Abstract
BIK is the founding member of the BH3-only family pro-apoptotic proteins. BIK is predominantly localized in the ER and induces apoptosis through the mitochondrial pathway by mobilizing calcium from the ER to the mitochondria and remodeling the mitochondrial cristae. BIK-mediated apoptosis is mediated by selective activation of BAX. BIK also induces non-apoptotic cell death in certain cell types by unknown mechanisms. BIK is non-essential for animal development, but appears to be functionally redundant for certain developmental functions with BIM. BIK is implicated in selection of mature B cells in humans. BIK is a pro-apoptotic tumor suppressor in several human tissues and its expression in cancers is prevented by chromosomal deletions encompassing the Bik locus or by epigenetic silencing. BIK appears to be a critical effector in apoptosis induced by toxins, cytokines and virus infection. Several anti-cancer drugs transcriptionally activate Bik gene expression through transcriptional pathways dependent on factors such as E2F and p53 or by removal of epigenetic marks on the chromatin. BIK appears to be a prominent target for anti-cancer drugs that inhibit proteasomal functions. BIK has also been used as a therapeutic molecule in gene therapy based approaches to treat difficult cancers.
Keywords: BIK, BH3-only, Apoptosis, ER, Cancer, Viral pathogenesis
Introduction
BIK (BCL-2 interacting killer), was the first member of the BH3-only pro-apoptotic proteins (Boyd et al., 1995). In addition to BCL-2, it was also shown to strongly interact with other anti-apoptotic proteins, adenovirus E1B-19K, BCL-xL and EBV-BHRF1 and to exhibit a potent cell death activity when expressed autonomously. The cell death activity was suppressed by coexpression of BCL-2, BCL-xL, E1B-19K and EBV-BHRF1, suggesting that BIK might be a common target for cellular and viral anti-apoptosis proteins. Although other BH3-only proteins such as BNIP1, BNIP3 (Boyd et al., 1994) and BAD (Yang et al., 1995) were identified earlier as proteins that interacted with viral and cellular anti-apoptotic proteins in two hybrid screenings, BIK was the first protein in which the cell death activity was linked to the conserved BCL-2 homology domain 3 (BH3) (Boyd et al., 1995; Chittenden et al., 1995) and thus considered as the founding member of BH3-only family proteins. BIK was shown to share two domains, the BH3 domain and the trans-membrane domain with other BCL-2 family proteins and specific mutations within the BH3 domain strongly reduced the pro-apoptotic activity (Boyd et al., 1995; Chittenden et al., 1995). BIK was also subsequently identified as an E1B-19K interacting protein, where it was designated as NBK (Han et al., 1996). An ortholog of BIK referred as BLK (BIK-Like Killer) was identified from the mouse EST data bank (Hegde et al., 1998). Although BIK is highly conserved among primates, in other mammalian species the amino acid sequences outside the BH3 domain exhibit some sequence variations. However, based secondary structure predictions all mammalian BIK proteins appear to be structurally similar. The gene for human BIK is encoded in chromosome 22q13.3 (Verma et al., 2000). The human BIK protein is a phosphoprotein (Verma et al., 2001). BIK is predominantly localized in the ER (Germain et al., 2002) and appears to mediate apoptosis signaling to the mitochondria (Germain et al., 2005). The mechanisms by which BIK induces cell death and its role in normal cells and in cells undergoing pathological stress including cancer are reviewed here.
BIK protein
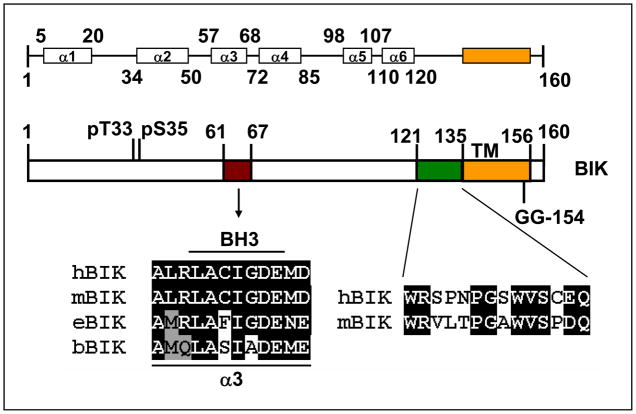
BIK is a 160 aa protein and contains a trans-membrane domain (TM), in addition to the BH3 domain (Fig. 1). Predictions based on primate genome sequence analyses have suggested a larger isoform of 202 residues (XB_525617). However, the existence of such an isoform remains to be demonstrated. Secondary structure prediction suggested that BIK might form six α helices with exposed BH3 domain (McDonnell et al., 1999). Predictions based on the analysis of mean hydrophobicity and mean net charge suggested that BIK may have a well-defined structure while most other BH3-only members (except BID) appear to be unstructured (Hinds et al., 2007). Phylogenetic analysis of BCL-2 family proteins suggested that BIK may be more closely related to BAK (Aouacheria et al., 2005). Mutational analyses indicated that the core BH3 domain was crucial for the apoptotic activity (Boyd et al., 1995; Chittenden et al., 1995; Elangovan and Chinnadurai, 1997; Mathai et al., 2002). The TM domain was dispensable for the apoptotic activity, at least under conditions of transient overexpression (Elangovan and Chinnadurai, 1997). In addition to the BH3 domain, a C-terminal domain adjacent to the TM was also important for maximal cell death activity in transient assays. The C-terminal motif resembles the substrate recognition motif present in several caspases (Elangovan and Chinnadurai, 1997) and a sequence element in bacterial hemolysins. This domain is conserved only in human and murine BIK proteins and its functional significance is not clear. hBIK was shown to be phosphorylated on Thr(33) and Ser(35) by a casein kinase II-like kinase and mutations that prevented phosphorylation reduced the cell death activity and interaction with anti-apoptosis proteins (Verma et al., 2001). Mutations that mimicked phosphorylation (Thr, Ser→Asp) enhanced the cell death activity of BIK and interaction with BCL-xL and BCL-2 (Li et al., 2003). hBIK was reported to be localized predominantly in the ER (Germain et al., 2002). However, mitochondrial localization of hBIK (Han et al., 1996) and mBIK (Hegde et al., 1998) has also been reported. Only modest steady state levels of BIK protein were generally observed in most cultured cell lines. Several reports have indicated that BIK was down regulated by proteasomal degradation (Marshansky et al., 2001; Nikrad et al., 2005; Zhu et al., 2005b). A recent report identified a serine protease of the Rhomboid family, RHBDD1 that released BIK by cleaving the C-terminal TM domain from the membrane at a GG (aa 153–154) motif (Wang et al., 2008). Although RHBDD1 was shown to localize predominantly in the mitochondria, it was suggested that it might be translocated to the ER to cleave ER-associated BIK. In cells treated with the proteasome inhibitor Bortizomib (Velcade) which was reported to increase the level of BIK (Li et al., 2008; Nikrad et al., 2005; Yeung et al., 2006; Zhu et al., 2005b), BIK appeared as a doublet (Hur et al., 2006; Wang et al., 2008), suggesting that the lower MW band was destined for proteasomal degradation. Depletion of RHBDD1 by the use of siRNA increased the level of full length BIK and apoptosis. These results suggested that RHBDD1 targeted BIK for proteasomal degradation by liberating BIK by regulated transmembrane proteolysis (Brown et al., 2000). Therapeutic intervention with proteasome-mediated down regulation of BIK was suggested as one of the modes of action of proteasome inhibitors that are currently in clinical trials to treat a variety of cancers (see below).
Figure 1. Domain structure of BIK protein.
Three domains of human BIK and their homologies to BIK proteins from different animal species are shown. The predicted secondary structure of hBIK (shown on the top) is based on McDonnell et al. (McDonnell et al., 1999). The α3 region encompassing the BH3 domain is highly conserved and the sequences of the α3 region of human, murine, equine and bovine BIK are indicated. The C-terminal domain (aa 121 to 135) that is required for maximal pro-apoptotic activity of hBIK is conserved only in mBIK. The phosphorylation sites at Thr33 and Ser35 and the RHBDD1 cleavage site (G153G154) in the transmembrane (TM) domain are indicated.
Regulation of BIK expression
Northern blot analysis showed a restricted pattern of expression of Bik in human tissues (Daniel et al., 1999). Higher levels of expression were seen in the kidneys and pancreas compared to other organs. Although Bik expression was not detectable in normal colon and lymphoid tissues, certain cell lines derived from these tumor tissues contained higher levels of Bik mRNA. In mouse, higher levels of Bik expression was observed in the hematopoietic compartment and in organs such as the kidney, liver, lung and heart. In general, Bik expression was increased in epithelial cells in both human and mouse. The sequence variations between the human and mouse Bik promoter regions may contribute to some the differential patterns of expression.
In addition to post-translational control of BIK protein expression at the level of protein stability, the expression of the Bik gene was shown to be regulated at the level of transcription under different contexts. Such a regulated expression appears to be consistent with the prediction that BIK might be constitutively active as a result of exposed BH3 domain (McDonnell et al., 1999). The human Bik gene was reported to be a transcriptional target for p53 (Mathai et al., 2002). Expression of one of the adenovirus E1A proteins that promotes accumulation of p53 (in addition to activation of E2F, see below) induced accumulation of Bik mRNA and protein. Further, infection of p53-null human epithelial cancer cells with an adenovirus vector that expressed wt p53 increased the levels of Bik mRNA and protein while the mutant p53 did not. Although stimuli such as γ radiation and treatment with the topoisomerase inhibitor doxorubicin (that up-regulate endogenous p53) were shown to activate BIK expression in human KB cells, the p53-dependency was not examined (Mathai et al., 2005). However, both agents were reported to enhance the levels of Bik mRNA in a p53-dependent fashion in MCF7 breast cancer cells by employing siRNA-mediated depletion of p53 by another group (Hur et al., 2006). Although the hBik promoter (Verma et al., 2000) contains degenerate p53 binding sites, in promoter assays no p53 response was detected (Hur et al., 2006; Mathai et al., 2002). It is possible that the degenerate p53-binding sites might be functional under conditions of p53 overexpression. Other studies have reported activation of Bik expression by genotoxic stress in cells that lacked functional p53. A rapid, albeit modest activation of Bik was observed in a p53-deficicent human lymphoma cell line treated with the DNA topoisomerase inhibitor, camptothecin (Paquet et al., 2004). An increase in Bik expression also occurred in a p53-null human colon cell line (HCT1116 p53−/−) treated with chemotherapeutic drugs adriamycin and cisplatin (Real et al., 2006). Thus, it appears that human Bik is activated by genotoxic stimuli through p53-dependent and –independent mechanisms (see below). In murine cells no Bik activation was observed as a result of genotoxic stress or by p53 overexpression (Coultas et al., 2004).
A gene profiling study of an estrogen-dependent breast cancer cell line revealed specific up-regulation of Bik expression during apoptosis caused by estrogen starvation or exposure to antiestrogens such as fulvestrant (Hur et al., 2004). Suppression of BIK expression by siRNA-mediated depletion diminished fulvestrant-induced apoptosis linking BIK to antiestrogen-induced apoptosis. The antiestrogen-induced up-regulation of Bik mRNA was linked to p53 since siRNA-mediated p53 knockdown as well as p53 dominant negative mutant abolished Bik mRNA accumulation (Hur et al., 2006). Surprisingly, this activity of p53 appeared to be unrelated to its DNA-binding activity since fulvestrant treatment did not enhance the DNA-binding activity of p53 in a gelshift assay with oligonucleotide probes containing p53 binding sites. A 2 kb Bik promoter region was also not responsive in the promoter assay. The mode of p53-mediated up-regulation of Bik mRNA accumulation in breast cancer cells exposed to antiestrogen remains to be elucidated.
In cells treated with adriamycin (Real et al., 2006) and in cells infected with adenovirus (Subramanian et al., 2007) Bik was shown to be transcriptionally activated by the E2F pathway. The hBik promoter contains an E2F-binding site at position -104. The direct involvement of E2F1 in transcriptional activation of Bik was demonstrated by promoter assays, by ChIP and electrophoretic mobility-shift (EMSA) assays. These results indicated that Bik was a pro-apoptotic target for E2F transcription factors that are activated during genotoxic stress and viral infection.
BIK protein expression was activated in human and murine epithelial cells treated with INFγ (Mebratu et al., 2008), a cytokine that mediates its effect through the transcription factor STAT1. In mouse STAT1−− airway epithelial cells that were treated with INFγ, BIK expression was reduced compared to STAT1+/+ cells, suggesting that BIK expression was increased as a result of STAT1 activation by INFγ. However, the mechanism of STAT1-dependent activation of BIK expression is unclear. Although the Bik promoter contains several STAT1-binding sites, reporter based promoter assays failed to detect response to INFγ treatment. The possibility that cooperation with other transcription factors and/or post transcriptional mechanisms may contribute to increased BIK expression in response to INFγ remains to be elucidated. Treatment of B-lymphoma cell lines with another cytokine, TGF-β resulted in increase in mRNA levels of Bik that was also accompanied by a reduction in the level of Bcl-xL mRNA (Saltzman et al., 1998; Spender et al., 2009). Bik was shown to be a direct transcriptional target for TGF-β via recruitment of the transcription factor Smad to the consensus Smad-binding sites in the Bik promoter (Spender et al., 2009). ChIP assays and EMSA assays revealed binding of the Smad 3/4 complex to a Smad binding site at -1055. These results indicated that BIK was a mediator of TGF-β-induced apoptosis. Increased expression of BIK was observed in human B cell lymphoma that underwent apoptosis as a result of ligation of surface IgM (Jiang and Clark, 2001). The increase in BIK expression was shown to be regulated both at transcriptional as well as at post-transcriptional level. The increase in Bik mRNA was abolished by treatment with cyclosporin A suggesting a calcium/calcineurin-dependent pathway while sustained BIK expression was dependent on both the calcium/calcineurin and the PI3K-dependent pathways. The regulated expression of BIK appears to be critical for the selection of mature B lymphocytes.
Mechanisms of BIK induced cell death
The mechanism of cell death induced by BIK has been investigated in epithelial cancer cell lines by different groups. Overexpression of hBIK via adenovirus vectors revealed that BIK induced apoptosis occurred through the activation of caspase-9 and the mitochondrial release cytochrome c (Germain et al., 2002; Gillissen et al., 2003; Tong et al., 2001). In transient transfection studies, the cell death activity of mBIK (BLK) was also shown to be mediated by the mitochondrial pathway since mBIK-induced cell death was inhibited by a dominant negative mutant of caspase-9. Thus, BIK induced cell death in epithelial cancer cells appears to follow the prototypical mitochondrial caspase-9-dependent pathway.
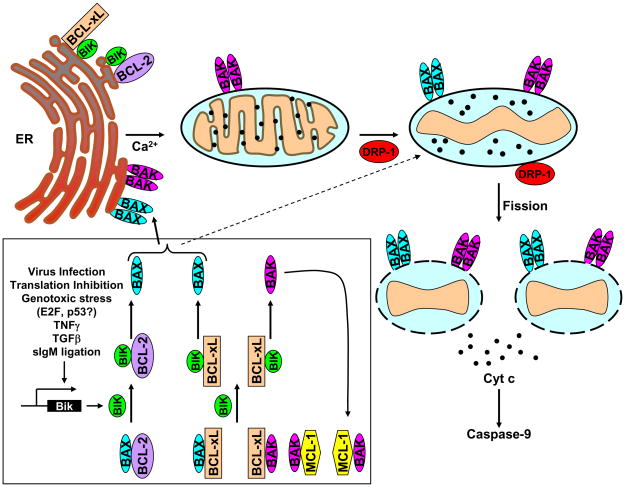
The mechanism of BIK-induced cell death in epithelial cancer cells has been further refined through studies by Shore and colleagues. They have shown that ER-localized BIK induced apoptosis via the mitochondrial pathway that resulted in cytochrome c release (Germain et al., 2005; Germain et al., 2002; Mathai et al., 2005). The release of ER Ca2+ mediated by BIK was dependent on localization of conformationally activated BAX/BAK in the ER and was deficient in BAX/BAK double knockout mouse kidney cells (Mathai et al., 2005). They demonstrated that BIK-induced Ca2+ release from the ER resulted in the recruitment of the mitochondrial fission protein DRP1 (Dynamin related protein 1) from the cytosol to the mitochondria and remodeling of the inner mitochondrial membrane cristae (Germain et al., 2005). BIK (wt) expression caused opening of the mitochondrial cristae tubules while expression of a BH3 mutant (L61G) of BIK was deficient in cristae opening linking the activity of the BH3 domain to cristae opening. BIK-induced cristae remodeling was inhibited by dominant negative DRP1 as well as by an inhibitor of mitochondrial Ca2+ uptake (Ru360). These results illuminated the essential role of the Ca2+-dependent GTPase activity of DRP1 in BIK-mediated opening of the cristae thereby mobilizing cytochrome c for cytosolic release via organelle fragmentation (Fig. 2). These results were consistent with previous studies that established the role of DRP1-mediated mitochondrial transformation in cytochrome c release during apoptosis (Frank et al., 2001; Scorrano et al., 2002). The mechanism of BIK-mediated cristae opening was different from that of tBID-mediated cristae remodeling that did not require the BH3 domain of tBID (Scorrano et al., 2002). The Ca2+-dependent release of cytochrome c was consistent with an in vitro study using isolated mitochondria where the release of cytochrome c mediated by recombinant BIK was suppressed by Ca2+ chelation (Shimizu and Tsujimoto, 2000). The Shore group also observed cooperation between BIK and the mitochondrial BH3-only member NOXA. Expression of hNOXA did not induce cytochrome c egress while coexpression of BIK and NOXA induced conformational activation of BAX and accelerated cytochrome c release. Although the mechanism of such cooperation between the two BH3-only proteins which localized at different organelles is not known, it is possible that it may be related to cooperative activation of BAX by BIK and liberation of BAK from the complex with MCL-1 by NOXA (Willis et al., 2005) (see below).
Figure 2. Model for BIK-induced apoptosis in epithelial cancer cells.
In the boxed area, various stimuli that transcriptionally activate the expression of endogenous Bik gene are indicated. The mode of BAX activation through interaction of BIK with BCL-xL and BCL-2 is also shown in the boxed panel. The mode of mitochondrial cristae remodeling, mitochondrial fission and the release of cytochrome c mediated by ER-associated BIK is illustrated and is based on Germain et al. (Germain et al., 2005). The mitochondrial uptake of Ca2+ released from the ER is depicted to recruit the cytosolic GTPase, DRP-1 to the mitochondria to mediate mitochondrial fission.
In contrast to the apoptotic cell death induced by BIK in epithelial cancer cells, BIK induced non-apoptotic cell death in other cell types. The cell death induced by BIK was independent of activation of common caspases such as caspase 6/7, caspase-9, and the release of cytochrome c in malignant glioma (Naumann et al., 2003) and melanoma (Oppermann et al., 2005) cell lines. In the case of glioma cell lines, BIK-induced cell death was suppressed by high concentrations of the broad spectrum caspase inhibitor zVAD-fmk and the anti-apoptosis protein XIAP suggesting an involvement of some caspase-related activities. The death activity in these cells was only partially suppressed by BCL-2/BCL-xL overexpression, raising the possibility that BIK-induced cell death might be independent of engagement of BCL-2/BCL-xL. The precise mechanism of cell death in these neuronal and ectodermal cancer cells remains unknown.
A form of non-apoptotic death was also observed in Bcl-2−/− mouse embryo fibroblasts (Rashmi et al., 2008). Overexpression of BIK in Bcl-2−/− cells resulted in enhanced cell death compared to Bcl-2+/+ cells in the absence of activation of caspase-9 and 3 and detectable release of cytochrome c from the mitochondria. The extent of BIK-induced cell death was augmented by treatment with the pancaspase inhibitor, zVAD-fmk in these cells. The cell death induced by BIK exhibited certain autophagic features such as cytosolic vacuoles, punctate distribution of LC3 and enhanced expression of Beclin-1 and was inhibited by pharmacological inhibitors of autophagy. Although the mechanism of this specific mode of cell death induced by BIK is not known, the possibility that BIK may selectively liberate Beclin-1 from BCL-xL remains to be investigated.
BAX-dependent apoptosis signaling by BIK
There is an obligate requirement of BAX and BAK for the manifestation of the pro-apoptotic activity of various BH3-only proteins. Mouse embryo fibroblasts (MEFs) doubly deficient for both BAX and BAK were shown to be resistant to apoptosis induced by ectopic expression of various BH3-only proteins such as BID, BIM and NOXA while MEFs singly deficient for either BAX or BAK were not (Cheng et al., 2001; Wei et al., 2001; Zong et al., 2001). Although the functional redundancy model for BAX and BAK is widely accepted, the pro-apoptotic activity of BIK was shown to be dependent only on BAX in several human epithelial cancer cell lines (Gillissen et al., 2003; Theodorakis et al., 2002). The exclusive BAX-dependency for BIK-induced cell death was not due to any functional deficiency in BAK expression as ectopic overexpression of BAK did not confer sensitivity to BIK in BAX-null cells (Theodorakis et al., 2002). Further, these cells exhibited sensitivity to a BAK-dependent pro-apoptotic protein BCL-xS (Gillissen et al., 2007). A potential mechanism underlying the BAX selectivity for BIK-induced apoptosis was recently reported (Fig. 2, boxed panel). In healthy cells, BAK was shown to be sequestered by BCL-xL and MCL-1 (Willis et al., 2005). Gillissen et al. showed that in coimmunoprecipitation studies BIK expressed from an adenovirus vector interacted with endogenous BCL-xL and not with MCL-1 and thus failed to disrupt the BAK-MCL-1 complex. Further, the stability of MCL-1 was also enhanced in BIK expressing cells. Displacement of the BH3 domain containing E3 ligase, MULE (Zhong et al., 2005) from MCL-1 by other BH3 ligands appeared to be a contributing factor in MCL-1 stabilization (Warr et al., 2005). In light of the inability of BIK to interact with MCL-1 in the above experiments, it appeared that BAK liberated from the BCL-xL complex by BIK might contribute to increased accumulation of MCL-1. The role of MCL-1 in dictating the BAX-dependent mode of action was further substantiated by siRNA mediated depletion of MCL-1 in BAX-null cells resulting in sensitivity to BIK. Since BIK was also shown to efficiently interact with BCL-2 (Boyd et al., 1995; Elangovan and Chinnadurai, 1997), it is likely that BIK might displace BAX from the BCL-2/BAX complex as well. The result of Gillissen et al. in which overexpresed BIK in BAX-null human cancer cells (DU145) did not interact with endogenous MCL-1 is different from the results of Shimazu et al. who reported interaction between transfected BIK and endogenous MCL-1 in 293 cells (Shimazu et al., 2007). Since 293 cells express E1B-19K that has been demonstrated to be in constitutive complex with BAK (Lomonosova et al., 2002; Sundararajan et al., 2001), it is possible that a fraction of MCL-1 is not complexed with BAK in these cells. Thus, the available evidence suggests that BIK may activate BAX by the ‘indirect mode’ i.e., interaction with pro-survival proteins (see an accompanying Chapter by Giam et al.) by selective displacement of BAX from BCL-xL and BCL-2. A caveat to the above model of BAX activation by BIK is that most BAX molecules in normal cells were reported to be in free monomeric form (Willis et al., 2005). Since BAX was originally identified as a BCL-2-binding protein (Oltvai et al., 1993), the possibility that at least a fraction of BAX might be present in complex with BCL-2/BCL-xL cannot be ruled out. Although the data of Gillissen et al is consistent with the ‘displacement’ model, other potential mechanism(s) of BAX activation by BIK cannot be ruled out.
BIK in physiological and pathological cell death
Mice with homozygous deletion in the Bik locus were shown to be viable without overt developmental defects (Coultas et al., 2004), possibly reflecting functional redundancy with other BH3-only members. For example, concomitant deletion of Bik and Bim was shown to arrest spermatogenesis in mice suggesting both BH3-only members play a redundant role in normal testicular development (Coultas et al., 2005). In humans, BIK appears to play a critical role in B-cell homeostasis. In a gene expression profiling study of B cell germinal center reaction, activation of Bik, in addition to other pro-apoptotic genes such as FAS was detected during naive B cell to centroblast transition and remained up-regulated in the memory B cells (Klein et al., 2003). As discussed earlier, in studies using B-lymphoma cells, increased expression of BIK was observed in cells that underwent apoptosis as a result of ligation of surface IgM (Jiang and Clark, 2001). These studies suggested that BIK may be important in apoptosis selection of mature human B lymphocytes.
Critical roles for BIK during certain pathological insults have been reported. The cytokine IFNγ which has been shown to induce apoptosis to resolve inflammation-induced airway hyperplasia was shown to mediate its pro-apoptotic activity through the action of BIK (Mebratu et al., 2008). Treatment with IFNγ induced increase in Bik mRNA and protein in human and murine airway epithelial cells. The apoptotic activity of IFNγ was diminished in cells transduced with Bik shRNA and in murine cells derived from Bik−/− animals. The cell death activity of BIK was attributed to its ability to interact with activated ERK1/2 in a BH3-dependent fashion and to inhibit nuclear translocation of ERK1/2. These results suggested that BIK may be important in elimination of supernumerary of inflammation-damaged cells in the airway.
Infection of mammalian cells with bacterial and viral pathogens generally inhibit cellular protein synthesis resulting in apoptosis of infected cells. One of the toxins produced by Escherichia coli, MazF that inhibited protein synthesis by cleavage of cellular mRNA induced apoptosis through BIK (Shimazu et al., 2007). The use of cells derived from mutant mice deficient in various BH3-only members such as Bik, Bim, Puma and Noxa revealed that MazF-induced cell death was dependent only on Bik. Similar effects were observed when cells were treated with a general translation inhibitor, cycloheximde. These results highlight the general importance of BIK in apoptosis induced by pathogens and toxins.
The results of Shimazu et al. (Shimazu et al., 2007) also suggested that BIK may be important in viral pathogenesis since shutoff of host cell protein synthesis is a prominent feature in cells infected with several different viruses. The authors showed that infection of mouse kidney cells from Bik−/− mice with an apoptogenic mutant of adenovirus type 5 did not manifest the apoptotic cytopathic effect. A different independent study identified prominent transcriptional activation of Bik during adenovirus induced apoptosis in human cells (Subramanian et al., 2007). This study demonstrated that the transcriptional activation of Bik was mediated by the E2F pathway. Specific depletion of BIK in adenovirus virus infected cells reduced the level of apoptosis induced by an apoptogenic viral mutant that lacked the anti-apoptosis protein E1B-19K. Although it is not known whether Bik was activated in adenovirus-infected mouse cells by an E2F-dependent mechanism, the conservation of E2F-binding sites in both human and mouse promoters might be an indication of a common mode of activation. In light of the results from the two groups, it would be interesting to directly determine the role of BIK in apoptosis induced by other viruses. It should be noted that BIK was shown to interact with the viral anti-apoptosis protein EBV-BHRF1 (Boyd et al., 1995), in addition to E1B-19K (Boyd et al., 1995; Han et al., 1996).
While viruses appear to target certain BH3-only members such as BIK to inhibit apoptosis through sequestration by their anti-apoptosis proteins, the pathogen Chlamydia trachomatis was shown to target BH3-only proteins for degradation in infected cells (Dong et al., 2005). It is known that infection with C. trachomatis, a leading cause for blindness and sexually transmitted diseases in certain parts of the world, results in an inflammatory response without significant cellular apoptotic response. Chlamydial infection was also shown to confer resistance to heterologous apoptotic stimuli (Fan et al., 1998). In human cells infected with C. trachomatis, the BH3-only proteins BIK, BIM and PUMA were degraded without any effect on some other BH3-only members (BID and BAD that are normally activated by post-transcriptional modifications). There was also no change in the multi-domain pro-apoptotic (BAX and BAK) and anti-apoptotic proteins (BCL-2 and BCL-xL). Although the specific consequence of degradation of BH3-only proteins in Chlamydial pathogenesis remains to be established, the above study suggested a novel mode of suppression of apoptotic response by pathogenic bacteria.
BIK and human cancer
Bik as a tumor suppressor
There are several reports suggesting that the Bik gene may serve as a pro-apoptotic tumor suppressor in specific tissues. Bik was shown to be expressed at high levels in normal kidney epithelia (Daniel et al., 1999) while in renal cell carcinomas (RCC) the expression of the Bik gene was inactivated by loss of heterozygosity at the Bik locus in chromosome 22q13.2 and by epigenetic promoter silencing (Sturm et al., 2006). A genome-wide analysis of genetic changes in human gliomas identified a deleted segment in chromosome 22q encompassing the Bik gene (Bredel et al., 2005). Short chromosomal deletions of the region containing the Bik locus were also reported in colorectal cancers (Castells et al., 1999) and in head and neck cancers (Reis et al., 2002). The Bik gene was shown to be frequently mutated in peripheral B-cell lymphomas (Arena et al., 2003). Mutations in both intronic and exonic regions of the Bik gene were identified. Although no mutation was detected in exon 3 coding for the BH3 domain, other exonic mutations were concentrated in the region coding for BIK between amino acids 43 and 134 that was previously shown to be important for maximal pro-apoptotic activity of BIK (Elangovan and Chinnadurai, 1997).
A microarray analysis showed prominent activation of Bik mRNA expression among over 350 apoptosis-related genes in four different human lung, prostate and renal carcinoma cell lines that were treated with a DNA methyltransferase 1 (DNMT1) inhibitor (5′-Aza-2′-deoxycytidine) and a histone deacetylase (HDAC) inhibitor (Depsipeptide) (Dai et al., 2006). These drugs had a synergistic effect in augmenting the level of BIK protein expression. A different study also reported increase in Bik mRMA and protein in human cancer cells that were treated with DNMT1 antisense oligonucleotides (Milutinovic et al., 2004). A microarray analysis of a multiple myeloma cell line treated with the demethylating drug zebularine also provided evidence that the Bik gene was epigenetically silenced (Pompeia et al., 2004). Although it is unclear whether the effect of the inhibitors of epigenetic modifications is directly related to changes at the Bik promoter or through indirect effects, the above studies suggested that the transcription of the Bik gene was silenced in different cancer cell lines. As discussed earlier, the expression of Bik was reported to be silenced in estrogen-dependent breast cancer cells and was activated by treatment with antiestrogens, resulting in BIK-mediated apoptosis and that cells resistant to antiestrogen-induced apoptosis did not express Bik (Hur et al., 2006; Hur et al., 2004). Several reports have shown that in cancer cells that constitutively express Bik mRNA, the BIK protein was actively targeted by the proteasomal machinery (Li et al., 2008; Marshansky et al., 2001; Nikrad et al., 2005; Yeung et al., 2006; Zhu et al., 2005b). Thus, genomic deletions at the Bik locus, selective silencing of the Bik gene expression and/or post-transcriptional down-regulation of BIK protein by proteasomal degradation and efficient apoptosis upon BIK reexpression reinforce the notion that Bik is a tumor suppressor.
Bik as a prognostic marker
Paradoxically, Bik expression was found to be high in certain sporadic breast tumors (Garcia et al., 2005). Also poor prognosis of non-small cell lung cancers (NSCLC) was shown to correlate with high expression of Bik (Lu et al., 2006). In the case of NSCLC, the increase in Bik expression also coincided with increase in Bcl-2 expression. It is possible that the pro-apoptotic activity of BIK might be kept in check by BCL-2 during tumorigenesis and chronic low level cell death mediated by runaway BIK may lead to tumor cell adaptation and evolution of aggressive tumor cells. Since overexpression of Bik and Bcl-2 in NSCLC was also accompanied by overexpression of caspases (caspase 8 and caspase 10) associated with inflammation and immune response, it is possible that the battle between the pro-apoptotic (BIK) and anti-apoptotic (BCL-2) molecules may lead to inflammatory responses and tumor progression. Interestingly, overexpression of another BH3-only member, Bnip3 was also reported to be associated with poor prognosis in breast cancers (Sowter et al., 2003) and in NSCLC (Giatromanolaki et al., 2004) (see Chapter by Chinnadurai, Vijayalingam and Gibson).
BIK in anticancer strategies
Since activation of BIK expression induced efficient cell death in estrogen-responsive breast cancer cell lines (Hur et al., 2006; Hur et al., 2004), modulating BIK expression could prove to be the basis of an interventional approach to treat estrogen receptor-positive human breast cancers. As mentioned earlier, treatment with various chemotherapeutic agents such as adriamycin (Real et al., 2006), camptothecin (Paquet et al., 2004) and doxorubicin (Panaretakis et al., 2002) was reported to activate BIK expression in p53-positive as well as in p53-negative cells. Treatment with DNMT1 inhibitors and HDAC inhibitors which activate Bik expression from the endogenous (silenced) Bik locus and the use of proteasomal inhibitors that stabilize BIK protein (in addition to other BH3-only members such as BIM and NOXA) appear to be highly promising anticancer approaches. The efficacy of proteasome inhibitors was also reported to be enhanced in combination with other chemotherapeutic agents such as TRAIL (Nikrad et al., 2005; Zhu et al., 2005a), cisplatin (Li et al., 2008) and inositol hexakisphosphate (Diallo et al., 2008).
Several reports linked the lack of BIK expression to drug resistance during chemotherapeutic treatments. While many of the conventional drug therapy fails to overcome the impasse of chemoresistance, using novel interventional strategies such as overexpression of BIK via exogenously introduced transgene expression could constitute an attractive approach to treat drug resistant cancers. BIK overexpression in drug resistant breast cancer cells that were deficient in the mitochondrial apoptosis pathway resulted in reversion of chemoresistance (Radetzki et al., 2002). Vector-mediated BIK expression in a TALL (acute lymphoblastic/lymphocytic leukemia) cell line conferred sensitivity to cortico-steroid induced cell death (Daniel et al., 1999). Adenovirus-mediated BIK expression was shown to inhibit tumor formation by chemoresistant human prostate and colon cancer cell lines in a mouse xenotransplant model (Tong et al., 2001). As pointed out earlier, in vitro studies have suggested potential BIK-based cancer gene therapy approaches to kill difficult cancers such as melanoma (Oppermann et al., 2005) and glioblastoma (Naumann et al., 2003). Busulfan (Bu) is a major conditioning chemotherapeutic agent used during bone marrow transplantation in patients with chronic or acute myelogenous leukemia. In Bu-resistant leukemic cell lines and in clinical samples expression of Bik among other apoptotic genes such as Bnip3 was found to be down-regulated while anti-apoptotic genes such as Bcl-2 and Bcl-xL were up-regulated (Valdez et al., 2008). Therapeutic approaches to activate the pro-apoptotic BH3-only molecules including Bik and/or gene therapy based approaches may improve the clinical outcome of chemotherapy treatments.
Systemic administration of wt Bik gene or a phosphomimetic mutant version (T33S35→D, BikDD) (that exhibited enhanced apoptotic activity) via cationic liposome particles was shown to inhibit growth and metastasis of human breast cancer cells in the mouse orthotopic xenograft model (Li et al., 2003; Zou et al., 2002). A similar approach using a tumor-specific BikDD expression construct was shown to exhibit significant anti-tumor effects on pancreatic cancer and prolong survival in xenograft and orthotopic mouse models of pancreatic tumors without toxicity (Xie et al., 2007).
Conclusions
Since the discovery of BIK, a number of BH3-only members have been identified and their physiological role as effectors of the canonical animal cell apoptosis pathway has been established. Although BIK deficiency does not appear to affect the normal developmental processes in the small animal model (possibly as a result of functional redundancy with other BH3-only cousins such as BIM) BIK is implicated in certain normal and pathological functions in human cells. Particularly, the potential role of BIK in viral pathogenesis is intriguing and could be a novel target in antiviral strategies. Since BIK appears to function as a tumor suppressor in human and appears to be activated by small molecule drugs that reactivate epigenetically silenced Bik gene or by other chemotherapeutic drugs that activate p53-indepdent transcriptional pathways, anti-cancer strategies focused on augmenting BIK expression appear to be attractive. Since proteasomal inhibitors have been shown to dramatically increase BIK protein accumulation, the combination of transcriptional modulators and proteasomal inhibitors appear to be very promising. The BIK-based gene therapy approaches also hold promise for treatment of difficult cancers such as brain and pancreatic cancers.
Acknowledgments
The authors were supported by grants CA-33616 and CA-73803 and from the National Cancer Institute. GC is grateful to Drs. Thomas Chittenden and Robert Lutz (Immunogen Inc.) for valuable discussions that were critical in functional characterization of BIK. GC expresses his gratitude to Dr. Walter Blattler (Immunogen Inc.) for his support and discussions. The authors thank Dr. Marc Germain for his comments on this review.
References
- Aouacheria A, Brunet F, Gouy M. Phylogenomics of life-or-death switches in multicellular animals: Bcl-2, BH3-Only, and BNip families of apoptotic regulators. Mol Biol Evol. 2005;22:2395–2416. doi: 10.1093/molbev/msi234. [DOI] [PubMed] [Google Scholar]
- Arena V, Martini M, Luongo M, Capelli A, Larocca LM. Mutations of the BIK gene in human peripheral B-cell lymphomas. Genes Chromosomes Cancer. 2003;38:91–96. doi: 10.1002/gcc.10245. [DOI] [PubMed] [Google Scholar]
- Boyd JM, Gallo GJ, Elangovan B, Houghton AB, Malstrom S, Avery BJ, et al. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11:1921–1928. [PubMed] [Google Scholar]
- Boyd JM, Malstrom S, Subramanian T, Venkatesh LK, Schaeper U, Elangovan B, et al. Adenovirus E1B 19 kDa and Bcl-2 proteins interact with a common set of cellular proteins. Cell. 1994;79:341–351. doi: 10.1016/0092-8674(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Bredel M, Bredel C, Juric D, Harsh GR, Vogel H, Recht LD, et al. High-resolution genome-wide mapping of genetic alterations in human glial brain tumors. Cancer Res. 2005;65:4088–4096. doi: 10.1158/0008-5472.CAN-04-4229. [DOI] [PubMed] [Google Scholar]
- Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- Castells A, Ino Y, Louis DN, Ramesh V, Gusella JF, Rustgi AK. Mapping of a target region of allelic loss to a 0.5-cM interval on chromosome 22q13 in human colorectal cancer. Gastroenterology. 1999;117:831–837. doi: 10.1016/s0016-5085(99)70341-0. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Chittenden T, Flemington C, Houghton AB, Ebb RG, Gallo GJ, Elangovan B, et al. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. Embo J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Bouillet P, Loveland KL, Meachem S, Perlman H, Adams JM, et al. Concomitant loss of proapoptotic BH3-only Bcl-2 antagonists Bik and Bim arrests spermatogenesis. Embo J. 2005;24:3963–3973. doi: 10.1038/sj.emboj.7600857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Bouillet P, Stanley EG, Brodnicki TC, Adams JM, Strasser A. Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed death. Mol Cell Biol. 2004;24:1570–1581. doi: 10.1128/MCB.24.4.1570-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Liu S, Marcucci G, Sadee W. 5-Aza-2′-deoxycytidine and depsipeptide synergistically induce expression of BIK (BCL2-interacting killer) Biochem Biophys Res Commun. 2006;351:455–461. doi: 10.1016/j.bbrc.2006.10.055. [DOI] [PubMed] [Google Scholar]
- Daniel PT, Pun KT, Ritschel S, Sturm I, Holler J, Dorken B, et al. Expression of the death gene Bik/Nbk promotes sensitivity to drug-induced apoptosis in corticosteroid-resistant T-cell lymphoma and prevents tumor growth in severe combined immunodeficient mice.PG - 1100-7. Blood. 1999;94:1100–1107. [PubMed] [Google Scholar]
- Diallo JS, Betton B, Parent N, Peant B, Lessard L, Le Page C, et al. Enhanced killing of androgen-independent prostate cancer cells using inositol hexakisphosphate in combination with proteasome inhibitors. Br J Cancer. 2008;99:1613–1622. doi: 10.1038/sj.bjc.6604730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Pirbhai M, Xiao Y, Zhong Y, Wu Y, Zhong G. Degradation of the proapoptotic proteins Bik, Puma, and Bim with Bcl-2 domain 3 homology in Chlamydia trachomatis-infected cells. Infect Immun. 2005;73:1861–1864. doi: 10.1128/IAI.73.3.1861-1864.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangovan B, Chinnadurai G. Functional dissection of the pro-apoptotic protein Bik. Heterodimerization with anti-apoptosis proteins is insufficient for induction of cell death. J Biol Chem. 1997;272:24494–24498. doi: 10.1074/jbc.272.39.24494. [DOI] [PubMed] [Google Scholar]
- Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, et al. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998;187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Garcia N, Salamanca F, Astudillo-de la Vega H, Curiel-Quesada E, Alvarado I, Penaloza R, et al. A molecular analysis by gene expression profiling reveals Bik/NBK overexpression in sporadic breast tumor samples of Mexican females. BMC Cancer. 2005;5:93. doi: 10.1186/1471-2407-5-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain M, Mathai JP, McBride HM, Shore GC. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. Embo J. 2005;24:1546–1556. doi: 10.1038/sj.emboj.7600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain M, Mathai JP, Shore GC. BH-3-only BIK functions at the endoplasmic reticulum to stimulate cytochrome c release from mitochondria. J Biol Chem. 2002;277:18053–18060. doi: 10.1074/jbc.M201235200. [DOI] [PubMed] [Google Scholar]
- Giatromanolaki A, Koukourakis MI, Sowter HM, Sivridis E, Gibson S, Gatter KC, et al. BNIP3 expression is linked with hypoxia-regulated protein expression and with poor prognosis in non-small cell lung cancer. Clin Cancer Res. 2004;10:5566–5571. doi: 10.1158/1078-0432.CCR-04-0076. [DOI] [PubMed] [Google Scholar]
- Gillissen B, Essmann F, Graupner V, Starck L, Radetzki S, Dorken B, et al. Induction of cell death by the BH3-only Bcl-2 homolog Nbk/Bik is mediated by an entirely Bax-dependent mitochondrial pathway. Embo J. 2003;22:3580–3590. doi: 10.1093/emboj/cdg343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillissen B, Essmann F, Hemmati PG, Richter A, Oztop I, Chinnadurai G, et al. Mcl-1 determines the Bax dependency of Nbk/Bik-induced apoptosis. J Cell Biol. 2007;179:701–715. doi: 10.1083/jcb.200703040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Sabbatini P, White E. Induction of apoptosis by human Nbk/Bik, a BH3-containing protein that interacts with E1B 19K. Mol Cell Biol. 1996;16:5857–5864. doi: 10.1128/mcb.16.10.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde R, Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES. Blk, a BH3-containing mouse protein that interacts with Bcl-2 and Bcl-xL, is a potent death agonist. J Biol Chem. 1998;273:7783–7786. doi: 10.1074/jbc.273.14.7783. [DOI] [PubMed] [Google Scholar]
- Hinds MG, Smits C, Fredericks-Short R, Risk JM, Bailey M, Huang DC, et al. Bim, Bad and Bmf: intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets. Cell Death Differ. 2007;14:128–136. doi: 10.1038/sj.cdd.4401934. [DOI] [PubMed] [Google Scholar]
- Hur J, Bell DW, Dean KL, Coser KR, Hilario PC, Okimoto RA, et al. Regulation of expression of BIK proapoptotic protein in human breast cancer cells: p53-dependent induction of BIK mRNA by fulvestrant and proteasomal degradation of BIK protein. Cancer Res. 2006;66:10153–10161. doi: 10.1158/0008-5472.CAN-05-3696. [DOI] [PubMed] [Google Scholar]
- Hur J, Chesnes J, Coser KR, Lee RS, Geck P, Isselbacher KJ, et al. The Bik BH3-only protein is induced in estrogen-starved and antiestrogen-exposed breast cancer cells and provokes apoptosis. Proc Natl Acad Sci U S A. 2004;101:2351–2356. doi: 10.1073/pnas.0307337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A, Clark EA. Involvement of Bik, a proapoptotic member of the Bcl-2 family, in surface IgM-mediated B cell apoptosis. J Immunol. 2001;166:6025–6033. doi: 10.4049/jimmunol.166.10.6025. [DOI] [PubMed] [Google Scholar]
- Klein U, Tu Y, Stolovitzky GA, Keller JL, Haddad J, Jr, Miljkovic V, et al. Gene expression dynamics during germinal center transit in B cells. Ann N Y Acad Sci. 2003;987:166–172. doi: 10.1111/j.1749-6632.2003.tb06045.x. [DOI] [PubMed] [Google Scholar]
- Li C, Li R, Grandis JR, Johnson DE. Bortezomib induces apoptosis via Bim and Bik up-regulation and synergizes with cisplatin in the killing of head and neck squamous cell carcinoma cells. Mol Cancer Ther. 2008;7:1647–1655. doi: 10.1158/1535-7163.MCT-07-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Wen Y, Zhou BP, Kuo HP, Ding Q, Hung MC. Enhancement of Bik antitumor effect by Bik mutants. Cancer Res. 2003;63:7630–7633. [PubMed] [Google Scholar]
- Lomonosova E, Subramanian T, Chinnadurai G. Requirement of BAX for Efficient Adenovirus-Induced Apoptosis. J Virol. 2002;76:11283–11290. doi: 10.1128/JVI.76.22.11283-11290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Lemon W, Liu PY, Yi Y, Morrison C, Yang P, et al. A gene expression signature predicts survival of patients with stage I non-small cell lung cancer. PLoS Med. 2006;3:e467. doi: 10.1371/journal.pmed.0030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshansky V, Wang X, Bertrand R, Luo H, Duguid W, Chinnadurai G, et al. Proteasomes modulate balance among proapoptotic and antiapoptotic Bcl-2 family members and compromise functioning of the electron transport chain in leukemic cells.PG - 3130-42. J Immunol. 2001;166:3130–3142. doi: 10.4049/jimmunol.166.5.3130. [DOI] [PubMed] [Google Scholar]
- Mathai JP, Germain M, Marcellus RC, Shore GC. Induction and endoplasmic reticulum location of BIK/NBK in response to apoptotic signaling by E1A and p53. Oncogene. 2002;21:2534–2544. doi: 10.1038/sj.onc.1205340. [DOI] [PubMed] [Google Scholar]
- Mathai JP, Germain M, Shore GC. BH3-only BIK regulates BAX,BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J Biol Chem. 2005;280:23829–23836. doi: 10.1074/jbc.M500800200. [DOI] [PubMed] [Google Scholar]
- McDonnell JM, Fushman D, Milliman CL, Korsmeyer SJ, Cowburn D. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell. 1999;96:625–634. doi: 10.1016/s0092-8674(00)80573-5. [DOI] [PubMed] [Google Scholar]
- Mebratu YA, Dickey BF, Evans C, Tesfaigzi Y. The BH3-only protein Bik/Blk/Nbk inhibits nuclear translocation of activated ERK1/2 to mediate IFNgamma-induced cell death. J Cell Biol. 2008;183:429–439. doi: 10.1083/jcb.200801186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milutinovic S, Brown SE, Zhuang Q, Szyf M. DNA methyltransferase 1 knock down induces gene expression by a mechanism independent of DNA methylation and histone deacetylation. J Biol Chem. 2004;279:27915–27927. doi: 10.1074/jbc.M312823200. [DOI] [PubMed] [Google Scholar]
- Naumann U, Schmidt F, Wick W, Frank B, Weit S, Gillissen B, et al. Adenoviral natural born killer gene therapy for malignant glioma. Hum Gene Ther. 2003;14:1235–1246. doi: 10.1089/104303403767740777. [DOI] [PubMed] [Google Scholar]
- Nikrad M, Johnson T, Puthalalath H, Coultas L, Adams J, Kraft AS. The proteasome inhibitor bortezomib sensitizes cells to killing by death receptor ligand TRAIL via BH3-only proteins Bik and Bim. Mol Cancer Ther. 2005;4:443–449. doi: 10.1158/1535-7163.MCT-04-0260. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Oppermann M, Geilen CC, Fecker LF, Gillissen B, Daniel PT, Eberle J. Caspase-independent induction of apoptosis in human melanoma cells by the proapoptotic Bcl-2-related protein Nbk/Bik. Oncogene. 2005;24:7369–7380. doi: 10.1038/sj.onc.1208890. [DOI] [PubMed] [Google Scholar]
- Panaretakis T, Pokrovskaja K, Shoshan MC, Grander D. Activation of Bak, Bax and BH3-only proteins in the apoptotic response to doxorubicin. J Biol Chem. 2002;21:21. doi: 10.1074/jbc.M205273200. [DOI] [PubMed] [Google Scholar]
- Paquet C, Schmitt E, Beauchemin M, Bertrand R. Activation of multidomain and BH3-only pro-apoptotic Bcl-2 family members in p53-defective cells. Apoptosis. 2004;9:815–831. doi: 10.1023/B:APPT.0000045791.55282.91. [DOI] [PubMed] [Google Scholar]
- Pompeia C, Hodge DR, Plass C, Wu YZ, Marquez VE, Kelley JA, et al. Microarray analysis of epigenetic silencing of gene expression in the KAS-6/1 multiple myeloma cell line. Cancer Res. 2004;64:3465–3473. doi: 10.1158/0008-5472.CAN-03-3970. [DOI] [PubMed] [Google Scholar]
- Radetzki S, Kohne CH, von Haefen C, Gillissen B, Sturm I, Dorken B, et al. The apoptosis promoting Bcl-2 homologues Bak and Nbk/Bik overcome drug resistance in Mdr-1-negative and Mdr-1-overexpressing breast cancer cell lines.PG - 227-38. Oncogene. 2002;21:227–238. doi: 10.1038/sj.onc.1205010. [DOI] [PubMed] [Google Scholar]
- Rashmi R, Pillai SG, Vijayalingam S, Ryerse J, Chinnadurai G. BH3-only protein BIK induces caspase-independent cell death with autophagic features in Bcl-2 null cells. Oncogene. 2008;27:1366–1375. doi: 10.1038/sj.onc.1210783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real PJ, Sanz C, Gutierrez O, Pipaon C, Zubiaga AM, Fernandez-Luna JL. Transcriptional activation of the proapoptotic bik gene by E2F proteins in cancer cells. FEBS Lett. 2006;580:5905–5909. doi: 10.1016/j.febslet.2006.08.088. [DOI] [PubMed] [Google Scholar]
- Reis PP, Rogatto SR, Kowalski LP, Nishimoto IN, Montovani JC, Corpus G, et al. Quantitative real-time PCR identifies a critical region of deletion on 22q13 related to prognosis in oral cancer. Oncogene. 2002;21:6480–6487. doi: 10.1038/sj.onc.1205864. [DOI] [PubMed] [Google Scholar]
- Saltzman A, Munro R, Searfoss G, Franks C, Jaye M, Ivashchenko Y. Transforming growth factor-beta-mediated apoptosis in the Ramos B-lymphoma cell line is accompanied by caspase activation and Bcl-XL downregulation. Exp Cell Res. 1998;242:244–254. doi: 10.1006/excr.1998.4096. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- Shimazu T, Degenhardt K, Nur EKA, Zhang J, Yoshida T, Zhang Y, et al. NBK/BIK antagonizes MCL-1 and BCL-XL and activates BAK-mediated apoptosis in response to protein synthesis inhibition. Genes Dev. 2007;21:929–941. doi: 10.1101/gad.1522007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Tsujimoto Y. Proapoptotic BH3-only Bcl-2 family members induce cytochrome c release, but not mitochondrial membrane potential loss, and do not directly modulate voltage-dependent anion channel activity. Proc Natl Acad Sci U S A. 2000;97:577–582. doi: 10.1073/pnas.97.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowter HM, Ferguson M, Pym C, Watson P, Fox SB, Han C, et al. Expression of the cell death genes BNip3 and NIX in ductal carcinoma in situ of the breast; correlation of BNip3 levels with necrosis and grade. J Pathol. 2003;201:573–580. doi: 10.1002/path.1486. [DOI] [PubMed] [Google Scholar]
- Spender LC, O’Brien DI, Simpson D, Dutt D, Gregory CD, Allday MJ, et al. TGF-beta induces apoptosis in human B cells by transcriptional regulation of BIK and BCL-X(L) Cell Death Differ. 2009 doi: 10.1038/cdd.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm I, Stephan C, Gillissen B, Siebert R, Janz M, Radetzki S, et al. Loss of the tissue-specific proapoptotic BH3-only protein Nbk/Bik is a unifying feature of renal cell carcinoma. Cell Death Differ. 2006;13:619–627. doi: 10.1038/sj.cdd.4401782. [DOI] [PubMed] [Google Scholar]
- Subramanian T, Vijayalingam S, Lomonosova E, Zhao LJ, Chinnadurai G. Evidence for involvement of BH3-only proapoptotic members in adenovirus-induced apoptosis. J Virol. 2007;81:10486–10495. doi: 10.1128/JVI.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan R, Cuconati A, Nelson D, White E. Tumor necrosis factor-alpha induces Bax-Bak interaction and apoptosis, which is inhibited by adenovirus E1B 19K. J Biol Chem. 2001;276:45120–45127. doi: 10.1074/jbc.M106386200. [DOI] [PubMed] [Google Scholar]
- Theodorakis P, Lomonosova E, Chinnadurai G. Critical requirement of BAX for manifestation of apoptosis induced by multiple stimuli in human epithelial cancer cells. Cancer Res. 2002;62:3373–3376. [PubMed] [Google Scholar]
- Tong Y, Yang Q, Vater C, Venkatesh LK, Custeau D, Chittenden T, et al. The pro-apoptotic protein, Bik, exhibits potent antitumor activity that is dependent on its BH3 domain. Mol Cancer Therapeutics. 2001;1:95–102. [PubMed] [Google Scholar]
- Valdez BC, Murray D, Ramdas L, de Lima M, Jones R, Kornblau S, et al. Altered gene expression in busulfan-resistant human myeloid leukemia. Leuk Res. 2008;32:1684–1697. doi: 10.1016/j.leukres.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Budarf ML, Emanuel BS, Chinnadurai G. Structural analysis of the human pro-apoptotic gene Bik: chromosomal localization, genomic organization and localization of promoter sequences. Gene. 2000;254:157–162. doi: 10.1016/s0378-1119(00)00276-6. [DOI] [PubMed] [Google Scholar]
- Verma S, Zhao LJ, Chinnadurai G. Phosphorylation of the pro-apoptotic protein BIK: mapping of phosphorylation sites and effect on apoptosis. J Biol Chem. 2001;276:4671–4676. doi: 10.1074/jbc.M008983200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guan X, Fok KL, Li S, Zhang X, Miao S, et al. A novel member of the Rhomboid family, RHBDD1, regulates BIK-mediated apoptosis. Cell Mol Life Sci. 2008 doi: 10.1007/s00018-008-8452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr MR, Acoca S, Liu Z, Germain M, Watson M, Blanchette M, et al. BH3-ligand regulates access of MCL-1 to its E3 ligase. FEBS Lett. 2005;579:5603–5608. doi: 10.1016/j.febslet.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Xia W, Li Z, Kuo HP, Liu Y, Ding Q, et al. Targeted expression of BikDD eradicates pancreatic tumors in noninvasive imaging models. Cancer Cell. 2007;12:52–65. doi: 10.1016/j.ccr.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Yeung BH, Huang DC, Sinicrope FA. PS-341 (bortezomib) induces lysosomal cathepsin B release and a caspase-2-dependent mitochondrial permeabilization and apoptosis in human pancreatic cancer cells. J Biol Chem. 2006;281:11923–11932. doi: 10.1074/jbc.M508533200. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Zhu H, Guo W, Zhang L, Wu S, Teraishi F, Davis JJ, et al. Proteasome inhibitors-mediated TRAIL resensitization and Bik accumulation. Cancer Biol Ther. 2005a;4:781–786. doi: 10.4161/cbt.4.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Zhang L, Dong F, Guo W, Wu S, Teraishi F, et al. Bik/NBK accumulation correlates with apoptosis-induction by bortezomib (PS-341, Velcade) and other proteasome inhibitors. Oncogene. 2005b;24:4993–4999. doi: 10.1038/sj.onc.1208683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Peng H, Zhou B, Wen Y, Wang SC, Tsai EM, et al. Systemic tumor suppression by the proapoptotic gene bik. Cancer Res. 2002;62:8–12. [PubMed] [Google Scholar]