Abstract
Elastic tendons can act as muscle power amplifiers or energy-conserving springs during locomotion. We used an in situ muscle-tendon preparation to examine the mechanical function of tendons during lengthening contractions, when muscles absorb energy. Force, length, and power were measured in the lateral gastrocnemius muscle of wild turkeys. Sonomicrometry was used to measure muscle fascicle length independently from muscle-tendon unit (MTU) length, as measured by a muscle lever system (servomotor). A series of ramp stretches of varying velocities was applied to the MTU in fully activated muscles. Fascicle length changes were decoupled from length changes imposed on the MTU by the servomotor. Under most conditions, muscle fascicles shortened on average, while the MTU lengthened. Energy input to the MTU during the fastest lengthenings was −54.4 J/kg, while estimated work input to the muscle fascicles during this period was only −11.24 J/kg. This discrepancy indicates that energy was first absorbed by elastic elements, then released to do work on muscle fascicles after the lengthening phase of the contraction. The temporary storage of energy by elastic elements also resulted in a significant attenuation of power input to the muscle fascicles. At the fastest lengthening rates, peak instantaneous power input to the MTU reached −2,143.9 W/kg, while peak power input to the fascicles was only −557.6 W/kg. These results demonstrate that tendons may act as mechanical buffers by limiting peak muscle forces, lengthening rates, and power inputs during energy-absorbing contractions.
Keywords: muscle lengthening, muscle damage, energy absorption
skeletal muscles transmit forces via tendons that behave mechanically like springs. For a number of locomotor activities, the importance of this springlike function is well known. During jumping, tendons can act as power amplifiers, storing the energy of muscular work slowly, then releasing it quickly to power a rapid increase in the body's kinetic energy (1, 7, 27). Tendons play a different role in walking and running, where fluctuations in mechanical energy are recycled via elastic energy storage and recovery to reduce muscular work and improve metabolic economy (12, 28). These mechanisms are familiar to most biomechanists, yet the significance of tendon function undoubtedly extends well beyond these “showcase” examples. Tendons stretch and store energy whenever muscles produce force, and their elastic behavior affects the pattern of muscle length change for virtually all contractions. Thus it is likely that, for many activities, the functional significance of the springlike behavior of tendons remains to be described.
One activity for which the role of series elastic elements is relatively unexplored is energy absorption. Muscles absorb (dissipate) mechanical energy by producing force while lengthening, thus providing a controlled means of reducing the kinetic or potential energy of the body. This function is important for activities such as deceleration, downhill running, or when landing from a jump. Energy absorption by muscles and tendons is also a part of the cyclic exchange of energy that occurs during walking and running (12, 32).
The role of tendons in energy-absorbing activities remains unclear. One potentially important role was observed in recent ultrasound measurements of fascicle movements during eccentric contractions in humans. Reeves and Narici (26) tracked fascicle length changes in the tibialis anterior while applying an eccentric load via an ankle dynamometer. Results from this study showed that, regardless of the rate of muscle-tendon unit (MTU) lengthening, muscle fascicles remained nearly isometric. The results suggested that most of the length change during lengthening was accommodated by the series elastic tendon. Using an isolated muscle preparation in cats, Griffiths (15) found that muscle fascicles actually shortened during contractions in which the MTU was lengthened simultaneous with activation. This mechanical behavior suggests a possible protective mechanism of tendon, as the damaging effects of eccentric contractions are well established (21, 25). Thus, for eccentric contractions, the springlike function of tendons may serve as a “mechanical buffer” (15, 26).
The goal of the present study was to follow the flow of mechanical energy in an isolated MTU to investigate the role of the tendon in eccentric contractions. The idea that a tendon can act as a mechanical buffer by limiting muscle fascicle lengthening during eccentric contractions is appealing, but incomplete. In any energy absorbing contraction, muscle fascicles must ultimately forcefully lengthen to dissipate energy. Tendons can store energy temporarily, but they cannot dissipate it. We hypothesized that the benefit of tendon stretch in an energy-absorbing contraction rests in tendons’ capacity to decouple the timing of muscle work from the mechanical event and reduce the peak rate of energy input to muscle fascicles. We predicted that, in an energy-absorbing contraction, energy stored rapidly in tendon during a muscle stretch is then released to do work on muscle fibers at a rate slower than the initial rate of energy absorption by the MTU.
We used an in situ muscle preparation to determine the effect of series elastic elements on the rate of lengthening and energy absorption in muscle fascicles during an imposed lengthening. The lateral gastrocnemius muscle of wild turkeys provides a good system for in situ studies (4, 24) and can ultimately provide information on muscle length change and force output in vivo (14, 28). We instrumented the lateral gastrocnemius with sonomicrometer transducers, to measure fascicle length, and attached the free tendon of the muscle to a servomotor, to measure MTU length and force. We imposed a series of stretches that varied in velocity. The timing of these contractions was chosen so that the MTU underwent lengthening during muscle activation, followed by an isometric period during which force declined. This pattern was chosen to approximate the expected pattern of length change and activation for a high-power, energy-absorbing activity, such as landing from a jump. We tested several hypotheses related to the possible effects of tendon elasticity in eccentric contractions. First, we hypothesized that, during the period of MTU stretch, the rate of fascicle lengthening would be lower than that of the MTU. Our second hypothesis was that the reduced rate of fascicle lengthening, relative to the MTU, would reduce peak forces and rates of force rise in a contraction. This hypothesis is based on the observation that the rate of force rise in a contraction is dependent on the length trajectory of the fascicles, due to both force-velocity effects as well as activation/deactivation processes (11, 13). Our third hypothesis was motivated by the idea that tendons can act as power attenuators. We hypothesized that peak power input to the MTU would be greater than the peak power input to the muscle fascicles.
METHODS
Adult female wild turkeys, Meleagris gallopavo, were obtained from a licensed breeder. Animals were housed in an enclosed space in the Brown University Animal Care Facility and maintained on a commercial diet. Food and water were provided ad libitum. All animal procedures were performed with the approval of the Brown University Institutional Animal Care and Use Committee.
Surgery and muscle instrumentation.
All procedures were carried out when animals were deeply anesthetized with inhalable isoflurane, 1.5–3%. Animals were warmed by a heating pad and a heat lamp, and body temperature was monitored with a thermocouple probe.
The in situ muscle preparation was similar to previously reported procedures (24). The lateral gastrocnemius muscle was stimulated via a stimulating electrode cuff on the tibial branch of the sciatic nerve. The nerve was isolated, and surrounding tissues were removed by careful dissection. The nerve cuff was fabricated from flexible tubing that surrounded the nerve, placing it in contact with two silver wire electrodes mounted in the tubing. Electrodes were positioned ∼1 mm apart. Once the nerve cuff was in position, the nerve was severed proximal to the cuff. A small sheet of Teflon was folded around the cuff to prevent conduction of stimuli to surrounding tissues. Mineral oil was added to the area before skin incision closure to help maintain electrical isolation of the nerve cuff.
Servomotor measurements of MTU force and length required a secure connection between the distal tendon and the servomotor lever, as well as a rigid anchor for the proximal attachment (origin) of the muscle. The tendon of the lateral gastrocnemius was severed and attached to the servomotor lever with a light-weight, custom-fabricated clamp and light-weight wire rope (aircraft cable). Affixing the clamp to the ossified region of the distal tendon provided a connection that was rigid and avoided problems of slipping or tearing that are common with soft tendon clamping. To anchor the muscle's origin, the femur was fixed in position by one of two methods. For some experiments, two stainless steel screws were used to attach a robust aluminum plate to the femur. The screws were mounted in the femur and firmly tightened to the aluminum plate using nuts. For two of the experiments, the femur was gripped firmly in two large stainless steel clamps. In either case, the clamps or aluminum plate were then affixed to a large, custom-fabricated aluminum and steel frame. This robust and rigid frame also provided the mount for the servomotor. The compliance of the entire system was determined by clamping a 1-cm thick aluminum bar between the tendon clamp and the stainless steel clamps used to mount the femur. Force and length were then measured by the servomotor system, while the lever was moved. The measured compliance was <0.01 mm/N.
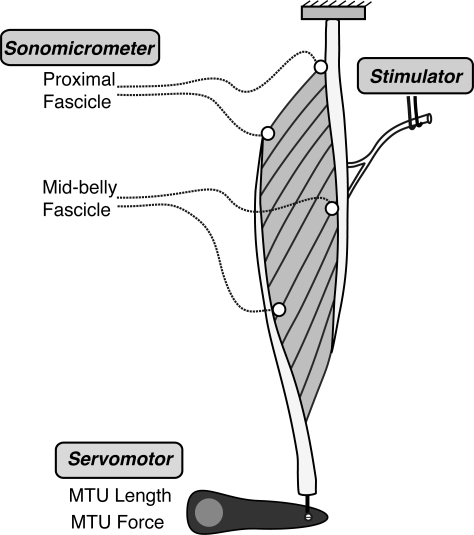
Sonomicrometer measurements were used to determine length changes in muscle fascicles, as distinct from the MTU length changes measured by the servomotor. Two pairs of small, 2-mm sonomicrometer crystals (Sonometrics, London, Ontario, CA) were placed along fascicles at two locations in the lateral gastrocnemius (Fig. 1). The first pair was placed along proximal fascicles, where the course of superficial fascicles can be easily visualized. This approach ensures good alignment of crystals along the fascicle trajectory. Overlying fascia and epimysium were carefully dissected away along a fascicle. Near the proximal end of the fascicle, an incision ∼2 mm long and 2 mm deep was made with a sapphire knife. The sonomicrometer crystal was inserted into this small hole and secured in place with a small drop of Vetbond adhesive. This procedure was repeated at the distal end of the same fascicle. Another pair of crystals was inserted at approximately the midbelly of the muscle. For these fascicles, one crystal was inserted in the superficial aspect of the muscle, and one crystal was inserted in the deep aspect of the muscle. The gastrocnemius is pennate, and it was impossible to visualize the course of the fascicles in the midbelly. To locate crystal pairs along the fascicle axis, the position of the deep crystal was determined from the position of the superficially placed crystal and the known length of the fascicles (as measured in the proximal part of the muscle; fascicle lengths are relatively uniform throughout the gastrocnemius). Once crystals were in place, leads were routed to the exterior and connected to the sonomicrometry equipment (Fig. 1).
Fig. 1.
Schematic of the in situ muscle preparation. The lateral gastrocnemius was instrumented with two pairs of sonomicrometer crystals to measure muscle fascicle length changes. Muscle-tendon unit (MTU) length changes were measured with a muscle servomotor system. The muscle servomotor applied ramp lengthenings to the muscle simultaneously with maximal stimulation via the tibial nerve.
Measurements, contraction protocol, and analysis.
Measurements of MTU length changes and force were taken with a servomotor-based muscle lever system (Model 310B-LR, Aurora Scientific, London, Ontario). Fascicle length measurements from the proximal and midbelly pairs of crystals were recorded with a sonomicrometer system (Sonometrics, Ontario, CA). Signals from the muscle servomotor and sonomicrometer system were recorded to a computer with a 16-bit analog-to-digital converter (PCI-MIO-16, National Instruments, Austin, TX), controlled by Labview software (National Instruments). The stimulus signal was also recorded. The analog-to-digital board and Labview software were used to generate the signal that controlled muscle servomotor position during eccentric contraction protocols. Signals were sampled at 1,000 Hz.
Before recordings of eccentric contractions, the stimulus voltage and muscle twitch length-tension curves were determined. The nerve was stimulated with a Grass S48 stimulator operating with a stimulus duration of 0.2 ms and, for tetanic contractions, a stimulus frequency of 100 pulses/s. The voltage necessary to achieve maximal stimulation was determined with a series of twitches over increasing voltages. Stimulus voltage was increased in 1-V increments until there was no further increase in twitch force with increase in voltage. The voltage at which there was no increase in force from the previous twitch was selected as the stimulus voltage for the experiment (this supramaximal stimulus voltage ranged from 3 to 5 V). Once supramaximal stimulus voltage was identified, the muscle's length-tension curve was characterized. Force, from the servomotor, and length, from the sonomicrometer crystals, were recorded over a series of twitches at different initial muscle lengths. A passive and active length-tension relationship was determined for each muscle, to determine lengths over which to make eccentric contraction measurements. All contractions were begun at lengths just below the muscle's optimal length for force production (Lo). The operating lengths of the muscles over all of the contraction conditions ranged from 0.75 Lo to 1.1 Lo.
For eccentric contractions, a constant velocity ramp lengthening was applied to the muscle-tendon by the servomotor. The primary independent variable of interest was MTU velocity. To get a range of velocities, the duration of the ramp lengthening was fixed, and the amplitude varied, for most contractions (Table 1). For most lengthening events, the ramp duration was 100 ms. The maximum rate of lengthening was ultimately limited by the maximum excursion range of the servomotor. To get faster rates of lengthening at the limit of the ergometer excursion, the ramp duration was decreased, to 80 and 70 ms for the second-fastest and fastest contractions, respectively (Table 1). The onset of stimulation was simultaneous with the start of the ramp lengthening. This timing was meant to mimic eccentric activities during locomotion, which often involve the near simultaneous onset of muscle activation and MTU lengthening (e.g., joint flexion and muscle activation both begin at toe-down in a jump landing). Stimulus duration was fixed at 50 ms for all contractions. This duration was chosen because it resulted in an approximate match between the duration of the period of force rise and the duration of the ramp lengthening (given typical muscle activation/deactivation dynamics, force typically continues to rise for 30–50 ms after the end of stimulation).
Table 1.
Range of contraction conditions used
| Lever Excursion, mm | Lever Excursion, %Lo | Ramp Duration, ms | Velocity, mm/s | Velocity, Lo/s |
|---|---|---|---|---|
| −0.5 | −2.0 | 100 | −5 | −0.2 |
| −1 | −3.9 | 100 | −10 | −0.4 |
| −2 | −7.8 | 100 | −20 | −0.8 |
| −4 | −15.7 | 100 | −40 | −1.6 |
| −6 | −23.5 | 100 | −60 | −2.4 |
| −8 | −31.4 | 100 | −80 | −3.1 |
| −8 | −31.4 | 80 | −100 | −3.9 |
| −8 | −31.4 | 70 | −114 | −4.5 |
Negative values indicate lengthening applied to the muscle-tendon. Lo, optimal length.
All data analysis was performed in the software program Igor Pro (Wavemetrics, Lake Oswego, OR). High-frequency components of recorded position signals from the muscle servomotor and sonomicrometer signals were removed with a quintic spline interpolation (SD = 0.01). This routine applied smoothing approximately equivalent to a 55-Hz finite-impulse response filter and removed only high-frequency components of the signal. MTU velocity and muscle fascicle velocities were calculated by differentiating the servomotor position and sonomicrometer length signals, respectively. Values reported for MTU velocity are the average velocity recorded over the period of MTU lengthening. All velocities are reported as negative for lengthening and positive for shortening.
Muscle fascicle power was calculated as the product of muscle fascicle velocity, as measured by sonomicrometry, and MTU force, as measured by the servomotor system. This value was divided by muscle mass to obtain the muscle-mass-specific value. Muscle fascicle power was calculated as the product of muscle fascicle velocity and force measured by the servomotor system, which assumes that velocities of measured fascicles are representative of all of the muscle fascicles. Calculating power from the product of servomotor force and fascicle velocity will result in an underestimate of muscle fascicle power, because, in a pennate muscle, the total force at the level of fascicles exceeds the force measured at the tendon. This effect is likely variable, depending on force (4). Data from shortening muscles indicate that, at very high levels of force, the effective gearing of muscle fascicles in this muscle is close to unity (4). For this reason, no attempt was made to correct for the effect of fascicle pennation on power calculations. Work, or energy absorbed, was calculated by taking the time integral of power over the time period of interest.
For some analyses, the contraction was divided into different periods to calculate variables of interest. Average muscle velocities were calculated from the beginning of muscle stimulation to the point of peak force, because it is only during this period that the fascicle length patterns can affect the force level reached. Work absorbed by the muscle fascicles was also calculated for this period, to determine the influence of work absorbed by the tendon on the work absorbed by the fascicles during the initial part of the contraction. To determine the peak rate of fascicle lengthening due to tendon recoil, and the peak power release from the tendon to the muscle, we analyzed the period of the contraction following MTU lengthening, when the MTU was isometric. A pair-wise t-test was used to compare the velocity, power, and work of the MTU to that of the fascicles across the range of contraction velocities.
RESULTS
Peak force output of the lateral gastrocnemius, measured during isometric tetanus, was 279.2 ± 30.6 N, which corresponded to a peak stress of 33.8 ± 2.29 N/cm2. The average resting length (Lo) measured for proximal fibers was 25.5 ± 1.8 mm, and average muscle mass was 23.6 ± 1.4 g.
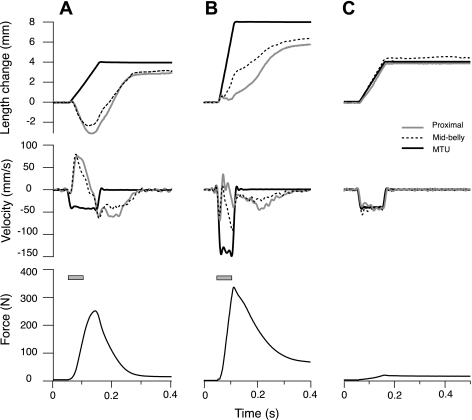
Fascicle length change patterns during eccentric contractions were qualitatively different from the length change imposed on the MTU by the servomotor (note: the term eccentric contraction is used here to refer to a contraction in which there is net lengthening and energy absorption for the MTU and fascicles over the course of the entire contraction, even if there are periods of instantaneous fascicle isometry or shortening). Representative contractions illustrate the typical pattern (Fig. 2). Immediately on muscle stimulation, an isovelocity lengthening ramp was imposed on the MTU by the servomotor. During the first part of MTU lengthening, fascicles shortened or lengthened slowly. Fascicle lengthening and negative fascicle velocities occurred later in the contraction, during the period of force decline. In active contractions, most of the fascicle lengthening occurred when the MTU was isometric (Fig. 2). Length measurements were validated with passive stretches, where agreement between fascicle length changes and MTU length changes were observed (Fig. 2C). Forces are low in passive stretches; thus tendon length changes are negligible and fascicle length changes track MTU length changes.
Fig. 2.
Sample contractions demonstrate the relationship between MTU and fascicle length changes. Active contractions at intermediate (A) and fast MTU lengthening rates (B) demonstrate the lack of correlation between MTU length changes (solid line) and length changes in the proximal (shaded line) and midbelly (dashed line) fascicles. Stimulation duration (shaded rectangle) was 50 ms for all active contractions. C: in contrast to the active conditions, we find good agreement between the length changes of the MTU and fascicles during constant-velocity passive stretches, where forces are too low to significantly stretch series elastic structures.
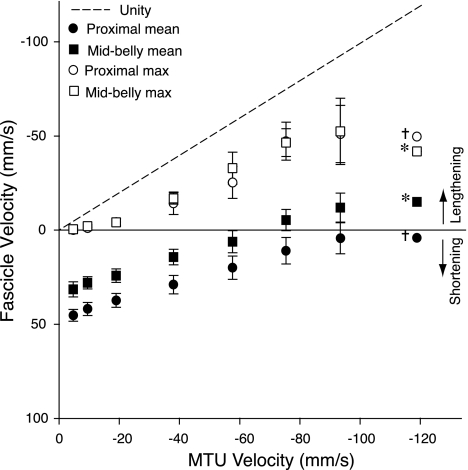
Over a range of lengthening velocities, MTU lengthening was largely decoupled from lengthening of the muscle fascicles. Figure 3 shows the average velocities as well as peak instantaneous lengthening velocities of the muscle fascicles during the force-rise period of the lengthening contraction, when the MTU underwent isovelocity lengthening. If series elasticity or fiber pennation had no effect, the fascicle velocity would be equal to the MTU velocity applied by the servomotor, as indicated by the dotted line in Fig. 3. Average fascicle velocities for both the proximal (P < 0.001) and middle region (P < 0.001) of the muscle during force rise were significantly different from the muscle-tendon velocity. Under most conditions, fascicles shortened, on average, while the MTU lengthened. Fascicle shortening velocities were reduced with increasing MTU lengthening velocities. The peak rate of muscle fascicle lengthening measured instantaneously during the force rise period increased with increasing MTU lengthening velocity. Peak instantaneous lengthening rates were closer to the MTU lengthening rate, but were still significantly lower in both the proximal (P = 0.003) and middle region (P = 0.006).
Fig. 3.
The average velocity of the muscle fibers (solid symbols) was much lower than the velocity applied to the MTU by the servomotor during the force rise period of the contraction. The line of unity (dashed) represents the fascicle velocity expected, if there were no effects of series elasticity or fiber pennation. The effects of series elastic elements are apparent in that, under most conditions, fibers shortened on average, while the MTU was lengthened. Peak velocities (open symbols) experienced during lengthening were higher than average velocities, but still well below the velocity applied to the MTU. Values are means ± SE; N = 5, unless otherwise noted (†N = 3; *N = 2).
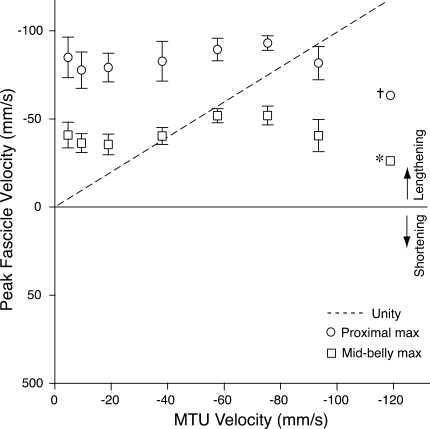
Over the course of an entire contraction, lengthening applied to the MTU must ultimately result in lengthening of muscle fascicles. In the contractions studied here, most of the fascicle lengthening occurred during the period following MTU lengthening, when the MTU was isometric (e.g., Fig. 2). Fascicle lengthening measured when the MTU was isomeric was the result of series elastic recoil. While fascicle velocity during MTU lengthening was lower (or opposite in sign) than the MTU velocity, fascicle velocity during the period following MTU lengthening was often greater than the velocity initially imposed by the servomotor. This trend is illustrated by the peak instantaneous lengthening velocities measured during the MTU isometric period of the contraction (Fig. 4). The most conspicuous feature of these data is that the peak rate of lengthening of the muscle fascicles is independent of the initial rate of lengthening applied to the MTU. Therefore, no significant relationship was found between peak MTU velocity and peak instantaneous velocity for either the proximal (P = 0.44) or midbelly (P = 0.78) fascicles. As a result, peak fascicle lengthening velocity exceeds MTU lengthening velocity at slower rates of lengthening and falls below the MTU lengthening rate for the more rapid lengthenings.
Fig. 4.
The maximum velocity of fascicle lengthening during the postlengthening, isometric portion of the contraction was relatively consistent across conditions, independent of the initial lengthening velocity applied to the MTU. Fascicle lengthening during this period results from the recoil of the series elastic element. For the contractions with the fastest rates of MTU lengthening, the maximum rate of fascicle lengthening was lower than the MTU lengthening rate. The line of unity (fascicle velocity = prior MTU lengthening velocity; dashed line) is provided for reference. Values are means ± SE; N = 5, unless otherwise noted (†N = 3; *N = 2).
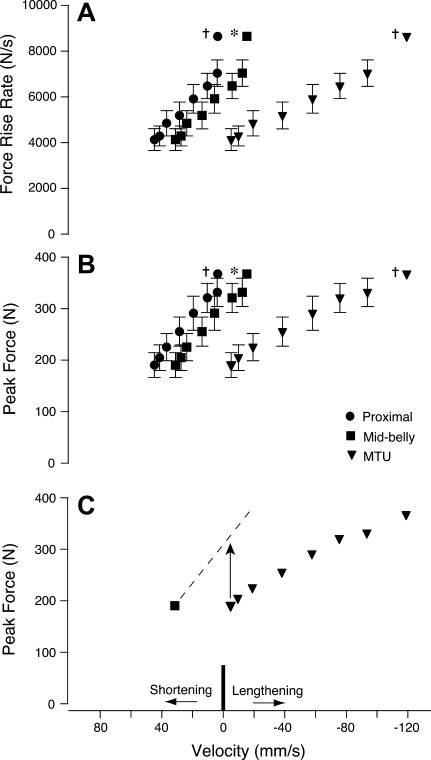
The force developed in a contraction was dependent on the rate of lengthening applied to the MTU (Fig. 5). Force was developed more rapidly (Fig. 5A), and higher peak forces were developed (Fig. 5B) with increasing MTU lengthening velocity. Because under most conditions fascicles shortened during the period of force rise, the increase in force output resulted from a decreasing average shortening velocity at the level of muscle fascicles. Higher forces and more rapid rates of force development occurred as average fascicle velocity decreased, from significant shortening (positive) velocities during slow MTU lengthenings to near isometric (on average) contractions for rapid MTU lengthenings. These data show the influence of series elasticity on the peak force developed in a lengthening contraction (Fig. 5C). A comparison of the force output when fascicles were near isometric and the force output at the slowest rate of MTU lengthening illustrates that the decoupling of fascicle and MTU velocity has a significant effect on force in these contractions. This comparison indicates that, if the rate of lengthening of the MTU had been applied directly to the fascicles, the slowest lengthening condition would have resulted in substantially higher forces (Fig. 5C).
Fig. 5.
Force increased at a much faster maximum rate (A) and reached a higher peak force (B) as the velocity of lengthening of the MTU (▾) increased. Force as a function of fascicle velocity, averaged over the period of force rise, for the same contractions is indicated for proximal fascicles (●) and midbelly fascicles (■). C: the effect of series elasticity on the peak force output in lengthening contractions can be represented as the difference between the force developed at a slow lengthening of the MTU and the force developed when the same lengthening velocity occurs in the fascicles (vertical arrow). Values are means ± SE; N = 5, unless otherwise noted (†N = 3; *N = 2).
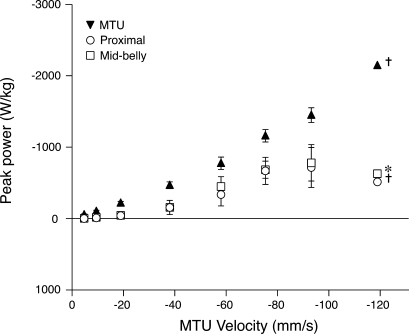
The lengthening contractions studied here resulted in a substantial rate of energy absorption by the MTU (Fig. 6). The peak negative power (i.e., rate of energy input) for the MTU under the fastest lengthening condition was −2,143.9 W/kg muscle. For reference, the peak isotonic power output of the turkey lateral gastrocnemius has been measured at 341.7 W/kg (24). To determine the influence of tendon elasticity on muscle fascicle power, we calculated peak power input during the force rise period of the contraction, when series elastic elements stretch and absorb energy. The peak power inputs, as measured from both the proximal (P = 0.015) and midbelly (P = 0.016) fascicles, were significantly lower than that of the MTU during force rise. The peak power input calculated from values for proximal fascicles length changes reached a value of −557.6 ± 57.0 W/kg at MTU lengthening rates of 75 mm/s and did not change significantly from this value for the two faster lengthening conditions. For the fastest rate of lengthening, the maximum rate of energy input to the MTU was −2,143.9 W/kg, more than three times the maximum rate of energy input to muscle fascicles.
Fig. 6.
Peak power input to the MTU (▾), and peak power input to the muscle fascicles as calculated from midbelly (□) and proximal (○) fascicle velocities during the period of force rise. Values were measured from the peak instantaneous power that occurred between the onset of activation and the time of peak force. The peak rate of energy absorption was much higher in the MTU compared with the muscle fascicles (as calculated from fascicle velocities), indicating that rapid energy absorption during force rise was accommodated by elastic elements. Units of power are watts per kilogram of muscle. Values are means ± SE; N = 5, unless otherwise noted (†N = 3; *N = 2).
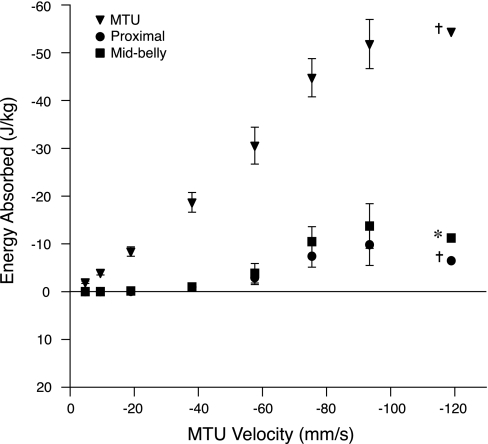
The total energy absorbed by the muscle over the course of an entire contraction (from the beginning of active force production to the end) can be measured from the work done by the muscle servomotor. Total energy absorbed in the contractions studied here increased linearly with MTU lengthening velocity, and it was substantial at rapid rates of lengthening (Fig. 7). Nearly all of this energy must ultimately be dissipated by muscle fascicles, as energy stored in series elastic elements is released, with very little loss, over the course of a contraction. Most of this energy was dissipated by fascicles during the period when the MTU was isometric. The negative work done by both the proximal (P = 0.005) and midbelly (P = 0.0035) fascicles during the force rise period of muscle lengthening was significantly lower than the MTU. Differences between calculated work values in the MTU and muscle belly (calculated from fascicles) result from the stretch of tendon and also possibly the effects of fiber pennation.
Fig. 7.
Very little of the work done by the servomotor on the MTU (▾) was absorbed by muscle fascicles during the force-rise portion of the contraction. Negative work in the muscle as calculated from proximal (●) and midbelly (■) fascicles during the period of force rise was nonexistent at the slow lengthening velocities, and it represented only a small fraction of the servomotor work at the higher lengthening velocities. The difference between MTU work and calculated fascicle work approximates the energy stored in elastic elements during force rise. This energy is released to do work on muscle fascicles during the second half of the contraction. Work units are Joules per kilogram muscle. Values are means ± SE; N = 5, unless otherwise noted (†N = 3; *N = 2).
DISCUSSION
Tendons as power attenuators.
Our results support the hypothesis that series elastic tendons can act as power attenuators during eccentric contractions. In the contractions studied here, the shuttling of energy through tendon allowed active muscle fascicles to absorb energy at a rate that was lower than the maximum power input to the MTU. For the fastest lengthenings, the maximum rate of energy absorption into the MTU was more than three times the maximum rate measured in muscle fascicles (Fig. 6). The trajectory of the data suggests that this discrepancy would have been even greater at higher rates of lengthening. Thus it seems that the tendons may allow muscle tendons to absorb energy very rapidly, even if the contractile elements’ maximum capacity for energy absorption is limited.
How rapidly can a muscle absorb mechanical energy? The maximum rate of energy production in muscle has been the focus of many studies, as it is considered a defining contractile property and a key determinant of locomotor performance (17, 18, 29, 30). By contrast, to our knowledge, the maximum performance of skeletal muscle as an energy absorber is unknown. Yet energy absorbing activities in terrestrial movement are arguably as common as energy-producing activities, and the maximum performance for many activities, such as deceleration or maneuvering, may, in part, be determined by muscle's energy-absorbing capacity. The scarcity of studies of the range of muscle performance during lengthening contractions may, in part, be due to a dominant view of muscles as motors for producing mechanical energy. Furthermore, the behavior of muscle on the lengthening side of the force-velocity relationship is more difficult to define, as it is not as deterministic as the behavior on the shortening side (19). Behavior of muscle during lengthening is also difficult to study (23) because lengthening (eccentric) contractions cause muscle damage (25). It is from studies of eccentric muscle damage that values can be gleaned for peak performance of muscle as an energy absorber. Some reported values are quite high. Brooks and Faulkner (9) measured ∼8,000 W/kg input into mouse extensor digitorum longus during rapid lengthening. This value is high relative to this muscle's peak rate of power output (164 W/kg; Ref. 8). The results from the present study suggest that instantaneous power measurements for muscles, such as the extensor digitorum longus, must be viewed cautiously, as they may reflect the behavior of tendon and not the contractile element. Although it has not been well characterized, skeletal muscles must have an intrinsic power input limit, and tendons may allow MTUs to operate instantaneously beyond this limit.
Limitations of the in situ muscle measurements.
Our calculations of power at the level of the fascicles are subject to some inaccuracies due to the fact that they were calculated from lengths measured at the level of fascicles and forces measured at the tendon. Due to the effects of muscle fiber pennation, the forces at the level of the fascicles will be greater than force at the tendon, as measured by the muscle servomotor system. For this reason, our calculations of power and work at the level of the fascicles are likely underestimates. Some of the discrepancy between MTU power and calculated fascicle power is likely due to this effect of fiber pennation. Both measured values of the effects of pennation angle during shortening contractions (4) and calculations suggest that this effect should be relatively small, on the order of 10–20%. Nevertheless, it should be noted that, while our data indicate a clear role for series elastic elements in uncoupling fascicle mechanics from MTU mechanics, the effects of fascicle gearing resulting from their pennation angle likely explain some of the discrepancy between instantaneous MTU power values and powers calculated from fascicle measurements.
Measurements of muscle power and work also assume that the length changes measured along a group of fascicles are representative of the entire muscle. We measured fascicle length changes in two different regions of the muscle, and these measurements indicate that, under many conditions, there were significant differences in shortening velocity in different portions of the muscle. Variability in fiber strain and velocity has been observed in other muscles in both in situ preparations (2) and in vivo (16). There is, of course, only one value for the total power or work produced by the muscle belly, and our estimates of power output are subject to error due to observed regional heterogeneity in fascicle shortening, and unknown heterogeneity in fascicle stress. Nevertheless, the observation that the powers calculated from fascicle length changes in two different regions of the muscle are similar (Fig. 6) is reassuring in that these length changes are representative. Furthermore, the very large difference between MTU power and power calculated from muscle fascicles suggests that, while our estimate of muscle belly power is subject to error, this error is unlikely to affect the conclusion that MTU power input is greater than peak power input to the muscle fibers.
How applicable are measurements on turkey gastrocnemius to other muscles and other species? If turkey tendons were unusually compliant, our results might exaggerate the effects of tendon elasticity. We believe that our results actually tend to underestimate the effects of tendon elasticity in many muscles, as the amount of series elastic tendon in our in situ preparation is reduced from the muscles’ in vivo condition. The free tendon of the turkey gastrocnemius just distal to the aponeurosis is calcified and has material properties similar to that of bone (6). Because it provides a reliable attachment point, we attached the servomotor lever to the bony region of the tendon, thus eliminating some of the series elastic compliance distal to this region. As a result, the compliance in our preparation represents the elastic behavior of the aponeurosis. Values for turkey aponeurosis material properties fall within the range measured for other tendons (5). Thus we expect our results are relevant for muscles with significant aponeuroses, a category that includes most limb muscles.
In our study, little work was done directly by the servomotor on the muscle fascicles. Work done by the servomotor was stored by the elastic element, and active lengthening of fascicles resulted from the subsequent recoil of the tendon. More in vivo data will be required to know whether this shuttling of energy via the tendon is a common mechanism during energy-absorbing activities. Several characteristics of our lengthening protocol would tend to maximize the role of tendon in attenuating power absorption by the active muscle. In our contractions, the muscle was maximally activated, and the period of muscle activation corresponded to the period of lengthening. Reducing the level of muscle activity would have increased the amount of fascicle lengthening during the lengthening ramp, because lower forces would have resulted in less tendon stretch. Extending the period of lengthening beyond the period of muscle activation also would have likely led to more fascicle lengthening during MTU lengthening. An extended period of stretch would have resulted in a period of MTU lengthening during muscle relaxation. During relaxation, force falls and tendon recoil tends to stretch muscle fascicles. If relaxation coincided with MTU lengthening, both the tendon and the servomotor would act to lengthen fascicles. This would have resulted in lengthening velocities that actually exceeded that of the MTU (we observed this pattern for some of our contractions, when the lengthening time exceeded force-rise time). Just as the power amplification function of tendon works only under the appropriate patterns of load and activation (3, 27), the power-attenuating function of tendon is also likely to operate effectively only with the right combination of activation and loading. The pattern used in this study, where MTU lengthening begins simultaneous with activation, and continues during the period of force rise, appears to be particularly well-suited to the power attenuating function of tendons. We predict that rapid energy absorbing activities in vivo (e.g., landing from a jump) will be similar to the pattern used in this study.
Decoupling of MTU velocity and fascicle velocity during lengthening.
Our results supported the hypothesis that fascicle lengthening velocity would be lower than the rate of MTU lengthening velocity during a lengthening imposed simultaneous with the onset of muscle activation. The pattern of fascicle length change during MTU lengthening in our study is consistent with previous measurements of cat gastrocnemius (15) and human tibialis anterior (26). These researchers found that muscle fascicles remained “quasi-isometric” (26) or shortened (15) during contractions in which the MTU was lengthened, regardless of the rate of lengthening. Simulations of MTUs undergoing sinusoidal length changes also show relatively constant-length fibers during MTU lengthening (33). In our study, the fascicles shortened on average during MTU lengthening and were close to isometric for the fastest lengthening contractions. Why are muscles so resistant to active lengthening when arranged in series with a compliant tendon? The tendency of the contractile element to resist lengthening may result from the force-velocity behavior of muscle. Any tendency of MTU lengthening to stretch the contractile element will tend to increase muscle force output, which, in turn, stretches the series elastic element. Higher rates of lengthening result in higher muscle forces and more series elastic element lengthening. Thus the dynamic interaction of the tendon and muscle contractile element can make it very difficult to lengthen muscle fibers, particularly during activation. The extent to which tendons protect muscle fibers from initial lengthening is likely variable among muscles and may depend on the fiber architecture, relative tendon compliance, and fiber type of the muscle tendon.
Tendon power attenuation as a protective mechanism.
What is the physiological significance of the springlike function of tendons during eccentric contractions? Our results point to several features of tendon behavior that may tend to protect muscle contractile elements from damage due to eccentric loading. In evaluating the possible protective function of tendons, it is important to remember that tendons are very efficient springs and, therefore, have negligible capacity to dissipate energy (20). Any protection provided by tendons must result from their ability to alter the timing of muscle lengthening and work absorption, because ultimately nearly all (accounting for a small loss of energy due to <100% tendon efficiency) of the energy absorbed by the MTU must be dissipated by the muscle. In the contractions studied here, the work done by the servomotor on the MTU is absorbed by the muscle fascicles, but only after it is temporarily stored in tendons as elastic strain energy. Is there an advantage to having the tendon temporarily store energy and then do work on the muscle contractile element? Our results suggest three possible protective functions provided by this mechanism.
In our study, the action of the series elastic element resulted in fascicle lengthening velocities that were much reduced, or eliminated, in the period of the contraction when force was rising. Because the rate of force development in an active muscle fiber is tightly coupled to its length change (13), the tendency of the tendon to accommodate most of the length changes during activation can act to limit the peak force developed in the contraction. This was the basis for our hypothesis that tendon lengthening would tend to reduce rate of force rise and peak force in a contraction. This hypothesis was supported by results demonstrating a clear trend of increased force with increased fascicle lengthening rate (Fig. 5). In vivo, a reduction in peak forces may reduce the chances of muscle damage. The elastic mechanism observed in the present study may also provide protection to musculoskeletal structures beyond the sarcomere. Because the forces developed in active muscles ultimately determine the forces on bones, joints and ligaments, the tendency of elastic mechanisms to limit peak muscle forces in actively lengthening muscle may provide an important protective mechanism for the musculoskeletal system in instances where mechanical energy must be rapidly absorbed.
The temporary storage and release of energy in elastic elements may also provide a protective mechanism by limiting peak lengthening velocities of active muscle fibers. In our study, most of the muscle fascicle lengthening occurred during the period when the MTU was isometric, and tendon recoil did work on muscle fascicles. This provided a rate of loading on the fascicles that was ultimately limited and consistent from contraction to contraction, because it was a function of the relaxation kinetics of the muscle. If eccentric muscle damage is in some way correlated with rate of muscle lengthening, it is possible that the action of a series elastic power attenuator provides rates of lengthening that are predictable and within a safe threshold to avoid muscle damage. The potentially beneficial role of tendons in limiting muscle fiber lengthening strain and velocity has been demonstrated in forward dynamics simulations of hamstring muscles during the swing phase of sprinting. Thelen and coworkers (31) demonstrated that the peak rates of fiber lengthening, as well as fiber strains in the hamstrings, were reduced due to the stretch of simulated series elastic elements in late swing. They proposed that a reduction in the rate and magnitude of fiber lengthening may be an important protective mechanism for the injury-prone hamstring muscles (31).
While our study suggests several ways in which the effects of series elasticity may buffer the mechanical loading of active muscles, a challenge in interpreting the significance of these effects is that the mechanical factors that determine eccentric muscle damage are not fully understood (25). Some studies suggest that damage is related to peak forces or velocities (34), others suggest that it is strain amplitude, not force, that determines damage (22), and still others indicate that total work absorbed is a good predictor of muscle damage, as it is a measure that combines strain amplitude and force (10). Given that the action of the turkey gastrocnemius tendon reduced lengthening velocity, force, and power, it seems likely that at least one of these functions has a protective effect.
Conclusions.
During lengthening contractions, the action of series elastic tendon in the turkey gastrocnemius results in reduced peak forces, reduced fascicle lengthening velocities, and reduced peak power input to active muscle fascicles. Most of the energy absorbed by the muscle fascicles in these contractions was first stored by the rapid stretch of tendon. Peak power input to the tendon was, in many cases, much greater than the peak rate of energy input to muscle fascicles. We propose that this power-attenuating function of tendons during energy absorbing activities provides a protective mechanism and may allow muscles to operate beyond the mechanical bounds set by intrinsic muscle contractile properties.
GRANTS
This work was supported by National Institutes of Health Grants AR055295 to T. J. Roberts and F32AR054246 to E. Azizi.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Greg Sawicki and Greg Halenda for assistance with experiments. Nicolai Konow provided helpful comments on the manuscript.
REFERENCES
- 1. Aerts P. Vertical jumping in Galago senegalensis: the quest for an obligate mechanical power amplifier. Philos Trans R Soc Lond B Biol Sci 353: 1607–1620, 1997 [Google Scholar]
- 2. Ahn AN, Monti RJ, Biewener AA. In vivo and in vitro heterogeneity of segment length changes in the semimembranosus muscle of the toad. J Physiol 549: 877–888, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander RM. Leg design and jumping technique for humans, other vertebrates and insects. Philos Trans R Soc Lond B Biol Sci 28: 235–248, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Azizi E, Brainerd EL, Roberts TJ. Variable gearing in pennate muscles. Proc Natl Acad Sci U S A 105: 1745–1750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Azizi E, Halenda GM, Roberts TJ. Mechanical properties of the gastrocnemius aponeurosis in wild turkeys. Integr Comp Biol 49: 51–58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bennett MB, Stafford JA. Tensile properties of calcified and uncalcified avian tendons. J Zool Lond 214: 343–351, 1988 [Google Scholar]
- 7. Bobbert MF. Dependence of human squat jump performance on the series elastic compliance of the triceps surae: a simulation study. J Exp Biol 204: 533–542, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Brooks SV, Faulkner JA. Maximum and sustained power of extensor digitorum longus muscles from young, adult, and old mice. J Gerontol 46: B28–B33, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Brooks SV, Faulkner JA. Severity of contraction-induced injury is affected by velocity only during stretches of large strain. J Appl Physiol 91: 661–666, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Brooks SV, Zerba E, Faulkner JA. Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. J Physiol 488: 459–469, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown IE, Loeb GE. Measured and modeled properties of mammalian skeletal muscle. III. The effects of stimulus frequency on stretch-induced force enhancement and shortening-induced force depression. J Muscle Res Cell Motil 21: 21–31, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Cavagna GA, Saibene FP, Margaria R. Mechanical work in running. J Appl Physiol 19: 249–256, 1964 [DOI] [PubMed] [Google Scholar]
- 13. Edman KA, Josephson RK. Determinants of force rise time during isometric contraction of frog muscle fibres. J Physiol 580: 1007–1019, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gabaldon AM, Nelson FE, Roberts TJ. Relative shortening velocity in locomotor muscles: turkey ankle extensors operate at low V/Vmax. Am J Physiol Regul Integr Comp Physiol 294: R200–R210, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Griffiths RI. Shortening of muscle fibers during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J Physiol 436: 219–236, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higham TE, Biewener AA, Wakeling JM. Functional diversification within and between muscle synergists during locomotion. Biol Lett 4: 41–44, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill AV. The dimensions of animals and their muscular dynamics. Sci Prog 38: 209–230, 1950 [Google Scholar]
- 18. Josephson RK. Contraction dynamics and power output of skeletal muscle. Annu Rev Physiol 55: 527–546, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Josephson RK, Stokes DR. The force-velocity properties of a crustacean muscle during lengthening. J Exp Biol 202: 593–607, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Ker RF. Dynamic tensile properties of the plantaris tendon of sheep (Ovis aries). J Exp Biol 93: 283–302, 1981 [DOI] [PubMed] [Google Scholar]
- 21. Lieber RL, Friden J. Mechanisms of muscle injury gleaned from animal models. Am J Phys Med Rehabil 81: S70–S79, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Lieber RL, Fridén J. Muscle damage is not a function of muscle force but active muscle strain. J Appl Physiol 74: 520–526, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Lindstedt SL, LaStayo PC, Reich TE. When active muscles lengthen: properties and consequences of eccentric contractions. News Physiol Sci 16: 256–261, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Nelson FE, Gabaldon AM, Roberts TJ. Force-velocity properties of two avian hindlimb muscles. Comp Biochem Physiol A Mol Integr Physiol 137: 711–721, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol 537: 333–345, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reeves ND, Narici MV. Behavior of human muscle fascicles during shortening and lengthening contractions in vivo. J Appl Physiol 95: 1090–1096, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Roberts TJ, Marsh RL. Probing the limits to muscle-powered accelerations: lessons from jumping bullfrogs. J Exp Biol 206: 2567–2580, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Roberts TJ, Marsh RL, Weyand PG, Taylor CR. Muscular force in running turkeys: the economy of minimizing work. Science 275: 1113–1115, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Rome LC. Some advances in integrative muscle physiology. Comp Biochem Physiol B Biochem Mol Biol 120: 51–72, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Swoap SJ, Johnson TP, Josephson RK, Bennett AF. Temperature, muscle power output and limitations on burst locomotor performance of the lizard Dipsosaurus Dorsalis. J Exp Biol 174: 185–197, 1993 [Google Scholar]
- 31. Thelen DG, Chumanov ES, Best TM, Swanson SC, Heiderscheit BC. Simulation of biceps femoris musculotendon mechanics during the swing phase of sprinting. Med Sci Sports Exerc 37: 1931–1938, 2005 [DOI] [PubMed] [Google Scholar]
- 32. van Ingen Schenau GJ, Bobbert MF, de Haan A. Does elastic energy enhance work and efficiency in the stretch-shortening cycle. J Appl Biomech 13: 389–415, 1997 [Google Scholar]
- 33. van Leeuwen J. Muscle function in locomotion, In: Mechanics of Animal Locomotion, edited by Alexander RM. New York: Springer-Verlag, 1992 [Google Scholar]
- 34. Warren GL, Lowe DA, Hayes DA, Karwoski CJ, Prior BM, Armstrong RB. Excitation failure in eccentric contraction-induced injury of mouse soleus muscle. J Physiol 468: 487–499, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]