Abstract
Glutaredoxin 1 (Glrx1) is a small dithiol protein that regulates the cellular redox state and redox-dependent signaling pathways via modulation of protein glutathionylation. IκB kinase (IKK), an essential enzyme for NF-κB activation, can be subjected to S-glutathionylation leading to alteration of its activity. However, the role of Glrx1 in cigarette smoke (CS)-induced lung inflammation and chromatin modifications are not known. We hypothesized that Glrx1 regulates the CS-induced lung inflammation and chromatin modifications via differential regulation of IKKs by S-glutathionylation in mouse lung. Glrx1 knockout (KO) and wild-type (WT) mice were exposed to CS for 3 days and determined the role of Glrx1 in regulation of proinflammatory response in the lung. Neutrophil influx in bronchoalveolar lavage fluid and proinflammatory cytokine release in lung were increased in Glrx1 KO mice compared with WT mice exposed to CS, which was associated with augmented nuclear translocation of RelA/p65 and its phospho-acetylation. Interestingly, phosphorylated and total levels of IKKα, but not total and phosphorylated IKKβ levels, were increased in lungs of Glrx1 KO mice compared with WT mice exposed to CS. Ablation of Glrx1 leads to increased CS-induced IKKβ glutathionylation rendering it inactive, whereas IKKα was activated resulting in increased phospho-acetylation of histone H3 in mouse lung. Thus, targeted disruption of Glrx1 regulates the lung proinflammatory response via histone acetylation specifically by activation of IKKα in response to CS exposure. Overall, our study suggests that S-glutathionylation and phosphorylation of IKKα plays an important role in histone acetylation on proinflammatory gene promoters and NF-κB-mediated abnormal and sustained lung inflammation in pathogenesis of chronic inflammatory lung diseases.
Keywords: NF-κB, glutathionylation, chronic obstructive pulmonary disease, oxidants, chromatin, glutathione
cigarette smoke (CS) contains numerous reactive oxygen species (∼1015 to 1017 oxidants/free radicals per puff), reactive nitrogen species, reactive aldehydes, and quinones (8), which are involved in pathogenesis of chronic lung inflammatory diseases. The airway system is susceptible to local redox imbalance due to direct exposure to exogenous inhaled oxidants, such as air pollutants, particulates, and CS (21). We and others have shown that the glutathione (GSH) redox status is altered in lung of smokers and patients with COPD (27, 32) and in lungs of mouse asthma model (42), as well as in airway epithelial cells in response to inflammatory stimuli (31, 50). It is well known that CS exposure causes inflammation in the lung by activation of redox-sensitive NF-κB via phosphorylation/acetylation of its subunit, RelA/p65 (11, 39, 54). IκB kinase (IKK), a heterotrimer including IKKα, IKKβ, and NEMO (also known as IKKγ), is involved in activation of NF-κB by degrading inhibitory molecule IκBα (5). IKKβ is shown to phosphorylate NH2-terminal serines on IκBα, whereas IKKα regulates non-canonical pathway of NF-κB in response to a variety of inflammatory stimuli (33, 43, 53). We and others have shown that IKKα is an important regulator of histone modifications in addition to its role on NF-κB activation in response to a variety of stimuli including CS and oxidants (15, 30, 52, 54). However, the mechanism underlying redox regulation of IKKs by CS is not well known.
S-glutathionylation, a process for protein-SSG formation, is a reversible redox-sensitive posttranslational modification that has been shown to regulate the function (activation or deactivation) of certain proteins, including NF-κB, interferon regulatory factor 3, and IKKβ (9, 36, 41, 45). Glutaredoxins (Glrxs) have been identified as the repair enzymes to regulate the redox status via deglutathionylation (17, 44). Glrxs are small dithiol proteins with disulfide exchange reaction on oxidized protein thiols, which regulate redox-dependent signaling pathways (23, 48). Glrxs act in defense against oxidative stress by catalyzing disulfide reduction via GSH-dependent catalysis. There are two dithiol Glrxs in human, cytosolic Glrx1 and mitochondrial Glrx2 (23, 32), which reduce S-glutathionylated proteins, restoring the protein cysteine residue (3). For example, S-glutathionylation of IKKβ specifically inhibits NF-κB pathway in lung epithelial cells (40, 41) by losing the DNA binding activity via S-glutathionylation of NF-κB subunits (p50 and RelA/p65) (35, 46). Thus, Glrx1 governs the redox posttranslational modifications of NF-κB signaling pathway thereby regulating inflammation (37, 45–47). However, the role of Glrx1 in regulation of NF-κB signaling proteins and subsequently in regulation of chromatin modification involved in lung inflammation is not known.
The level of Glrx1 is decreased in alveolar macrophages and bronchial epithelium of lungs in patients with chronic obstructive pulmonary disease (COPD) and interstitial lung diseases (31, 32). No studies on the role of Glrx1 in regulation of CS-induced lung inflammation and chromatin modifications (histone acetylation) are available. We therefore hypothesized that Glrx1 regulates CS-induced lung inflammation and histone acetylation via differential regulation of IKKs by S-glutathionylation in mouse lung. To test this hypothesis, we exposed Glrx1 knockout (KO) and wild-type (WT) mice to CS, and the lung inflammatory responses as well as posttranslational modifications of IKKs and histones were assessed in the lung.
MATERIALS AND METHODS
Materials.
Unless otherwise stated, all biochemical reagents used in this study were purchased from Sigma (St. Louis, MO). Antibodies against phosphorylated IκBα (Ser32/36) (#9246), phosphorylated RelA/p65 (Ser276) (#3037), phosphorylated RelA/p65 (Ser536) (#3036), acetylated RelA/p65 (Lys310) (#3035), phosphorylated serine (#9606), acetylated and phosphorylated histone H3 (Lys9/Ser10) (#9711), histone H3 (#9715), acetylated histone H4 (Lys12) (#2591), and histone H4 (#2592) were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against Glrx1 (sc-32943), IκBα (sc-847), NF-κB RelA/p65 (sc-372), IKKα (sc-7606), IKKβ (sc-34673), IKKα/β (sc-7607), and lamin B (sc-6216) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Glrx1 (AF3119), anti-GSH for determination of protein-S-glutathionylation (101-A), and anti-actin (CP01) antibodies were purchased from R&D Systems (Minneapolis, MN), Virogen (Watertown, MA), and Calbiochem (La Jolla, CA), respectively.
Mice.
Glutaredoxin 1 KO mice (Glrx1−/−) on a genetic background of C57BL/6J were kindly provided by Dr. Y. S. Ho (Wayne State Univ., Detroit, MI) (25). WT mice (C57BL/6J) were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were bred and maintained under specific pathogen-free condition in the Vivarium Facility of the University of Rochester. All experimental protocols were performed in accordance with the standards established by the U.S. Animal Welfare Acts, as set forth by the National Institutes of Health guidelines, and the research protocol for these studies was approved by the University of Rochester Committee on Animal Research.
CS exposure to mice.
Eight to twelve weeks of age WT and Glrx1−/− were used for 3 days of CS exposure as described previously (57). In brief, mice were placed in an individual compartment of a wire cage, which was placed inside a closed plastic box connected to the smoke source. Research grade cigarettes [2R4F (total particulate matter per cubic meter of air, TPM, concentration 11.7 mg/cigarette, tar 9.7 mg/cigarette, nicotine 0.85 mg/cigarette), University of Kentucky, Lexington, KY] were used to generate smoke, and mice received two 1-h exposures per day, 1 h apart, according to the Federal Trade Commission protocol (1 puff/min of 2-s duration and 35-ml volume) for 3 days using a Baumgartner-Jaeger CSM2072i automatic cigarette smoking machine (CH Technologies, Westwood, NJ) (58, 59). Mainstream CS was diluted with filtered air and directed into the exposure chamber. The smoke exposure (TPM) was monitored in real-time with a MicroDust pro-aerosol monitor (Casella CEL, Bedford, UK) and verified daily by gravimetric sampling. The smoke concentration was set at a value of ∼300 mg/m3 TPM (corresponding to human consumption of 1–1.5 packs/day) by adjusting the flow rate of the diluted medical air. Control mice were exposed to filtered air in an identical chamber according to the same protocol described for CS exposure. Carbon monoxide concentration in the chamber was 290–300 ppm. The dosimetry of carbon monoxide in CS was estimated by measuring blood carboxyhemoglobin levels. Mice tolerated CS without evidence of toxicity (carboxyhemoglobin levels ∼17%, and no significant loss in body weight) (57).
Differential cell count in bronchoalveolar lavage fluid.
Mice were killed at 24 h after the last exposure by an intraperitoneal injection of pentobarbital sodium (100 mg/kg; Abbott Laboratories, Abbott Park, IL) followed by exsanguination. The lungs were lavaged three times with 0.5 ml of saline via a cannula inserted into the trachea. The aliquots were combined and centrifuged, and the bronchoalveolar lavage (BAL) inflammatory cell pellet was resuspended in saline. The total cell number was determined by counting on a hemocytometer, and cytospin slides (Thermo Shandon, Pittsburgh, PA) were prepared using 50,000 cells/slide. Differential cell counts (∼500 cells/slide) were performed on cytospin-prepared slides stained with Diff-Quik (Dade Behring, Newark, DE).
Protein extraction from lung tissue.
One-hundred milligrams of lung tissue was mechanically homogenized in 0.5 ml of buffer A [10 mM HEPES (pH 7.8), 10 mM KCl, 2 mM MgCl2, 1 mM dithiothreitol, 0.1 M EDTA, 0.2 mM NaF, 0.2 mM sodium orthovanadate, 1% (vol/vol) Nonidet P-40, and 0.6 mM phenylmethylsulfonyl fluoride] on ice. The homogenate was centrifuged at 2,000 g in a benchtop centrifuge for 30 s at 4°C to remove cellular debris. The supernatant was then transferred to a 1.7-ml ice-cold Eppendorf tube and further centrifuged for 30 s at 13,000 g at 4°C. The supernatant was collected as a cytoplasmic extract. The pellet was resuspended in 200 μl of buffer C [50 mM HEPES (pH 7.8), 50 mM KCl, 300 mM NaCl, 0.1 M EDTA, 1 mM dithiothreitol, 10% (vol/vol) glycerol, 0.2 mM NaF, 0.2 mM sodium orthovanadate, and 0.6 mM phenylmethylsulfonyl fluoride] and placed on the rotator in the cold room for 30 min. After centrifugation at 13,000 g in an Eppendorf tube for 5 min, the supernatant was collected as the nuclear extract and kept frozen at −80°C. For extraction of histone protein, pellets from the nuclear extraction were resuspended in 150 μl of deionized water containing 0.2 N HCl and 0.36 N H2SO4. The histone proteins were precipitated from the supernatant, agitated overnight at 4°C, and then centrifuged at 13,000 g for 10 min, and the supernatant was transferred into a fresh tube. Ice-cold acetone precipitation samples were incubated overnight at −80°C and centrifuged, and the air-dried pellets were resuspended in 50 μl of deionized water. Whole cell lysate was extracted from lung tissue after homogenization in RIPA buffer (50 mM Tris·HCl, 150 mM NaCl, 1 mM EDTA, 0.25% deoxycholate, 1 mM sodium orthovanadate, 1 mM NaF, 1% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride). Protein level in lung samples was measured by bicinchoninic acid (BCA) colorimetric assay (Thermo Scientific, Rockford, IL) using BSA as a standard.
Cytokine analysis.
The levels of proinflammatory mediators, such as monocyte chemotactic protein (MCP)-1, keratinocyte-derived cytokine (KC), and interferon-inducible protein (IP)-10 in lung homogenates were measured by ELISA using respective duo-antibody kits (R&D Systems) according to the manufacturer's instructions. The results were expressed in the samples as pg/mg protein.
Western blot analysis.
Proteins from lung tissue homogenates, including cytoplasmic and nuclear fractions, and histone extracts were separated on a 6.5–14% SDS-polyacrylamide gel. Separated proteins were electroblotted onto nitrocellulose membranes (Amersham, Arlington Heights, IL) and blocked for 1 h at room temperature with 5% BSA. The membranes were then probed with a specific primary antibody (1:1,000 dilution in PBS containing 0.1% Tween 20) at 4°C for overnight. After three washing steps (10 min each), the levels of protein were detected by probing with secondary anti-rabbit, anti-mouse, or anti-goat antibody (1:10,000 dilution in PBS containing 0.1% Tween 20) linked to horseradish peroxidase for 1 h, and bound complexes were detected using the enhanced chemiluminescence method (Perkin Elmer, Waltham, MA). Equivalent loading of the gel was determined by quantitation of protein as well as by reprobing membranes for actin, lamin B, histone H3, or histone H4.
Immunohistochemical localization of Glrx1 and IKKα/β.
The levels of Glrx1 were measured in the fixed lung sections (4 μm thick) by immunohistochemical staining using Glrx1 rabbit polyclonal antibody (1:100 dilution) with avidin-biotin-peroxidase complex (ABC) method followed by hematoxylin counterstaining. Appearance of dark brown color represents the presence of Glrx1 in lung tissue. In brief, the formalin-fixed, paraffin-embedded lung sections were deparaffinized and rehydrated by passing through a series of xylene and graded alcohol. Endogenous peroxidase activity was quenched by exposure to 3% H2O2 in methanol for 30 min. Nonspecific binding of antibodies to the tissue sections was blocked by incubating with 10% normal goat serum (Invitrogen, Carlsbad, CA) for 1 h. Tissue sections were incubated with Glrx1 antibody overnight at 4°C. After being washed, tissue sections were incubated with secondary antibody for 30 min. 3,3′-Diaminobenzidine (Vector Laboratories, Burlingame, CA) was used as peroxidase substrate. In each instance, sections from different groups were processed together, with equal time for color development. The positive cells in lung sections were counted manually at ×100 magnification (18, 54, 57). Similarly, anti-IKKα and anti-IKKβ antibody at a titer of 1:100 was used for the staining of IKKα- and IKKβ-positive cells in mouse lung.
Immunoprecipitation.
A total of 250 μg of proteins in mouse lung tissue homogenate was incubated with 2 μg of specific antibodies in RIPA buffer at 4°C for overnight. Then, 20 μl of protein A/G agarose beads (Santa Cruz Biotechnology) were added and incubated at 4°C on a rotating device for 2 h. After immunoprecipitation, the precipitates were washed at least three times with RIPA buffer with spinning at 1,500 g for 30 s at 4°C. The precipitants were resuspended in 50 μl of Laemmli sample buffer to a final concentration of 1× sample buffer and heated at 95°C for 5 min. The collected supernatants (immunoprecipitants) were run on 6.5% SDS-PAGE.
Labeling of protein reactive thiols.
Cysteine residues were labeled using the thiol-specific reagent EZ-link Maleimide-PEO2-Biotin (Thermo) as per the manufacturer's instructions. In brief, the immunoprecipitated proteins were incubated with 400 μM maleimide-PEO2-biotin for 2 h in the dark at 4°C. Samples were then separated on SDS-PAGE gels, transferred onto nitrocellulose membrane, and probed with streptavidin-HRP (Invitrogen) with the enhanced chemiluminescence method (51).
In situ detection of S-glutathionylated proteins in lung tissue following Glrx1 catalyzed cysteine derivatization.
In situ analysis of protein S-glutathionylation in lung tissue was determined as described previously (2) with slight modifications. In brief, the formalin-fixed, paraffin-embedded lung sections were deparaffinized and rehydrated by passing through a series of xylene and graded alcohol. Free thiol groups were blocked using a buffer [25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.4, 0.1 mM EDTA, pH 8.0, 0.01 mM neocuproine, 40 mM N-ethylmaleimide, and 1% Triton] for 30 min. S-glutathionylated cysteine groups were reduced by incubation with 13.5 μg/ml human Glrx1 (American Research Products, Belmont, MA), 35 μg/ml GSSG reductase (Roche, Indianapolis, IN), 1 mM GSH, 1 mM NADPH, 18 μM EDTA, and 137 mM Tris·HCl, pH 8.0, for 20 min. After being washed with PBS, newly reduced cysteine residues were labeled with 1 mM N-(3-maleimidylpropionyl) biocytin (MPB) (Invitrogen) for 1 h before incubation with 1 μg/ml streptavidin-conjugated Alexa Fluor 568 (Invitrogen) for 30 min. Tissue sections were then mounted with anti-fade DAPI fluoromount (Southern Biotech, Birmingham, AL) and viewed under a fluorescence microscope.
Reduced GSH assay.
GSH level was measured with a colorimetric assay kit (MBL international, Woburn, MA) according to the manufacturer's instructions (37a, 57). The assay is based on the GSH recycling system by 5–5′′-dithio-bis(2-nitrobenzoic acid) (DTNB) and GSH reductase. The results were expressed in the samples as μg/mg protein.
Chromatin immunoprecipitation.
ChIP was performed by using the EpiQuik Tissue Chromatin Immunoprecipitation Kit (Epigentek, Brooklyn, NY) (20). Briefly, mouse lung tissue was formaldehyde fixed and in vivo cross-linked, followed by tissue disaggregation. The cells were lysed, and the nucleoprotein complexes were sonicated to reduce the sizes of DNA fragments to 1,000–200 bp. Normal mouse IgG was used as the negative control and genomic and input chromatin as positive controls. Acetylated histone H3 and H4 antibodies (Millipore, Billerica, MA) were used for each immunoprecipitation. The immunoprecipitated DNA was eluted in a total volume of 20 μl, and 4 μl were used for PCR that was performed using a PTC-200 DNA engine (M. J. Research, Waltham, MA) under the following conditions: 94°C for 3 min; 32 cycles at 94°C for 45 s, 60°C for 1 min, and 72°C for 1 min; and final elongation at 72°C for 10 min. The following primer was used in PCR: IL-6, 5′-GAC ATG CTC AAG TGC TGA GTC AC-3′ (sense) and 5′-AGA TTG CAC AAT GTG ACG TCG-3′ (antisense), and PCR products were analyzed on a 1.8% agarose gel.
Statistical analysis.
Results are expressed as means ± SE. Statistical analysis of significance was calculated by one-way ANOVA followed by Fisher's protected least significant difference post hoc test for multigroup comparisons (StatView 5.0; SAS Institute, Cary, NC). Statistical significance is indicated in the figure legends.
RESULTS
Glrx1 level was decreased in mouse lung in response to CS exposure.
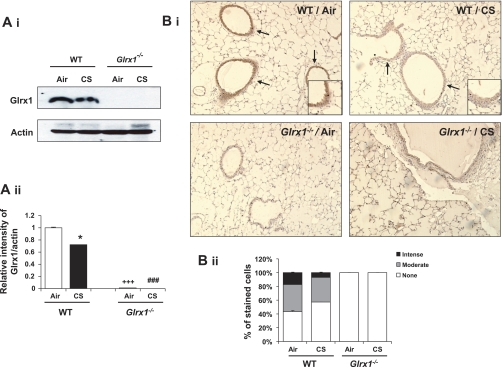
Mammalian Glrx1 is known as a redox modulatory enzyme, which restores the protein cysteine to sulfhydryl group (13). Glrx1 levels are decreased in lungs of COPD patients compared with nonsmokers (32). Therefore, we hypothesized that the expression of Glrx1 is regulated by CS in mouse lungs. To test this hypothesis, the level of Glrx1 was measured using immunoblot and immunohistochemistry in lungs of mice exposed to CS. Total Glrx1 level was significantly decreased in CS-exposed mouse lung homogenates (Fig. 1A), demonstrating that redox changes imposed by CS led to reduction of endogenous Glrx1. Immunostaining studies revealed that Glrx1-positive cells were decreased predominantly in the bronchial epithelium in lungs of mouse exposed to CS (Fig. 1B). We then verified the levels of Glrx1 in mice disrupted for Glrx1 gene in lung homogenates and lung sections. Glrx1 protein level was completely abolished in Glrx1−/− mice (Fig. 1).
Fig. 1.
Expression of Glutaredoxin 1 (Glrx1) in lung of Glrx1 wild-type (WT) and knockout (Glrx1−/−) mice. Deficiency of Glrx1 in lungs of Glrx1−/− mice was confirmed by immunoblotting and immunohistochemistry. A: Glrx1 protein was measured in lung homogenates of WT and Glrx1−/− mice in response to air or cigarette smoke (CS) exposure. Immunoblot pictures (i) and histograms (ii) showing relative intensity of Glrx1 vs. actin bands. B: Glrx1-positive cells were identified by dark brown immunohistochemical staining (i). Insets are magnified airway regions showing decreased Glrx1 staining in response to CS compared with air exposure. ii: immunostaining scores for Glrx1 per cell in airway regions of the lung. The assessment of immunostaining intensity was performed semiquantitatively and in a blinded fashion. Solid bars, intense staining; shaded bars, moderate/weak staining; open bars, no staining. Original magnification: ×100. *P < 0.05, significant compared with respective air-exposed mice. ###P < 0.001, significant compared with CS-exposed WT mice. +++P < 0.001, significant compared with air-exposed WT mice.
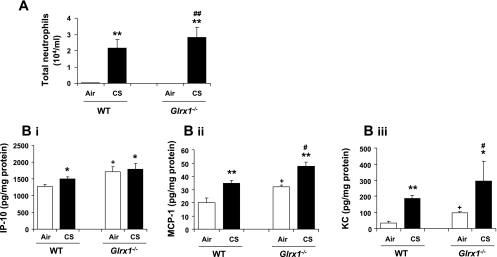
Genetic ablation of Glrx1 enhanced lung inflammatory cell influx and proinflammatory mediators release in response to CS exposure.
To determine the role of Glrx1 on CS-induced lung inflammation, Glrx1 KO (Glrx1−/−) and WT mice were exposed to CS, and inflammatory cell influx into BAL fluid was assessed by Diff-Quik staining. WT mice showed significant neutrophil influx in BAL fluid in response to CS exposure, which was augmented in Glrx1−/− mice (Fig. 2A). The number of total cells and macrophages were not significantly altered in BAL fluid of both WT and Glrx1−/− mice after 3 days of CS exposure (data not shown). Similar data of augmented neutrophil influx in BAL were obtained in response to lipopolysaccharide (LPS; 1 mg/ml) aerosolization in Glrx1−/− vs. saline-treated Glrx1−/− mice and LPS-treated WT mice (unpublished observations).
Fig. 2.
Increased lung inflammatory responses in Glrx1−/− mice in response to CS exposure. A: neutrophil influx was assessed by Diff-Quik staining in cytospin slides, which were prepared using bronchoalveolar lavage fluid collected by lung lavage in air- or CS-exposed WT and Glrx1−/− mice. B: the levels of proinflammatory mediators, such as IP-10(i), MCP-1 (ii), and KC (iii), were measured by ELISA in lung homogenates of air- and CS-exposed WT and Glrx1−/− mice. Data are shown as means ± SE (n = 3–5/group). *P < 0.05, **P < 0.01, significant compared with respective air-exposed mice. #P < 0.05, ##P < 0.01, significant compared with CS-exposed WT mice. +P < 0.05, significant compared with air-exposed WT mice.
CS-mediated recruitment of inflammatory cells into the lung tissue is due to the increased release of proinflammatory mediators either from epithelium or inflammatory cells (54, 57). To confirm the increased inflammatory responses in Glrx1−/− mice exposed to CS, the levels of proinflammatory mediators (IP-10, MCP-1, and KC) were measured in lung homogenate. Lung of Glrx1−/− mice showed increased basal levels of IP-10, MCP-1, and KC compared with WT (Fig. 2B). CS exposure significantly increased the levels of IP-10, MCP-1, and KC in WT mice compared with air-exposed group (P < 0.05) (Fig. 2B). Furthermore, Glrx1−/− mice show augmented levels of cytokines in lungs of mouse exposed to CS compared with CS-exposed WT mice. These data confirmed that Glrx1−/− mice are more susceptible than WT mice to incite CS-mediated lung inflammatory response. However, overexpression of Glrx1 (overexpressing/transgenic mice Glrx1+/+) on lung-specific surfactant protein C (SP-C) promoter in alveolar epithelium (25) did not affect either neutrophil influx or cytokine release in the lung in response to CS exposure or by LPS aerosolization compared with WT mice exposed to CS or LPS (unpublished observations). These results indicate that localized overexpression of Glrx1 in alveolar epithelial cells was not sufficient to protect against CS-induced lung inflammation.
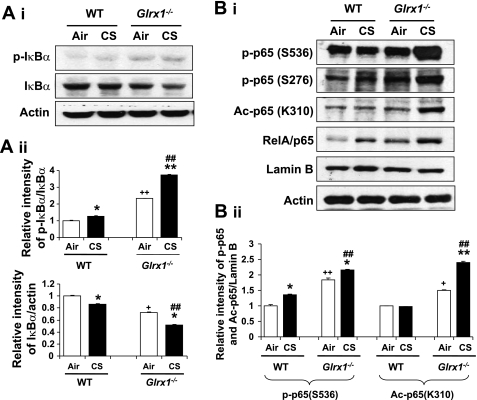
Genetic ablation of Glrx1 led to increased degradation of IκBα and posttranslational modifications of RelA/p65 in response to CS exposure.
Activation of NF-κB is controlled by phosphorylation and degradation of IκBα protein leading to enhanced nuclear translocation of RelA/p65 (38). It is well known that NF-κB activation plays a key role in CS-mediated lung inflammation (55, 58). Hence, the nuclear translocation of RelA/p65 and IκBα degradation in cytosol were determined by Western blot in lung of WT and Glrx1−/− mice to study the role of Glrx1 in CS-induced activation of NF-κB. Immunoblot analysis showed that IκBα level was decreased in lung of WT mice in response to CS along with the corresponding increase in phosphorylation of IκBα level and nuclear translocation of RelA/p65 (Fig. 3A). In addition, basal level of IκBα in Glrx1−/− mice was decreased, and disruption of Glrx1 further decreased the level of IκBα compared with WT mice in response to CS exposure. These data demonstrate that IκBα degradation and subsequent nuclear translocation of RelA/p65 were further increased in lung of Glrx1−/− mice compared with WT mice when exposed to CS.
Fig. 3.
Targeted disruption of Glrx1 increased degradation of IκBα and posttranslational modifications of RelA/p65 in response to CS exposure. A: p-IκBα and IκBα levels were measured in lung cytosolic fractions of WT and Glrx1−/− mice (i). ii: histograms show relative intensity of IκBα bands vs. controls. B: posttranslational modification of RelA/p65 was assessed by measuring phosphorylation (Ser276 and Ser536) and acetylation (Ac) (Lys310) of RelA/p65 in nuclear fractions of lung by immunoblotting (i). ii: histograms show relative intensity of p65 bands vs. controls. Gel pictures shown are representative of at least 3 separate experiments. *P < 0.05, **P < 0.01, significant compared with respective air-exposed mice. ##P < 0.01, significant compared with CS-exposed WT mice. +P < 0.05, ++P < 0.01, significant compared with air-exposed WT mice.
Apart from the nuclear translocation, posttranslational modifications of RelA/p65, such as site-specific phosphorylation and acetylation, play an important role in CS-induced lung inflammation (6, 7, 29). To further investigate the role of Glrx1 in NF-κB activation and posttranslational modifications of RelA/p65, we then determined the nuclear levels of phosphorylated (Ser276 and Ser536) and acetylated (Lys310) RelA/p65 in lungs of WT and Glrx1−/− mice exposed to CS. CS exposure increased the phosphorylation of nuclear RelA/p65 on Ser276 and Ser536 residues, and acetylation on Lys310 in lungs of WT mice, which were further increased in Glrx1−/− mice (Fig. 3B). In addition, basal levels of phosphorylation and acetylation of RelA/p65 were also increased in Glrx1−/− mice compared with air-exposed WT mice. These results suggested that augmented phosphorylation and acetylation of RelA/p65 are important contributing factors in CS-mediated NF-κB-dependent proinflammatory cytokine release in Glrx1 KO mouse lung. In contrast, there was no protection against CS-induced RelA/p65 nuclear translocation and its phosphorylation/acetylation in lung of Glrx1 overexpressing/transgenic mice (unpublished observations). These data further substantiate that alveolar epithelial cells do not play a critical role in CS-induced Glrx1-mediated lung inflammation.
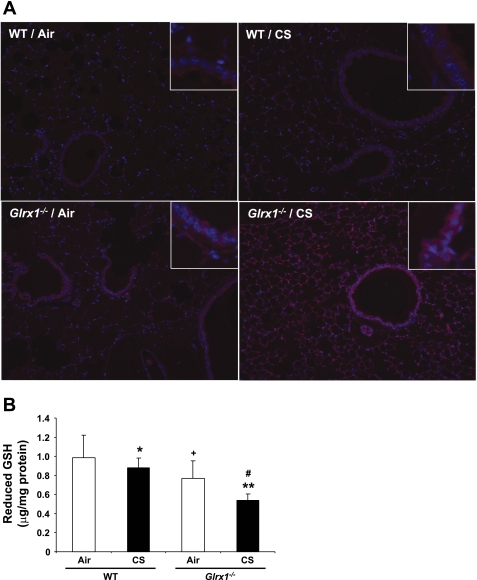
Deficiency of Glrx1 increased the protein glutathionylation in mouse lungs.
Significant increase in protein S-glutathionylation (PSSG) was reported in lungs of acute injury models using a method of Glrx1-catalyzed derivatization (2, 42). Increased PSSG level by oxidative stimuli is controlled by Glrx1-mediated deglutathionylation reaction (44). To investigate whether Glrx1 regulates CS-induced increase of PSSG levels in mouse lung, we examined PSSG reactivity using the Glrx1 catalyzed cysteine derivatization method. Consistent with decreased level of Glrx1 in response to CS (Fig. 1), PSSG levels were increased in lung of WT mice (Fig. 4A). Targeted disruption of Glrx1 further increased the level of PSSG in both bronchial and alveolar epithelium of mouse lung in response to CS exposure. Furthermore, lung of Glrx1−/− mice showed increased basal level of PSSG compared with corresponding WT mice. These data indicate that increased lung PSSG levels are associated with Glrx1 inactivation/deficiency in response to CS exposure.
Fig. 4.
Protein S-glutathionylation was increased in lung of Glrx1−/− mice in response to CS exposure. A: protein S-glutathionylation (PSSG) reactivity in mouse lung tissue was evaluated using Glrx1-based cysteine derivatization. Red, PSSG reactivity; blue, DNA content. Original magnification, ×100. Insets are magnified airway regions showing increased PSSG reactivity in response to CS. B: level of reduced GSH was measured in lungs of air- and CS-exposed WT and Glrx1−/− mice. Data are shown as means ± SE (n = 3–5/group). *P < 0.05, **P < 0.01, significant compared with respective air-exposed mice. #P < 0.05, significant compared with CS-exposed WT mice. +P < 0.05, significant compared with air-exposed WT mice.
It is possible that increased S-glutathionylation is associated with depletion of GSH in Glrx1−/− mice compared with WT mice. Our data showed that reduced GSH level in lung homogenates was decreased in air-exposed Glrx1−/− mice compared with air-exposed WT mice. Exposure to CS resulted in a significant decrease in reduced GSH level in lung of WT mice, which was further decreased in CS-exposed Glrx1−/− mice (Fig. 4B). These results suggested that CS-induced depletion of GSH is associated with increased S-glutathionylation and subsequent accumulation of more extensive specific S-glutathionylated protein in mouse lung.
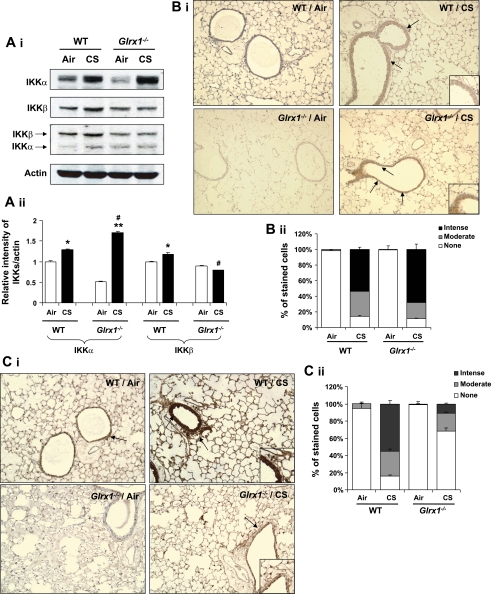
Differential regulation of IKKα and IKKβ in lung of Glrx1-deficient mice in response to CS exposure.
It is reported that NF-κB activation is regulated by IKK proteins, and IKKβ glutathionylation affects its own activity (inhibition of IKKβ activity) (34, 41). Hence, we hypothesized that increased NF-κB activation is due to augmented activation of IKKs in Glrx1−/− mice exposed to CS. We first investigated the levels of IKKα and IKKβ in mouse lung in response to CS exposure using immunoblot and immunohistochemical analyses. We found that CS exposure increased the level of IKKα in both lung homogenates and lung sections of WT mice, which was further increased in Glrx1−/− mice (Fig. 5, A and B). In contrast, Glrx1−/− mice showed significant decrease in IKKβ protein expression compared with WT mice exposed to CS (Fig. 5, A and C). Consistent with this observation, immunostaining studies revealed that IKKβ-positive cells were not increased in bronchial epithelium region of CS-exposed Glrx1−/− mice compared with WT (Fig. 5C). In contrast, overexpression of Glrx1 in type II alveolar epithelial cells did not affect the increased lung IKKα level compared with Glrx1−/− mice, which confirmed that type II cells were not responsible for Glrx1-mediated lung inflammatory response (unpublished observations). These findings suggest that CS regulates differential activation of IKKs, and increased IKKα level is associated with augmented CS-induced lung inflammation in Glrx1−/− mice.
Fig. 5.
Differential regulation of IKKα and IKKβ in lung of Glrx1−/− mice in response to CS exposure. A: IKKs were blotted by anti-IKKα, anti-IKKβ, and anti-IKKα/β antibody, respectively, in lung of WT and Glrx1−/− mice (i). ii: histograms showing relative intensity of IKKs vs. actin bands. Gel pictures shown are representative of at least 3 separate experiments. *P < 0.05, **P < 0.01, significant compared with respective air-exposed mice. #P < 0.05, significant compared with CS-exposed WT mice. IKKα (B) and IKKβ (C) positive cells were identified by dark brown immunohistochemical staining (i). ii: immunostaining scores for IKKs per cell in alveolar and airway regions of the lung. The assessment of immunostaining intensity was performed semiquantitatively and in a blinded fashion. Insets are magnified airway regions showing increased IKKα (B) and IKKβ (C) staining in response to CS compared with air-expressed WT and Glrx1-/- mice. Solid bars, intense staining; shaded bars, moderate/weak staining; open bars, no staining. Original magnification, ×100.
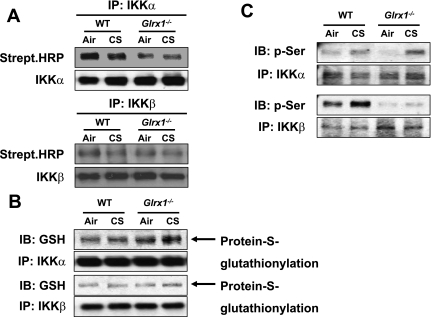
Modifications of IKKα were target for CS-mediated lung inflammation in Glrx1−/− mice.
It has been shown that S-glutathionylation of cysteine residue of IKKβ by oxidants regulates NF-κB signaling pathway (41, 45). Glrx1 catalyzes disulfide reductions, which is known to control S-glutathionylation of target proteins (36, 56). As a first step in clarifying the IKK modification by CS, we investigated IKK thiol reactivity using the irreversible thiol-biotinylating agent, maleimide-PEO2-biotin. Modification of IKKα and IKKβ protein with maleimide-biotin occurred in air-exposed WT mice. These modifications were attenuated in lungs of CS-exposed WT mice that were further decreased in Glrx1−/− mice, suggesting that reactive thiols of IKKs were regulated by Glrx1 in response to CS exposure (Fig. 6A). Moreover, IKKα and IKKβ immunoprecipitations were carried out under nonreducing conditions of immunoblotting with anti-GSH antibody that detects S-glutathionylated protein thiols (51). As expected, we found that IKKβ was S-glutathionylated in its inactive form in CS-exposed WT mouse lung, which was consistent with previous studies showing that oxidants increase S-glutathionylation of IKKβ (41). KO of Glrx1 further increased S-glutathionylated IKKβ level in lungs of mouse exposed to CS (Fig. 6B). Similarly, increase in S-glutathionylated IKKα level was observed in Glrx1−/− mice compared with WT mice exposed to CS. These data suggest that CS-induced increase of S-glutathionylated IKKα and IKKβ is perhaps not directly associated with NF-κB-mediated lung inflammation in Glrx1−/− mice. Rather, some other modifications on IKKs would regulate the activation of NF-κB and CS-mediated lung inflammation in Glrx1−/− mice.
Fig. 6.
Inactivation of IKKβ and accumulation of phosphorylated IKKα in Glrx1−/− mice in response to CS exposure. A: lung homogenates were subjected to immunoprecipitation with IKKα and IKKβ antibodies, and immunoblotted with streptavidin-conjugated HRP (top) and IKKα and IKKβ (lower) antibodies. B: IKKα and IKKβ immunoprecipitates separated under nonreducing conditions, and then immunoblotted with anti-GSH (top) and IKKα and IKKβ (lower) antibodies. C: immunoprecipitated IKKα and IKKβ were analyzed for phosphorylation of serine residues using specific phospho-serine antibody, respectively. Gel pictures shown are representative of at least 3 separate experiments.
As mentioned above, glutathionylated IKKβ inactivates its own activity (41); however, it is not known whether IKKα activity is also altered upon S-glutathionylation. Therefore, we determined another key posttranslational modification, i.e., phosphorylation of IKKα and IKKβ in lungs of WT and Glrx1−/− mice in response to CS exposure since their phosphorylation pattern reflects active forms of IKKs. CS exposure increased the level of phosphorylated IKKα in lung of WT mice, which was further increased in Glrx1−/− mice (Fig. 6C). Interestingly, phosphorylation of IKKβ in lung was not altered in Glrx1−/− mice (presumably due to the extensive glutathionylation of IKKβ) compared with WT mice exposed to CS. These findings suggest that IKKα is activated by phosphorylation despite it is S-glutathionylated and contributed to increased IKK activity leading to NF-κB-mediated lung inflammation in Glrx1−/− mice.
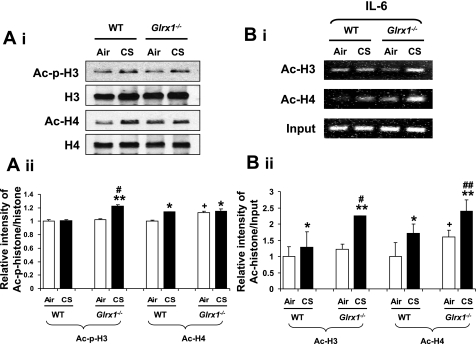
Targeted disruption of Glrx1 altered CS-mediated histone modification.
IKKα is thought to regulate phospho-acetylation of histone H3 and acetylation of histone H4, thereby inducing chromatin remodeling (30, 54). In the present study, we found that CS exposure also induced chromatin modifications including phospho-acetylation of histone H3 (Ser10/Lys9) and acetylation of histone H4 (Lys12) in lung of WT mice (Fig. 7A).Targeted disruption of Glrx1 further increased the degree of histone modifications compared with WT mice in response to CS exposure. However, the overexpression of Glrx1 in alveolar epithelial cells did not contribute in protection against CS-induced histone acetylation in mouse lung, although there was a slight reduction in histone H4 acetylation, but that was statistically insignificant between Glrx1 transgenic and WT mice exposed to CS (unpublished observations). ChIP assay is the most powerful tool for identifying proteins associated with specific promoter regions. We examined the CS-induced acetylation of histone H3 and H4 on IL-6 promoter by the ChIP assay in mouse lung homogenates. We found that the levels of acetylated histones H3 and H4 on IL-6 promoter were increased in CS-exposed WT mice, which was further increased in Glrx1−/− mice (Fig. 7B). These results suggest that the proinflammatory responses seen in lung of Glrx1−/− mice are associated with increased histone modification/acetylation via increased phosphorylation of IKKα and increased NF-κB binding on promoters of various proinflammatory genes.
Fig. 7.
Deficiency of Glrx1 led to increased histone modification in mouse lungs in response to CS exposure. A: histone modifications were assessed by measuring phosphorylation (Ser10) and acetylation (Lys9) of histone H3, and acetylation of histone H4 (Lys12) in lung by immunoblotting using acid-extracted nuclear histone fraction (i). B: acetylated histone H3 and H4 on the IL-6 gene promoter in mouse lung were analyzed by ChIP assay. Lung homogenates were immunoprecipitated with anti-acetylated histone H3 and H4 antibodies, and chromatin modification on the promoter region of proinflammatory cytokine was detected by PCR using the primers for IL-6. Gel pictures shown are representative of at least 3 separate experiments.*P < 0.05, **P < 0.01, significant compared with respective air-exposed mice. #P < 0.05, ##P < 0.01, significant compared with CS-exposed WT mice. +P < 0.05 significant compared with air-exposed WT mice.
DISCUSSION
Glrx1 is a GSH-dependent reductase that is involved in redox regulation of various signaling proteins by catalyzing reversible protein S-glutathionylation (4, 22, 26, 44). It has been shown that Glrx1 is mainly localized in bronchial epithelium in mouse lung (2, 42), and prominent expression of Glrx1 was observed in alveolar macrophages in human (31). Our data show decreased abundance of Glrx1 in bronchial epithelium in lungs of mouse exposed to CS suggesting that Glrx1 is indeed localized in bronchial epithelium and it is reduced in response to CS exposure in mouse lung.
We determined whether the loss of Glrx1 augments the CS-mediated lung inflammation via redox posttranslational modifications of IKKα and IKKβ in mouse lung. Acute CS exposure resulted in increased lung inflammatory neutrophil influx and proinflammatory mediator release associated with reduced Glrx1 expression in WT mice, which was further enhanced in Glrx1−/− mice. Similarly, proinflammatory response was also seen in Glrx1-deficient mice in response to oxidants in lens and heart (25, 26). However, Glrx1−/− mice were not susceptible to ischemia/reperfusion and hyperoxia (17). Furthermore, Glrx1 deficiency led to attenuation of redox-sensitive transcription factor NF-κB activation in lung epithelial cells in response to LPS challenge (41). The reason for these discrepancies compared with our findings is not known, but it may be due to different stimuli used in different tissues.
In this study, the susceptibility of Glrx1−/− mice to CS-mediated inflammation is confirmed by increased NF-κB nuclear translocation and posttranslational modifications of NF-κB subunit, RelA/p65. Our data revealed that CS exposure induced the degradation of IκBα in WT, which was augmented in lungs of Glrx1−/− mice associated with RelA/p65 nuclear translocation. Additionally, we observed that genetic deficiency of Glrx1 led to further increased phosphorylation of RelA/p65 on Ser276 and Ser536 in lung compared with WT mice in response to CS. Recent evidence showed that phosphorylation of Ser276 is required for its association with CBP/p300 (60), whereas Ser536 is a prerequisite for acetylation of RelA/p65 on Lys310, which plays an important role in proinflammatory gene transcription (7, 55). Consistent with these reports, CS exposure induced the acetylation of RelA/p65 on Lys310 in WT mice, which was augmented in Glrx1−/− mice. Overall, the proinflammatory response including cytokine release and NF-κB activation were enhanced in lung of Glrx1 KO mice compared with WT mice in response to CS. These findings indicate that Glrx1 regulates NF-κB activation possibly by reversible protein S-glutathionylation; however, this control is lost in Glrx1−/− mice leading to augmented activation of NF-κB-dependent lung inflammatory response. Interestingly, overexpression of Glrx1 in lung type II cells had no effect on CS-induced lung inflammation and NF-κB activation. This suggests that Glrx1 in bronchial epithelium, rather than alveolar epithelium, plays an important role in regulation of CS-induced lung inflammation.
Glrx1 functions as a redox switch by catalyzing reversible protein S-glutathionylation (24, 26). S-glutathionylation alters the function of many proteins, such as kinases and redox-sensitive transcription factors. A previous study has shown that Glrx1 deficiency led to reduced NF-κB activity in lung epithelial cells through S-glutathionylation of IKKβ in response to LPS (41). Overexpression of Glrx1 led to increased NF-κB-mediated adhesion molecule expression in retinal cells (45). Our study shows increased NF-κB activity in lungs of Glrx1 KO mice in response to CS. This suggests that LPS and CS may have differential effects in triggering NF-κB-mediated lung inflammatory response involving protein S-glutathionylation when the lung level of Glrx1 is decreased. Thus, our findings provide the information on the role for Glrx1 in protection against CS-induced lung inflammation.
IKKs directly phosphorylate IκBα in response to proinflammatory stimuli. IKK contains two major catalytic subunits, IKKα and IKKβ (10, 33). Glutathionylation of IKKβ modulates its activity and/or regulates cellular signaling pathway, thereby influencing the transactivation potential of NF-κB (19). In this study, we found that S-glutathionylation of IKKα and IKKβ along with in situ protein S-glutathionylation were increased in lung of Glrx1−/− mice compared with WT mice in response to CS exposure. S-glutathionylation of IKKβ has been reported to impinge inhibitory effect on IKK leading to inactivation of NF-κB particularly using in vitro systems (41, 45, 46). On the contrary, we observed that NF-κB-mediated proinflammatory response was further increased despite increased S-glutathionylation of IKKβ in lung of Glrx1−/− mice compared with that of WT mice exposed to CS. The reason for this differential in vitro and in vivo effect is not well known. One possible explanation would be the involvement of another posttranslational modification, such as phosphorylation of IKKs. More than one type of posttranslational modification occurs on proteins (12). For example, phosphorylation and glycosylation occur on a different site of signal transducer and activator of transcriptions (STATs) (14, 16). Therefore, it is possible that S-glutathionylation on cysteine residues could also be subject to phosphorylation of serine, threonine, and tyrosine residues on signaling proteins (44). We showed for the first time that S-glutathionylation of IKKs in Glrx1−/− mice was increased compared with WT mice in response to CS, and the phosphorylated IKKα contributed to increased NF-κB-mediated lung inflammation in Glrx1−/− mice. Once IKKα is activated by phosphorylation, it leads to sustained gene transcription of proinflammatory mediators via the increase in phospho/acetylation of RelA/p65 and histones H3 and H4 (54). In contrast, no phosphorylation on IKKβ was found in Glrx1−/− mice lung in response to CS. The relative interplay between S-glutathionylated and phosphorylated IKKα and glu kinase activity) is critical in phospho/acetylation of histone H3, and acetylation of Lys310 on RelA/p65 on proinflammatory gene promoters (54). Our findings corroborated with the above studies showing that acetylation of histones H3 and H4 along with RelA/p65 were augmented in lung of Glrx1−/− mice compared with WT mice in response to CS. Our data further showed that acetylated H3 and H4 on IL-6 gene promoter were increased in lungs of Glrx1−/− mice compared with CS-exposed WT mice. These observations suggest that S-glutathionylation of IKKα plays an important role in histone acetylation on proinflammatory gene promoters resulting in sustained lung inflammation.
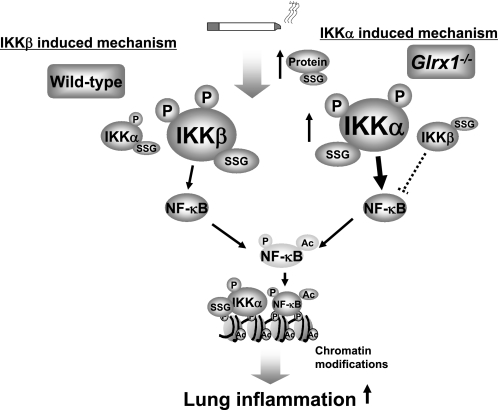
In summary, our findings provide a novel role for Glrx1 in lung inflammation in response to CS exposure. Enhanced inflammatory responses and RelA/p65 posttranslational modifications were observed in lungs of Glrx1−/− mice exposed to CS. In addition, not only S-glutathionylation but also phosphorylation of IKKα was observed in Glrx1 KO lung, which may be the underlying mechanism for abnormal and sustained inflammatory response in lung of these mice exposed to CS (Fig. 8). These posttranslational modifications on IKKα act as a key regulator of CS-mediated lung inflammation via histone acetylation on proinflammatory genes in Glrx1−/− mice. Our findings not only demonstrate the important role of Glrx1 in NF-κB-mediated abnormal lung inflammation by oxidants/CS, but also provide a new insight into the molecular mechanisms in redox-mediated posttranslational modifications of NF-κB signaling pathways and chromatin modifications, particularly in pathogenesis of chronic inflammatory lung diseases. Thus, regulation of this key redox enzyme, Glrx1, may provide better insight in understanding the pathogenesis of CS-mediated inflammatory lung diseases and devising better redox-based therapeutic strategies.
Fig. 8.
A schematic diagram for the role of Glrx1 in regulation of IKKα and IKKβ in response to CS. Targeted disruption of Glrx1 leads to increased lung proinflammatory response, which is associated with NF-κB activation through the differential regulation of IKKs, including glutathionylation and phosphorylation in response to CS exposure. Glrx1 deficiency leads to increased IKKα and IKKβ activation in response to CS associated with S-glutathionylation. IKKβ-S-glutathionylation leads to its inactivation and hence inhibition of NF-κB (dotted lines), whereas posttranslational modifications (S-glutathionylation and phosphorylation) on IKKα cause NF-κB activation and chromatin modifications (histone acetylation) on proinflammatory genes. IKKα and IKKβ modifications are reversible, and the inflammatory pathway is predominantly mediated by IKKβ.
GRANTS
This study was supported by the Institute for Science and Health and National Institutes of Health Grants R01-HL-085613 and P30-ES-01247.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Saravanan Rajendrasozhan for technical assistance, Dr. Michael Bulger for help in ChIP assay, and Dr. Vuokko Kinnula, University of Helsinki, Finland, for useful discussions.
REFERENCES
- 1.Adenuga D, Yao H, March TH, Seagrave J, Rahman I. Histone deacetylase 2 is phosphorylated, ubiquitinated, and degraded by cigarette smoke. Am J Respir Cell Mol Biol 40: 464–473, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aesif SW, Anathy V, Havermans M, Guala AS, Ckless K, Taatjes DJ, Janssen-Heininger YM. In situ analysis of protein S-glutathionylation in lung tissue using glutaredoxin-1-catalyzed cysteine derivatization. Am J Pathol 175: 36–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anathy V, Aesif SW, Guala AS, Havermans M, Reynaert NL, Ho YS, Budd RC, Janssen-Heininger YM. Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas. J Cell Biol 184: 241–252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol 71: 551–564, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Chariot A. The NF-kappaB-independent functions of IKK subunits in immunity and cancer. Trends Cell Biol 19: 404–413, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J 21: 6539–6548, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol 25: 7966–7975, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect 64: 111–126, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dailianis S, Patetsini E, Kaloyianni M. The role of signalling molecules on actin glutathionylation and protein carbonylation induced by cadmium in haemocytes of mussel Mytilus galloprovincialis (Lmk). J Exp Biol 212: 3612–3620, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science 284: 309–313, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Di Stefano A, Caramori G, Oates T, Capelli A, Lusuardi M, Gnemmi I, Ioli F, Chung KF, Donner CF, Barnes PJ, Adcock IM. Increased expression of nuclear factor-kappaB in bronchial biopsies from smokers and patients with COPD. Eur Respir J 20: 556–563, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Dunphy JT, Linder ME. Signalling functions of protein palmitoylation. Biochim Biophys Acta 1436: 245–261, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal 6: 63–74, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Gewinner C, Hart G, Zachara N, Cole R, Beisenherz-Huss C, Groner B. The coactivator of transcription CREB-binding protein interacts preferentially with the glycosylated form of Stat5. J Biol Chem 279: 3563–3572, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Gloire G, Horion J, El Mjiyad N, Bex F, Chariot A, Dejardin E, Piette J. Promoter-dependent effect of IKKalpha on NF-kappaB/p65 DNA binding. J Biol Chem 282: 21308–21318, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hanover JA. Glycan-dependent signaling: O-linked N-acetylglucosamine. FASEB J 15: 1865–1876, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Ho YS, Xiong Y, Ho DS, Gao J, Chua BH, Pai H, Mieyal JJ. Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic Biol Med 43: 1299–1312, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue K, Takano H, Kaewamatawong T, Shimada A, Suzuki J, Yanagisawa R, Tasaka S, Ishizaka A, Satoh M. Role of metallothionein in lung inflammation induced by ozone exposure in mice. Free Radic Biol Med 45: 1714–1722, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Janssen-Heininger YM, Poynter ME, Aesif SW, Pantano C, Ather JL, Reynaert NL, Ckless K, Anathy V, van der Velden J, Irvin CG, van der Vliet A. Nuclear factor kappaB, airway epithelium, and asthma: avenues for redox control. Proc Am Thorac Soc 6: 249–255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson CE, Huang YY, Parrish AB, Smith MI, Vaughn AE, Zhang Q, Wright KM, Van Dyke T, Wechsler-Reya RJ, Kornbluth S, Deshmukh M. Differential Apaf-1 levels allow cytochrome c to induce apoptosis in brain tumors but not in normal neural tissues. Proc Natl Acad Sci USA 104: 20820–20825, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinnula VL, Vuorinen K, Ilumets H, Rytila P, Myllarniemi M. Thiol proteins, redox modulation and parenchymal lung disease. Curr Med Chem 14: 213–222, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem 267: 4928–4944, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim Biophys Acta 1780: 1304–1317, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Lofgren S, Fernando MR, Xing KY, Wang Y, Kuszynski CA, Ho YS, Lou MF. Effect of thioltransferase (glutaredoxin) deletion on cellular sensitivity to oxidative stress and cell proliferation in lens epithelial cells of thioltransferase knockout mouse. Invest Ophthalmol Vis Sci 49: 4497–4505, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Malik G, Nagy N, Ho YS, Maulik N, Das DK. Role of glutaredoxin-1 in cardioprotection: an insight with Glrx1 transgenic and knockout animals. J Mol Cell Cardiol 44: 261–269, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Meyer LM, Lofgren S, Ho YS, Lou M, Wegener A, Holz F, Soderberg P. Absence of glutaredoxin1 increases lens susceptibility to oxidative stress induced by UVR-B. Exp Eye Res 89: 833–839, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Morrison D, Rahman I, Lannan S, MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. Am J Respir Crit Care Med 159: 473–479, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis 27: 811–819, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park GY, Christman JW. Nuclear factor kappa B is a promising therapeutic target in inflammatory lung disease. Curr Drug Targets 7: 661–668, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Park GY, Wang X, Hu N, Pedchenko TV, Blackwell TS, Christman JW. NIK is involved in nucleosomal regulation by enhancing histone H3 phosphorylation by IKKalpha. J Biol Chem 281: 18684–18690, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peltoniemi M, Kaarteenaho-Wiik R, Saily M, Sormunen R, Paakko P, Holmgren A, Soini Y, Kinnula VL. Expression of glutaredoxin is highly cell specific in human lung and is decreased by transforming growth factor-beta in vitro and in interstitial lung diseases in vivo. Hum Pathol 35: 1000–1007, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Peltoniemi MJ, Rytila PH, Harju TH, Soini YM, Salmenkivi KM, Ruddock LW, Kinnula VL. Modulation of glutaredoxin in the lung and sputum of cigarette smokers and chronic obstructive pulmonary disease. Respir Res 7: 133, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 8: 49–62, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene 25: 6717–6730, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Pineda-Molina E, Klatt P, Vazquez J, Marina A, Garcia de Lacoba M, Perez-Sala D, Lamas S. Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry 40: 14134–14142, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Prinarakis E, Chantzoura E, Thanos D, Spyrou G. S-glutathionylation of IRF3 regulates IRF3-CBP interaction and activation of the IFN beta pathway. EMBO J 27: 865–875, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qanungo S, Starke DW, Pai HV, Mieyal JJ, Nieminen AL. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. J Biol Chem 282: 18427–18436, 2007 [DOI] [PubMed] [Google Scholar]
- 37a.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1: 3159–3165, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Rahman I, MacNee W. Regulation of redox glutathione levels and gene transcription in lung inflammation: therapeutic approaches. Free Radic Biol Med 28: 1405–1420, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Rajendrasozhan S, Yang SR, Edirisinghe I, Yao H, Adenuga D, Rahman I. Deacetylases and NF-kappaB in redox regulation of cigarette smoke-induced lung inflammation: epigenetics in pathogenesis of COPD. Antioxid Redox Signal 10: 799–811, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci USA 101: 8945–8950, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EF, Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci USA 103: 13086–13091, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynaert NL, Wouters EF, Janssen-Heininger YM. Modulation of glutaredoxin-1 expression in a mouse model of allergic airway disease. Am J Respir Cell Mol Biol 36: 147–151, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitz ML, Bacher S, Kracht M. I kappa B-independent control of NF-kappa B activity by modulatory phosphorylations. Trends Biochem Sci 26: 186–190, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein S-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal 7: 348–366, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Shelton MD, Distler AM, Kern TS, Mieyal JJ. Glutaredoxin regulates autocrine and paracrine proinflammatory responses in retinal glial (muller) cells. J Biol Chem 284: 4760–4766, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shelton MD, Kern TS, Mieyal JJ. Glutaredoxin regulates nuclear factor kappa-B and intercellular adhesion molecule in Muller cells: model of diabetic retinopathy. J Biol Chem 282: 12467–12474, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Shelton MD, Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cells 25: 332–346, 2008 [PMC free article] [PubMed] [Google Scholar]
- 48.Sykes MC, Mowbray AL, Jo H. Reversible glutathiolation of caspase-3 by glutaredoxin as a novel redox signaling mechanism in tumor necrosis factor-alpha-induced cell death. Circ Res 100: 152–154, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Torres L, Sandoval J, Penella E, Zaragoza R, Garcia C, Rodriguez JL, Vina JR, Garcia-Trevijano ER. In vivo GSH depletion induces c-myc expression by modulation of chromatin protein complexes. Free Radic Biol Med 46: 1534–1542, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Woo HJ, Bae CH, Song SY, Kim YW, Lee HM, Kim YD. Expression of glutaredoxin-1 in nasal polyps and airway epithelial cells. Am J Rhinol Allergy 23: 288–293, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Xie Y, Kole S, Precht P, Pazin MJ, Bernier M. S-glutathionylation impairs signal transducer and activator of transcription 3 activation and signaling. Endocrinology 150: 1122–1131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature 423: 655–659, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol 291: L46–L57, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Yang SR, Valvo S, Yao H, Kode A, Rajendrasozhan S, Edirisinghe I, Caito S, Adenuga D, Henry R, Fromm G, Maggirwar S, Li JD, Bulger M, Rahman I. IKK alpha causes chromatin modification on pro-inflammatory genes by cigarette smoke in mouse lung. Am J Respir Cell Mol Biol 38: 689–698, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol 292: L567–L576, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Yang Y, Jao S, Nanduri S, Starke DW, Mieyal JJ, Qin J. Reactivity of the human thioltransferase (glutaredoxin) C7S, C25S, C78S, C82S mutant and NMR solution structure of its glutathionyl mixed disulfide intermediate reflect catalytic specificity. Biochemistry 37: 17145–17156, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol 294: L1174–L1186, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Yao H, Edirisinghe I, Yang SR, Rajendrasozhan S, Kode A, Caito S, Adenuga D, Rahman I. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am J Pathol 172: 1222–1237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao H, Yang SR, Edirisinghe I, Rajendrasozhan S, Caito S, Adenuga D, O'Reilly MA, Rahman I. Disruption of p21 attenuates lung inflammation induced by cigarette smoke, LPS, and fMLP in mice. Am J Respir Cell Mol Biol 39: 7–18, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell 9: 625–636, 2002 [DOI] [PubMed] [Google Scholar]