Abstract
Factors that stimulate mitochondrial biogenesis in skeletal muscle include AMP-activated protein kinase (AMPK), calcium, and circulating free fatty acids (FFAs). Chronic treatment with either 5-aminoimidazole-4-carboxamide riboside (AICAR), a chemical activator of AMPK, or increasing circulating FFAs with a high-fat diet increases mitochondria in rat skeletal muscle. The purpose of this study was to determine whether the combination of chronic chemical activation of AMPK and high-fat feeding would have an additive effect on skeletal muscle mitochondria levels. We treated Wistar male rats with a high-fat diet (HF), AICAR injections (AICAR), or a high-fat diet and AICAR injections (HF + AICAR) for 6 wk. At the end of the treatment period, markers of mitochondrial content were examined in white quadriceps, red quadriceps, and soleus muscles, predominantly composed of unique muscle-fiber types. In white quadriceps, there was a cumulative effect of treatments on long-chain acyl-CoA dehydrogenase, cytochrome c, and peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) protein, as well as on citrate synthase and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activity. In contrast, no additive effect was noted in the soleus, and in the red quadriceps only β-HAD activity increased additively. The additive increase of mitochondrial markers observed in the white quadriceps may be explained by a combined effect of two separate mechanisms: high-fat diet-induced posttranscriptional increase in PGC-1α protein and AMPK-mediated increase in PGC-1α protein via a transcriptional mechanism. These data show that chronic chemical activation of AMPK and a high-fat diet have a muscle type specific additive effect on markers of fatty acid oxidation, the citric acid cycle, the electron transport chain, and transcriptional regulation.
Keywords: AICAR, fiber type, mitochondrial biogenesis, PGC-1α, PPARδ
factors known to stimulate mitochondrial biogenesis in skeletal muscle include AMP-activated protein kinase (AMPK) activity and circulating free fatty acids (FFAs). In response to an endurance exercise training bout both AMPK activity in skeletal muscle and circulating FFAs are elevated (42, 60), raising the question as to whether these two factors could work together to induce mitochondrial biogenesis. Rats treated with 5-aminoimidazole-4-carboxamide riboside (AICAR) for 4 wk have increased levels of certain mitochondrial markers in skeletal muscle (62). Furthermore, models of reduced AMPK activity have less skeletal muscle mitochondrial proteins compared with controls (35, 56). High-fat feeding has also been shown to increase fatty acid oxidative capacity and mitochondrial content in skeletal muscle, likely due to elevated circulating FFAs (25, 39–41, 48, 57). Treating rats with both heparin, which increases circulating FFAs, and a high-fat diet results in mitochondrial biogenesis (21). In contrast, treating diabetics for 7 days with acipimox, which decreases circulating FFAs, results in decreased mRNA levels of a number of mitochondrial proteins and transcription regulators (7). These data show that circulating FFAs play an important role in mitochondrial biogenesis in skeletal muscle (21).
The mechanisms through which AMPK and circulating FFAs induce mitochondrial biogenesis appear to be somewhat distinct. For example, both are believed to induce mitochondrial biogenesis by increasing the ability of peroxisome proliferator-activated receptor-gamma coactivator-1 α (PGC-1α) to coactivate transcription factors through different mechanisms. AMPK regulates PGC-1α at both the gene and protein level. Constitutively activating AMPK by mutating a regulatory subunit of AMPK increases both the protein and mRNA levels of PGC-1α (22). AMPK can increase PGC-1α mRNA levels by regulating binding of transcription factors to regions in the PGC-1α gene promoter (29, 32, 34). Additionally, it has been reported that AMPK activates PGC-1α by phosphorylating PGC-1α (33, 34) and via activation of sirtuin 1 (SIRT1) (12, 30). Together these studies show that AMPK increases PGC-1α abundance and/or binding activity through multiple mechanisms.
High-fat feeding is believed to influence mitochondrial gene transcription by regulating PGC-1α through a different mechanism. Raising circulating FFAs increases peroxisome proliferator-activated receptor-δ (PPARδ) binding to the muscle carnitine palmitoyltransferase (mCPT) promoter in rat epitrochlearis (21). FFAs activate PPARδ by binding tightly inside the ligand binding domain causing movement of the COOH-terminal helix, a region involved in the binding of PPARδ coactivators (18, 20). Interestingly, inducing high levels of PPARδ protein in rat skeletal muscle increases PGC-1α protein but not mRNA abundance (25, 38). A similar pattern is also observed when rats are treated with a high-fat diet for 5 wk, which elevates PPARδ protein content (25, 39). Since increasing PPARδ protein levels induces similar effects as raising circulating FFAs and FFAs are ligands of PPARδ, this suggests that FFAs increase skeletal muscle mitochondria by activating PPARδ. In summary, it is believed that high-fat feeding can stimulate mitochondrial biogenesis by raising circulating FFAs that activate PPARδ, and over a period of a few weeks, leads to a posttranscriptional increase in PGC-1α protein (25).
The availability of blood FFAs and AMPK protein abundance vary considerably between skeletal muscle fiber types. Muscles predominantly composed of type IIa and type I fibers receive greater blood flow, and consequently receive more FFAs than muscles mostly composed of type IIb fibers (23). The protein abundance of one of the two catalytic subunits of AMPK, AMPKα1, is greater in muscles predominantly composed of type I and/or type IIa fibers compared with those predominantly composed of type IIb fibers (47, 61). Conflicting reports exist on the relative abundance of the other catalytic subunit, AMPK α2, in different muscle fiber types (47, 61). The AMPK γ3 subunit, believed to be the only γ-subunit bound to heterotrimeric AMPK complexes in skeletal muscle that is activated by exercise (8), is higher in the red quadriceps and soleus compared with white quadriceps(16). Because blood FFA availability and the expression of AMPK subunits differ between the muscle fiber types, elevation of circulating FFA or AMPK activity would also likely not have the same degree of an effect on mitochondria content.
The purpose of this study was to determine if chronic AMPK activation in skeletal muscle and elevated FFAs in the blood have an additive effect on mitochondrial content of skeletal muscle. In addition, we examined three different muscles to determine if responses to treatments were muscle type specific. In the future, these findings could be applied to better understanding the mechanisms involved in exercise training-induced mitochondrial biogenesis.
MATERIALS AND METHODS
Animal care.
All experimental procedures used were approved by the Institutional Animal Care Committee of Brigham Young University. Wistar male rats were kept in a temperature-controlled and well-ventilated room with a 12:12-h light-dark cycle. Rats were fed rodent laboratory chow diet, 8604 Harlan Teklad Rodent Diet, and water ad libitum.
Treatments lasted 6 wk. Rats were treated with either AICAR injections (AICAR) (n = 8), a high-fat diet (HF) (n = 11), AICAR injections and a high-fat diet (HF + AICAR) (n = 10), or nothing (control) (n = 9). To examine the effect of our treatments on different muscle types we measured mitochondrial markers in white quadriceps, red quadriceps, and soleus. The fiber type composition of these three muscles is as follows (fiber type-population%): 1) white quadriceps—rectus femoris (type I-1%, type IIa-25%, type IIb-74%), vastus lateralis (type I-0%, type IIa-3%, type IIb-97%); 2) red quadriceps—rectus femoris (type I-7%, type IIa-53%, type IIb-40%), vastus lateralis (type I-9%, type IIa-56%, type IIb-35%); and 3) soleus (type I-87%, type IIa-13%, type IIb-0%) (4).
AICAR injections.
AICAR treatment was given by subcutaneous injection at a dose of 0.5 mg AICAR/g body weight (BW) dissolved in 0.9% NaCl each morning of the treatment period. AICAR was injected into two regions, axillary and between the scapulas. To distinguish the acute response from chronic adaptations to the AICAR treatment, half the rats in the AICAR and HF + AICAR groups were injected with AICAR 1 h before dissection while the other half were not injected on the day of dissections.
To equalize the stress associated with the AICAR treatment, rats from control and HF groups were handled daily at the time the rats treated with AICAR were handled. Further, rats in the control and HF groups were injected with comparable volume of saline to the AICAR injection.
High-fat diet.
Rats were fed the high-fat diet ad libitum for 6 wk. Of the calories in the high-fat diet, 60% came from fat with the fat coming from flax seed (40% of calories) and olive oil (20% of calories), a diet described previously (21). These fats were chosen to maximize the potential activation of PPARδ through the binding of fatty acids to PPARδ because unsaturated FAs are most effective at activating PPARδ (18). The composition of the diet was as follows (g/kg of food): 116.3 g olive oil, 232.7 g flax seed oil, 87.2 g sugar, 174.6 g starch, 226.6 g casein, 4.5 g methionine, 30.7 g gelatin, 51.2 g wheat bran, 22.5 g vitamin mix (Harlan Teklad, AIN76A), 52.2 g mineral mix (Harlan Teklad, AIN76), 1.4 g choline chloride.
Dissections.
Rats were anesthetized with 65 mg/kg pentobarbital sodium. Tissue extraction began once rats were fully sedated. Red (approximately 200–300 mg) and white (approximately 130–200 mg) sections of quadriceps and soleus were removed quickly and clamp frozen with liquid nitrogen chilled metal tongs then wrapped in aluminum foil and stored at −90°C. Blood was drawn from the abdominal vena cava and placed in heparinized Eppendorf tubes, centrifuged for 10 min at 3,000 g, and supernatant was stored at −90°C. Omental, epididymal, and retroperitoneal fat pads were removed and weighed to determine abdominal fat pad weight.
Homogenization.
Frozen muscle was pulverized in liquid nitrogen, weighed, and homogenized in 19× homogenization buffer (50 mM Tris·HCl, 250 mM mannitol, 50 mM NaF, 5 mM sodium pyrophosphate, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 50 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM DTT, 1 mM benzamidine, 0.1 mM PMSF, 5 μg/ml soybean trypsin inhibitor, pH 7.4). Homogenate was stored at −90°C.
Citrate synthase assay.
Whole raw homogenates, obtained using methods as described above, that had been freeze-thawed three times to disrupt the mitochondria were diluted in 100 mM Tris buffer, pH 8.0, and citrate synthase activity was measured using the method described by Srere (50).
β-Hydroxyacyl-CoA dehydrogenase activity assay.
β-Hydroxyacyl-CoA dehydrogenase (β-HAD) activity was measured as described previously (62) with the exception that the supernatant spun at 1,200 g was used instead of whole raw homogenate. Briefly, potassium phosphate buffer, pH 7.5, NADH, and supernatant were added to a cuvette. The cuvette was incubated for 10 min at 30°C. Acetoacetyl-CoA was added to the cuvette, and activity was measured at 340 nm.
Nonesterified free fatty acids assay.
Nonesterified free fatty acids (NEFA) were measured using a commercially available assay, NEFA-HR(2) (WAKO Diagnostics, Richmond, VA).
RT-qPCR.
RNA was isolated by homogenizing frozen ground muscle in Trizol reagent (Invitrogen, Carlsbad, CA; cat. no. 15596-026) using an Ultra Turrax T10 homogenizer and then using the Qiagen mini kit (Qiagen, Alencia, CA; cat. no. 74104). Isolated RNA was quantified using NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA) using 260/280 absorbance ratio. RNA was reverse transcribed using the Invitrogen Superscript III kit (cat. no. 18080-051) according to manufacturer's protocol, and cDNA was stored at 4°C. For quantified PCR (qPCR), cDNA, forward and reverse primers, and Syber green were added to wells of a clear polypropylene plate (Bio-Rad, Hercules, CA; cat. no. MLL9601) in triplicate, sealed with Microseal B Film (Bio-Rad; cat. no. MSB1001), centrifuged to remove air bubbles, and placed in a C1000 Thermal Cycler (Bio-Rad). Eight control, six AICAR, nine HF, and eight HF + AICAR white quadriceps were used to measure PGC-1α mRNA. Samples were subjected to 2 min at 50°C, 8.5 min at 95°C, and 40 cycles of 15 s at 95°C and 60°C for 1 min. The melt curve was obtained by changing temperature from 55°C to 90°C at 10-s, 0.5°C increments. Bio-Rad CFX manager software was used. We used the quantification cycle (Cq) values provided by the software to quantify the relative expression of mRNA using the Livak method. Samples from the qPCR plate post qPCR reaction diluted in 6× loading dye solution (Fermentas, Burlington, Ontario, Canada; cat. no. R0611) and Perfect Size 50-bp ladder (5 Prime, Gaithersburg, MD; cat. no. 2500320) were loaded into the wells of a 2% ethidium bromide gel. cDNA bands were visualized with a UV light. Only one amplicon for each primer set, which appeared at their predicted weights, was detected (see Supplemental Fig. S1, available with the online version of this article). To verify RNA stability we ran the RNA samples diluted in 2× sample loading buffer (Fisher Scientific, Rockford, IL; cat. no. BP2812500), which contained ethidium bromide and Riboladder 1-kb RNA standard (Fisher BioReagents BP281150) in a 1.5% agarose gel. RNA bands were visualized with a UV light and 18S and 28S bands were checked to verify RNA stability (see Supplemental Fig. S2, available with the online version of this article).
Primer sequences (5′ to 3′) were as follows: 18S mRNA (Invitrogen), forward GTGCATGGCCGTTCTTAGTTG and reverse GCCACTTGTCCCTCTAAGAAGTTG; PGC-1α mRNA (Biosynthesis, Lewisville, TX), forward CGATGACCCTCCTCACACCA and reverse TTGGCTTGAGCATGTTGCG.
Western blot.
Muscle homogenates obtained as described above were freeze-thawed three times and then centrifuged for 10 min at 1,000 g. Muscle homogenates were freeze-thawed three times to disrupt mitochondria. Protein concentration was measured on the supernatant fraction using the DC protein assay method (Bio-Rad). An aliquot of the supernatant fraction was dissolved in 2× Laemmli buffer and then subjected to SDS-PAGE. Membranes were blocked for 1 h at room temperature in 5% nonfat dry milk dissolved in TBST, incubated in the appropriate primary antibody overnight, rinsed 4× 5 min in TBST, incubated for 1 h at room temperature in the appropriate secondary antibody dissolved in 1% nonfat dry milk dissolved in TBST, and again rinsed 4 × 5 min in TBST. Protein bands were visualized on autoradiographic film (Classic Blue Sensitive, Midwest Scientific, St. Louis, MO) using ECL PLUS (GE Healthcare, Piscataway, NJ) and quantified by densitometry using AlphaEaseFC software (Alpha Innotech, San Leandro, CA). In our lab we have identified the PGC-1α protein band at 110 kDa by running recombinant PGC-1α protein (a gift from John O. Holloszy, Washington Univ. School of Medicine, St. Louis, MO) and brown adipose tissue homogenate on a gel along with skeletal muscle homogenates. Brown adipose tissue was loaded because it has a high level of PGC-1α protein. A representative blot is available in the supplemental section of a recently published paper from our lab (54). In the supplemental section evidence is also provided for the specificity of the Custom PPARδ rat-specific antibody (provided by Dong-Ho Han, Washington Univ. School of Medicine, St. Louis, MO) that we used (see supplemental Fig. S3, available with the online version of this article).
Primary antibodies were as follows: cytochrome c (Santa Cruz, Santa Cruz, CA; cat. no. sc-13156), hexokinase II (Santa Cruz; cat. no. sc-6521), long-chain acyl-CoA dehydrogenase (LCAD) (a gift from Daniel P. Kelly, Burnham Institute for Medical Research-Lake Nona, Orlando, FL); PGC-1α (Calbiochem, La Jolla, CA; cat. no. 516557), PPARδ (a gift from Dong-Ho Han, Washington Univ. School of Medicine, St. Louis, MO), uncoupling protein 3 (UCP3) (Affinity BioReagents, Golden, CO; cat. no. PA1-055), phospho-AMPKα (pAMPKα) (Cell Signaling, Beverly, MA; cat. no. 2535L), total AMPKα (Cell Signaling; cat. no. 2532L), phospho-acetyl-CoA carboxylase (pACC) (Upstate, Lake Placid, NY; cat. no. 07-303), acetyl-CoA carboxylase (ACC), and steptavidin horseradish peroxidase (GE Healthcare; cat. no. RPN1231V).
Secondary antibodies were as follows: donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), donkey anti-mouse IgG (Santa Cruz; cat. no. sc-2314), donkey anti-goat IgG (Santa Cruz; cat. no. sc-2020).
Statistics.
Significant differences between groups were determined using two-way ANOVA (high fat × AICAR) and Bonferonni post hoc test for multiple comparisons. The statistical software SigmaStat (Systat Software, San Jose, CA) was used. Statistical significance is defined as P < 0.05. Results are presented as means ± SE.
RESULTS
Treatment response to AICAR and high-fat feeding.
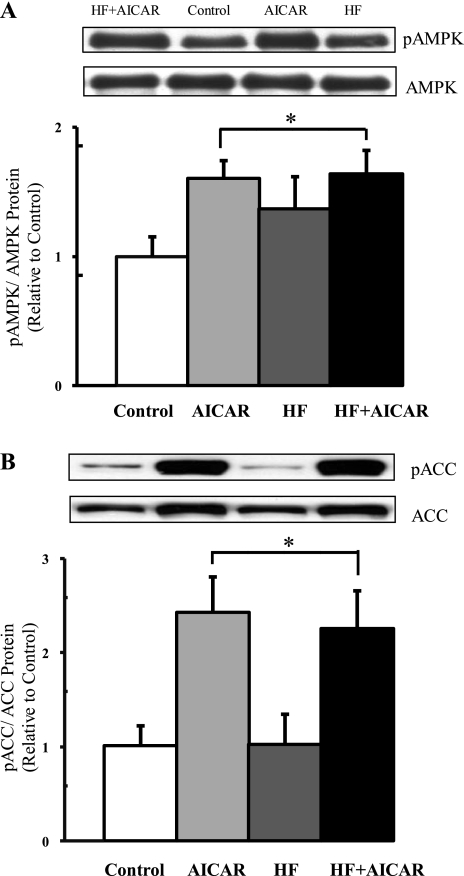
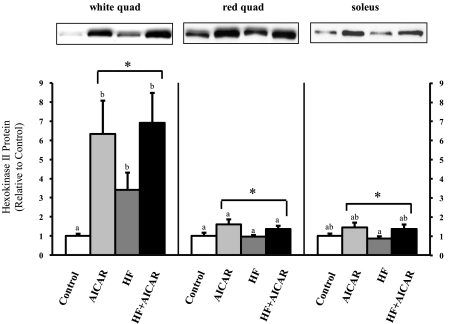
Acute AMPK activation by AICAR was verified by measuring the phosphorylation of AMPK and ACC in rats from the AICAR and HF + AICAR groups injected with AICAR on the day of dissection. One hour after AICAR injection, there was an increase in pAMPK in the white quadriceps (Fig. 1). Further, in the white quadriceps (Fig. 1), red quadriceps, and soleus the AICAR treatment significantly elevated pACC levels. Together these data verify that AICAR acutely increased AMPK activation in skeletal muscle. The effectiveness of the chronic activation of AMPK was also confirmed by examining the abundance of hexokinase II protein. Previously, a similar duration of AICAR treatment as used in our study, albeit at a higher dose (1 mg/g BW), resulted in increased hexokinase II protein expression (62). It has also been demonstrated that this increase in hexokinase II in response to AICAR is dependent on the presence of AMPKα2 (35). Using the lower dose of AICAR (0.5 mg/g BW) that we used in this study also resulted in increased hexokinase II protein expression (Fig. 2). This lower dose was used to limit potential side effects of AICAR on rats.
Fig. 1.
AMP-activated protein kinase (AMPK) activity increased with acute 5-aminoimidazole-4-carboxamide riboside (AICAR) treatment. Muscles from AICAR-treated rats were removed 1 h after the daily AICAR injection. A: phospho-AMPK (pAMPK)/total AMPK protein level in white quadriceps (n = 3–4). B: phospho-acetyl-CoA carboxylase (pACC)/total ACC protein level in white quadriceps (n = 4–5). *Main treatment effect (P < 0.05). HF, high-fat diet; AICAR, AICAR injections; HF + AICAR, high-fat diet and AICAR injections.
Fig. 2.
Hexokinase II protein levels increased with chronic AICAR treatment in skeletal muscle (n = 6–10). Letters are used to represent significance; same letter means no significant difference (P < 0.05). AICAR and HF were not compared. *Main treatment effect (P < 0.05). Quad, quadriceps.
The high-fat diet was effective in elevating circulating FFA levels as there was a significant main effect of high-fat feeding on the elevation of circulating levels of FFAs (Table 1). The duration of the high-fat diet used in this study was not sufficient to cause a significant increase in BW compared with control (Table 1). As expected, abdominal fat was significantly increased with high-fat feeding (Table 1).
Table 1.
Average body weight, liver weight, and abdominal fat pad weight at end of treatment
| Control | AICAR | HF | HF + AICAR | |
|---|---|---|---|---|
| Body weight, g | 345 ± 8a | 310 ± 3b | 348 ± 6a† | 341 ± 10a† |
| Liver weight, g | 14.9 ± 0.3a | 15.2 ± 0.6a | 14.6 ± 0.4a | 15.8 ± 0.7a |
| Abdominal fat pads, fat pads weight g/g body wt | 0.028 ± 0.002a | 0.028 ± 0.002a | 0.049 ± 0.003b† | 0.044 ± 0.004b† |
| NEFA in plasma, mmol/l | 0.254 ± 0.02a | 0.274 ± 0.05a | 0.444 ± 0.05b† | 0.336 ± 0.03ab† |
Values expressed are means ± SE (n = 8–11). Two-way ANOVA was used to determine significance. Groups with different letters are significantly different from each other (P < 0.05). HF, high-fat diet; AICAR, 5-aminoimidazole-4-carboxyamide riboside (AICAR) injection; HF + AICAR, high-fat diet and AICAR injection; NEFA, nonesterified free fatty acids. AICAR and HF were not compared.
There was a main effect of high-fat feeding (P < 0.05).
Mitochondrial enzyme activities in response to chronic AMPK activation and high-fat feeding.
To assess the combined effect of chronic AMPK activation and elevated circulating FFAs in response to high-fat feeding on mitochondrial enzyme activities, the activity of citrate synthase, a marker of the Krebs cycle, and β-HAD, a marker of FA metabolism, were measured. As might be expected, changes in markers of mitochondrial content were proportionally smaller and more difficult to discern in muscles with high oxidative capacity in response to chronic AMPK activation and/or high-fat feeding. In the white quadriceps muscle, predominantly composed of fast-twitch glycolytic fibers, AICAR treatment and high-fat feeding had an additive effect on citrate synthase and β-HAD activity (Tables 2 and 3). Further, an additive effect was also observed for β-HAD activity in red quadriceps (Table 3). While there was a tendency for the treatments to have an additive effect on citrate synthase activity in the red quadriceps, this difference was not consistent enough to reach statistical significance (P = 0.07, observed power = 0.66) (Table 2). Interestingly, in the soleus, a predominantly slow-twitch muscle with high oxidative capacity, each treatment increased citrate synthase activity; however, an additive effect was not observed (Table 2). β-HAD activity was only increased in response to high-fat feeding in the soleus. Thus only in muscles predominantly composed of fast-twitch fibers was a clear combined effect of AICAR and high-fat feeding on citrate synthase and β-HAD activity observed.
Table 2.
Citrate synthase activity
| Control | AICAR | HF | HF + AICAR | |
|---|---|---|---|---|
| White quadriceps | 18.1 ± 0.8a | 28.1 ± 1.7b* | 23.6 ± 0.8b† | 38.0 ± 1.6c*† |
| Red quadriceps | 72.5 ± 2.7a | 78.8 ± 2.7a* | 85.8 ± 1.7b† | 91.9 ± 2.3b*† |
| Soleus | 42.1 ± 1.1a | 47.1 ± 1.6a | 49.0 ± 2.0b† | 50.9 ± 1.7b† |
Values are expressed as μmol·g−1·min−1 ± SE (n = 7–11). Two-way ANOVA was used to determine significance. Groups with different letters are significantly different from each other (P < 0.05). AICAR and HF were not compared.
There was a main effect of the AICAR treatment (P < 0.05).
There was a main effect of high-fat feeding (P < 0.05).
Table 3.
β-HAD activity
| Control | AICAR | HF | HF+AICAR | |
|---|---|---|---|---|
| White quadriceps | 4.4 ± 0.17a | 5.7 ± 0.35b* | 6.5 ± 0.28b† | 8.6 ± 0.38c*† |
| Red quadriceps | 28.8 ± 1.68a | 30.3 ± 0.78a* | 39.0 ± 1.59b† | 43.8 ± 0.86c*† |
| Soleus | 22.6 ± 0.91a | 24.6 ± 1.06a | 29.9 ± 1.36b† | 30.6 ± 1.03b† |
Values are expressed as μmol·g−1·min−1 ± SE (n = 7–10). Two-way ANOVA was used to determine significance. Groups with different letters are significantly different from each other (P < 0.05). AICAR and HF were not compared. β-HAD, β-hydroxyacyl-CoA dehydrogenase.
There was a main effect of the AICAR (P < 0.05).
There was a main effect of high-fat feeding (P < 0.05).
Mitochondrial protein expression in response to chronic AMPK activation and high-fat feeding.
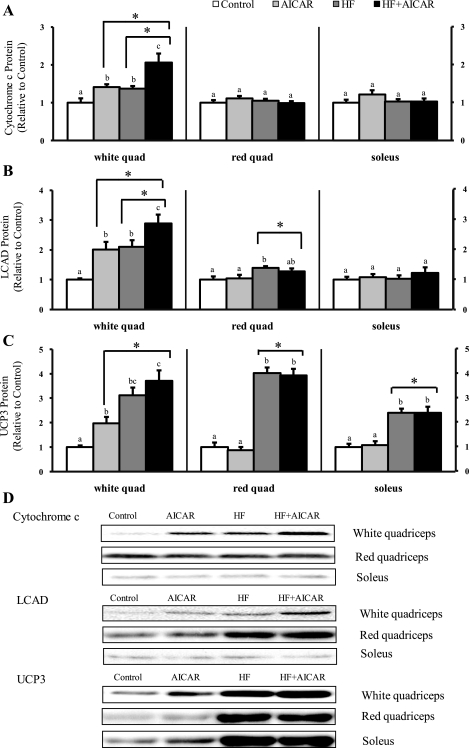
To determine the combined effect of chronic AMPK activation and elevated circulating FFAs on mitochondrial protein expression, we measured the protein abundance of a classic marker of mitochondria content (cytochrome c) and two proteins involved in fatty acid metabolism (LCAD and UCP3). As seen with the changes observed with citrate synthase activity, AICAR injections and a high-fat diet had an additive effect on cytochrome c protein in the white quadriceps (Fig. 3). In the red quadriceps and soleus muscles, cytochrome c protein was not significantly elevated in any of the treatment groups (Fig. 3). There was an additive effect of treatments on LCAD protein abundance in the white quadriceps (Fig. 3). This is consistent with the effect of treatments seen with β-HAD activity. In the red quadriceps LCAD protein levels were only increased by high-fat feeding (Fig. 3). LCAD protein expression in the soleus was not different between any of the groups (Fig. 3). In the white quadriceps, abundance of UCP3 protein was increased with AICAR treatment and high-fat feeding; however, no additive effect was observed (Fig. 3). UCP3 protein levels in the red quadriceps and soleus were only elevated with high-fat feeding (Fig. 3).
Fig. 3.
Long-chain acyl-CoA dehydrogenase (LCAD) and cytochrome c protein levels greater in animals fed a high-fat diet and given AICAR than either individual treatment in the white quadriceps. A: cytochrome c protein levels in skeletal muscle (n = 7–10). B: LCAD protein levels in skeletal muscle (n = 7–11). C: uncoupling protein 3 (UCP3) protein levels in skeletal muscle (n = 6–10). D: representative Western blots. Letters are used to represent significance; same letter means no significant difference (P < 0.05). AICAR and HF were not compared. *Main treatment effect (P < 0.05).
PPARδ protein expression in response to chronic AMPK activation and high-fat feeding.
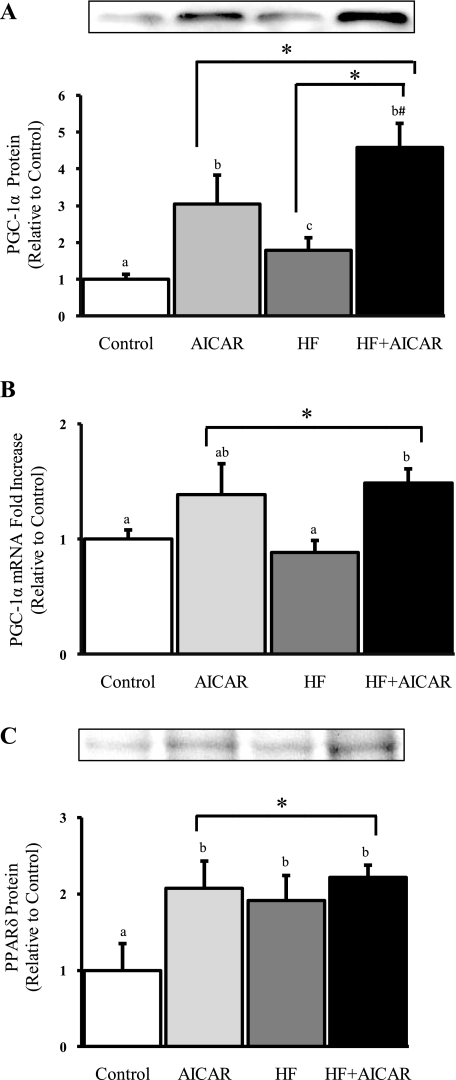
PPARδ is a transcription factor known to be involved in the transcription of a number of mitochondrial proteins especially those involved in fatty acid oxidation. Since it has been previously shown that high-fat feeding elevates PPARδ content in skeletal muscle it was no surprise that PPARδ protein content was elevated in the white quadriceps in HF (Fig. 4). These data suggest that PPARδ activity is up with high-fat feeding. An interesting observation we made was that the protein content was also elevated in response to AICAR (Fig. 4). Further, there was no additive effect of chronically activating AMPK and elevating circulating FFAs on PPARδ content (Fig. 4). These data suggest that PPARδ may be involved in the elevation of some of the mitochondrial markers that we measured.
Fig. 4.
Peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α) protein is greater in HF + AICAR than either individual treatment, and AICAR treatment elevates PGC-1α mRNA in the white quadriceps. A: PGC-1α protein levels in white quadriceps (n = 8–10). B: PGC-1α mRNA fold difference in white quadriceps (n = 6–9). C: PPARδ protein levels in white quadriceps (n = 4–6). Letters are used to represent significance; same letter means no significant difference (P < 0.05). AICAR and HF were not compared. *Main treatment effect (P < 0.05). #Greater than AICAR (P = 0.05).
PGC-1α protein and mRNA expression in response to chronic AMPK activation and high-fat feeding.
PGC-1α is a known coactivator of some of the transcription factors that regulate mitochondria protein expression. Because AMPK and elevated circulating FFAs are known to increase PGC-1α binding activity and/or abundance, we evaluated whether a combined effect would be observed in our model. In the white quadriceps, AICAR treatment and high-fat feeding had an additive effect on PGC-1α protein expression (Fig. 4). In the red quadriceps and soleus, PGC-1α protein abundance was not elevated in AICAR, HF, or HF + AICAR compared with control (data not included). Further, a significant increase in PGC-1α mRNA was only observed in response to AICAR treatment (Fig. 4). Together this confirms distinct mechanisms for AMPK and circulating FFA regulation of PGC-1α protein expression. These data suggest a possible mechanism for how chronic activation of AMPK and elevated circulating FFAs induce additive effects on mitochondrial content.
DISCUSSION
The purpose of this study was to determine the combined effect of chronic AMPK activation and elevated circulating FFAs on mitochondrial content of skeletal muscle. Rats were treated for 6 wk with AICAR injections, a high-fat diet, or both AICAR injections and a high-fat diet. The effect of the combined treatments on mitochondria content was examined in three muscles predominantly composed of unique fiber types [white quadriceps (type IIb), red quadriceps (type IIa), and soleus (type I)] (4). Particularly in muscles with low oxidative capacity we report an additive increase in mitochondrial markers in response to chronic AMPK activation and high-fat feeding. These data show that chronically activating AMPK and elevating circulating FFAs with a high-fat diet has muscle type-specific additive effects on markers of FA metabolism, the citric acid cycle, the electron transport chain, and transcriptional regulation.
Under physiological conditions, such as exercise, in which AMPK activity and circulating FFAs are elevated, both AMPK and FFAs may have varying degrees of effect on mitochondria, depending on the muscle type being examined. As previously reported, a 4-wk AICAR treatment increases mitochondrial markers to a much greater degree in the white quadriceps compared with red quadriceps (62). In agreement with these results, we observed that the response of mitochondrial markers to AICAR was smaller in red quadriceps and soleus compared with white quadriceps. This may be due to a higher average 24-h level of AMPK activity in the red quadriceps and soleus compared with the white quadriceps in the absence of any treatment. Thus greater increases in AMPK activity are likely required to stimulate mitochondrial biogenesis in the red quadriceps and soleus muscles. Elevating circulating FFAs also have muscle type-specific effects on mitochondria content. This could be due to the difference in availability of blood FFAs between skeletal muscle fiber types. Muscles predominantly composed of type IIa and/or type I fibers receive greater blood flow and consequently would receive more FFAs than those predominantly composed of type IIb fibers (23). The higher FFA availability to the red quadriceps and soleus would likely result in lower sensitivity to the effects of elevated circulating FFAs on mitochondria content in these muscles compared with muscles that receive less blood flow and have more limited capacity for fatty acid oxidation such as the white quadriceps muscle. In addition, it may be difficult to discern changes in mitochondrial proteins in muscles that are already rich in mitochondria. The mitochondrial markers we measured are mostly consistent with this predicted pattern with the exception of UCP3 protein abundance.
UCP3 protein expression follows a unique pattern compared with the other measurements made. The role of UCP3 is not well understood, but recent evidence suggests UCP3 limits reactive oxygen species (ROS) production or plays a role in the regulation of fatty acid oxidation (5). UCP3 expression is regulated by the PPAR transcription factors, predominantly PPARδ in skeletal muscle, and PGC-1α, which coactivates PPARδ (37, 49). The 5′-flanking region of the human UCP3 gene contains peroxisome proliferator response elements present, which PPARδ likely binds to regulate UCP3 gene expression (1). Knocking out PPARδ in cardiac muscle results in reduced levels of both UCP3 mRNA and protein content in mouse hearts (14). Further, in primary myotubes PPARδ short hairpin RNA (shRNA) completely blocks the GW501516 (a chemical activator of PPARδ)-induced increase in UCP3 mRNA, strongly supporting the idea that PPARδ directly regulates UCP3 gene expression (37). As would be expected since FFAs directly activate PPARδ, UCP3 mRNA level in skeletal muscle is also increased in response to elevated circulating FFAs (49, 59). Therefore, the rise in UCP3 protein content that we observed when circulating FFAs were elevated strongly suggests that PPARδ activity was increased in the muscles in rats fed the high-fat diet. PGC-1α protein abundance could also help explain the interesting pattern of UCP3 protein expression. Since PGC-1α coactivates PPARδ, the elevation of PGC-1α protein in the white quadriceps in response to either AICAR injections or a high-fat diet could explain why UCP3 protein content is only increased in response to both treatments in the white quadriceps. These data suggest that in muscles with a large proportion of oxidative fibers, circulating FFAs may have a much larger role in elevating UCP3 protein abundance than AMPK activation in conditions that elevate both factors, such as exercise.
We then asked what mechanism(s) may be responsible for the additive increase in mitochondrial content observed with chronic activation of AMPK and elevating circulating FFAs. To do this we measured PGC-1α protein, a transcription factor coactivator that can induce mitochondrial biogenesis. We observed an additive effect of chronic AMPK activation and high-fat feeding on PGC-1α protein abundance in the white quadriceps. We also noted that PGC-1α mRNA was elevated in response to chronic AMPK activation but was not elevated by high-fat feeding consistent with a previous report using the same dietary treatment (25). Together, these data support our hypothesis that the additive increase in PGC-1α protein abundance was a combined effect of high-fat feeding induced posttranscriptional and AMPK-dependent transcriptional increases in PGC-1α protein expression.
Exercise training also increases PGC-1α protein and mRNA expression (6, 24, 31, 45, 53). AMPK-dependent increases in PGC-1α mRNA and protein are thought to be important in the exercise-induced elevation in mitochondrial content. Exercise increases the binding activity of PGC-1α in skeletal muscle by initially activating PGC-1α protein and later increasing PGC-1α protein expression (63). The MEF and CRE binding sites on the PGC-1 gene promoter are essential for contraction-induced PGC-1α gene transcription (2, 3). Recently, it was discovered that AMPK activates members of the CREB family (55), which regulate CRE promoter regions, and this may result in increased binding to the PGC-1α gene promoter CRE sites (55). AMPK is known to increase PGC-1α mRNA levels by regulating the binding of transcription factors to the MEF and CRE sites in the PGC-1α gene promoter (29, 32, 34), which are the same sites regulated by muscle contraction. Further, AMPK increases PGC-1α binding activity via phosphorylation (33, 34) and less direct mechanisms such as increasing the activity of SIRT1 resulting in deacetylation of PGC-1α (12).
Exercise training and high-fat feeding both regulate PPARδ. The protein abundance of PPARδ, a transcription factor coactivated by PGC-1α, is increased in skeletal muscle after 3 wk of exercise (38). Exercise training and high-fat feeding increase expression of proteins regulated by PPARδ, such as PDK4 (44). High-fat feeding also increases PPARδ protein abundance in skeletal muscle (25). We also measured an increase in PPARδ protein content in the white quadriceps of rats fed a high-fat diet. These similarities are not surprising since both exercise and high-fat feeding increase circulating levels of FFAs, which are ligands for PPARδ.
Conditions that raise FFAs, such as a high-fat diet or lipid infusion, cause insulin resistance (13, 25, 52), which has been associated with reduced mitochondrial content in skeletal muscle (9, 36, 43). If mitochondrial content and insulin resistance are causally related, this could be a confounding factor in our study. However, experimental models where insulin resistance would be expected or was measured have not confirmed this relationship (21, 25, 57). As demonstrated previously, feeding rats the high-fat diet used in this study causes insulin resistance (25). While high-fat feeding is known to induce insulin resistance, AMPK activation has been linked to increased insulin sensitivity (17, 19, 46, 51). If insulin resistance caused by high-fat feeding and the expected insulin sensitivity from AICAR treatment were confounding factors in our study, we might expect to see a reduction in skeletal muscle mitochondrial levels in the HF group compared with the control group. Also, the values for the high-fat-fed group that was chronically treated with AICAR would likely be somewhere between the values of the HF and AICAR groups. In contrast, we observed an additive effect of AICAR and high-fat feeding on mitochondrial marker expression in a number of instances. Further, we did not observe HF to be less than control in any of the measurements. Therefore, any negative effect that insulin resistance may be having on mitochondria content does not appear to be confounding our results.
It is well known that circulating FFAs are elevated during and/or after prolonged exercise bouts (42). Our findings suggest that this elevation in circulating FFAs may contribute to exercise training-induced mitochondrial biogenesis. Furthermore, it is reasonable to consider that increases in skeletal muscle mitochondria capacity could be enhanced if training were performed under conditions that further elevated the levels of circulating FFAs. This idea is not new. A number of studies have examined the effect of a high-fat diet combined with training on endurance capacity. Feeding rats a high-fat diet has been reported to enhance endurance exercise capacity and increase mitochondrial markers in skeletal muscle (40, 48). In contrast, some human studies have failed to demonstrate a beneficial effect on endurance capacity that might be expected when training is combined with a high-fat diet for either 4 or 8 wk (27, 28). Further, a 7-wk high-fat diet combined with exercise training induces a comparable increase in citrate synthase activity compared with those trained but fed a carbohydrate-rich diet (26). The difference in results may be due to the fat composition of the rat control diet being about half that of the human control diets. It should be noted that the control diet used in our study and the rat training studies just mentioned consisted of 10% of the calories from fat, while the typical American diet consists of 33–34% fat (58). It is possible that since the fat content of American diets is already high, further elevating dietary fat would be less likely to enhance exercise training-induced mitochondrial biogenesis than if the fat composition of the regular diet was closer to the control diet in our study. Regardless, consuming a high-fat diet is not suggested since it has numerous deleterious health effects, including impaired cardiovascular system function, insulin resistance, and inflammation (for a review, see Refs. 10, 11, 15).
In conclusion, chronically activating AMPK and elevating circulating FFAs for 6 wk has a muscle type-specific additive effect on markers of fatty acid metabolism, the citric acid cycle, the electron transport chain, and transcriptional regulation. The additive effect on mitochondrial content was most prominent in the white quadriceps, predominantly composed of type IIb fibers. These data support our hypothesis that chronically activating AMPK activity in skeletal muscle and increasing circulating FFAs has an additive effect on mitochondria levels in skeletal muscle. They also suggest that both the exercise-induced increase in AMPK activity in skeletal muscle and elevation in circulating FFAs could be simultaneously contributing to exercise training-induced mitochondrial biogenesis in skeletal muscle. Future work needs to be done to determine whether this is in fact occurring.
GRANTS
This research was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-051928 (W. W. Winder).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Acin A, Rodriguez M, Rique H, Canet E, Boutin JA, Galizzi JP. Cloning and characterization of the 5′ flanking region of the human uncoupling protein 3 (UCP3) gene. Biochem Biophys Res Commun 258: 278–283, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Akimoto T, Li P, Yan Z. Functional interaction of regulatory factors with the Pgc-1 alpha promoter in response to exercise by in vivo imaging. Am J Physiol Cell Physiol 295: C288–C292, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akimoto T, Sorg BS, Yan Z. Real-time imaging of peroxisome proliferator-activated receptor-gamma coactivator-1alpha promoter activity in skeletal muscles of living mice. Am J Physiol Cell Physiol 287: C790–C796, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat 171: 259–272, 1984 [DOI] [PubMed] [Google Scholar]
- 5. Azzu V, Brand MD. The on-off switches of the mitochondrial uncoupling proteins. Trends Biochem Sci 35: 298–307, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16: 1879–1886, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Bajaj M, Medina-Navarro R, Suraamornkul S, Meyer C, DeFronzo RA, Mandarino LJ. Paradoxical changes in muscle gene expression in insulin-resistant subjects after sustained reduction in plasma free fatty acid concentration. Diabetes 56: 743–752, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Birk JB, Wojtaszewski JF. Predominant alpha2/beta2/gamma3 AMPK activation during exercise in human skeletal muscle. J Physiol 577: 1021–1032, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50: 790–796, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brindley DN, Kok BP, Kienesberger PC, Lehner R, Dyck JR. Shedding light on the enigma of myocardial lipotoxicity: The involvement of known and putative regulators of fatty acid storage and mobilization. Am J Physiol Endocrinol Metab 298: E897–E908, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Bullo M, Casas-Agustench P, Amigo-Correig P, Aranceta J, Salas-Salvado J. Inflammation, obesity and comorbidities: the role of diet. Public Health Nutr 10: 1164–1172, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD(+) metabolism and SIRT1 activity. Nature 458: 1056–1060, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chavez AO, Kamath S, Jani R, Sharma LK, Monroy A, Abdul-Ghani MA, Centonze VE, Sathyanarayana P, Coletta DK, Jenkinson CP, Bai Y, Folli F, Defronzo RA, Tripathy D. Effect of short-term free Fatty acids elevation on mitochondrial function in skeletal muscle of healthy individuals. J Clin Endocrinol Metab 95: 422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, Chen YE, Yang Q. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med 10: 1245–1250, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 111: 1448–1454, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Durante PE, Mustard KJ, Park SH, Winder WW, Hardie DG. Effects of endurance training on activity and expression of AMP-activated protein kinase isoforms in rat muscles. Am J Physiol Endocrinol Metab 283: E178–E186, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 282: E18–E23, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA 94: 4312–4317, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fujii N, Ho RC, Manabe Y, Jessen N, Toyoda T, Holland WL, Summers SA, Hirshman MF, Goodyear LJ. Ablation of AMP-activated protein kinase alpha2 activity exacerbates insulin resistance induced by high-fat feeding of mice. Diabetes 57: 2958–2966, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fyffe SA, Alphey MS, Buetow L, Smith TK, Ferguson MA, Sorensen MD, Bjorkling F, Hunter WN. Reevaluation of the PPAR-beta/delta ligand binding domain model reveals why it exhibits the activated form. Mol Cell 21: 1–2, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Garcia-Roves P, Huss JM, Han DH, Hancock CR, Iglesias-Gutierrez E, Chen M, Holloszy JO. Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc Natl Acad Sci USA 104: 10709–10713, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcia-Roves PM, Osler ME, Holmstrom MH, Zierath JR. Gain-of-function R225Q mutation in AMP-activated protein kinase gamma3 subunit increases mitochondrial biogenesis in glycolytic skeletal muscle. J Biol Chem 283: 35724–35734, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Gorski J, Hood DA, Terjung RL. Blood flow distribution in tissues of perfused rat hindlimb preparations. Am J Physiol Endocrinol Metab 250: E441–E448, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Goto M, Terada S, Kato M, Katoh M, Yokozeki T, Tabata I, Shimokawa T. cDNA Cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun 274: 350–354, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 105: 7815–7820, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Helge JW, Kiens B. Muscle enzyme activity in humans: role of substrate availability and training. Am J Physiol Regul Integr Comp Physiol 272: R1620–R1624, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Helge JW, Richter EA, Kiens B. Interaction of training and diet on metabolism and endurance during exercise in man. J Physiol 492: 293–306, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Helge JW, Wulff B, Kiens B. Impact of a fat-rich diet on endurance in man: role of the dietary period. Med Sci Sports Exerc 30: 456–461, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Holmes BF, Sparling DP, Olson AL, Winder WW, Dohm GL. Regulation of muscle GLUT4 enhancer factor and myocyte enhancer factor 2 by AMP-activated protein kinase. Am J Physiol Endocrinol Metab 289: E1071–E1076, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab 34: 465–472, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Irrcher I, Adhihetty PJ, Sheehan T, Joseph AM, Hood DA. PPARgamma coactivator-1alpha expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am J Physiol Cell Physiol 284: C1669–C1677, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Irrcher I, Ljubicic V, Kirwan AF, Hood DA. AMP-activated protein kinase-regulated activation of the PGC-1alpha promoter in skeletal muscle cells. PLoS One 3: e3614, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 104: 12017–12022, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jensen TE, Wojtaszewski JF, Richter EA. AMP-activated protein kinase in contraction regulation of skeletal muscle metabolism: necessary and/or sufficient? Acta Physiol (Oxf) 196: 155–174, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Jorgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, Richter EA. Role of AMPKalpha2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab 292: E331–E339, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Kleiner S, Nguyen-Tran V, Bare O, Huang X, Spiegelman B, Wu Z. PPARδ agonism activates fatty acid oxidation via PGC-1α but does not increase mitochondrial gene expression and function. J Biol Chem 284: 18624–18633, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luquet S, Lopez-Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M, Grimaldi PA. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J 17: 2299–2301, 2003 [DOI] [PubMed] [Google Scholar]
- 39. McAinch AJ, Lee JS, Bruce CR, Tunstall RJ, Hawley JA, Cameron-Smith D. Dietary regulation of fat oxidative gene expression in different skeletal muscle fiber types. Obes Res 11: 1471–1479, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Miller WC, Bryce GR, Conlee RK. Adaptations to a high-fat diet that increase exercise endurance in male rats. J Appl Physiol 56: 78–83, 1984 [DOI] [PubMed] [Google Scholar]
- 41. Nemeth PM, Rosser BW, Choksi RM, Norris BJ, Baker KM. Metabolic response to a high-fat diet in neonatal and adult rat muscle. Am J Physiol Cell Physiol 262: C282–C286, 1992 [DOI] [PubMed] [Google Scholar]
- 42. Nikolaidis MG, Mougios V. Effects of exercise on the fatty-acid composition of blood and tissue lipids. Sports Med 34: 1051–1076, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100: 8466–8471, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pilegaard H, Neufer PD. Transcriptional regulation of pyruvate dehydrogenase kinase 4 in skeletal muscle during and after exercise. Proc Nutr Soc 63: 221–226, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 546: 851–858, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, Flyvbjerg A, Fujii N, Goodyear LJ, Gotfredsen CF, Brand CL, Lund S. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes 54: 928–934, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Putman CT, Martins KJ, Gallo ME, Lopaschuk GD, Pearcey JA, MacLean IM, Saranchuk RJ, Pette D. Alpha-catalytic subunits of 5′AMP-activated protein kinase display fiber-specific expression and are upregulated by chronic low-frequency stimulation in rat muscle. Am J Physiol Regul Integr Comp Physiol 293: R1325–R1334, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Simi B, Sempore B, Mayet MH, Favier RJ. Additive effects of training and high-fat diet on energy metabolism during exercise. J Appl Physiol 71: 197–203, 1991 [DOI] [PubMed] [Google Scholar]
- 49. Son C, Hosoda K, Matsuda J, Fujikura J, Yonemitsu S, Iwakura H, Masuzaki H, Ogawa Y, Hayashi T, Itoh H, Nishimura H, Inoue G, Yoshimasa Y, Yamori Y, Nakao K. Up-regulation of uncoupling protein 3 gene expression by fatty acids and agonists for PPARs in L6 myotubes. Endocrinology 142: 4189–4194, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Srere PA. Citrate synthase. Methods Enzymol 13: 3–6, 1969 [Google Scholar]
- 51. Steinberg GR, Jorgensen SB. The AMP-activated protein kinase: role in regulation of skeletal muscle metabolism and insulin sensitivity. Mini Rev Med Chem 7: 519–526, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Storlien LH, James DE, Burleigh KM, Chisholm DJ, Kraegen EW. Fat feeding causes widespread in vivo insulin resistance, decreased energy expenditure, and obesity in rats. Am J Physiol Endocrinol Metab 251: E576–E583, 1986 [DOI] [PubMed] [Google Scholar]
- 53. Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun 296: 350–354, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Thomson DM, Hancock CR, Evanson BG, Kenney SG, Malan BB, Mongillo AD, Brown JD, Hepworth S, Fillmore N, Parcell AC, Kooyman DL, Winder WW. Skeletal muscle dysfunction in muscle-specific LKB1 knockout mice. J Appl Physiol 108: 1775–1785 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thomson DM, Herway ST, Fillmore N, Kim H, Brown JD, Barrow JR, Winder WW. AMP-activated protein kinase phosphorylates transcription factors of the CREB family. J Appl Physiol 104: 429–438, 2008 [DOI] [PubMed] [Google Scholar]
- 56. Thomson DM, Porter BB, Tall JH, Kim HJ, Barrow JR, Winder WW. Skeletal muscle and heart LKB1 deficiency causes decreased voluntary running and reduced muscle mitochondrial marker enzyme expression in mice. Am J Physiol Endocrinol Metab 292: E196–E202, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 56: 2085–2092, 2007 [DOI] [PubMed] [Google Scholar]
- 58. US Department of Agriculture/Agricultural Research Service. Nutrient Intakes from Food: Mean Amounts and Percentages of Calories from Protein, Carbohydrate, Fat, and Alcohol, One Day, 2005–2006. Washington, DC: USDA, 2008. http://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/0506/Table_.2_NIF_05.pdf) [Google Scholar]
- 59. Weigle DS, Selfridge LE, Schwartz MW, Seeley RJ, Cummings DE, Havel PJ, Kuijper JL, Beltran del Rio H. Elevated free fatty acids induce uncoupling protein 3 expression in muscle: a potential explanation for the effect of fasting. Diabetes 47: 298–302, 1998 [DOI] [PubMed] [Google Scholar]
- 60. Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol Endocrinol Metab 270: E299–E304, 1996 [DOI] [PubMed] [Google Scholar]
- 61. Winder WW, Hardie DG, Mustard KJ, Greenwood LJ, Paxton BE, Park SH, Rubink DS, Taylor EB. Long-term regulation of AMP-activated protein kinase and acetyl-CoA carboxylase in skeletal muscle. Biochem Soc Trans 31: 182–185, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol 88: 2219–2226, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem 282: 194–199, 2007 [DOI] [PubMed] [Google Scholar]