Abstract
Given previous demonstrations of both selective and divided attention deficits in Alzheimer’s disease (AD) patients, understanding how declines in the integrity of component processes of selective attention in these patients interact with impairments to executive processes mediating dual-task performance has both theoretical and practical relevance. To address this issue, healthy elderly and AD patients performed computerized tasks of spatial orienting, Simon response interference, and visual search both in isolation and while simultaneously engaged in a visuomotor tracking task (i.e., maintaining car position within a simulated driving environment). Results from the single-task conditions confirmed previous demonstrations of selective attention deficits in AD. Dual-task conditions produced in AD patients (but not healthy elderly) a change in the efficiency of the selective attention mechanisms themselves, as reflected in differential effects on cue or display conditions within each task. Rather than exacerbating the selective attention deficits observed under single-task conditions, however, dual-task conditions produced an apparent diminution of these deficits. We suggest this diminution is due to the combination of deficient top-down inhibitory processes along with a decrease in the attention-capturing properties of cue information under dual-task conditions in AD patients. These findings not only increase our understanding of the nature of the attentional deficits in AD patients, but also have implications for understanding the processes mediating attention in neurologically intact individuals.
Keywords: divided attention, dementia, executive control, spatial orienting, visual search, response interference
Introduction
Attentional disturbances are frequently the first nonmemory impairment to appear in patients with Alzheimer’s disease (AD), and a growing number of investigations have served to identify the specific aspects of attention that are affected in the disease (for reviews, see Amieva, Phillips, Della Sala, & Henry, 2004; Parasurman, Greenwood, & Sunderland, 2002; Parasurman & Haxby, 1993; Perry & Hodges, 1999). In their comprehensive review, Perry and Hodges (1999) classified the pattern of impairments observed in AD in terms of three broad subtypes of attention: 1) selective attention (the ability to focus on a single relevant stimulus or process while ignoring irrelevant stimuli); 2) divided attention (the ability to distribute attention across multiple stimuli or processes); and 3) sustained attention (the ability to maintain attentional focus over time). Based on the then-available evidence, the authors concluded that while sustained attention was relatively well-preserved early in AD (but see a more recent study by Berardi, Parasuraman, & Haxby, 2005), aspects of both selective attention and divided attention were particularly susceptible to disruption. Specifically, disengagement and set-shifting abilities at both the sensory- and response-selection levels in selective attention tasks seemed to be affected, as well as the ability to effectively distribute and coordinate attentional efforts necessary to perform multiple tasks or attend to multiple stimuli simultaneously in divided attention tasks.
The majority of studies investigating the nature of the divided attention deficit in AD have utilized dual-task paradigms in which tasks are performed both singly and in combination (e.g., Baddeley, Bressi, Della Sala, Logie, & Spinnler, 1991; Baddeley, Baddeley, Bucks, &Wilcock, 2001; Grober & Sliwinski, 1991; Logie, Cocchini, Della Sala, & Baddeley, 2004; MacPherson, Della Sala, Logie, & Wilcock, 2007; Nestor, Parasuraman, & Haxby, 1991a; Nestor, Parasuraman, Haxby, & Grady, 1991b; Ramsden, Kinsella, Ong, & Storey, 2008; Sebastian, Menor, & Elosua, 2006); these studies have consistently found AD patients to demonstrate a disproportionate impairment performing two tasks simultaneously relative to healthy elderly controls (but see Crossley, Hiscock, & Foreman, 2004; Lonie, Tierney, Herrmann, Donaghey, O’Carroll, Lee, & Ebmeier, 2008). This dual-task impairment has been observed even when single-task difficulty is adjusted to match performance levels across the groups. Indeed, a recent study (Logie et al., 2004) found that while increasing the demands of the single task produced comparable effects on performance decrements in AD and healthy elderly control groups, AD patients nevertheless showed a disproportionate decrement in performance under dual-task conditions across both easy and difficult single-task demand levels. Healthy elderly, in contrast, have been found to display either no dual-task impairments relative to young controls under similar conditions, or slight age-related costs under conditions in which the tasks share perceptual modalities or require the concomitant generation and execution of similar motor programs (see Riby, Perfect, Stollery, 2004 for review).
Taken together, these findings suggest that the dual-task deficit in AD may be due more to a specific inability to effectively coordinate processing across attentional networks (Baddeley et al., 2001; Logie et al., 2004) than to a general deficit in cognitive function or reduction in available attentional resources (Crossley et al., 2004). This impairment has been interpreted as a deficit in a specific dual-task coordination function of the central executive component of working memory as proposed by Baddeley and his colleagues (Baddeley & Hitch, 1974; Repovs & Baddeley, 2006). The presence of a specific dual-task coordination deficit in AD patients also suggests that the ability to execute concomitantly two competing tasks may prove to be a particularly sensitive marker of subtle changes in cognitive status associated with AD.
Given that AD is associated with impairments in selective attention as well as divided attention, it is critical to understand how declines in the component processes of selective attention (e.g., spatial attention, perceptual filtering, and inhibitory control) interact with the impairments in the executive processes coordinating dual-task performance in these patients. To date, however, there have been relatively few studies that have directly examined interactions between different attentional mechanisms in AD (e.g., Fernandez-Duque & Black, 2006; Festa-Martino, Ott, & Heindel, 2004; Nestor et al., 1991a, b; Parasuraman, Greenwood, & Alexander, 2000; Tales, Snowden, Brown, & Wilcock, 2006), with only two of these studies specifically comparing attentional effects in single-task to dual-task conditions (Nestor et al., 1991a, b).
Behaviorally, dual-task impairments on selective attention tasks could be manifested in different ways. First, dual-task conditions could lead to an overall increase in error rate and/or response time due to difficulty in coordinating and executing performance of the selective attention task under dual-task conditions. Based on their previously documented dual-task deficits, it is anticipated that AD patients would display both types of impairment relative to healthy elderly across a range of selective attention tasks. In addition, dual-task conditions could lead to a change in the efficiency of the selective attention mechanisms themselves, as reflected by a differential effect on different cue or display conditions within a given task. In a covert orienting task, for example, the magnitude of the spatial orienting effect can (regardless of the presence of overall slowing) either remain unchanged or increase (or decrease) due to the differential effect of dual-task conditions on response times to validly- versus invalidly-cued trials. To the degree that specific selective attention mechanisms are impaired in AD patients, the corresponding cue or stimulus conditions should be differentially affected in these patients relative to healthy elderly under dual-task conditions.
It is important to note that while there may be specific cognitive control mechanisms concerned with the coordination of multiple tasks, successful dual-task performance is ultimately an emergent property of effective processing both within and across multiple attentional and working memory components. Consistent with this notion, Lavie and colleagues (Lavie, 1995; Lavie & de Fockert, 2005; Lavie, Hirst, de Fockert, & Viding, 2004) have developed a general cognitive framework for understanding the interplay between cognitive control, working memory, and selective attention processes. These authors propose two mechanisms of selective attention within their load theory: 1) a relatively early and passive perceptual selection mechanism that excludes irrelevant stimuli under conditions of high perceptual load, based simply upon insufficient processing capacity; and 2) a relatively late attentional control mechanism that actively inhibits irrelevant stimuli when they are perceived under conditions of low perceptual load.
In support of this view, a number of empirical studies have found that conditions of high perceptual load that exhaust perceptual capacity lead to a reduction in interference from distracting stimuli, while conditions of low perceptual load lead to an increase in the demands placed on inhibitory control processes (e.g., Lavie, 1995; Lavie & Cox, 1997; Lavie & Fox, 2000). Load theory has also been used to examine age-related changes in selective attention, with older adults displaying greater interference from distracting information than younger adults under low perceptual load conditions, but displaying a more rapid reduction of interference with increasing perceptual load than younger adults (Maylor & Lavie, 1998). Based on these findings, Maylor and Lavie proposed the presence of two age-related deficits in attention: 1) a decline in inhibitory control reflected in increased interference under low perceptual loads; and 2) a decline in perceptual capacity reflected in greater improvement in selective attention with increasing perceptual load.
More recently, Lavie and colleagues have extended their findings to examine the effects of changes in cognitive control load as well as perceptual load on selective attention (Lavie & de Fockert, 2003; Lavie et al., 2004). In contrast to the more passive early selection mechanisms associated with high perceptual load, low perceptual load conditions require active cognitive control mechanisms to effectively inhibit irrelevant but perceived stimuli. In a series of studies, Lavie et al. (2004) examined the effects of two types of cognitive control load (working memory and dual-task coordination) on a selective attention task; in both cases, increased cognitive control load produced increased sensitivity to irrelevant distractor information. Thus, increases in perceptual load and cognitive load produce opposite effects on selective attention: While increases in perceptual load lead to decreased interference, increases in cognitive control load lead to increased interference due to an inability to actively inhibit irrelevant information. Moreover, these findings also indicated that both increased working memory load and dual-task coordination load were effective in increasing distractor interference on selective attention tasks.
Based both on these findings and on the previous demonstrations of a dual-task coordination deficit in AD patients, one could predict that the impairments that AD patients exhibit on selective attention tasks under single-task conditions would be further exacerbated under dual-task conditions. However, other findings suggest that dual-task conditions may not in fact increase the selective attention deficits observed in AD patients, but rather may produce an apparent amelioration or “normalization” of these deficits. While increases in perceptual load have consistently been found to produce decreased interference effects in selective attention tasks as predicted by load theory, increases in cognitive control load have proved to be more variable, producing either increases or decreases in interference depending on the relationship between the nature of the selective attention task and the nature of the cognitive control mechanism being manipulated. Park, Kim and Chun (2007), for example, found that working memory load can either impair or facilitate selective attention depending on whether the working memory items shared processing resources with the targets or distracters, respectively. Similarly, increases in spatial and executive working memory load, but not object working memory load, have been found to impair the efficiency of visual search mechanisms (Han & Kim, 2004; Oh & Kim, 2004; Woodman & Luck, 2004; Woodman, Vogel & Luck, 2001). Finally, recent studies have found that attention capture associated with abrupt onset exogenous orienting cues is reduced under dual-task conditions involving a concurrent monitoring task (Boot, Brockmole & Simons, 2005; Santangelo, Belardinelli & Spence, 2007). Taken together, these findings suggest that dual-task conditions may instead reduce the magnitude of the selection attention deficits observed in AD patients. Because these findings were all based on the performance of healthy young adults, however, it remains unclear exactly how impaired selective attention mechanisms will specifically interact with the dual-task coordination deficits present in AD patients.
The purpose of the present study is therefore to understand the differential effect of dual- versus single-task conditions on AD patients’ performance on tasks that assess different aspects of selective attention. To address this issue, we compared the performance of healthy elderly and AD patients on a set of computerized tasks designed to systematically assess different components of visual attention within the framework of a single basic task paradigm. Specifically, component attentional processes were assessed within tasks involving orienting of spatial attention, Simon response interference, and visual search; these tasks were performed both in isolation and while simultaneously engaged in a visuomotor tracking task (i.e., maintaining car position within a simulated driving environment). We chose to incorporate visuomotor tracking as our secondary task both because of its use in previous studies concerned with dual-task performance in AD (e.g., Logie et al., 2004), and because it continuously engages on-line monitoring and coordination processes while placing minimal demands upon working memory per se. Similarly, the selective attention tasks utilized in this study were designed to be driven primarily by automatic, bottom-up processes while involving minimal engagement of top-down control processes. In this way, we are specifically targeting the interaction between dual-task coordination load and the automatic, stimulus-driven attentional mechanisms previously demonstrated to be impaired in AD.
Methods
Participants
A total of 130 elderly drivers participated in this study: 89 AD patients and 41 elderly controls without cognitive impairment (EC). All participants were between the ages of 55 and 90 years of age and had valid drivers’ licenses. All patients were recruited from the outpatient practices of the Memorial Hospital and Rhode Island Hospital Memory Disorder programs as part of a larger longitudinal investigation of drivers with dementia. Control participants were recruited from patients’ family and friends. All participants signed a document of informed consent approved by Memorial Hospital and Rhode Island Hospital human subjects committee before participation.
Alzheimer disease diagnoses were performed by the study neurologist based on the NINCDS/ADRDA criteria (McKhann, Drachman, Folstein, Katzman, Price, & Stadloan, 1984). Of the 89 AD patients, 54 had very mild dementia and 34 had mild dementia (Clinical Dementia Ratings [CDR; Morris, 1993] of 0.5 and 1, respectively), and 66 were diagnosed with probable AD and 23 were diagnosed with possible AD. All healthy control participants had a CDR=0 and a Mini-Mental State Examination (MMSE) score greater than 26. Participants with mild cognitive impairment but no impairment in activities of daily living were excluded. Diagnostic laboratory tests performed on all patients, exclusion criteria, and methods for the office assessment have been described in detail in several reports from this longitudinal research project (Bhalla, Papandonatos, Stern, & Ott, 2007; Ott, Festa, Amick, Grace, Davis, & Heindel, 2008a; Ott, Heindel, Papandonatos, Festa, Davis, Daiello, & Morris, 2008b).
Eighteen of the 130 participants did not achieve accuracy levels of at least 67% on each of the selective attention tasks under single-task conditions, and were therefore excluded from further analyses. Demographic data for the remaining study sample of 112 participants are presented in Table 1. As expected, there were significant differences between patients and controls for MMSE and education (controls more highly educated).
Table 1.
Demographic information of participants.
| Controls (n=40) | Patients (n=72) | p | |
|---|---|---|---|
| Age, y (SD) | 75.2 (6.7) | 76.8 (6.4) | 0.21 |
| Sex (male/female) | 17/23 | 43/29 | 0.11 |
| Education, y (SD) | 15.4 (3.1) | 13.7 (3.5) | 0.02 |
| MMSE* (SD) | 29.0 (1.1) | 24.8 (2.9) | 0.001 |
MMSE = Mini-mental state examination
Tracking Task
As an experimental analog of driving, participants performed a compensatory visuomotor tracking task in which they used a steering wheel to maintain the position of a car hood within the center lane of a straight three-lane highway displayed on a computer monitor (Figure 1). The center lane measured 19.5° along the bottom edge of the display. The car hood was in the shape of a trapezoid with the dimensions of 5.3° (bottom edge) by 3.8° (top edge) by 3.5° (sides). As they performed the task, the position of the car hood moved continuously and unpredictably to the left and right across the highway at the bottom of the display. The direction and relative magnitude of the force applied to the car hood was determined by a complex low frequency signal composed of three superimposed sine waves (3.75F, 7.5F, 15F) with different base amplitudes (0.1°, 0.5°, 0.2° of visual angle, respectively) at randomized phases (0–360°).
Figure 1.
Illustration of the three selective attention stimulus displays under dual-task conditions. The dotted white line was not part of the display, but rather is a schematic representation of the randomized amplitude and direction of the forcing function that was applied to the car hood over the course of the task.
Single-Task Condition
While the tracking task was performed in isolation, the amplitude of the forcing function was individually adjusted to the value at which each participant could successfully maintain the car in the center lane 88–92% of the time. Participants first practiced moving the car from lane to lane without the forcing function to become comfortable using the steering wheel. Participants then performed the compensatory tracking task with the forcing function set at a constant amplitude for each 1½ minute trial. Across eight successive trials, the amplitude of the forcing function was multiplied by factors of 2.0 to 4.25 and then decreased back down to 2.0 with a step-size of 0.75. The initial 30 seconds of each trial served as practice for the chosen amplitude. The amount of time the car was successfully maintained within the center lane during the final minute of each trial was recorded. The largest amplitude at which the participants could successfully maintain the time-on-target accuracy within the 88–92% criterion range was chosen as the threshold measure of each participant’s compensatory tracking ability, and was then used in the subsequent dual-task conditions. The mean amplitude threshold value for the tracking task (established individually for each participant) did not differ significantly between the EC and AD groups [3.35 vs. 3.13; t(110)=1.42, p=0.16].
Dual-Task Condition
After performing the tracking task alone in order to determine the amplitude of the forcing function, participants then performed the tracking task in conjunction with each of three selective attention tasks (Figure 1). Participants were asked to maintain the car within the center lane in the same manner as they had done in the single task while they performed the cognitive task that was presented at the horizon of the three-lane highway. Each cognitive task was first performed by itself on the static highway display and was then immediately performed in conjunction with the compensatory tracking task. During the initial 30 seconds of the dual-task condition, participants performed the compensatory task alone, after which the cognitive task was initiated. Time-on-target accuracy for the compensatory tracking task under dual-task conditions was measured from the onset of the cognitive task until its completion.
Spatial Orienting
The majority of studies concerned with spatial orienting in AD have utilized modifications of the precuing paradigm developed by Posner and colleagues (Posner, 1980; Posner, Cohen, & Rafal, 1982), in which participants are presented with cues that provide valid, invalid, or neutral (i.e., non-predictive) information regarding the spatial location of a subsequent target stimulus. Valid cues have been found to produce a benefit (i.e., decreased response time) in detecting a target at the cued location compared to an uncued or neutral location, whereas invalid cues produce a cost (i.e., increased response time) in detecting a target at an uncued or neutral location. The difference between response times to the invalidly-and validly-cued targets (i.e., the orienting effect) reflects participants’ efficiency both in automatically shifting spatial attention in response to a valid peripheral cue, and in disengaging attention from an invalid (i.e., “distracting”) peripheral cue. Under single-task conditions, AD patients have previously been found to display an increase in exogenous spatial orienting relative to healthy elderly (e.g., Festa-Martino et al. 2004; Parasuraman, Greenwood, Haxby, & Grady, 1992; Tales, Muir, Bayer, & Snowden, 2002b, Tales et al., 2006).
In the present task, participants focused on a fixation point at the horizon of the three-lane highway which was flanked on the left and right by two square boxes that measured 2.9° on each side. The fixation point was positioned approximately 16.5° from the bottom of the display. The center of each box was 5.0° from fixation. After a variable delay interval between 1,000 and 1,500 ms, the outline of one, both, or neither of the boxes thickened for 100 ms. After a variable inter-stimulus interval (ISI) ranging from 160 to 240 ms (mean ISI=200 ms +/−20%), the warning cue was followed by the presentation of a target at the center of one of the two boxes. The target was a black circle with a diameter of 0.6°. Participants indicated which box contained the target by pressing one of two response keys on the back of the steering wheel with their left or right hands as quickly as possible. Targets remained on the display until a response was made. Response time and accuracy were recorded for each trial.
Participants were given a practice block of 6 trials, followed by a block of 96 trials in which the following four trial types were presented 26 times each in a randomized order: (a) double cue trials, in which both boxes brightened simultaneously; (b) no cue trials, in which neither of the boxes brightened; (c) valid cue trials, in which the box correctly predicting the location of the target brightened; and (d) invalid cue trials, in which the box opposite the subsequent target location brightened. Because there was an equal number of each trial type, all cue conditions were equally probable and therefore of no predictive value. Moreover, the target appeared equally often in the left and right boxes across trials.
Simon Response Interference
While the spatial orienting task examined the effect of dual-task conditions on sensory-level selective attention, the Simon interference task (e.g., Simon & Berbaum, 1990) examined selection primarily at the response level. In a typical Simon interference task, participants are presented with a spatial word (e.g., “left” or “right”) in either the same (i.e., congruent) or different (i.e., incongruent) relative spatial location indicated by the semantic meaning of the spatial word. Participants are asked to respond to the meaning of the spatial word, and to ignore the spatial location at which it was shown. Studies in healthy adults (see Lu & Proctor, 1995; Simon, 1990 for reviews) have shown that response latencies are longer when the word and spatial location are incongruent (e.g., the word “left” shown on the right) than when they are congruent (e.g., the word “left” shown on the left). This increased latency presumably reflects the difficulty inhibiting the prepotent response associated with the corresponding spatial location of the word when trying to select the response corresponding to the semantic meaning of the word. Castel, Balota, Hutchison, Logan, & Yap (2007) found that both healthy elderly and AD patients showed disproportionately larger costs when the stimulus and response were in conflict relative to the costs of healthy young adult, with AD patients additionally producing more errors relative to healthy elderly on incongruent trials.
In the present task, participants focused on a fixation point at the horizon of the three-lane highway (approximately 16.5° from the bottom of the display); the fixation point was flanked on the left and right by two rectangular boxes that measured 5.5° by 2.0°. The center of each box was 6.0° from fixation. After a variable delay interval between 1,000 and 1,500 ms, a target spatial word (LEFT or RIGHT) was displayed at the center of one of the two boxes. Participants indicated the semantic meaning of the spatial word by pressing one of two response keys on the back of the steering wheel with their left or right hands as quickly as possible. Participants were given a practice block of 8 trials, immediately followed by a block of 72 trials in which each of the four possible spatial word/spatial location combinations were presented in equal numbers in a randomized order. Response time and accuracy were recorded for each trial.
Visual Search
In a typical visual search task, participants must search for and detect the presence of a predefined target embedded within an array of irrelevant distractor items. Search efficiency is most often measured by the degree to which target detection response times increase as a function of increasing number of distractor items (i.e., the slope), although other measures such as target detection sensitivity (d-prime) and response criterion shifts (β) are used as well. In highly efficient searches, response times are relatively unaffected by the number of distractor items in the display, while inefficient searches are characterized by a monotonic increase in response times with distractor items. Older adults display significantly steeper slopes compared to young adults under inefficient search conditions (e.g., Humphrey & Kramer, 1997; Madden, Pierce, & Allen, 1996; Plude & Doussard-Roosevelt, 1989), and recent studies have also demonstrated poorer performance in AD patients relative to healthy elderly under conditions of decreased target saliency and increased task complexity (Foldi, Schaefer, White, Johnson, Berger, Carney, & Macina, 2005; Foster, Behrmann, & Stuss, 1999; Greenwood, Parasuraman, & Alexander, 1997; Neargarder & Cronin-Golumb, 2005; Parasuraman et al., 2000; Rösler, Mapstone, Hays, Mesulam, Rademaker, Gitelman, & Weintraub, 2000; Rösler, Mapstone, Hays-Wicklund, Gitelman, & Weintraub, 2005; Tales, Butler, Fossey, Gilchrist, Jones, & Troscianko, 2002a, Tales, Muir, Jones, Bayer, & Snowden, 2004).
The search display in the present task consisted of three, five, or nine colored geometric figures in random positions around the perimeter of a gray box centered at the horizon of the three-lane highway. The items in the display were of three possible shapes (circle, square, triangle) in three possible colors (red, green, blue). The target item was always a red triangle; the distractor items varied across trials with half the items always matching the target in either shape or color but differing on the other dimension (e.g., blue triangles or red circles). The other half of the distractor items were unique from the target on both dimensions (e.g., green squares). This combined-feature search was utilized in order to provide sufficient difficulty under single-task conditions, while also ensuring that AD patients were able to successfully perform the visual task under dual-task conditions with an error rate acceptable for response time analysis. Although the target within this array is always unique on one dimension, search is less efficient than a standard single feature search since a heterogeneous distractor set is used and the dimension (color or form) distinguishing the target from the distractor varies across trials.
Each trial consisted of a fixation cross appearing at the center of the gray box for 500 ms, which was followed by a search display presented for 1000 ms and then a blank gray box for 1500 ms. Each side of the gray box measured 8.7°. The fixation point was positioned approximately 16.5° from the bottom of the display. Each trial began immediately following the 1500 ms blank gray box display of the previous trial. Participants were asked to press a response key on the back of the steering wheel only when the target appeared in the display. They had a maximum time limit of 2500 ms (the combined time of the presentation of the search display and the blank gray box) to respond.
Participants were given six practice trials, immediately followed by a single block of 96 trials. Within the block, the target was present in half of the search displays, and an equal number of search displays at each set size were presented. The position of the target was randomly but equally distributed across the 12 positions around the perimeter of the gray box. Target-present trials in which no response was made were classified as misses, while target-absent trials in which a response was made were classified as false alarms. Response times to target-present trials and accuracy across target-present and target-absent trials were recorded.
Results
Overall Task Accuracy
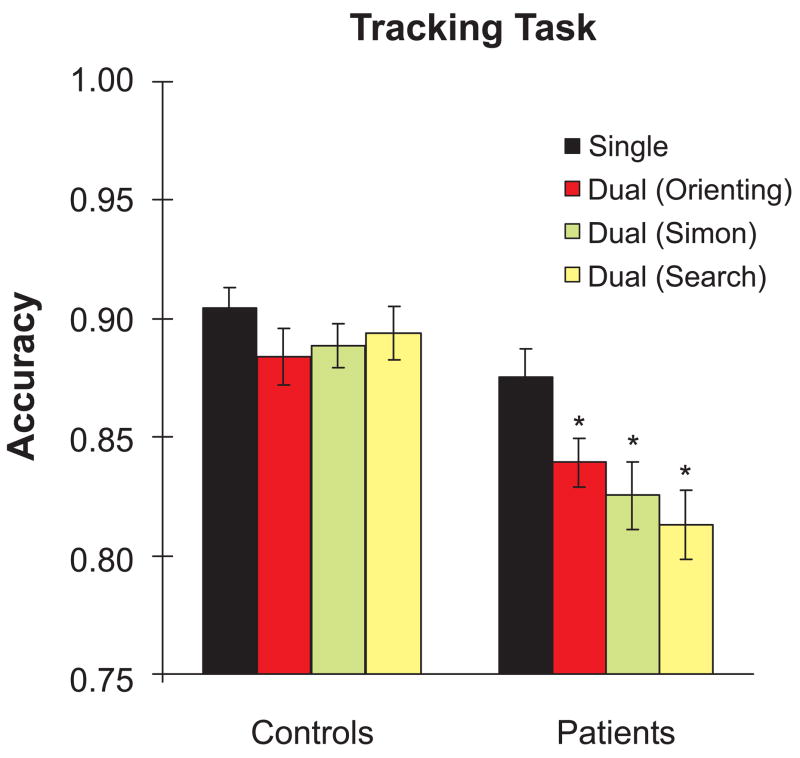
The mean percent time on target for the tracking task in the single-task condition and in the three dual-task condition are shown in Figure 2. A two-way ANOVA with Group (AD, EC) and Task Condition (Single, Dual/Orienting, Dual/Simon, Dual/Search) as factors revealed significant main effects of Task Condition [F(3,330)=6.26, p<0.001, ε=0.89, ηp2=0.06] and Group [F(1,110)=13.51, p<0.001, ηp2=0.11], as well as a significant Task Condition X Group interaction [F(3,330)=2.80, p<0.05, ε=0.89, ηp2=0.03]. Pairwise comparisons further revealed that: a) dual-task tracking accuracy was significantly worse than single-task tracking accuracy under all three dual-task conditions in the AD group [ts(71)>2.69, ps<0.01], but was marginally worse only under the dual-task orienting condition in the EC group [t(39)=1.99, p=0.054]; and b) the AD group was significantly less accurate than the EC group under all three dual-task conditions [ts(110)>2.75, ps<0.008], but not under the single-task condition [t(110)=1.71, p=0.09]. Thus, despite matching tracking performance across groups under single-task conditions, only the AD group displayed substantial performance decrements under all three dual-task conditions, consistent with the presence of a specific dual-task deficit in the AD group.
Figure 2.
Mean time-on-target accuracy rates for the tracking task under single-task and dual-task conditions for each group.
Overall mean accuracy rates for the three selective attention tasks performed under single- and dual-task conditions are shown in Table 2. For all three tasks, two-way ANOVAs with Group (AD, EC) and Task Condition (Single, Dual) as factors revealed significant main effects of Group [Fs(1,110)>8.30, ps<0.005, ηp2s:0.07–0.09] and Task Condition [Fs(1,110)>11.95, ps<0.001, ηp2s:0.10–0.18], as well as significant Group X Task Condition interactions [F(1,110)>3.90, ps<0.05, ηp2s:0.04–0.06]. Within-group comparisons indicated that the AD group performed significantly worse under dual-task than single-task conditions on all three selective attention tasks [ts(71)>4.15, ps<0.001], while the EC group performed significantly worse under dual-task than single-task conditions on the spatial orienting and Simon interference tasks [ts(39)>3.70, ps<0.001] but not the Visual Search task [t(39)=0.94, p=0.35]. Finally, between-group comparisons revealed that, on the spatial orienting and visual search tasks, the AD group performed comparably to the EC group under single-task conditions [ts(110)=1.34, ps>0.18], but performed significantly worse than the EC group under dual-task conditions [ts(110)>2.72, ps<0.01]. On the Simon interference task, the AD group performed significantly worse than the EC group under both single- and dual-task conditions [Single: t(110)=2.11, p<0.04; Dual: t(110)=2.86, p<0.005].
Table 2.
Overall accuracy proportions under single- and dual-task conditions for the three cognitive tasks.
| Spatial Orienting | Simon Interference | Visual Search | ||||
|---|---|---|---|---|---|---|
| Single | Dual | Single | Dual | Single | Dual | |
| Controls | 0.94 (0.004) | 0.91 (0.01) | 0.93 (0.004) | 0.91 (0.01) | 0.94 (0.01) | 0.93 (0.01) |
| Patients | 0.93 (0.01) | 0.86 (0.01) | 0.91 (0.01) | 0.84 (0.02) | 0.93 (0.01) | 0.86 (0.02) |
Note: Numbers in parentheses indicate the standard error of the means.
Spatial Orienting
Mean trimmed response times (removing outliers above and below 2 standard deviations) to correct responses for the different cue and task conditions are shown in Table 3. A three-way ANOVA with Group (AD, EC), Task Condition (Single, Dual) and Cue Type (Valid, Invalid) as factors revealed significant main effects of Group [F(1,110)=7.33, p<0.01, ηp2=0.06], Task Condition [F(1,110)=34.36, p<0.001, ηp2=0.24], and Cue Type [F(1,110)=166.60, p<0.001, ηp2=0.60], as well as a significant Group X Task Condition interaction [F(1,110)=6.15, p<0.02, ηp2=0.05] and a marginally significant Group X Task Condition X Cue Type interaction [F(1,110)=3.58, p=0.06, ηp2=0.03]. These results indicate that response times were: a) slower overall for the AD than the EC group; b) slower for invalid than valid cues for both groups; and c) slower under dual-task than single-task conditions for both groups, but more so for the AD group. In addition, the marginal three-way interaction suggests that dual-task conditions affected response times to valid and invalid cues differently across the groups.
Table 3.
Mean reaction times for the different cue conditions in the spatial orienting task.
| Single | Dual | |||||
|---|---|---|---|---|---|---|
| Valid | Invalid | Double | Valid | Invalid | Double | |
| Controls | 541 (13) | 595 (14) | 552 (13) | 566 (13) | 638 (18) | 591 (12) |
| Patients | 561 (12) | 644 (12) | 597 (11) | 652 (20) | 720 (21) | 666 (21) |
Note: Reaction times are presented in millisecond units. Numbers in parentheses indicate the standard error of the means.
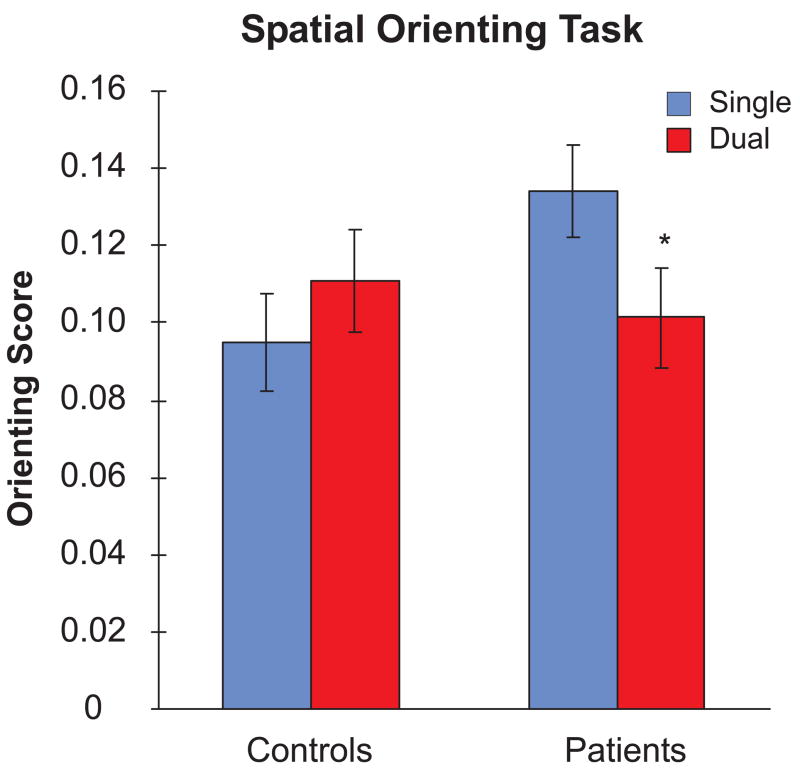
Orienting Scores were calculated by taking the difference between the mean response times for the invalid and valid cue conditions, and dividing these differences by the overall mean response time for each participant in order to adjust for differences in overall slowing (Figure 3). A two-way ANOVA with Group (AD, EC) and Task Condition (Single, Dual) as factors revealed no significant main effects (ps>0.32), but a significant Group X Condition interaction [F(1,110)=4.09, p<0.05, ηp2=0.04]. Pairwise comparisons further revealed that: a) Orienting Scores were comparable under single- and dual-task conditions for the EC group [t(39)=0.99, p=0.33], but were significantly greater in the single-task condition than in the dual-task condition for the AD group [t(71)=2.10, p<0.04]; and b) Orienting Scores were significantly greater in the AD group than the EC group in the single-task condition [t(110)=2.11, p<0.04], but not in the dual-task condition [t(110)=0.47, p=0.64].
Figure 3.
Mean orienting scores under single-task and dual-task conditions for each group. The orienting score was defined as the difference between the response times of the invalid and valid cue conditions, divided by the overall mean response time.
The Orienting Score was separated into Cost and Benefit indexes by computing the differences in mean response times between the invalid and double cue conditions (cost of shifting attention to invalid spatial location) and between the double and valid cue conditions (benefit of shifting attention to a valid spatial location) and dividing these differences by the overall mean response time. A two-way ANOVA on the Cost index with Group (AD, EC) and Task Condition (Single, Dual) as factors revealed no significant effects (ps>0.64), indicating the Cost did not differ across the groups and was unaffected by the dual-task conditions. The two-way ANOVA on the Benefit index, however, revealed a significant Group X Task Condition interaction [F(1,110)=7.16, p<0.01, ηp2=0.06]. Pairwise comparisons further revealed that: a) Benefits were comparable under single- and dual-task conditions for the EC group [0.02 vs. 0.04; t(39)=1.52, p=0.14], but were significantly greater in the single-task condition than in the dual-task condition for the AD group [0.06 vs. 0.02; t(71)=2.61, p<0.01]; and b) Benefits were significantly greater in the AD group than the EC group in the single-task condition [t(110)=2.91, p<0.01], but not in the dual-task condition [t(110)=1.10, p=0.27].
Simon Response Interference
Mean trimmed response times (removing outliers above and below 2 standard deviations) to correct responses for the different trial and task conditions are shown in Table 4. A three-way ANOVA with Group (AD, EC), Task Condition (Single, Dual) and Trial Type (Congruent, Incongruent) as factors revealed significant main effects of Group [F(1,110)=8.23, p<0.005, ηp2=0.07], Task Condition [F(1,110)=79.85, p<0.001, ηp2=0.42], and Trial Type [F(1,110)=99.79, p<0.001, ηp2=0.48], as well as a significant Group X Trial Type interaction [F(1,110)=4.66, p<0.03, ηp2=0.04]. Separate ANOVAs for each group further revealed significant main effects of Task Condition [AD: F(1,71)=51.82, p<0.001, ηp2=0.42; EC: F(1,39)=44.14, p<0.001, ηp2=0.53] and Trial Type [AD: F(1,71)=77.20, p<0.001, ηp2=0.52; EC: F(1,39)=61.99, p<0.001, ηp2=0.61], and a marginally significant Task Condition X Trial Type interaction for the AD group [F(1,71)=3.31, p=0.07, ηp2=0.05]. These results indicate that response times were: a) slower overall for the AD than the EC group; b) slower for incongruent than congruent trials for both groups, but more so for the AD group; and c) slower overall under dual-task than single-task conditions for both groups. Moreover, the difference in response times between the congruent and incongruent trials was marginally decreased under dual-task conditions for the AD group.
Table 4.
Mean reaction times for different conditions in the Simon interference task.
| Single | Dual | |||
|---|---|---|---|---|
| Congruent | Incongruent | Congruent | Incongruent | |
| Controls | 780 (15) | 831 (17) | 924 (23) | 971 (27) |
| Patients | 837 (14) | 931 (20) | 1013 (31) | 1072 (28) |
Note: Reaction times are presented in millisecond units. Numbers in parentheses indicate the standard error of the means.
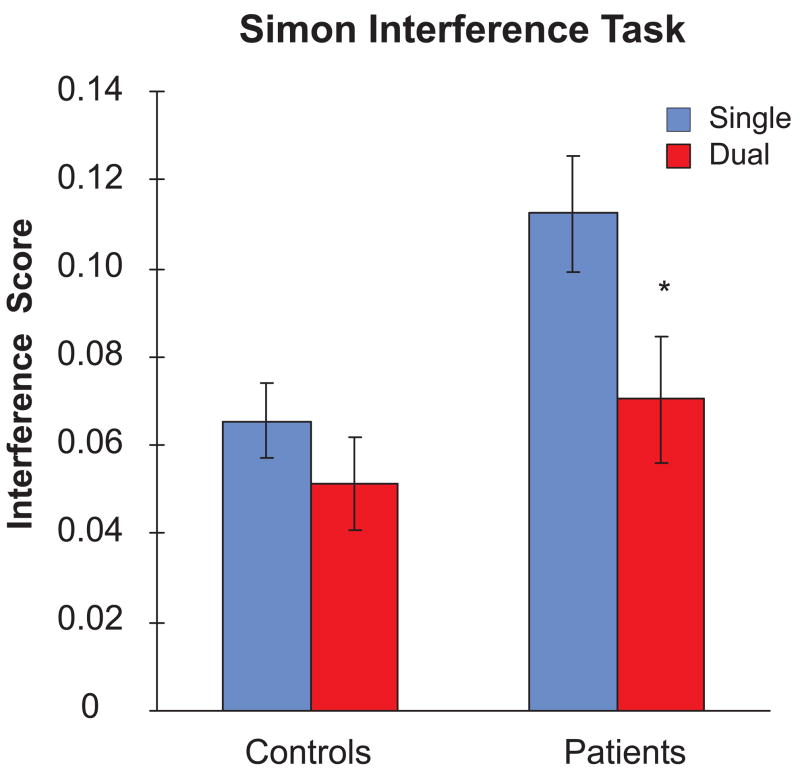
Response Interference Scores were calculated by taking the difference between the mean response time for the congruent and incongruent conditions, and then dividing this difference by the mean response time in the congruent condition for each participant (Figure 4). A two-way ANOVA with Group (AD, EC) and Task Condition (Single, Dual) as factors revealed significant main effects of Group [F(1,110)=5.74, p<0.02, ηp2=0.05] and Condition [F(1,110)=4.05, p<0.05, ηp2=0.04], but no significant Group X Task Condition interaction [F(1,110)=0.98, p=0.32, ηp2=0.009]. Planned comparisons, however, indicated that: a) while Interference Scores were comparable under single- and dual-task conditions for the EC group [0.07 vs. 0.05; t(39)=1.10, p=0.28], they were significantly greater in the single-task condition than in the dual-task condition for the AD group [0.11 vs. 0.07; t(71)=2.15, p<0.04]; and b) Interference Scores were significantly greater in the AD group than the EC group in the single-task condition [t(110)=2.53, p<0.01], but not in the dual-task condition [t(110)=0.93, p=0.36].
Figure 4.
Mean response interference scores under single-task and dual-task conditions for each group. The interference score was defined as the difference between the response times of the congruent and incongruent conditions, divided by the response time of the congruent condition.
In order to investigate which trial type (congruent vs. incongruent) was differentially affected by the dual-task conditions in the AD group, change scores for response times for the congruent and incongruent trials were calculated by taking the difference between the mean response times in the single- and dual-task conditions for each trial type, and then dividing these differences by the mean response times for each trial type in the single-task condition for each participant. Pairwise comparisons indicated that the change score was significantly greater for the congruent trials than the incongruent trials in the AD group [0.21 vs. 0.16; t(71)=2.28, p<0.03], but not in the EC group [0.19 vs. 0.17; t(39)=1.03, p=0.31]. These results indicate that Simon response interference is affected more by dual-task conditions in the AD group than the EC group, and that this reduction in interference under dual-task conditions is due to a greater increase in response times to the congruent than the incongruent trials.
Visual Search
Signal detection d-prime and β criterion parameters were derived from the hit and false alarm rates on target detection within the visual search task (Table 5). While β criterion values did not differ across groups or task conditions [ps>0.64], d-prime values decreased for the AD group in the dual-task condition [t(71)>5.44, p<0.001], indicating that their dual-task performance decrement was due to a reduction in sensitivity to detecting the target rather than to a shift in response criterion.
Table 5.
Mean signal detection values and slopes under single- and dual-task conditions for the visual search task.
| d-prime | β criterion | Slope (ms/item) | ||||
|---|---|---|---|---|---|---|
| Single | Dual | Single | Dual | Single | Dual | |
| Controls | 3.87 (0.06) | 3.78 (0.07) | 1.02 (0.10) | 0.97 (0.09) | 5.42 (1.03) | 6.18 (1.78) |
| Patients | 3.78 (0.06) | 3.28 (0.09) | 0.97 (0.08) | 0.92 (0.08) | 7.26 (1.28) | 5.80 (1.86) |
Note: Numbers in parentheses indicate the standard error of the means.
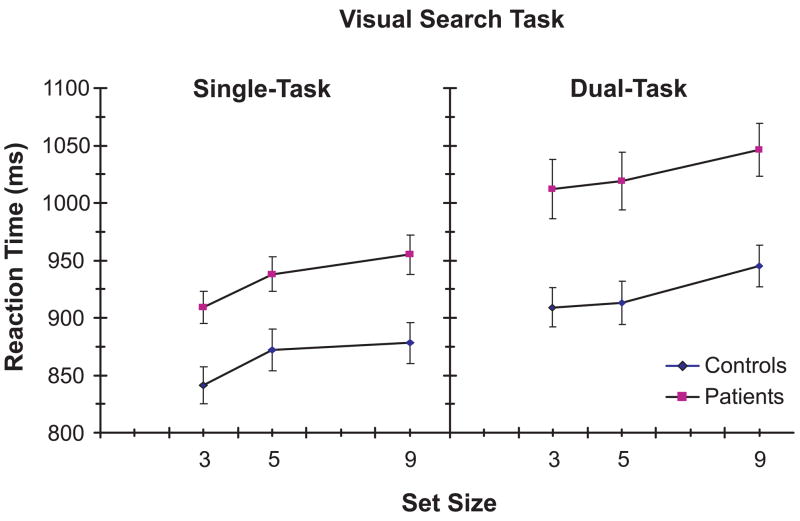
Mean trimmed response times (removing outliers above and below 2 standard deviations) to correct responses on target-present trials for the different set size and task conditions are shown in Figure 5. A three-way ANOVA with Group (AD, EC), Task Condition (Single, Dual) and Set Size (3, 5, 9) as factors revealed significant main effects of Group [F(1,110)=11.92, p<0.001, ηp2=0.10], Task Condition [F(1,110)=26.26, p<0.001, ηp2=0.19], and Set Size [F(2,220)=24.83, p<0.001, ε=0.91, ηp2=0.18], and a marginally significant Task Condition X Set Size interaction [F(2, 220)=2.89, p=0.06, ε=0.92, ηp2=0.03]. Planned single-group ANOVAs confirmed the presence of significant main effects of Task Condition [AD: F(1,71)=21.32, p<0.001, ηp2=0.23; EC: F(1,39)=12.79, p<0.001, ηp2=0.25] and Set Size [AD: F(2,142)=15.54, p<0.001, ε=0.93, ηp2=0.18; EC: F(2,78)=15.57, p<0.001, ε=0.80, ηp2=0.29] for both AD and EC groups, but no significant Task Condition X Set Size interaction in either group.
Figure 5.
Mean response times to target-present trials on the visual search task as a function of set size, under single-task and dual-task conditions, for each group.
Taken together, these results indicate that: a) the AD group was slower overall compared to the EC group; b) response times were longer overall under dual-task than single-task conditions for both groups; c) response times increased with increasing set size for both groups; and d) the increase in response time associated with increased set size was not affected by the dual-task condition in either group.
To further analyze the increase in response time as a function of set size, linear regression analyses were used to determine the slopes of the best fitting lines for each participant (Table 5). A two-way ANOVA with Group (AD, EC) and Task Condition (Single, Dual) as factors revealed no significant main effects or interaction (ps>0.51), indicating that the slopes did not differ between the groups and were not affected by the dual-task condition. While the slope values were small, comparisons indicated that they were significantly greater than zero for both groups under both single- and dual-task conditions [AD: ts(71)>3.13, ps<0.003; EC: ts(39)>3.48, ps<0.001].
Discussion
The results from the single-task conditions of the present study confirm previous demonstrations of selective attention deficits in AD patients. First, we found an increased exogenous spatial orienting effect in AD patients relative to age-matched controls similar to that observed in other studies (Festa-Martino et al., 2004; Parasuraman et al., 1992; Tales et al., 2002b, 2006). While the underlying cause of the increased orienting effect under exogenous conditions is not entirely clear, the present findings do support the notion that AD patients derive an abnormally increased benefit from validly-cued location under these conditions, perhaps due to the recruitment of top-down compensatory mechanisms. Second, we found an increased Simon response interference effect in AD patients relative to healthy elderly similar to that observed by Castel et al. (2007), and consistent with other studies demonstrating increased response-level interference in AD patients. On the combined-feature visual search task, the AD and EC groups were both influenced by the number of items in the display, with slope measures indicating efficient and comparable search performance across the two groups. These findings are consistent with those of Tales et al. (2002a, 2004) showing comparable performance across the groups under conditions that utilize highly salient targets and relatively low task complexity.
Of greater interest, the pattern of results observed under dual-task conditions provides further support for a specific dual-task coordination impairment in AD patients (e.g., Baddeley et al., 2001; Logie et al., 2004; Nestor et al., 1991a, b) under conditions that minimized working memory demands per se. First, the AD group displayed significantly greater decrements on the visuomotor tracking task under all three dual-task conditions than did the elderly group, despite the fact that tracking performance levels were matched under single-task conditions by individually adjusting tracking difficulty. In contrast, the dual-task tracking accuracy of the EC group was not significantly different from their single-task tracking in two of the dual-task conditions and only marginally worse in the third condition. Second, while the elderly group displayed slight reductions in overall accuracy on two of the selective attention tasks, the AD group displayed substantially greater decrements across all three selective attention tasks. Third, although overall response times increased under dual-task conditions for both groups, this increase was substantially greater for the AD group on all tasks except the highly efficient search task. Taken together, these results indicate that AD patients are particularly sensitive to the effect of dual-task conditions, and therefore provide support for the presence a specific coordination deficit in AD patients.
The main novel finding from the present study is the demonstration that dual-task conditions produced, in the AD but not the EC group, a change in efficiency of the selective attention mechanisms themselves across all three tasks, as reflected in differential effects on cue or display conditions within each task. Specifically, spatial orienting, Simon response interference, and visual search target detection sensitivity (d-prime) were all significantly reduced under dual-task conditions in the AD but not the EC group. The spatial orienting and Simon interference measures were both found to be significant larger in AD patients than healthy elderly under single-task conditions: Rather than exacerbating these selective attention deficits, dual-task conditions produced an apparent “normalization” of both measures in AD patients. Moreover, this reduction appeared to be due in both cases to a decrease in the benefit derived from valid sensory or congruent response information provided in the tasks, rather than to a decrease in cost associated with conflicting information (i.e., invalid cue or incongruent response conditions).
Thus, these findings do not support the more straightforward prediction derived from load theory (Lavie, 1995) that increased cognitive control load (i.e., increased dual-task coordination load) will necessarily lead to an increased sensitivity to distracting information, and therefore to an exacerbation of the selective attention deficits that are observed in AD patients under single-task conditions. Rather, these findings provide more support for the notion that cognitive control load (unlike perceptual load) can either increase or decrease sensitivity to interference depending on the relationship between the nature of the selective attention task and the nature of the cognitive load manipulation (Park et al., 2007). In the present study, an interaction between exogenous selective attention mechanisms and dual-task coordination processes produced the perhaps surprising effect of a reduction in AD patients’ sensitivity to interference. However, it is possible that other experimental conditions, such as the use of endogenous rather than exogenous selective attention mechanisms or the manipulation of working memory load rather than dual-task coordination load, would have produced an exacerbation rather than a reduction of the selective attention deficits in AD; studies designed to directly examine the effects of these different task conditions on AD patients’ selective attention performance are currently being conducted in our laboratory.
Somewhat analogous to Maylor and Lavie’s (1998) explanation for age-related changes in selective attention observed under different levels of perceptual load, we propose that the pattern of performance observed by AD patients on selective attention tasks under different levels of cognitive control load can be accounted for by the presence of two competing AD-related deficits in attention: 1) an impairment in inhibitory control that is reflected in the AD patients’ selective attention deficit under single-task conditions (i.e., low cognitive control load); and 2) a depletion of available attentional resources associated with a specific dual-task coordination deficit that is reflected in the reduction of the AD patients’ selective attention deficit under dual-task conditions. In this view, the presence of a specific dual-task coordination deficit in AD forces these patients to devote the majority of their available pool of attentional resources to performing two tasks concurrently under dual-task conditions, leaving little or no attentional resources available for allocation in response to the exogenous selective attention cues. Thus, although the sensory information provided by the selective attention cues does not differ across the single- and dual-task conditions, the “normalization” in selective attention under dual-task conditions results from a reduction in the attention-capturing properties of the cue information in these tasks. Moreover, this reduction in sensitivity to cue information under dual-task conditions in AD patients would be particularly apparent in the reduction to the benefits associated with valid and congruent cues in the Orienting and Simon tasks, respectively, since the concomitant reduction to the costs associated with invalid and incongruent cues would be attenuated by the impaired inhibitory control process that are also present in these patients. The finding of a reduction in target detection sensitivity under dual-task conditions in the combined-feature visual search task is also consistent with a decrease in the attention-capturing properties of the cue information in AD patients.
While the degree to which exogenous or stimulus-driven selective attention cues can be considered truly automatic remains an issue of considerable debate (e.g., Folk, Remington & Johnston, 1992; Yantis & Jonides, 1990; Van der Lubbe & Postma, 2005), several recent studies with healthy young adults have demonstrated decreased attentional capture with abrupt-onset exogenous cues under highly demanding dual-task conditions (Boot et al., 2005; Santangelo et al., 2007) similar to that observed in the present study. Decreased attentional capture from exogenous cues has also been observed in other conditions which minimize the pool of available attentional resources (e.g., Du & Abrams, 2009). Thus the present findings of reduced selective attention effects in AD patients under dual-task conditions are not only consistent with findings from these previous studies, but also serve to provide additional support for the notion that, at least under some conditions, exogenous cues are sensitive to the modulation of top-down or attentional processes.
The lack of a significant effect of dual-task conditions on the different selective attention measures in the healthy elderly is notable given the findings from several recent studies showing changes in selective attention processes with increased cognitive load under dual-task conditions in healthy young adults (Boot et al., 2005; Lavie & de Fockert, 2005; Lavie et al., 2004; Woodman & Luck, 2004). Lavie and de Fockert (2005), for example, found increased attentional capture by an irrelevant color distractor item with increasing working memory load. Although Boot et al. (2005) also found changes in attentional capture under dual-task conditions, the direction of that change depended on the nature of the distractor item: Attentional capture increased with irrelevant color singletons but decreased with irrelevant abrupt-onset items under dual-task conditions, suggesting a differential effect of top-down modulation on sustained versus transient irrelevant distractors. The lack of dual-task effects on selective attention in the healthy elderly may be attributable at least in part to nature of the secondary task: The tracking task used in the present study may have primarily engaged bottom-up visuomotor processes and placed relatively few demands on working memory load. The overall slowing in our elderly group suggests an additive cost associated with dual-task coordination function, but the lack of any change in selective attention efficiency under dual-task conditions suggests that, at least under the conditions used in the present study, this specific coordination function (intact in healthy elderly) did not interact with these selective attention processes.
Taken together, findings from the present study both confirmed the presence of selective attention and divided attention deficits in AD patients, and helped elucidate the ways in which dual-task coordination processes interact with component selective attention processes in these patients. Rather than exacerbating the selective attention deficits observed under single-task conditions, dual-task conditions instead produced an apparent diminution of these deficits. We suggest that this diminution is due to the combination of deficient top-down control processes along with a decrease in the attention-capturing properties of cue information under dual-task conditions in AD patients. These findings not only serve to increase our understanding of the nature of the attentional deficits in AD patients, but also have implications for our understanding of the processes mediating attention in neurologically intact individuals.
Acknowledgments
This work was supported by the National Institutes of Health [AG16335 to B.R.O.]. Portions of this work were presented at the Cognitive Neuroscience Society Meeting (May 2007) and the International Conference of Alzheimer’s Disease (July 2008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amieva H, Phillips LH, Della Sala S, Henry JD. Inhibitory functioning in Alzheimer’s disease. Brain. 2004;127:949–964. doi: 10.1093/brain/awh045. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Baddeley HA, Bucks RS, Wilcock GK. Attentional control in Alzheimer’s disease. Brain. 2001;124:1492–1508. doi: 10.1093/brain/124.8.1492. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. The decline of working memory in Alzheimer’s disease: A longitudinal study. Brain. 1991;114:2521–2542. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ. Working memory. In: Bower GA, editor. Recent advances in learning and motivation. Vol. 8. New York: Academic Press; 1974. pp. 47–90. [Google Scholar]
- Berardi AM, Parasuraman R, Haxby JV. Sustained attention in mild Alzheimer’s disease. Developmental Neuropsychology. 2005;28:507–537. doi: 10.1207/s15326942dn2801_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla RK, Papandonatos GD, Stern RA, Ott BR. Anxiety of Alzheimer’s disease patients before and after a standardized on-road driving test. Alzheimer’s & Dementia. 2007;3:33–39. doi: 10.1016/j.jalz.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot WR, Brockmole JR, Simons DJ. Attention capture is modulated in dual-task situations. Psychonomic Bulletin & Review. 2005;12:662–668. doi: 10.3758/bf03196755. [DOI] [PubMed] [Google Scholar]
- Castel AD, Balota DA, Hutchison KA, Logan JM, Yap MJ. Spatial attention and response control in healthy younger and older adults and individuals with Alzheimer’s disease: Evidence for disproportionate selection impairments in the Simon task. Neuropsychology. 2007;21:170–182. doi: 10.1037/0894-4105.21.2.170. [DOI] [PubMed] [Google Scholar]
- Crossley M, Hiscock M, Foreman JB. Dual-task performance in early stage dementia: Differential effects for automatized and effortful processing. Journal of Clinical & Experimental Neuropsychology. 2004;26:332–346. doi: 10.1080/13803390490510068. [DOI] [PubMed] [Google Scholar]
- Du F, Abrams RA. Onset capture requires attention. Psychonomic Bulletin & Review. 2009;16:537–541. doi: 10.3758/PBR.16.3.537. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D, Black SE. Attentional networks in normal aging and Alzheimer’s disease. Neuropsychology. 2006;20:133–143. doi: 10.1037/0894-4105.20.2.133. [DOI] [PubMed] [Google Scholar]
- Festa-Martino E, Ott BR, Heindel WC. Interactions between phasic alerting and spatial orienting: Effects of normal aging and Alzheimer’s disease. Neuropsycholgy. 2004;18:258–268. doi: 10.1037/0894-4105.18.2.258. [DOI] [PubMed] [Google Scholar]
- Foldi NS, Schaefer LA, White REC, Johnson R, Berger JT, Carney MT, Macina LO. Effects of graded levels of physical similarity and density on visual selective attention in patients with Alzheimer’s disease. Neuropsychology. 2005;19:5–17. doi: 10.1037/0894-4105.19.1.5. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception & Performance. 1992;18:1030–1044. [PubMed] [Google Scholar]
- Foster JK, Behrmann M, Stuss DT. Visual attention deficits in Alzheimer’s disease: Simple versus conjoined feature search. Neuropsychology. 1999;13:223–245. doi: 10.1037//0894-4105.13.2.223. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R, Alexander GE. Controlling the focus of spatial attention during visual search: Effects of advanced aging and Alzheimer disease. Neuropsychology. 1997;11:3–12. doi: 10.1037//0894-4105.11.1.3. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski MJ. Dual-task performance in demented and nondemented elderly. Journal of Clinical & Experimental Neuropsychology. 1991;13:667–676. doi: 10.1080/01688639108401081. [DOI] [PubMed] [Google Scholar]
- Han S-H, Kim M-S. Visual search does not remain efficient when executive working memory is working. Psychological Science. 2004;15:623–628. doi: 10.1111/j.0956-7976.2004.00730.x. [DOI] [PubMed] [Google Scholar]
- Humphrey DG, Kramer AF. Age differences in visual search for feature, conjunction, and triple-conjunction targets. Psychology & Aging. 1997;12:704–717. doi: 10.1037//0882-7974.12.4.704. [DOI] [PubMed] [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. Journal of Experiemntal Psychology: Human Perception & Performance. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Lavie N, Cox S. On the efficiency of attentional selection: Efficient visual search results in inefficient rejection of distraction. Psychological Science. 1997;8:395–398. [Google Scholar]
- Lavie N, de Fockert JW. Contrasting effects of sensory limits and capacity limits in visual selective attention. Perception & Psychophysics. 2003;65:202–212. doi: 10.3758/bf03194795. [DOI] [PubMed] [Google Scholar]
- Lavie N, de Fockert JW. The role of working memory in attentional capture. Psychonomic Bulletin & Review. 2005;12:669–674. doi: 10.3758/bf03196756. [DOI] [PubMed] [Google Scholar]
- Lavie N, Fox E. The role of perceptual load in negative priming. Journal of Experimental Psychology: Human Perception & Performance. 2000;26:1038–1052. doi: 10.1037//0096-1523.26.3.1038. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Logie RH, Cocchini G, Della Sala S, Baddeley AD. Is there a specific executive capacity for dual task coordination? Evidence from Alzheimer’s disease. Neuropsychology. 2004;18:504–513. doi: 10.1037/0894-4105.18.3.504. [DOI] [PubMed] [Google Scholar]
- Lonie JA, Tierney KM, Herrmann LL, Donaghey C, O’Carroll RE, Lee A, Ebmeier KP. Dual task performance in early Alzheimer’s disease, amnestic mild cognitive impairment and depression. Psychological Medicine. 2008;1:1–19. doi: 10.1017/S0033291708003346. [DOI] [PubMed] [Google Scholar]
- Lu CH, Proctor RW. The influence of irrelevant location information on performance: A review of the Simon and spatial Stroop effects. Psychonomic Bulletin & Review. 1995;2:174–207. doi: 10.3758/BF03210959. [DOI] [PubMed] [Google Scholar]
- MacPherson SE, Della Sala S, Logie RH, Wilcock GK. Specific AD impairment in concurrent performance of two memory tasks. Cortex. 2007;43:858–865. doi: 10.1016/s0010-9452(08)70685-3. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Pierce TW, Allen PA. Adult age differences in the use of distractor homogeneity during visual search. Psychology & Aging. 1996;11:454–474. doi: 10.1037//0882-7974.11.3.454. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Lavie N. The influence of perceptual load on age differences in selective attention. Psychology & Aging. 1998;13:563–573. doi: 10.1037//0882-7974.13.4.563. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadloan E. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Workgroup under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Neargarder SA, Cronin-Golomb A. Characteristics of visual target influence detection of change in naturalistic scenes in Alzheimer disease. Cognitive Behavioral Neurology. 2005;18:151–158. doi: 10.1097/01.wnn.0000178229.39068.9b. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Parasuraman R, Haxby JV. Speed of information processing and attention in early Alzheimer’s dementia. Developmental Neuropsychology. 1991a;7:243–256. [Google Scholar]
- Nestor PG, Parsuraman R, Haxby JV, Grady CL. Divided attention and metabolic brain dysfunction in mild dementia of the Alzheimer’s type. Neuropsychologia. 1991b;29:379–387. doi: 10.1016/0028-3932(91)90026-5. [DOI] [PubMed] [Google Scholar]
- Oh SH, Kim MS. The role of spatial working memory on visual search. Psychonomic Bulletin & Review. 2004;11:275–281. doi: 10.3758/bf03196570. [DOI] [PubMed] [Google Scholar]
- Ott BR, Festa EK, Amick MM, Grace J, Davis JD, Heindel WC. Computerized maze navigation and on-road performance by drivers with dementia. Journal of Geriatric Psychiatry. 2008;21:18–25. doi: 10.1177/0891988707311031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott BR, Heindel WC, Papandonatos GD, Festa EK, Davis JD, Daiello LA, Morris JC. A longitudinal study of drivers with Alzheimer’s disease. Neurology. 2008;70:1171–1178. doi: 10.1212/01.wnl.0000294469.27156.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Alexander GE. Alzheimer disease constricts the dynamic range of spatial attention in visual search. Neuropsychologia. 2000;38:1126–1135. doi: 10.1016/s0028-3932(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Haxby JV, Grady CL. Visuospatial attention in dementia of the Alzheimer type. Brain. 1992;115:711–733. doi: 10.1093/brain/115.3.711. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Sunderland T. The apolipoprotein E gene, attention, and brain function. Neuropsychology. 2002;16:254–274. doi: 10.1037//0894-4105.16.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman R, Haxby J. Attention and brain function in Alzheimer’s disease: A review. Neuropsychology. 1993;7:242–272. [Google Scholar]
- Park S, Kim MS, Chun MM. Concurrent working memory load can facilitate selective attention: Evidence for specialized load. Journal of Experimental Psychology: Human Perception & Performance. 2007;33:1062–1075. doi: 10.1037/0096-1523.33.5.1062. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease: A critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Plude DJ, Doussard-Roosevelt JA. Aging, selective attention, and feature integration. Psychology & Aging. 1989;4:98–105. doi: 10.1037/0882-7974.4.1.98. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y, Rafal RD. Neural systems control of spatial orienting. Philosphical Transactions of the Royal Society of London. 1982;298:187–198. doi: 10.1098/rstb.1982.0081. [DOI] [PubMed] [Google Scholar]
- Ramsden CM, Kinsella GJ, Ong B, Storey E. Performance of everyday actions in mild Alzheimer’s disease. Neuropsychology. 2008;22:17–26. doi: 10.1037/0894-4105.22.1.17. [DOI] [PubMed] [Google Scholar]
- Repovš G, Baddeley A. The multi-component model of working memory: Explorations in experimental cognitive psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Riby LM, Perfect TJ, Stollery BT. The effects of age and task domain on dual task performance: A meta-analysis. European Journal of Cognitive Psychology. 2004;16:863–891. [Google Scholar]
- Rösler A, Mapstone ME, Hays AK, Mesulam MM, Rademaker A, Gitelman DR, Weintraub S. Alterations of visual search strategy in Alzheimer’s disease and aging. Neuropsychology. 2000;14:398–408. doi: 10.1037//0894-4105.14.3.398. [DOI] [PubMed] [Google Scholar]
- Rösler A, Mapstone ME, Hays-Wicklund AK, Gitelman DR, Weintraub S. The “zoom lens” of focal attention in visual search: Changes in aging and Alzheimer’s disease. Cortex. 2005;41:512–519. doi: 10.1016/s0010-9452(08)70191-6. [DOI] [PubMed] [Google Scholar]
- Santangelo V, Belardinelli MO, Spence C. The suppression of reflexive visual and auditory orienting when attention is otherwise engaged. Journal of Experimental Psychology: Human Perception & Performance. 2007;33:137–148. doi: 10.1037/0096-1523.33.1.137. [DOI] [PubMed] [Google Scholar]
- Sebastian MV, Menor J, Elosua MR. Attentional dysfunction of the central executive in AD: Evidence from dual task and perseveration errors. Cortex. 2006;42:1015–1020. doi: 10.1016/s0010-9452(08)70207-7. [DOI] [PubMed] [Google Scholar]
- Simon JR. The effects of an irrelevant directional cue on human information processing. In: Proctor RW, Reeve TG, editors. Stimulus-response compatibility: An integrated perspective. Amsterdam; North-Holland: 1990. pp. 31–86. [Google Scholar]
- Simon JR, Berbaum K. Effect of conflicting cues: the “Stroop effect” vs. the “Simon effect. Acta Psychologica. 1990;73:159–170. doi: 10.1016/0001-6918(90)90077-s. [DOI] [PubMed] [Google Scholar]
- Tales A, Butler SR, Fossey J, Gilchrist ID, Jones RW, Troscianko T. Visual search in Alzheimer’s disease: A deficiency in processing conjunctions of features. Neuropsychologia. 2002a;40:1849–1857. doi: 10.1016/s0028-3932(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Tales A, Muir JL, Bayer A, Snowden RJ. Spatial shifts in visual attention in normal ageing and dementia of the Alzheimer type. Neuropsychologia. 2002b;40:2000–2012. doi: 10.1016/s0028-3932(02)00057-x. [DOI] [PubMed] [Google Scholar]
- Tales A, Muir J, Jones R, Bayer A, Snowden RJ. The effects of saliency and task difficulty on visual search performance in ageing and Alzheimer’s disease. Neuropsychologia. 2004;42:335–345. doi: 10.1016/j.neuropsychologia.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Tales A, Snowden RJ, Brown M, Wilcock G. Alerting and orienting in Alzheimer’s disease. Neuropsychology. 2006;20:752–756. doi: 10.1037/0894-4105.20.6.752. [DOI] [PubMed] [Google Scholar]
- Van der Lubbe RHJ, Postma A. Interuption from irrelevant auditory and visual onsets even when attention is in a focused state. Experimental Brain Research. 2005;164:464–471. doi: 10.1007/s00221-005-2267-0. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Visual search is slowed when visuospatial working memory is occupied. Psychonomic Bulletin & Review. 2004;11:269–274. doi: 10.3758/bf03196569. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Vogel EK, Luck SJ. Visual search remains efficient when visual working memory is full. Psychological Science. 2001;12:219–224. doi: 10.1111/1467-9280.00339. [DOI] [PubMed] [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: Voluntary versus automatic allocation. Journal of Experimental Psychology: Human Perception & Performance. 1990;16:121–134. doi: 10.1037//0096-1523.16.1.121. [DOI] [PubMed] [Google Scholar]