Abstract
C2 spinal hemisection (C2HS) interrupts ipsilateral bulbospinal pathways and induces compensatory increases in contralateral spinal and possibly supraspinal respiratory output. Our first purpose was to test the hypothesis that after C2HS contralateral respiratory motor outputs become resistant to vagal inhibitory inputs associated with lung inflation. Bilateral phrenic and contralateral hypoglossal (XII) neurograms were recorded in anesthetized and ventilated rats. In uninjured (control) rats, lung inflation induced by positive end-expired pressure (PEEP; 3–9 cmH2O) robustly inhibited both phrenic and XII bursting. At 2 wk post-C2HS, PEEP evoked a complex response associated with phrenic bursts of both reduced and augmented amplitude, but with no overall change in the mean burst amplitude. PEEP-induced inhibition of XII bursting was still present but was attenuated relative to controls. However, by 8 wk post-C2HS PEEP-induced inhibition of both phrenic and XII output were similar to that in controls. Our second purpose was to test the hypothesis that vagal afferents inhibit ipsilateral phrenic bursting, thereby limiting the incidence of the spontaneous crossed phrenic phenomenon in vagal-intact rats. Bilateral vagotomy greatly enhanced ipsilateral phrenic bursting, which was either weak or absent in vagal-intact rats at both 2 and 8 wk post-C2HS. We conclude that 1) compensatory increases in contralateral phrenic and XII output after C2HS blunt the inhibitory influence of vagal afferents during lung inflation and 2) vagal afferents robustly inhibit ipsilateral phrenic bursting. These vagotomy data appear to explain the variability in the literature regarding the onset of the spontaneous crossed phrenic phenomenon in spontaneously breathing (vagal intact) vs. ventilated (vagotomized) preparations.
Keywords: plasticity, positive end-expired pressure, vagotomy, crossed phrenic phenomenon, hypoglossal nerve
spinal cord hemisection injury at C2 (C2HS) interrupts ipsilateral bulbospinal pathways to the phrenic motor nucleus, thereby inactivating ipsilateral phrenic motoneurons and paralyzing the hemidiaphragm (22, 23, 59). Inspiratory bursting in the ipsilateral phrenic neurogram and/or diaphragm electromyogram (EMG) can be induced after C2HS via physiological stimulation (e.g., hypoxia) as well as various pharmacological treatments (16, 19, 27, 57). The removal of spinal sensory inputs via cervical dorsal rhizotomy can also reveal or enhance ipsilateral phrenic output after C2HS (15, 24). This induced recovery of ipsilateral phrenic output has been termed the crossed phrenic phenomenon (23, 44). In addition to the induced crossed phrenic phenomenon, a gradual and spontaneous return of ipsilateral diaphragm EMG activity can be observed over weeks to months after injury (41, 43). The relative degree of phrenic motoneuron activation associated with the spontaneous appearance of the crossed phrenic phenomenon is minimal and thus does not approximate “normal” phrenic output (14, 32).
Lung inflation is associated with activation of slowly adapting receptors (SARs), which are located primarily in the intrapulmonary airways (48). In spinal-intact animals, lung inflation and SAR activation via positive end-expired pressure (PEEP) inhibit phrenic inspiratory motor output (37). However, it is not known whether chronic cervical spinal cord injury can alter these vagally mediated respiratory reflexes. For example, the compensatory increase in contralateral phrenic motor output after C2HS (41, 45) may attenuate or “override” the normal vagally mediated inhibition that occurs during lung inflation. Changes in lung volume and/or compliance (49) after cervical spinal cord injury also have the potential to alter vagal reflexes. Since compensatory respiratory control mechanisms following C2HS appear to occur at both supraspinal (20, 58) and spinal (20, 42, 45) levels, our first purpose was to test the hypothesis that contralateral phrenic and hypoglossal (XII) motor output become more resistant to vagal inhibitory inputs associated with lung inflation following chronic C2HS.
The postinjury time interval required for the spontaneous crossed phrenic phenomenon to become evident is highly variable across published reports (see Refs. 41, 47 for commentary). Most spontaneously breathing rats do not have inspiratory ipsilateral diaphragm EMG activity during eupneic breathing as late as 4 wk post-C2HS (1, 41, 43). On the other hand, under normoxic, normocapnic conditions [i.e., arterial partial pressure of O2 (PaO2) ∼100 mmHg, arterial partial pressure of CO2 (PaCO2) ∼35–40 mmHg], ipsilateral phrenic inspiratory bursting is readily apparent in >60% of mechanically ventilated, vagotomized rats by 14 days after injury (11, 14, 16, 47). By 1 mo post-C2HS, close to 100% of vagotomized, ventilated rats show ipsilateral phrenic inspiratory bursting (11, 14, 16, 18). A common finding is that the incidence of the spontaneous crossed phrenic phenomenon and the magnitude of ipsilateral bursting can be increased by raising the overall level of respiratory drive (10, 11, 14, 16, 18). Therefore, processes that actively inhibit ipsilateral phrenic motoneurons during spontaneous breathing may “mask” the recovery of ipsilateral phrenic bursting. There is one report from 1948 in which the potential vagal influence on the crossed phrenic phenomenon was investigated by observing diaphragm movements in rabbits following acute C2–C3 hemisection (7). It was noted that section of the vagus nerves evoked movement of the previously paralyzed hemidiaphragm (7). Accordingly, we reasoned that the discrepancy in the literature regarding the onset of the spontaneous crossed phrenic phenomenon in rats with chronic C2HS could be explained by the integrity of the vagus nerves. Our second purpose was to test the hypothesis that vagal afferents attenuate expression of ipsilateral phrenic bursting after chronic C2HS, and therefore acute vagotomy can reveal or enhance ipsilateral phrenic motor output.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats obtained from Harlan (Indianapolis, IN) were divided into the following groups: control (uninjured): n = 7, 373 ± 28 g, age 123 ± 18 days; sham operated (laminectomy only): n = 2, 425 and 440 g, age of both animals 144 days; 2 wk post-C2HS: n = 9, 321 ± 22 g, age 107 ± 5 days; and 8 wk post-C2HS: n = 7, 380 ± 39 g, age 145 ± 7 days (data are expressed as means ± SD). There were no significant differences in respiratory or cardiovascular responses between the uninjured animals and those that received sham surgery. The data from these animals were therefore combined as a single control group. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida.
Spinal Cord Injury
Rats were injured at 90 ± 1 days (mean ± SE) of age by previously described methods (10, 11, 14, 47). Briefly, the animals were anesthetized by injection of xylazine (10 mg/kg sq) and ketamine (140 mg/kg ip, Fort Dodge Animal Health). A dorsal cervical incision was made from the base of the skull to the C3 segment, followed by C2 laminectomy and left C2 dural section. A left C2HS was performed by microscalpel incision and aspiration. The dura and overlying muscles were sutured with 9-0 sutures (Ethicon) and 4-0 polyglycolic acid sutures (Webster Veterinary), respectively. The skin was subsequently closed with stainless steel wound clips (Stoelting), and the animal received an injection of yohimbine (1.2 mg/kg sq; Lloyd) to reverse the effect of xylazine. After surgery, animals were given an analgesic (buprinorphine, 0.03 mg/kg sq; Hospira, Lake Forest, IL) and sterile lactated Ringer solution (5 ml sq). The postsurgical care protocol included injection of lactated Ringer solution (5 ml/day sq) and oral supply of Nutri-cal supplements (1–3 ml, Webster Veterinary), which were given until adequate volitional drinking and eating resumed.
Neurophysiology Preparation
Many aspects of these procedures have been published previously (10, 11, 14, 47). Isoflurane anesthesia (3–4%) was initially induced in a closed chamber and then maintained via nose cone (2–3%). After tracheotomy rats were mechanically ventilated (model 683, Harvard Apparatus, South Natick, MA) with an oxygen-nitrogen mixture [inspired O2 fraction (FiO2) = 0.5–0.6; volume = 7 ml/kg; frequency = 60–65/min] throughout the experiment. The femoral vein was catheterized (PE-50) to permit conversion to urethane anesthesia (1.6 g/kg iv; Sigma, St. Louis, MO) and injection of a paralytic drug (pancuronium bromide, 2.5 mg/kg iv; Hospira). Another catheter was inserted into the femoral artery and connected to a pressure transducer (Statham P-10EZ pressure transducer, CP122 AC/DC strain gauge amplifier, Grass Instruments, West Warwick, RI) for pressure measurements. The tracheal pressure was monitored by a pressure transducer (Statham P-10EZ pressure transducer, CP122 AC/DC strain gauge amplifier, Grass Instruments) connected to the inspired line of the ventilator. The partial pressure of end-tidal CO2 (PetCO2) was analyzed with a Capnogard neonatal CO2 monitor placed on the expiratory line of the ventilator circuit (Novametrix Medical Systems, Wallingford, CT) (10, 11, 47) and was maintained at 50 mmHg by adjusting inspired CO2. Rectal temperature was monitored by an electrical thermometer and maintained at 37.5 ± 1°C by a servo-controlled heating pad (model TC-1000, CWE, Ardmore, PA).
The phrenic nerves were isolated in the cervical region via a ventral approach and sectioned distally. The right XII nerve was exposed and cut peripherally after removal of the diagastric muscle (34, 36, 37). Nerve activity was recorded with monopolar or bipolar hook silver electrodes and then amplified (1,000×, model 1700, A-M Systems, Carlsborg, WA), band-pass filtered (0.3–10 kHz), and integrated (time constant 100 ms; model MA-1000, CWE). The raw and integrated neural signals were digitized by the CED Power 1401 data acquisition interface and recorded on a PC with Spike2 software (Cambridge Electronic Design, Cambridge, UK). The sampling frequency of all signals was 100 Hz, with the exception of the raw neurograms, which were sampled at 10 KHz.
Experimental Protocols
Two separate protocols were performed in each animal. In the first protocol, lung inflation was induced by changing the PEEP under vagal-intact status. The purpose of this protocol was to examine whether phrenic and/or XII efferent bursting during lung inflation were altered after C2HS. Baseline was established with PEEP at 0 cmH2O. After stable nerve recordings were obtained, different levels of PEEP (3, 6, and 9 cmH2O) were randomly applied once by inserting the outlet tubing of the ventilator into a graduated cylinder of water. Each condition was maintained for 10 s, and then PEEP was returned to 0 cmH2O until nerve activity returned to baseline values. In the second protocol, rats were bilaterally vagotomized in the midcervical region after a prolonged (5 min) period of baseline recordings. This protocol was designed to test whether ipsilateral phrenic inspiratory bursting would be revealed after removal of vagal afferent inputs. During the baseline period, PetCO2 was maintained at 50 mmHg and no PEEP was applied. Different levels of PEEP (3, 6, and 9 cmH2O) were subsequently applied again to confirm that the effects of PEEP in the first protocol were mediated by the vagus nerves.
Spinal Cord Histology
Our procedures have been published along with detailed histological examples of the C2HS lesion (33, 47). At the conclusion of the neurophysiology experiments, rats, which were already deeply anesthetized with urethane (see above), were euthanized by systemic saline perfusion followed by 4% paraformaldehyde (Sigma). The cervical spinal cord was removed, cryoprotected, and sectioned at 40 μm via vibrotome. The spinal cord tissue sections were serially mounted on glass slides (Fisher Scientific, Pittsburgh, PA), stained with cresyl violet, and then evaluated by light microscopy. Two rats were removed from the analysis after their hemilesions were assessed to be incomplete.
Data Analyses
Neurograms, blood pressure, and tracheal pressure were analyzed with Spike 2 software. The integrated phrenic neurogram (∫Phr) was used to calculate inspiratory (Ti) and expiratory (Te) duration as previously described (34, 38). Briefly, the Ti was defined as the period between inspiratory phrenic burst onset and the time when ∫Phr amplitude declined by 50% of the peak value. The overall respiratory frequency was calculated as 60/(Ti + Te). Phrenic and XII inspiratory activity were defined as the peak height of the integrated neurograms. Although phrenic motor output was generally inhibited during application of PEEP, the peak ∫Phr signal was also sporadically augmented during PEEP (e.g., Figs. 1 and 2). These augmented discharge patterns were identified when the peak ∫Phr was greater than 110% of the baseline value. The characteristics of these bursts did not match previously published descriptions of “augmented breaths” in rats (14, 17). Augmented breaths are characterized by a distinct two-phase pattern. The first phase is similar to the preceding breath pattern. The second phase is characterized by a steeper rate of rise in the inspiratory flow trace or diaphragm EMG pattern (8). The augmented discharges observed during PEEP in our study (Fig. 2) did not show the characteristic two-phase pattern, but rather consisted of a single phase in which the rate of rise in the peak ∫Phr signal was greater than the previous breaths.
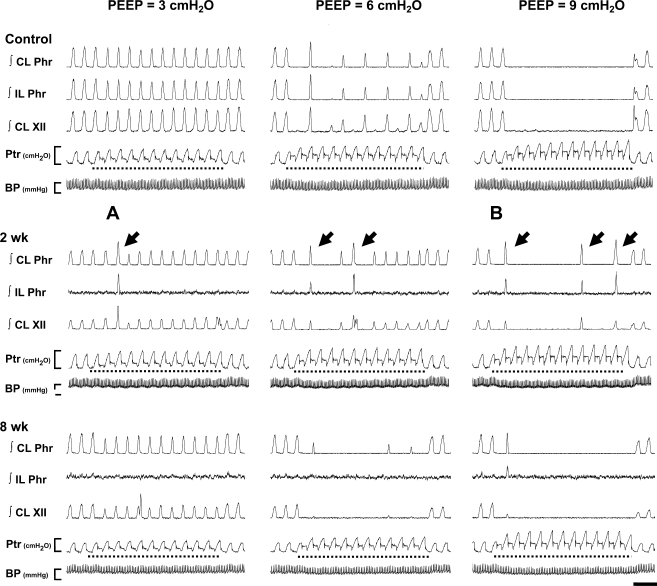
Fig. 1.
Representative neurograms demonstrating the impact of positive end-expired pressure (PEEP) on spinal (phrenic) and supraspinal (XII) respiratory motor output in control (uninjured) and C2 spinal hemisection (C2HS) rats. Figure depicts integrated (∫) phrenic (∫Phr) and hypoglossal (∫XII) bursting during 3 (left), 6 (center), or 9 (right) cmH2O PEEP in a control animal and 2 C2HS rats (2 and 8 wk after injury). Application of PEEP (dashed lines in each panel) was always accompanied by an increase in tracheal pressure (Ptr). At 2 wk post-C2HS, PEEP-induced inhibition of phrenic and XII output is less than in the control rat. Arrows indicate an “augmented phrenic burst.” See text for a more complete description. Sections marked with “A” and “B” are shown at an expanded timescale in Fig. 2. CL, contralateral (i.e., uninjured or right side); IL, ipsilateral (i.e., injured or left side); BP, arterial blood pressure (scale bar indicates 50–150 mmHg). Ptr scale bar indicates 0–10 cmH2O; time scale bar indicates 2 s.
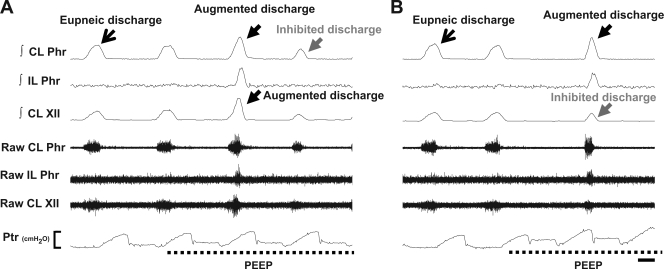
Fig. 2.
Representative neurograms depicting an augmented phrenic discharge accompanied by an augmented XII discharge (A) and an inhibited XII discharge (B) during PEEP application. This figure provides expanded timescale traces of the augmented phrenic discharges indicated by “A” and “B” in Fig. 1. The augmented phrenic discharge was accompanied by a similarly augmented XII discharge in A, but B shows that XII discharge could also be reduced under these conditions. Labels are as in Fig. 1. Ptr scale bar represents 0–10 cmH2O; time scale bar represents 0.25 s.
In the first protocol, data were averaged over 10 s at each level of PEEP. In the second protocol, data were averaged over 30 s before and after bilateral cervical vagotomy. The peak amplitude of the ∫Phr and ∫XII neurograms was expressed relative to the maximum PEEP response (% max) in the first protocol. In the second protocol, changes in efferent bursting following vagotomy were represented as a percentage of peak response (% peak) after vagotomy. A two-way repeated measurement analysis of variance (ANOVA) and Student-Newman-Keuls post hoc test (Sigma Stat 2.03, Jandel Scientific, St. Louis, MO) were used to compare differences across conditions (e.g., control vs. injured group at different levels of PEEP; ipsilateral vs. contralateral phrenic amplitude before and after vagotomy). All data are presented as means ± SE. A P value < 0.05 was considered statistically significant for all analyses.
RESULTS
Effects of PEEP in Vagal-Intact Rats
Phrenic and XII output.
Representative examples depicting phrenic and XII activity during different levels of PEEP in control and C2HS rats are shown in Fig. 1. Both phrenic and XII activity were reduced by increasing PEEP in control animals. In all C2HS rats, both the contralateral phrenic and XII nerves showed clear rhythmic inspiratory activity during baseline conditions (0 cmH2O PEEP) with intact vagus nerves (see bursting before PEEP application, Fig. 1). However, inspiratory bursting in the ipsilateral phrenic nerve during baseline was either very weak or absent in vagal-intact C2HS rats at both 2 and 8 wk after injury. There was an indication of a time-dependent recovery of spontaneous ipsilateral phrenic activity in vagal-intact rats (43). Specifically, ipsilateral phrenic bursting was observed in 33% (3/9) of rats at 2 wk post-C2HS and 71% (5/7) of rats at 8 wk post-C2HS. Of note, the 2 wk data are remarkably similar to those in a recent report showing a 30% incidence of ipsilateral diaphragm EMG activity in spontaneously breathing and unanesthetized rats at 2 wk post-C2HS (41).
PEEP evoked two distinct responses in the contralateral peak ∫Phr neurogram (or the right ∫Phr signal of control rats): 1) suppression of phasic inspiratory bursts and 2) augmented discharges associated with enhanced ∫Phr amplitude (141 ± 4% baseline) and reduced Ti (75 ± 4% baseline; P < 0.01, t-test). In both control and 8 wk post-C2HS rats, the appearance of augmented phrenic discharges was relatively rare (Table 1). Accordingly, in these groups increasing PEEP caused a reduction of the contralateral peak ∫Phr burst (injured rats) or bilateral ∫Phr bursts (controls) (P < 0.01, Figs. 1 and 3A). In contrast, the augmented phrenic discharges occurred more frequently in 2 wk post-C2HS animals (Table 1), and therefore the contralateral peak ∫Phr amplitude was maintained as PEEP was increased in these rats (P > 0.05, Fig. 3A). When augmented discharges were excluded from the analyses, the inhibitory effects of PEEP on contralateral activity were still attenuated in 2 wk post-C2HS animals (data not shown). Interestingly, the augmented discharges observed in the contralateral phrenic nerve were usually associated with activation of crossed phrenic pathways. Thus ipsilateral phrenic bursting could be observed during augmented contralateral phrenic discharges evoked by PEEP (Fig. 2). The onset of augmented discharges in the ipsilateral phrenic nerve always occurred later than for contralateral augmented discharges in C2HS animals (Fig. 2). Specifically, the onset difference between ipsilateral and contralateral augmented phrenic discharges was 78 ± 10 ms and 50 ± 36 ms at 2 and 8 wk post-C2HS, respectively.
Table 1.
Augmented discharges in phrenic nerve during PEEP application
| PEEP |
|||
|---|---|---|---|
| 3 cmH2O | 6 cmH2O | 9 cmH2O | |
| 2 wk | |||
| Animals | 44% | 88% | 56% |
| Breaths | 6 | 12 | 7 |
| 8 wk | |||
| Animals | 29% | 29% | 14% |
| Breaths | 2 | 2 | 1 |
| Control | |||
| Animals | 33% | 33% | 0% |
| Breaths | 3 | 3 | 0 |
The percentage of rats showing augmented discharges in each experimental group (e.g., 2 wk, 8 wk, or control) at each level of positive end-expired pressure (PEEP) is shown. In addition, the total number of augmented discharges at each level of PEEP (breaths) is shown.
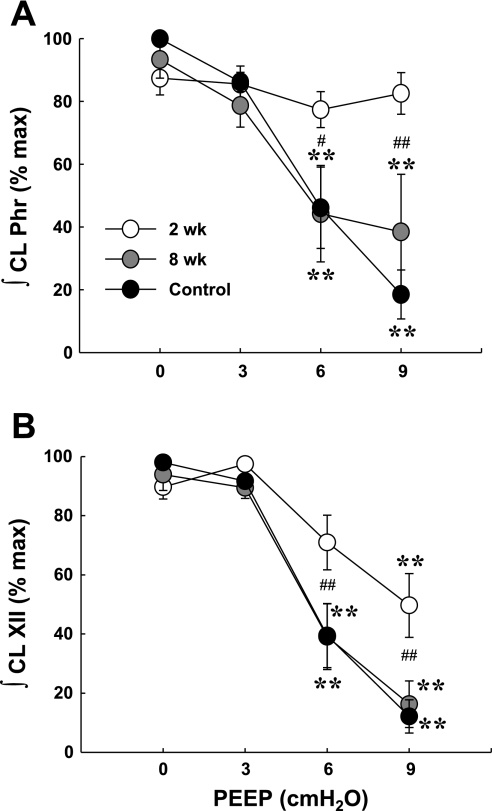
Fig. 3.
Effects of PEEP on contralateral phrenic (A) and XII (B) burst amplitude. PEEP of 6–9 cmH2O reduced peak contralateral ∫Phr in both control and 8 wk post-C2HS animals. In contrast, this response was not observed at 2 wk after injury. PEEP reduced peak ∫XII in all groups; however, the effects of PEEP were attenuated at 2 wk after injury. **Different from baseline condition of PEEP = 0 cmH2O (P < 0.01); #P < 0.05, ##P < 0.01, significant difference between the 2 wk postinjury group and both the 8 wk postinjury and control groups.
The responses of the contralateral XII neurogram during PEEP were similar to what was observed in the contralateral phrenic neurogram. The extent of XII inhibition during PEEP was indistinguishable between control and 8 wk postinjury rats (P > 0.05, Fig. 3B), whereas the inhibitory effects of PEEP on XII activity were attenuated 2 wk post-C2HS (P < 0.01, Fig. 3B). Occasionally, XII motor output demonstrated a similarly augmented burst coinciding with the augmented phrenic discharges evoked by PEEP (Fig. 2A). On average, the increase in peak ∫XII discharge (121 ± 11%) associated with augmented bursting was lower than observed in the ∫Phr output (141 ± 4% baseline; P < 0.01, t-test on ranks). The augmented XII response was observed in 18 of 36 cases of augmented phrenic discharges (50%) recorded in all animals. In the remaining cases, the XII burst was either unchanged or reduced during augmented phrenic discharges (Fig. 2B). Thus the increased respiratory drive associated with augmented phrenic discharges was not always transmitted to inspiratory upper airway motor output.
After bilateral vagotomy, PEEP no longer induced changes in phrenic or XII bursting in control or C2HS rats (data not shown). Accordingly, the inhibitory effects of PEEP on inspiratory motor output were mediated by vagal afferent neurons.
Respiratory cycle and frequency.
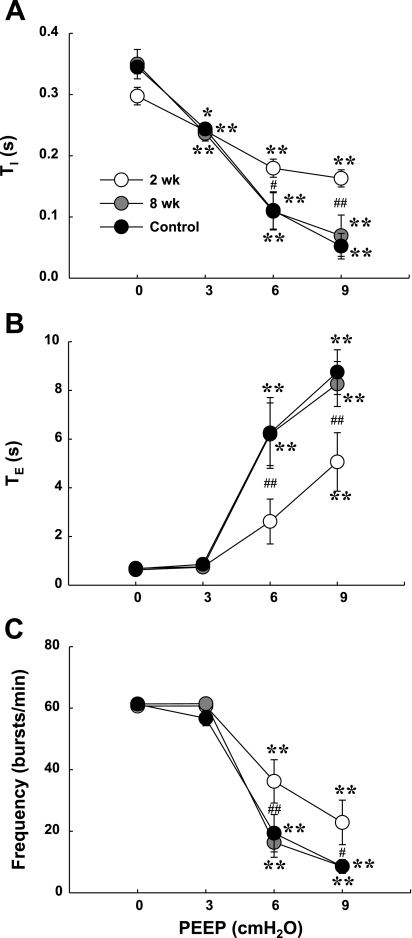
Increasing PEEP caused a gradual decrease in Ti in all groups (P < 0.01, Fig. 4A). On the other hand, Te was significantly increased when PEEP was set at 6–9 cmH2O (P < 0.01, Fig. 4B), and this resulted in a reduction in respiratory frequency (P < 0.01, Fig. 4C). Interestingly, PEEP-induced changes in respiratory cycle duration and frequency were significantly less in the 2 wk post-C2HS animals compared with control and 8 wk post-C2HS animals (P < 0.05, Fig. 4).
Fig. 4.
Effects of PEEP on inspiratory (Ti, A) and expiratory (Te, B) duration and respiratory frequency (C) in control and C2HS rats. Elevation of PEEP induced a typical lung inflation reflex resulting in reduction of Ti, elongation of Te, and a decline in respiratory frequency (bursts/min) in all groups. However, the extent of inhibition during PEEP was attenuated in the 2 wk post-C2HS animals. *P < 0.05, **P < 0.01 vs. baseline value; #P < 0.05, ##P < 0.01 significant difference between the 2 wk postinjury group and both the 8 wk postinjury and control groups.
Tracheal pressure.
As PEEP increased from 0 to 9 cmH2O, the peak tracheal pressure associated with lung inflation increased as expected (P < 0.01, Table 2). There were no significant differences in tracheal pressure between C2HS and control rats (P > 0.01, Table 2). This finding indicates that the degree of lung inflation induced by PEEP was similar in all groups.
Table 2.
Effects of PEEP on tracheal pressure
| PEEP |
||||
|---|---|---|---|---|
| 0 cmH2O | 3 cmH2O | 6 cmH2O | 9 cmH2O | |
| 2 wk | 11 ± 1 | 12 ± 1 | 15 ± 1 | 17 ± 1 |
| 8 wk | 10 ± 1 | 12 ± 1 | 15 ± 1 | 19 ± 1 |
| Control | 10 ± 1 | 11 ± 1 | 14 ± 1 | 19 ± 1 |
Tracheal pressure values (in cmH2O) are means ± SE.
Mean arterial blood pressure and heart rate.
Similar to prior reports (37), mean arterial blood pressure declined when PEEP was elevated to 6 and 9 cmH2O in control animals (P < 0.05, Table 3). Application of PEEP caused a slight reduction in mean arterial pressure in control and 2 wk post-C2HS rats. The mean arterial pressure tended to be lower in 8 wk post-C2HS rats at baseline (P = 0.059 vs. control), and PEEP did not cause a further reduction in mean arterial pressure in these rats (Table 3). With one exception, heart rate (HR) was unchanged during PEEP: the 8 wk postinjury group showed a small but significant decrease in HR during 9 cmH2O PEEP (P < 0.05, Table 3).
Table 3.
Effect of PEEP on mean arterial pressure and heart rate
| PEEP |
||||
|---|---|---|---|---|
| 0 cmH2O | 3 cmH2O | 6 cmH2O | 9 cmH2O | |
| MAP, mmHg | ||||
| 2 wk | 135 ± 5 | 133 ± 4 | 128 ± 4† | 126 ± 4† |
| 8 wk | 116 ± 7 | 115 ± 7 | 112 ± 7 | 112 ± 7 |
| Control | 135 ± 6 | 132 ± 5 | 129 ± 5* | 125 ± 5† |
| HR, beats/min | ||||
| 2 wk | 456 ± 9 | 455 ± 9 | 454 ± 10 | 452 ± 10 |
| 8 wk | 437 ± 8 | 436 ± 8 | 433 ± 8 | 430 ± 11† |
| Control | 445 ± 9 | 444 ± 9 | 442 ± 9 | 443 ± 8 |
Values are means ± SE. MAP, mean arterial pressure; HR, heart rate.
P < 0.05,
P < 0.01 compared with values during baseline (PEEP = 0 cmH2O).
Phrenic and XII Activity Following Bilateral Vagotomy
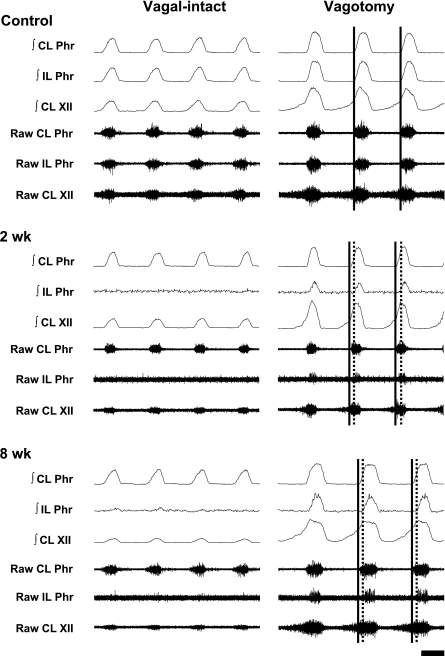
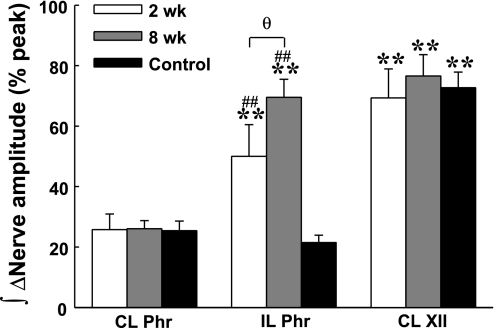
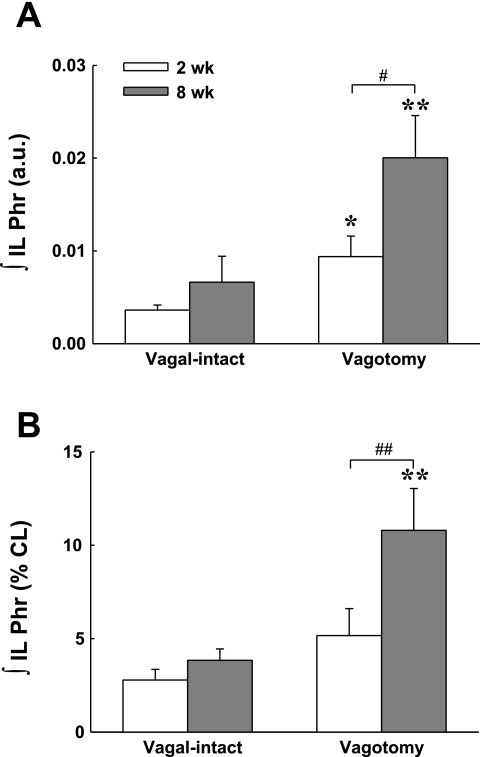
Vagotomy induced similar increases in peak bilateral ∫Phr in the control group (P > 0.05, Figs. 5 and 6). In contrast, the increase in peak ipsilateral ∫Phr amplitude was greater than the increase in contralateral amplitude in C2HS animals at both 2 and 8 wk after injury (P < 0.01, Figs. 5 and 6). Additionally, the number of C2HS rats showing spontaneous ipsilateral phrenic activity increased after vagotomy. Specifically, after vagotomy rhythmic ipsilateral phrenic activity occurred in 78% (7/9) of rats at 2 wk post-C2HS and in all rats at 8 wk post-C2HS. Moreover, evidence of time-dependent (i.e., postinjury) recovery of ipsilateral bursting was more robust after vagotomy (Fig. 7). After vagotomy, peak ipsilateral ∫Phr burst amplitude was greater at 8 wk vs. 2 wk post-C2HS when data were expressed either as arbitrary units (P < 0.05) or relative to the contralateral peak ∫Phr amplitude (% contralateral, P < 0.01) (Fig. 7). Although rhythmic bursting in the ipsilateral phrenic nerve was enhanced after vagotomy, the onset of the ipsilateral phrenic burst was delayed with respect to the contralateral phrenic nerve by 82 ± 11 ms and 72 ± 8 ms at 2 wk and 8 wk post-C2HS, respectively. Vagotomy caused a robust increase in XII activity in control rats as previously reported (38). The increase in contralateral peak ∫XII amplitude following vagotomy was similar in control and C2HS rats (Fig. 6).
Fig. 5.
Representative neurograms demonstrating the impact of bilateral vagotomy on spinal (phrenic) and supraspinal (XII) respiratory motor output in control (uninjured) and C2HS rats. Respiratory bursting is shown with vagus nerves intact (left) and shortly after bilateral vagotomy (right). In control rats (top), vagotomy induced the expected slowing of burst frequency and increase in both phrenic and XII burst amplitude. Note also that the onset of inspiratory bursting is similar between the contralateral and ipsilateral phrenic nerves in the control rat (vertical solid lines). At 2 (middle) and 8 (bottom) wk post-C2HS, ipsilateral phrenic inspiratory bursting was typically absent or very weak when the vagus nerves were intact. After vagotomy, however, very clear ipsilateral phrenic bursting was present, and the onset of bursting was slightly delayed relative to the contralateral phrenic burst (compare solid vs. dashed vertical lines). After vagotomy, ipsilateral phrenic bursting was more robust in rats that were 8 vs. 2 wk post-C2HS. Labels are as in Fig. 1. Time scale bar is 0.5 s.
Fig. 6.
Average changes (Δ) in peak integrated phrenic and XII burst amplitude following bilateral vagotomy. Peak contralateral ∫Phr and ∫XII burst amplitudes showed similar increases after vagotomy in control and C2HS rats. However, vagotomy induced a relatively greater increase in the ipsilateral ∫Phr burst amplitude after C2HS. In addition, the increase in the ipsilateral ∫Phr burst amplitude after vagotomy was greater at 8 compared with 2 wk post-C2HS. **P < 0.01 vs. contralateral phrenic amplitude; ##P < 0.01 vs. control group; θP < 0.05, significant difference between 2 and 8 wk post-C2HS groups.
Fig. 7.
Mean ipsilateral phrenic inspiratory burst amplitude at 2 and 8 wk post-C2HS. Peak ipsilateral ∫Phr burst amplitude was quantified as an absolute voltage [i.e., arbitrary units (a.u.), A] and as % of the burst amplitude in the contralateral phrenic nerve (% CL, B). Both analyses suggest a progressive increase in ipsilateral phrenic burst amplitude over 2–8 wk after C2HS. Differences between 2 and 8 wk post-C2HS rats were statistically significant only after vagotomy. *P < 0.05, **P < 0.01 vagal-intact vs. vagotomized; ##P < 0.01, significant different between 2 and 8 wk post-C2HS groups.
Respiratory frequency (min−1) was similar in vagal-intact control and C2HS rats because of the entrainment effects of the mechanical ventilator; however, some differences in timing were noted between groups (Table 4). Most notably, Ti was significantly less 2 wk after injury compared with control rats. After vagotomy, respiratory frequency was significantly reduced in control and 2 wk post-C2HS rats. Ti was significantly lower in 2 wk post-C2HS versus control rats (P < 0.01, Table 4).
Table 4.
Effects of vagotomy on respiratory cycle and frequency
| Ti, s |
Te, s |
Respiratory Frequency, bursts/min |
||||
|---|---|---|---|---|---|---|
| Vagal-intact | Vagotomized | Vagal-intact | Vagotomized | Vagal-intact | Vagotomized | |
| 2 wk | 0.29 ± 0.01† | 0.27 ± 0.01‡ | 0.70 ± 0.02 | 0.86 ± 0.04* | 60 ± 1 | 54 ± 2* |
| 8 wk | 0.35 ± 0.01 | 0.33 ± 0.02 | 0.61 ± 0.01 | 0.71 ± 0.04 | 62 ± 0 | 58 ± 2† |
| Control | 0.34 ± 0.01 | 0.37 ± 0.02 | 0.62 ± 0.02 | 0.81 ± 0.07* | 62 ± 1 | 52 ± 2* |
Values are means ± SE. Ti, inspiratory duration; Te, expiratory duration.
P < 0.01 compared with values under vagal-intact condition;
P < 0.05,
P < 0.01 compared with control group.
DISCUSSION
This study provides the first comprehensive analyses of the impact of vagal afferents on phrenic and XII motor output following chronic cervical spinal cord injury. These data demonstrate that the PEEP-induced inhibition of contralateral phrenic and XII burst amplitude and timing normally observed in neurologically intact animals is attenuated at 2 wk post-C2HS. However, this attenuated inhibition is no longer evident at 8 wk post-C2HS, indicating that vagally mediated lung inflation reflexes are altered only transiently after unilateral high cervical spinal cord injury. Additionally, these data indicate that ipsilateral phrenic burst amplitude becomes considerably more robust after bilateral vagotomy at both 2 and 8 wk post-C2HS. In contrast, rhythmic inspiratory activity in the ipsilateral phrenic nerve was weak or absent entirely in vagal-intact animals at both time points. Thus vagal afferents have persistent inhibitory effects on ipsilateral phrenic motor output after C2HS. We suggest that vagally mediated inhibition of phrenic output contributes to the delayed appearance of the crossed phrenic phenomenon in spontaneously breathing, vagal-intact animals compared with ventilated and vagotomized animals.
Critique of Methods
Our data indicate that the functional impact of activating vagal afferent neurons during PEEP is transiently changed after C2HS. This interpretation, however, is subject to caveats. Lung volume was manipulated by applying PEEP to the expiratory line of the mechanical ventilator (9, 13, 35, 37, 46), and it is possible that the relative degree of lung inflation differed between experimental groups. For example, decreased lung compliance has been reported after cervical spinal cord injury in humans (reviewed in Ref. 49), and altered compliance may influence the extent of lung inflation during PEEP. However, the tracheal pressure during PEEP application was similar between C2HS and control animals (Table 2), suggesting similar lung compliance between groups. Additionally, animals in the present study were paralyzed, and therefore end-expiratory lung volume could not change due to recruitment of expiratory muscles during PEEP application. Accordingly, we suggest that the changes in PEEP-induced reflexes following C2HS do not reflect differential lung inflation compared with control animals.
Application of PEEP will activate different populations of pulmonary vagal receptors including SARs, rapidly adapting receptors (RARs), and bronchial C-fiber receptors (40, 56). Consequently, the vagally mediated reflexes evoked in this study cannot be specifically attributed to activation of a single type of vagal afferent neuron. Another prospect not addressed in this study is that the sensitivity of pulmonary vagal receptors may change after chronic spinal cord injury. We are unaware of any data directly addressing this possibility, and this will be an interesting topic for future studies. Finally, the PetCO2 in the present study (50 mmHg) was maintained ∼10 mmHg above the CO2 recruitment threshold for inspiratory phrenic activity as reported in prior studies (10, 11, 14, 16, 19). We felt it was important to maintain PetCO2 well above the recruitment threshold to ensure robust contralateral bursting, and to confirm that the weak ipsilateral phrenic bursting in vagal intact rats was not due to low CO2 values. The potential interaction between arterial CO2 values and vagal afferents in modulating phrenic output after C2HS represents another interesting topic for future studies.
Influence of PEEP on Contralateral Phrenic and XII Activity
Both phrenic and XII responses indicated that the classic Hering-Breuer inflation reflex was evoked by PEEP in control rats as previously reported (37). In contrast, PEEP-induced phrenic and XII reflexes were transiently attenuated after C2HS. The attenuated inhibition of respiratory motor output evident at 2 wk after injury appears to reflect the sum of two potentially independent processes. Specifically, PEEP evoked a decrease in the amplitude of the typical “eupneic” bursts but also induced the occurrence of augmented phrenic bursts. As a result, the overall contralateral phrenic burst amplitude was not reduced during PEEP. The augmented phrenic bursts during PEEP do not appear to be identical to the previously described “augmented breaths” that occur during spontaneous breathing (8, 17). First, the pattern of the augmented discharges observed in the contralateral phrenic nerve did not match the two-phase pattern of the typical augmented breath (8). Second, two prior studies have demonstrated that while augmented breaths occur more frequently after C2HS, this effect is not evident until ∼3 mo after injury (14, 17). The occurrence of the augmented discharges may counteract the inhibitory impact of PEEP on “eupneic activity,” thereby enabling C2HS animals to maintain ventilation during activation of inhibitory vagal inputs.
Several mechanisms could underlie the altered contralateral phrenic output during lung inflation after C2HS. We initially hypothesized that compensatory increases in contralateral respiratory output would blunt vagally mediated inhibition. However, if that were the case, we would have expected the attenuated inhibitory effects of PEEP to persist at 8 wk after injury because ipsilateral phrenic motor output remained very low in vagal-intact rats (i.e., compensatory contralateral output was presumably still required). The augmented phrenic discharges during PEEP were also observed in ipsilateral output. Thus the mechanisms driving this response are not unique to contralateral compensation per se, and this response likely reflects plasticity within the brain stem neurons and/or networks activated by vagal afferent neurons. Pulmonary vagal afferents terminate primarily in the nucleus tractus solitarii (NTS), which integrates peripheral sensory inputs and initiates reflexes to modulate ventilation (3, 4, 31). The rapid shallow breathing pattern induced by C2HS (21) will likely be associated with persistent changes in vagal inputs to the NTS, and this in turn may trigger changes in neural connectivity within brain stem respiratory neural circuits. Prior work indicates that both short-term and long-term plasticity in NTS neurons can be induced under experimental or pathophysiological conditions (4, 29).
Ipsilateral Phrenic Motor Output and Vagotomy
A time-dependent, spontaneous recovery of ipsilateral phrenic activity occurred over 2–8 wk post-C2HS (e.g., Fig. 7). This observation is consistent with the results of many previous investigations (1, 10, 11, 14, 16, 19, 20, 41, 42). Evaluation of these prior studies indicates that the appearance of ipsilateral diaphragm EMG activity in spontaneously breathing, vagal-intact rats after C2HS usually occurs later than the return of ipsilateral phrenic bursting recorded in ventilated, vagotomized rats (14, 16, 43, 54). For example, Nantwi et al. (43) and Vinit et al. (54) reported that inspiratory EMG activity of the ipsilateral diaphragm was not observed until 1–3 mo post-C2HS. In contrast, inspiratory bursting in the ipsilateral phrenic nerve of vagotomized, ventilated rats occurs as early as 2 wk post-C2HS (14, 16). We observed robust increases in ipsilateral phrenic bursting after vagotomy at both 2 and 8 wk post-C2HS. In light of these results, we suggest that the status of the vagus nerves contributes to differences in the time course of ipsilateral phrenic versus diaphragm recovery across prior studies.
The increase in ipsilateral phrenic bursting after vagotomy most likely reflects a decrease in vagally mediated inhibitory inputs to brain stem respiratory centers with a resultant increase in depolarizing inputs to ipsilateral phrenic motoneurons/premotor neurons. Indeed it is well established that the overall level of “respiratory drive” can increase crossed phrenic activity (16, 19, 27, 58). Since tracheal pressure was held constant before and after vagotomy, it is unlikely that changes in lung mechanics contributed to the changes in phrenic bursting. Interestingly, a previous report showed that recovery of ipsilateral phrenic bursting was accelerated in C2HS rats with chronic carotid body excision (2). Accordingly, both mechanical and/or chemical-sensitive vagal afferent inputs may inhibit ipsilateral phrenic motor output following C2HS and partially mask the onset of the crossed phrenic phenomenon.
There are a few caveats related to our suggestion that vagal inhibition at least partially explains the discrepancy in the onset of the spontaneous crossed phrenic phenomenon among prior studies. First, ipsilateral phrenic neurograms may not necessarily predict EMG activity in the ipsilateral diaphragm. However, since both are strongly influenced by phrenic motoneuron activity, it is reasonable to speculate that a strong relationship will exist between these two variables. Second, differences in arterial blood gases across published reports make it difficult to compare the results directly. While a wide range of arterial CO2 values has been reported for spontaneously breathing, anesthetized, spinal-intact rats [∼33–49 mmHg (21, 25, 28)], there is considerably less information about blood gases after chronic C2HS in unventilated animals. Golder et al. (21) found that anesthetized, spontaneously breathing rats studied at 2 mo post-C2HS are slightly hypoxic (PaO2 ∼82 mmHg) with PaCO2 values of ∼49 mmHg. These blood gases would seem to favor crossed phrenic activity compared with values typically maintained in ventilated preparations [e.g., PaO2 > 100 mmHg, PaCO2 = 35–40 mmHg (10, 11, 14, 16, 19)]. In any case, here we document that when CO2 is held constant at a level associated with robust contralateral phrenic activity, activation of vagal afferents will dramatically inhibit the crossed phrenic phenomenon.
Ipsilateral vs. Contralateral Phrenic Motor Output
The vagotomy-induced enhancement of burst amplitude was considerably greater for the ipsilateral phrenic nerve compared with the contralateral nerve. This result could reflect the minimal output of the ipsilateral phrenic nerve before vagotomy. Assuming a similar change in motoneuron discharge frequencies, an increase from 5 to 15 active ipsilateral phrenic motoneurons (200% increase) following vagotomy may manifest as a much larger response compared with an increase from 75 to 100 contralateral phrenic motoneurons (33% increase). Another possibility is that the intrinsic cellular properties of ipsilateral motoneurons are altered after injury. For example, ipsilateral phrenic motoneuron soma size decreases after C2HS (41), and changes in synaptic morphology have also been described (51). Ipsilateral phrenic motoneurons may therefore be more excitable after chronic C2HS (23, 41), and this adaptation might affect their responses to vagotomy. It also appears that ipsilateral and contralateral phrenic motoneurons have different bursting patterns after C2HS. Studies in animals indicate that inspiratory phrenic activity is generated by cells that burst at the onset of inspiration [early inspiratory (early-I)] and during the middle of the inspiration [late inspiratory (late-I)] (30, 38, 52). A previous study demonstrated that most phrenic motoneurons burst with a late-I pattern immediately following C2HS (12). Similarly, we have observed that the majority of active ipsilateral phrenic motoneurons discharge as the late-I type following chronic C2HS (K.-Z. Lee and D. D. Fuller, unpublished results). At least one prior report (26) indicates that late-I phrenic motoneuron discharge is more strongly modulated by lung inflation compared with early-I bursting. Hence, the enhancement of ipsilateral versus contralateral phrenic motor output following vagotomy may reflect a shift in the distribution of ipsilateral phrenic motoneurons toward the late-I phenotype. This alteration of phrenic motoneuron distribution may also explain why bursting in the ipsilateral phrenic nerve after C2HS is usually initiated slightly after the inspiratory burst in the contralateral phrenic nerve (Fig. 5; Ref. 14). Finally, the responses of ipsilateral versus contralateral phrenic motoneurons to vagotomy may reflect a difference in descending inputs to these cells. Phrenic premotor neurons are primarily located in the rostral ventral respiratory group (rVRG) and the Bötzinger complex. These brain stem regions project primarily excitatory (rVRG) and inhibitory (Bötzinger) inputs to phrenic motoneurons (50, 53). However, in contrast to the bilateral nature of rVRG projections, Bötzinger complex neurons primarily innervate the ipsilateral phrenic nucleus (5). Hence, ipsilateral phrenic motoneurons may receive fewer descending inhibitory inputs after C2HS and may therefore be more easily excited after vagotomy.
Functional Implications
There are several implications of our data for respiratory control following high cervical spinal cord injury. First, we suggest that the blunted inhibition of phrenic burst frequency during PEEP and the increase in the number of augmented phrenic discharges are both compensatory responses that enable ventilation to be maintained in the face of inhibitory inputs in the initial days and weeks following injury. Second, these results confirm that ipsilateral phrenic motor output is relatively weak or even absent in vagal-intact rats over 2–8 wk post-C2HS (1, 43). These results are consistent with the notion that ipsilateral phrenic bursting does not make a substantial contribution to tidal volume during “eupneic” breathing after C2HS (14, 18). Third, both the PEEP and vagotomy data support the idea that magnitude of crossed phrenic activity correlates with the overall intensity of central respiratory drive (16, 39) and indicate that vagal afferents have a particularly strong inhibitory influence on ipsilateral phrenic motor output. The potential influence of vagal afferents on assessment of functional respiratory outcomes should be considered in studies of rehabilitation or other interventions after C2HS (see, e.g., Refs. 16, 19, 55). Finally, persons with cervical spinal cord injury experiencing respiratory failure often require mechanical ventilator support involving relatively large tidal volumes and PEEP (6). These ventilator settings help to maintain alveolar ventilation, but they may limit activation of spinal (phrenic) motoneurons and any associated activity-dependent plasticity that could otherwise facilitate phrenic motor recovery.
GRANTS
Support for this work was provided by National Institutes of Health Grants 1R01-HD-052682-01A1 (D. D. Fuller) and 1R01-NS-054025 (P. J. Reier). Support was also provided by the Oscar and Anne Lackner Chair in Medicine (P. J. Reier). K.-Z. Lee was supported by grants from the University of Florida and the Paralyzed Veterans of America Research Foundation (no. 2691).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Elisa Gonzalez-Rothi for editing the manuscript.
REFERENCES
- 1. Alilain WJ, Goshgarian HG. Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp Neurol 212: 348–357, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bae H, Nantwi KD, Goshgarian H. Effects of carotid body excision on recovery of respiratory function in C2 hemisected adult rats. Exp Neurol 195: 140–147, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Bailey EF, Fregosi RF. Modulation of upper airway muscle activities by bronchopulmonary afferents. J Appl Physiol 101: 609–617, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Bonham AC, Chen CY, Sekizawa S, Joad JP. Plasticity in the nucleus tractus solitarius and its influence on lung and airway reflexes. J Appl Physiol 101: 322–327, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Boulenguez P, Gauthier P, Kastner A. Respiratory neuron subpopulations and pathways potentially involved in the reactivation of phrenic motoneurons after C2 hemisection. Brain Res 1148: 96–104, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care 51: 853–868, 2006 [PMC free article] [PubMed] [Google Scholar]
- 7. Chatfield PO, Mead S. The role of the vagi in the crossed phrenic phenomenon. Fed Proc 7: 20, 1948 [PubMed] [Google Scholar]
- 8. Cherniack NS, von Euler C, Glogowska M, Homma I. Characteristics and rate of occurrence of spontaneous and provoked augmented breaths. Acta Physiol Scand 111: 349–360, 1981 [DOI] [PubMed] [Google Scholar]
- 9. Coleridge HM, Coleridge JCG. Reflexes evoked from tracheobronchial tree and lungs. In: Handbook of Physiology. The Respiratory System. Control of Breathing. Bethesda, MD: Am. Physiol. Soc., 1986, sect. 3, vol. II, p. 395–429 [Google Scholar]
- 10. Doperalski NJ, Fuller DD. Long-term facilitation of ipsilateral but not contralateral phrenic output after cervical spinal cord hemisection. Exp Neurol 200: 74–81, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Doperalski NJ, Sandhu MS, Bavis RW, Reier PJ, Fuller DD. Ventilation and phrenic output following high cervical spinal hemisection in male vs. female rats. Respir Physiol Neurobiol 162: 160–167, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. El-Bohy AA, Goshgarian HG. The use of single phrenic axon recordings to assess diaphragm recovery after cervical spinal cord injury. Exp Neurol 156: 172–179, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Ezure K, Tanaka I, Saito Y. Activity of brainstem respiratory neurones just before the expiration-inspiration transition in the rat. J Physiol 547: 629–640, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol 211: 97–106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fuller DD, Johnson SM, Johnson RA, Mitchell GS. Chronic cervical spinal sensory denervation reveals ineffective spinal pathways to phrenic motoneurons in the rat. Neurosci Lett 323: 25–28, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci 23: 2993–3000, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Golder FJ, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Augmented breath phase volume and timing relationships in the anesthetized rat. Neurosci Lett 373: 89–93, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci 23: 2494–2501, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci 25: 2925–2932, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci 21: 8680–8689, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Golder FJ, Reier PJ, Davenport PW, Bolser DC. Cervical spinal cord injury alters the pattern of breathing in anesthetized rats. J Appl Physiol 91: 2451–2458, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Goshgarian HG. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir Physiol Neurobiol 169: 85–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol 94: 795–810, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol 72: 211–225, 1981 [DOI] [PubMed] [Google Scholar]
- 25. Goshgarian HG, Moran MF, Prcevski P. Effect of cervical spinal cord hemisection and hemidiaphragm paralysis on arterial blood gases, pH, and respiratory rate in the adult rat. Exp Neurol 93: 440–445, 1986 [DOI] [PubMed] [Google Scholar]
- 26. Hwang JC, St John WM, Bartlett D., Jr Influence of pulmonary inflations on discharge patterns of phrenic motoneurons. J Appl Physiol 63: 1421–1427, 1987 [DOI] [PubMed] [Google Scholar]
- 27. James E, Nantwi KD. Involvement of peripheral adenosine A2 receptors in adenosine A1 receptor-mediated recovery of respiratory motor function after upper cervical spinal cord hemisection. J Spinal Cord Med 29: 57–66, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janssen PL, Fregosi RF. No evidence for long-term facilitation after episodic hypoxia in spontaneously breathing, anesthetized rats. J Appl Physiol 89: 1345–1351, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Kline DD. Plasticity in glutamatergic NTS neurotransmission. Respir Physiol Neurobiol 164: 105–111, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kong FJ, Berger AJ. Firing properties and hypercapnic responses of single phrenic motor axons in the rat. J Appl Physiol 61: 1999–2004, 1986 [DOI] [PubMed] [Google Scholar]
- 31. Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 101: 618–627, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends Neurosci 31: 538–547, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol 511: 692–709, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee KZ, Fuller DD. Preinspiratory and inspiratory hypoglossal motor output during hypoxia-induced plasticity in the rat. J Appl Physiol 108: 1187–1198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee KZ, Fuller DD, Lu IJ, Ku LC, Hwang JC. Pulmonary C-fiber receptor activation abolishes uncoupled facial nerve activity from phrenic bursting during positive end-expired pressure in the rat. J Appl Physiol 104: 119–129, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Lee KZ, Fuller DD, Lu IJ, Lin JT, Hwang JC. Neural drive to tongue protrudor and retractor muscles following pulmonary C-fiber activation. J Appl Physiol 102: 434–444, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Lee KZ, Fuller DD, Tung LC, Lu IJ, Ku LC, Hwang JC. Uncoupling of upper airway motor activity from phrenic bursting by positive end-expired pressure in the rat. J Appl Physiol 102: 878–889, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Lee KZ, Reier PJ, Fuller DD. Phrenic motoneuron discharge patterns during hypoxia-induced short-term potentiation in rats. J Neurophysiol 102: 2184–2193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lewis LJ, Brookhart JM. Significance of the crossed phrenic phenomenon. Am J Physiol 166: 241–254, 1951 [DOI] [PubMed] [Google Scholar]
- 40. Ma A, Bravo M, Kappagoda CT. Responses of bronchial C-fiber afferents of the rabbit to changes in lung compliance. Respir Physiol Neurobiol 138: 155–163, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Mantilla CB, Sieck GC. Neuromuscular adaptations to respiratory muscle inactivity. Respir Physiol Neurobiol 169: 133–140, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol 79: 1640–1649, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Nantwi KD, El-Bohy A, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehabil Neural Repair 13: 225–234, 1999 [Google Scholar]
- 44. Rosenblueth A, Ortiz T. The crossed respiratory impulses to the phrenic. Am J Physiol 117: 495–513, 1936 [Google Scholar]
- 45. Rowley KL, Mantilla CB, Sieck GC. Respiratory muscle plasticity. Respir Physiol Neurobiol 147: 235–251, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Saito Y, Ezure K, Tanaka I. Difference between hypoglossal and phrenic activities during lung inflation and swallowing in the rat. J Physiol 544: 183–193, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sandhu MS, Dougherty BJ, Lane MA, Bolser DC, Kirkwood PA, Reier PJ, Fuller DD. Respiratory recovery following high cervical hemisection. Respir Physiol Neurobiol 169: 94–101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schelegle ES, Green JF. An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respir Physiol 125: 17–31, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Schilero GJ, Spungen AM, Bauman WA, Radulovic M, Lesser M. Pulmonary function and spinal cord injury. Respir Physiol Neurobiol 166: 129–141, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Schreihofer AM, Stornetta RL, Guyenet PG. Evidence for glycinergic respiratory neurons: Botzinger neurons express mRNA for glycinergic transporter 2. J Comp Neurol 407: 583–597, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Sperry MA, Goshgarian HG. Ultrastructural changes in the rat phrenic nucleus developing within 2 h after cervical spinal cord hemisection. Exp Neurol 120: 233–244, 1993 [DOI] [PubMed] [Google Scholar]
- 52. St John WM, Bartlett D., Jr Comparison of phrenic motoneuron responses to hypercapnia and isocapnic hypoxia. J Appl Physiol 46: 1096–1102, 1979 [DOI] [PubMed] [Google Scholar]
- 53. Stornetta RL, Sevigny CP, Guyenet PG. Inspiratory augmenting bulbospinal neurons express both glutamatergic and enkephalinergic phenotypes. J Comp Neurol 455: 113–124, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Vinit S, Gauthier P, Stamegna JC, Kastner A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J Neurotrauma 23: 1137–1146, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol 169: 210–217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yu J, Pisarri TE, Coleridge JC, Coleridge HM. Response of slowly adapting pulmonary stretch receptors to reduced lung compliance. J Appl Physiol 71: 425–431, 1991 [DOI] [PubMed] [Google Scholar]
- 57. Zhou SY, Goshgarian HG. 5-Hydroxytryptophan-induced respiratory recovery after cervical spinal cord hemisection in rats. J Appl Physiol 89: 1528–1536, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Zimmer MB, Goshgarian HG. Spinal cord injury in neonates alters respiratory motor output via supraspinal mechanisms. Exp Neurol 206: 137–145, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the neural regulation of respiratory function. Exp Neurol 209: 399–406, 2008 [DOI] [PubMed] [Google Scholar]