analyses with a p-value ≤0.10 were included in a multivariate Cox proportional hazards regression model. We included CD4 cell count results in a separate multivariate Cox proportional hazards regression model for the 213 participants with CD4 cell count results. In these models the lowest risk stratification for mortality was used as the reference against which the highest risk stratification was compared.
Study procedures were approved by the University of Zambia's Research Ethics Committee in Lusaka, the Institutional Review Boards of Emory University, the University of Alabama at Birmingham, and the University of California at San Francisco, and the Office of Protection from Research Risks of the National Institutes of Health, Bethesda, MD. Written informed consent was obtained from all study participants and the human experimentation guidelines of all respective institutions involved in conducting the study were followed.
Results
We enrolled 1479 HIV-discordant couples (750 with an HIV-infected female and 729 with an HIV-infected male) with a combined 7305 person-years of follow-up. HIV-infected participants (n = 1479) had a median follow-up of 21 months [interquartile range (IQR) 11–41 months]. Among HIV-infected participants over the 9-year study period, there were 333 (23% of total HIV-infected participants) confirmed deaths. A total of 442 (30%) HIV-infected participants were still active in the cohort when follow-up was censored on December 31, 2004. An additional 352 (24%) participants had voluntarily withdrawn from the study, 184 (12%) had been lost to follow-up, and 168 participants (11%) were dropped from the overall study due to separation from or death of their HIV-negative partner. Among HIV-negative participants who remained HIV negative during the study (n = 1204), there was a median follow-up of 20 months (IQR: 9–33) and 32 (3%) confirmed deaths. Among HIV-negative participants who seroconverted during the study (n = 275), there was a median follow-up of 26 HIV-infected months (IQR: 12–56) and 24 (9%) confirmed deaths.
All couples were in stable cohabiting relationships with 14% in a legal marriage and 86% in a common law union. Overall baseline demographic features were similar among the cohort of HIV-infected participants and their HIV-negative partners (Table 1). The median enrollment age was 30 years (IQR: 25–36) for both HIV-infected participants and their HIV-negative partners. HIV-infected participants, however, were more likely to report previous hospitalizations, a history of tuberculosis, and chronic diarrhea at study enrollment. HIV-infected participants also had lower baseline median hematocrit and higher median ESR results. HIV-infected women reported a mean of 3.2 previous pregnancies and 100 (13%) of 750 HIV-infected women were pregnant at the time of enrollment.
Table 1.
Baseline Characteristics Comparing HIV-Infected and HIV-Negative Adults (n = 2958)
| Baseline characteristics | HIV-infected adults (n = 1479) | HIV-negative adults (n = 1479) | p-valuea |
|---|---|---|---|
| Female, n (%) | 750 (51%) | 729 (49%) | 0.44 |
| Median age, years (IQRb) | 30 (25–36) | 30 (25–36) | 0.56 |
| Literate (in English), n (%) | 387 (28%) | 432 (31%) | 0.18 |
| Income >$30/month, n (%) | 509 (36%) | 503 (35%) | 0.77 |
| Median BMI, kg/m2 (IQR) | 20.3 (18.8–22.0) | 20.8 (19.1–22.9) | <0.0001 |
| Previous hospitalization (past 5 years), n (%) | 525 (36%) | 345 (24%) | <0.0001 |
| Tuberculosis (past 5 years), n (%) | 135 (9%) | 30 (2%) | <0.0001 |
| Chronic diarrhea at enrollment, n (%) | 91 (6%) | 28 (2%) | <0.0001 |
| Median hematocrit, % (IQR) | 39 (35–44) | 43 (39–46) | <0.0001 |
| Median ESR, mm/h (IQR) | 65 (35–93) | 25 (10–46) | <0.0001 |
Chi-square test (dichotomous variables) and Wilcoxon rank-sum test (continuous variables) used.
IQR, interquartile range.
At enrollment, 895 (61%) HIV-infected participants had either MKC stage 1 or 2 (Fig. 1). A total of 446 (30%) and 138 (9%) HIV-infected participants had MKC stage 3 and 4, respectively. Women (n = 480; 64%) were more likely than men (n = 415; 57%) to be MKC stage 1 or 2 (p < 0.01). Men (n = 91; 13%) were more likely than women (n = 47; 6%) to be MKC stage 4 (p < 0.01).
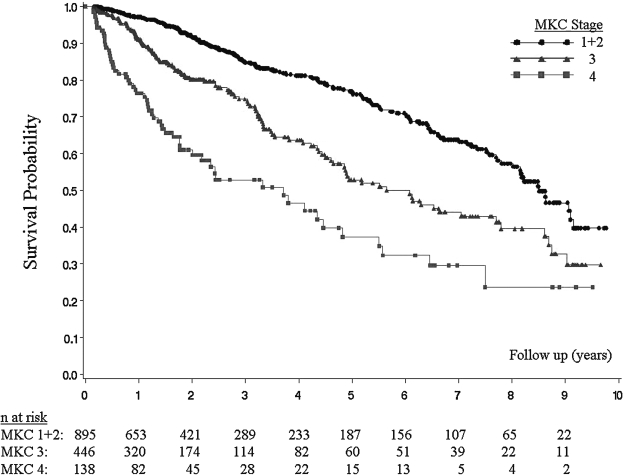
Overall, the median survival time for HIV-infected participants was 7.7 years (IQR: 7.0–8.5). When stratified by baseline MKC stage (Fig. 2), the median survival for HIV-infected participants with MKC stage 1 or 2 disease was 8.5 years (IQR: 8.0–9.1). Participants with MKC stage 3 disease had a median survival of 5.6 years (IQR: 4.7–7.7) (p < 0.0001 compared to MKC 1 or 2 disease by log-rank test). Participants with MKC stage 4 disease had a median survival of 3.7 years (IQR: 2.2–4.8) (p < 0.0001 compared to MKC 1 or 2 disease and MKC 3 disease by log-rank test). Survival rates at 3 years were 85% (±3%) with MKC stage 1 or 2 disease, 74% (±6%) with MKC stage 3 disease, and 51% (±11%) with MKC stage 4 disease. In comparison, survival rates at 3 years were 94% (±2%) for HIV-negative participants and 92% (±4%) for participants who seroconverted during follow-up.
FIG. 2.
Survival among HIV-infected Zambian adults stratified by MKC stage (n = 1479).
In univariate analysis MKC stage 3 and stage 4 disease, clinical stage 3 and stage 4 disease, male gender, and age ≥35 years were predictive of mortality (Table 2). In multivariate analysis, MKC stage 4 disease (HR = 3.7, 95% CI = 2.7–5.0) remained a significant predictor of mortality after adjusting for clinical stage, gender, and age.
Table 2.
Cox Proportional Hazard Ratios (HR) for Mortality among HIV-Infected Zambian Participants (N = 1479)
| n (%) | Univariate HR (95% CI) | p-value | Multivariate HR (95% CI) | p-value | |
|---|---|---|---|---|---|
| MKC stage | |||||
| 1 + 2 | 895 (61%) | Ref. | — | — | — |
| 3 | 446 (30%) | 1.9 (1.5–2.4) | <0.0001 | 2.0 (1.6–2.7) | <0.0001 |
| 4 | 138 (9%) | 3.8 (2.8–5.2) | <0.0001 | 4.7 (2.8–7.9) | <0.0001 |
| Clinical stage | |||||
| 1 + 2 | 819 (56%) | Ref. | — | — | — |
| 3 | 390 (26%) | 1.5 (1.1–1.9) | 0.0028 | 1.3 (1.0–1.7) | 0.07 |
| 4 | 270 (18%) | 2.5 (1.9–3.2%) | <0.0001 | 0.8 (0.5–1.3) | 0.44 |
| Agea (years) | |||||
| ≤24 | 323 (22%) | Ref. | — | — | — |
| 25–29 | 372 (25%) | 1.3 (0.9–1.8) | 0.1362 | — | — |
| 30–34 | 314 (21%) | 1.2 (0.8–1.7) | 0.3612 | — | — |
| ≥35 | 462 (32%) | 2.1 (1.5–2.9) | <0.0001 | 1.7 (1.4–2.2) | <0.0001 |
| Gender | |||||
| Female | 750 (51%) | Ref. | — | — | — |
| Male | 729 (49%) | 1.4 (1.1–1.7) | 0.0026 | 1.1 (0.9–1.4) | 0.27 |
Eight participants were missing age data.
A total of 213 participants (14% of HIV-infected participants) had CD4+ cell count testing performed in a cross-sectional substudy. Participants with CD4+ cell count results were more likely to be male (p = 0.03) and were more likely (p = 0.055) to have MKC 1 or 2 disease (141/213, 66%) compared to participants who did not have CD4+ cell count testing (750/1266, 59%). The median CD4+ cell count was 333 cells/μl (IQR 229–461 cells/μl) and 39 (18%) participants had a CD4+ cell count ≤200 cells/μl. Among these participants with ≤200 cells/μl, 23 had MKC stage 1 or 2 disease, 14 had MKC stage 3 disease, and only 2 had MKC stage 4 disease. Conversely, among participants with MKC stage 1 or 2 disease (n = 141, 66% of the participants with CD4+ cell count results) 63 (45%) had a CD4+ cell count ≥350 cells/μl, 55 (39%) had a CD4+ cell count from 201 to 349 cells/μl, and 23 (16%) had a CD4+ cell count ≤200 cells/μl. In multivariate analysis (Table 3) both MKC stage 4 disease (HR = 2.8, 95% CI = 1.2–6.4) and CD4+ cell count ≤200 cells/μl (HR = 3.8, 95% CI = 2.0–7.1) remained significant predictors of mortality.
Table 3.
Cox Proportional Hazard Ratios (HR) for Mortality among the Subset of HIV-Infected Zambian Participants Who Had CD4 Cell Count Testing Performed (N = 213)
| n (%) | MultivariateaHR (95% CI) | p-value | |
|---|---|---|---|
| MKC stage | |||
| 1 + 2 | 141 (66%) | — | — |
| 3 | 58 (27%) | 1.7 (1.0–2.8) | 0.046 |
| 4 | 14 (7%) | 2.8 (1.2–6.4) | 0.016 |
| CD4+ cell count (cells/μl) | |||
| >350 | 94 (44%) | — | — |
| 201–350 | 80 (38%) | 2.1 (1.2–3.7) | 0.008 |
| ≤200 | 39 (18%) | 3.8 (2.0–7.1) | <0.0001 |
Age and gender were also included in the multivariate model.
Discussion
In this prospective cohort study of HIV-discordant couples in Lusaka, Zambia, MKC staging was a powerful, low-cost tool to predict risk for mortality in HIV-infected adults. Overall, HIV-infected adults with MKC stage 4 disease had a high rate of mortality after 3 years of follow-up compared to HIV-infected participants with MKC stage 1 or 2 disease who had a low rate of mortality. MKC staging also remained an independent predictor of mortality even when controlling for clinical stage and CD4+ cell count.
CD4+ cell count and viral load testing are well established tests3,7,23 that can identify HIV-infected individuals at high risk for mortality, but access to these technologies in many African countries can be limited (although it has increased markedly since 2004). WHO clinical staging is an equally important method to identify HIV-infected individuals at high risk for mortality and provides information that is independently predictive.9–13,24 WHO staging, however, can be hampered in many resource-limited settings as the technology to diagnose stage 4 opportunistic infections is often not available. Furthermore stage 4 symptoms, such as HIV wasting syndrome (defined as weight loss >10% of body weight, plus either unexplained chronic diarrhea or chronic weakness and unexplained prolonged fever), can be prevalent even among HIV-negative African adults.21
In our cohort MKC staging was a stronger predictor of mortality than clinical staging alone. MKC staging incorporates objective measurements of wasting (BMI), anemia, and inflammation (ESR), which have all been independently associated with HIV-related mortality.3,25–28 These measurements are low cost and can be easily performed in a resource-constrained clinical setting. The exact mechanism by which a low BMI, anemia, and an elevated ESR contribute to HIV-related mortality is unknown. Abnormalities in any of these measurements may result from an opportunistic infection or may reflect advanced HIV infection. Regardless of the mechanism, however, access to these low-cost measurements could improve decisions on when to initiate antiretrovirals.
MKC staging also identified HIV-infected adults who were at low risk for short-term (3 year) mortality. Although the 3-year mortality rate with MKC stage 1 or 2 disease was low, it was higher than the observed mortality of HIV-negative participants and HIV-infected participants with recent HIV seroconversion. This difference in mortality rates is explained by the observation that 17% of HIV-infected participants with MKC stage 1 or 2 disease had a CD4 cell count ≤200 cells/μl. Therefore although HIV-infected participants with MKC stage 1 or 2 have a high probability of 3-year survival, there is still a need to increase access to CD4+ cell count testing to identify individuals with mild clinical symptoms but advanced disease.
A limitation of our study was that CD4 cell count data were available for only a subset (14%) of participants. In this subset, however, MKC staging remained an independent predictor of mortality when controlling for CD4+ cell count. We would, therefore, recommend using MKC staging as an adjuvant to CD4+ cell count testing when available because each test provides independent information on risk of mortality. In situations where CD4+ cell count testing is not available we would recommend using MKC staging as a primary method to evaluate prognosis because it is a stronger predictor of mortality than clinical staging alone.
Another limitation of our study was a significant rate of loss to follow-up, which could result in an underestimation of mortality. We minimized underestimations of mortality, however, by having a social worker visit the participant's home after a missed study visit. Missed visits due to hospitalization or illness were subsequently followed up by a clinical officer and any deaths that occurred at home were investigated by a trained nurse who performed a verbal autopsy with the surviving family members.
In conclusion, our study demonstrates the utility of incorporating ESR, hematocrit, and BMI with clinical staging. The MKC staging system remains independently predictive of mortality when controlling for either clinical stage or CD4+ cell count. MKC staging is therefore a powerful, low-cost tool to identify HIV-infected adults at high risk for mortality who require HAART.
Appendix 1
Modifications to the WHO clinical staging:
Modified clinical stage I: Same as WHO clinical stage I.
Modified clinical stage II: Same as WHO clinical stage II, but body mass index between 19 kg/m2 and 21 kg/m2 is substituted for weight loss.
Modified clinical stage III: Same as WHO clinical stage III, but with the following changes: (1) body mass index of 19 kg/m2 or less is substituted for weight loss and (2) oral candidiasis and pulmonary tuberculosis are not included.
Modified clinical stage IV: Same as WHO clinical stage IV, but with the following changes: (1) substitution of body mass index of 19 kg/m2 or less for weight loss, (2) addition of chronic (>1 month) oral or genital ulcer, and (3) addition of oral candidiasis and pulmonary tuberculosis.
Laboratory results were stratified as either stage A (normal) or stage B (an abnormal hematocrit or ESR result). For HIV-infected Zambians in this cohort, an abnormal hematocrit was defined as a result less than or equal to the sex-specific 25th percentile (male: ≤38%, female: ≤34%) and an abnormal ESR was defined as a result greater than or equal to the sex-specific 75th percentile (male: ≥82 mm/h, female: ≥100 mm/h).
Acknowledgments
The investigators would like to thank all of the couples who participated, all of the staff at the Zambia-Emory HIV Research Project (ZEHRP), and the project management group who made this study possible. Data were presented in abstract form (#30) at the 13th Conference on Retroviruses and Opportunistic Infections, Denver, CO, February 5–8, 2006. This study has been funded in whole or in part with federal funds from the U.S. National Institutes of Health under Grants RO1 HD 40125, RO1 MH 66767, RO1 AI23980, 40951, and 51231, the Fogarty AIDS International Training and Research Program (AITRP) FIC 2D43 TW001042, Emory Center for AIDS Research (CFAR) P30 AI050409, and the International AIDS Vaccine Initiative (IAVI).
References
- 1.UNAIDS. Epidemiological Fact Sheets on HIV/AIDS, Sexually Transmitted Infections: Zambia, 2004 Update. http://data.unaids.org/Publications/Fact-Sheets01/zambia_EN.pdf. [Aug 20;2007 ]. http://data.unaids.org/Publications/Fact-Sheets01/zambia_EN.pdf
- 2.Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: A collaborative re-analysis. Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet. 2000;355:1131–1137. [PubMed] [Google Scholar]
- 3.Lyles RH. Munoz A. Yamashita TE, et al. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis. 2000;181:872–880. doi: 10.1086/315339. [DOI] [PubMed] [Google Scholar]
- 4.Morgan D. Mahe C. Mayanja B. Okongo JM. Lubega R. Whitworth JA. HIV-1 infection in rural Africa: Is there a difference in median time to AIDS and survival compared with that in industrialized countries? AIDS. 2002;16:597–603. doi: 10.1097/00002030-200203080-00011. [DOI] [PubMed] [Google Scholar]
- 5.Leroy V. Four year natural history. J Acquir Immune Defic Syndr. 1995;9:415–421. [PubMed] [Google Scholar]
- 6.Crampin AC. Long-term follow-up of HIV-positive and HIV-negative individuals in rural Malawi. AIDS. 2002;16:1545–1550. doi: 10.1097/00002030-200207260-00012. [DOI] [PubMed] [Google Scholar]
- 7.Lavreys L. Baeten JM. Chohan V, et al. Higher set point plasma viral load and more-severe acute HIV type 1 (HIV-1) illness predict mortality among high-risk HIV-1-infected African women. Clin Infect Dis. 2006;42:1333–1339. doi: 10.1086/503258. [DOI] [PubMed] [Google Scholar]
- 8.Whittle H. Clinical and laboratory predictors of survival in Gambian patients with symptomatic HIV-1 or HIV-2 infection. AIDS. 1992;6:685–689. doi: 10.1097/00002030-199207000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Kaleebu P. French N. Mahe C, et al. Effect of human immunodeficiency virus (HIV) type 1 envelope subtypes A and D on disease progression in a large cohort of HIV-1-positive persons in Uganda. J Infect Dis. 2002;185:1244–1250. doi: 10.1086/340130. [DOI] [PubMed] [Google Scholar]
- 10.Malamba SS. Morgan D. Clayton T. Mayanja B. Okongo M. Whitworth J. The prognostic value of the World Health Organization staging system for HIV infection and disease in rural Uganda. AIDS. 1999;13:2555–2562. doi: 10.1097/00002030-199912240-00009. [DOI] [PubMed] [Google Scholar]
- 11.Teck R. Ascurra O. Gomani P, et al. WHO clinical staging of HIV infection and disease, tuberculosis and eligibility for antiretroviral treatment: Relationship to CD4 lymphocyte counts. Int J Tuberc Lung Dis. 2005;9:258–262. [PubMed] [Google Scholar]
- 12.Zachariah R. Teck R. Ascurra O. Humblet P. Harries AD. Targeting CD4 testing to a clinical subgroup of patients could limit unnecessary CD4 measurements, premature antiretroviral treatment and costs in Thyolo District, Malawi. Trans R Soc Trop Med Hyg. 2006;100:24–31. doi: 10.1016/j.trstmh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Diomande FV. Bissagnene E. Nkengasong JN, et al. The most efficient use of resources to identify those in need of antiretroviral treatment in Africa: Empirical data from Cote d'Ivoire's Drug Access Initiative. AIDS. 2003;17(Suppl. 3):S87–93. doi: 10.1097/00002030-200317003-00012. [DOI] [PubMed] [Google Scholar]
- 14.Severe P. Leger P. Charles M, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353:2325–2334. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 15.Stringer JS. Zulu I. Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: Feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 16.Lifson AR. Allen S. Wolf W, et al. Classification of HIV infection and disease in women from Rwanda. Evaluation of the World Health Organization HIV staging system and recommended modifications. Ann Intern Med. 1995;122:262–270. doi: 10.7326/0003-4819-122-4-199502150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Lau B. Sharrett AR. Kingsley LA, et al. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006;166:64–70. doi: 10.1001/archinte.166.1.64. [DOI] [PubMed] [Google Scholar]
- 18.Drain PK. Kupka R. Msamanga GI. Urassa W. Mugusi F. Fawzi WW. C-reactive protein independently predicts HIV-related outcomes among women and children in a resource-poor setting. AIDS. 2007;21:2067–2075. doi: 10.1097/QAD.0b013e32826fb6c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenna SL. Muyinda GK. Roth D, et al. Rapid HIV testing and counseling for voluntary testing centers in Africa. AIDS. 1997;11(Suppl. 1):S103–110. [PubMed] [Google Scholar]
- 20.Fideli US. Allen SA. Musonda R, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses. 2001;17:901–910. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modjarrad K. Zulu I. Karita E. Kancheya N. Funkhouser E. Allen S. Predictors of HIV serostatus among HIV discordant couples in Lusaka, Zambia and female antenatal clinic attendants in Kigali, Rwanda. AIDS Res Hum Retroviruses. 2005;21:5–12. doi: 10.1089/aid.2005.21.5. [DOI] [PubMed] [Google Scholar]
- 22.Paxton H. Pins M. Denton G. McGonigle AD. Meisner PS. Phair JP. Comparison of CD4 cell count by a simple enzyme-linked immunosorbent assay using the TRAx CD4 test kit and by flow cytometry and hematology. Clin Diagn Lab Immunol. 1995;2:104–114. doi: 10.1128/cdli.2.1.104-114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anastos K. Kalish LA. Hessol N, et al. The relative value of CD4 cell count and quantitative HIV-1 RNA in predicting survival in HIV-1-infected women: Results of the women's interagency HIV study. AIDS. 1999;13:1717–1726. doi: 10.1097/00002030-199909100-00016. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance: African region. World Health Organization; Geneva, Switzerland: 2005. [Google Scholar]
- 25.Moss AR. Bacchetti P. Osmond D, et al. Seropositivity for HIV and the development of AIDS or AIDS related condition: Three year follow up of the San Francisco General Hospital cohort. Br Med J (Clin Res Ed) 1988;296:745–750. doi: 10.1136/bmj.296.6624.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefrere JJ. Salmon D. Doinel C, et al. Sedimentation rate as a predictive marker in HIV infection. AIDS. 1988;2:63–64. [PubMed] [Google Scholar]
- 27.Schwartlander B. Bek B. Skarabis H. Koch J. Burkowitz J. Koch MA. Improvement of the predictive value of CD4+ lymphocyte count by beta 2-microglobulin, immunoglobulin A and erythrocyte sedimentation rate. The Multicentre Cohort Study Group. AIDS. 1993;7:813–821. [PubMed] [Google Scholar]
- 28.Lindan CP. Allen S. Serufilira A, et al. Predictors of mortality among HIV-infected women in Kigali, Rwanda. Ann Intern Med. 1992;116:320–328. doi: 10.7326/0003-4819-116-4-320. [DOI] [PubMed] [Google Scholar]