Abstract
Increasing either genioglossus muscle activity (GG) or end-expiratory lung volume (EELV) improves airway patency but not sufficiently for adequate treatment of obstructive sleep apnea (OSA) in most patients. The mechanisms by which these variables alter airway collapsibility likely differ, with increased GG causing airway dilation, whereas increased EELV may stiffen the airway walls through caudal traction. We sought to determine whether the airway stabilizing effect of GG activation is enhanced when EELV is increased. To investigate this aim, 15 continuous positive airway pressure (CPAP)-treated OSA patients were instrumented with an epiglottic catheter, intramuscular GG-EMG electrodes, magnetometers, and a nasal mask/pneumotachograph. Subjects slept supine in a sealed, head-out plastic chamber in which the extra-thoracic pressure could be lowered (to raise EELV) while on nasal CPAP with a variable deadspace to allow CO2 stimulation (and GG activation). The pharyngeal critical closing pressure (PCRIT) was measured by sudden reduction of CPAP for three to five breaths each minute during non-rapid eye movement (NREM) sleep in 4 conditions: a) baseline, b) 500 ml increased EELV, c) 50% increased GG, and d) conditions b and c combined. PCRIT was found to be reduced from 2.2 ± 0.7 cmH2O at baseline to −1.0 ± 0.5 with increased EELV, 0.6 ± 0.7 with increased GG and −1.6 ± 0.7 when both variables were raised (P < 0.001). The slope of the PCRIT curves remained unchanged in all conditions (P = 0.05). However, the CPAP level at which flow limitation developed was lower in both increased EELV conditions (P = 0.001). These findings indicate that while both increased GG and EELV improve airway collapsibility, the combination of both variables has little additional effect over increasing EELV alone.
Keywords: obstructive sleep apnea, pharyngeal critical closing pressure, upper airway dilator muscles
obstructive sleep apnea (OSA) is a common disorder of repetitive upper airway collapse during sleep that has important cardiovascular and neurocognitive consequences (1). Although there are several different treatments available for OSA, some are poorly tolerated, whereas others are only effective in a subset of individuals with the disorder. Thus a considerable number of OSA patients are poorly treated. This burden of partially treated disease has led to the interest in developing new treatment strategies for OSA.
There have been considerable advances in our understanding of the multiple causes of OSA in recent years. In all patients some form of pharyngeal anatomical compromise appears to be present (24). However, during wakefulness the airway is held open by the activity of the upper airway dilator muscles. As the patient falls asleep the dilator muscle activity is reduced (30). End-expiratory lung volume (EELV) also likely falls at sleep onset in OSA patients as it does in healthy nonobese individuals (2, 12), reducing caudal tracheal traction on the airway. How much EELV falls is yet to be determined in obese patients with OSA (13, 25). Reductions in both muscle activity and lung volume likely contribute to the sleep-related airway collapse.
Current treatments for OSA predominantly manipulate the airway anatomy. Electrical stimulation of the hypoglossal nerve or the genioglossus muscle directly has been shown to improve airway patency and reduce the pharyngeal critical closing pressure (PCRIT) in several studies (16, 19, 21). However, complete therapeutic treatment of OSA was often not achieved (19). Pharmacologic stimulation of the airway dilator muscles with paroxetine or mirtazapine has also been attempted in several studies (4, 18) with mixed effects on apnea hypopnea index (AHI), although the actual effect on muscle activity was only measured in one study (4). More recently, when end-expiratory lung volume has been manipulated during sleep (10, 11, 26) and anesthesia (28), upper airway function has improved. Although significant improvements in the AHI occurred when lung volume was increased, no subjects had their AHI reduced below 5 events/h (11). Thus both increased genioglossus activity and increased lung volume improve airway function and reduce the severity of OSA but by themselves are inadequate treatments (at least at the levels previously tested).
The mechanisms by which increased dilator muscle activity and increased lung volume influence airway collapsibility are incompletely understood. However, recent data suggest that activation of the genioglossus muscle results in anterior movement of the tongue (8) such that the retroglossal airspace is dilated. In contrast, increased lung volume is thought to result in caudal movement of the diaphragm such that traction is applied on the mediastinal structures and in turn the upper airway, stiffening the airway walls (29). Given these likely different mechanisms of action, we hypothesized that the combined effect of increased lung volume and increased genioglossus muscle activity would be greater than either factor alone and that when combined, airway collapsibility may be reduced to a level that would abolish OSA. To test these hypotheses we measured airway collapsibility (PCRIT) during NREM sleep at baseline, with increased lung volume alone, with increased genioglossus muscle activity alone, and with simultaneous increases in lung volume and genioglossus muscle activity in patients with OSA.
METHODS
Subjects
Fifteen continuous positive airway pressure (CPAP)-treated (>3 mo) OSA patients (4 women) were recruited. No subject smoked and all subjects were free of neurologic, cardiorespiratory, and sleep disorders other than OSA. Five subjects took antihypertensives, three took allergy medications, one took a cholesterol lowering drug, and another used a treatment for acid reflux. Subjects were aged 20–65 yr and premenopausal women (n = 3) were studied in the follicular menstrual phase. The study conformed to the standards set by the latest revision of the Declaration of Helsinki. All subjects gave informed written consent, and the study was approved by the Institutional Review Board of the Brigham and Women's Hospital.
Instrumentation and Measurements
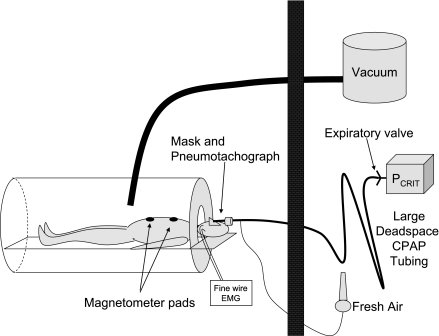
Subjects arrived in the laboratory 2 h before their usual bedtime. After a physician evaluation, subjects were instrumented with electroencephalogram (C3-A2, Oz-A2), left and right electrooculogram, and submental electromyogram (Nihon Kohden, Foothill Ranch, CA) for sleep staging and arousal scoring. Next, magnetometer pads (EOL Eberhard, Oberwil, Switzerland) were placed on the chest and abdomen for quantification of changes in EELV. To assess airway pressures and identify breaths with flow limitation, a pressure tipped catheter (MPC-550, Millar Instruments, Houston, TX) was inserted to the level of the epiglottis (∼1.5 cm below the tongue base) through one nostril after nasal decongestion (oxymetazoline HCl) and surface anesthesia (lidocaine HCl). The activity of the genioglossus muscle was determined by recording from intramuscular fine wire electrodes inserted per-orally after surface anesthesia. One electrode was placed on either side of the frenulum to a depth of ∼15 mm (17). The raw EMG signal was rectified and moving time averaged (100 ms) before being expressed as a percentage of the maximum level observed during swallow, tongue protrusion, or deep breaths. Subjects then lay supine inside a sealed head-out plastic shell (Portalung, Denver CO), and a nasal mask was applied. The mask was attached to a pneumotachograph (model 3700A, Hans Rudolph, Kansas City, MO) and CPAP tubing without an expiration valve. Fresh air was bled into the CPAP tubing upstream of the pneumotachograph at a rate sufficient to flush the tubing and prevent rebreathing. During the night, the flow rate of air bled into the circuit could be reduced to allow rebreathing [and genioglossus (GG) activation] that remained constant as CPAP level was manipulated. Pressure in the mask was continuously monitored (Validyne, Northridge, CA) and end-tidal CO2 (PetCO2) levels were sampled from a catheter positioned inside the subject's nostril (model 17630 Vacumetrics, Ventura, CA). Arterial oxygen saturation and electrocardiogram were monitored for safety purposes. A schematic of the setup is shown in Fig. 1.
Fig. 1.
Experimental setup. Patient lay with his/her body within and head out of the sealed plastic shell in the bedroom. He/she wore a nasal mask/pneumotachograph that was connected to continuous positive airway pressure (CPAP) tubing without an expiratory leak valve but with a fresh air input bleed line in place. To maintain eucapnia, high flow rates of medical air were introduced into the CPAP tubing flushing expiratory gas out of the tubing. However, when hypercapnia was required, the fresh air flow rate could be reduced such that the subjects partially rebreathed their own expired air. Thus the degree of hypercapnia could be maintained throughout changes in CPAP pressure. The subjects also wore magnetometers placed on the chest and abdomen such that changes in EELV, which were induced by applying negative pressure to the sealed chamber, could be documented.
Protocol
Once the subjects were comfortable and relaxed in the plastic shell, 5 min of data were recorded during wakefulness before the lights were turned off and the subjects were allowed to fall asleep. After sleep onset, the level of CPAP was adjusted to abolish any residual flow limitation (if present) and 5 min of data during sleep were collected for measurement of sleeping PetCO2 and GG muscle activity level. PCRIT was then measured in the standard manner (5) by multiple, sudden, three-to-five breath drops in CPAP level every minute in the following four conditions, which were performed in random order.
Baseline.
At the subject's natural levels of GG activity and EELV while sleeping on CPAP.
Fifty percent increased GG activity.
The flow rate of air bled into the tubing was reduced to raise the peak inspiratory GG activity 50% above the average value observed on fully therapeutic CPAP during the minute immediately preceding the trial.
Five hundred milliliter increased EELV.
The extrathoracic pressure was reduced until EELV was increased by 500 ml above the level on fully therapeutic CPAP measured in the minute immediately prior to reducing extrathoracic pressure.
Combined 50% increased GG activity and 500 ml increase in EELV.
The amount of fresh air bled into the inspiratory line was reduced to raise peak inspiratory GG activity 50% simultaneously as the lung volume was increased 500 ml by reducing extrathoracic pressure (both relative to the levels of GG and EELV on fully therapeutic CPAP measured in the minute immediately prior to the trial).
Each condition was repeated one to four times across the night (as many times as possible) such that multiple PCRIT values were determined in each condition. At least 3 min of room air breathing with atmospheric extrathoracic pressure separated each run of each condition. If a subject briefly aroused at any stage the conditions were not altered and PCRIT measurement did not resume until the subject returned to stable sleep. However, when a full awakening occurred, the run of that condition was aborted until the subject had returned to stable sleep. All manipulations and measurements were made during NREM sleep (stages 2–4). All data were acquired using a 1401plus analog-to-digital converter and Spike software (CED, Cambridge, UK).
Data Analysis
Custom-written software was used to calculate breath timing, tidal volume, peak inspiratory flow, mask pressure at end expiration, peak inspiratory and expiratory tonic genioglossus muscle activity, PetCO2, and the end-expiratory ribcage and abdominal distances. PetCO2 values were only used while on fully therapeutic CPAP because during the CPAP drops required for PCRIT measurement expired airflow often did not plateau, indicating that the gas sampled was not alveolar gas. PCRIT was determined by plotting the peak inspiratory flow vs. end-expiratory mask pressure on all flow-limited breaths during pressure drops but not apneas (zero flow). If not all of the breath was flow limited (transient peaks in flow are sometimes observed, particularly early in inspiration) then the peak flow during the flow-limited segment of inspiration was used instead of peak inspiratory flow. A linear regression was then performed and the regression extrapolated to zero flow for PCRIT determination. If there were only two CPAP levels in a trial with flow-limited breaths, or the range in flow levels was less than 0.1 l/s, the trial was not used for PCRIT determination. The multiple PCRIT values within a condition in each subject were averaged to give a single value.
EELV was calculated according to Banzett et al.(3). Briefly, a calibration constant (x) was determined at the beginning of the night using the pneumotachograph derived volume during breaths with a range of tidal volumes. The x constant was then applied throughout the night with the differences in end-expiratory abdominal and rib cage distances used to calculate the change in EELV according to the following formula: ΔEELV= x[4RC+ABD].
Statistical Analysis
The PCRIT, EELV, genioglossus muscle activity, and respiratory variables in the four conditions were analyzed with one-way repeated measures ANOVA with Greenhouse-Geisser correction for asphericity. Dunn-Sidak corrected Student's t-tests were used in post hoc analyses. Means ± SE are presented.
RESULTS
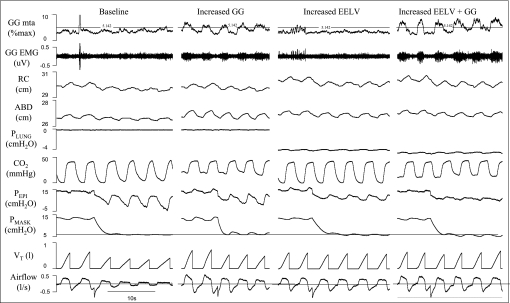
Six subjects did not sleep adequately to obtain PCRIT measurements in all four conditions. These subjects did not differ from those in whom all data were obtained with regard to age, BMI, severity of OSA, or prescribed nightly CPAP. Only the results from the nine subjects with full data are reported. These nine subjects were middle aged, moderately overweight, and all but one patient had severe OSA (Table 1). On average 3.0 ± 0.4 PCRIT measurements were averaged in each subject at baseline, 1.4 ± 0.2 with increased GG activity, 1.9 ± 0.3 with increased EELV, and 1.6 ± 0.2 with both GG and EELV increased. Examples of raw data in each condition in one subject are shown in Fig. 2.
Table 1.
Anthropometric data in the 9 subjects
| Mean | Range | |
|---|---|---|
| Men:Women | 7:2 | |
| Age, yr | 46 ± 4 | 20–60 |
| BMI, kg/m | 33.6 ± 2.3 | 25.8–45.5 |
| AHI, events/h | 55.5 ± 7.6 | 13.1–87.4 |
| CPAP, cmH2O | 8.6 ± 0.8 | 4–12 |
BMI, body mass index; AHI, apnea hypopnea index; CPAP, continuous positive airway pressure.
Fig. 2.
Raw data in all 4 conditions in 1 subject. Airflow, tidal volume (VT), mask pressure (PMASK), epiglottic pressure (PEPI), carbon dioxide (CO2) level, pressure inside the plastic shell (PLUNG), abdominal diameter (AB), rib cage diameter (RC), and both the raw (EMG) and rectified/moving time averaged (MTA) genioglossal (GG) EMG signals are shown in 1 subject in all 4 conditions: baseline (BL), 50% increased genioglossus activity (Inc GG), 500 ml increased end-expiratory lung volume (Inc EELV), and both 500 ml increased end-expiratory lung volume and 50% increased genioglossal activity (Inc GG+Inc EELV). In each condition a pressure drop to ∼6 cmH2O was performed with varying degrees of flow limitation resulting.
The respiratory changes that occurred during increased GG and EELV (while on therapeutic CPAP) are presented in Table 2. During hypercapnia to stimulate GG activity, tidal volume, minute ventilation, and peak flow increased above baseline levels. Although the mean value of peak inspiratory GG activity was increased ∼50%, there was considerable variability between subjects (range 16–73% increase), which was consistent across hypercapnic trials. EELV did not change during hypercapnia-induced GG activation. When EELV was raised, minute ventilation, peak flow, and GG activity remained unchanged. Although the target was a 500-ml increase in EELV, the actual increase in EELV was closer to 600 ml. Importantly, however, when GG activity and EELV were increased simultaneously, the changes in GG and EELV were not different from the trials in which each variable was manipulated independently.
Table 2.
Respiratory variables in the 4 experimental conditions
| Baseline | Inc GG | Inc EELV | Inc GG + Inc EELV | |
|---|---|---|---|---|
| FB, breaths/min | 14.3 ± 0.9 | 14.9 ± 1.1 | 14.5 ± 0.9 | 14.8 ± 1.1 |
| VT, liter | 0.56 ± 0.02 | 0.89 ± 0.07* | 0.52 ± 0.03# | 0.81 ± 0.08* |
| VI, l/min | 7.8 ± 0.3 | 12.7 ± 0.7* | 7.2 ± 0.3# | 11.5 ± 0.9* |
| PIF, l/s | 0.48 ± 0.03 | 0.72 ± 0.05* | 0.49 ± 0.02# | 0.71 ± 0.05* |
| PETCO2, mmHg | 41.1 ± 1.2 | 44.5 ± 1.5* | 40.8 ± 1.3# | 44.2 ± 1.3* |
| PEPI, cmH2O | −3.1 ± 0.6 | −3.4 ± 0.5# | −2.2 ± 0.5 | −1.9 ± 0.3 |
| GG peak, %pretrial baseline | 96.9 ± 8.4 | 150.8 ± 6.4* | 101.4 ± 10.1 | 156.3 ± 14.2* |
| EELV, ml change from pretrial baseline | −33 ± 89 | 38 ± 34 | 589 ± 38* | 645 ± 59* |
FB, breathing frequency; VT, tidal volume; VI, inspired minute ventilation; PIF, peak inspiratory flow rate; PETCO2, end-tidal CO2 levels; PEPI, epiglottic pressure; GG Peak, peak inspiratory genioglossus EMG activity; EELV, end-expiratory lung volume in the 4 experimental conditions: baseline (BL), 50% increased genioglossus activity (Inc GG), 500 ml increased end-expiratory lung volume (Inc EELV), and with both 500 ml increased end-expiratory lung volume and 50% increased genioglossal activity (Inc GG+Inc EELV).
Significantly different from baseline;
significantly different from Inc GG + Inc EELV.
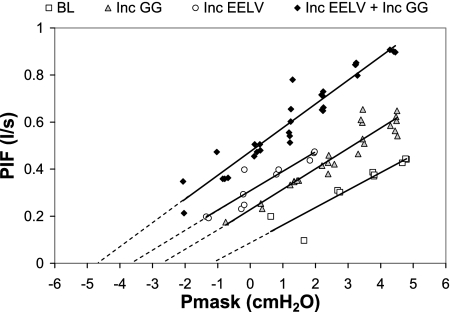
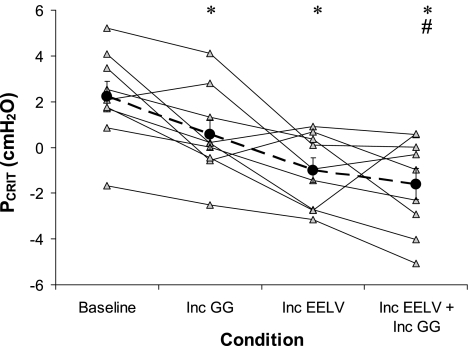
PCRIT curves in all four conditions in one subject are shown in Fig. 3. The group data (Fig. 4, ANOVA P < 0.001) follow a similar pattern and show that PCRIT is reduced from baseline with increased GG activity (P = 0.03) and slightly more (not significantly more) when EELV is increased (P = 0.1 compared with increased GG but P = 0.003 compared with baseline). When both GG and EELV are simultaneously increased, the reduction in PCRIT is not greater than with EELV alone (P = 0.7), although it is significantly greater than when GG activity is increased alone (P = 0.04) and significantly lower than baseline (P = 0.003). Thus there is minimal additional benefit from stimulating both factors simultaneously over that of increased EELV alone.
Fig. 3.
An example of pharyngeal critical closing pressure (PCRIT) curves in the 4 conditions in 1 subject. The CPAP level (PMASK) and peak inspiratory flow rate (PIF) are shown for all flow-limited breaths during PCRIT measurement (3rd to 5th breaths after CPAP drop only) in the 4 experimental conditions: BL, Inc GG, Inc EELV, and Inc GG+Inc EELV. Linear regression lines, extrapolated to zero flow are shown in each condition.
Fig. 4.
Individual and group mean PCRIT in each subject across the 4 conditions: BL, Inc GG, Inc EELV, and Inc GG+Inc EELV. Group mean values (±SE) are shown in the large black circles. *Significantly lower than BL; #significantly lower than Inc GG.
The inverse slope of the PCRIT curve (known as upstream resistance) was unchanged (P = 0.05) in all four conditions (14.8 ± 1.5 at baseline, 15.8 ± 3.6 with increased GG, 17.5 ± 1.6 with increased EELV, and 12.4 ± 1.3 cmH2O/l with both increased GG and EELV). However, the range of CPAP levels over which flow limitation occurred did differ between conditions (P = 0.001 by ANOVA). At baseline, breaths became flow limited at 9.5 ± 0.9 cmH2O and this was unchanged with increased GG activity (9.6 ± 1.1 cmH2O, P = 0.9). However, when EELV was increased either alone, or in combination with increased GG, inspiratory airflow did not become flow limited until CPAP was reduced to 7.2 ± 0.9 and 7.6 ± 0.8 cmH2O, respectively (P = 0.02 and 0.048 relative to baseline). Similarly, the theoretical airflow at atmospheric pressure (y intercept) was below zero at baseline and with increased GG activity (−0.2 ± 0.07 and −0.05 ± 0.07 l/s, respectively) indicating that no airflow would occur without CPAP in these conditions. In contrast, when EELV was raised alone or in the combined condition, airflow at atmospheric pressure was positive (0.05 ± 0.03 and 0.1 ± 0.06 l/s, respectively, P = 0.01 compared with baseline).
DISCUSSION
This study highlighted that combining different factors that promote airway patency has complex effects on airway collapsibility. As we anticipated, increasing both genioglossus activity and lung volume reduces the collapsibility of the pharyngeal airway, but simultaneously altering both variables has minimal additional benefit over increasing lung volume alone. Airway collapsibility is determined by several factors including the airway cross-sectional area (smaller airways tend to collapse), stiffness of the pharyngeal walls (floppy walls more easily collapse), the driving pressure across the airway (higher negative intraluminal pressures predispose to collapse) and extraluminal tissue pressure (high pressure outside the airway will predispose to collapse). We are unable to determine which of these factors were altered by increasing GG with CO2 [although in rabbits genioglossus stimulation does not change extraluminal tissue pressure despite reducing airway resistance (15)] or raising EELV and, as such, we are unable to determine why these two factors failed to have a synergistic effect. However, as mentioned in the introduction, increasing genioglossus activity is thought to dilate the airway, whereas raising EELV is thought to stiffen the airway walls. As such, there are several likely explanations for our finding that are outlined below.
First, it is possible that increasing lung volume alters the anatomical relationship between airway structures, such that the genioglossus muscle is now mechanically disadvantaged. The length/tension relationship of the GG has been minimally studied in humans, but may be a function of prevailing airway mechanics and geometry (6). Oliven and Odeh (20) reported that caudal tracheal traction in dogs increased the length of the genioglossus at baseline but this increased the magnitude of shortening of the muscle in response to chemical and electrical stimulation. Whether a similar relationship between EELV and genioglossus activation exists in humans is unclear. A similar, but alternative hypothesis is that airway wall stiffness (tube law) affects the ability of the GG to dilate the airway, i.e., the GG is ineffective at airway dilation when airway walls are stiff (from increased EELV). However, against this, data in cats show that physical tongue protrusion reduced PCRIT when caudal traction was applied but did not alter PCRIT without the traction (23). It is also possible that a maximum level of airway dilation was already reached when lung volume was raised alone. We think such a ceiling effect is unlikely as all patients reduce their closing pressures to quite low values during wakefulness, although the mechanisms are not entirely clear. Finally, it is possible that increasing lung volume and increasing GG activity influence the airway through common mechanisms. For example, raising lung volume may dilate the airway in the same way the genioglossus does, rather than stiffen the airway as has been traditionally hypothesized (29). Alternatively, raising GG activity with CO2 may stiffen the airway walls (22). In either case, increasing GG and EELV together would have redundant effects on the airway. We think this is unlikely because the CPAP level at which flow limitation begins was not different than baseline with increased GG activity but was reduced by ∼2 cmH2O when lung volume was increased. Although the exact significance of the change in CPAP at which flow limitation starts is unknown, this would suggest that the mechanisms of altering airway collapsibility differ between conditions.
The potential mechanism of reduced collapsibility during increased lung volume deserves further comment as this has been debated, with several theories having emerged from prior studies. These theories include caudal traction on the airway (stiffening the airway walls), reflex activation of upper airway dilator muscles, reductions in tissue pressure surrounding the pharyngeal airway, and airway dilation through mechanical forces (29). If increasing lung volume simply stiffened the airway walls, a greater pressure change would be expected to deform the airway and impede airflow. Therefore, the slope of the pressure/flow relationship would be expected to be reduced. However, the pressure/flow slope remained unchanged in this study. The genioglossus muscle activity was not increased during the raised lung volume condition in the current or prior studies from our laboratory (26), suggesting neuromuscular reflex responses are not likely to explain the reduction in airway collapsibility. Thus we believe it is likely that increasing lung volume dilates the airway directly or reduces the tissue pressure surrounding the airway (14) rather than stiffening the airway as we originally hypothesized. However, further investigation is clearly required. Regardless of the mechanism, our results do suggest that therapeutic strategies to treat OSA by combining mechanical and neuromuscular approaches (as done in the present study) may be only partially effective in OSA patients as a group. However, it should be noted that there was considerable variability in patient responses and four individuals did show at least a 1 cmH2O further reduction in PCRIT with the combined EELV-GG stimuli (Fig. 4). These four individuals tended to be younger (39.8 vs. 50.8 yr, P = 0.3), have lower BMIs (30.2 vs. 36.3 kg/m2, P = 0.2), and were less severe (AHI = 37.5 vs. 69.9, P = 0.03) and prescribed lower CPAP levels (6.5 vs. 10.5, P = 0.02) than the five patients with minimal/no further improvement in PCRIT when both EELV and genioglossus activity were raised. Thus the possibility remains that some individual patients would be amenable to a combined lung volume, genioglossus stimulation treatment. Whether the subset of patients who might respond to the combined lung volume augmentation/genioglossus activation approach can be identified based on polysomnographic features or demographic variables requires further research.
A number of investigators have attempted to improve pharyngeal mechanics using either pharmacological or electrical dilator muscle stimulation strategies (4, 16, 19). Such approaches have not yielded consistent clinical benefits to date, at least at the levels of stimulation tested. Some have suggested that a combined approach (e.g., hypoglossal and phrenic stimulation to activate GG and raise EELV, respectively) may yield further improvements in pharyngeal mechanics compared with either intervention alone. However, our results provide some caution regarding this possible approach given our observed lack of benefit to the combined strategy. Despite this, we cannot rule out the possibility that greater than 50% increases in genioglossus activity when combined with more than 500-ml increases in EELV may prove useful, at least for some patients. Pharmacological targets have also been developed to stimulate output to the upper airway and to the diaphragm pump muscles concurrently. Again, such an approach will need to be tested rigorously given the limited benefits that we might anticipate in light of our new findings. In contrast to our study however, Oliven et al. (21) recently stimulated the GG in the setting of mandibular advancement and found that activation of the GG was more effective at reducing PCRIT when the mandibular splint was in place. Thus other combinations of treatments may be beneficial in treating OSA, although further study is required.
EELV Influence on Airway Collapsibility
The effect of increasing EELV was found to be quite varied between our patients with OSA who were studied during natural sleep. A nearly 600-ml increase in EELV resulted in reductions of PCRIT between 0.8 and 6.8 cmH2O in individual patients. Given we only had full data in nine subjects, we were underpowered to conduct meaningful regression analyses to determine whether the PCRIT change more closely related to the actual EELV change or other factors such as BMI. Of note, however, PCRIT was reduced below −2.5 cmH2O in three of nine severe OSA patients in this study during the increased EELV condition. PCRIT values of −4cmH2O are typical of patients who snore or have only mild hypopnea (9) and thus a 600-ml increase in EELV alone in these subjects may have been nearing the level required to treat their OSA. Although clearly using a Portalung to raise EELV in the home is not feasible, alternate treatments to raise EELV may prove to be beneficial in some patients.
Limitations
Despite its strengths, we acknowledge a number of limitations of our study. First, given the intensive nature of our physiological studies and the poor quality of sleep in some subjects, our final sample size was limited to nine subjects. As a result, we may have been underpowered for some of our comparisons. However, we calculated a sample size of 150 patients would be required to yield statistical significance (e.g., for increased EELV vs. increased EELV plus increased GG) with the mean and standard deviation observed in this study. Given 40% of subjects studied did not sleep sufficiently for PCRIT measurement in all four conditions, 260 enrolled subjects would be required. Such a large study is clearly not feasible in a physiology laboratory. In addition, the study was not sufficiently powered to perform subgroup analyses, which may be important. For example, sex effects on fat distribution may influence the EELV contribution to the upper airway. Further study will be required to elucidate the potential influence of sex on such relations.
Second, the magnetometers provide an accurate means to assess acute EELV changes, but are not useful for the quantification of absolute values of lung volume. As a result, we cannot determine whether our manipulations are yielding peak inflation lung volumes close to TLC or whether further elevations in EELV are possible. Our prior experience suggests that very large elevations in lung volume frequently lead to arousal; however, we cannot assess whether our participants in the present study would have benefited further from greater EELV increases.
Third, genioglossus activity was increased 50% above the baseline value in asleep subjects on therapeutic CPAP. CPAP and sleep both decrease genioglossus activity and as a result baseline levels of GG were very low (average 1.0 ± 0.2% maximum level observed during tongue protrusion while awake). Thus a 50% increase in GG activity was actually a small absolute increase in genioglossus activity. However, PetCO2 needed to be raised 3.5 mmHg on average to achieve this level of genioglossus increase, which does represent a considerable respiratory stimulus. This degree of hypercapnia would result in much more marked increases in genioglossus activity while awake and off CPAP and, even at this modest level, several subjects failed to sleep adequately for PCRIT measurement. However, it remains quite possible that if genioglossus activity were increased more than 50%, perhaps through other means, then further reductions in PCRIT may occur. It should also be noted that CO2 rebreathing likely stimulated numerous airway muscles and the observed effects on PCRIT may not be a result of genioglossus stimulation alone.
Fourth, we recognize that CO2 has a number of influences on the cardiorespiratory system in addition to activation of GG. We acknowledge that CO2 may be contributing to catecholamine release, increased cardiac output, etc. (7). However, we were using CO2 manipulation as a standardized respiratory/dilator muscle stimulus, but cannot determine with confidence whether other means of GG activation would yield similar results. In the study of Oliven et al. (21) electrical stimulation of the GG did reduce the pressure at which flow limitation developed, suggesting that electrical stimulation of the GG may differ somewhat from activation by CO2.
Finally, we are aware that negative extrathoracic pressure has more influences than just increasing lung volume. In theory, venous return from the structures outside the sealed chamber could be affected by our approach, which could in turn reduce neck fluid volumes (27). We doubt this effect importantly influenced our results because our neck cuff surrounds the upper airway and we do not believe that we are creating a pressure gradient from the neck to the chest.
Conclusion
This study demonstrated that increasing both end-expiratory lung volume and genioglossus muscle activity improves airway collapsibility in patients with OSA but simultaneously increasing both variables has little additional benefit over increased lung volume alone. A treatment strategy aiming to raise these two variables simultaneously to the levels that were studied in this experiment is unlikely to treat OSA adequately. However, raising EELV alone may be useful in some patients.
GRANTS
The study was funded by National Heart, Lung, and Blood Institute Grants HL-048531, HL-60292, and RR-01032 and American Heart Association 0840159N, 0635318N. The modified CPAP machine was provided by Philips Respironics.
DISCLOSURES
A. S. Jordan and D. J. Eckert consult for Apnex Medical (<$20,000 per year). A. Malhotra consults for several companies, including Philips Respironics (<$20,000 per year). D. P. White is Chief Medical Officer for Philips Respironics. While a number of the authors have some industry affiliations, related to the treatment of apnea, the current study was a physiological investigation designed and performed by the investigators independently. The study was funded by peer-reviewed grant mechanisms.
ACKNOWLEDGMENTS
We thank our sleep technicians Karen E. Stevenson, Scott A. Smith, and Lauren Hess for their invaluable assistance in obtaining these data.
Dr. Jordan's current address is Sleep Laboratory, Psychological Sciences, The University of Melbourne, Grattan St, Parkville, VIC, 3010, Australia.
REFERENCES
- 1. Al Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis 51: 285–293, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Ballard RD, Irvin CG, Martin RJ, Pak J, Pandey R, White DP. Influence of sleep on lung volume in asthmatic patients and normal subjects. J Appl Physiol 68: 2034–2041, 1990 [DOI] [PubMed] [Google Scholar]
- 3. Banzett RB, Mahan ST, Garner DM, Brughera A, Loring SH. A simple and reliable method to calibrate respiratory magnetometers and Respitrace. J Appl Physiol 79: 2169–2176, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Berry RB, Yamaura EM, Gill K, Reist C. Acute effects of paroxetine on genioglossus activity in obstructive sleep apnea. Sleep 22: 1087–1092, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Boudewyns A, Punjabi N, Van de Heyning PH, De Backer WA, O'Donnell CP, Schneider H, Smith PL, Schwartz AR. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest 118: 1031–1041, 2000 [DOI] [PubMed] [Google Scholar]
- 6. BuSha BF, Strobel RJ, England SJ. The length-force relationship of the human genioglossus in patients with obstructive sleep apnea. Respir Physiol Neurobiol 130: 161–168, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Carvalho CR, Barbas CS, Medeiros DM, Magaldi RB, Lorenzi Filho G, Kairalla RA, Deheinzelin D, Munhoz C, Kaufmann M, Ferreira M, Takagaki TY, Amato MB. Temporal hemodynamic effects of permissive hypercapnia associated with ideal PEEP in ARDS. Am J Respir Crit Care Med 156: 1458–1466, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Cheng S, Butler JE, Gandevia SC, Bilston L. Movement of the tongue during normal breathing in awake healthy humans. J Physiol 586: 4283–4294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis 143: 1300–1303, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Heinzer RC, Stanchina ML, Malhotra A, Fogel RB, Patel SR, Jordan AS, Schory K, White DP. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med 172: 114–117, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heinzer RC, Stanchina ML, Malhotra A, Jordan AS, Patel SR, Lo Y, Wellman A, Schory K, Dover L, White DP. Effect of increased lung volume on sleep disordered breathing in sleep apnoea patients. Thorax 61: 435–439, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hudgel DW, Devadatta P. Decrease in functional residual capacity during sleep in normal humans. J Appl Physiol 57: 1319–1322, 1984 [DOI] [PubMed] [Google Scholar]
- 13. Jordan AS, Yim S, Schory K, Smith SA, Malhotra A, White DP. The reduction in end-expiratory lung volume at sleep onset in obstructive sleep apnea [Abstract]. Sleep 30: A137, 2007 [Google Scholar]
- 14. Kairaitis K, Byth K, Parikh R, Stavrinou R, Wheatley JR, Amis TC. Tracheal traction effects on upper airway patency in rabbits: the role of tissue pressure. Sleep 30: 179–186, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Kairaitis K, Verma M, Fish V, Wheatley JR, Amis TC. Pharyngeal muscle contraction modifies peri-pharyngeal tissue pressure in rabbits. Respir Physiol Neurobiol 166: 95–101, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi I, Perry A, Rhymer J, Wuyam B, Hughes P, Murphy K, Innes JA, McIvor J, Cheesman AD, Guz A. Inspiratory coactivation of the genioglossus enlarges retroglossal space in laryngectomized humans. J Appl Physiol 80: 1595–1604, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Lo Y, Jordan AS, Malhotra A, Wellman A, Heinzer RC, Schory K, Dover L, Fogel RB, White DP. Genioglossal muscle response to CO2 stimulation during NREM sleep. Sleep 29: 470–477, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marshall NS, Yee BJ, Desai AV, Buchanan PR, Wong KK, Crompton R, Melehan KL, Zack N, Rao SG, Gendreau RM, Kranzler J, Grunstein RR. Two randomized placebo-controlled trials to evaluate the efficacy and tolerability of mirtazapine for the treatment of obstructive sleep apnea. Sleep 31: 824–831, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oliven A, O'Hearn DJ, Boudewyns A, Odeh M, De Backer W, van de Heyning P, Smith PL, Eisele DW, Allan L, Schneider H, Testerman R, Schwartz AR. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol 95: 2023–2029., 2003 [DOI] [PubMed] [Google Scholar]
- 20. Oliven A, Odeh M. Effect of positional changes of anatomic structures on upper airway dilating muscle shortening during electro- and chemostimulation. J Appl Physiol 101: 745–751, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Oliven R, Tov N, Odeh M, Gaitini L, Steinfeld U, Schwartz AR, Oliven A. Interacting effects of genioglossus stimulation and mandibular advancement in sleep apnea. J Appl Physiol 106: 1668–1673, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Olson LG, Strohl KP. Non-muscular factors in upper airway patency in the rabbit. Respir Physiol 71: 147–155, 1988 [DOI] [PubMed] [Google Scholar]
- 23. Rowley JA, Permutt S, Willey S, Smith PL, Schwartz AR. Effect of tracheal and tongue displacement on upper airway airflow dynamics. J Appl Physiol 80: 2171–2178, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Sforza E, Petiau C, Weiss T, Thibault A, Krieger J. Pharyngeal critical pressure in patients with obstructive sleep apnea syndrome. Clinical implications. Am J Respir Crit Care Med 159: 149–157, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Stadler DL, Catcheside PG, Paul D, Bradley J, McEvoy RD. Changes in lung volume and upper airway dilator muscle activity at sleep onset in obese male obstructive sleep apnea patients [Abstract]. Am J Respir Crit Care Med 179: A5405, 2009 [Google Scholar]
- 26. Stanchina ML, Malhotra A, Fogel RB, Trinder J, Edwards JK, Schory K, White DP. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep 26: 851–856, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Su MC, Chiu KL, Ruttanaumpawan P, Shiota S, Yumino D, Redolfi S, Haight JS, Bradley TD. Lower body positive pressure increases upper airway collapsibility in healthy subjects. Respir Physiol Neurobiol 161: 306–312, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Tagaito Y, Isono S, Remmers JE, Tanaka A, Nishino T. Lung volume and collapsibility of the passive pharynx in patients with sleep-disordered breathing. J Appl Physiol 103: 1379–1385, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol 65: 2124–2131, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol 85: 908–920, 1998 [DOI] [PubMed] [Google Scholar]