Abstract
Although nitric oxide (NO) delivery systems have been fabricated with sol−gel-based materials, remote control of such systems with light has not been achieved. In this work, a fiber optic-based NO delivery system is described in which the photoactive metal-nitrosyl, [Mn(PaPy3)(NO)]ClO4 (1), has been employed in a sol−gel material. The material (1·FO) contains the manganese-nitrosyl, which releases NO upon illumination with visible light. The NO-releasing capacity of 1·FO has been measured with an NO-sensitive electrode, and the spatial diffusion of NO in solution has been visualized using the Griess reaction. The utility of 1·FO has been demonstrated in effective reduction of bacterial loads of Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and methicillin-resistant S. aureus. The results suggest that a device that releases NO via illumination by optical fiber may have clinical applications in combating infections with both Gram-positive, Gram-negative, and to some degree antibiotic-resistant bacteria.
Keywords: Nitric oxide, fiber optic-based device, photoactive metal nitrosyl, bacterial infection, antibiotic
Nitric oxide (NO) plays an important role in the immune response to bacterial infection.1 The invasion of pathogens leads to activation of NO synthase in host cells at the infected locale. The elevated levels of NO along with other reactive oxygen species (ROS) promote the oxidation of lipids, deactivation of enzymes, and deamination of DNA leading to bactericidal effects. Enhancement of this innate/nonspecific immune response by delivery of exogenous NO would be, in some cases, preferable to the systemic administration of antibiotics, as the emergence of antibiotic-resistant strains of bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), is of great concern.2,3 Resistant bacteria are especially prevalent in the intensive care unit.4−6 Recent studies indicate that, in the United States, nearly 60% of hospital-acquired S. aureus isolates are methicillin-resistant.5 Antibiotic-resistant Gram-negative bacteria, including Pseudomonas aeruginosa and Escherichia coli, are of increasing concern. Specifically, S. aureus, P. aeruginosa, and E. coli are opportunistic pathogens found commonly in infections of the lungs4−6 and urinary tract.7,8
Instances where NO delivery may be preferable to traditional antibiotic treatment may include the treatment of urinary tract infections (UTI) and ventilator-associated pneumonia, where the infected site is accessible to a catheter or endoscope. Design and assembly of a suitable endoscope could deliver high doses of NO to selectively treat the infected area and avoid overuse of antibiotics and concomitant emergence of antibiotic-resistant strains of bacteria in many of these cases.
Ghaffari et al. have successfully applied NO gas in antibiotic studies against a number of bacteria and yeast.9,10 In their investigations, exposure to 200 ppm NO gas for approximately 4 h resulted in complete bacterial death for S. aureus, MRSA, E. coli, group B Streptococcus, P. aeruginosa, and the yeast Candida albicans. These experiments were conducted within a specialized chamber for applications in the improvement of wound healing. Toward the goal of reducing implant-associated infection/fouling, Schoenfish and co-workers have prepared a variety of materials incorporating diazeniumdiolate NO donors.11−14 Bacterial adhesion to these materials was greatly reduced, and in a recent study, Schoenfish et al. were able to decrease bacterial survival by ≥99% by treating biofilms of P. aeruginosa, E. coli, S. aureus, Staphylococcus epidermidis, and C. albicans with NO-releasing silica nanoparticles.11 Additionally, the degradation of the cell walls of P. aeruginosa and E. coli in response to NO of varying concentrations and exposure times was investigated by atomic force microscopy.13 This study demonstrated that these bacteria were more damaged by short bursts of NO at a high concentration than longer, lower doses.
In previous work, we reported the light-sensitive NO donor, [Mn(PaPy3)(NO)]ClO4 [1, PaPy3H = N,N-bis(2-pyridylmethyl) amine-N-ethyl-2-pyridine-2-carboxamide],15 which has been successfully employed to deliver NO to biological targets such as cytochrome c oxidase,16 papain (a cysteine protease),17 and soluble guanylate cyclase.18 Scheme 1 illustrates light-induced release of NO from the cation of 1 followed by binding of a water (solvent) molecule to the coordination site on Mn, vacated by NO. In a later account, a hybrid material, 1·HM, in which 1 had been sealed within a sol−gel matrix with a polyurethane coating, was also reported.19 This material was then used to deliver NO to the heme of the protein myoglobin (Mb) in vitro. In addition, spatial release of NO was controlled by using a photomask to shield areas where NO release was not desired. A poly(2-hydroxyethyl methacrylate)-based material was later employed in antibiotic studies, where the photoreleased NO from 1, in combination with singlet oxygen or hydrogen peroxide, demonstrated bactericidal effects against the Gram-negative bacteria P. aeruginosa and E. coli.20 Herein, we report the fabrication of a prototype fiber optic NO delivery system based on 1·HM and demonstrate its utility in remote-controlled, localized delivery of NO and the results of antibiotic studies in the absence of other growth attenuators.
Scheme 1. Scheme of Light-Induced NO Release from [Mn(PaPy3)(NO)]ClO4 (1) (Top) and Photograph of 1·FO before (Left, Green Tip) and after (Right, Yellow Tip) Illumination (and NO Release) via the Optical Fiber (Bottom).
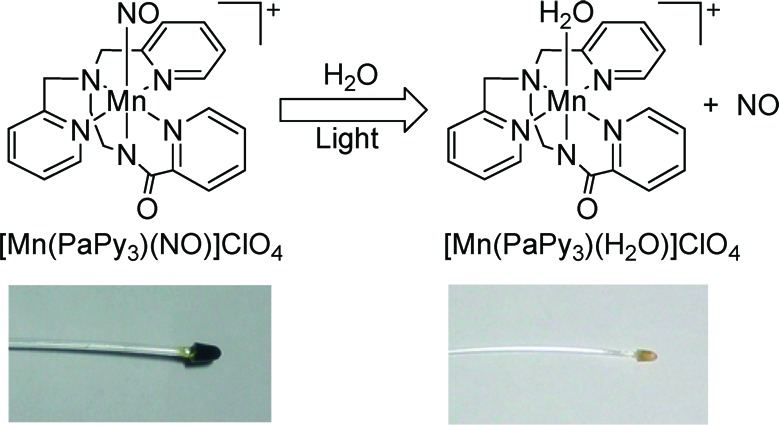
The NO delivery prototype units (1·FO) were made from 1·HM and poly(methyl methacrylate) optical fiber (South Coast Fiber Optic, Inc.). One end of 40 cm long pieces of the optical fiber (1.5 mm core diameter) was manually polished into cones 2.5 mm in height. The cone-tipped ends were then equipped with 1·HM by casting the sol−gel in eppendorf tubes into which the fibers had been positioned. Following unmolding, the modified fiber tips were coated with polyurethane (Tecoflex SG-80) in THF. The monoliths of 1·HM at the tips of 1·FO measured 4 mm in diameter and 5 mm in length (Scheme 1, bottom left). Before use, 1·FO was rinsed copiously with 100% ethanol followed by sterile-filtered Milli-Q-purified water.
The release of micromolar concentrations of NO from 1·FO was quantitatively monitored using an NO sensitive electrode (Figure 1a). The concentrations of NO released from 1·FO increased with illumination time. Illumination for 30 s resulted in a peak NO concentration of 1.5 μM, while an illumination for 60 s resulted in a peak NO concentration of 5 μM. Sustained illumination (5 min) gave a relatively stable concentration of NO around ∼4.5 μM (Figure S1 of the Supporting Information).
Figure 1.
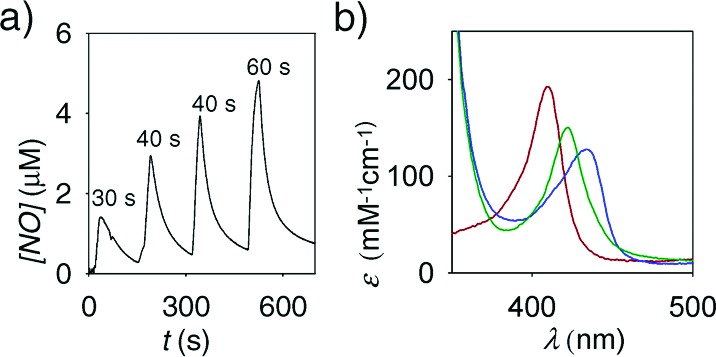
(a) Amperogram of NO release from 1·FO in response to pulses of light (time denoted in s). (b) Absorbance spectra of Mb (red line), reduced Mb (FeII, blue line), and Mb−NO adduct (FeII−NO, green line) following NO capture.
NO delivery to Mb in vitro was monitored by absorbance spectroscopy. The addition of dithionite to a 1.3 μM solution of Mb (Soret band, λmax = 410 nm) resulted in a spectral change corresponding to the reduction of Mb (λmax = 432 nm).21 Over a 25 min illumination time (∼1 nmol) of NO was transferred to the FeII of the heme of Mb (λmax = 420 nm, ε = 147 cm−1 mM−1). The results indicate that the fiber optic delivery system may be useful in studies/applications where low micromolar concentrations of NO are desired at specific locations. In such delivery, complications arising from the presence of the photoproducts are obviated by their effective confinement within the polymer tip.
We employed the Griess assay to investigate the fate of the NO released from 1·FO to solution under aerobic conditions. The modified end of 1·FO was pictured in a 3 mL sample of Greiss reagents (Figure 2a). The released NO was oxidized to NO2−, which then reacted with sulfanilic acid, the product of which upon further reaction formed a pink azo dye (λmax = 548 nm). This color was apparent after 4 min of illumination of 1·FO via the optical fiber, indicating an apparent 1−2 min lifetime of the photoreleased NO in the acidic solution (Figure 2b). In the absence of stirring, the appearance of coloration at distances removed from the tip of 1·FO indicated that NO diffused through the aerobic solution before oxidation to NO2− and reaction with the Griess reagents. Upon further illumination (10 min total), the pink color deepened (Figure 2c,d) and only distributed upon stirring (Figure 2f).
Figure 2.
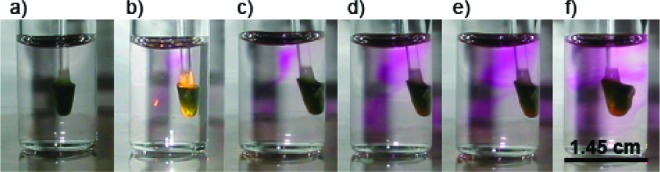
(a) Fiber optic NO delivery system (1·FO) in a solution of Griess reagents before illumination. (b) Following 4 min of illumination, the azo dye (pink) appeared upon reaction of Griess reagents with NO2− (oxidized NO). The pink color intensifies upon illumination for (c) 5, (d) 9, and (e) 10 min. (f) Distribution of the azo dye after using 1·FO to stir the solution.
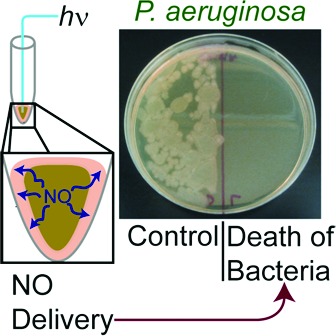
Finally, the antibiotic efficacy of photoreleased NO was qualitatively investigated against P. aeruginosa, E. coli, S. aureus, and MRSA. The tip of 1·FO was placed in 10 μL samples of bacterial culture as shown in Figure 3. Cultures with 105 colony-forming units per mL were used, as this concentration of bacteria in urine represents the putative lower limit in the diagnosis of UTI.22 Light guided through 1·FO caused release of NO from the tip as discussed previously. Following illumination (Electrore Optics Corp. IL 410 Illumination System; light power at the tip, 0.6 mW cm−2), the cultures were plated side by side with control cultures for direct comparison. The control cultures were maintained in the presence of 1·FO (but no light).
Figure 3.
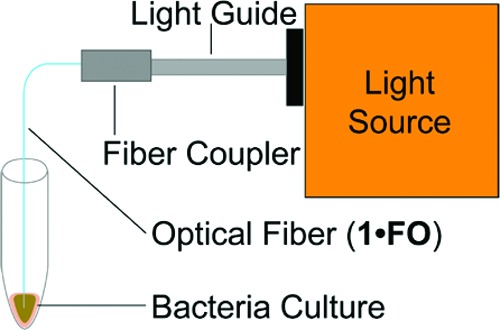
Experimental setup for the antibiotic experiments.
The results shown in Figure 4 demonstrate that 10 min of exposure to NO completely eradicates P. aeruginosa (Figure 4a, right). The corresponding culture maintained with 1·FO in the absence of illumination via the fiber grew to near confluence (Figure 4a, left). In the case of E. coli, the bacterial load was greatly reduced (Figure 4b, right) as compared to the control (Figure 4b, left) following 5 min of illumination with 1·FO. Similarly, dark control cultures of S. aureus maintained in the presence of 1·FO without illumination, when plated, produced multitudinous small, white colonies (Figure 4c, left). In contrast, the survival of S. aureus exposed to NO for 10 min via 1·FO was greatly diminished (Figure 4c, right). Along these lines, even modest reduction in MRSA survival was observed after 10 min of exposure to NO (Figure 4d). We anticipate that effective eradication of MRSA could be achieved by increasing the loading of 1 at the tip of the endoscope and/or the illumination time. That light alone did not cause the bacterial death has been checked via exposure of samples to light from the fiber optic line with no 1 at the tip. In each case, light had a minimal effect on the colony-forming ability of the light-exposed microorganism.
Figure 4.
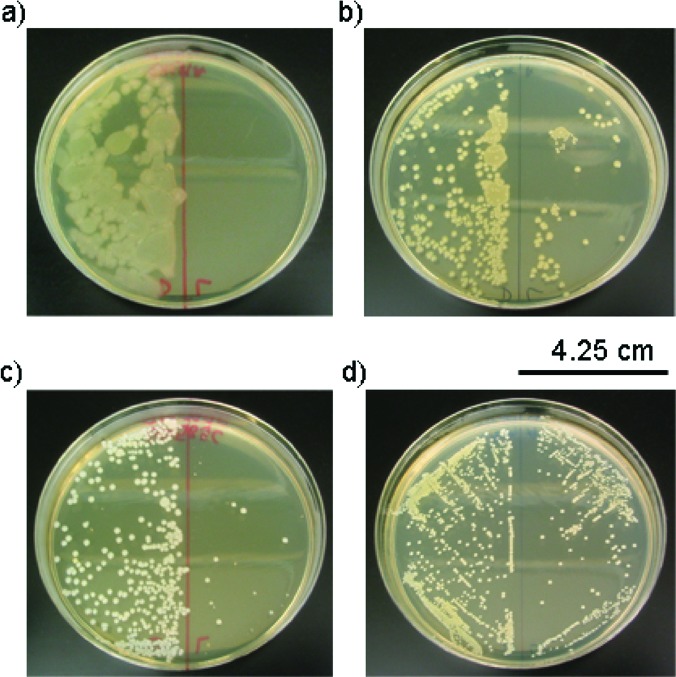
(a) Left, P. aeruginosa incubated with 1·FO 10 min. Right, P. aeruginosa incubated with 1·FO with illumination for 10 min. (b) Left, E. coli incubated with 1·FO 5 min. Right, E. coli incubated with 1·FO and illumination for 5 min. (c) Left, S. aureus incubated with 1·FO for 10 min. Right, S. aureus incubated with 1·FO and illumination for 10 min. (d) Left, MRSA incubated with 1·FO for 10 min. Right, MRSA incubated with 1·FO and illumination for 10 min.
In conclusion, the results of the present work suggest that an endoscopic device that releases NO from its tip via illumination through an optical fiber may have clinical applications in reducing the bacterial load in the treatment of infection with both Gram-positive, Gram-negative, and to some degree antibiotic-resistant bacteria. Advantages of this technique are described as follows: (1) This method avoids the problems associated with using NO, a toxic gas; (2) following delivery of NO, all of the photoproducts are withdrawn from the infected site, and as a result, no side effects arising from the photoproducts should be evident; and finally, (3) this mode of NO delivery can be used in body cavities accessible to such a device. In a recent study, Av-Gay and co-workers have demonstrated that release of high doses (5 mM to 166 μM) of gaseous NO from NO-impregnated Foley urinary catheters over long periods of time prevent bacterial colonization and biofilm formation on their surfaces as well as inhibit bacterial growth of E. coli within the surrounding media.23 In the present work, much lower doses of NO (∼5 μM), delivered from a sol−gel matrix via light triggering, have resulted in drastic reduction of various bacterial loads. Because normal human cells are quite tolerant of such low levels of NO,9,10 the present endoscopic delivery of NO deserves further attention.
Experimental Procedures
The synthesis of [Mn(PaPy3)(NO)]ClO4 (1) was carried out following previously published procedures.15 Optical fibers [poly(methyl methacrylate, 1.5 mm core diameter, South Coast Fiber Optics, Inc.] were cut into 40 cm segments and manually polished into cones (extending 2.5 mm) at one end using aluminum oxide fiber polishing film (Fiber Network Tools). The sol was prepared by stirring 750 μL of tetramethyl orthosilicate with 400 μL of Milli-Q water until homogeneous and then an additional hour (∼2 h total). The sol (25 μL) was delivered to each 500 μL eppendorf tube into which 2 mg of 1 had previously been weighed. The cone ends of the optical fibers were forced through tight holes pierced in the cap of the eppendorf tubes and agitated to suspend 1. The tip of the fiber extended down to approximately 0.75 mm from the bottom of the eppendorf tube. The fiber was stabilized in the center of the eppendorf tube using a plug of adhesive putty. The sol−gels were cured for 12 h before unmolding. The monoliths were then twice coated in Tecoflex SG-80 (Lubrizol Advanced Materials, Inc., 1 g in 10 mL of THF) 0.5 mm up the optical fiber, drying in a stream of nitrogen between coats. The finished monoliths were dark green cones 4 mm in diameter and 5 mm in height. Control fibers were fabricated by the same method except with the omission of 1. Before use, each was rinsed copiously with ethanol and then Milli-Q purified water. Bacteria (E. coli ATCC 25922, P. aeruginosa ATCC 27853, S. aureus ATCC 25923, and MRSA ATCC BAA-44) were maintained according to standard procedures. Bacteria were diluted to 105 colony-forming units mL−1 in trypticase soy broth and allocated into 10 μL samples. Samples were then incubated for 5 and 10 min in the presence of 1·FO in the dark or with illumination (visible light, 0.6 mW cm−2). Each experiment was replicated (a minimum of three times), and control experiments were conducted with control fibers fabricated without 1 (to check the effects of light only). The samples were then plated onto trypticase soy agar and incubated for 16 h. Bacterial survival in each sample was assessed by comparing the area of colonies that grew versus the dark control.
Supporting Information Available
Sustained release of NO from the tip of 1·FO upon illumination (visible light) for 5 min (Figure S1). This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by the National Science Foundation (CHE-0553405) and National Institute of Health (GM-61636).
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- DeGroote M. A.; Fang F. C.. Antimicrobial Properties of Nitric Oxide. In Nitric Oxide and Infection; Fang F. C., Ed.; Kluwer Academic/Plenum Publishers: New York, 1999; pp 231−261. [Google Scholar]
- Barrett C. T.; Barrett J. F. Antibacterials: Are the New Entries Enough to Deal with the Emerging Resistance Problems?. Curr. Opin. Biotechnol. 2003, 14, 621–626. [DOI] [PubMed] [Google Scholar]
- Wenzel R. P.; Bearman G.; Edmond M. B. Community-Acquired Methicillin-Resistant Staphylococcus aureus (MRSA): New Issues for Infection Control. Int. J. Antimicrob. Agents 2007, 30, 210–212. [DOI] [PubMed] [Google Scholar]
- Bearman G. M. L.; Munro C.; Sessler C. N.; Wenzel R. P. Infection Control and the Prevention of Nosocomial Infections in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2006, 27, 310–324. [DOI] [PubMed] [Google Scholar]
- Chastre J. Evolving Problems with Resistant Pathogens. CMI 2008, 14Suppl. 33–14. [DOI] [PubMed] [Google Scholar]
- Bodmann K. F. Current Guidelines for the Treatment of Severe Pneumonia and Sepsis. Chemotherapy 2005, 51, 227–233. [DOI] [PubMed] [Google Scholar]
- Carson C.; Naber K. G. Role of Fluoroquinolones in the Treatment of Serious Bacterial Urinary Tract Infections. Drugs 2004, 64, 1359–1373. [DOI] [PubMed] [Google Scholar]
- Wagenlehner F. M. E.; Naber K. G. Current Challenges in the Treatment of Complicated Urinary Tract Infections and Prostatitis. Clin. Microbiol. Infect. 2006, 12, 67–80. [DOI] [PubMed] [Google Scholar]
- Ghaffari A.; Neil D. H.; Ardakani A.; Road J.; Ghahary A.; Miller C. C. A Direct Nitric Oxide Gas Delivery System for Bacterial and Mammalian Cell Cultures. Nitric Oxide 2005, 12, 129–140. [DOI] [PubMed] [Google Scholar]
- Ghaffari A.; Miller C. C.; McMullin B.; Ghahary A. Potential Application of Gaseous Nitric Oxide as a Topical Antimicrobial Agent. Nitric Oxide 2006, 14, 21–29. [DOI] [PubMed] [Google Scholar]
- Hetrick E. M.; Schoenfisch M. H. Reducing Implant-Related Infections: Active Release Strategies. Chem. Soc. Rev. 2006, 35, 780–789. [DOI] [PubMed] [Google Scholar]
- Shin J. H.; Schoenfisch M. E. Improving the Biocompatibility of in vivo Sensors via Nitric Oxide Release. Analyst 2006, 131, 609–615. [DOI] [PubMed] [Google Scholar]
- Hetrick E. M.; Shin J. H.; Paul H. S.; Schoenfisch M. H. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 2009, 30, 2782–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deupree S. M.; Schoenfisch M. H. Morphological Analysis of the Antimicrobial Action of Nitric Oxide on Gram-Negative Pathogens Using Atomic Force Microscopy. Acta Biomater. 2009, 5, 1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K.; Eroy-Reveles A. A.; Avila B.; Holman T. R.; Olmstead M. M.; Mascharak P. K. Reactions of NO with Mn(II) and Mn(III) Centers Coordinated to Carboxamido Nitrogen: Synthesis of a Manganese Nitrosyl with Photolabile NO. Inorg. Chem. 2004, 43, 2988–2997. [DOI] [PubMed] [Google Scholar]
- Szundi I.; Rose M. J.; Sen I.; Eroy-Reveles A. A.; Mascharak P. K.; Einarsdóttir Ó. A New Approach for Studying Fast Biological Reactions Involving Nitric Oxide: Generation of NO Using Photolabile Ruthenium and Manganese NO Donors. Photochem. Photobiol. 2006, 82, 1377–1384. [DOI] [PubMed] [Google Scholar]
- Afshar R. K.; Patra A. K.; Mascharak P. K. Light-Induced Inhibition of papain by a {Mn-NO}6 Nitrosyl: Identification of Papain-SNO Adduct by Mass Spectrometry. J. Inorg. Biochem. 2005, 99, 1458–1464. [DOI] [PubMed] [Google Scholar]
- Madhani M.; Patra A. K.; Miller T. W.; Eroy-Reveles A. A.; Hobbs A. J.; Fukuto J. M.; Mascharak P. K. Biological Activity of Designed Photolabile Metal Nitrosyls: Light-Dependent Activation of Soluble Guanylate Cyclase and Vasorelaxant Properties in Rat Aorta. J. Med. Chem. 2006, 49, 7325–7330. [DOI] [PubMed] [Google Scholar]
- Eroy-Reveles A. A.; Leung Y.; Mascharak P. K. Release of Nitric Oxide from a Sol-Gel Hybrid Material Containing a Photoactive Manganese Nitrosyl upon Illumination with Visible Light. J. Am. Chem. Soc. 2006, 128, 7166–7167. [DOI] [PubMed] [Google Scholar]
- Halpenny G. M.; Steinhardt R. C.; Okialda K. A.; Mascharak P. K. Characterization of pHEMA-Based Hydrogels that Exhibit Light-Induced Bactericidal Effect via Release of NO. J. Mater. Sci:. Mater. Med. 2009, 20, 2353–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romberg R. W.; Kassner R. J. Nitric Oxide and Carbon Monoxide Equilibria of Horse Myoglobin and (N-Methylinidazole)protoheme. Evidence for Steric Interaction with the Distal Residues. Biochemistry 1979, 18, 5387–5392. [DOI] [PubMed] [Google Scholar]
- Franz M.; Hörl W. H. Common Errors in Diagnosis and Management of Urinary Tract Infection. I: Pathophysiology and Diagnostic Techniques. Nephrol., Dial., Transplant. 1999, 14, 2746–2753. [DOI] [PubMed] [Google Scholar]
- Regev-Shoshani G.; Ko M; Miller C.; Av-Gay Y. Slow Release of Nitric Oxide from Charged Catheters and Its Effect on Biofilm Formation by Escherichia coli. Antimicrob. Agents Chemother. 2010, 54, 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


