Abstract
Muscle metaboreflex activation during dynamic exercise induces a substantial increase in cardiac work and oxygen demand via a significant increase in heart rate, ventricular contractility, and afterload. This increase in cardiac work should cause coronary metabolic vasodilation. However, little if any coronary vasodilation is observed due to concomitant sympathetically induced coronary vasoconstriction. The purpose of the present study is to determine whether the restraint of coronary vasodilation functionally limits increases in left ventricular contractility. Using chronically instrumented, conscious dogs (n = 9), we measured mean arterial pressure, cardiac output, and circumflex blood flow and calculated coronary vascular conductance, maximal derivative of ventricular pressure (dp/dtmax), and preload recruitable stroke work (PRSW) at rest and during mild exercise (2 mph) before and during activation of the muscle metaboreflex. Experiments were repeated after systemic α1-adrenergic blockade (∼50 μg/kg prazosin). During prazosin administration, we observed significantly greater increases in coronary vascular conductance (0.64 ± 0.06 vs. 0.46 ± 0.03 ml·min−1·mmHg−1; P < 0.05), circumflex blood flow (77.9 ± 6.6 vs. 63.0 ± 4.5 ml/min; P < 0.05), cardiac output (7.38 ± 0.52 vs. 6.02 ± 0.42 l/min; P < 0.05), dP/dtmax (5,449 ± 339 vs. 3,888 ± 243 mmHg/s; P < 0.05), and PRSW (160.1 ± 10.3 vs. 183.8 ± 9.2 erg·103/ml; P < 0.05) with metaboreflex activation vs. those seen in control experiments. We conclude that the sympathetic restraint of coronary vasodilation functionally limits further reflex increases in left ventricular contractility.
Keywords: ventricular function, pressure-volume relationship, coronary blood flow
during exercise, when oxygen demand by the active skeletal muscle is greater than oxygen supply, metabolites accumulate stimulating chemosensitive afferents (33, 44, 50–53), eliciting a pressor response termed the muscle metaboreflex (1, 4, 50). In contrast to other cardiovascular reflexes that raise arterial pressure primarily via peripheral vasoconstriction (e.g., the arterial and cardiopulmonary baroreflexes) (8, 21, 43), during submaximal exercise involving a large muscle mass the muscle metaboreflex-induced pressor response occurs virtually solely via increases in cardiac output (6, 17, 56). Raising the total flow available for perfusion is the only effective strategy to substantively increase skeletal muscle blood flow during exercise because the vast majority of cardiac output is already directed to this vascular bed (46). Vasoconstriction of inactive vascular beds has little potential to improve skeletal muscle blood flow in this setting (40). Thus this reflex has been described as a flow-sensitive, flow-raising reflex (6, 45, 50). Muscle metaboreflex activation increases cardiac output by raising heart rate (HR) and ventricular contractility (9, 48). Left ventricular preload is sustained via substantial central blood volume mobilization (49), thereby allowing the chronotropic and inotropic responses to maintain steady-state increases in cardiac output. This substantial increase in cardiac work (large increases in cardiac output pumped against a much higher arterial pressure) would be expected to elicit marked metabolic coronary vasodilation (10, 24). Furthermore, the large increase in sympathetic activity could elicit significant β-mediated feedforward vasodilation (13, 14). However, the reflex rise in sympathetic activity to the heart may also activate vascular α1-adrenergic receptors (16). Previous studies from our laboratory showed that, despite the marked increase in cardiac work, no coronary vasodilation occurred when the reflex was activated during submaximal dynamic exercise (3). The potent vasoconstrictor impetus of this reflex was revealed when the marked increase in cardiac work did not or could not occur. In these settings, actual coronary vasoconstriction was observed with metaboreflex activation [as seen in normal animals during severe exercise when cardiac output is already maximal (3, 6) and during mild exercise after β-adrenergic blockade with acute ventricular pacing, which causes acute ventricular dysfunction (3), and after induction of chronic heart failure (2)]. In contrast, when the metaboreflex was activated after blockade of coronary vascular α1-adrenergic receptors, substantial coronary vasodilation occurred with the large increases in cardiac work (42). Together, these studies support the concept that increases in cardiac sympathetic nerve activity simultaneously engender both coronary vasodilation (due to the substantial increase in cardiac work and possible β-mediated feedforward vasodilation) as well as neurogenic vasoconstriction (via activation of coronary α1-adrenergic receptors), with the resulting level of coronary vasomotor tone dependent on the level of activation of each mechanism.
To what extent this functional metaboreflex-induced coronary vasoconstriction limits the ability to improve ventricular function and therefore ultimately limits the ability to increase cardiac output and improve oxygen delivery to the active muscle is unknown. Gwirtz et al. (16) have shown that α1-adrenergic blockade accentuates the increase in coronary blood flow and left ventricular performance (dP/dt and myocardial segment dL/dt) observed during moderate exercise. These data indicate that, even during moderate dynamic exercise, the vasoconstrictor effects of increases in cardiac sympathetic nerve activity limit increases in myocardial performance. To what extent this change in segment performance translates into increases in global cardiac function is unclear. Previous to this, Heyndrickx et al. (19) showed no increase in left ventricular dP/dt during exercise after systemic infusion of prazosin. Notably, Gwirtz et al. (16) used intracoronary infusion of prazosin resulting in unaltered loading conditions, which may explain the different findings in dP/dt. O'Leary et al. (42) have shown that metaboreflex activation after systemic α1-adrenergic blockade resulted in larger increases in cardiac output. Whether the higher cardiac output was due to an increased cardiac contractility brought about by the greater coronary vasodilation vs. the lower left ventricular afterload caused by systemic vasodilation caused by the α1-adrenergic blockade is unknown.
In the present study, we tested whether this restraint of coronary vasodilation by the metaboreflex-induced increase in cardiac sympathetic nerve activity functionally limits the ability to increase left ventricular contractility. We assessed left ventricular contractility via analysis of changes in the pressure-volume relationship. We hypothesized that blockade of α1-adrenergic receptors would now allow coronary vasodilation during metaboreflex activation and that the increase in coronary blood flow would further the reflex increase in left ventricular contractility.
METHODS
All of the methods and procedures were reviewed and approved by the Wayne State University Institutional Animal Care and Use Committee. The experiments were conducted on mongrel dogs (n = 9), weighing 22.7 ± 2.02 kg. The dogs were selected for their willingness to exercise on a motor-driven treadmill. Although no selection was made for gender, by random availability of laboratory dogs, all animals were female. Our group (29) has previously shown that gender has little or no effect on metaboreflex responses in dogs.
The medications and surgical preparations used have been described in detail previously (2, 3, 48). Briefly, a 20-mm flow transducer was placed around the aortic root to assess cardiac output. Hydraulic vascular occluders were placed on the superior and inferior vena cavae to manipulate preload. Two pairs of sonomicrometry crystals were implanted in the endocardium of the left ventricle, to measure the long axis and the short axis, which were used to estimate ventricular volume. A catheter was placed in the left ventricle, and its telemeter-pressure transducer was implanted subcutaneously for left ventricular pressure. A 3-mm flow transducer was placed on the circumflex artery to assess coronary flow. Arterial and central venous catheters were placed to measure systemic blood pressures. In a retroperitoneal abdominal approach, a vascular occluder was placed about the terminal aorta. Just proximal to this occluder, a 10-mm flow transducer was placed around the aorta to measure hindlimb blood flow (HLBF). The animals were allowed at least 7 days for recovery before the experiments were conducted.
Experimental protocol.
Each dog was directed to stand on the treadmill for 10–15 min while all equipment was connected and adequacy of the signals verified. All data were recorded on digital recording systems.
We obtained 1 min of steady-state resting data with the dog standing on the treadmill. Steady-state data and data during transient vena caval occlusions (for variably loaded pressure-volume loops) were recorded during the conditions of rest, mild exercise (3.2 km/h), and mild exercise with muscle metaboreflex activation. The reflex was activated by partially inflating the vascular occluder on the terminal aorta to reduce HLBF to ∼50% of the normal value during mild exercise. The experiments were performed with and without α1-adrenergic blockade (20–50 μg/kg iv prazosin 30 min before exercise). In each experiment, the dose of prazosin was sufficient to abolish any pressor response to 4 μg/kg of phenylephrine for the duration of the experiment.
Data analysis.
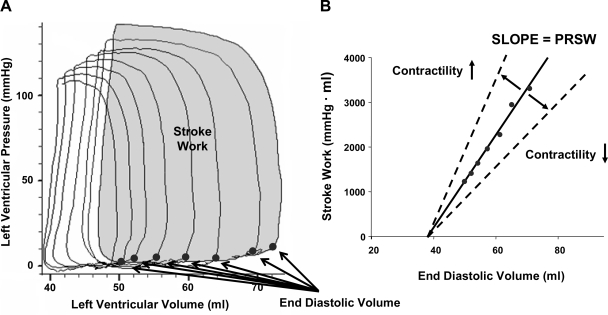
We calculated left ventricular volume using a modified ellipsoid equation: LVV = (π/6) × SA2 × LA, where LVV is the left ventricle volume, short axis (SA) represents the distance between the anterior and posterior crystals, and long axis (LA) represents the distance between the crystals placed on the base and apex of the left ventricle (30). The pressure-volume loops were plotted for each condition. PRSW and ±dP/dt were calculated. PRSW is the slope of the relationship between stroke work and the left ventricular end-diastolic volume (illustrated in Fig. 1). An increased slope reflects an increased contractility, as a decreased slope reflects a decrease in contractility (12, 22, 31). Cardiac power was calculated as the product of stroke work and HR. The integral of the cardiac output wave was calculated to give stroke volume (SV). Coronary vascular conductance (CVC) was calculated as CBF/(MAP − CVP), where CBF is coronary blood flow, MAP is mean arterial pressure, and CVP is central venous pressure. Systemic vascular conductance to all nonischemic areas (e.g., all areas except the hindlimbs) is termed nonischemic vascular conductance (NIVC) and was calculated as (cardiac output − HLBF)/(MAP − CVP). A repeated-measures factorial ANOVA was used for the main effects analyses, and a pair-wise comparison was used for post hoc analyses using the test for simple effects. Statistical significance was defined as P < 0.05. Regression analyses were conducted with CVC with respect to cardiac power for each animal, and the slopes were compared between control and α1-blockade by repeated-measures one-way ANOVA.
Fig. 1.
A: example of pressure-volume loop during preload reductions, illustrating stroke work of a single loop (shaded) and the end-diastolic volume point (●) for each loop. B: example of how the end-diastolic points and corresponding stroke work for each loop is used to illustrate preload recruitable stroke work (PRSW) and how it can be used to assess contractility.
RESULTS
Table 1 shows the levels of HLBF at rest, during exercise, and during metaboreflex activation before and after α1-adrenergic blockade. Prazosin caused a small but significant increase in HLBF over control values during exercise. HLBF was reduced to the same values in both conditions for activation of the muscle metaboreflex.
Table 1.
HLBF at rest, during exercise, and during metaboreflex activation before and after α1-adrenergic blockade
| HLBF, l/min | |||
|---|---|---|---|
| Rest | Exercise | Exercise + MMA | |
| Control | 0.58 ± 0.05 | 1.00 ± 0.09† | 0.52 ± 0.04 |
| α1-Blockade | 0.61 ± 0.06 | 1.07 ± 0.09*† | 0.55 ± 0.04 |
Values are means ± SE. HLBF, hindlimb blood flow; MMA, muscle metaboreflex activation.
Significant effect of α1-blockade (P < 0.05);
significant effect of exercise (P < 0.05).
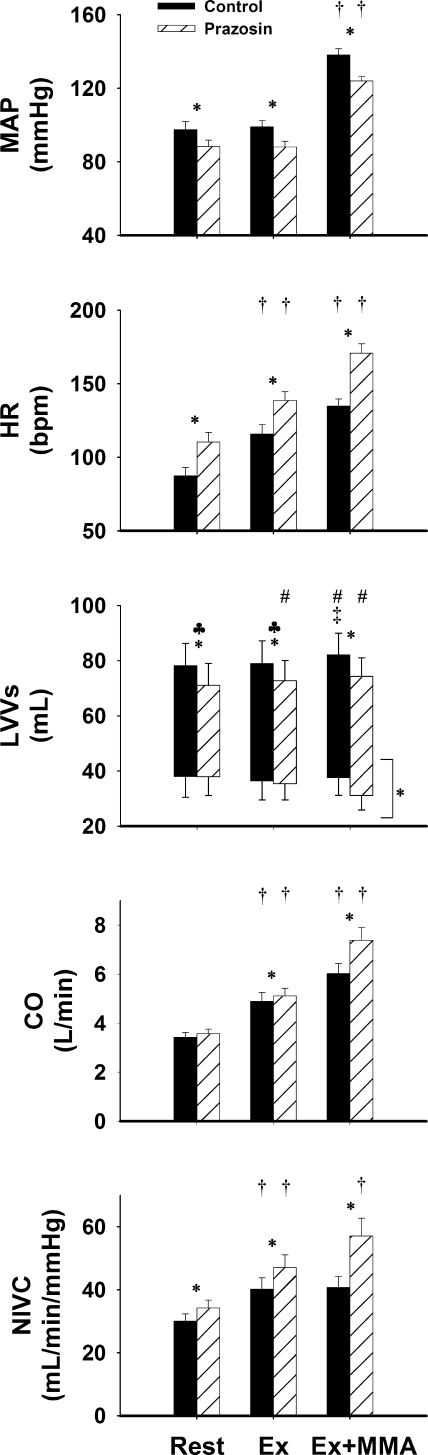
Figure 2 shows the mean steady-state values of MAP, HR, left ventricular end-diastolic and end-systolic volumes, cardiac output, and NIVC at rest, mild exercise, and during exercise with metaboreflex activation in control and after α1-adrenergic blockade. In control animals, there was no change in MAP or SV from rest to mild exercise; however, HR, cardiac output, and NIVC were increased. Imposed reductions in HLBF caused muscle metaboreflex-induced increases in MAP, HR, SV, and cardiac output. No significant change in NIVC occurred with metaboreflex activation. At rest, α1-adrenergic blockade caused a significant decrease in MAP, marked tachycardia, and reduced SV, due to a reduced end-diastolic volume. Responses to mild exercise were similar to those in controls, with the exception that now SV was slightly increased. Metaboreflex activation caused a significant though lesser increase in MAP and a significant increase in SV. End-diastolic volume was still reduced compared with results shown in controls; however, end-systolic volume was also reduced, resulting in a comparable SV between control and α1-adrenergic blockade. A greater reflex increase in HR and cardiac output than that shown in control and a significant increase in NIVC occurred. Left ventricular end-systolic volume was significantly different across workloads but had no significant difference between conditions (control vs. α1-adrenergic blockade) and no significant interaction; therefore, a pair-wise comparison could not be calculated.
Fig. 2.
Hemodynamic responses. Shown are mean arterial pressure (MAP), heart rate (HR) in beats/min (bpm), left ventricular volumes (LVVs), cardiac output (CO), and nonischemic vascular conductance (NIVC) responses during rest, mild exercise (Ex), and mild exercise with muscle metaboreflex activation (Ex+MMA) settings in control (solid bars) and α1-adrenergic blockade conditions (hatched bars). All parameters showed significance across workload settings, as well as significance between control and prazosin conditions (P < 0.05), with the exception of stroke volume and left-ventricular end-systolic volume (which were only significant across workload settings). All parameters had a significant interaction between the 2 independent variables, with the exception of left ventricular end-systolic volume. *Between 2 bars, signifies a significant pair-wise comparison (P < 0.05). †Significant increase from the previous setting. ♣Above a specific setting, signifies a significant pair-wise comparison in left ventricle stroke volume (P < 0.05). ‡Significant increase in left ventricular end-diastolic volume. #Significant increase in stoke volume from the previous workload (P < 0.05). *Next to bracket, indicates a significance between left ventricular end-systolic volume across workloads but not between control and α1-adrenergic blockade conditions.
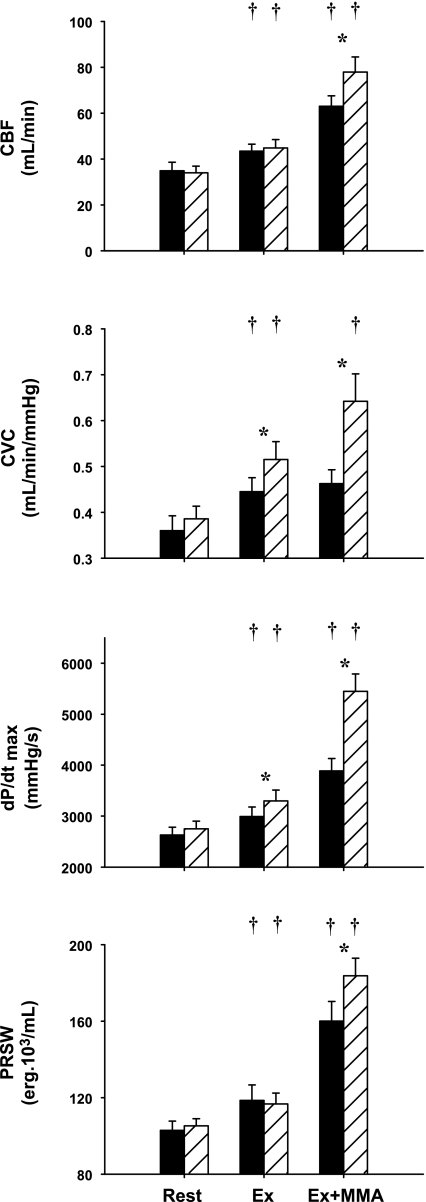
Figure 3 shows left ventricular hemodynamic and inotropic responses to mild exercise and metaboreflex activation before and after α1-adrenergic blockade. In control, there was a significant increase from rest to mild exercise in coronary blood flow, CVC, dP/dtmax, and PRSW. Metaboreflex activation increased coronary blood flow and left ventricular contractility; however, no vasodilation occurred in the coronary circulation as there was no significant increase in CVC. Thus all of the increase in coronary blood flow was due to the increase in perfusion pressure. Under α1-adrenergic blockade, there was also a significant increase in all-illustrated parameters from rest to mild exercise, which were statistically greater in CVC and dP/dtmax, vs. that shown with control conditions. After α1-adrenergic blockade, activation of the muscle metaboreflex now elicited significantly greater increases in coronary blood flow. Although the rise in perfusion pressure was smaller, substantial coronary vasodilation occurred. Metaboreflex activation in this setting caused significantly greater increases in both indexes of myocardial contractility.
Fig. 3.
Left ventricular hemodynamic and function responses. Shown are coronary blood flow (CBF), coronary vascular conductance (CVC), maximal rate of left ventricular pressure change (dP/dtmax), and PRSW during rest, EX, and Ex+MMA settings, in control (solid bars) and α1-adrenergic blockade conditions (hatched bars). All parameters showed significance across workload settings, as well as significance between control and prazosin conditions (P < 0.05). All parameters had a significant interaction between the 2 independent variables. *Above a specific setting, signifies a significant pair-wise comparison (P < 0.05). †Significant increase from the previous setting (P < 0.05).
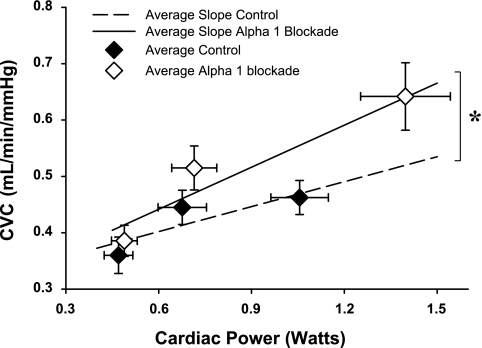
After α1-adrenergic blockade, the slope of the relationship between CVC and cardiac power (used as an index of myocardial oxygen consumption) was significantly increased. Furthermore, this relationship was extended over a significantly greater range, as both CVC and cardiac power were significantly greater during muscle metaboreflex stimulation after α1-adrenergic blockade (Fig. 4).
Fig. 4.
CVC plotted as a function of cardiac power. The broken regression line represents the average relationship between CVC and cardiac power in control, whereas the solid regression line represents the corresponding average relationship during α1-adrenergic blockade. ♦, Averaged values in control condition. ◊, Averaged values during α1-adrenergic blockade. Bracket and asterisk signify the significant difference between the 2 slopes (P < 0.05).
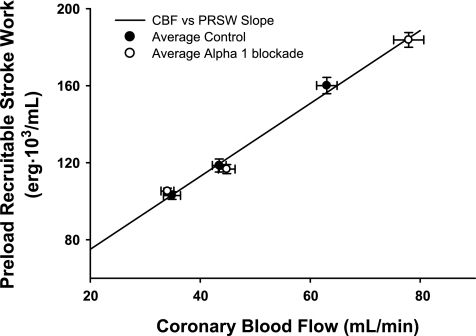
Figure 5 shows the relationship between PRSW and coronary blood flow. There was no difference between the slope of the relationship between control and after α1-adrenergic blockade; therefore, the data were combined into one regression. After α1-adrenergic blockade, greater increases in coronary blood flow occurred with metaboreflex activation, which also elicited substantially greater increases in ventricular contractility.
Fig. 5.
Contractility indicated by PRSW with respect to CBF. As no significant difference between control and α1-adrenergic blockade was found (P > 0.05), a single relationship is represented by a single line. ●, Averaged values in controls. ○, Averaged values during α1-adrenergic blockade.
DISCUSSION
This is the first study to show that, during dynamic exercise, the sympathetically induced restraint of coronary vasodilation during muscle metaboreflex activation impairs increases in left ventricular contractility. During metaboreflex activation, a “push-pull” situation likely exists as a result of the increase in sympathetic activity to the heart. The increase in metabolic vasodilation, coupled with possible vascular β-mediated feedforward vasodilation, is opposed by direct α-mediated vasoconstriction. The direct vasoconstrictor drive limits vasodilation, and the restrained increase in blood flow limits increases in ventricular performance. Suppressing the increase in ventricular contractility likely limits the ability to raise cardiac output and thereby functionally limits the ability of the muscle metaboreflex to improve blood flow to the active skeletal muscles.
Coronary perfusion/dilation and ventricular performance: cause and effect.
The complex relationship between coronary perfusion and ventricular performance can make it difficult to discern the difference between cause and effect. Changes in flow can elicit changes in function, and changes in function can elicit metabolic coronary vasodilation. Because flow will vary with changes in both vessel caliber and perfusion pressure, vasodilation can only be assessed via changes in conductance [or resistance, but we prefer conductance (40)]. Ventricular function is likely limited by blood flow [or oxygen delivery (39)] rather than vasodilation per se [e.g., flow can change solely due to changes in perfusion pressure (2, 3)]. We addressed this in two distinct ways. Figure 4 shows that the relationship between cardiac power and CVC was shifted upward, with a significantly steeper slope after α1-adrenergic blockade. This shows that, with metaboreflex activation, greater vasodilation occurs after α1-adrenergic blockade at any level of metabolic stimuli for vasodilation (as indexed by cardiac power). We based this analysis on that done by Huang and Feigl (20), who showed that the relationship between coronary blood flow and myocardial oxygen consumption is linear but that the slope of the relationship during exercise increases after regional α1-receptor blockade. In that study (20), perfusion pressure was not different with coronary α1-receptor blockade; therefore, changes in blood flow will be proportionally equivalent to changes in conductance and flow is a valid index of vasodilation/vasoconstriction. In our study, perfusion pressure was different both before and after α1-receptor blockade and markedly so between exercise and metaboreflex activation; therefore, differences in vasomotor tone must be addressed via changes in conductance (40). For example, in the control experiments, large increases in coronary blood flow occurred with metaboreflex activation, yet this was not due to vasodilation inasmuch as conductance remained unchanged. All of the increase in flow was due to an increase in perfusion pressure.
Whether due to increased perfusion pressure or vasodilation, increases in blood flow may allow increases in ventricular function by providing more oxygen delivery (39). Oxygen extraction in the coronary circulation is already near maximal under basal conditions; therefore, increases in myocardial oxygen consumption with exercise occur predominately via increases in coronary blood flow (24). In addition, mild exercise and metaboreflex activation in this model elicit minimal increases in arterial oxygen content (∼5%) (41); therefore, increases in oxygen delivery occur via increases in blood flow. We found that the relationship between ventricular contractility (PRSW) and blood flow was exceedingly linear. α1-Adrenergic blockade only extended the range of this relationship and did not affect the slope. With metaboreflex activation in the control experiments, all of the increase in coronary blood flow and therefore oxygen delivery occurred via the increase in perfusion pressure; no vasodilation occurred (no significant increase in conductance), as previously observed by our group (3, 42). In contrast, after prazosin, much larger increases in coronary blood flow occurred because of the combined effect of substantial vasodilation coupled with increased perfusion pressure; in addition, increases in PRSW were greater. Collectively, we interpret these data as indicating that, during metaboreflex activation, the increases in sympathetic activity prevent coronary vasodilation and therefore restrains increases in coronary blood flow to only that which occurs via increases in perfusion pressure (3, 42). α1-Adrenergic blockade revealed substantial coronary vasodilation during metaboreflex activation, which now coupled with the rise in perfusion pressure provided for much greater increases in coronary blood flow. The increased blood flow and oxygen delivery thereby elicited a greater increase in ventricular contractility. Gwirtz and colleagues (15, 16, 27) showed that blockade of coronary α1-adrenergic receptors increased coronary blood flow during moderate exercise in dogs. This was also accompanied by higher myocardial oxygen consumption and regional ventricular dynamics (increased maximal velocity of segment shortening). Thus the rise in sympathetic activity that normally occurs with moderate exercise likely functionally restrains coronary vasodilation and ventricular function. One possible beneficial effect of this vasoconstriction may be to preserve endocardial blood flow (20), inasmuch as the epicardium is vasoconstricted to a greater extent than the endocardium, which would act to redistribute coronary blood flow toward the inner layers of the ventricle. This greater vasodilation with α1-adrenergic blockade could be revealing both metabolic vasodilation and β-mediated feedforward vasodilation (14).
Muscle metaboreflex activation either during exercise or during postexercise circulatory occlusion causes marked increases in cardiac work; however, little if any coronary vasodilation is observed (3, 37, 42). Similar results are observed with strong static muscle contractions (32, 38). Previous studies from our laboratory have shown that metaboreflex activation during submaximal dynamic exercise caused no coronary vasodilation despite marked increases in HR and ventricular contractility. Cardiac output increased substantially and was pumped against a much higher afterload, yet all of the increase in coronary blood flow occurred via increases in perfusion pressure rather than vasodilation (3). These results indicated that a “push-pull” situation exists between the vasodilatory drives and the vasoconstrictor effects of the increased sympathetic activity. If the increase in cardiac work during metaboreflex activation is reduced, actual coronary vasoconstriction is seen (3). Similarly, during maximal exercise when HR and cardiac output are already at maximal levels and little further steady-state increases in ventricular work occur, metaboreflex activation causes coronary vasoconstriction (3). Finally, in heart failure, little or no metaboreflex increases in contractility occur, and the reflex increase in cardiac sympathetic activity causes frank coronary vasoconstriction (2). To what extent this actual coronary vasoconstriction contributes to the inability to raise ventricular contractility and cardiac output during metaboreflex activation in heart failure is unknown.
Baroreflex vs. metaboreflex.
We used systemic α1-adrenergic blockade rather than injection into a coronary artery because we wanted to assess the effects on total ventricular function rather than on only an individual ventricular segment, which is more susceptible to changes in loading conditions (23, 30). After prazosin administration, MAP was lower due to the peripheral vasodilation, which raises the question as to what extent the enhanced increases in cardiac output and ventricular contractility reflect baroreflex responses. We feel this is unlikely for several reasons. Heyndrickx et al. (19) previously showed that, during exercise after systemic infusion of prazosin, whereas arterial plasma levels of norepinephrine were increased, there was no increase in norepinephrine release at the heart itself despite a large decrease in MAP. In the present study, after prazosin administration, cardiac output and PRSW were not higher than control levels, neither at rest nor during mild exercise (a small rise in dP/dt did occur, which may reflect changes in preload and/or afterload) (23). In addition, coronary blood flow was unchanged; the small increase in coronary conductance was offset by the small reduction in perfusion pressure. Thus, whereas MAP was lower after α1-adrenergic blockade, which would elicit a baroreflex response (tachycardia), this resulted in no significant increase in cardiac output or ventricular contractility, as SV fell with the rise in HR. The fall in SV with this rise in rate is very similar to that observed with merely increasing pacing rate within this range, which also elicits little if any increase in cardiac output (54). We have recently shown that this increase in rate by itself would have very little direct effect on ventricular contractility (Treppe effect) in this model (7). In contrast, a similar tachycardia induced by activation of the muscle metaboreflex causes large increases in cardiac output and ventricular contractility (48). Furthermore, in both dogs (8) and humans (43), carotid baroreceptor unloading during exercise causes little steady-state increases in cardiac output. The baroreflex pressor response is mediated via increases in peripheral resistance (8, 43). After α1-adrenergic blockade, only when the metaboreflex was activated did cardiac output, ventricular contractility, CVC, and coronary blood flow all rise above levels observed during the control experiments, whereas the difference in MAP was similar to that at rest and during mild exercise. We feel this is compelling evidence that the response was indeed metaboreflex in nature as the major effects on cardiac output and PRSW were only observed when the metaboreflex was activated and not at rest or during exercise when pressure was similarly lowered with α1-adrenergic blockade.
The arterial baroreflex normally acts to buffer the metaboreflex (26). Whether the rise in sympathetic activity that occurred with metaboreflex activation was greater after α1-adrenergic blockade because MAP did not rise to the same extent cannot be discounted. However, we recently showed that, after removal of the buffering effects of the arterial baroreflex (sinoaortic arterial baroreflex denervation), the much larger metaboreflex pressor response occurs via increased peripheral vasoconstriction. Indeed, the rise in cardiac output is if anything slightly smaller after baroreceptor denervation (25). Furthermore, the higher slope of the relationship between coronary conductance and cardiac power indicates that greater vasodilation occurs with α1-adrenergic blockade, as power increases during metaboreflex activation. Thus, even at the same cardiac power, larger coronary vasodilation occurs. Similarly, the overlap of the data relating PRSW to coronary blood flow indicates that, if the rise in coronary blood flow was the same after α1-adrenergic blockade, then ventricular contractility would have risen to the same extent.
Limitations.
Cardiac power is a relatively novel measure of cardiac function (11, 34) and, in the present study, was used as an index of myocardial oxygen consumption. Previous studies performed in humans used cardiac power calculated as product of cardiac output and MAP. Khouri et al. (24) previously used a similar calculation, and they referred to it as cardiac work or left ventricular work. However, power is work performed over time so we feel cardiac power is the correct term, especially so as we calculated cardiac power as stroke work (work/beats) times HR (beats/min), resulting in work/minute. Khouri et al. (24) showed an excellent correlation between this and myocardial oxygen consumption. Cardiac power has been shown to be a strong indicator of prognosis in chronic heart failure (55) and a strong predictor of mortality due to cardiogenic shock (11). Most recently, there has been evidence to suggest that cardiac power can be a very useful prognostic tool across a broad spectrum of acute cardiac diseases (34).
PRSW has been shown to be a very robust index of cardiac contractility (22). However, our technique used to estimate left ventricular volume has limitations. On average, the left ventricular volume values calculated from the sonomicrometry crystals underestimated the SV obtained by integrating the signal from cardiac output flow probe placed on the ascending aorta. We showed previously that this underestimation is highly linear within each animal (48). Similarly, low SV values for dogs of this size were reported by others using sonomicrometery (30, 47). To our knowledge, our studies are the only in which SV was measured simultaneously via these two techniques. This discrepancy between the SV values calculated using sonomicrometry vs. cardiac output likely occurred due to the number of crystals used and their placement on the left ventricle. In our study, only two pairs of crystals were used, to limit any damage made to the myocardium. In two animals, we simultaneously measured left ventricular volumes via sonomicrometry as well as echocardiography while also monitoring cardiac output via the implanted blood flow transducer. As we suspected, values for end-diastolic volume for echocardiography and sonomicrometry were very similar, whereas the values for SV were very similar between echocardiography and those calculated from the ascending aortic flow probe. Therefore, we believe that the error in the sonomicrometry value for SV resides in overestimating end-systolic volume. Therefore, for the volume data shown in Fig. 2, we used the end-diastolic volume obtained from sonomicrometry and SV from the aortic flow signal. These calculations yield reasonable estimates of other parameters such as ejection fraction.
In the present study, systemic vascular conductance to all areas except the hindlimbs (NIVC) also increased with metaboreflex activation after α1-receptor blockade. In a limited number of previous experiments, this systemic vasodilation was abolished by propranolol (42). NIVC reflects mostly skeletal muscle (26). Thus, this vasodilation likely is within skeletal muscle and may occur via epinephrine release from the adrenal glands (28). This may explain why with metaboreflex activation vasoconstriction is seen in select vascular beds, but no global change in NIVC is observed (5, 6, 17, 35, 36). It is possible that a portion of the coronary vasodilation seen after α1-adrenergic blockade was due to β2-adrenergic receptor stimulation via an increase in circulating epinephrine in addition to the marked increase in ventricular work (18).
In summary, muscle metaboreflex activation increases sympathetic tone to α1-adrenergic receptors and functionally restricts coronary vasodilation. This impedes blood flow to the myocardium and limits the increase in left ventricular performance. This likely limits the ability of the reflex to raise cardiac output and therefore restore blood flow to the ischemic muscles.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-55473 and HL-095819.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGEMENTS
We thank Erin Welsh-Krengel, Jody Helme-Day, and Janine Mattei for superior technical assistance.
REFERENCES
- 1. Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ansorge EJ, Augustyniak RA, Perinot RL, Hammond RL, Kim JK, Sala-Mercado JA, Rodriguez J, Rossi NF, O'Leary DS. Altered muscle metaboreflex control of coronary blood flow and ventricular function in heart failure. Am J Physiol Heart Circ Physiol 288: H1381–H1388, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Ansorge EJ, Shah SH, Augustyniak R, Rossi NF, Collins HL, O'Leary DS. Muscle metaboreflex control of coronary blood flow. Am J Physiol Heart Circ Physiol 283: H526–H532, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Asmussen E, Nielsen M. Experiments on nervous factors controlling respiration and circulation during exercise employing blocking of the blood flow. Acta Physiol Scand 60: 103–111, 1964 [DOI] [PubMed] [Google Scholar]
- 5. Augustyniak RA, Ansorge EJ, O'Leary DS. Muscle metaboreflex control of cardiac output and peripheral vasoconstriction exhibit differential latencies. Am J Physiol Heart Circ Physiol 278: H530–H537, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O'Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Chen X, Mukkamala R, Sala-Mercado JA, Hammond RL, Ichinose M, Soltani S, O'Leary DS. Dynamic control of maximal ventricular elastance in conscious dogs before and after pacing-induced heart failure. Conf Proc IEEE Eng Med Biol Soc 2009: 5328–5331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collins HL, Augustyniak RA, Ansorge EJ, O'Leary DS. Carotid baroreflex pressor responses at rest and during exercise: cardiac output vs. regional vasoconstriction. Am J Physiol Heart Circ Physiol 280: H642–H648, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJS, Concu A, Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc 35: 221–228, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Feigl EO. Coronary physiology. Physiol Rev 63: 1–205, 1983 [DOI] [PubMed] [Google Scholar]
- 11. Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, LeJemtel TH, Cotter G. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol 44: 340–348, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DC, Jr, Rankin JS. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation 71: 994–1009, 1985 [DOI] [PubMed] [Google Scholar]
- 13. Gorman MW, Tune JD, Richmond KN, Feigl EO. Quantitative analysis of feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol 89: 1903–1911, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Gorman MW, Tune JD, Richmond KN, Feigl EO. Feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol 89: 1892–1902, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Gwirtz PA, Dodd-OJM, Downey HF, Mass HJ, Barron BA, Williams AG, Jr, Jones CE. Effects of a coronary α1-constriction on transmural left ventricular flow and contractile function. Am J Physiol Heart Circ Physiol 262: H965–H972, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Gwirtz PA, Overn SP, Mass HJ, Jones CE. α1-Adrenergic constriction limits coronary flow and cardiac function in running dogs. Am J Physiol Heart Circ Physiol 250: H1117–H1126, 1986 [DOI] [PubMed] [Google Scholar]
- 17. Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Hein TW, Zhang C, Wang W, Kuo L. Heterogeneous β2-adrenoceptor expression and dilation in coronary arterioles across the left ventricular wall. Circulation 110: 2708–2712, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Heyndrickx GR, Vilaine JP, Moerman EJ, Leusen I. Role of prejunctional α2-adrenergic receptors in the regulation of myocardial performance during exercise in conscious dogs. Circ Res 54: 683–693, 1984 [DOI] [PubMed] [Google Scholar]
- 20. Huang AH, Feigl EO. Adrenergic coronary vasoconstriction helps maintain uniform transmural blood flow distribution during exercise. Circ Res 62: 286–298, 1988 [DOI] [PubMed] [Google Scholar]
- 21. Johnson JM, Rowell LB, Niederberger M, Eisman MM. Human splanchnic and forearm vasoconstrictor responses to reductions of right atrial and aortic pressures. Circ Res 34: 515–524, 1974 [DOI] [PubMed] [Google Scholar]
- 22. Karunanithi MK, Michniewicz J, Copeland SE, Feneley MP. Right ventricular preload recruitable stroke work, end-systolic pressure volume, and dp/dt(max)-end-diastolic volume relations compared as indexes of right ventricular contractile performance in conscious dogs. Circ Res 70: 1169–1179, 1992 [DOI] [PubMed] [Google Scholar]
- 23. Kass DA, Maughan WL, Guo ZM, Kono A, Sunagawa K, Sagawa K. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical-analysis based on pressure-volume relationships. Circulation 76: 1422–1436, 1987 [DOI] [PubMed] [Google Scholar]
- 24. Khouri EM, Gregg DE, Rayford CR. Effect of exercise on cardiac output, left coronary flow and myocardial metabolism in the unanesthetized dog. Circ Res 17: 427–437, 1965 [DOI] [PubMed] [Google Scholar]
- 25. Kim JK, Sala-Mercado JA, Hammond RL, Rodriguez J, Scislo TJ, O'Leary DS. Attenuated arterial baroreflex buffering of muscle metaboreflex in heart failure. Am J Physiol Heart Circ Physiol 289: H2416–H2423, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Kim JK, Sala-Mercado JA, Rodriguez J, Scislo TJ, O'Leary DS. Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. Am J Physiol Heart Circ Physiol 288: H1374–H1380, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Kim SJ, Kline G, Gwirtz PA. Limitation of cardiac output by a coronary α1-constrictor tone during exercise in dogs. Am J Physiol Heart Circ Physiol 271: H1125–H1131, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Kitchen AM, Scislo TJ, O'Leary DS. NTS purinoceptor activation elicits hindlimb vasodilation primarily via a β-adrenergic mechanism. Am J Physiol Heart Circ Physiol 278: H1775–H1782, 2000 [DOI] [PubMed] [Google Scholar]
- 29. LaPrad SL, Augustyniak RA, Hammond RL, O'Leary DS. Does gender influence the strength and mechanisms of the muscle metaboreflex during dynamic exercise in dogs? Am J Physiol Regul Integr Comp Physiol 276: R1203–R1208, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Little WC. The left-ventricular dp/dtmax-end-diastolic volume relation in closed-chest dogs. Circ Res 56: 808–815, 1985 [DOI] [PubMed] [Google Scholar]
- 31. Little WC, Cheng CP, Mumma M, Igarashi Y, Vinten-Johansen J, Johnston WE. Comparison of measures of left ventricular contractile performance derived from pressure-volume loops in conscious dogs. Circulation 80: 1378–1387, 1989 [DOI] [PubMed] [Google Scholar]
- 32. Longhurst JC, Aung-Din R, Mitchell JH. Static exercise in anesthetized dogs, a cause of reflex alpha-adrenergic coronary vasoconstriction. Basic Res Cardiol 76: 530–535, 1981 [DOI] [PubMed] [Google Scholar]
- 33. MacLean DA, Imadojemu VA, Sinoway LI. Interstitial pH, K+, lactate, and phosphate determined with MSNA during exercise in humans. Am J Physiol Regul Integr Comp Physiol 278: R563–R571, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Mendoza DD, Cooper HA, Panza JA. Cardiac power output predicts mortality across a broad spectrum of patients with acute cardiac disease. Am Heart J 153: 366–370, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Mittelstadt SW, Bell LB, O'Hagan KP, Clifford PS. Muscle chemoreflex alters vascular conductance in nonischemic exercising skeletal muscle. J Appl Physiol 77: 2761–2766, 1994 [DOI] [PubMed] [Google Scholar]
- 36. Mittelstadt SW, Bell LB, O'Hagan KP, Sulentic JE, Clifford PS. Muscle chemoreflex causes renal vascular constriction. Am J Physiol Heart Circ Physiol 270: H951–H956, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Momen A, Gahremanpour A, Mansoor A, Kunselman A, Blaha C, Pae W, Leuenberger UA, Sinoway LI. Vasoconstriction seen in coronary bypass grafts during handgrip in humans. J Appl Physiol 102: 735–739, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Momen A, Mascarenhas V, Gahremanpour A, Gao Z, Moradkhan R, Kunselman A, Boehmer JP, Sinoway LI, Leuenberger UA. Coronary blood flow responses to physiological stress in humans. Am J Physiol Heart Circ Physiol 296: H854–H861, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nozawa T, Cheng CP, Noda T, Little WC. Relation between left ventricular oxygen consumption and pressure-volume area in conscious dogs. Circulation 89: 810–817, 1994 [DOI] [PubMed] [Google Scholar]
- 40. O'Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol Heart Circ Physiol 260: H632–H637, 1991 [DOI] [PubMed] [Google Scholar]
- 41. O'Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1399–H1403, 1999 [DOI] [PubMed] [Google Scholar]
- 42. O'Leary DS, Sala-Mercado JA, Hammond RL, Ansorge EJ, Kim JK, Rodriguez J, Fano D, Ichinose M. Muscle metaboreflex-induced increases in cardiac sympathetic activity vasoconstrict the coronary vasculature. J Appl Physiol 103: 190–194, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988 [DOI] [PubMed] [Google Scholar]
- 45. Rowell LB. Neural control of muscle blood flow: importance during dynamic exercise. Clin Exp Pharmacol Physiol 24: 117–125, 1997. [DOI] [PubMed] [Google Scholar]
- 46. Rowell LB, O'Leary DS, Kellogg DL., Jr Integration of cardiovascular control systems in dynamic exercise. New York: Oxford, 1996, p. 770–838 [Google Scholar]
- 47. Sala-Mercado JA, Hammond RL, Kim JK, McDonald PJ, Stephenson LW, O'Leary DS. Heart failure attenuates muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 292: H2159–H2166, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O'Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 290: H751–H757, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Sheriff DD, Augustyniak RA, O'Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Sheriff DD, Wyss CR, Rowell LB, Scher AM. Does inadequate oxygen delivery trigger pressor response to muscle hypoperfusion during exercise? Am J Physiol Heart Circ Physiol 253: H1199–H1207, 1987 [DOI] [PubMed] [Google Scholar]
- 51. Sinoway LI, Rea RF, Mosher TJ, Smith MB, Mark AL. Hydrogen ion concentration is not the sole determinant of muscle metaboreceptor responses in humans. J Clin Invest 89: 1875–1884, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sinoway LI, Smith MB, Enders B, Leuenberger U, Dzwonczyk T, Gray K, Whisler S, Moore RL. Role of diprotonated phosphate in evoking muscle reflex responses in cats and humans. Am J Physiol Heart Circ Physiol 267: H770–H778, 1994 [DOI] [PubMed] [Google Scholar]
- 53. Victor RG, Bertocci LA, Pryor SL, Nunnally RL. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest 82: 1301–1305, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. White S, Patrick T, Higgins CB, Vatner SF, Franklin D, Braunwald E. Effects of altering ventricular rate on blood flow distribution in conscious dogs. Am J Physiol 221: 1402–1407, 1971 [DOI] [PubMed] [Google Scholar]
- 55. Williams SG, Cooke GA, Wright DJ, Parsons WJ, Riley RL, Marshall P, Tan LB. Peak exercise cardiac power output; a direct indicator of cardiac function strongly predictive of prognosis in chronic heart failure. Eur Heart J 22: 1496–1503, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983. [DOI] [PubMed] [Google Scholar]