Abstract
Ventilatory long-term facilitation (vLTF) is a form of respiratory plasticity induced by acute intermittent hypoxia (AIH). Although vLTF has been reported in unanesthetized animals, little is known concerning the effects of vigilance state on vLTF expression. We hypothesized that AIH-induced vLTF is preferentially expressed in sleeping vs. awake male Lewis rats. Vigilance state was assessed in unanesthetized rats with chronically implanted EEG and nuchal EMG electrodes, while tidal volume, frequency, minute ventilation (V̇e), and CO2 production were measured via plethysmography, before, during, and after AIH (five 5-min episodes of 10.5% O2 separated by 5-min normoxic intervals), acute sustained hypoxia (25 min of 10.5% O2), or a sham protocol without hypoxia. Vigilance state was classified as quiet wakefulness (QW), light and deep non-rapid eye movement (NREM) sleep (l-NREM and d-NREM sleep, respectively), or rapid eye movement sleep. Ventilatory variables were normalized to pretreatment baseline values in the same vigilance state. During d-NREM sleep, vLTF was observed as a progressive increase in V̇e post-AIH (27 ± 5% average, 30–60 min post-AIH). In association, V̇e/V̇co2 (36 ± 2%), tidal volume (14 ± 2%), and frequency (7 ± 2%) were increased 30–60 min post-AIH during d-NREM sleep. vLTF was significant but less robust during l-NREM sleep, was minimal during QW, and was not observed following acute sustained hypoxia or sham protocols in any vigilance state. Thus, vLTF is state-dependent and pattern-sensitive in unanesthetized Lewis rats, with the greatest effects during d-NREM sleep. Although the physiological significance of vLTF is not clear, its greatest significance to ventilatory control is most likely during sleep.
Keywords: breathing, respiratory plasticity
acute intermittent hypoxia (AIH) elicits plasticity in respiratory motor control (34, 41), including long-term facilitation (LTF) of respiratory motor output (15, 32). LTF was originally described as a prolonged increase in respiratory-related discharge in the phrenic nerve (phrenic LTF) of anesthetized cats or rats that persists for minutes to hours following episodic stimulation of the carotid sinus nerve (16, 18, 29–31) or episodic hypoxia (4, 18). AIH also induces LTF of pulmonary ventilation [ventilatory LTF (vLTF)] in unanesthetized spontaneously breathing dogs (11), goats (45), ducks (33), rats (25–28, 38), and mice (21, 44). In general, vLTF in unanesthetized animals is more variable, requires more hypoxic episodes, and is of shorter duration than phrenic LTF in anesthetized preparations (for review see Ref. 32). Breathing frequency (f) often contributes more to vLTF in unanesthetized animals [vs. amplitude in anesthetized preparations (9)], raising some question as to whether vLTF and phrenic LTF share a common mechanism (41). At least some inconsistencies in vLTF expression can be attributed to differences in the experimental preparation and/or protocol, such as poikilocapnia vs. isocapnia (38), the prevailing arterial Pco2 during baseline conditions (17, 19), differences in vagal feedback (23), or details of the hypoxic exposure protocol (25, 40).
Vigilance state (i.e., wakefulness vs. sleep) might also play a critical role in vLTF expression. vLTF is not observed after ten 5-min hypoxic episodes in normal human subjects during wakefulness (20, 24) but is observed following the same AIH protocol in sleeping human subjects with inspiratory flow limitation (1–3) or following a more intense AIH protocol (∼15 <1-min hypoxic episodes) in normal sleeping human subjects (40). By contrast, vLTF is expressed in unanesthetized mice during wakefulness and non-rapid eye movement (NREM) sleep, but there are differences in the pattern of vLTF expression between vigilance states (44).
Our primary goal in the present study was to determine the impact of vigilance state on vLTF in unanesthetized rats, the species used most frequently in studies concerning cellular/synaptic mechanisms of phrenic LTF (for reviews see Refs. 15, 22, 32). Experiments were performed on Lewis rats, a strain with high constitutive phrenic LTF expression in anesthetized preparations (10). After acclimatization to the plethysmography chamber, ventilation was measured in rats exposed to AIH or acute sustained hypoxia (ASH) while vigilance state was monitored. Since rats cycle through vigilance states fairly rapidly, measurements of ventilation during quiet wakefulness (QW) and light NREM and deep NREM (l-NREM and d-NREM) sleep were measured in the same protocol for individual rats before and after hypoxic exposures during d-NREM sleep, thus allowing state-dependent comparisons of vLTF within the same rats. Vigilance state had a profound influence on vLTF expression, with robust vLTF and greater tidal volume (Vt) responses during d-NREM sleep than l-NREM sleep or QW. Thus, vLTF may be of greatest significance to ventilatory control during sleep or anesthesia.
METHODS
Experimental animals.
Twelve adult, male Lewis rats (Harlan) were studied. The Animal Care and Use Committee of the School of Veterinary Medicine, University of Wisconsin, approved all experimental procedures.
Surgical preparation.
At least 1 wk prior to initiation of an experimental protocol, rats were anesthetized with isoflurane in 100% O2. An EEG-nuchal EMG (EEG/nEMG) appliance was attached to the rat's head, and a temperature telemeter (Mini-Mitter, Sun River, OR) was inserted into the rat's peritoneal cavity under aseptic conditions.
EEG and nEMG electrodes were placed as described previously (46). Briefly, while the animals were in a stereotaxic apparatus, the muscles of the neck were exposed and displaced to allow skull access. Five 1/64th-in. holes were drilled for implantation of stainless steel screw EEG electrodes, which were soldered to Teflon-coated stainless steel wires (no. AS634, Cooner Wire, Chatsworth, CA). An exposed (1–2 mm) end of Teflon-coated stainless steel wire was sutured into a nuchal muscle with 4-0 silk. The wires were connected to a skullcap, which was securely mounted with dental acrylic. The wound was sutured closed around the skullcap. Skull electrodes were placed at the following coordinates: 1) two electrodes were placed 1 mm lateral to the midline and 1 and 4 mm rostral to lambda to enable recordings of hippocampal theta waves, 2) two electrodes were placed 4 mm lateral to midline and 1 and 4 mm rostral to lambda to enable recordings of cortical waves, and 3) one ground electrode was placed 2 mm rostral and 4 mm lateral to lambda.
After EEG and nEMG electrode implantation, a sterilized temperature transmitter (Mini-Mitter) was inserted into the peritoneal cavity to enable continuous measurements of body temperature. At the end of surgery, triple-antibiotic ointment was applied to incisions, and analgesic (buprenorphine) was administered (0.03 mg/kg sc) at 12-h intervals for 48 h postsurgery. Rats were visually monitored and weighed daily, and topical triple-antibiotic ointment was continued twice daily, as needed. Experiments were not initiated until rats resumed normal weight gain.
Ventilatory measurements.
Flow-through, whole body plethysmography was used to measure ventilation and metabolism, as described previously (38). Gas flowed through the chamber at 2 l/min (20.9% inspired O2, 100% humidity). Pressure changes relative to a reference chamber were measured (model PM15E, Statham Instruments, Hato Rey, Puerto Rico) and used to calculate f, Vt, and minute ventilation (V̇e). Rat body temperature was measured continuously with the telemeters and was used to calculate volumes, as described previously (38). Chamber temperature was 23.5–24.5°C. O2 and CO2 concentrations in the chamber inflow and outflow gases were continuously monitored [model FCX-MV, Fujikura, Tokyo, Japan (O2 sensor) and model LB-2, Beckman, Fullerton, CA (CO2 sensor)], and the rates of O2 consumption and CO2 production (V̇co2) were calculated at 10-min intervals.
Assessment of sleep-wake states.
Sleep-wake (vigilance) states were determined (before analysis of ventilation) by visual inspection of nEMG and EEG signals by an experienced analyst. Vigilance state was classified as follows: QW, l-NREM sleep, d-NREM sleep, or rapid eye movement (REM) sleep. Wakefulness was defined as a desynchronized, low-voltage, high-frequency EEG with large-amplitude EMG. Sniffing and other activity distorted the plethysmograph signals, and these records were not included in the analyses. d-NREM sleep was defined as a continuous, synchronized, low-frequency (≤2 Hz) EEG with an amplitude two to three times greater than observed in QW. Periods of l-NREM sleep occurred during transitions between wakefulness and d-NREM sleep. REM sleep was characterized by desynchronized, low-voltage, high-frequency EEG with the lowest observed EMG amplitude. Periods when significant artifacts were observed in the EEG/nEMG recordings (<3% of recording time), which made the sleep-wake state difficult to determine, were omitted from analysis.
Experimental design.
Once normal body mass increases resumed following surgery (>7 days), a sham experimental protocol was performed. At 7 AM on the experimental day, the rat was placed in the plethysmograph, loosely tethered to the EEG/nEMG recoding equipment, and allowed to acclimate. Pretreatment data acquisition began at ∼10 AM. After at least three bouts of d-NREM sleep were confirmed (∼2 h later), sham treatment commenced during a period of d-NREM sleep. The sham protocol involved alternating flow between two tanks, each containing 20.9% O2 (i.e., normoxia); gases were alternated using the pattern described for intermittent hypoxia (see below). Sham experiments acclimated rats to experimental procedures and demonstrated a lack of ventilatory drift during continuous normoxia.
At ≥6 days after the initial sham study, 10 of the original 12 rats were exposed to AIH or ASH on a random basis. After ≥6 days, 9 of these 10 rats were exposed to the opposite protocol (e.g., AIH rats were exposed to ASH). The major reason rats were excluded from subsequent studies was deterioration of the electrode skullcap; skullcap viability improved once housing was modified to minimize contact between the skullcap and protuberances in their home cage. The exclusion of data from those rats did not affect the conclusions from this study (data not shown).
As with the sham protocols, rats were placed in the plethysmograph at 7 AM; pretreatment data acquisition began at ∼10 AM. At least three bouts of d-NREM sleep were confirmed prior to initiation of hypoxic exposure protocols (∼2 h later). Hypoxia was always initiated during a d-NREM sleep cycle. Although rats generally aroused (i.e., QW) during the first hypoxic episode, arousals were less frequent in the second episode and were rarely observed in the third through fifth hypoxic episodes. The AIH protocol consisted of five 5-min episodes of 10.5% O2 interspersed with 5 min of 20.9% O2. ASH consisted of a continuous 25-min exposure to 10.5% O2, which is equal to the total cumulative duration of hypoxia during the AIH protocol.
Data analyses.
In each rat, data were grouped in 5-min periods during each sleep-wake state (QW and l-NREM, d-NREM, and REM sleep). Because minimal time (<10% of total) was spent in REM sleep, we do not report ventilatory data during this sleep state. Data obtained during the pretreatment period in each of the other vigilance states were used to calculate their respective baseline values. Posttreatment ventilatory data (V̇e, Vt, f, and V̇e/V̇co2) in each vigilance state were averaged from 0 to 20, 20 to 40, and 40 to 65 min posttreatment and then expressed as a ratio of the baseline specific to each vigilance state.
Data were analyzed by two-way ANOVA with a repeated-measures design and Fisher's least significant difference post hoc tests to make statistical inferences concerning individual differences among treatment groups (AIH, ASH, and sham protocols) in each vigilance state (d-NREM sleep, l-NREM sleep, and QW). Ventilatory measurements (V̇e, Vt, f, and V̇e/V̇co2) were compared among vigilance states during baseline conditions by one-way ANOVA followed by Fisher's least significant difference post hoc tests to compare individual groups. All data are expressed as means ± SE. Statistical significance was accepted as P < 0.05.
RESULTS
Sleep-wake architecture.
Table 1 summarizes the relative time spent in each vigilance state during normoxia before and after treatment protocols (AIH, ASH, and sham). The average length of the baseline period (132 ± 9, 126 ± 14, and 104 ± 10 min in AIH, ASH, and sham, respectively) differed with each animal, because hypoxic stimulation was always initiated during d-NREM sleep (some rats took longer than others to reach that sleep state). Time spent in active wakefulness (e.g., sniffing, moving) is not included in the analysis, since we could not make reliable ventilatory measurements when the rats were active. After AIH, the fraction of time in d-NREM and REM sleep increased, whereas QW time decreased (all P < 0.05). There were no significant changes in time spent in these vigilance states following ASH or sham protocols (P > 0.05), suggesting that sleep architecture may be sensitive to the pattern of hypoxic exposure. However, we are hesitant to draw firm conclusions from these results, since there were unexpected differences in d-NREM sleep time during pretreatment baseline conditions among groups (43 ± 6%, 37 ± 4%, and 28 ± 6% in sham, ASH, and AIH, respectively, P < 0.05).
Table 1.
Percentage of time spent in each vigilance state before and after treatment
| n | QW | l-NREM | d-NREM | REM | |
|---|---|---|---|---|---|
| AIH | 10 | ||||
| Pre | 46 ± 11 | 23 ± 7 | 28 ± 6 | 3 ± 1 | |
| Post | 29 ± 6* | 22 ± 7 | 42 ± 5* | 8 ± 2* | |
| ASH | 9 | ||||
| Pre | 44 ± 3 | 16 ± 5 | 37 ± 4 | 2 ± 1 | |
| Post | 37 ± 6 | 25 ± 10 | 29 ± 8 | 8 ± 5 | |
| Sham | 12 | ||||
| Pre | 35 ± 4 | 18 ± 6 | 43 ± 6 | 4 ± 2 | |
| Post | 44 ± 7 | 13 ± 3 | 37 ± 9 | 6 ± 1 |
Values are means ± SE. QW, quite wakefulness; l-NREM, transition between QW and deep non-rapid eye movement (NREM) sleep; d-NREM, deep non-REM sleep; AIH, acute intermittent hypoxia (five 5-min hypoxic episodes separated by 5-min normoxic intervals); ASH, acute sustained hypoxia (a single 25-min hypoxic exposure); sham, continuous normoxia for an equivalent duration. Each treatment protocol was separated by ≥6 days in the same rat. Period during prehypoxic stimulation differed with each animal (132 ± 9, 126 ± 14, and 104 ± 10 min for AIH, ASH, and sham, respectively), and period during posthypoxic stimulation in all protocols was 65 min.
Significantly different from Pre (P < 0.05).
Differences in ventilation among vigilance states.
Ventilation during pretreatment baseline conditions differed among vigilance states (Fig. 1). Specifically, V̇e was lower in d-NREM and REM sleep than in QW and l-NREM sleep (P < 0.05); V̇e was not different between d-NREM and REM sleep (P > 0.05). Decreases in Vt and f contributed to decreased V̇e during d-NREM and REM sleep relative to QW (both P < 0.05), whereas only decreases in f contributed to decreased V̇e during d-NREM sleep relative to l-NREM sleep (P < 0.05; Fig. 1). We were unable to determine whether V̇e differences among vigilance states were associated with changes in metabolic rate, since V̇co2 measurements were made in 10-min epochs because of design limitations in our experimental setup.
Fig. 1.
Absolute values of ventilation (V̇e) and its components tidal volume (Vt) and frequency (f) in each vigilance state: quiet wakefulness (QW), light and deep non-rapid eye movement (NREM) sleep (l-NREM and d-NREM sleep), and rapid eye movement (REM) sleep. Values were obtained during the ∼2-h normoxic period prior to treatments (i.e., baseline). Significant differences are indicated with brackets and associated P values. All ventilatory variables exhibited a similar pattern: QW > l-NREM > d-NREM = REM. Pretreatment ventilatory data were used as baseline measurements for comparison with posttreatment values.
Body temperature regulation.
Pretreatment body temperatures were similar on each test day (37.9°C). During AIH and ASH protocols, body temperature progressively declined, reaching a nadir 10–20 min posthypoxia. Peritoneal temperature reached a nadir ∼0.6°C below baseline values during AIH protocols and >0.9°C below baseline during ASH protocols (Fig. 2). By 20 min posthypoxia, peritoneal temperature began to return toward baseline, but recovery was not complete even at 60 min posthypoxia (0.3 and 0.5°C below baseline for AIH and ASH, respectively, P < 0.05). There were no significant changes in body temperature during sham protocols.
Fig. 2.
Time course of rat peritoneal temperature (in 5-min averages, expressed as change from baseline) during and following sham (●), acute intermittent hypoxia (AIH, ○), or acute sustained hypoxia (ASH, ▼) protocol. Peritoneal temperatures prior to any of the 3 treatments were not significantly different; overall baseline peritoneal temperature was 37.9 ± 0.16°C (mean ± SE, n = 12). During AIH and ASH, peritoneal temperature significantly decreased relative to sham, and these values had not returned to baseline 1 h posttreatment.
An important implication of these data is that failure to correct for changes in body temperature at the time of volume measurement (during or after AIH or ASH) will introduce systematic calibration (and, therefore, measurement) errors. For example, if volumes were calibrated using a body temperature measured before hypoxia or 65 min posthypoxia (when the rat was removed from the plethysmograph), volumes reported shortly following hypoxia would overestimate the actual Vt, as described in the equations of Drorbaugh and Fenn (13).
V̇e increases following AIH but not ASH.
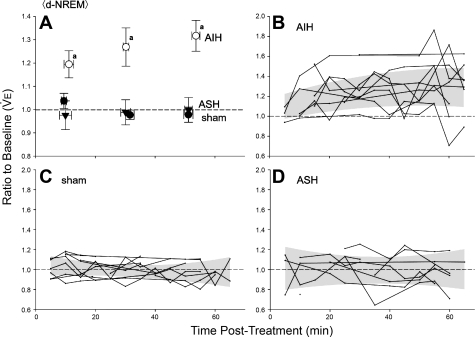
Posttreatment measurements of normalized V̇e are shown for AIH, ASH, and sham-treated rats during d-NREM sleep in Fig. 3; these values are expressed as a fraction of baseline measurements during d-NREM sleep. AIH significantly increased V̇e from pretreatment, baseline values during d-NREM sleep, and V̇e remained above baseline at the end of our observation period (i.e., 65 min post-AIH; P < 0.05). Thus, vLTF was observed for ≥65 min post-AIH during d-NREM sleep. On the other hand, neither ASH nor sham treatment consistently affected posttreatment V̇e during d-NREM sleep (P > 0.05). Thus, vLTF during d-NREM sleep is sensitive to the pattern of hypoxia, similar to pattern sensitivity of phrenic LTF in anesthetized and vagotomized rats (5).
Fig. 3.
Time course of normalized V̇e during d-NREM sleep following sham (●), AIH (○), or ASH (▼) protocol. A: average V̇e for 0–20, 20–40, and 40–65 min of plethysmograph measurements during d-NREM sleep following each of the 3 gas exposure protocols. Error bars are standard errors (SE) for mean V̇e (vertical bars) and average time of d-NREM measurements made in each time interval (horizontal bars). B–D: V̇e for each individual rat during each 5-min measurement period post-AIH (n = 10), post-sham (n = 12), and post-ASH (n = 9). Shaded area designates 95% confidence limits of quadratic regression relating V̇e with time. Baseline V̇e was determined during d-NREM sleep in the ∼2-h period prior to the experimental protocol. aSignificant difference (P < 0.05) relative to 1.0 (i.e., baseline) and to sham and ASH in the same time period.
vLTF results from increased Vt and f.
Figure 4 shows the time course of Vt during d-NREM sleep; these values are normalized to pretreatment baseline values in the same sleep state. During d-NREM sleep, Vt was elevated immediately following AIH (P < 0.05), but not ASH or sham treatments (P > 0.05), thus confirming that vLTF is due in part to increased Vt in spontaneously breathing (and poikilocapnic) Lewis rats during d-NREM sleep. During d-NREM sleep, f slowly and progressively increased following AIH (Fig. 5; P < 0.05), but not after ASH or sham treatment (P > 0.05). Thus, Vt is the dominant contributor to vLTF shortly after AIH, whereas the relative contribution of increased f increases progressively until Vt and f make approximately equal contributions 40–65 min post-AIH (cf. Fig. 4A with Fig. 5A).
Fig. 4.
Time course of normalized tidal volume (Vt) during d-NREM sleep following sham (●), AIH (○), or ASH (▼) protocol. A: average Vt for 0–20, 20–40, and 40–65 min of plethysmograph measurements during d-NREM sleep following each of the 3 gas exposure protocols. Error bars are standard errors (SE) for mean Vt (vertical bars) and average time of d-NREM measurements made in each time interval (horizontal bars). B–D: Vt for each individual rat during 5-min measurement periods post-AIH (n = 10), post-sham (n = 12), and post-ASH (n = 9). Shaded area designates 95% confidence limits of quadratic regression relating Vt with time. Baseline Vt was determined during d-NREM sleep in the ∼2-h period prior to the experimental protocol. aSignificant difference (P < 0.05) relative to 1.0 and to sham and ASH in the same time period.
Fig. 5.
Time course of normalized respiratory frequency (f) during d-NREM sleep following sham (●), AIH (○), or ASH (▼) protocol. A: average f for 0–20, 20–40, and 40–65 min plethysmograph measurements during d-NREM sleep following each of the 3 gas exposure protocols. Error bars are standard errors (SE) for mean f (vertical bars) and average time of d-NREM measurements made in each time interval (horizontal bars). B–D: f for each individual rat during 5-min measurement periods post-AIH (n = 10), post-sham (n = 12), and post-ASH (n = 9). Shaded area designates 95% confidence limits of quadratic regression relating f with time. Baseline f was determined during d-NREM sleep in the ∼2-h period prior to the experimental protocol. aSignificant difference (P < 0.05) relative to 1.0 and to sham and ASH in the same time period. bSignificant (P < 0.05) difference relative to 1.0. dSignificant (P < 0.05) difference relative to ASH. eSignificant (P < 0.05) difference relative to 0–20 min post-AIH.
vLTF is state-dependent.
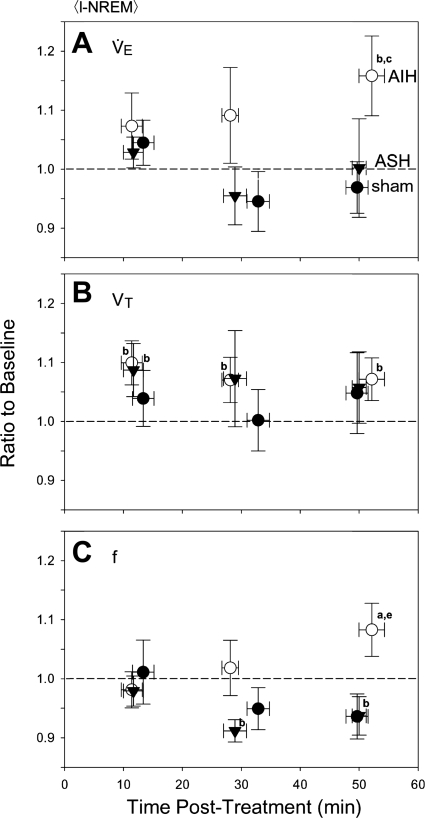
In l-NREM sleep, normalized V̇e was significantly greater than baseline by 40–65 min post-AIH (P < 0.05), indicative of vLTF (Fig. 6A). However, vLTF was smaller and more variable during l-NREM than d-NREM sleep (cf. Fig. 3A with Fig. 6A) and was significant only during the last measurement period; no changes in V̇e were observed following ASH or sham treatment during l-NREM sleep (P > 0.05). Increased V̇e during l-NREM sleep following AIH appeared to be due to modest increases in f and Vt (both P < 0.05), although increased Vt following AIH was not significantly different from Vt responses following ASH or sham treatment at the same time points (P > 0.05). Thus, although present during l-NREM sleep, vLTF is smaller and more variable and results from a ventilatory pattern that is different (i.e., greater relative f contribution) from that of d-NREM sleep.
Fig. 6.
Time course of normalized mean V̇e, Vt, and f during l-NREM sleep for sham (●), AIH (○), and ASH (▼) protocol. Values were averaged between 0–20, 20–40, and 40–65 min posttreatment. aSignificant differences (P < 0.05) relative to 1.0 (i.e., baseline) and to sham and ASH during the same time interval. bSignificant (P < 0.05) difference relative to 1.0 (i.e., baseline) only. cSignificant (P < 0.05) difference relative to sham only. eSignificant (P < 0.05) difference relative to 0–20 min post-AIH.
In QW, normalized V̇e (Fig. 7A) was not significantly elevated above baseline at any time posttreatment (AIH, ASH, or sham; P > 0.05), although there was a small trend for values to be elevated following AIH alone. Vt was slightly increased following AIH relative to baseline (P < 0.05; Fig. 7B), but this effect was small and not significantly different from values following ASH or sham treatment. There were no apparent changes in f at any time post-AIH during QW. Thus, vLTF in male Lewis rats is robust in d-NREM sleep, less robust in l-NREM sleep, and minimal or absent during QW, suggesting a profound state dependence of vLTF in this experimental model.
Fig. 7.
Time course of normalized mean V̇e, Vt, and f during quiet wakefulness (QW) for sham (●), AIH (○), or ASH (▼) protocol. Values were averaged between 0–20, 20–40, and 40–65 min posttreatment. bSignificant (P < 0.05) difference relative to 1.0 only. eSignificant (P < 0.05) difference relative to 0–20 min post-AIH.
V̇e/V̇co2 exhibits LTF during d-NREM sleep.
Since vigilance state-dependent changes in vLTF (Figs. 3, 6, and 7) could conceivably be caused by uncontrolled changes in metabolic rate caused by the experimental treatments, V̇e/V̇co2 was assessed before and after AIH, ASH, and sham treatment. During d-NREM sleep, V̇e/V̇co2 was significantly elevated 30–60 min post-AIH (36 ± 2%, P < 0.05), confirming that vLTF causes hyperventilation; however, V̇e/V̇co2 was not increased following ASH or sham treatment during d-NREM sleep (data not shown; P > 0.05). Thus, vLTF during d-NREM sleep is independent of treatment-induced changes in metabolism.
Increases in V̇e/V̇co2 associated with vLTF suggest that arterial Pco2 decreased and, subsequently, minimized vLTF expression due to inhibitory chemoreceptor feedback (38). The apparent vLTF is expected to have been greater if arterial isocapnia had been maintained, as reported previously in unanesthetized Sprague-Dawley rats (38). In addition, since ASH was not associated with changes in V̇e/V̇co2, vLTF pattern sensitivity is independent of differential effects on metabolic rate. There were no significant changes in estimates of V̇e/V̇co2 in l-NREM sleep or QW, confirming that changes in metabolic rate do not account for state-dependent differences in vLTF expression.
DISCUSSION
Our results confirm that AIH elicits pattern-sensitive vLTF in unanesthetized, spontaneously breathing rats (25, 26, 38), further demonstrating that LTF of respiratory motor output is not a unique feature of anesthetized, paralyzed, vagotomized, and ventilated animals (9, 15, 32). More importantly, we demonstrate that vLTF exhibits profound state dependence in unanesthetized rats, with the greatest response during deep, slow-wave (i.e., d-NREM) sleep. Thus, vLTF may be of considerable relevance to ventilatory control during sleep, possibly ensuring adequate alveolar ventilation in conditions where the risk of repetitive (central or obstructive) apneas is high. However, the specific role of vLTF in modifying breathing during sleep, particularly in rats, remains to be determined (22).
Vigilance state dependence of baseline ventilation.
As expected, breathing was inhibited during sleep, with d-NREM and REM sleep causing the greatest depression (Fig. 1). Historically, the effects of sleep on breathing have been discussed in terms of a “wakefulness drive,” which is diminished on the transition from wakefulness to sleep (39), but neural mechanisms contributing to the wakefulness drive are not known. Nevertheless, ventilation is reduced ∼33% on the transition from QW to d-NREM and REM sleep.
Vigilance state dependence of vLTF.
Previous studies suggest that vLTF is more difficult to elicit, is of shorter duration, and tends to involve greater frequency (vs. amplitude) responses in unanesthetized than anesthetized rats (cf. Refs. 9, 32). Although the rats were reported to be awake in each of these earlier studies, vigilance state was not explicitly monitored (25–28, 38), which may have increased observed variability. For example, if some animals had been awake while others were in l-NREM sleep during measurement periods, the apparent vLTF magnitude would be misleadingly high because of the combined data from wakefulness and l-NREM sleep. To the extreme, if the predominant vigilance state during baseline measurements is d-NREM sleep, whereas post-AIH measurements reflect predominantly QW, vLTF magnitude will be substantially overestimated. This overestimate would result from a normalization artifact induced when vLTF is calculated against the lower baseline value characteristic of d-NREM sleep (Fig. 1). Conversely, if baseline measurements predominantly reflect QW, whereas post-AIH measurements reflect a greater fraction of time spent in d-NREM sleep, the magnitude of vLTF will be underestimated because of an artificially high relative baseline ventilation characteristic of QW (i.e., 33% higher than d-NREM; Fig. 1). By explicitly measuring vLTF during documented vigilance states in the present study, we presumably minimized variability attributable to heterogeneous vigilance states or normalization artifacts due to vigilance state differences between baseline and posttreatment measurement periods, although we cannot rule out subtle differences within our defined sleep states (e.g., shifts toward lighter d-NREM sleep after intermittent hypoxia).
The reasons that vLTF was not observed during wakefulness in this study on Lewis rats is not clear. The same plethysmograph and experimental protocol were used by us in the first published study demonstrating vLTF in awake Sprague-Dawley rats (38), suggesting that the methods are sufficiently sensitive to detect vLTF if it is present. Possible contributors to the lack of vLTF during wakefulness in the present study include strain differences. There are major differences in phrenic LTF expression among rat strains (9, 10), with Lewis rats exhibiting greater phrenic LTF than Sprague-Dawley, Fischer, or Brown Norway rats. There may be similar strain differences in the state sensitivity of vLTF in unanesthetized rats or in the ventilatory pattern adopted during vLTF (see below). Alternately, since AIH was initiated during d-NREM sleep, this may confer state specificity to subsequent vLTF. Finally, since none of the published accounts of vLTF in “awake” Sprague-Dawley rats documented vigilance state, some of the vLTF in these studies may have actually occurred during l-NREM sleep (25–28, 38). Although we do not know this to be the case, there is strong selection bias toward making awake ventilatory measurements in quiet rats that are not moving because of the limits of whole body plethysmography. It is difficult to discriminate between QW and l-NREM sleep in these conditions. Although we cannot make firm conclusions concerning the reasons that vLTF was not observed during QW in the present study, our data clearly demonstrate that vLTF expression is vigilance state-dependent in Lewis rats.
Although vLTF has not been observed in human subjects during normal wakefulness (20, 24), vLTF can be induced in awake humans with modest hypercapnia (17). AIH-induced vLTF is also observed during sleep in humans (1–3, 40). From the perspective of ventilatory stability, AIH improves stability during wakefulness in normal human subjects (35) but may destabilize breathing when applied during sleep (12).
In contrast, awake and sleeping mice exhibit vLTF following AIH, although there are time-dependent differences in the ventilatory patterns giving rise to vLTF (44). For example, during the first 20 min post-AIH in d-NREM sleep, vLTF largely results from increased Vt with minimal change in f. In contrast, the same AIH protocol elicits vLTF via increased f during QW, with only minimal change in Vt (44). However, by 40 min post-AIH, differences in ventilatory pattern among vigilance states are no longer evident, and vLTF is expressed predominantly as increased f in both states.
Our finding that unanesthetized Lewis rats in d-NREM sleep exhibit vLTF following AIH complements the report of Terada and colleagues (44) in mice, although Vt contributions to vLTF are larger and persist for a longer period in Lewis rats. However, our finding that vLTF is virtually absent during QW contrasts with the report of vLTF during QW in mice (44). These differing results may be due to genetic differences (species or strain), differences in the details of vigilance state analysis, or differences in chemoreceptor feedback that restrain the manifestation of vLTF. Terada and colleagues did not distinguish between l-NREM and d-NREM sleep, whereas this distinction was made in the present study. By using 10-s epochs to classify vigilance state, Terada and colleagues were able to carefully define each time epoch; thus, some of the time included as l-NREM sleep in our study may have been represented as QW in their study. With subtle differences in state composition, some of the vLTF reported during l-NREM sleep in the present study may have been apportioned to QW in their study on mice. Since l-NREM is often transitional and difficult to classify, it is not clear which analysis more accurately portrays the vigilance state dependence of vLTF. Another factor that may exaggerate or diminish the apparent Vt contribution to vLTF is difference in chemoreceptor feedback elicited by the hypocapnic conditions characteristic of spontaneously breathing animals. For example, in rats, the contribution of Vt to vLTF is greatly diminished by hypocapnia attendant to vLTF (38). Since there are differences in the magnitude of hypercapnic ventilatory responses among mouse (43) and rat (42) strains, chemoreceptor feedback restraint of Vt during vLTF may differ among species or even strains of a given species.
Unfortunately, we were unable to quantify vLTF during REM sleep because of the limited duration of REM episodes in our protocol. The existence of vLTF during REM sleep is of considerable interest, since ventilatory instability is a frequent problem during REM sleep. Further investigation of vLTF in REM sleep is warranted, particularly in an animal model with a greater proportion of REM sleep.
Ventilatory patterns during vLTF in d-NREM sleep.
Our finding that vLTF during d-NREM sleep in Lewis rats was manifested as changes in f and Vt contrasts with most other studies in unanesthetized rats, which primarily report frequency changes (for review see Ref. 9). The distinction between Vt and f facilitation can be critical in the identification of underlying mechanisms (9, 41). For example, phrenic LTF in anesthetized rats is primarily expressed as increased nerve burst amplitude (a neural correlate of Vt) and has been suggested to result from facilitation of synaptic inputs to respiratory motor neurons (15, 22, 32). Indeed, AIH elicits a cascade of cellular events operating at the level of the cervical spinal cord to elicit phrenic LTF (7, 8). By contrast, the most common manifestation of vLTF in earlier studies of unanesthetized rats (presumed to be awake) is increased f (for review see Ref. 9); f LTF is generally attributed to facilitation of brain stem respiratory neurons that generate respiratory rhythm (32, 41), although plasticity in synaptic pathways from primary afferent neurons to brain stem rhythm-generating neurons cannot be excluded with currently available data (9).
We suggest that Vt LTF in unanesthetized Lewis rats during d-NREM sleep is due, at least in part, to cellular/synaptic mechanisms similar to phrenic LTF in anesthetized rats, i.e., facilitation of spinal synaptic inputs to phrenic motor neurons. Thus, mechanistic studies of phrenic LTF in anesthetized rats likely pertain to unanesthetized, spontaneously breathing animals, particularly during sleep. Factors distinguishing vLTF capacity during d-NREM sleep and QW remain to be explored.
Pattern sensitivity of vLTF.
A hallmark of respiratory LTF is its pattern sensitivity; phrenic LTF is induced by intermittent, but not sustained, hypoxia of the same cumulative duration (5, 6). In unanesthetized animals, Turner and Mitchell (45) demonstrated that 10 hypoxic episodes elicit vLTF in awake goats, whereas sustained hypoxia does not (14). Subsequently, Olson and colleagues (38) confirmed vLTF pattern sensitivity in unanesthetized rats (i.e., AIH, but not ASH, induced vLTF), similar to more recent reports in mice (44). Here, we confirm that AIH, but not ASH, induces vLTF during d-NREM sleep in Lewis rats.
Conclusions.
vLTF in unanesthetized Lewis rats is exquisitely sensitive to changes in vigilance state. During d-NREM sleep, vLTF expression is similar to phrenic LTF in anesthetized rats, suggesting that vLTF and phrenic LTF are different manifestations of the same fundamental mechanism. Investigations concerning neural mechanisms of phrenic LTF are likely to be relevant in unanesthetized animals, particularly during sleep. Any consideration of vLTF and its physiological significance must consider its state dependence.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-80209 and HL-65383 and Training Grant HL-07654.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank T. Kuwaki and T. Baker-Herman for their thoughtful critiques during the preparation of the manuscript and S. Mahamed for advice concerning data processing.
Present address of A. Nakamura: Departments of Autonomic Physiology and Respirology, Chiba University Graduate School of Medicine, Chiba, Japan.
Some data from this study were presented in preliminary form (36, 37).
REFERENCES
- 1. Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol 91: 2751–2757, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep 21: 709–716, 1998 [PubMed] [Google Scholar]
- 3. Babcock M, Shkoukani M, Aboubakr SE, Badr MS. Determinants of long-term facilitation in humans during NREM sleep. J Appl Physiol 94: 53–59, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol 529: 215–219, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker TL, Fuller DD, Zabka AG, Mitchell GS. Respiratory plasticity: differential actions of continuous and episodic hypoxia and hypercapnia. Respir Physiol 129: 25–35, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6244, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol 162: 8–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baker-Herman TL, Bavis RW, Dahlberg JM, Mitchell AZ, Wilkerson JE, Golder FJ, Macfarlane PM, Watters JJ, Behan M, Mitchell GS. Differential expression of respiratory long-term facilitation among inbred rat strains. Respir Physiol Neurobiol 170: 260–267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J Appl Physiol 73: 2083–2088, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol 108: 369–377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–87, 1955 [PubMed] [Google Scholar]
- 14. Dwinell MR, Janssen PL, Bisgard GE. Lack of long-term facilitation of ventilation after exposure to hypoxia in goats. Respir Physiol 108: 1–9, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fregosi RF, Mitchell GS. Long term facilitation of inspiratory intercostal nerve activity following repeated carotid sinus nerve stimulation in cats. J Physiol 477: 469–479, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol 291: R1111–R1119, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol Regul Integr Comp Physiol 265: R811–R819, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Janssen PL, Fregosi RF. No evidence for long-term facilitation after episodic hypoxia in spontaneously breathing, anesthetized rats. J Appl Physiol 89: 1345–1351, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Jordan AS, Catcheside PG, O'Donoghue FJ, McEvoy RD. Long-term facilitation of ventilation is not present during wakefulness in healthy men or women. J Appl Physiol 93: 2129–2136, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Kline DD, Overholt JL, Prabhakar NR. Mutant mice deficient in NOS-1 exhibit attenuated long-term facilitation and short-term potentiation in breathing. J Physiol 539: 309–315, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92: 27–37, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Mateika JH, Fregosi RF. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J Appl Physiol 82: 419–425, 1997 [DOI] [PubMed] [Google Scholar]
- 24. McEvoy RD, Popovic RM, Saunders NA, White DP. Effects of sustained and repetitive isocapnic hypoxia on ventilation and genioglossal and diaphragmatic EMGs. J Appl Physiol 81: 866–875, 1996 [DOI] [PubMed] [Google Scholar]
- 25. McGuire M, Zhang Y, White DP, Ling L. Effect of hypoxic episode number and severity on ventilatory long-term facilitation in awake rats. J Appl Physiol 93: 2155–2161, 2002 [DOI] [PubMed] [Google Scholar]
- 26. McGuire M, Zhang Y, White DP, Ling L. Chronic intermittent hypoxia enhances ventilatory long-term facilitation in awake rats. J Appl Physiol 95: 1499–1508, 2003 [DOI] [PubMed] [Google Scholar]
- 27. McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol 286: R334–R341, 2004 [DOI] [PubMed] [Google Scholar]
- 28. McGuire M, Ling L. Ventilatory long-term facilitation is greater in 1- vs. 2-mo-old awake rats. J Appl Physiol 98: 1195–1201, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol 41: 87–103, 1980 [DOI] [PubMed] [Google Scholar]
- 30. Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol 42: 171–188, 1980 [DOI] [PubMed] [Google Scholar]
- 31. Millhorn DE, Eldridge FL. Role of ventrolateral medulla in regulation of respiratory and cardiovascular systems. J Appl Physiol 61: 1249–1263, 1986 [DOI] [PubMed] [Google Scholar]
- 32. Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Intermittent hypoxia and respiratory plasticity. J Appl Physiol 90: 2466–2475, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Mitchell GS, Powell FL, Hopkins SR, Milsom WK. Time domains of the hypoxic ventilatory response in awake ducks: episodic and continuous hypoxia. Respir Physiol 124: 117–128, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358–374, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Morris KF, Gozal D. Persistent respiratory changes following intermittent hypoxic stimulation in cats and human beings. Respir Physiol Neurobiol 140: 1–8, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Nakamura A, Wenninger JM, Olson EB, Bisgard GE, Mitchell GS. Ventilatory long-term facilitation in sleeping Lewis rats (Abstract). FASEB J 19: A1284, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakamura A, Wenninger JM, Olson EB, Jr, Bisgard GE, Mitchell GS. Ventilatory long-term facilitation following intermittent hypoxia is state-dependent in rats (Abstract). J Physiol Sci 56: S75, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Olson EB, Jr, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchel GS. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol 91: 709–716, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Phillipson EA, Bowes G. Control of breathing during sleep. In: Handbook of Physiology. The Respiratory System. Control of Breathing. Bethesda, MD: Am. Physiol. Soc., 1986, sect. 3, vol. II, pt. 2, p. 649–689 [Google Scholar]
- 40. Pierchala LA, Mohammed AA, Grullon K, Mateika JH, Badr MS. Ventilatory long-term facilitation in non-snoring subjects during NREM sleep. Respir Physiol Neurobiol 160: 259–266, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Strohl KP, Thomas AJ, St Jean P, Schlenker EH, Koletsky RJ, Schork NJ. Ventilation and metabolism among rat strains. J Appl Physiol 82: 317–323, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Tankersley CG, Fitzgerald RS, Kleeberger SR. Differential control of ventilation among inbred strains of mice. Am J Physiol Regul Integr Comp Physiol 267: R1371–R1377, 1994 [DOI] [PubMed] [Google Scholar]
- 44. Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, Kuwaki T. Ventilatory long-term facilitation in mice can be observed both during sleep and wake periods and depends on orexin. J Appl Physiol 104: 499–507, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Turner DL, Mitchell GS. Long term facilitation of ventilation following repeated hypoxic episodes in awake goats. J Physiol 499: 453–460, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wenninger JM, Olson EB, Wang Z, Keith IM, Mitchell GS, Bisgard GE. Carotid sinus nerve responses and ventilatory acclimatization to hypoxia in adult rats following two weeks of postnatal hyperoxia. Respir Physiol Neurobiol 150: 155–164, 2006 [DOI] [PubMed] [Google Scholar]