Abstract
Induction of effective inflammation in the lung in response to environmental and microbial stimuli is dependent on cooperative signaling between leukocytes and lung tissue cells. We explored how these inflammatory networks are modulated by diesel exhaust particles (DEP) using cocultures of human monocytes with epithelial cells. Cocultures, or monoculture controls, were treated with DEP in the presence or absence of LPS or flagellin. Production of cytokines was explored by Western blotting and ELISA; cell signaling was analyzed by Western blotting. Here, we show that responses of epithelial cells to DEP are amplified by the presence of monocytes. DEP amplified the responses of cellular cocultures to very low doses of TLR agonists. In addition, in the presence of DEP, the responses induced by LPS or flagellin were less amenable to antagonism by the physiological IL-1 antagonist, IL-1ra. This was paralleled by the uncoupling of IL-1 production and release from monocytes, potentially attributable to an ability of DEP to sequester or degrade extracellular ATP. These data describe a model of inflammation where DEP amplifies responses to low concentrations of microbial agonists and alters the nature of the inflammatory milieu induced by TLR agonists.
Keywords: cocultures, inflammation, interleukin-1, Toll-like receptors
environmental exposure to microbial products through the inhalation of bacterial fragments, such as LPS, has long been associated with exacerbations of airway disease, and levels of LPS within the environment can correlate with severity of asthma (30, 38). Asthma and COPD are commonly exacerbated by bacterial and viral infections (3, 24) that can often coexist and synergize with pollutant exposure (8, 46). Inhalation of small particulate matter is also a major cause of airway inflammation, and rising air pollution levels are associated with elevated mortality and increased hospital admissions in individuals with respiratory diseases such as asthma and COPD (2, 10, 45).
Diesel exhaust particles (DEP) are the major particulate matter of air pollution and comprise a nanoparticular carbonaceous core, multiple organic components such as polyaromatic hydrocarbons (PAH), transition metals, and adsorbed materials such as pollen and dust. Their small size (5–20 nm; 100-nm aggregates) means that they are often denoted nanoparticles and favors their deposition in the lung (7, 11). Acute and chronic inhalation of DEP in healthy individuals, and those with preexisting respiratory disease, results in respiratory toxicity with consequent development of lung edema, infiltration of polymorphonuclear leukocytes, and the production of proinflammatory cytokines and reactive oxygen species (ROS) (5, 36, 44, 47).
The active inflammation that occurs in asthma and COPD leads to the recruitment of monocytes to the alveolar wall (28). Furthermore, urban particulate matter has been shown to cause the release of monocytes from the bone marrow (17, 23). We have previously shown that effective inflammatory responses to bacterial-derived stimuli are mediated by cooperative signaling between monocytes and tissue cells (31, 32, 39, 50). The direct effects of DEP, with the production of proinflammatory cytokines and ROS in single cell types, are well established (4, 9, 25, 27, 29, 37, 43, 49), but less is known about their actions in models of the cell networks that underpin inflammatory responses. It is also likely that air pollutants such as DEP will coexist in the environment with microbial products, and that these pollution particulates can act as carriers for microbes and allergens, delivering them to sites in the airways. The coexistence of DEP and microbial products such as LPS and flagellin may modulate the inflammatory response to these pathogenic stimuli.
In this study, we show that DEP exposure induced a modest proinflammatory response in models of airway inflammation when monocytes were cocultured with airway epithelial cells. DEP also caused a potentiation of the responses of epithelial cell/monocyte cocultures to microbial stimuli. We have shown previously that IL-1 is crucial to the induction of inflammation in cocultures stimulated with microbial stimuli (31, 32), but importantly, here we reveal that DEP altered the inflammatory milieu such that targeting of the IL-1 pathway was markedly less able to reduce inflammatory responses when DEP were present.
MATERIALS AND METHODS
Materials.
Reagents were purchased from Sigma-Aldrich or Invitrogen (Paisley, UK), except where specified. OptiPrep density gradient was from Axis Shield (Oslo, Norway). The Monocyte Isolation Kit II was from Miltenyi Biotec (Bergisch Gladbach, Germany). Purified LPS (LPS; Escherichia coli serotype R515) was from Alexis (Nottingham, UK). Endotoxin-free flagellin from Pseudomonas aeruginosa was a generous gift from Dr. Yolanda Sanchez (GlaxoSmithKline, King of Prussia, PA), and flagellin purified from Salmonella typhimurium was purchased from Invivogen (Toulouse, France). Both flagellin types exhibited similar in vitro properties. IL-1ra was from National Biological Standards Board (NIBSC, Potters Bar, UK), and hydrocortisone was purchased from PromoCell (Heidelberg, Germany). The immortalized epithelial cell line BEAS-2B cells were purchased from American Type Culture Collection (Manassas, VA). Matched ELISA antibody pairs were from R&D Systems (Abingdon, UK). PE-conjugated anti-human TLR4 or CD14 and appropriate isotype controls were purchased from eBioscience (San Diego, CA). The Hyband-c-extra nitrocellulose membrane and EZ-ECL system were from Amersham Pharmacia Biotech (Buckinghamshire, UK). The anti-phospho-p38 antibody was from Promega (Southampton, UK). The anti-phospho IκBα, human IL-1β mouse monoclonal antibody, and anti-rabbit HRP-conjugated secondary antibody were from Cell Signaling Technologies. Anti-actin was from Sigma-Aldrich. A soluble TNFα receptor fusion protein was purchased from R&D Systems.
DEP.
In these studies we have used a standardized DEP, SRM 2975 from National Institute of Standards and Technology (Dept. of Commerce, United States). The DEP has been prepared from an industrial fork-lift truck and has the advantage that the particulate matter has been analyzed and evaluated for PAH concentrations, mass fraction, and thus controlling for variables such as nitric oxide, ozone, and sulfur dioxide content. Diesel particles are typically of the order of 60 nm in diameter; however, they agglomerate in the environment and in culture. Particles were prepared by ultrasonication, but cells were exposed to a mixture of agglomerates of various sizes and single nanoparticles.
Maintenance of BEAS-2B cell line.
BEAS-2B epithelial cells were maintained in 75-cm2 flasks (Nunc, Denmark) in RPMI 1640 with 10% BioWhittaker or Promocell low endotoxin (<0.01 EU/ml) FBS and 1% penicillin G (100 U/ml) and streptomycin (100 μg/ml). The composition of cell culture media was the same throughout, unless stated otherwise. BEAS-2B cells routinely tested negative for mycoplasma.
Monocyte preparation.
Peripheral venous blood was taken with written informed consent from healthy volunteers in accordance with a protocol approved by the South Sheffield Research Ethics Committee. Whole blood was anticoagulated with 3.8% trisodium citrate. Plasma and platelets were removed by centrifugation, and following dextran sedimentation, peripheral blood mononuclear cells (PBMCs) were separated from granulocytes by centrifugation over density gradients using either plasma/Percoll or OptiPrep as previously described (19). Monocytes were further purified by negative magnetic selection using the Monocyte Isolation Kit II from Miltenyi Biotec (Bergisch Gladbach, Germany) to a purity of 91 ± 0.5% (means ± SE) CD14+ cells.
Coculture models.
BEAS-2B cells were stimulated in 24-well plates when ∼80% confluent. At the time of incubation, the cells were washed with PBS, and the cell culture media was replaced. DEP SRM 2975 were suspended in PBS at 5 mg/ml. Before use, stock suspensions of DEP were sonicated twice for two periods of 10 min. Cocultures of BEAS-2B cells with 5,000 purified monocytes per well, or the equivalent monoculture controls, were stimulated with varying concentrations of DEP in the presence or absence of LPS or flagellin (at the indicated concentrations). In subsequent experiments, 50 μg/ml DEP was used, and, in some experiments, 10 μg/ml IL-1ra, 1 μg/ml hydrocortisone, or 0.5 μg/ml TNFα receptor fusion protein (sTNFR1) were added to the cell cultures at the same time as the DEP. Final volumes in each well of a 24-well plate always totaled 500 μl during experiments. Stimulated cells were incubated for 24 h at 37°C and humidified 5% CO2.
ELISA.
Cell-free supernatants were prepared and stored at −80°C until CXCL8 or IL-1β cytokine generation was determined by ELISA using matched pairs of antibodies at optimized concentrations as previously described (31). Absorbance was measured at 450 nm using an MRX plate reader (Thermo Labsystems, Vantaa, Finland) and Biolinx software version 2.20 (Frankfurt am Main, Germany). Samples were typically diluted so that the optical density fell within the optimal portion of a log-linear standard curve. Limits of detection (LD) for the ELISAs were 31.25 pg/ml. Samples whose values were below the LD were assigned the LD value for analysis.
Western blotting.
BEAS-2B cells or monocytes alone were stimulated in 12-well plates with 50 μg/ml DEP, 1 pg/ml LPS, or both DEP and LPS for 15, 30, and 60 min. Protein was obtained by lysing samples as previously described (40) and analyzed by SDS gel electrophoresis blotted onto Hyband-c-extra nitrocellulose membrane. Samples were probed with 1:1,000 anti-p38 monoclonal IgM (rabbit), 1:5,000 anti-actin (rabbit), or 1:1,000 anti-IL-1β (mouse) monoclonal antibody. Binding was detected using a 1:2,000 anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibody, respectively, and enhanced chemiluminescence detected with Amersham ECL detection reagents. Quantitative signals were derived by densitometric analysis using NIH image 1.62 analysis software, and data displayed as ratio compared with actin loading control.
Flow cytometry.
Cells were stimulated with 50 μg/ml DEP, 1 pg/ml LPS, or both DEP and LPS for 1, 4, or 24 h. BEAS-2B cells were detached with non-enzymatic cell dissociation solution, pelleted by centrifugation (1,000 g), and resuspended in buffer (PBS, 0.25% bovine serum albumin, 10 mM HEPES). Monocytes were harvested by gentle pipetting. Cells were stained with 1:10 PE-conjugated anti-human TLR4 or CD14 antibody or their relevant 1:10 PE-conjugated mouse IgG2a and IgG1 kappa isotype controls, for 30 min in the dark on ice. Antibody binding was detected in the FL-2 channel of a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA) and quantified as specific mean fluorescence after subtraction of the respective isotype control. Data analysis was performed using FlowJo software (Tree Star, Ashland, OR) and quantified as specific mean fluorescence.
ATP determination.
1 μM ATP was suspended in dH2O, with or without 50 μg/ml DEP, for 3 h. The mixture was centrifuged at 1,000 g for 3 min to mimic the method used to obtain cell-free supernatants and then serially diluted. Samples were detected using an ATP determination kit (Invitrogen, Paisley, UK) as per the manufacturer's instructions. The ATP Determination Kit is a bioluminescence assay for quantitative determination of ATP with recombinant firefly luciferase and its substrate d-luciferin. Luminescence was determined using a Packard Biosciences Fusion universal microplate analyzer (PerkinElmer, Beaconsfield, UK).
Statistics.
Data were analyzed using GraphPad Prism v5 (San Diego, CA). Three or more groups of data were compared using two-way analysis of variance with the appropriate posttests with a statistically significant value of P < 0.05.
RESULTS
We have previously shown that mechanisms of airway inflammation can be dissected using cocultures of epithelial cells and monocytes, and have revealed the existence of IL-1-dependent inflammatory networks potentially amenable to therapeutic targeting (31, 32, 39, 50). Active inflammation in COPD and asthma delivers monocytes to the airway wall, and, accordingly, we initially investigated whether DEP pollution activated epithelial cell/monocyte cocultures and compared the inflammatory potential of these particles to microbial stimuli. We show here that DEP cause modest inflammatory responses in isolation, but potentiate responses to pathogenic stimuli and change the phenotype of airway inflammation.
DEP activates cocultures of epithelial cells and monocytes.
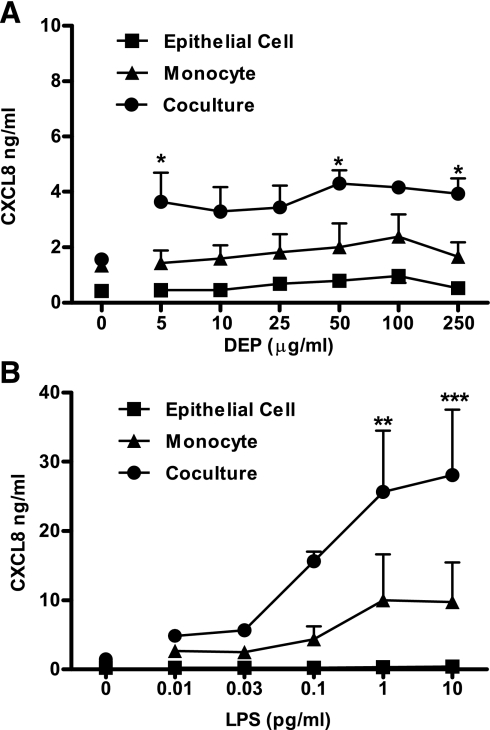
To investigate activation of proinflammatory pathways by DEP and/or TLR agonists, we examined production of CXCL8 from stimulated cells. CXCL8 is the dominant mediator responsible for neutrophil recruitment, and hence is highly relevant to the pathology of asthma and COPD. Moreover, this chemokine is classically induced by TLR activation, and production is primarily dependent on activation of NF-κB and p38 MAPKs, making it an ideal marker for activation of pathways associated with engagement of the innate immune system (6, 33). Stimulation of epithelial cells or monocyte monocultures for 24 h with DEP at concentrations of up to 250 μg/ml did not result in induction of inflammation as measured by CXCL8 production (Fig. 1A). We therefore established cocultures of epithelial cells and monocytes in 24-well plates and stimulated these for 24 h. In these cocultures, we observed that DEP induced significant CXCL8 production (Fig. 1A). However, this response was modest compared with a classic microbial inflammatory activator, LPS (Fig. 1B).
Fig. 1.
Cooperation between epithelial cells and monocytes results in the synergistic production of CXCL8 in response to diesel exhaust particles (DEP) or LPS. The epithelial cell line BEAS-2B (epithelial cell) was grown to 80% confluence in 24-well plates, and cocultures were created through the addition of 5,000 monocytes/well. Monocultures and cocultures were stimulated with DEP (A) or LPS (B) at the indicated concentrations, in the presence or absence of 5,000 monocytes/well. After 24 h, levels of CXCL8 in the supernatant were determined by ELISA. Data shown are means ± SE of n = 3 replicates. Each replicate was performed at a separate passage with freshly prepared monocytes from different donors. Significant differences between monolayer and epithelial cell/monocyte cocultures are indicated by *P < 0.05, **P < 0.01, and ***P < 0.001 as measured by 2-way ANOVA and Bonferroni's posttest.
DEP potentiates the proinflammatory response of cocultures to the TLR agonists LPS and flagellin.
Individuals with high DEP exposure are also likely to be inhaling various amounts of environmental contaminants such as LPS, and patients with COPD may have chronic or recurrent bacterial infections delivering microbial stimuli to the airway. Flagellin is the building block of flagellae, used by many bacteria for motility, and a potent stimulus of epithelial cell activation (12, 16).
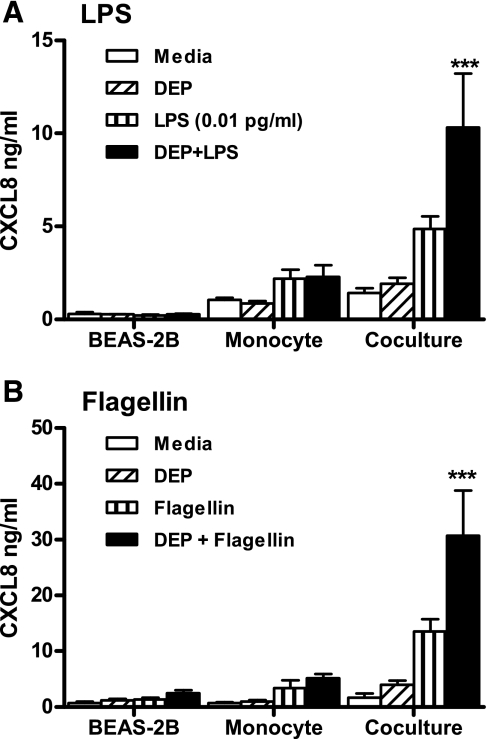
We therefore determined whether responses to flagellin were amplified in coculture models, and whether LPS or flagellin effects were amplified in the presence of DEP. For the experiments shown in Fig. 2, we selected a low concentration of LPS (0.01 pg/ml) that alone had minimal ability to activate cocultures. Stimulation of cocultures for 24 h with LPS and DEP in combination caused a synergistic increase in CXCL8 release (Fig. 2A). This synergistic increase in CXCL8 was also evident when lower concentrations of DEP were utilized (Supplementary Fig. S1. Supplemental data for this article is available online at the AJP-Lung web site.). Responses to flagellin were also greater in cocultures of epithelial cells and monocytes than the relevant monoculture controls, and again these responses were enhanced in the presence of DEP (Fig. 2B). The ability of DEP to amplify responses to endotoxin was confined to low concentrations of endotoxin causing only modest activation of epithelial cell/monocyte cocultures. Where cocultures generated ≥15 ng/ml CXCL8 in response to tested concentrations of LPS, these responses were not potentiated in the presence of DEP (data not shown).
Fig. 2.
DEP potentiates the proinflammatory response of LPS and flagellin in epithelial cell and monocyte cocultures. Epithelial cell/monocyte cocultures were established as described in Fig. 1. Cells were stimulated with 50 μg/ml DEP in the presence or absence of 0.01 pg/ml LPS (A) or 0.5 μg/ml S. typhimurium flagellin (B). After 24 h, levels of CXCL8 in the supernatant were determined by ELISA. Data shown are means ± SE of n = 5–6 replicates. Each replicate was performed at a separate passage with freshly prepared monocytes from different donors. Significant differences in CXCL8 are indicated by ***P < 0.001 compared with DEP or LPS alone, as measured by 2-way ANOVA and Bonferroni's posttest.
TLR engagement results in rapid activation of signaling to MAPKs and NF-κB. Accordingly, we investigated whether cotreatment of monocytes or epithelial cells with TLR agonists and DEP resulted in enhanced activation of these pathways. Although exposure of cocultures to DEP resulted in enhancement of cytokine generation in response to LPS, DEP did not induce measurable enhancement of TLR-associated intracellular signaling in monocytes or epithelial cells, specifically p38 MAPK phosphorylation (Supplementary Fig. S2) or NF-κB as measured by phosphorylation of IκBα (data not shown), over a 1-h time course. We next examined whether DEP might exert effects on TLR signaling over a longer time course (24 h), to investigate the possibility that DEP exposure might increase the expression of TLRs and accessory signaling molecules. We found that monocyte TLR4 and CD14 expression was not enhanced, but in fact was significantly reduced, by exposure to DEP over 24 h (Supplementary Fig. S3). Expression of TLR4 and CD14 was not detected on BEAS-2B cells by flow cytometry, and was not altered by DEP exposure (data not shown).
DEP exposure alters the role of IL-1 in the initiation of inflammation.
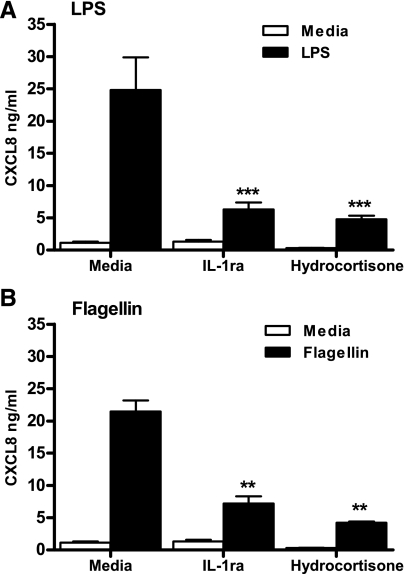
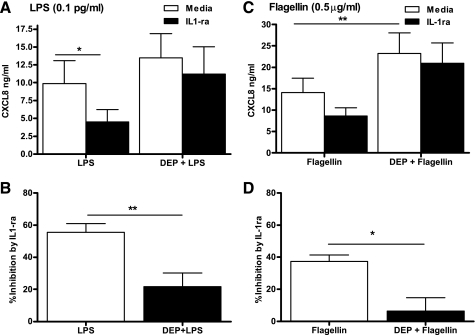
We have previously shown that effective activation of cocultures by LPS required IL-1 production from the monocytes (32). Figure 3A confirms this finding and also shows that activation of cocultures can be inhibited by the steroid hydrocortisone. Figure 3B shows that this dependence on IL-1 for activation of cocultures was also evident in responses to flagellin, with the addition of either the IL-1 receptor antagonist (IL-1ra) or hydrocortisone inhibiting CXCL8 production. These data provide further evidence for the importance of this IL-1-regulated inflammatory network. Our subsequent experiments determined whether the synergistic interactions of DEP and LPS or flagellin altered the dependence of this system on IL-1. We therefore constituted cocultures of epithelial cells and monocytes, stimulated these for 24 h with LPS or flagellin, in the presence or absence of DEP or IL-1ra, and measured CXCL8 production at 24 h. Strikingly, IL-1ra lost its ability to effectively inhibit cytokine generation induced by either LPS or flagellin when DEP were also present (Fig. 4).
Fig. 3.
Cooperative production of CXCL8 by epithelial cell/monocyte cocultures in response to TLR agonists is significantly inhibited by IL-1ra and hydrocortisone. Epithelial cell/monocyte cocultures were established as described in Fig. 1. Cells were stimulated with 10 ng/ml LPS (A) or 1 μg/ml P. aeruginosa flagellin (B), in the presence or absence of IL-1ra (10 μg/ml) or hydrocortisone (1 μg/ml). After 24 h, levels of CXCL8 in the supernatants were determined by ELISA. Data shown are means ± SE of n = 3 replicates. Each replicate was performed at a separate passage with freshly prepared monocytes from a different donor. Significant inhibition of TLR agonist CXCL8 production by IL-1ra and hydrocortisone are indicated by **P < 0.01 and ***P < 0.001, measured by 2-way ANOVA and Bonferroni's posttest.
Fig. 4.
DEP prevents inhibition of TLR-stimulated epithelial cell/monocyte coculture responses by IL-1ra. Epithelial cell/monocyte cocultures were established as described in Fig. 1. Cells were stimulated with 0.1 pg/ml LPS (A) or 0.5 μg/ml S. typhimurium flagellin (C), in the presence or absence of 50 μg/ml DEP and/or IL-1ra (10 μg/ml). After 24 h, levels of CXCL8 in the supernatant were determined by ELISA. Data shown are means ± SE of n = 6–10 replicates. Each replicate was performed at a separate passage with freshly prepared monocytes from a different donor. Significant differences comparing media and addition of IL-1ra are indicated by *P < 0.05 and **P < 0.01 for A and C, as measured by 2-way ANOVA and Bonferroni's posttest. In B and D, data were recalculated to show the degree to which IL-1ra inhibited the induction of CXCL8 in cocultures treated with LPS or DEP+LPS. Significant differences between percentage inhibition by IL-1ra in the presence of DEP are indicated by *P < 0.05 and **P < 0.01, measured by paired t-test for B and D.
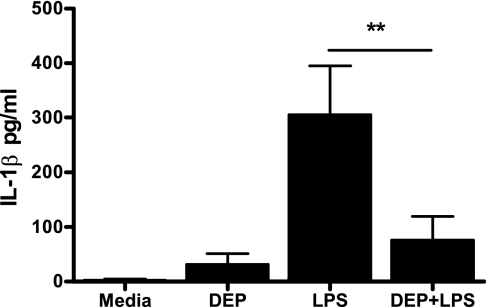
We investigated the mechanisms by which DEP exposure might alter the efficacy of IL-1ra. We determined that this was not the result of absorption of IL-1ra by DEP, since IL-1ra incubated with DEP still retained biological activity when tested in other CXCL8 assays (Supplementary Fig. S4). Figure 5 shows that DEP exposure resulted in a marked decrease in detectable IL-1β release in cocultures stimulated with LPS. Once again, we confirmed that this was not due to IL-1β absorption by DEP (data not shown), and we therefore investigated why IL-1β levels were reduced in activated cocultures when DEP were present.
Fig. 5.
LPS-induced extracellular IL-1β release is reduced in epithelial cell/monocyte cocultures stimulated in the presence of DEP. Epithelial cell/monocyte cocultures were established as described in Fig. 1. Cells were stimulated with 50 μg/ml DEP, 1 pg/ml LPS, or both DEP and LPS. After 24 h, levels of IL-1β in the supernatants were determined by ELISA. Data shown are means ± SE of n = 7 replicates. Each replicate was performed at a separate passage with freshly prepared monocytes from a different donor. Significant differences are indicated by **P < 0.01, measured by 1-way ANOVA and Tukey's posttest.
IL-1 signaling is dependent on a complex sequence of events. IL-1β is produced in a pro-form by inflammatory stimuli. Pro-IL-1β is predominantly cleaved by caspase 1, the proteolytic component of the inflammasome. Release of mature IL-1β occurs via a mechanism involving microvesicle shedding from the cell membrane (26). IL-1β release is heavily dependent on the activation of P2X7 by its cognate ligand, ATP, which results in efflux of potassium, activation of the inflammasome, and release of active IL-1 protein (34).
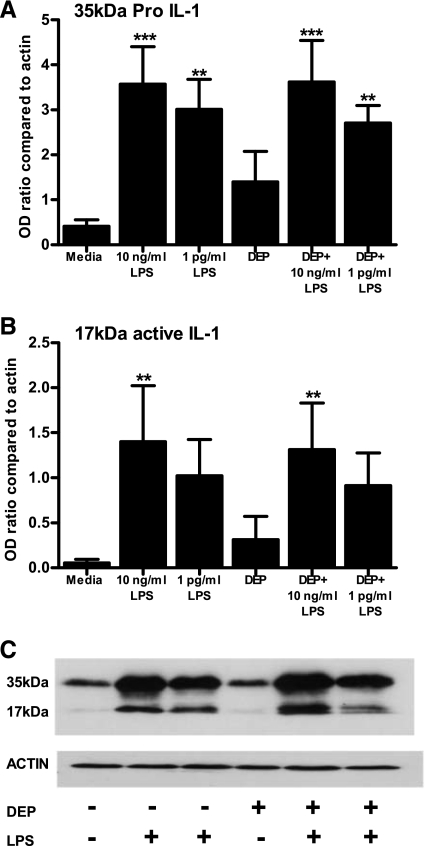
We had established that IL-1β levels were reduced in cocultures stimulated with LPS in the presence of DEP, and that this was not consequent on absorption of IL-1β by DEP. We therefore determined whether IL-1β production was maintained in LPS-stimulated monocytes when DEP were present. Examination of IL-1β release into the supernatants of stimulated cocultures was performed at 24 h to capture total IL-1β produced; however, IL-1β is a classic “early” cytokine, produced rapidly when TLRs are activated. We therefore examined IL-1β intracellular production and processing in primary human monocytes stimulated for 6 h with LPS in the presence or absence of DEP. Figure 6 shows that LPS induced intracellular production of the 35-kDa pro-IL-1β, and also the production of mature 17-kDa processed IL-1β. The presence of DEP over this time course did not significantly alter the production or cleavage of IL-1β.
Fig. 6.
A and B: LPS-induced intracellular IL-1 production is maintained in monocytes stimulated in the presence of DEP. Five-hundred thousand monocytes were stimulated in 12-well plates with the indicated agonists for 6 h. Cell lysates were analyzed for pro- and active IL-1β or actin expression by Western blot. Quantitative signals were derived by densitometric analysis using NIH image 1.62, and data displayed as ratio compared with actin loading control. Data shown are means ± SE of n = 4 replicates, each experiment being performed with freshly prepared monocytes from different donors. A representative blot is shown in C. Significant differences compared with unstimulated monocytes are indicated by **P < 0.01 and ***P < 0.001 as measured by 1-way ANOVA and Tukey's posttest.
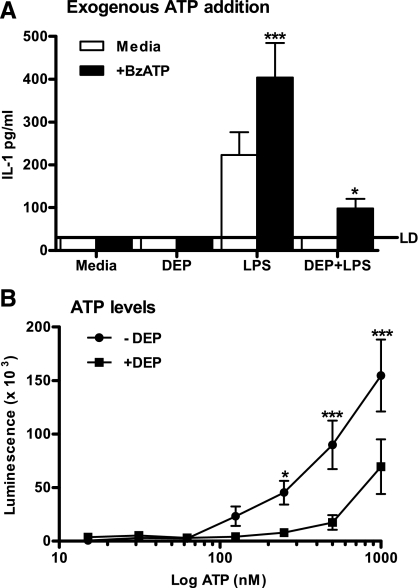
We next determined whether IL-1β produced in monocytes in the presence of DEP was able to be released. We returned to our coculture model and stimulated epithelial cell/monocyte cocultures for 24 h with LPS, in the presence or absence of DEP. In the final 30 min, we added BzATP, a stable and potent activator of P2X7. This induced IL-1β release from cocultures whether or not they had been stimulated with DEP, although in keeping with our earlier data, the cocultures that had been stimulated with DEP showed a much lower baseline level of IL-1β (Fig. 7A).
Fig. 7.
Epithelial cell/monocyte cocultures stimulated with combined DEP and LPS can still release IL-1 in response to exogenous ATP addition, but DEP reduces recoverable ATP levels. A: epithelial cell/monocyte cocultures were established as described in Fig. 1. Cells were then stimulated with 50 μg/ml DEP, 1 ng/ml LPS, or dual DEP and LPS for 23.5 h. Exogenous ATP (300 μM BzATP) was added for the final 30 min of culture. After 24 h, levels of IL-1 in the supernatant were determined by ELISA. LD denotes the limit of detection. Data shown are means ± SE of n = 5 experiments. Each experiment was performed at a separate passage of BEAS-2B cells with freshly prepared monocytes from different donors. Significant differences with or without BzATP are indicated by *P < 0.05 and ***P < 0.001, as measured by 2-way ANOVA and Bonferroni's posttest. B: 1 μM ATP was suspended in dH2O with or without 50 μg/ml DEP, for 3 h. The mixture was centrifuged at 1,000 g for 3 min to mimic the method used to obtain cell-free supernatants and then serially diluted. Samples were detected using an ATP determination kit as per the manufacturer's instructions. Luminescence was determined using a Packard Biosciences Fusion universal microplate analyzer. Data shown are means ± SE of n = 3 replicates. Significant differences are indicated by *P < 0.05 and ***P < 0.001 as measured by 2-way ANOVA and Bonferroni's posttest.
IL-1β release is thought to be largely dependent on P2X7 signaling (13, 34, 52). To investigate if DEP might interfere with IL-1β release by inhibiting signaling through this pathway, we incubated ATP in water or with DEP for 3 h in a cell-free system and measured the amount of recoverable ATP. We observed that treatment of ATP with DEP resulted in a marked loss in recoverable ATP (Fig. 7B).
Cooperative responses of epithelial cells and monocytes to LPS and combined DEP and LPS can be inhibited by a neutralizing soluble TNFα receptor fusion protein.
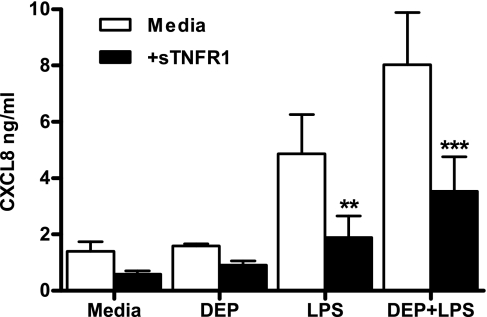
TNFα has been implicated as an important mediator of proinflammatory cytokine release from bronchial epithelial cells (22). In a previous study from our group examining cocultures of airway smooth muscle cells and monocytes, we observed that inhibition of TNFα with sTNFR1 did not impair LPS-mediated activation of CXCL8 production. We considered that the altered inflammatory response seen when DEP was added to our epithelial cell/monocyte cocultures might be reflected by altered roles for TNFα. Both DEP, and to a lesser extent LPS, caused an increase in TNFα in our coculture system (Supplementary Fig. S5), and we observed that sTNFR1 was able to partially inhibit CXCL8 production in BEAS-2B/monocyte cocultures stimulated either with LPS alone or with both DEP and LPS (Fig. 8).
Fig. 8.
Cooperative production of CXCL8 by epithelial cell/monocyte cocultures in response to LPS or combined DEP and LPS is significantly inhibited by a neutralizing soluble TNFα receptor fusion protein (sTNFR1). Epithelial cell/monocyte cocultures were established as described in Fig. 1. Cells were stimulated with 0.01 pg/ml LPS, in the presence or absence of 50 μg/ml DEP and/or sTNFR1 (100 ng/ml). After 24 h, levels of CXCL8 in the supernatants were determined by ELISA. Data shown are means ± SE of n = 3 replicates. Each replicate was performed at a separate passage with freshly prepared monocytes from a different donor. Significant inhibition of TLR agonist responses are indicated by **P < 0.01 and ***P < 0.001, measured by 2-way ANOVA and Bonferroni's posttest.
DISCUSSION
We have previously shown that effective induction of inflammation in the lung requires cooperative responses between monocytes and tissue cells (31, 32, 39, 50). In this study, we show that DEP exert a modest inflammatory action on their own, which also requires cooperative signaling between monocytes and epithelial cells. We show that, in our models of monocyte/epithelial cell cocultures, DEP can also enhance the responses to very low levels of TLR agonists. Finally, we show that the presence of DEP causes a reduction in IL-1β production in LPS-activated cocultures, potentially consequent upon reduced IL-1β release from monocytes. These data reveal considerable complexity in the proinflammatory mechanisms by which DEP may modulate lung function.
The direct toxicity of DEP with the production of proinflammatory cytokines and ROS in single cell types is well established (25, 27, 29, 37, 43, 49). Our data demonstrate that DEP alone are, however, a relatively modest stimulus of inflammation. Activation of CXCL8 generation from epithelial cells by DEP required the presence of monocytes, but in contrast to the responses induced by LPS or flagellin, DEP induced little CXCL8, and showed no evidence of a concentration-dependent induction of inflammatory responses. Similar cooperative interactions between human epithelial cells and alveolar macrophages with enhanced production of GM-CSF and IL-6 but not CXCL8, IL-1β, and TNFα, have been described in response to urban particulate matter (EHC-93) (15).
Although this cytokine response is modest compared with that of TLR agonists, a greater significance can be attributed to our finding that varied concentrations of DEP are capable of accentuating the cellular responses of very small doses of LPS that in their own right have only modest or no actions in our coculture system. This is consistent with in vivo studies that have demonstrated a synergistic interaction between DEP and endotoxin with the enhanced production of lung free radicals (1), proinflammatory cytokines, and the subsequent neutrophil infiltration into the lungs (21, 48). Thus low levels of pollution exposure may enhance the proinflammatory potential of very small concentrations of microbial products, with implications for the pathology of airway inflammation in asthma and COPD. Our data suggest that determining the safe levels of environmental pollution exposure may be complicated, and that in inflamed tissues, very low levels of pollutants may impact on disease more significantly than expected through these cooperative networks.
The lung environment is constantly exposed to inhaled microbial products that are effectively handled and eliminated by the host. In addition to LPS, many other microbial agonists may reach the lung through inhalation or local infection. Indeed, flagellin is in its own right a potent stimulus of epithelial cell activation (12, 16), and here we show for the first time that flagellin responses (from 2 different bacteria, demonstrating that this is a generic response) were also amplified when epithelial cells and monocytes are allowed to interact. Our work also reveals that DEP is capable of potentiating proinflammatory cytokine release in response to flagellin, showing that exposure to pollution in the form of DEP can exert a potentially important amplification of responses to a range of microbial stimuli.
The mechanism of this potentiation may well involve ROS generation, since DEP are known to induce ROS, and ROS is known to prime and enhance signaling of pathways such as that regulating NF-κB (14, 42). We were unable to prevent the ability of DEP to enhance responses to LPS with the ROS scavenger, N-acetyl cysteine (data not shown), or the NADPH oxidase inhibitor, diphenylene iodonium (DPI). However, DPI proved toxic to BEAS-2B cells, and we were unable to test it at optimal concentrations (data not shown).
Both LPS- and flagellin-induced activation of epithelial cell/monocyte cocultures are inhibited by neutralization of IL-1 signaling. This work provides increased evidence of a generic mechanism of cooperative signaling in response to TLR agonists, and has implications for therapeutic targeting in patients with COPD who often have recurrent bacterial infections and can be colonized by flagellated bacteria. In our model, activation of monocytes by LPS or flagellin causes IL-1 production, which in turn activates the tissue cells to release large quantities of inflammatory mediators (these data and Refs. 31, 32, 50). Strikingly, TLR coculture responses become markedly less dependent on IL-1 signaling in the presence of DEP. This represents a major change in the underlying inflammatory response when DEP is present.
In our coculture system, monocyte-derived IL-1 is largely responsible for subsequent activation of the tissue cells and effective production of proinflammatory mediators such as CXCL8. However, we failed to detect IL-1β in epithelial cell/monocyte cocultures stimulated with LPS and DEP in combination. Addition of BzATP at the end of the culture period still caused IL-1β release even in DEP/LPS-treated cells, indicating that IL-1β release could be achieved and that the release pathways were functional. We found that incubation of DEP with IL-1ra or IL-1β did not inhibit their recovery or biological actions. However, incubation of DEP with ATP rapidly reduced the measurable levels of ATP. The processing and release of IL-1β has been shown to be dependent on ATP signaling via P2X7 in monocytes and macrophages (34), with recent data indicating that monocytes have constitutively active caspase 1 and constitutive release of ATP (34), facilitating IL-1β production. This remains a contentious and complicated area, since P2X7-independent release of IL-1β has been described by ourselves (51) and others (18).
Our data would therefore be consistent with a model in which ATP secretion in cocultures, perhaps from the epithelial cells, amplifies monocyte IL-1β production; however, in the presence of DEP sequestration or catalyzed breakdown of ATP reduces total IL-1β production and release.
On the contrary, TLR coculture responses in the presence of DEP were partially inhibited by the soluble TNFα receptor antagonist (sTNFR1). This demonstrates a major change in the nature of the inflammatory response when DEP is present, whereby IL-1β is no longer a key initiator of inflammatory responses, and the proinflammatory properties of DEP are partially maintained by the production of TNFα. We have also demonstrated that LPS-induced activation of epithelial cell/monocyte cocultures are in part dependent on the production of TNFα. In the models shown here, we have selected LPS concentrations that are at the lower end of the effective dose range, to reveal and explore the potential for DEP to interact with these agonists. Where previously we used greater LPS concentrations in coculture models of airway smooth muscle cells, we observed no role for TNFα in induction of inflammation (32). Furthermore, when LPS is used to activate cocultures of monocytes and endothelial cells, TNFα production is actually suppressed (20, 50). Our data indicate that TNFα may therefore exert roles either selectively in the activation of epithelial cells by monocytes or more likely play roles when modest inflammatory responses are induced by low concentrations of agonists; but, when higher concentrations of agonists are used, IL-1β signaling may dominate.
We appreciate that cell lines are only a model of human primary epithelial cells, and we believe the study of primary cells is important. Here we have utilized the BEAS-2B cell line, as opposed to other cell lines, because they closely resemble bronchial epithelial cells by electron microscopy with the presence of keratin, formation of tight junctions, and mucin production (35, 41). We are currently working to establish reliable cultures of primary bronchial epithelial cells to study our models in more detail. However, we have discovered that hydrocortisone, at the concentration present in standard commercial BEC culture media, profoundly dampens the responses of monocytes to LPS, as measured in terms of CXCL8 production. We have also observed that the presence of FCS facilitates endotoxin responses of monocytes without inducing their baseline activation, but FCS markedly activates primary BEC, and this activation of BEC is not completely suppressed by hydrocortisone. In addition, bovine pituitary extract, an important growth factor in primary epithelial cell media, activates primary human monocytes. We are continuing to try to determine experimental conditions to allow optimal study of primary epithelial cell/monocyte cocultures to reliably extend our observations to primary cells.
In conclusion, our data proposes a model of DEP-modulated inflammation, in which IL-1β is no longer a key initiator of inflammatory responses, and in which the proinflammatory roles of DEP take the place of IL-1β in the initiation of inflammation. Effective targeting of DEP-modulated inflammation may be achievable in part by targeting the TNFα or ROS axes of inflammation.
GRANTS
These studies were funded by Sheffield Hospitals Charitable Trust Grant 7853 and by a Medical Research Council Senior Clinical Fellowship (G116/170) to I. Sabroe.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. H. L. Wilson for helpful discussion.
REFERENCES
- 1.Arimoto T, Kadiiska MB, Sato K, Corbett J, Mason RP. Synergistic production of lung free radicals by diesel exhaust particles and endotoxin. Am J Respir Crit Care Med 171: 379–387, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Atkinson RW, Anderson HR, Strachan DP, Bland JM, Bremner SA, Ponce de Leon A. Short-term associations between outdoor air pollution and visits to accident and emergency departments in London for respiratory complaints. Eur Respir J 13: 257–265, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Ball P, Harris JM, Lowson D, Tillotson G, Wilson R. Acute infective exacerbations of chronic bronchitis. QJM 88: 61–68, 1995 [PubMed] [Google Scholar]
- 4.Bayram H, Devalia JL, Sapsford RJ, Ohtoshi T, Miyabara Y, Sagai M, Davies RJ. The effect of diesel exhaust particles on cell function and release of inflammatory mediators from human bronchial epithelial cells in vitro. Am J Respir Cell Mol Biol 18: 441–448, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Behndig AF, Mudway IS, Brown JL, Stenfors N, Helleday R, Duggan ST, Wilson SJ, Boman C, Cassee FR, Frew AJ, Kelly FJ, Sandstrom T, Blomberg A. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur Respir J 27: 359–365, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Berube J, Bourdon C, Yao Y, Rousseau S. Distinct intracellular signaling pathways control the synthesis of IL-8 and RANTES in TLR1/TLR2, TLR3 or NOD1 activated human airway epithelial cells. Cell Signal 21: 448–456, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Chan TL, Lee PS, Hering WE. Pulmonary retention of inhaled diesel particles after prolonged exposures to diesel exhaust. Fundam Appl Toxicol 4: 624–631, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Chauhan AJ, Inskip HM, Linaker CH, Smith S, Schreiber J, Johnston SL, Holgate ST. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet 361: 1939–1944, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devalia JL, Bayram H, Abdelaziz MM, Sapsford RJ, Davies RJ. Differences between cytokine release from bronchial epithelial cells of asthmatic patients and non-asthmatic subjects: effect of exposure to diesel exhaust particles. Int Arch Allergy Immunol 118: 437–439, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med 329: 1753–1759, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Donaldson K, Tran L, Jimenez LA, Duffin R, Newby DE, Mills N, MacNee W, Stone V. Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibr Toxicol 2: 10, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaves-Pyles T, Murthy K, Liaudet L, Virag L, Ross G, Soriano FG, Szabo C, Salzman AL. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: I kappa B alpha degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J Immunol 166: 1248–1260, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176: 3877–3883, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med 166: S4–S8, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Fujii T, Hayashi S, Hogg JC, Mukae H, Suwa T, Goto Y, Vincent R, van Eeden SF. Interaction of alveolar macrophages and airway epithelial cells following exposure to particulate matter produces mediators that stimulate the bone marrow. Am J Respir Cell Mol Biol 27: 34–41, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 167: 1882–1885, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Goto Y, Ishii H, Hogg JC, Shih CH, Yatera K, Vincent R, van Eeden SF. Particulate matter air pollution stimulates monocyte release from the bone marrow. Am J Respir Crit Care Med 170: 891–897, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Grahames CB, Michel AD, Chessell IP, Humphrey PP. Pharmacological characterization of ATP- and LPS-induced IL-1beta release in human monocytes. Br J Pharmacol 127: 1915–1921, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haslett C, Guthrie LA, Kopaniak MM, Johnston RB, Jr, Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol 119: 101–110, 1985 [PMC free article] [PubMed] [Google Scholar]
- 20.Houston DS, Carson CW, Esmon CT. Endothelial cells and extracellular calmodulin inhibit monocyte tumor necrosis factor release and augment neutrophil elastase release. J Biol Chem 272: 11778–11785, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Inoue K, Takano H, Yanagisawa R, Hirano S, Sakurai M, Shimada A, Yoshikawa T. Effects of airway exposure to nanoparticles on lung inflammation induced by bacterial endotoxin in mice. Environ Health Perspect 114: 1325–1330, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii H, Fujii T, Hogg JC, Hayashi S, Mukae H, Vincent R, van Eeden SF. Contribution of IL-1β and TNF-α to the initiation of the peripheral lung response to atmospheric particulates (PM10). Am J Physiol Lung Cell Mol Physiol 287: L176–L183, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Ishii H, Hayashi S, Hogg JC, Fujii T, Goto Y, Sakamoto N, Mukae H, Vincent R, van Eeden SF. Alveolar macrophage-epithelial cell interaction following exposure to atmospheric particles induces the release of mediators involved in monocyte mobilization and recruitment. Respir Res 6: 87, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O'Toole S, Myint SH, Tyrrell DA, Holgate ST. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 310: 1225–1229, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li N, Wang M, Oberley TD, Sempf JM, Nel AE. Comparison of the pro-oxidative and proinflammatory effects of organic diesel exhaust particle chemicals in bronchial epithelial cells and macrophages. J Immunol 169: 4531–4541, 2002 [DOI] [PubMed] [Google Scholar]
- 26.MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 15: 825–835, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Matsuzaki T, Amakawa K, Yamaguchi K, Ishizaka A, Terashima T, Matsumaru A, Morishita T. Effects of diesel exhaust particles on human neutrophil activation. Exp Lung Res 32: 427–439, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW, Seeger W, Welte T, Lohmeyer J. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol 35: 227–235, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Mazzarella G, Ferraraccio F, Prati MV, Annunziata S, Bianco A, Mezzogiorno A, Liguori G, Angelillo IF, Cazzola M. Effects of diesel exhaust particles on human lung epithelial cells: an in vitro study. Respir Med 101: 1155–1162, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Michel O, Kips J, Duchateau J, Vertongen F, Robert L, Collet H, Pauwels R, Sergysels R. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med 154: 1641–1646, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Morris GE, Parker LC, Ward JR, Jones EC, Whyte MK, Brightling CE, Bradding P, Dower SK, Sabroe I. Cooperative molecular and cellular networks regulate Toll-like receptor-dependent inflammatory responses. FASEB J 20: 2153–2155, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Morris GE, Whyte MK, Martin GF, Jose PJ, Dower SK, Sabroe I. Agonists of toll-like receptors 2 and 4 activate airway smooth muscle via mononuclear leukocytes. Am J Respir Crit Care Med 171: 814–822, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Mukaida N, Okamoto S, Ishikawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol 56: 554–558, 1994 [PubMed] [Google Scholar]
- 34.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, van der Meer JW, Dinarello CA. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 113: 2324–2335, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noah TL, Yankaskas JR, Carson JL, Gambling TM, Cazares LH, McKinnon KP, Devlin RB. Tight junctions and mucin mRNA in BEAS-2B cells. In Vitro Cell Dev Biol Anim 31: 738–740, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Nordenhall C, Pourazar J, Ledin MC, Levin JO, Sandstrom T, Adelroth E. Diesel exhaust enhances airway responsiveness in asthmatic subjects. Eur Respir J 17: 909–915, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Ohyama M, Otake T, Adachi S, Kobayashi T, Morinaga K. A comparison of the production of reactive oxygen species by suspended particulate matter and diesel exhaust particles with macrophages. Inhal Toxicol 19, Suppl 1: 157–160, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Park JH, Gold DR, Spiegelman DL, Burge HA, Milton DK. House dust endotoxin and wheeze in the first year of life. Am J Respir Crit Care Med 163: 322–328, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Parker LC, Prestwich EC, Ward JR, Smythe E, Berry A, Triantafilou M, Triantafilou K, Sabroe I. A phosphatidylserine species inhibits a range of TLR- but not IL-1beta-induced inflammatory responses by disruption of membrane microdomains. J Immunol 181: 5606–5617, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker LC, Whyte MK, Vogel SN, Dower SK, Sabroe I. Toll-like receptor (TLR)2 and TLR4 agonists regulate CCR expression in human monocytic cells. J Immunol 172: 4977–4986, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, Brash DE, Park JB, Rhim JS, Harris CC. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res 48: 1904–1909, 1988 [PubMed] [Google Scholar]
- 42.Ryan KA, Smith MF, Jr, Sanders MK, Ernst PB. Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-kappa B and interleukin-8 expression. Infect Immun 72: 2123–2130, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito Y, Azuma A, Kudo S, Takizawa H, Sugawara I. Effects of diesel exhaust on murine alveolar macrophages and a macrophage cell line. Exp Lung Res 28: 201–217, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Salvi S, Blomberg A, Rudell B, Kelly F, Sandstrom T, Holgate ST, Frew A. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med 159: 702–709, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Schwartz J, Slater D, Larson TV, Pierson WE, Koenig JQ. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis 147: 826–831, 1993 [DOI] [PubMed] [Google Scholar]
- 46.Spannhake EW, Reddy SP, Jacoby DB, Yu XY, Saatian B, Tian J. Synergism between rhinovirus infection and oxidant pollutant exposure enhances airway epithelial cell cytokine production. Environ Health Perspect 110: 665–670, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stenfors N, Nordenhall C, Salvi SS, Mudway I, Soderberg M, Blomberg A, Helleday R, Levin JO, Holgate ST, Kelly FJ, Frew AJ, Sandstrom T. Different airway inflammatory responses in asthmatic and healthy humans exposed to diesel. Eur Respir J 23: 82–86, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Takano H, Yanagisawa R, Ichinose T, Sadakane K, Yoshino S, Yoshikawa T, Morita M. Diesel exhaust particles enhance lung injury related to bacterial endotoxin through expression of proinflammatory cytokines, chemokines, and intercellular adhesion molecule-1. Am J Respir Crit Care Med 165: 1329–1335, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Takizawa H. Diesel exhaust particles and their effect on induced cytokine expression in human bronchial epithelial cells. Curr Opin Allergy Clin Immunol 4: 355–359, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Ward JR, Francis SE, Marsden L, Suddason T, Lord GM, Dower SK, Crossman DC, Sabroe I. A central role for monocytes in Toll-like receptor-mediated activation of the vasculature. Immunology 128: 58–68, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward JR, West PW, Ariaans MP, Parker LC, Francis SE, Crossman DC, Sabroe I, Wilson HL. Temporal IL-1β secretion from primary human peripheral blood monocytes by P2X7-independent and P2X7-dependent mechanisms. J Biol Chem. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson HL, Francis SE, Dower SK, Crossman DC. Secretion of intracellular IL-1 receptor antagonist (type 1) is dependent on P2X7 receptor activation. J Immunol 173: 1202–1208, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.