Abstract
Whereas ethanol has behavioral actions consistent with increased GABAergic function, attempts to demonstrate a direct enhancement of GABA-gated currents by ethanol have produced mixed results. Recent work has suggested that a part of the GABAergic profile of ethanol may result from enhanced GABA release from presynaptic terminals. The present study examines the effect of ethanol on GABA release in several brain regions to assess the regional nature of ethanol-induced GABA release. Whole-cell voltage clamp recording of spontaneous inhibitory postsynaptic currents (sIPSCs) from mechanically dissociated neurons and miniature inhibitory postsynaptic currents (mIPSCs) and paired-pulse ratio (PPR) from a slice preparation were used to quantify GABA release. Ethanol produced a concentration-dependent increase in the frequency of sIPSCs recorded from mechanically dissociated cerebellar Purkinje neurons and mIPSCs from substantia nigra neurons without having an effect on sIPSCs recorded from lateral septal or cerebrocortical neurons. This regional difference in the effect of ethanol on GABA release was confirmed with PPR recording from brain slices. These data indicate that ethanol can act on presynaptic terminals to increase GABA release in some brain regions while having little or no effect on GABA release in others. This regional difference is consistent with earlier in vivo studies in which ethanol affected neural activity and sensitivity to GABA in some, but not all, brain sites.
Effects of ethanol on mood, coordination, and sedation are similar to those of the benzodiazepines and barbiturates (Frye et al., 1979); furthermore, benzodiazepines substitute for ethanol in drug discrimination tests (Grant et al., 2000). Because benzodiazepines and barbiturates are known to act by enhancing the inhibitory action of GABA (Martz et al., 1983), it has been assumed that at least some of the behavioral actions of ethanol are mediated by an effect of ethanol on GABA function (Criswell and Breese, 2005; Siggins et al., 2005; Weiner and Valenzuela, 2006). In support of this view, early studies found that ethanol enhanced the effects of GABA in synaptoneurosomes and cultured neurons (Suzdak et al., 1986; Aguayo, 1990; Reynolds et al., 1992), and it enhanced the effect of GABA on neuronal discharge rate in vivo (Bloom and Siggins, 1987; Givens and Breese, 1990). Based on these results, a general conclusion was that ethanol acted postsynaptically on GABAA receptor function. How-ever, a number of studies failed to find a direct effect of ethanol on GABA-gated currents from neurons (Sigel et al., 1993; Peoples and Weight, 1999; Mori et al., 2000; Criswell et al., 2003; Borghese et al., 2006). More recently, effects of low concentrations of ethanol on tonic currents mediated by GABAA receptors containing the α4, βx, δ or α6, βx, δ subunit combination have been reported (Wallner et al., 2003, 2006; Chandra et al., 2006). However, even this finding has raised controversy (Borghese et al., 2006; Botta et al., 2007a,b). Irrespective of any postsynaptic action ethanol may possess, earlier in vivo extracellular recordings indicated that ethanol reliably enhances GABA function in some brain regions while being ineffective in others (McCown et al., 1985; Bloom and Siggins, 1987; Givens and Breese, 1990; Ebert et al., 1994).
An emerging consensus indicates that ethanol can act presynaptically to enhance GABA function by increasing GABA release (Crowder et al., 2002; Roberto et al., 2003; Ziskind-Conhaim et al., 2003; Criswell and Breese, 2005; Siggins et al., 2005; Ming et al., 2006; Zhu and Lovinger, 2006). However, few studies have directly compared effects of ethanol on GABA release across brain regions. Differential sensitivity to the presynaptic action of ethanol to release GABA could contribute to a regional effect of ethanol on GABA function, which could influence specific behaviors (Mc-Cown et al., 1985). The present study uses whole-cell voltage clamp recording of GABA-mediated inhibitory postsynaptic currents from mechanically dissociated neurons or neurons in a slice preparation and paired-pulse ratio (PPR) recording from a slice preparation to examine the effect of ethanol on presynaptic GABA release in selected brain regions.
Materials and Methods
Preparation of Brain Slices
Sprague-Dawley rats, 13 to 20 days old, were anesthetized with an i.p. injection of 75% urethane and decapitated. The brains were rapidly removed and placed in ice-cold bicarbonate-buffered artificial cerebrospinal fluid (ACSF) of the following composition: 124 mM NaCl, 3.25 mM KCl, 1.25 mM KH2PO4, 2 mM CaCl2, 20 mM NaHCO3, 2 mM MgSO4, and 10 mM glucose. Parasagittal slices (400 μm) through the cerebellum, or coronal sections through the lateral septum, substantia nigra, and medial frontal cortex, were cut with a vibrating tissue slicer (Vibratome, series 1000; Vashaw Scientific, Inc., Norcross, GA). The slices were equilibrated in a beaker containing ACSF gassed with 95% O2/5% CO2 for at least 1 h at room temperature before initiating electrophysiological recording.
Preparation and Properties of Mechanically Dissociated Neurons
Slices, as described above, were transferred to a recording chamber containing a HEPES-buffered ACSF (145 mM NaCl, 5 mM KCl, 10 mM HEPES, 2 mM CaCl2, and 10 mM glucose, pH 7.4; 340 mOsM/l). A 0.3-mm probe was touched to the surface of the submerged slice over the cells to be dissociated and vibrated using a piezoelectric transducer (~0.5-mm amplitude at 10–50 Hz for 2 min). The slice was then removed from the chamber, and the mechanically dissociated neurons were allowed to settle to the bottom of the recording chamber.
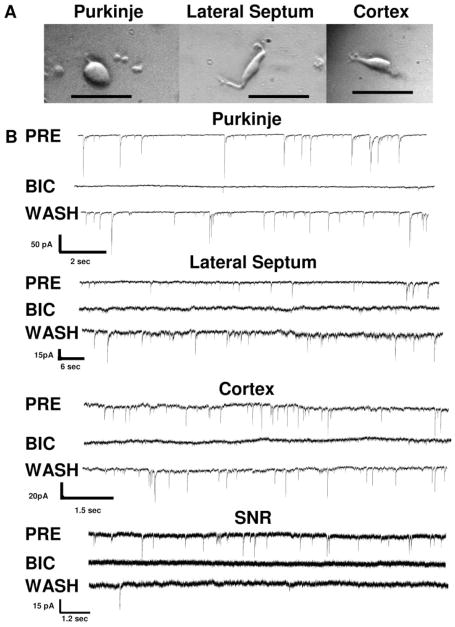
The mechanical dissociation of neurons strips the dendritic tree and most of the axon from neurons. The micrographs in Fig. 1 illustrate representative neurons dissociated from the cerebellum, lateral septum, and medial frontal cortex. While the axon and dendritic tree are removed during the dissociation, many of the boutons from presynaptic neurons remain attached to the soma (Akaike and Moorhouse, 2003). As a result of the presynaptic terminal being present, spontaneous inhibitory postsynaptic currents (sIPSCs) can be recorded in the absence of inputs from other neurons.
Fig. 1.
Morphology and GABA-gated currents of mechanically dissociated neurons. A, a photomicrograph of a mechanically dissociated cerebellar Purkinje neuron surrounded by granule neurons is shown on the left. Mechanically dissociated neurons from the lateral septum and cerebral cortex are depicted in the middle and right (top), respectively. The calibration bars are 50 μm. B, representative traces illustrating inhibition of sIPSCs by the GABA antagonist bicuculline (50 μM) are shown for each neuronal type. A pretest control (PRE) trace, a trace during application of bicuculline (BIC), and a trace after a 1-min washout (WASH) are shown for a cerebellar Purkinje neuron (Purkinje), a lateral septal neuron (Lateral Septum), a cerebro-cortical neuron (Cortex), and a substantia nigra reticulata neuron (SNR). Note the differing time and current calibration bars for each brain region. Overall, bicuculline decreased the frequency of sIPSCs by the following: 97 ± 1.0% for Purkinje neurons (n = 11); 98 ± 1.8% for lateral septal neurons (n = 6); 97 ± 2.7% for cerebrocortical neurons (n = 6); and 100 ± 0% for substantia nigra reticulata neurons (n = 4). Substantia nigra neurons were recorded in a slice preparation while all other neurons were mechanically dissociated.
Electrophysiological Recording
Electrophysiological studies were performed under voltage clamp [−60 mV for cortical, substantia nigra reticulata (SNR), and lateral septal neurons, and −70 mV for cerebellar Purkinje neurons] in the whole-cell configuration using an Axopatch-1D amplifier (Molecular Devices, Sunnyvale, CA). Recording pipettes were fabricated from N 51A capillary glass (Drummond Scientific, Broomall, PA). The internal solution included 150 mM KCl, 3.1 mM MgCl2, 15 mM HEPES, 2 mM K-ATP, 5 mM EGTA, 15 mM phosphocreatine, and 50 U/ml creatine phosphokinase, adjusted to pH 7.4 with KOH with an osmolality of 310 mOsM/l. Seals were formed on the neurons with electrodes having a tip resistance of 2 to 4 MΩ. Data were displayed on an oscilloscope, digitized at either 2 or 5 kHz, and stored on a personal computer. Recordings were performed at room temperature in a bath where the neurons were superfused at 0.5 to 1 ml/min.
Recording of sIPSCs from Mechanically Dissociated Cells
Neurons mechanically dissociated from the cerebral cortex, cerebellum, and lateral septum were bathed in the HEPES-buffered ACSF at a rate of 0.5 ml/min. Control solution or drugs were applied by an array of fused silica tubes (200-μm tip diameter moved by a micro-manipulator) or by a perfusion pencil (250-μm tip diameter; Automate Scientific, Inc., Sarasota, FL) placed approximately 200-μm upstream from the cell.
Recording of Miniature Inhibitory Postsynaptic Currents and Paired-Pulse Ratio from Brain Slices
Brain slices from the cerebellum, lateral septum, or medial frontal cortex were prepared as described above. Brain slices were then placed in a recording chamber and perfused with bicarbonate-buffered ACSF at a rate of 1 ml/min. Neurons were visualized using an infrared camera under Namarski or oblique illumination. For miniature inhibitory postsynaptic current (mIPSC) recordings, tetrodotoxin (TTX; 500 nM) and 6-cyano-7-nitroquinoxaline-2,3-dione (10 μM) were applied to the recording site by a drug application pencil (250-μm diameter) to block sodium currents and excitatory glutamate-gated currents, respectively. Because spontaneous N-methyl-D-aspartate (NMDA)-gated currents are not observed under the present recording conditions, AP-5 was not included. Whole-cell voltage-clamp recording was established, and mIPSCs were recorded for 1-min intervals in the presence and absence of ethanol (25–100 mM).
PPR was recorded in the absence of TTX. Platinum-iridium-stimulating electrodes were lowered into the vicinity (150 μm) of a cell to be recorded, and paired-pulse stimulation (0.2-ms duration and 50-ms interstimulus interval) was delivered at 0.1 Hz. After placement of the stimulating electrodes, whole-cell patch was established as described for the isolated cells, and after a 5-min adaptation period to the 2-amino-5-phosphonovaleric acid (50 μM) and 6-cyano-7-nitroquinoxaline-2,3-dione (10 μM), PPR recording was performed. After a minimum of five PPR records obtained at 10-s intervals, ethanol (25, 50, or 100 mM in bicarbonate-buffered ACSF) was delivered by the drug pencil, and 5 min later a second series of PPR values was recorded. The ethanol was washed out for 5 min, and a final set of PPR values was obtained after washout. Data from only one neuron was collected from each slice.
Analysis
Rate, amplitude, and decay time of sIPSCs were analyzed using Mini Analysis (version 5.6.4; Synaptosoft, Decatur, GA). Numerical data are given as mean ± S.E.M., and n represents the number of cells tested. Percentage change during ethanol administration was calculated as 100 times the value for ethanol divided by the mean of the pretest and washout values. Differences between means were compared with analysis of variance followed by Tukey’s Honestly Significant different test or Dunnett’s post hoc comparisons as appropriate. Linear trends were analyzed by the Pearson correlation. Calculated P values of less than 0.05 were accepted as evidence of a significant difference.
Results
Blockade of sIPSCs and mIPSCs by Bicucullilne
Both the sIPCSs recorded from mechanically dissociated neurons in the absence of TTX and the mIPSCs recorded from the slice preparation in the presence of TTX result from the spontaneous release of neurotransmitter from the presynaptic terminal (Akaike and Moorhouse, 2003; Zhu and Lovinger, 2006). When tested in a subset of neurons, the spontaneous currents recorded from SNR neurons in the slice preparation or from mechanically dissociated cerebellar Purkinje, lateral septal, or cerebrocortical neurons were blocked by bicuculline, indicating that they were mediated by GABAA receptors (Fig. 1B). Thus, the frequency of mIPSCs from neurons in the SNR slice or sIPSCs from the mechanically dissociated neurons gives a good index of spontaneous release of GABA from presynaptic terminals.
Effects of Ethanol on GABAergic (sIPSCs) from Mechanically Dissociated Purkinje Neurons
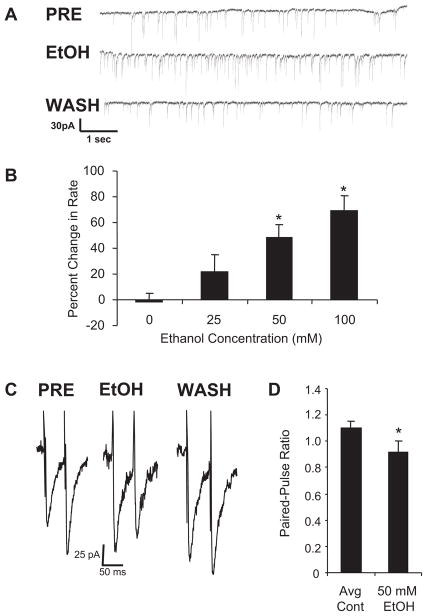
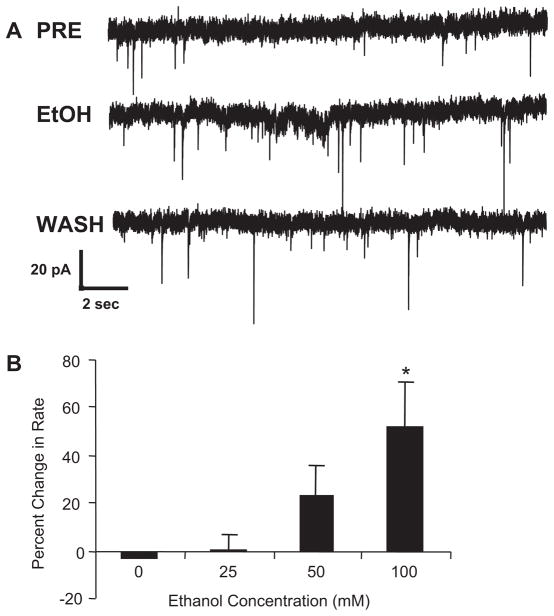
Figure 2A illustrates that 50 mM ethanol increased the rate of sIPSCs recorded from a mechanically dissociated cerebellar Purkinje neuron with a return to baseline upon washout. Figure 2B demonstrates that this facilitation of sIPSCs by ethanol is concentration related, results similar to those obtained previously in cerebellar slices (Ming et al., 2006; Kelm et al., 2007). In contrast to the change in frequency of sIPSCs by ethanol, Table 1 shows that amplitude and decay times were unchanged by ethanol. These latter results indicate that ethanol does not have measurable postsynaptic effects on GABA-gated currents under the present conditions.
Fig. 2.
Ethanol increases frequency of sIPSCs and decreases PPR from cerebellar Purkinje neurons. A, when ethanol was applied to mechanically dissociated cerebellar Purkinje neurons, GABA release was increased as indicated by an increased rate of sIPSCs. Representative traces of sIPSCs are shown from a Purkinje neuron before ethanol administration (PRE), during ethanol administration (EtOH), and after washout (WASH). B, means for the percentage change in rate averaged across 10 to 18 neurons for varying concentrations of ethanol are shown. Control values represent repeated delivery of ACSF to the cell using the drug delivery pencil, whereas experimental values represent the effect of 25, 50, and 100 mM ethanol delivered in ACSF. *, significantly different from control (P < 0.05, Dunnett test). C, the effect of ethanol on PPR is shown. When pulse pairs were applied at a 50-ms interstimulus interval under control conditions (PRE), the second pulse produced a greater response than the first (paired-pulse facilitation). During application of 50 mM ethanol (EtOH), the second stimulus produced a smaller effect than the first (paired-pulse depression). The decrease in paired-pulse ratio is consistent with an increase in stimulus-induced GABA release. On washout, the paired-pulse ratio returned to control values (WASH). Mean amplitude for the initial pulse was 65 ± 9.9; n = 9. D, the mean (n = 9) PPRs under control (mean of pretest and wash; Avg Cont) and ethanol (50 mM EtOH) conditions are illustrated. *, P < 0.05 compared with control.
TABLE 1. Effect of ethanol on amplitude and decay times across brain regions.
Amplitude for control sIPSCs or mIPSCs (CONT AMP) and during 100 mM ethanol (EtOH AMP) did not differ significantly at any of the three sites (P > 0.05). Similarly, the decay times under control conditions (CONT Decay) and during ethanol administration (EtOH Decay) did not differ significantly at any of the four sites (P > 0.05).
| Site | CONT AMP | EtOH AMP | CONT Decay | EtOH Decay |
|---|---|---|---|---|
| pA | ms | |||
| Purkinje | 31.4 ± 4.0 | 29.6 ± 4.2 | 6.1 ± 0.53 | 5.8 ± 0.41 |
| Cortex | 46.43 ± 6.7 | 40.85 ± 4.7 | 16.42 ± 2.7 | 13.39 ± 2.1 |
| Lateral septum | 23.9 ± 9.7 | 19.0 ± 5.17 | 13.29 ± 1.22 | 13.48 ± 1.15 |
| Substantia nigra | 87.5 ± 10.1 | 98.8 ± 13.7 | 13.54 ± 1.86 | 13.26 ± 1.62 |
CONT, control; AMP, amplitude; EtOH, ethonal.
Effects of Ethanol on PPR from Cerebellar Purkinje Neurons
To determine whether the effect of ethanol on spontaneous GABA release extended to evoked release, the ability of ethanol to increase GABA release onto cerebellar Purkinje neurons using PPR recording in slices was examined. Figure 2C shows a representative example of a paired-pulse recording from a cerebellar Purkinje neuron. Under control conditions, the response to the second pulse was greater than that to the first (paired-pulse facilitation). This result is consistent with previous work (Liu, 2007) that showed paired-pulse facilitation from cerebellar Purkinje neurons at this age. During exposure of the slice to 50 mM ethanol (Fig. 2C), the response to the second pulse was smaller than that seen with the first pulse (paired-pulse depression). Removal of the ethanol restored the original PPR (Fig. 2C). Figure 2D indicates that the mean PPR was significantly depressed by 50 mM ethanol when averaged across several cerebellar Purkinje neurons. This decrease in the ratio of the second response to the first during ethanol administration indicates an increase in stimulated GABA release (Siggins et al., 2005). Thus, the PPR data are consistent with the ethanol-induced increase in sIPSC frequency from the mechanically dissociated Purkinje neurons.
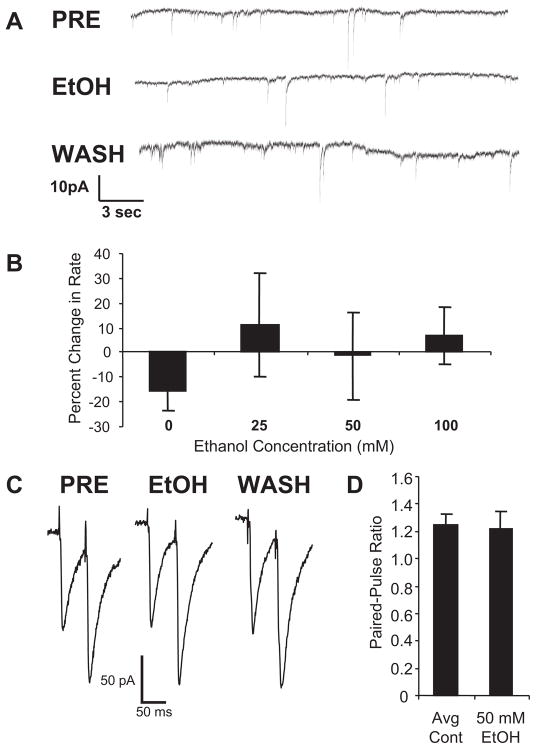
Effect of Ethanol on sIPSCs and PPR from Lateral Septal Neurons
Because earlier in vivo studies failed to find an effect of ethanol on enhancement of GABA responsiveness from lateral septal neurons (Givens and Breese, 1990), we next examined the effect of ethanol on sIPSC frequency from mechanically dissociated lateral septal neurons. Figure 3A provides a representative record from a lateral septal neuron illustrating the lack of effect of 50 mM ethanol on sIPSC frequency. A concentration-response relationship for the effect of ethanol on frequency of lateral septal sIPSCs is shown in Fig. 3B. There was no significant effect of any concentration of ethanol tested on lateral septal sIPSC frequency, including a concentration of 100 mM (P > 0.05). Likewise, as shown in Table 1, there was no effect of ethanol on amplitude or decay time of the sIPSCs from lateral septal neurons.
Fig. 3.
Ethanol does not have an effect on the frequency of the sIPSCs and the PPR from lateral septal neurons. A, when ethanol was applied to mechanically dissociated lateral septal neurons, GABA release was not affected as indicated by a lack of effect on rate of sIPSCs. Representative traces are shown from a lateral septal neuron before ethanol administration (PRE), during application of 50 mM ethanol (EtOH), and after washout (WASH). B, when data were averaged across 6 to 10 neurons, there was no effect of 25, 50, or 100 mM ethanol on sIPSC frequency (P > 0.1). C, representative paired-pulse traces from a lateral septal neuron are shown before ethanol administration (PRE), during application of 50 mM ethanol (EtOH), and after washout (WASH). Note that a similar paired-pulse facilitation was observed in each instance. Mean amplitude for the initial pulse was 84 ± 20 pA; n = 6. D, the mean PPR for lateral septal neurons in the presence (50 mM EtOH) and absence (mean of pretest and wash; Avg Cont) of ethanol is shown. There was no effect of the 50 mM ethanol on the PPR from the lateral septal neurons (P > 0.1; n = 6).
Because GABAB receptors on presynaptic terminals can interfere with the ability of drugs to increase GABA release (Ariwodola and Weiner, 2004; Zhu and Lovinger, 2006), we examined the effect of 100 mM ethanol on sIPSC frequency in the presence of a GABAB antagonist. Ethanol did not cause a significant increase in sIPSC frequency (21.3 ± 19.3%, n = 8; P > 0.05) in the presence of a GABAB antagonist (CGP-53432; 10 μM). These results indicate that stimulation of GABAB receptors was not responsible for the lack of an effect of ethanol on GABA release in the lateral septum.
To provide further evidence for this lack of effect of ethanol on GABA release, we conducted paired-pulse recording from lateral septal neurons. As shown in a representative trace from lateral septal neurons, Fig. 3C demonstrates that under control conditions, the response to the second pulse is considerably greater than that to the first (paired-pulse facilitation). Unlike the PPR data from cerebellar Purkinje neurons, ethanol did not alter the pattern of PPR of this lateral septal neuron. Figure 3D demonstrates the lack of effect of the 50 mM ethanol on PPR when averaged across several lateral septal neurons. These two approaches clearly indicate a lack of effect of behaviorally relevant concentrations of ethanol on stimulated GABA release from this brain region.
Effects of Ethanol on sIPSCs from Mechanically Dissociated Cerebrocortical Neurons
Mechanically dissociated neurons from the medial frontal cortex exhibit sIPSCs resulting from release of GABA by attached presynaptic terminals. However, when ethanol was applied to these neurons, the rate of sIPSCs was unchanged (Fig. 4). Figure 4A shows representative traces of sIPSC activity from a cerebrocortical neuron before, during, and after ethanol exposure. Figure 4B shows the concentration-response relationship for the effect of ethanol on sIPSC rate in these cortical neurons. These results demonstrate that ethanol did not increase GABA release from presynaptic terminals attached to these neurons. In addition, as shown in Table 1, ethanol had no effect on sIPSC amplitude or decay time from cerebrocortical neurons.
Fig. 4.
Ethanol does not have an effect on the frequency of the sIPSCs from medial frontal cortex neurons. A, representative traces of sIPSCs were recorded from a cerebrocortical neuron before ethanol administration (PRE), during application of 50 mM ethanol (EtOH), and after washout (WASH). B, when data were averaged across 10 to 12 neurons, there was no effect of 25, 50, or 100 mM ethanol on sIPSC frequency (P > 0.1).
Effect of Ethanol on mIPSCs Recorded from Neurons in a Substantia Nigra Reticula Slice
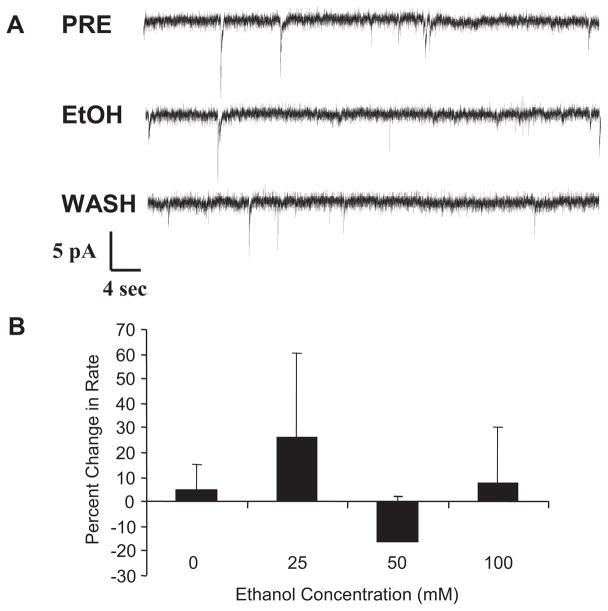
To demonstrate that effects of ethanol on GABAergic mIPSCs could be detected in a slice preparation as well as to examine another brain region, mIPSCs were recorded from SNR neurons in the slice. Figure 5A shows traces of mIPSC activity from a SNR neuron before, during, and after ethanol exposure. Figure 5B shows a concentration-related increase in mIPSC frequency by ethanol from SNR neurons. Under these recording conditions, ethanol did not alter either mIPSC amplitude or decay time from SNR neurons (Table 1).
Fig. 5.
Ethanol increases the frequency of mIPSCs from substantia nigra reticulata neurons. A, representative traces of mIPSCs recorded from a substantia nigra reticulata neuron before ethanol administration (PRE), during application of 50 mM ethanol (EtOH), and after washout (WASH). B, whereas there was a significant linear trend with an n of 9 to 13 per group (r = 0.48, n = 47), indicating a concentration-related effect of ethanol, only the 100 mM ethanol concentration produced a significant increase in mIPSC frequency (P < 0.05, Tukey’s honest square difference test). In the presence of a GABAB antagonist, the 50 mM ethanol concentration also produced a statistically reliable increase in frequency of mIPSCs (see text).
Because the effect of 50 mM ethanol on mIPSC frequency was not statistically reliable, we examined that same effect in the presence of a GABAB antagonist. In contrast to data from lateral septal neurons, the effect of 50 mM ethanol on mIPSC frequency in the presence of the GABAB agonist (CGP-53432; 32 10 μM) was significantly different from controls (41.4 ± 23.3% for the ethanol group versus −3.7 ± 3.8%; n = 13 for untreated controls). These data suggest that negative feedback from GABAB action at the presynaptic terminal may have decreased the effect of ethanol on GABA release in the SNR slice preparation.
Discussion
In agreement with previous work evaluating mIPSCs in the cerebellar slice preparation (Ming et al., 2006; Kelm et al., 2007), ethanol enhanced the frequency of sIPSCs, but it did not affect sIPSC amplitude or decay time. Although the mechanically dissociated neurons in the present study were tested in the absence of TTX, a similar increase in frequency of mechanically dissociated Purkinje cell mIPSCs was reported by Kelm et al. (2007) in the presence or absence of TTX. Thus, voltage-sensitive sodium channels on the preterminal axon or on the presynaptic terminal are not necessary for the effect of ethanol on mIPSCs or sIPSCs. The decrease in PPR during ethanol administration to cerebellar Purkinje neurons is consistent with the elevated frequency of sIPSCs and supports the concept that ethanol increases both spontaneous and stimulated GABA release. Likewise, ethanol enhanced the frequency of mIPSCs from neurons in the SNR slice without affecting mIPSC amplitude or decay time. These observations at these two brain sites are consistent with ethanol increasing release of GABA from presynaptic terminals onto the cerebellar Purkinje and SNR neurons with no measurable effect of ethanol on the postsynaptic receptor. This lack of an effect of ethanol on postsynaptic GABA receptor function is in agreement with earlier conclusions where ethanol did not alter the effect of direct application of GABA to neurons from these brain sites (Criswell et al., 2003). However, because this preparation was from young animals and was optimized for detection of presynaptic events, it may not be representative of in vivo neural function. Therefore, due to alterations in the internal environment of the postsynaptic neuron due to whole-cell patch recording, including dialysis of intracellular molecules and high chloride concentrations, these later results should be viewed with caution (Weiner and Valenzuela, 2006). Similar to previous findings in the cerebellum and the SNR, prior studies have demonstrated increased GABA release in several other regions of brain including the brainstem (Sebe et al., 2003), basolateral amygdala (Zhu and Lovinger, 2006), hippocampus (2006), and central amygdala (Roberto et al., 2003). Thus, these data collectively provide convincing data that ethanol can facilitate presynaptic release of GABA at several sites in the brain.
In contrast to the ability of ethanol to increase GABA release from presynaptic terminals in the cerebellum, SNR, and other brain sites, ethanol did not affect sIPSCs from lateral septal or cerebrocortical neurons. In addition, ethanol did not change the PPR in the lateral septum. These observations provide evidence that ethanol does not enhance spontaneous or stimulated GABA release from these sites at the ethanol concentrations tested. Furthermore, ethanol did not affect sIPSC amplitude or decay time at these latter brain sites, indicating that ethanol did not affect postsynaptic function. This latter finding in the lateral septum is also in agreement with previous data that ethanol does not affect postsynaptic receptors on neurons at this brain site (Criswell et al., 2003). However, as noted earlier, this preparation was optimized for detection of presynaptic events, and only a sample of easily patched neurons is represented. Thus, there may be specific neurons or conditions under which either presynaptic or postsynaptic effects of ethanol can be observed at these brain sites. Similar to the lack of effect of ethanol on presynaptic terminals in the lateral septum and cerebral cortex affecting GABA release, Proctor et al. (2006) failed to find an effect of ethanol on PPR from CA-1 hippocampal neurons in mice.
Taken together, these varying results provide evidence that ethanol is having a regionally specific effect on GABA release in brain. Whereas the mechanism underlying the effect of ethanol on presynaptic release of GABA from cerebellar Purkinje neurons has not been resolved, recent work has implicated the release of calcium from internal stores (Kelm et al., 2007). This observation provides a potential lead to determine whether this mechanism associated with calcium release from internal stores applies to other brain sites where ethanol increases GABA release. Likewise, it will be of interest to determine whether this mechanism is absent in neurons where ethanol does not influence GABA release.
Earlier work in the cerebellar slice preparation and in mechanically dissociated neurons from the amygdala found a similar enhancement of GABA release by ethanol in the presence of TTX (Ming et al., 2006; Zhu and Lovinger, 2006; Kelm et al., 2007). This indicates that an effect of ethanol on TTX-sensitive Na+ channels on the presynaptic terminal or preterminal axon is not required for an effect on GABA release. This conclusion was supported in the present investigation by the clear increase in mIPSC frequency by ethanol observed in the SNR slice in the presence of TTX (Fig. 5).
It is conceivable that ethanol could regulate GABA release by acting on the postsynaptic neuron to release a retrograde messenger such as nitric oxide (Shin and Linden, 2005) or an endogenous cannabinoid (Galante and Diana, 2004). However, the lack of NMDA receptors on cerebellar Purkinje neurons (Shin and Linden, 2005) argues against NMDA receptor-mediated nitric oxide release contributing to the effect of ethanol on GABA release at that site. Likewise, because the dendritic tree of the Purkinje neuron is removed during the mechanical dissociation, thereby eliminating the usual space-clamp problems, the voltage-clamp recording should minimize depolarization-induced cannabinoid release from mechanically dissociated neurons in the cerebellum or other brain sites (Galante and Diana, 2004). In support of this view, Kelm et al., (2007) demonstrated a similar increase in mIPSC frequency from cerebellar Purkinje neurons by ethanol in the presence of a CB1 receptor antagonist.
Presynaptic GABAB receptors can provide feedback inhibition of GABA release, and the activation of these receptors by endogenous GABA in some brain regions could decrease the ability of ethanol to elicit GABA release (Ariwodola and Weiner, 2004; Zhu and Lovinger, 2006). Therefore, differential GABAB receptor inhibition of GABA release represents a potential mechanism for the lack of effect of ethanol in some brain regions; moreover, we observed this effect in the SNR (Fig. 5). However, in the lateral septum, the observed lack of an effect of ethanol on GABA release in the presence of a GABAB antagonist argues against this explanation. In addition, GABAB-mediated feedback inhibition of increased GABA release would only be expected if the ethanol initially caused at least some increase in GABA release. Thus, a mechanism distinct from GABAB receptor inhibition must be sought to elucidate the mechanism of the regional specificity associated with the action of ethanol to release GABA from presynaptic neurons.
The noted regional specificity of ethanol to release GABA has considerable relevance to actions of ethanol on the CNS and to drugs that influence GABA function. The ability of ethanol to release GABA could account for a part of the behavioral GABAmimetic profile of ethanol resembling that of benzodiazepines and barbiturates (Frye and Breese, 1982), both of which are known to act primarily by a GABAergic mechanism (Martz et al., 1983). In addition, behavioral actions of ethanol are additive or superadditive with these GABAergic compounds, and they substitute for ethanol in discrimination studies (Frye and Breese, 1982; Frye et al., 1983; Martz et al., 1983; Grant et al., 2000). Although met with controversy (Wallner et al., 2003; Borghese et al., 2006), the ability of ethanol to enhance the action of GABA on receptors containing α6β3δ and α4β3δ subunits could be enhanced by the increased release of GABA by ethanol. Thus, in this case, “spill over” from increased GABA release by ethanol could have a direct effect on these extrasynaptic GABAA receptors to increase inhibitory tone (Wei et al., 2003, 2004). This scenario would be dependent upon a brain site where ethanol released GABA and where these ethanol-sensitive GABAA receptors are localized. Previous work has shown that substantia nigra and cerebellar Purkinje neurons are highly sensitive to enhancement of currents gated by exogenously applied GABA by neuroactive steroids (Criswell et al., 2003). Thus, there are receptors present that could respond to the increased GABA release by ethanol.
The regional differences in the ability of ethanol to release GABA could offer an explanation of earlier studies showing the regionally specific effects of ethanol on GABA function using in vivo extracellular recording (Bloom and Siggins, 1987; Givens and Breese, 1990; Criswell et al., 1993). Furthermore, this regional specificity for GABA release by ethanol may account for the selective effect of ethanol on specific behaviors. In other words, only behaviors mediated by a brain region sensitive to this action of ethanol would be modified by ethanol. A caution for this interpretation is that concentrations of ethanol below 50 mM did not induce a reliable increase in mIPSC frequency in vitro—a finding that might be expected for this action of ethanol to contribute to all aspects of its GABAmimetic profile (Frye et al., 1979). Future studies should be able to define the accuracy of the view that release of GABA from presynaptic terminals in specific brain regions is a critical contributor to the GABAmimetic profile of ethanol.
ABBREVIATIONS
- PPR
paired-pulse ratio
- ACSF
artificial cerebrospinal fluid
- sIPSC
spontaneous inhibitory postsynaptic current
- SNR
substantia nigra reticulate
- mIPSC
miniature inhibitory postsynaptic current
- TTX
tetrodotoxin
- NMDA
N-methyl-D-aspartate
- CGP-53432
3-[[(3,4-dichlorophenyl)methyl]amino]propyl]diethoxymethyl)phosphinic acid
References
- Aguayo LG. Ethanol potentiates the GABAA-activated Cl− current in mouse hippocampal and cortical neurons. Eur J Pharmacol. 1990;187:127–130. doi: 10.1016/0014-2999(90)90349-b. [DOI] [PubMed] [Google Scholar]
- Akaike N, Moorhouse AJ. Techniques: applications of the nerve-bouton preparation in neuropharmacology. Trends Pharmacol Sci. 2003;24:44–47. doi: 10.1016/s0165-6147(02)00010-x. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom FE, Siggins GR. Electrophysiological action of ethanol at the cellular level. Alcohol. 1987;4:331–337. doi: 10.1016/0741-8329(87)90031-0. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Storustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. The delta subunit of gamma-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- Botta P, Mameli M, Floyd KL, Radcliffe RA, Valenzuela CF. Ethanol sensitivity of GABAergic currents in cerebellar granule neurons is not increased by a single amino acid change (R100Q) in the alpha6 GABAA receptor subunit. J Pharmacol Exp Ther. 2007a;323:684–691. doi: 10.1124/jpet.107.127894. [DOI] [PubMed] [Google Scholar]
- Botta P, Radcliffe RA, Carta M, Mameli M, Daly E, Floyd KL, Deitrich RA, Valenzuela CF. Modulation of GABAA receptors in cerebellar granule neurons by ethanol: a review of genetic and electrophysiological studies. Alcohol. 2007b;41:187–199. doi: 10.1016/j.alcohol.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, et al. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci U S A. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsycho-pharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Griffith BL, Breese GR. Comparison of effect of ethanol on N-methyl-D-aspartate- and GABA-gated currents from acutely dissociated neurons: absence of regional differences in sensitivity to ethanol. J Pharmacol Exp Ther. 2003;304:192–199. doi: 10.1124/jpet.102.041590. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Simson PE, Duncan GE, McCown TJ, Herbert JS, Morrow AL, Breese GR. Molecular basis for regionally specific action of ethanol on gamma-aminobutyric acidA receptors: generalization to other ligand-gated ion channels. J Pharmacol Exp Ther. 1993;267:522–537. [PubMed] [Google Scholar]
- Crowder TL, Ariwodola OJ, Weiner JL. Ethanol antagonizes kainate receptor-mediated inhibition of evoked GABA(A) inhibitory postsynaptic currents in the rat hippocampal CA1 region. J Pharmacol Exp Ther. 2002;303:937–944. doi: 10.1124/jpet.102.038471. [DOI] [PubMed] [Google Scholar]
- Ebert B, Wafford KA, Whiting PJ, Krogsgaard-Larsen P, Kemp JA. Molecular pharmacology of gamma-aminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different alpha, beta, and gamma receptor subunit combinations. Mol Pharmacol. 1994;46:957–963. [PubMed] [Google Scholar]
- Frye GD, Breese GR. GABAergic modulation of ethanol-induced motor impairment. J Pharmacol Exp Ther. 1982;223:750–756. [PMC free article] [PubMed] [Google Scholar]
- Frye GD, Breese GR, Mailman RB, Vogel RA, Mueller RA. Similarities in the central actions of GABAmimetic drugs and ethanol. Br Res Bull. 1979;4:706. [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Differential sensitivity of ethanol withdrawal signs in the rat to gamma-aminobutyric acid (GABA)mimetics: blockade of audiogenic seizures but not forelimb tremors. J Pharmacol Exp Ther. 1983;226:720–725. [PubMed] [Google Scholar]
- Galante M, Diana MA. Group I metabotropic glutamate receptors inhibit GABA release at interneuron-Purkinje cell synapses through endocannabinoid production. J Neurosci. 2004;24:4865–4874. doi: 10.1523/JNEUROSCI.0403-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens BS, Breese GR. Site-specific enhancement of gamma-aminobutyric acid-mediated inhibition of neural activity by ethanol in the rat medial septal area. J Pharmacol Exp Ther. 1990;254:528–538. [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Waters CA, Green-Jordan K, Azarov A, Szeliga KT. Characterization of the discriminative stimulus effects of GABA(A) receptor ligands in Macaca fascicularis monkeys under different ethanol training conditions. Psychopharmacology (Berl) 2000;152:181–188. doi: 10.1007/s002130000510. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous gamma-aminobutyric acid release onto cerebellum Purkinje neurons. J Pharmacol Exp Ther. 2007;323:356–364. doi: 10.1124/jpet.107.126144. [DOI] [PubMed] [Google Scholar]
- Liu SJ. Biphasic modulation of GABA release from stellate cells by glutamatergic receptor sub-types. J Neurophysiol. 2007;98:550–556. doi: 10.1152/jn.00352.2007. [DOI] [PubMed] [Google Scholar]
- Martz A, Deitrich RA, Harris RA. Behavioral evidence for the involvement of gamma-aminobutyric acid in the actions of ethanol. Eur J Pharmacol. 1983;89:53–62. doi: 10.1016/0014-2999(83)90607-6. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Frye GD, Breese GR. Evidence for site specific ethanol actions in the CNS. Alcohol Drug Res. 1985;6:423–429. [PubMed] [Google Scholar]
- Ming Z, Criswell HE, Yu G, Breese GR. Competing presynaptic and postsynaptic effects of ethanol on cerebellar purkinje neurons. Alcohol Clin Exp Res. 2006;30:1400–1407. doi: 10.1111/j.1530-0277.2006.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Aistrup GL, Nishikawa K, Marszalec W, Yeh JZ, Narahashi T. Basis of variable sensitivities of GABA(A) receptors to ethanol. Alcohol Clin Exp Res. 2000;24:965–971. [PubMed] [Google Scholar]
- Peoples RW, Weight FF. Differential alcohol modulation of GABA(A) and NMDA receptors. Neuroreport. 1999;10:97–101. doi: 10.1097/00001756-199901180-00019. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Diao L, Freund RK, Browning MD, Wu PH. Synaptic GABAergic and glutamatergic mechanisms underlying alcohol sensitivity in mouse hippocampal neurons. J Physiol. 2006;575:145–159. doi: 10.1113/jphysiol.2006.112730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Prasad A, MacDonald JF. Ethanol modulation of GABA receptor-activated Cl− currents in neurons of the chick, rat and mouse central nervous system. Eur J Pharmacol. 1992;224:173–181. doi: 10.1016/0014-2999(92)90802-b. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebe JY, Eggers ED, Berger AJ. Differential effects of ethanol on GABA(A) and glycine receptor-mediated synaptic currents in brain stem motoneurons. J Neurophysiol. 2003;90:870–875. doi: 10.1152/jn.00119.2003. [DOI] [PubMed] [Google Scholar]
- Shin JH, Linden DJ. An NMDA receptor/nitric oxide cascade is involved in cerebellar LTD but is not localized to the parallel fiber terminal. J Neurophysiol. 2005;94:4281–4289. doi: 10.1152/jn.00661.2005. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Malherbe P. Recombinant GABAA receptor function and ethanol. FEBS Lett. 1993;324:140–142. doi: 10.1016/0014-5793(93)81380-i. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Schwartz RD, Skolnick P, Paul SM. Ethanol stimulates gamma-aminobutyric acid receptor-mediated chloride transport in rat brain synaptoneurosomes. Proc Natl Acad Sci U S A. 1986;83:4071–4075. doi: 10.1073/pnas.83.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low dose acute alcohol effects on GABA A receptor subtypes. Pharmacol Ther. 2006;112:513–528. doi: 10.1016/j.pharmthera.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spill-over in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol potentiates GABAergic synaptic transmission in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurophysiol. 2006;96:433–441. doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Gao BX, Hinckley C. Ethanol dual modulatory actions on spontaneous postsynaptic currents in spinal motoneurons. J Neurophysiol. 2003;89:806–813. doi: 10.1152/jn.00614.2002. [DOI] [PubMed] [Google Scholar]