Abstract
The role of systemic and local insulin-like growth factor I (IGF-I) in the development of prostate cancer is still controversial. Transgenic adenocarcinoma mouse prostate (TRAMP) mice express the SV40 T-antigen under the control of the probasin promoter, and spontaneously develop prostate cancer. We crossed TRAMP mice with liver IGF–deficient (LID) mice to produce LID-TRAMP mice, a mouse model of prostate cancer with low serum IGF-I, to allow us to study the effect of circulatory IGF-I levels on the development of prostate cancer. LID mice have a targeted deletion of the hepatic Igf1 gene but retain normal expression of Igf1 in extrahepatic tissues. Serum IGF-I and IGFBP-3 levels in LID and LID-TRAMP mice were measured using novel assays, which showed that they are ~10% and 60% of control L/L− mice, respectively. Serum growth hormone (GH) levels of LID-TRAMP mice were 3.5-fold elevated relative to L/L-TRAMP mice (P < 0.001), but IGFBP-2 levels were not different. Surprisingly, rates of survival, metastasis, and the ratio of genitourinary tissue weight to body weight were not significantly different between LID-TRAMP and L/L-TRAMP mice. There was also no difference in the pathologic stage of the prostate cancer between the two groups at 9 to 19 weeks of age. LID-TRAMP tumors displayed increased levels of GH receptors and increased Akt phosphorylation. These results are in striking contrast with the published model of the GH-deficient lit/lit-TRAMP, which has smaller tumors and improved survival, and indicate that the reduction in systemic IGF-I is not sufficient to inhibit prostate cancer tumor progression in the TRAMP model, which may require a reduction of GH levels as well.
Introduction
There is controversy as to whether serum insulin-like growth factor I (IGF-I) has an important role in the development of prostate cancer. IGF-I is well-described as a growth and survival factor for cultured tumor cells, including prostate cancer. Chan et al. reported that men in the Harvard Physician Health Study whose serum IGF-I levels were in the upper quartile of the reference range had a 4-fold increased risk of developing prostate cancer in subsequent years compared with those whose serum IGF-I levels were in the lower quartile (1). There are multiple reports that support the hypothesis that high serum IGF-I levels predisposes to prostate cancer (2–4). However, Severi et al. reported that prediagnostic circulating levels of IGF-I were not associated with the risk of prostate cancer (5). Other investigators similarly found no association between circulating levels of IGF-I and the risk of prostate cancer (6–12).
The TRAMP (transgenic adenocarcinoma mouse prostate) mouse is an established murine model of prostate cancer (13). We previously showed that serum IGF-I levels and prostate-specific Igf1 mRNA expression are increased during prostate cancer progression in TRAMP mice compared with nontransgenic controls (14). When TRAMP mice were crossed with lit/lit mice, which are homozygous for a mutated GHRH receptor gene and have very low levels of both serum growth hormone (GH) and IGF-I, these mice exhibited dramatically delayed progression of prostate cancer (15).
Liver IGF–deficient (LID) mice have been engineered to carry a liver-specific Igf1 gene deletion, but retain normal Igf1 expression in other tissues (16). Serum IGF-I levels of LID mice were reported to be one-fourth of those of L/L− controls (mice homozygous for loxP-flanked Igf1 gene lacking the cre-recombinase transgene; ref. 16). Because of the lack of negative feedback by IGF-I, serum GH levels of LID mice were reported to be 4-fold higher than those of L/L− controls (17).
It has been shown that the growth of certain cancers is impaired in LID mice. In a chemically induced mammary carcinoma model, tumor onset was 74 days in LID mice compared with 60 days in controls (18). In LID mice crossed with C3(1)/SV40 large T-antigen transgenic mice, the mean age of onset of mammary tumors was 30 weeks compared with 22 weeks in controls (18). In another study, colon 38 (mouse adenocarcinoma) tumors implanted in the cecum reached 1.2 g in LID mice and 1.6 g in L/L− littermates (19).
To elucidate the role of circulatory IGF-I in the development of prostate cancer, we crossed TRAMP and LID mice to produce LID-TRAMP, a murine model of prostate cancer with very low circulatory IGF-I. Contrary to previous studies which targeted GH expression in TRAMP mice indirectly leading to IGF-reduction (15, 20), we found no significant difference in survival, tumor growth, and metastasis between L/L-TRAMP (TRAMP mice homozygous for loxP-flanked Igf1 gene lacking the cre-recombinase transgene) and LID-TRAMP mice. These results suggest the possibility that either GH-induced, locally produced autocrine-paracrine IGF-I compensates for reduced systemic IGF, or that in addition to IGF-I, the direct actions of GH might also be important for the development of prostate cancer in the TRAMP model.
Materials and Methods
Transgenic mice
TRAMP mice (129S1/Sv and C57BL/6 background) and LID mice (FVB/N and C57BL/6 background) were produced as previously described (13, 16). TRAMP mice were purchased from Jackson Laboratory. Both TRAMP and LID mice were maintained in a C57BL/6 background. They were fed standard lab chow and maintained on a 12 h light/12 h dark cycle in the animal facility at University of California at Los Angeles (UCLA). Female TRAMP mice were crossed with male LID mice to produce an F1 generation heterozygous for a loxP-flanked Igf1 gene carrying both SV40 small and large T antigen driven by the rat probasin promoter (PB-Tag), and a cre-recombinase gene driven by the albumin promoter and enhancer (Alb-Cre). F1 mice carrying both the PB-Tag and Alb-Cre transgenes were crossed with L/L− mice, littermates of LID mice and homozygous for loxP-flanked Igf1 gene without any transgene, to generate F2 mice homozygous for loxP-flanked Igf1 gene carrying both Alb-Cre transgene and PB-Tag transgene (LID-TRAMP) and F2 mice homozygous for loxP-flanked Igf1 gene carrying PB-Tag transgene (L/L-TRAMP). LID-TRAMP mice of F2 and subsequent generations were crossed with their L/L− littermates (and vice versa) to produce LID-TRAMP and L/L-TRAMP mice. The genotypes of all offspring were analyzed by PCR with genomic DNA isolated from ear or tail clippings. PCR protocols were used as previously described to analyze loxP-flanked Igf1 gene, the Alb-Cre transgene (21) and the PB-Tag transgene. Only male LID-TRAMP and L/L-TRAMP of F4 or later generations were used for experiments. All mice studied carried a copy of the PB-Tag transgene.
Blood and tissue sampling
At 14 to 20 weeks of age, 18 male L/L-TRAMP mice and 23 male LID-TRAMP mice were euthanized and blood samples were collected by direct right ventricle puncture and centrifuged at 2,000 × g for 10 min at 4°C. Serum was stored at −80°C.
After blood collection, carcasses were perfused with cold 0.9% (w/v) NaCl solution by direct left ventricle puncture. Following perfusion, genitourinary tissues (GU) containing the bladder, prostate glands, part of urethra, bilateral seminal vesicles, and a small portion of bilateral ureters and vas deferens were dissected and weighed. For five 19-week-old male LID-TRAMP mice and five 19-week-old male L/L-TRAMP mice, GUs were quickly put into cold 0.9% (w/v) NaCL solution supplemented with protease and phosphatase inhibitor cocktails (Calbiochem). Each lobe of the prostate gland was dissected under a dissection microscope, weighed, and immediately frozen with liquid nitrogen or ethanol-dry ice. All procedures were approved by the Animal Care and Use Committee at UCLA.
Longitudinal survival
Seventy-seven male LID-TRAMP mice and 84 male L/L-TRAMP mice were observed until they died spontaneously or until the tumor grew above the size limit stipulated by UCLA Animal Research Committee guidelines (causing excessive suffering to the animals) and euthanasia was required.
At the time of death (from any cause), genitourinary tissues (prostate with or without tumor, bladder, and bilateral seminal vesicles; GU) were dissected and weighed. At the same time, periaortic lymph nodes, perirenal lymph nodes, liver, peritracheal lymph nodes, and lungs were macroscopically examined for the presence of metastases. Tumor samples from 36-week-old LID-TRAMP and L/L-TRAMP mice were also harvested and snap-frozen.
ELISA of hormone levels
IGF-I and IGFBP-3 levels were measured by in-house mIGF-I and mIGFBP-3 ELISAs, as previously described (22). Serum GH levels were measured by rat/mouse GH ELISA kit (ALPCO Diagnostics). Mouse-specific IGF-II and IGFBP-2 levels were measured by in-house ELISA assays using recombinant mouse proteins and monoclonal antibodies from R&D Systems.
Histology
Twenty-two LID-TRAMP and 23 L/L-TRAMP mice were euthanized at the age of 9 to 19 weeks. After blood samples were collected, the carcasses were perfused with 4% (w/v) paraformaldehyde. GUs were quickly dissected, weighed, and fixed with 4% paraformaldehyde at 4°C for 24 h. The tissues were embedded in paraffin and processed into 5-μm sections. The sections were stained with H&E and the stage of prostate cancer was evaluated by an experienced pathologist.
Western immunoblots
Frozen prostate or tumor was homogenized in protein lysis buffer and the protein concentration was measured by the Bradford method (Bio-Rad). Fifty micrograms of the lysate protein was separated by SDS-PAGE and transferred to polyvinylidene difluoride membrane. Membranes were blocked in 0.2% I-block (Applied BioSystems) in PBS containing 0.1% Tween 20 for 3 h at room temperature and then probed with the appropriate primary and secondary antibodies. Antibody-antigen complexes were visualized by Western Lightning Chemiluminescence reagents (Perkin-Elmer) and autoradiography. Primary antibodies were as follows: rabbit anti–IGF-IRβ antibody (1:2,000; Santa Cruz Biotechnology), rabbit anti–phosphorylated Akt (Ser473) antibody (1:2,000; Cell Signaling Technology), rabbit anti-Akt antibody (1:2,000; Cell Signaling Technology), rabbit anti-PTEN antibody (1:2,000; Cell Signaling Technology), goat anti-GHR (1:1,000; R&D Systems), chicken anti–IGF-2R (1:2,000; Abcam), goat anti-AR (1:1,000; Abcam), rabbit anti-ERK (1:5,000; Cell Signaling Technology), and phosphorylated ERK (1:2,000; Cell Signaling Technology). β-Actin (1:10,000; Sigma) was used as a loading control.
Statistics
Two-tailed Mann-Whitney U test was used for analyzing differences in serum levels of IGF-I, GH, and IGFBP-3, and histologic stage of tumors between L/L-TRAMP and LID-TRAMP mice. Log-rank test was used to analyze the difference in survival and the rate of metastasis between L/L-TRAMP and LID-TRAMP mice. ANOVA was used to analyze the difference in linear regression slope and Y-intercept.
Results
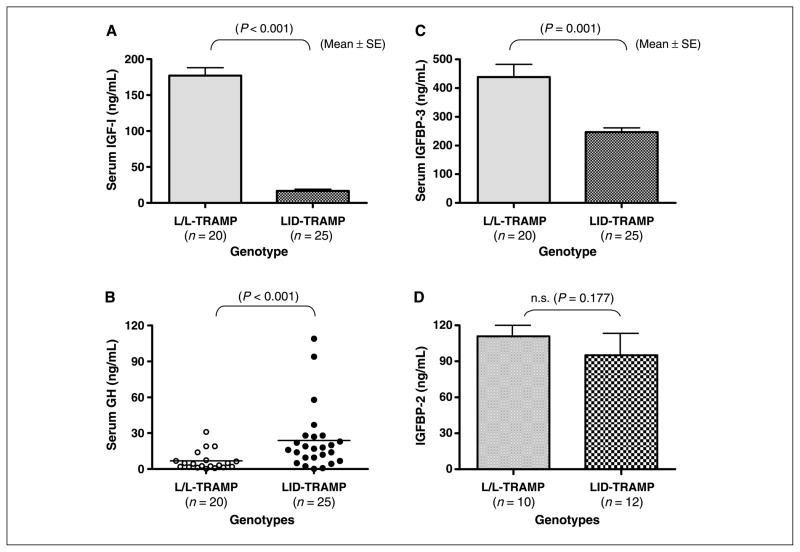
To assess the effects of deletion of the hepatic Igf1 gene on circulating IGF-I, GH, and IGFBP-3 levels, sera were harvested from 12- to 19-week-old male L/L-TRAMP and LID-TRAMP mice and assessed by specific ELISA assays. Mean ± SE serum IGF-I levels of L/L-TRAMP (n = 20) and LID-TRAMP mice (n = 25) were 177 ± 11 and 17 ± 2 ng/mL, respectively (P < 0.001; Fig. 1A), showing a 90% reduction in circulating IGF-I in LID-TRAMP mice compared with L/L-TRAMP. Consistent with reduced circulating IGF-I, GH levels were 3.5-fold elevated in LID-TRAMP mice compared with control. Mean (range) serum GH levels of 12- to 19-week-old male L/L-TRAMP mice (n = 20) and LID-TRAMP mice (n = 25) were 6.8 (<0.5–31.0) and 23.8 (<0.5–109.0) ng/mL, respectively (P < 0.001; Fig. 1B). Mean ± SE serum IGFBP-3 levels of 12- to 19-week-old male L/L-TRAMP mice (n = 20) and LID-TRAMP mice (n = 25) were 439 ± 44 and 241 ± 15 ng/mL, respectively (P = 0.001; Fig. 1C), showing that although reducing serum IGF-I levels does lead to a decrease in circulating IGFBP-3, the extent of this reduction is substantially less. IGFBP-2 levels were compared in Fig. 1D and were not different between strains.
Figure 1.
Characterization of the GH-IGF axis in the LID-TRAMP model. Sera were obtained from L/L-TRAMP and LID-TRAMP mice at 12 to 19 weeks of age. Levels of IGF-I (A), GH (B), IGFBP-3 (C), and IGFBP-2 (D) were assessed by mouse-specific ELISA assays. A, male LID-TRAMP mice at 12 to 19 weeks of age show a 90% reduction in serum IGF-I as compared with male L/L-TRAMP mice of the same age (P < 0.001). B, male LID-TRAMP mice at 12 to 19 weeks of age show 3.5-fold higher serum GH as compared with male L/L-TRAMP mice of the same age (P < 0.001). C, serum IGFBP3 levels of 12- to 19-week-old male LID-TRAMP mice were 60% of male L/L-TRAMP mice of the same age (P < 0.001). D, IGFBP-2 levels were not different between strains.
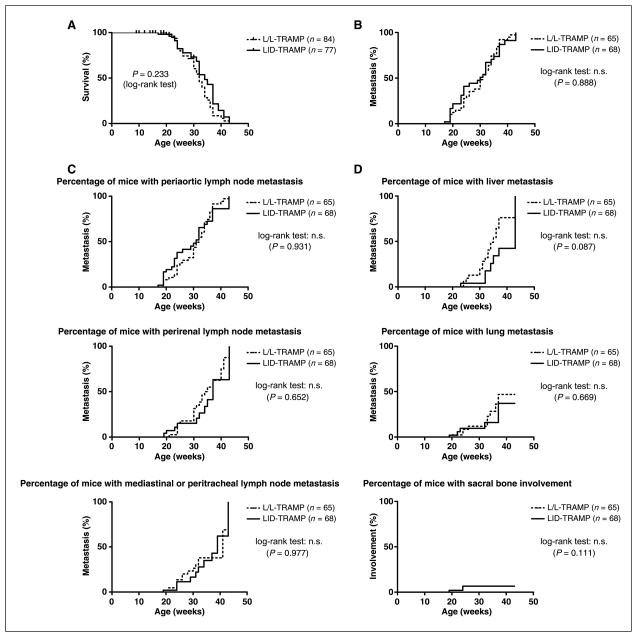
We compared the life span of LID-TRAMP mice to controls and observed no significant difference in the survival rate between male LID-TRAMP and L/L-TRAMP mice (all mice were dead by 43 weeks as a result of their cancer). The hazard ratio of male LID-TRAMP mice to male L/L-TRAMP mice was 0.736 (Fig. 2A). This suggests that the reduction in circulating IGF-I did not affect survival.
Figure 2.
Rates of survival and metastases in the LID-TRAMP model. Mice were analyzed at the time of death. A, survival curves for the two strains. There were no significant differences in the survival rates between male LID-TRAMP and L/L-TRAMP mice. The hazard ratio of male LID-TRAMP mice to male L/L-TRAMP mice was 0.736. B, percentage of mice with metastasis. Reducing the circulatory IGF-I level does not reduce the rate of metastasis in male LID-TRAMP mice. The hazard ratio of male LID-TRAMP mice to male L/L-TRAMP mice was 0.97. C, percentage of mice with macroscopic lymph node metastasis in three sites. No significant differences were seen. D, percentages of mice with macroscopic organ metastases for the three major sites of metastases.
In both strains of mice, macroscopic metastases (in lymph nodes, lungs, kidney, liver, and bone) appeared after the age of 19 weeks and reached 100% by 40 weeks of age. There was no significant difference in the rate or site of metastasis between male LID-TRAMP and L/L-TRAMP mice. The hazard ratio of male LID-TRAMP to male L/L-TRAMP mice was 0.97 (Fig. 2B). Analysis of specific lymph node metastases shown in Fig. 2C showed no differences between strains. Analysis of specific organ site metastases shown in Fig. 2D showed similar rates of lung metastases among the strains. Liver metastases showed an insignificant trend towards fewer metastases in the LID-TRAMP strain, whereas rare bone metastases were insignificantly less prevalent in this strain. Overall, however, metastases are not different in either model. Lytic bone lesions occurred in two LID-TRAMP mice and in none of the L/L TRAMP mice (out of 133 mice total; P = not significant).
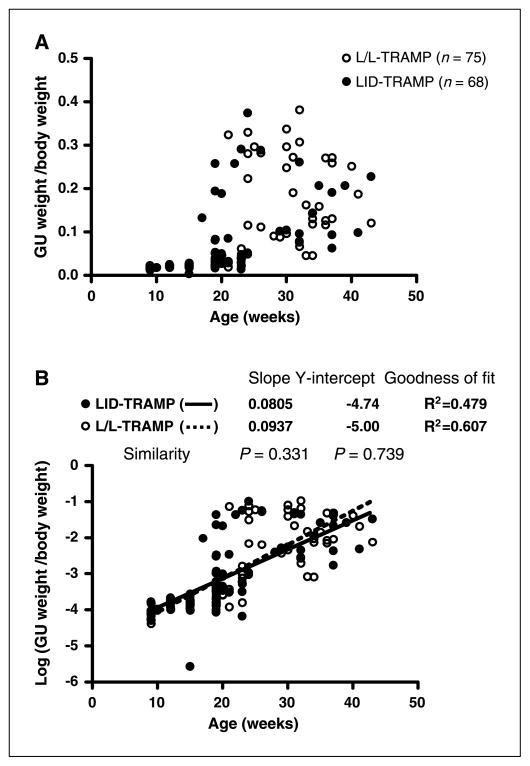
Relative GU weight (GU weight/body weight) remained low until the age of 15 weeks, but then rapidly increased in both LID-TRAMP and L/L-TRAMP mice (Fig. 3A). When the relative GU weights of male LID-TRAMP mice were log-transformed and plotted against age, linear regression was observed. The slope of the regression line was 0.0805, the Y-intercept was −4.74, and the r2 value was 0.479. When the relative GU weights of male L/L-TRAMP mice were log-transformed and plotted against age, the slope of the regression line was 0.0937, the Y-intercept −5.00, and r2 was 0.607. There was no significant difference in the slope and Y-intercept of regression lines between male LID-TRAMP and L/L-TRAMP mice (Fig. 3B). There were no correlations between the weights of GU tumors and IGFBP-2, IGF-I, IGFBP-3, or GH levels (data not shown).
Figure 3.
Genitourinary weights in the LID-TRAMP model. A, relative GU weight increased rapidly after the age of 15 weeks in both male LID-TRAMP and L/L-TRAMP mice. B, log-transformed GU weight. Reducing the circulatory IGF-I did not reduce the relative GU weight in male LID-TRAMP mice.
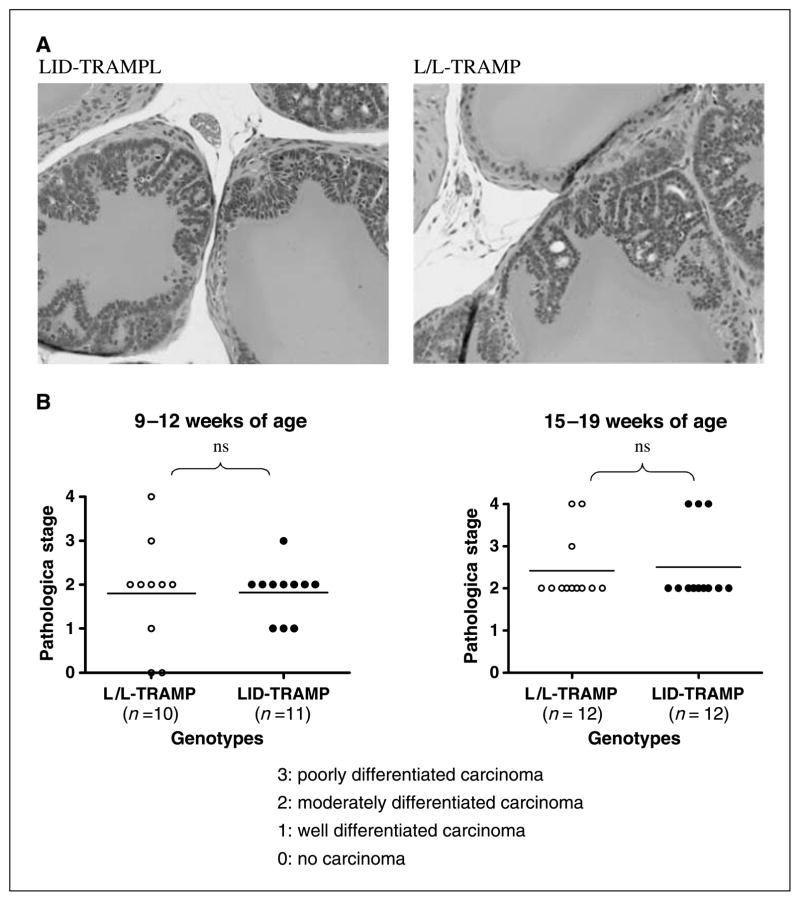
By 12 weeks of age, both LID-TRAMP and L/L-TRAMP mice had moderately differentiated prostate carcinoma (Fig. 4A). There was no difference in the histologic findings between LID-TRAMP and L/L-TRAMP mice either at early (9–12 weeks) or intermediate (15–19 weeks) stages of tumor development (Fig. 4B).
Figure 4.
Pathologic stage of prostate cancer in the LID-TRAMP model. A, H&E stains of prostate sections of a representative 12-week-old male LID-TRAMP mouse and a male L/L-TRAMP mouse of the same age. Well-differentiated prostate carcinoma was seen in both. B, comparison of progression of prostate cancer in TRAMP and LID-TRAMP mice. Staging was performed by a blinded expert pathologist in tumors obtained from early (9–12 weeks) and late (15–19 weeks) mice. There was no significant difference in histologic findings between male LID-TRAMP mice and L/L-TRAMP mice.
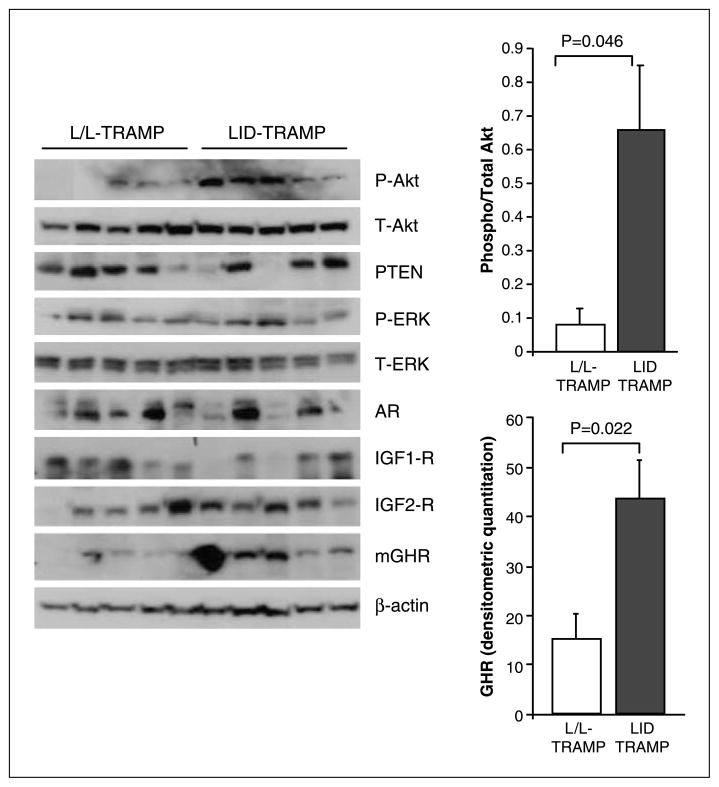
We examined the expression of various molecules within the GH-IGF axis using specific immunoblots (Fig. 5). A significant increase was observed in the levels of GH receptors in the LID-TRAMP model and these tumors also exhibited significantly higher levels of phosphorylated Akt. There was a trend towards lower PTEN levels in LID-TRAMP tumors (P = 0.2), however, this did not reach statistical significance. The levels of the type 1 and type 2 IGF receptors were not significantly different, nor were androgen receptor or phosphorylated ERK levels in the prostate glands of male 19-week-old LID-TRAMP compared with L/L-TRAMP.
Figure 5.
Expression of GH-IGF signaling pathway proteins in LID-TRAMP. Immunoblots from 10 matched tumors of LID-TRAMP and L/L-TRAMP mice at 19 weeks. Significantly higher GH receptor expression was seen in the tumors of male LID-TRAMP mice. Phosphorylation of AKT was also significantly higher in tumors from this strain.
IGF-I content in tumors (measured by ELISA) ranged from 10 to 300 pg/mg of protein. At 19 weeks, there was a trend for higher IGF-I levels in LID-TRAMP mice versus L/L TRAMP (115 ± 90 versus 50 ± 30; P = 0.2), but this trend was reversed in tumors from older mice, and overall, no significance was seen. IGF-II levels were below the limit of the detection of the assay (<0.1 ng/mg protein) in tumor extracts. The mRNA levels for AR, IGF-I, and IGF-II were measured by quantitative reverse transcription-PCR (Applied Biosystems) and were not different between strains (data not shown).
These data indicate that there is a differential molecular response to the circulating GH and IGF levels in LID-TRAMP and L/L-TRAMP tumors, whereby a low systemic IGF-I environment coupled with higher GH levels was associated with compensatory increases in the GH receptor and possibly higher tumor IGF levels and an accelerated loss of PTEN, all of which lead to higher levels of phosphorylated Akt in the LID-TRAMP tumors.
To address the question of the sensitivity of TRAMP tumors to IGF-blockade directly, we used a cell line derived from TRAMP tumors grown in the presence of 1 mmol/L of IGF-IR blocking antibody (a gift from Dr. Frank Calzone, Amgen, Thousand Oaks, CA) or control IgG for 24 hours and measured apoptosis using Death Detection ELISAPLUS for the determination of cytoplasmic histone-associated DNA fragments (Roche Applied Science) following the manufacturer’s instructions. IGF-IR blockade increased apoptosis by 30% (P < 0.01).
Discussion
We developed a prostate cancer mouse model with very low serum IGF-I to address the contribution of circulatory IGF-I to the development of prostate cancer. The serum IGF-I levels of LID-TRAMP mice we report here are significantly lower than those reported previously (16). The likely explanation for this is the enhanced specificity and sensitivity of the primary antibody used in our ELISA. In this assay, serum IGF-I levels of LID mice were comparable with LID-TRAMP (data not shown) measured using our in-house ELISA (22). We did not detect any differences in longevity, tumor size, metastasis ratio, or stage of prostate cancer between LID-TRAMP and L/L-TRAMP mice. These results suggest that, in the TRAMP model, circulatory IGF-I has little effect on the development of prostate cancer after 9 weeks of age (the equivalent of localized prostate cancer in men), when GH levels are elevated.
Two previously published mouse models of genetic disruptions in the GH-IGF axis showed delayed progression of prostate cancer (refs. 15, 20; Table 1). Both of these models, however, were associated with interrupted GH signaling, either by the elimination of GH secretion in the lit/lit mouse that harbors a mutation in the GHRH receptor (15) or by abolishing the action of GH in the GHR-null mouse (20). In both of these models, circulating IGF-I levels were also very low (similar to the LID mouse) and IGFBP-3 levels were even lower than in the LID model.3 In these two models, size was substantially reduced compared with wild-type animals, whereas the LID mouse displays only a small (10%) reduction in auxologic growth. Our data does not suggest that increased local regulation of IGF-IR accounts for this observation, however, an insignificant trend towards higher IGF-I content in prostate tumors from 19-week-old TRAMP mice suggests the possibility of increased local IGF-I, perhaps as a result of increased GH action. Another possible explanation for the differing results between the LID-TRAMP and the lit/lit TRAMP models are the differences in local GH-dependent effects in these different model systems, either in stromal or epithelial components, which can be assumed to be reduced in the GHR−/− and GHRHR−/− prostate cancer models but increased in the LID-TRAMP, especially in lieu of the increased tumor GH receptors levels coupled with increased serum GH levels. This possible explanation involves the direct actions of GH on the progression of prostate adenocarcinoma, which compensate for the lack of circulatory IGF-I. This would be consistent with previously reported data in LNCaP cells, showing the expression of GH receptors and a GH-induced increase in androgen receptor levels (23, 24).
Table 1.
Comparison of three prostate cancer models with GH-IGF disruption
| Group | Mice | Serum GH | Serum IGF-I | IGF-I effect on prostate |
GH effect on prostate | Progression of CaP | |
|---|---|---|---|---|---|---|---|
| Endocrine | Autocrine/paracrine | ||||||
| Montreal | TRAMP × GHRHR mut/mut | Low | Low | Low | ?? | Low | Delayed |
| UIC | C3(1)/Tag × Ghr−/− | NA | Low | Low | ?? | Low | Delayed |
| UCLA | TRAMP × liver Igf1−/− | High | Low | Low | ?? | High | No difference |
Nevertheless, there could be other differences between the LID-TRAMP and lit/TRAMP models, including the fact that the LID-mouse has elevated insulin levels, which may play a role in the pathogenesis of prostate cancer, and GH suppression may not be the growth stimulus in that model.
We showed that TRAMP cells in culture are susceptible to inhibition by IGF-IR blockade. This indicates that blocking IGF signaling acutely inhibits TRAMP tumors, but that in a long-term in vivo study, the reduction of systemic IGF-I allows the tumor to develop alternative adaptive growth pathways. At 19 weeks of age, prostate tissue from LID-TRAMP mice expressed more GH receptors and displayed higher levels of phosphorylated Akt. The possible contribution of GH activation of Akt phosphorylation (possibly via local IGF-I production) versus decreased PTEN activity (which showed a trend towards lower levels in our study; P = 0.2, but was not statistically significant) needs to be evaluated further. These results suggest that depletion of systemic IGF-I from the early stage of prostate cancer development may induce the development of a tumor that is less dependent on IGF-I for growth. Because many therapies involving the blockade of IGF-I signaling are currently being developed for prostate cancer including anti–IGF-I receptor monoclonal antibodies, which seem to be very promising in this disease (25), the role of the GH and IGF axis on the progress of prostate cancer should be continued to be thoroughly characterized. It is possible that future therapy for patients with prostate cancer may involve the blockade of both IGF-I and GH pathways.
It is, however, interesting that in one experimental model of prostate cancer of allografts implanted in rats, GH treatment actually increased survival, suggesting that inhibiting GH action may affect the host as much as the tumor (26). Future studies should examine additional models of genetic alterations of the GH-IGF axis in prostate cancer including prostate-specific blockade of GH action in tumors.
It is striking that the dramatic reduction of IGF in the circulation did not affect progression in the TRAMP model as recent studies showed the susceptibility of TRAMP tumors to various nutritional interventions, which may be related to the IGF system (27). Nevertheless, the role of GH in our model cannot be directly assessed. Indeed, in this mouse model, as in early clinical trials of IGF-IR blockade with IGF-IR neutralizing antibodies, GH levels increase secondarily to the low serum IGF-I or IGF-IR signaling. Therefore, the causative role of GH is speculative and only future studies in animal models and clinical trials will fully determine the relative value of IGF-I versus GH blockade or their combination.
In conclusion, data from this report indicate that reduction of systemic IGF-I in the face of elevated GH levels is insufficient to reduce the progression of prostate cancer in the TRAMP model and may be associated with a more aggressive molecular response to IGF deprivation in the tumor in the form of GHR and phosphorylated Akt up-regulation. Future studies of the GH-IGF system as a target for prostate cancer drug development should address the role of GH as an additional drug-able target using agents such as GH receptor antagonists.
Acknowledgments
Grant support: 1R01CA100938, R01HD047013, P50CA92131, DOD Idea Development Award (P. Cohen), and a DOD Fellowship Award (L.J. Cobb).
Footnotes
Cohen and Hwang, personal communication.
References
- 1.Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–6. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 2.Stattin P, Rinaldi S, Biessy C, Stenman UH, Hallmans G, Kaaks R. High levels of circulating insulin-like growth factor-I increase prostate cancer risk: a prospective study in a population-based nonscreened cohort. J Clin Oncol. 2004;22:3104–12. doi: 10.1200/JCO.2004.10.105. [DOI] [PubMed] [Google Scholar]
- 3.Harman SM, Metter EJ, Blackman MR, Landis PK, Carter HB Baltimore Longitudinal Study on Aging. Serum levels of insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-3, and prostate-specific antigen as predictors of clinical prostate cancer. J Clin Endocrinol Metab. 2000;85:4258–65. doi: 10.1210/jcem.85.11.6990. [DOI] [PubMed] [Google Scholar]
- 4.Chan JM, Stampfer MJ, Ma J, et al. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst. 2002;94:1099–106. doi: 10.1093/jnci/94.14.1099. [DOI] [PubMed] [Google Scholar]
- 5.Severi G, Morris HA, MacInnis RJ, et al. Circulating insulin-like growth factor-I and binding protein-3 and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1137–41. doi: 10.1158/1055-9965.EPI-05-0823. [DOI] [PubMed] [Google Scholar]
- 6.Lacey JV, Jr, Hsing AW, Fillmore CM, Hoffman S, Helzlsouer KJ, Comstock GW. Null association between insulin-like growth factors, insulin-like growth factor-binding proteins, and prostate cancer in a prospective study. Cancer Epidemiol Biomarkers Prev. 2001;10:1101–2. [PubMed] [Google Scholar]
- 7.Woodson K, Tangrea JA, Pollak M, et al. Serum insulin-like growth factor I: tumor marker or etiologic factor? A prospective study of prostate cancer among Finish men. Cancer Res. 2003;63:3991–4. [PubMed] [Google Scholar]
- 8.Morris JK, George LM, Wu T, Wald NJ. Insulin-like growth factors and cancer: no role in screening. Evidence from the BUPA study and meta-analysis of prospective epidemiological studies. Br J Cancer. 2006;95:112–7. doi: 10.1038/sj.bjc.6603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer F, Galan P, Douville P, et al. A prospective study of the insulin-like growth factor axis in relation with prostate cancer in the SU.VI. MAX trial Cancer. Epidemiol Biomarkers Prev. 2005;14:2269–72. doi: 10.1158/1055-9965.EPI-05-0303. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Lewis SK, Voigt L, Fitzpatrick A, Plymate SR, Weiss NS. Prostate carcinoma incidence in relation to prediagnostic circulating levels of insulin-like growth factor I, insulin-like growth factor binding protein 3, and insulin. Cancer. 2005;103:76–84. doi: 10.1002/cncr.20727. [DOI] [PubMed] [Google Scholar]
- 11.Platz EA, Pollak MN, Leitzmann MF, Stampfer MJ, Willett WC, Giovannucci E. Plasma insulin-like growth factor-1 and binding protein-3 and subsequent risk of prostate cancer in the PSA era. Cancer Causes Control. 2005;16:255–62. doi: 10.1007/s10552-004-3484-8. [DOI] [PubMed] [Google Scholar]
- 12.Stattin P, Bylund A, Rinaldi S, et al. Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. J Natl Cancer Inst. 2000;92:1910–7. doi: 10.1093/jnci/92.23.1910. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan PJ, Mohan S, Cohen P, foster BA, Greenberg NM. The insulin-like growth factor axis and prostate cancer: lessons from the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Cancer Res. 1999;59:2203–9. [PubMed] [Google Scholar]
- 15.Majeed N, Blouin MJ, Kaplan-Lefko PJ, et al. A germ line mutation that delays prostate cancer progression and prolongs survival in a murine prostate cancer model. Oncogene. 2005;24:4736–40. doi: 10.1038/sj.onc.1208572. [DOI] [PubMed] [Google Scholar]
- 16.Yakar S, Liu JL, Stannard B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–9. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yakar S, Liu JL, Fernandez AM, et al. Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes. 2001;50:1110–8. doi: 10.2337/diabetes.50.5.1110. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Cui K, Miyoshi K, et al. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res. 2003;63:4384–8. [PubMed] [Google Scholar]
- 19.Wu Y, Yakar S, Zhao L, Henninghausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62:1030–5. [PubMed] [Google Scholar]
- 20.Wang Z, Prins GS, Coschigano KT, et al. Disruption of growth hormone signaling retards early stages of prostate carcinogenesis in the C3(1)/T antigen mouse. Endocrinology. 2005;146:5188–96. doi: 10.1210/en.2005-0607. [DOI] [PubMed] [Google Scholar]
- 21.Liu JL, Grinberg A, Westphal H, et al. Insulin-like growth factor-I affects perinatal lethality and postnatal development in a gene dosage-dependent manner: manipulation using the Cre/loxP system in transgenic mice. Mol Endocrinol. 1998;12:1452–62. doi: 10.1210/mend.12.9.0162. [DOI] [PubMed] [Google Scholar]
- 22.Hwang DL, Lee PD, Cohen P. Quantitative ontogeny of murine insulin-like growth factor (IGF)-I, IGF-binding protein-3 and the IGF-related acid-labile subunit. Growth Horm IGF Res. 2008;18:65–74. doi: 10.1016/j.ghir.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss-Messer E, Merom O, Adi A, et al. Growth hormone (GH) receptors in prostate cancer: gene expression in human tissues and cell lines and characterization, GH signaling and androgen receptor regulation in LNCaP cells. Mol Cell Endocrinol. 2004;220:109–23. doi: 10.1016/j.mce.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Chopin LK, Veveris-Lowe TL, Philipps AF, Herington AC. Co-expression of GH and GHR isoforms in prostate cancer cell lines. Growth Horm IGF Res. 2002;12:126–36. doi: 10.1054/ghir.2002.0271. [DOI] [PubMed] [Google Scholar]
- 25.Wu JD, Haugk K, Coleman I, et al. Combined in vivo effect of A12, a type 1 insulin-like growth factor receptor antibody, and docetaxel against prostate cancer tumors. Clin Cancer Res. 2006;12:6153–60. doi: 10.1158/1078-0432.CCR-06-0443. [DOI] [PubMed] [Google Scholar]
- 26.Torosian MH. Growth hormone and prostate cancer growth and metastasis in tumor-bearing animals. J Pediatr Endocrinol. 1993;6:93–7. doi: 10.1515/jpem.1993.6.1.93. [DOI] [PubMed] [Google Scholar]
- 27.Raina K, Singh RP, Agarwal R, Agarwal C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res. 2007;67:5976–82. doi: 10.1158/0008-5472.CAN-07-0295. [DOI] [PubMed] [Google Scholar]