Abstract
The fetal heart is highly sensitive to changes in mechanical load. We have previously demonstrated that increased cardiac load can stimulate cell cycle activity and maturation of immature cardiomyocytes, but the effects of reduced load are not known. Sixteen fetal sheep were given either continuous intravenous infusion of lactated Ringer solution (LR) or enalaprilat, an angiotensin-converting enzyme inhibitor beginning at 127 days gestational age. After 8 days, fetal arterial pressure in the enalaprilat-infused fetuses (23.8 ± 2.8 mmHg) was lower than that of control fetuses (47.5 ± 4.7 mmHg) (P < 0.0001). Although the body weights of the two groups of fetuses were similar, the heart weight-to-body weight ratios of the enalaprilat-infused fetuses were less than those of the LR-infused fetuses (5.6 ± 0.5 g/kg vs. 7.0 ± 0.6 g/kg, P < 0.0001). Dimensions of ventricular myocytes were not different between control and enalaprilat-infused fetuses. However, there was a significant decrease in cell cycle activity in both the right ventricle (P < 0.005) and the left ventricle (P < 0.002) of the enalaprilat-infused fetuses. Thus, we conclude a sustained reduction in systolic pressure load decreases hyperplastic growth in the fetal heart.
Keywords: blood pressure, enalaprilat, hyperplasia, cardiomyocyte
fetal heart growth occurs through an increase in cardiomyocyte size (hypertrophy) and myocyte number (hyperplasia) (21). Early in gestation, most cardiomyocytes are mononucleated and capable of cell division. Mononucleated myocytes entering the cell cycle in the fetal sheep heart may either divide into two mononucleated cells by complete mitosis or become a single binucleated cell by karyokinesis without cytokinesis. These binucleated myocytes lose their ability to divide; thereafter, these terminally differentiated myocytes may only grow larger (4, 6). Thus, the proportion of the myocyte population that is binucleated is an indication of the maturational state of the developing myocardium. Sheep, like humans, are born with nearly all myocytes terminally differentiated (3, 15); accordingly, alterations in myocyte growth or maturation in utero may have long-term consequences in the form of adult cardiomyocyte endowment and enduring function.
We have previously demonstrated that fetal heart growth is sensitive to changes in mechanical load (4, 9, 11, 12). Increased systolic pressure load accelerates fetal heart growth by increasing both hypertrophic and hyperplastic processes, including terminal differentiation (4, 12). Thus, it is likely that tissue peripheral vascular resistance and placental vascular architecture are key determinants of load-sensitive myocardial growth patterns. However, the effects of reduced systolic pressure load on fetal cardiac growth are not known.
The renin-angiotensin system is an important determinant of arterial pressure in the fetus (1, 5, 8) and thus a significant contributor to cardiac afterload regulation. We reasoned that blockade of the conversion of ANG I to ANG II by angiotensin-converting enzyme would result in a significant and sustained reduction of arterial pressure and thus systolic cardiac load. On the basis of the current understanding of load-modulated fetal cardiac growth, we hypothesized that reduced systolic pressure load would decrease both hypertrophic and hyperplastic growth. To test this hypothesis, we lowered fetal arterial pressure by infusing the angiotensin-converting enzyme inhibitor, enalaprilat, over a period of 8 days into late-gestation fetal sheep. We then determined whether cardiac mass, cardiomyocyte size, cell cycle activity, and degree of terminal differentiation were affected by altered systolic loading conditions.
METHODS
Animal preparations.
Methods of anesthesia and surgery on pregnant ewes and their fetuses and all experimental procedures were approved by the Oregon Health and Science University Animal Care and Use Committee. Details have been published previously (11, 12). Time-bred ewes of mixed Western breeds were obtained from a commercial source. Sixteen fetal sheep (7 singletons and 9 twins) were instrumented in a sterile fashion at a gestational age of 119 to 122 days (mean 120 days). Anesthesia was induced by administering an intravenous mixture of diazepam (0.13 mg/kg) and ketamine (5 mg/kg). The ewe was intubated, and anesthesia was maintained using 1–2% isoflurane in a carrier gas mixture of 70:30 oxygen and nitrous oxide. The isoflurane concentration was adjusted as necessary, and additional doses of diazepam or ketamine were administered to ensure a surgical level of anesthesia in both the ewe and the fetus.
A catheter was placed in a carotid artery of the fetus, and the tip was advanced to the level of the aorta. A catheter was placed in a jugular vein, and the tip was advanced to a level just cranial of the right atrium. Catheters were also placed in the amniotic fluid space. All catheters were anchored to the fetal skin. The catheters exited the uterus, and the uterus was closed forming a tight seal of the amniotic cavity. The catheters were tunneled underneath the skin of the ewe, and the abdomen was closed. The catheters emerged at the ewe's flank and were stored in a pouch sewn into the skin. One million units of Penicillin G (Bristol-Meyers Squibb, Princeton, NJ) were instilled into the amniotic space at the conclusion of the procedure, anesthesia was terminated, and the ewe was allowed to recover. The ewes received routine postoperative pain medication (0.6 mg bupremorphine, twice a day) for 2 days.
Experimental protocol.
During the experiments, the ewes were housed in stanchions in the laboratory. Baseline experiments were initiated at a gestational age of 124 days. Arterial blood pressures, central venous blood pressures, and amniotic fluid pressures were continuously measured for the next 2 days. Fetal arterial blood samples were taken daily for determination of fetal blood gases, pH, hematocrit, hemoglobin, and oxygen content. At the end of the 2-day control period, the response of the fetal arterial blood pressure to an intravenous injection of ANG I was determined.
After the baseline data were collected, fetuses were randomly assigned to one of two protocols. In one group the angiotensin-converting enzyme inhibitor, enalaprilat (dissolved in lactated Ringer solution), was infused (345 μg/day in a volume of 229 ml/day) using a Gilson Minipuls 3 roller pump (Gilson, Middleton, WI). This daily dose of enalaprilat is ∼30-fold lower than that used in late-gestation fetal sheep by Giammattei et al. (10). The second group received an infusion of lactated Ringer solution at the same rate of 229 ml/day. There were eight fetuses in each group. Infusions continued for 8 days. At the end of the 8-day period, the response of the fetal arterial blood pressure to an intravenous bolus of ANG I was measured. Following completion of the day 8 measurements, the ewe was euthanized with an overdose of pentobarbital sodium, and fetal hearts were collected as described below.
Hemodynamic measurements.
Pressures were measured with Abbott Transpac pressure transducers (Abbott Park, IL) and a computerized recording system (AD Instruments, Colorado Springs, CO; Apple, Cupertino, CA). The system was calibrated against a mercury manometer to a scale value of better than 1% and rezeroed for drift before each measurement. Repeat calibrations established an accuracy of 0.5 mmHg. All fetal intravascular pressures were referred to amniotic fluid pressure as zero. Zeros were checked every morning, after which the data were taken over the next 60-min period were averaged and recorded for later analysis. Heart rates were obtained from arterial pressure tracings.
Blood gas measurements and plasma levels.
Arterial blood samples were anticoagulated with heparin or EDTA. Arterial blood gas partial pressures and pH were determined in a Radiometer ABL 720 blood gas analyzer corrected to 39°C. Hemoglobin concentrations and hemoglobin oxygen content were determined.
Cardiac myocyte analysis.
Fetal hearts were harvested, weighed, and enzymatically dissociated, as described in detail previously (12). Briefly, heparin (3000 U) and 3 ml saturated KCl solution were injected into the umbilical vein of the deeply anesthetized fetuses, stopping the fetal hearts in diastole. The hearts were hung from a perfusion apparatus via the aorta and perfused by a series of solutions through the coronary arteries. Collagenase and protease were used to dissociate the myocytes. Lengths and widths were measured for 50 binucleated and 50 mononucleated isolated, fixed cardiomyocytes from each ventricle using commercially available image analysis software (Image-Pro Plus, Media Cybernetics, Bethesda MD). Following analysis of cell dimensions, maturational state was determined by counting the number of mononucleated and binucleated cardiomyocytes in a random sample of 300 cells from each ventricle (12). Lastly, to evaluate cell cycle activity (12), cardiomyocytes were immunostained with the MIB-1 antibody (DAKO, Carpenteria, CA) against the Ki-67 antigen. A minimum of 500 myocytes per ventricle were scored for each fetus for Ki-67-positive staining, and results were expressed as a percentage of mononucleated cardiomyocytes.
Statistical methods.
All statistical analyses were performed with GraphPad Prism (GraphPad Software, San Diego, CA). Repeated-measures ANOVA with Dunnett's multiple-comparison test was used to compare daily measurement values to preinfusion values. Heart and body weights, myocyte size, percent binucleation, and cell cycle activity of the fetuses infused with enalaprilat were compared with those of fetuses infused with LR using an unpaired t-test. The effects of ANG I on fetal arterial pressure before and after infusion of enalaprilat or lactated Ringer solution were compared using a paired t-test. A level of P < 0.05 was required for any change to be considered statistically significant. Results are presented as means ± SD, except for the figures, which show means ± SE.
RESULTS
Hemodynamics and arterial blood gases.
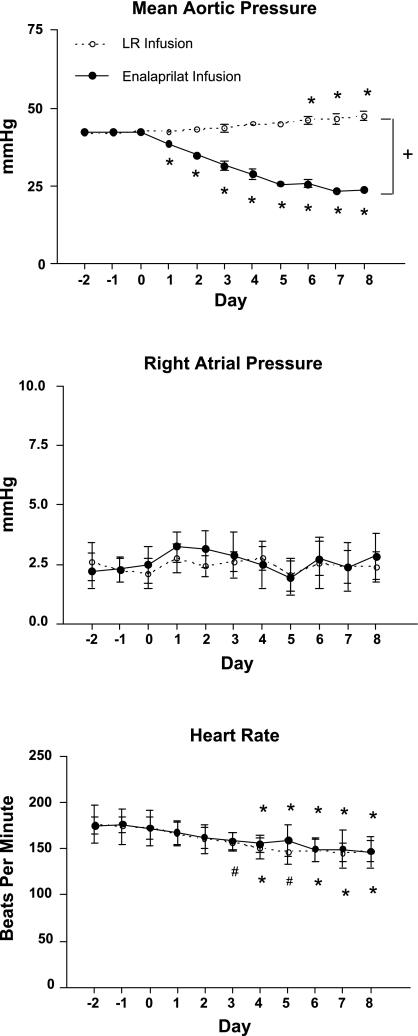
Inhibition of angiotensin-converting enzyme via enalaprilat produced a large decrease in fetal arterial blood pressure from a control value of 42.4 ± 2.3 mmHg on day 0 to 23.8 ± 2.8 mmHg on day 8 (P < 0.01). This decrease in arterial blood pressure was statistically significant as early as day 1 (Fig. 1). In contrast, the fetuses infused with lactated Ringer solution (LR group) showed the normal gestational increase in mean arterial pressure (42.6 ± 1.7 on day 0 and 47.5 ± 4.7 mmHg on day 8; P < 0.01). When comparing the two groups of fetuses at day 0, there were no statistically significant differences in arterial blood pressure. However, by day 8, the differences in arterial blood pressure were statistically significant (P < 0.0001; Fig. 1).
Fig. 1.
Effect of enalaprilat or lactated Ringer solution (LR) infusion on fetal hemodynamics. Infusions were begun on day 0. Values are expressed as means ± SE. #P < 0.05, *P < 0.01 comparing daily measurements to day 0 during enalaprilat or LR infusion. +P < 0.0001 comparing day 8 LR infusion to enalaprilat infusion.
Enalaprilat infusion had no detectable effect on either right atrial pressure over the 8-day duration of the experiment or compared with control fetuses (Fig. 1). Fetal heart rate showed a slight decline over time in the LR infusion group as is normal during gestation (24). Fetal heart rate decreased significantly in the enalaprilat infusion group starting at day 3 of the infusion and continued to decrease for the duration of the infusion (Fig. 1). However, fetal heart rates in the LR- and enalaprilat-infused fetuses did not differ.
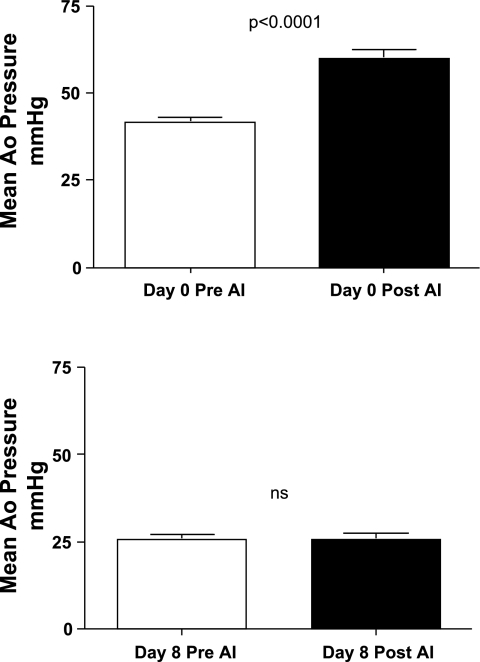
Adequate blockade of the angiotensin-converting enzyme by enalaprilat was tested by injecting bolus doses of ANG I. An average maximal dose of 5.3 ± 3.8 μg ANG I increased mean arterial pressure from 41.4 ± 4.3 mmHg to 59.9 ± 7.6 mmHg (P = 0.03) on day 0 before enalaprilat infusion (Fig. 2, top). After 8 days of enalaprilat infusion, an average maximal dose of 18.0 ± 16.7 μg ANG I had no effect on arterial pressure (25.6 ± 3.9 mmHg before and 25.8 ± 4.3 mmHg after ANG I) (Fig. 2, bottom). In the fetuses infused with lactated Ringer solution, 4.4 ± 1.5 μg of ANG I increased arterial blood pressure from 39.6 ± 4.1 mmHg to 59.5 ± 8.9 mmHg on day 0 (P < 0.006). After 8 days of infusion, arterial blood pressure increased from 46.1 ± 6.6 mmHg to 64.3 ± 4.3 mmHg (P < 0.0001) in response to 4.4 ± 1.2 μg of ANG I.
Fig. 2.
Effect of ANG I on mean arterial pressure before (top) and after (bottom) 8 days of enalaprilat infusion. Values are expressed as means ± SE. Ao, Aortic; AI, ANGI; NS, not significant.
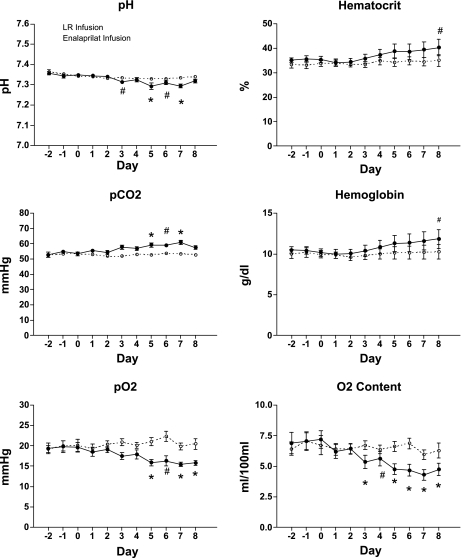
Arterial pH values were not significantly different from initial values at the end of the protocol in either group (Fig. 3). Arterial Pco2 increased in enalaprilat-infused fetuses over the treatment period (P < 0.05) but remained unchanged in controls (53.5 ± 3.6 on day 0 and 52.9 ± 2.6 on day 8). Po2 decreased significantly from 19.7 ± 3.1 mmHg to 15.8 ± 1.8 mmHg (P < 0.01) following 8 days of enalaprilat infusion along with O2 content (7.2 ± 2.0 ml O2/100 ml blood on day 0 and 4.7 ± 1.4 ml O2/100 ml blood on day 8; P < 0.01). Neither Po2 nor O2 content were significantly different on day 8 compared with day 0 values in LR-infused controls. Hematocrit remained statistically unchanged by LR (33.8% ± 3.6, day 0 and 35.0% ± 6.8, day 8), but was significantly increased by enalaprilat (35.4% ± 3.7 day 0 and 40.3% ± 9.4 day 8; P < 0.05) infusion. Hemoglobin levels were increased following 8 days of enalaprilat infusion (10.2 g/dl ± 1.3 day 0 and 11.8 g/dl ± 3.2 day 8; P < 0.05) and were not significantly altered by LR infusion (10.0 g/dl ± 1.0 day 0 and 10.3 g/dl ± 2.5 day 8). Daily changes are shown in Fig. 3.
Fig. 3.
Effect of enalaprilat or lactated Ringer solution (LR) infusion on fetal arterial blood pH, Pco2, Po2, hematocrit, hemoglobin, and oxygen content. Infusions were begun on day 0. Values are expressed as means ± SE. #P < 0.05, *P < 0.01 comparing daily measurements to day 0 during enalaprilat or LR infusion.
Morphometric measurements and cardiac myocyte characteristics.
The effects of reduced systolic load on fetal heart size are illustrated in Table 1. Fetal body weight was not statistically different between experimental and control groups. Hearts exposed to a reduced arterial pressure tended to be smaller (22.1 ± 3.0 vs. 27.1 ± 6.1 g). The heart weight-to-body weight ratio was 20% lower in the enalaprilat-infused fetuses compared with LR-infused fetuses (5.6 ± 0.5 vs. 7.0 ± 0.6 g/kg; P < 0.0001). There were no statistically significant differences in the right or left ventricular myocyte dimensions between enalaprilat-treated fetuses and LR-infused controls. Cell volumes were calculated assuming that the cardiomyocyte is cylindrical and using a correction factor for the spindle shape of the cell, as previously published (13). Enalaprilat treatment did not have a statistically significant effect on right or left myocyte cell volumes. Percent binucleation (used as an index of terminal differentiation) of fetal cardiomyocytes in either ventricle was not demonstrably affected by enalaprilat infusion. However, a sustained decrease in arterial pressure reduced cell cycle activity, as assessed by Ki-67 staining, by 80–90% in fetal cardiomyocytes from both left (P < 0.002) and right ventricles (P < 0.005) compared with LR controls.
Table 1.
Ventricular myocyte growth data for LR-infused and for enalaprilat-infused fetuses
| LR Infusion | Enalprilat Infusion | P value | |
|---|---|---|---|
| Fetal weight, kg | 3.9 ± 1.1 | 4.0 ± 0.6 | ns |
| Heart weight, g | 27.1 ± 6.1 | 22.1 ± 3.0 | ns |
| Heart/body weight ratio, g/kg | 7.0 ± 0.6 | 5.6 ± 0.5 | <0.0001 |
| Mononucleated RV myocyte | |||
| Length, μm | 74.2 ± 4.8 | 72.9 ± 4.7 | ns |
| Width, μm | 10.9 ± 0.4 | 10.6 ± 0.9 | ns |
| Volume, μm3 | 4025 ± 369 | 3796 ± 833 | ns |
| Binculeated RV myocyte | |||
| Length, μm | 93.6 ± 12.2 | 97.9 ± 6.6 | ns |
| Width, μm | 12.0 ± 1.7 | 11.8 ± 0.9 | ns |
| Volume, μm3 | 6439 ± 1977 | 6343 ± 1173 | ns |
| Mononucleated LV myocyte | |||
| Length, μm | 68.6 ± 4.3 | 66.4 ± 6.4 | ns |
| Width, μm | 9.5 ± 0.6 | 9.1 ± 1.2 | ns |
| Volume, μm3 | 2739 ± 422 | 2487 ± 903 | ns |
| Binculeated LV myocyte | |||
| Length, μm | 89.1 ± 6.1 | 86.7 ± 7.5 | ns |
| Width, μm | 10.6 ± 0.7 | 9.9 ± 0.6 | ns |
| Volume, μm3 | 4357 ± 693 | 3752 ± 689 | ns |
| Percent binucleated myocytes | |||
| RV Myocyte, % | 48.1 ± 11.4 | 50.7 ± 6.7 | ns |
| LV Myocyte, % | 48.1 ± 12.0 | 50.1 ± 8.6 | ns |
| Percent Ki67-positive mononucleated myocytes | |||
| RV Myocyte, % | 8.3 ± 5.1 | 2.0 ± 1.6 | <0.005 |
| LV Myocyte, % | 9.1 ± 5.7 | 1.1 ± 0.6 | <0.002 |
Values are expressed as means ± SD. LR, lactated Ringer; LV, left ventricle; RV, right ventricle; NS, not significant.
DISCUSSION
We found that a decrease in systolic load in the fetal sheep heart led to important changes in cardiac growth over an 8-day period. Heart weight relative to body weight and the number of cardiomyocytes in the cell cycle were both significantly decreased. Surprisingly, cardiomyocyte size and the state of maturation of the myocardium, as indicated by the proportion of binucleated cells, were not affected by the reduced load. Thus, reduced cardiac systolic load primarily affects hyperplastic growth, in the developing fetal heart.
Cell cycle activity and terminal differentiation.
It is known that cardiomyocyte cell cycle activity in the developing sheep heart is sensitive to sustained alterations in cardiac load (12). Cell cycle activity more than doubled in fetal cardiomyocytes following 4 to 8 days of intravascular plasma infusion in near-term fetal sheep, a model that produced an increase in arterial pressure of ∼40% above baseline (11, 12). Heart cell numbers increased by some 30% above control levels following 10 days of increased systolic load in the right ventricle (3). In the present study, a ∼40% decrease in arterial pressure below baseline for 8 days was associated with a near-complete cessation of cell cycle activity. These data confirm that cardiac-loading conditions are a powerful modulator of cardiac myocyte cell cycle activity during fetal development.
In near-term fetal sheep, mononucleated myocytes actively engaged in the cell cycle are capable of either division (hyperplasia) or binucleation (terminal differentiation). Alterations in cell cycle activity in cardiomyocytes may affect both heart growth and maturation by influencing hyperplasia and terminal differentiation, respectively. Thus, the reduced cell cycle activity observed in response to fetal hypotension may produce a general decrease in cardiomyocyte division, resulting in fewer cells overall, and/or it may lead to fewer cells becoming binucleated. As the ratio of binucleated to mononucleated myocytes did not differ between normotensive and hypotensive fetuses, we conclude that the proportion of mononucleated myocytes becoming terminally differentiated was not affected by reductions in systolic load of this duration.
This finding refines our view of cell cycle regulation. There are two scenarios that may explain the growth of the myocardium under reduced load conditions: 1) Fewer than normal cardiomyocytes were being generated by proliferation of mononucleated cells, while the usual fraction of this smaller pool was becoming terminally differentiated. In this situation, the proportion of mononucleated and binucleated cells is unchanged from controls, but the size of the myocyte population would be reduced compared with normally growing myocardium. 2) Mononucleated cells stopped dividing at the outset of the load reduction, and virtually no new myocytes were produced. In this case, the proportion of cells that were binucleated remained constant over the 8-day experiment. Distinguishing between these two possibilities awaits further study.
We observed that by 3 days of enalaprilat-induced hypotension the fetuses became hypoxemic compared with normotensive controls. Notably, the normal body weights of the hypotensive fetuses suggest that the decreases in Po2 and O2 content were insufficient to stunt somatic growth. We speculate that the decreased arterial Po2 resulted from a hypotension-induced reduction in placental perfusion (2). It is unlikely that the 4 days of mild hypoxemia observed in the enalaprilat-treated fetuses is responsible for the reduction in cell cycle activity observed in this study as 10 days of chronic and more severe hypoxemia induced by placental embolization did not significantly alter cardiomyocyte cell cycle activity (14).
Effect of reduced load on cardiac weight.
We have previously demonstrated that sustained elevations in cardiac load result in increased length and width of fetal cardiac myocytes (12). Decreases in myocyte length and width in response to unloading of the adult heart have been demonstrated using the heterotopically transplanted rat heart model (7, 17). In this study, we found that sustained reductions in cardiac load did not demonstrably affect cardiomyocyte size or volume in either fetal ventricle. Cardiac myocytes do not normally lengthen during the period of gestation covered in the present study (13). Thus, it is possible that a longer period of load reduction is necessary to detect inhibition of normal hypertrophic growth. Myocytes did not become shorter, however, in response to this period of reduced load.
The sustained decrease in systolic load resulted in a significant reduction in cardiac weight relative to body weight, while overall somatic growth was maintained in the developing fetus. As no reduction in cell size (hypotrophic growth) was observed, it is likely that a reduction in cell number (hypoplastic growth) is responsible for the relatively smaller hearts observed in the hypotensive fetuses. This reasoning is supported by the near-complete suppression of cardiomyocyte cell cycle activity associated with sustained hypotension.
Role of the renin-angiotensin system.
In addition to a reduction in systolic load, an inhibition of the activity of the renin-angiotensin system (RAS) may also lead to reductions in cardiomyocyte growth. It is difficult to isolate the effects of cardiac load from RAS activity in vivo as one often impacts the other. The role of ANG II in regulating cardiac growth and maturation in vivo is controversial, but several studies shed light on the issue. For example, blockade of ANG II activity does not inhibit the effect of an increase in load on cardiac growth in the fetus (19, 20). Increases in cardiac load resulting from plasma protein infusion lead to steep decreases in ANG II levels. Nevertheless, the myocardial response includes increased cardiac growth, cardiomyocyte hypertrophy, maturation, and hyperplasia (12). In culture, ANG II treatment of near-term fetal sheep cardiomyocytes stimulates cell cycle activity but does not stimulate hypertrophy (22). An increase in left ventricular weight-to-body weight ratio follows 8 days of ANG II infusion; however, in that study, there was also an elevation in systolic load (19). These findings suggest that the RAS is not the main effector of load-modulated cardiac growth in the fetus. In addition to inhibiting ANG II production, angiotensin-converting enzyme inhibition may increase angiotensin (1–7) levels. ANG (1–7) peptide has antihypertensive and antihypertrophic properties in the adult cardiovascular system and may also inhibit the mitogen-activated protein kinase pathway in adult cardiac myocytes (18, 23). In fetal sheep, ANG (1–7) does not alter arterial pressure, ANG I, ANG II, or renin activity (16). The direct role of ANG (1–7) in hypertrophic and hyperplastic growth of the fetal heart requires further study. Thus, blockade of the RAS via enalaprilat infusion may play a role in the myocardial response to reduced loading conditions but cannot solely explain the reduction in cardiac growth observed in the present study.
Consistent with our hypothesis, we have shown that a sustained decrease in systolic load results in reductions in cardiac myocyte cell cycle activity and relative heart weight in the near-term sheep fetus. As cardiomyocyte proliferation is nearly complete at birth in sheep and humans, a significant decrease in hyperplastic growth in utero could reduce the number of cardiomyocytes present in the heart at birth. Fewer myocytes at birth may adversely impact the capacity of the myocardium to adapt to conditions in adulthood, thus increasing susceptibility to heart disease later in life.
Perspectives and Significance
Functional adaptation of the fetal heart to changes in cardiac load allows the fetus to maintain adequate blood flow to its systemic and placental circulations, which is necessary for the well-being of the developing organism. Though a survival advantage during development, these adaptations may compromise the ability of the organism to respond to conditions after birth, increasing the risk for developing chronic cardiovascular disease in adulthood.
GRANTS
Perrie O'Tierney is a recipient of an American Heart Association Postdoctoral Fellowship (0720139Z). This research was funded by a National Institutes of Health Program Project Grant (P01HD34430), the Department of Veterans Affairs, and the M. Lowell Edwards Endowment.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We would like to acknowledge the pharmacologic consultation of Dr. Dennis R. Koop. We thank Mr. Robert Webber and Ms. Loni Socha for excellent surgical assistance.
REFERENCES
- 1.Anderson DF, Barbera A, Faber JJ. Substantial reductions in blood pressure after bilateral nephrectomy in fetal sheep. Am J Physiol Heart Circ Physiol 266: H17–H20, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Anderson DF, Faber JJ. Regulation of fetal placental blood flow in the lamb. Am J Physiol Regul Integr Comp Physiol 247: R567–R574, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Austin A, Fagan DG, Mayhew TM. A stereological method for estimating the total number of ventricular myocyte nuclei in fetal and postnatal hearts. J Anat 187: 641–647, 1995 [PMC free article] [PubMed] [Google Scholar]
- 4.Barbera A, Giraud GD, Reller MD, Maylie J, Morton MJ, Thornburg KL. Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am J Physiol Regul Integr Comp Physiol 279: R1157–R1164, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Binder ND, Anderson DF. Plasma renin activity responses to graded decreases in renal perfusion pressure in fetal and newborn lambs. Am J Physiol Regul Integr Comp Physiol 262: R524–R529, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Burrell JH, Boyn AM, Kumarasamy V, Hsieh A, Head SI, Lumbers ER. Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat Rec A Discov Mol Cell Evol Biol 274: 952–961, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Campbell SE, Korecky B, Rakusan K. Remodeling of myocyte dimensions in hypertrophic and atrophic rat hearts. Circ Res 68: 984–996, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Faber JJ, Anderson DF, Binder ND. Delayed vasoconstriction of the umbilico-placental circulation by angiotensin in fetal sheep. Placenta 19: 675–676, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Faber JJ, Anderson DF, Jonker SS, Davis LE, Giraud GD. Fetal infusions of plasma cause an increase in umbilical vascular resistance in sheep. Placenta 27: 876–881, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Giammattei CE, Strandhoy JW, Rose JC. Regulation of in vitro renin secretion by ANG II feedback manipulation in vivo in the ovine fetus. Am J Physiol Regul Integr Comp Physiol 277: R1230–R1238, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Giraud GD, Faber JJ, Jonker S, Davis L, Anderson DF. Intravascular infusions of plasma into fetal sheep cause arterial and venous hypertension. J Appl Physiol 99: 884–889, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Jonker SS, Faber JJ, Anderson DF, Thornburg KL, Louey S, Giraud GD. Sequential growth of fetal sheep cardiac myocytes in response to simultaneous arterial and venous hypertension. Am J Physiol Regul Integr Comp Physiol 292: R913–R919, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol 102: 1130–1142, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Louey S, Jonker SS, Giraud GD, Thornburg KL. Placental insufficiency decreases cell cycle activity and terminal maturation in fetal sheep cardiomyocytes. J Physiol 580: 639–648, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayhew TM, Pharaoh A, Austin A, Fagan DG. Stereological estimates of nuclear number in human ventricular cardiomyocytes before and after birth obtained using physical disectors. J Anat 191: 107–115, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moritz KM, Campbell DJ, Wintour EM. Angiotensin-(1–7) in the ovine fetus. Am J Physiol Regul Integr Comp Physiol 280: R404–R409, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Rakusan K, Heron MI, Kolar F, Korecky B. Transplantation-induced atrophy of normal and hypertrophic rat hearts: effect on cardiac myocytes and capillaries. J Mol Cell Cardiol 29: 1045–1054, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Santos RA, Ferreira AJ, Simoes E, Silva AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1–7)-Mas axis. Exp Physiol 93: 519–527, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Segar JL, Dalshaug GB, Bedell KA, Smith OM, Scholz TD. Angiotensin II in cardiac pressure-overload hypertrophy in fetal sheep. Am J Physiol Regul Integr Comp Physiol 281: R2037–R2047, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Segar JL, Scholz TD, Bedell KA, Smith OM, Huss DJ, Guillery EN. Angiotensin AT1 receptor blockade fails to attenuate pressure-overload cardiac hypertrophy in fetal sheep. Am J Physiol Regul Integr Comp Physiol 273: R1501–R1508, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Smolich JJ, Walker AM, Campbell GR, Adamson TM. Left and right ventricular myocardial morphometry in fetal, neonatal, and adult sheep. Am J Physiol Heart Circ Physiol 257: H1–H9, 1989 [DOI] [PubMed] [Google Scholar]
- 22.Sundgren NC, Giraud GD, Stork PJ, Maylie JG, Thornburg KL. Angiotensin II stimulates hyperplasia but not hypertrophy in immature ovine cardiomyocytes. J Physiol 548: 881–891, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol 289: H1560–H1566, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Walker AM, Cannata J, Dowling MH, Ritchie B, Maloney JE. Sympathetic and parasympathetic control of heart rate in unanaesthetized fetal and newborn lambs. Biol Neonate 33: 135–143, 1978 [DOI] [PubMed] [Google Scholar]