Abstract
The purpose of this review is to delineate the general features of endocrine regulation of the baroreceptor reflex, as well as specific contributions during pregnancy. In contrast to the programmed changes in baroreflex function that occur in situations initiated by central command (e.g., exercise or stress), the complex endocrine milieu often associated with physiological and pathophysiological states can influence the central baroreflex neuronal circuitry via multiple sites and mechanisms, thereby producing varied changes in baroreflex function. During pregnancy, baroreflex gain is markedly attenuated, and at least two hormonal mechanisms contribute, each at different brain sites: increased levels of the neurosteroid 3α-hydroxy-dihydroprogesterone (3α-OH-DHP), acting in the rostral ventrolateral medulla (RVLM), and reduced actions of insulin in the forebrain. 3α-OH-DHP appears to potentiate baroreflex-independent GABAergic inhibition of premotor neurons in the RVLM, which decreases the range of sympathetic nerve activity that can be elicited by changes in arterial pressure. In contrast, reductions in the levels or actions of insulin in the brain blunt baroreflex efferent responses to increments or decrements in arterial pressure. Although plasma levels of angiotensin II are increased in pregnancy, this is not responsible for the reduction in baroreflex gain, although it may contribute to the increased level of sympathetic nerve activity in this condition. How these different hormonal effects are integrated within the brain, as well as possible interactions with additional potential neuromodulators that influence baroreflex function during pregnancy and other physiological and pathophysiological states, remains to be clearly delineated.
Keywords: 3α-hydroxy-dihydroprogesterone, angiotensin II, insulin, paraventricular nucleus of the hypothalamus
normal pregnancy is characterized by profound changes in fluid and electrolyte balance and blood pressure regulation. Blood volume and cardiac output increase by 30–50%, but arterial pressure falls due to even greater decreases in systemic vascular resistance (120, 138, 189). These hemodynamic alterations are evident early in pregnancy, precede the increases in uterine blood flow, and are sustained until parturition (127, 138, 189). Considerable data implicate increases in relaxin and nitric oxide (NO) in the systemic vasodilation induced by pregnancy (20, 127, 128, 177, 196). In addition, peripheral vascular sensitivity to circulating vasoconstrictors including vasopressin, angiotensin II (ANG II), and norepinephrine (NE) is decreased (50, 71, 154).
Unlike the hemodynamic changes, which likely subserve adequate fetal development, an adverse consequence of normal pregnancy is a marked impairment of the arterial baroreceptor reflex. This change was first noted by Humphreys and Joels (97, 98) in an extensive series of studies in anesthetized pregnant rabbits, in which reductions in carotid sinus pressure failed to elicit normal increases in arterial pressure and peripheral vascular resistance. Since then, arterial baroreflex impairment has been documented in several species studied in the conscious state, including dogs (23), rats (24, 124), rabbits (21, 97, 162), goats (152), sheep (104, 123), and humans (16, 79, 122, 164, 175, 193). Reflex responses to atrial stretch are also blunted (51, 94, 95, 103), which may contribute to retention of the expanded blood volume. The depressed baroreflex function observed during normal pregnancy appears to worsen in pregnancy-associated diseases such as preeclampsia (69, 129, 137, 175, 195). Interestingly, early gestational measurements of depressed baroreflex sensitivity in conjunction with reduced uterine perfusion have been proposed as a means to predict the ultimate development of this disease (194).
The consequences of depressed baroreflex function are serious. Pregnant women are prone to orthostatic hypotension, due to blunted reflex activation of the sympathetic nervous system and inadequate peripheral vasoconstriction (56, 122, 132). Moreover, in many species, pregnancy interferes with the normal ability to maintain arterial pressure during hemorrhage (26, 36, 99, 152, 186). Hemodynamic studies have revealed that in pregnant animals, arterial pressure falls with less blood loss due to both a failure to maintain cardiac output and also inadequate peripheral vasoconstriction (22, 36). Importantly, 11–22% of maternal mortality is attributed to peripartum hemorrhage (156, 166), a common event during vaginal delivery and C-section and after delivery.
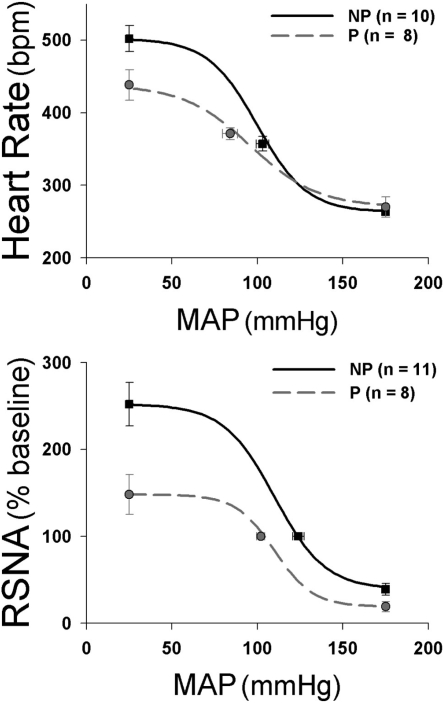
Given the potentially deleterious effects of impaired baroreflex function during pregnancy, considerable effort has been devoted to identifying the mechanisms. Experiments have assessed the site(s) within the baroreflex arc that are functionally depressed. Initial studies documented depressed baroreflex control of multiple efferents, including heart rate (5, 21, 24, 123, 175, 193), renal and muscle sympathetic nerve activity (79, 89, 148, 149), and hormones such as vasopressin and ACTH/glucocorticoids (26, 104). Multiple approaches have been used to assess the effect of pregnancy on reflex control of the autonomic nervous system (for reviews, see Refs. 69, 163), including infusion of vasoactive drugs to generate complete sigmoidal baroreflex relationships between arterial pressure and heart rate or sympathetic nerve activity. Three aspects of these curves are particularly attenuated: the midpoint of the baroreflex function curve relative to MAP or the “set point,” the maximal level of heart rate or sympathetic activity achieved when arterial pressure is lowered, and the maximal gain or sensitivity of the most linear segment of the curve (24, 47, 89, 123, 149) (Fig. 1). Pharmacological blockade of the sympathetic or parasympathetic nervous systems has revealed that the sympathetic contribution to heart rate baroreflex curves is particularly blunted (21, 123). In addition, pregnancy-induced decreases in baroreflex function in humans and rats have been successfully detected via measurement of spontaneous baroreflex sensitivity, which identifies brief spontaneous baroreflex-mediated ramps in which systolic pressure and pulse interval both simultaneously increase or decrease (5, 24, 175, 193). This measurement is restricted to the more linear segment of baroreflex curves close to basal values and highlights in particular the impact of rapid changes in parasympathetic control of the heart, documenting that pregnancy depresses these aspects of baroreflex function as well.
Fig. 1.
Pregnancy impairs baroreflex control of heart rate (bpm, beats per min) and renal sympathetic nerve activity (RSNA) in conscious rats. Arterial pressure was increased and decreased by infusion of increasing doses of phenylephrine or nitroprusside, respectively, while continuously recording arterial pressure, heart rate, and RSNA. Sigmoidal relationships between arterial pressure and heart rate or RSNA were determined by fitting a 4-parameter equation from which maximal gain was calculated, and parameters were statistically compared between pregnant (P) and nonpregnant (NP) rats (24, 124). In terms of baroreflex control of heart rate, pregnancy decreased the maximum level of heart rate, the heart rate range, and the maximal baroreflex gain and increased the range over which arterial pressure is regulated. Pregnancy also decreased the RSNA range, RSNA maximum, and RSNA minimum; the slope of RSNA responses to decreases in arterial pressure was also decreased. [Adapted from Brooks et al. (24) and Masilamani and Heesch (124).]
Several studies have determined whether pregnancy impairs specifically the responsiveness of baroreflex afferent pathways. In pregnant rats, the aortic baroreceptor discharge function curve is shifted to the lower prevailing arterial pressure level, suggesting that the parallel shift in baroreflex curves is secondary to pressure-dependent resetting of arterial baroreceptors (92, 115). On the other hand, the sensitivity of arterial baroreceptors to increments in pressure (gain), at least to short-term pressure changes, is preserved (92, 115). In contrast, differently from arterial baroreceptor afferent input, which is well maintained, in response to atrial stretch discharge of the high frequency subgroup of cardiac vagal afferent fibers is greatly reduced in pregnant rats (51, 183). Thus depressed atrial afferent responsiveness may play a role in the blunted volume-regulating reflexes observed during pregnancy.
The preservation of arterial baroreceptor afferent function suggests that the decrease in baroreflex sensitivity that occurs during pregnancy must be due to a large extent to changes within the brain. To determine potential sites within the medullary baroreflex pathway that may be altered, Curtis et al. (43) evaluated expression of Fos protein in response to sustained hypotension in the brain stem of pregnant compared with nonpregnant rats. Although in both groups arterial blood pressure was decreased to a level that would produce maximum baroreflex sympathoexcitation, activation of the rostral ventrolateral medulla (RVLM), a brain stem region important to reflex sympathetic control, was less in pregnant rats, suggesting that RVLM neurons that are normally activated by baroreceptor unloading are less responsive in pregnancy. Similarly, Deng and Kaufman (51) found, also using the c-fos method, that there is much less activation of neurons in the paraventricular nucleus of the hypothalamus (PVN) in response to atrial distention in pregnant animals compared with nonpregnant animals. Thus the function of multiple cardiovascularly relevant brain regions is depressed during pregnancy.
If brain control of arterial pressure is altered during pregnancy, then what causes this change? This has been a challenging question to answer, since the endocrine milieu of pregnancy is complex. Multiple hormones known to influence the cardiovascular system and baroreflex regulation are increased, including steroids such as estrogen and progesterone, pressor hormones such as ANG II and aldosterone, ovarian hormones such as relaxin, placental hormones such as corticotrophin-releasing hormone, pituitary hormones such as oxytocin, adipokines such as leptin, and inflammatory factors such as TNF-α and IL-6. Longitudinal studies during pregnancy indicate that the depression of baroreflex function does not correlate temporally with hemodynamic changes (e.g., blood volume, blood pressure, or cardiac output), at least in rats (24, 178) and rabbits (47, 147, 162), suggesting that different factors are involved. Moreover, estrogen is likely not an initiating factor, since levels are not increased significantly in some species such as rabbit and sheep, and since several studies have demonstrated that this steroid enhances arterial baroreflex function via an action in the hindbrain (135, 155, 169). In this article, we review evidence for increased actions of 3α-hydroxy-dihydroprogesterone (3α-OH-DHP), and decreased actions of insulin, as contributing factors to central nervous system (CNS) changes in baroreflex function associated with pregnancy. Before discussing these factors in detail, we begin with a brief description of the central pathways that subserve the arterial baroreflex, followed by an outline of the general features of hormonal regulation of baroreflex function.
Central Baroreflex Pathways and Their Modulation by Hormones
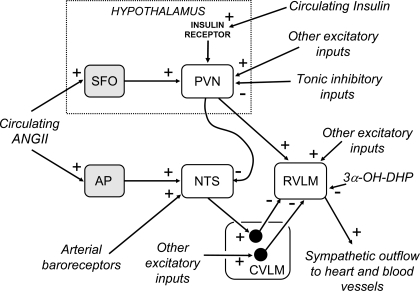
The essential features of the central pathways subserving the arterial baroreflex are now well established (46, 81) and are illustrated in Fig. 2. Primary baroreceptor afferent fibers terminate in the nucleus tractus solitarii (NTS), mainly in its dorsomedial portion. Second-order neurons within the NTS, many of which receive direct monosynaptic inputs from primary baroreceptor afferent fibers (7), project to and excite neurons in the caudal ventrolateral medulla (CVLM), which contains a group of interneurons that project to and inhibit sympathetic premotor neurons in the RVLM.
Fig. 2.
Schematic diagram showing the essential pathways that subserve the baroreflex control of the sympathetic outflow to the heart and blood vessels and possible sites of action and mechanisms by which changes in the activity of the hormones angiotensin II (ANG II), 3α-hydroxy-dihydroprogesterone (3α-OH-DHP; a metabolite of progesterone), and insulin can alter the baroreflex. Note that in addition to the inhibitory input to rostral ventrolateral medulla (RVLM) sympathetic premotor neurons from barosensitive neurons in the caudal ventrolateral medulla (CVLM), there is a baroreceptor-independent inhibitory input to the RVLM neurons arising from other CVLM neurons that receive excitatory inputs from neurons in unknown brain regions. NTS, nucleus tractus solitarii; AP, area postrema; PVN, paraventricular nucleus; SFO, subfornical organ. + indicates an excitatory effect, and − indicates an inhibitory effect.
The main transmitter released by primary baroreceptor afferent fibers within the NTS is glutamate, which excites second-order NTS neurons primarily via non-N-methyl-d-aspartate (non-NMDA) receptors (77). Second-order barosensitive neurons that terminate in the CVLM also release glutamate, which acts on NMDA receptors on the GABAergic inhibitory neurons that project to the RVLM (76). Also, independent of the baroreflex pathway, there are other GABAergic projections from the CVLM to the RVLM that inhibit sympathetic outflow and that may influence reflex responses (170) (Fig. 2). The RVLM sympathetic premotor neurons are glutamatergic (184) and directly excite sympathetic pathways to the heart and blood vessels (46, 81) (Fig. 2). The RVLM neurons are also a site of convergence of inputs from many other sources, including both peripheral receptors and other brain nuclei (46, 81). In addition, second-order neurons within the NTS directly innervate and excite cardiac vagal preganglionic neurons in the nucleus ambiguus.
Studies of the baroreflex in conscious animals and humans have shown that the reflex is continuously modulated, depending on the prevailing behavioral and physiological conditions. For example, during exercise and psychological stress, baroreflex control of heart rate and vasomotor activity is reset in the short term such that it continues to regulate both these variables but over a higher operating range of arterial pressure compared with resting conditions (102, 130, 165). Rather than being caused by adaptation of the baroreceptors themselves, the resetting of the baroreceptor reflex that occurs in association with these relatively short-term challenges is a consequence of “central command,” i.e., is often driven by centers in the cortex and other forebrain regions (44). In the case of exercise, feedback from peripheral receptors, particularly from muscle receptors (so-called “ergoreceptors”) also contributes to the acute resetting of the baroreflex (165). The effect of this resetting is that the baroreflex continues to regulate arterial pressure at all times but at a level that is appropriate for the particular behavioral conditions.
Circulating hormones can also modulate baroreflex function via a variety of brain sites and mechanisms. First, some hormones [e.g., progesterone (191)] cross the blood-brain barrier and therefore can directly access any of the critical neurons. Second, other hormones [e.g., insulin (8)] gain access to the brain via specific transport systems. Third, some hormones [e.g., ANG II (70, 192)] act on receptors on neurons in circumventricular organs (CVO) that are located outside the blood-brain barrier. Activation of these neurons can then influence brain function via the projections of the hormone-sensitive CVO neurons to particular target nuclei within the brain. Fourth, some circulating hormones [e.g., ANG II or 3α-OH-DHP (61, 72, 160, 190)] are also synthesized de novo in the brain, where they serve as local neuromodulators. Finally, some hormones (e.g., ANG II again) may act on receptors in cerebral blood vessels, triggering events that lead to the production of neuromodulatory compounds, such as NO, within the local brain tissue (159).
The central mechanisms that cause changes in the operating characteristics of the baroreflex during pregnancy or other physiological or pathophysiological conditions associated with endocrine changes may therefore be due to effects that occur at one or more of the critical central synapses that subserve the reflex. Figure 2 illustrates some of the different ways that circulating hormones such as ANG II could cause such effects, either directly or indirectly. In particular, the NTS has long been regarded as a prime site at which baroreflex modulation can occur (1). It receives direct inputs from the area postrema and subfornical organ (46) and therefore can be influenced by hormones such as ANG II that act on AT1 receptors on neurons within these CVOs. There is also a major direct input to the NTS from neurons in the PVN, which itself receives inputs from the subfornical organ (46). Finally, the NTS has been proposed as a site at which circulating ANG II stimulates NO production via endothelial nitric oxide synthase (NOS) in blood vessels (159). In the NTS, the increased level of NO in turn enhances GABA release, impairing baroreflex function (159).
The RVLM also receives synaptic inputs from the PVN (46) and so is also a potential site at which circulating hormones that affect PVN neurons may modulate baroreflex function. Furthermore, there is a direct input to this region from the area postrema (17). Also, as noted above, hormones such as progesterone (or their metabolites) that cross the blood-brain barrier can potentially affect neurons within the CVLM and RVLM, as well as the NTS or in any other brain regions that project to these nuclei. Finally, substances produced within the brain parenchyma, such as ANG II or 3α-OH-DHP, may act directly on neurons in several brain nuclei that influence baroreflex function, such as the NTS, PVN, and RVLM (45, 63, 85).
Since circulating hormones may affect neurons in many different sites, the overall effect on the baroreflex of an increase in the level of a hormone could be the consequence of several different effects operating simultaneously and could vary. For example, increased ANG II levels appear to support or increase the arterial pressure level at which the baroreflex operates in some states, such as hypertension (78, 88, 108, 109, 185), sodium and water deprivation (18, 54, 199), and pregnancy (27, 149), whereas in other states, including heart failure (53, 143, 144), this action is minimal. Similarly, the effect of ANG II to decrease baroreflex gain is revealed only in a subset of states with elevated levels, such as heart failure (53, 144, 145) and some forms of hypertension (96, 108, 109). Since on a cellular level brain ANG II is modulatory, it is likely that the presence or absence of other inputs determines the ultimate effect of this neurohormone (45).
In summary, there are several potential mechanisms that could explain the fact that ANG II or other hormones can produce different effects on the baroreflex control of heart rate, RSNA, or other outputs, depending on the physiological or pathophysiological condition. In pregnancy, it is also very important to consider the effects of chronic changes in hormones, especially those whose levels are greatly altered in this condition. For example, a sustained increase (or decrease) in the activity of a hormone may result in genomic changes that in turn may lead to a wide range of effects that would not occur acutely, such as changes in the expression of receptors or the synthesis of neurotransmitters or enzymes that affect synaptic transmission.
Apart from the arterial baroreflex, there are other important reflexes that contribute to circulatory control and are also profoundly altered in pregnancy. In particular, receptors in the right atrium have a critical role in regulating blood volume. Activation of these receptors results in a rather selective reflex inhibition in renal sympathetic nerve activity (121, 200). The central pathways mediating this reflex have not been fully determined but include a critical synapse in the PVN (121, 200). On the basis of various observations, it is thought that signals from atrial receptors, mediated via the NTS, excite GABAergic neurons within the PVN that in turn inhibit other PVN neurons that directly or indirectly regulate the renal sympathetic outflow (51, 200). As described above, activation of neurons in the PVN in response to atrial distention is blunted in pregnant compared with nonpregnant animals (51). Very little information is available, however, on the possible central mechanisms that cause the inhibition of this reflex. Therefore, the following sections of this review focus primarily on the endocrine factors that modulate the arterial baroreflex during pregnancy.
Roles of ANG II and NO in Baroreflex Modulation During Pregnancy
The levels of circulating ANG II and vascular (endothelial) NO are elevated during pregnancy (3, 176, 177, 196) and can impair baroreflex function via a central action (85, 126). Indeed, ANG II and/or NO have been implicated in the baroreflex dysfunction associated with hypertension and heart failure (53, 109, 146, 159). Therefore, both substances were considered excellent candidates as potential modulators of baroreflex function during pregnancy. However, neither systemic nor central blockade of ANG II AT1 receptors and of NO production, alone or together, improved the gain of arterial baroreflex control of renal sympathetic nerve activity or heart rate (27, 41, 48, 149). These data suggest that a central action of ANG II and/or NO is not responsible for the decrease in baroreflex gain during pregnancy, at least via a rapidly reversible mechanism.
On the other hand, baseline sympathetic tone is increased in pregnancy in both humans and animals (2, 21, 37, 79, 124), and changes in CNS actions of ANG II may contribute. Compared with nonpregnant animals, pregnant animals are more dependent on ANG II for maintenance of baseline arterial pressure (27, 149). Moreover, acute administration of an AT1 receptor antagonist, either intravenously or intracerebroventricularly, caused a leftward shift in both HR and RSNA baroreflex function curves, effectively reducing SNA at any given arterial pressure level (27, 48, 149). It has been suggested that the elevated baseline sympathetic activity in pregnant rats is a consequence of increased activity of PVN sympathoexcitatory neurons which in turn is due, at least in part, to the increased level of circulating ANG II that occurs (112) (Fig. 2). Consistent with this hypothesis, chronic infusion of ANG II has been shown to cause activation of PVN neurons (49).
It is also possible that reductions in neuronal NOS (nNOS) activity specifically in the PVN could contribute to elevated baseline sympathetic outflow in pregnancy. In the PVN, NO increases GABA release and results in inhibition of autonomic-related parvocellular neurons (119). Decreased nNOS activity, and a consequent reduction in GABA release in the PVN, has been associated with augmented sympathoexcitation in other conditions, such as hypertension and heart failure (157). Therefore, it is interesting that in near-term pregnant rats, PVN nNOS expression and activity (91) and baseline GABAergic inhibition (25, 112) are both decreased. However, further study is required to determine the physiological significance of these changes in the PVN in pregnancy.
Although an increased level of NO does not appear to be involved in the depression of arterial baroreflex gain in pregnancy, it may contribute to impaired function of volume-dependent atrial reflexes. In pregnant animals, administration of the NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME) completely restored the appearance of Fos immunoreactivity in both parvo- and magnocellular regions of the PVN, and the resulting natriuresis, in response to atrial distension (187, 188). These findings emphasize the potential complexity of endocrine control of baroreflex function in a state such as pregnancy.
Insulin Resistance: a Contributor to Pregnancy-Induced Decreases in Baroreflex Gain?
Multiple conditions besides pregnancy are associated with arterial baroreflex impairment, including heart failure, obesity, hypertension, Alzheimer's disease, and aging. Intriguingly, a common feature of all these conditions is insulin resistance, which results from blunted intracellular signaling following binding of insulin to its receptor. One consequence can be elevated plasma glucose levels, since a major action of insulin is to stimulate glucose uptake from plasma into skeletal muscle and adipocytes. During pregnancy, the development of insulin resistance is teleologically beneficial, since elevated plasma glucose levels would favor the movement of this nutrient into the placental-fetal compartments.
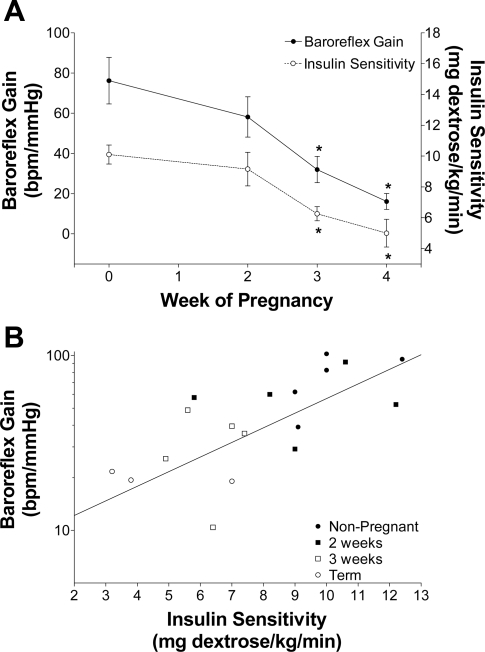
This common association between arterial baroreflex dysfunction and insulin resistance led to the hypothesis that the two are causally related during pregnancy. In support of this hypothesis, decreases in insulin sensitivity (40, 47, 100, 114, 141, 167) and baroreflex gain (16, 24, 47, 122) exhibit the same time course during gestation in humans, rabbits (Fig. 3), and rats. Interesting, preeclampsia produces further reductions in insulin sensitivity (100, 180) and, as indicated above, is associated with further baroreflex impairment. More importantly, when pregnant rabbits were treated throughout gestation with the insulin-sensitizing drug rosiglitazone, insulin sensitivity improved and, to the same extent, baroreflex gain increased, without altering other aspects of the sigmoidal baroreflex curve (47). Collectively, these data implicate insulin resistance as a factor involved in the baroreflex impairment induced by pregnancy.
Fig. 3.
Insulin sensitivity and baroreflex gain decreased in parallel during pregnancy in rabbits, and the changes were well correlated (r2 = 0.65 ± 0.08, n = 5). [From Daubert et al. (47).]
If pregnancy-induced insulin resistance and baroreflex impairment are mechanistically linked, then what is the link? One possible mechanism involves the actions of insulin in the brain (Fig. 2). Insulin receptors are present in numerous but discrete sites throughout the brain, including regions directly or indirectly involved in central pathways regulating the cardiovascular system: hypothalamus (PVN, arcuate nucleus, supraoptic nucleus, dorsomedial and ventromedial hypothalamus) and brain stem (NTS, the raphe nuclei and nucleus ambiguus) (171, 197). Although insulin, a large polypeptide, cannot penetrate the blood-brain barrier unassisted, a specific and saturable transport system moves insulin from the plasma compartment into brain interstitial fluid via receptor-mediated endocytosis through vascular endothelial cells (for reviews, see Refs. 8, 62, 74, 198). Kinetic studies suggest that insulin passes from plasma to brain interstitial fluid and then into cerebral spinal fluid (198). The rate of passage of insulin varies throughout the brain, with the highest levels recorded in the medulla and hypothalamus (8, 12, 171).
Once plasma insulin enters the brain, it binds to insulin receptors to affect cardiovascular function (Fig. 2). It is well established that insulin is sympathoexcitatory, via a central action (117, 142). In addition, acute and chronic increases in brain insulin increase the gain of baroreflex control of both heart rate and sympathetic activity (150, 161). Interestingly, however, the rate of transport of insulin into brain, and therefore brain insulin levels, can be altered; indeed, insulin resistance is associated with impaired transport of insulin into brain (62, 74, 198). For example, adiposity in dogs reduces brain insulin transport (101), and brain insulin levels are decreased in Zucker obese rats (13) and in individuals with Alzheimer's disease (8, 74). Although the influence of pregnancy on transport of insulin into the brain has not been investigated, recent studies indicate that levels of insulin in cerebrospinal fluid are reduced (47). Because brain insulin supports or enhances baroreflex gain, decreases in brain insulin levels or in its actions could attenuate baroreflex function during pregnancy. In support of this hypothesis, it has been shown that intracerebroventricular infusion of insulin normalizes gain of baroreflex control of heart rate in conscious pregnant rats (6). Yet, similar to the effects of rosiglitazone, increases in brain insulin did not alter the baroreflex in virgin rats and did not reverse the depressed maximal level of heart rate in pregnant rats. These results have several important implications. First, brain insulin levels must be sufficiently high in normal conscious animals to support optimal heart rate baroreflex function. Second, pregnancy-induced decreases in brain insulin contribute to the baroreflex impairment. Third, other factors must underlie the reduced ability of pregnant animals to respond to severe hypotension with maximal increases in heart rate or sympathetic activity.
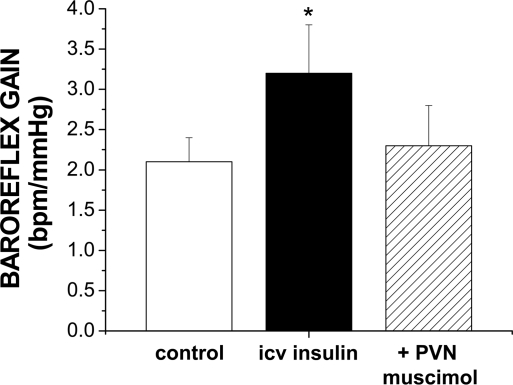
Recent studies have explored the brain sites and mechanisms by which insulin in brain increases baroreflex gain. As described above, insulin receptors are concentrated in many forebrain and hindbrain regions known to influence neural control of the circulation. However, infusion of insulin via the fourth cerebral ventricle failed to alter baroreflex control of heart rate or lumbar sympathetic nerve activity, suggesting that insulin initiates its effect at a forebrain site when infused via the lateral ventricles (161). Among potential hypothalamic sites, the PVN is a likely candidate, since this region is enriched with insulin receptors (171, 197) (Fig. 2) and is known to influence baroreflex gain (35, 55, 158). In support of this hypothesis, it was found that blockade of PVN, secondary to PVN microinjection of the GABAA agonist muscimol, reversed the effect of insulin to increase baroreflex gain (Fig. 4). Thus the PVN is involved. Nevertheless, several questions remain. Is the PVN the site at which insulin initiates its action or merely a relay station? Does the role of PVN change during pregnancy or other insulin-resistant states? What downstream pathways and brain stem sites effect the action of forebrain insulin to increase gain?
Fig. 4.
Local blockade of the paraventricular nucleus (PVN) with bilateral microinjection of muscimol reversed the effect of intracerebroventricular (icv) insulin infusion to increase baroreflex gain (n = 4). In contrast, in control animals not receiving insulin, bilateral PVN muscimol did not alter baroreflex gain (1.1 ± 0.3 bpm/mmHg, control; 1.1 ± 0.3 bpm/mmHg, icv artificial cerebrospinal fluid or no infusion; 1.2 ± 0.3 bpm/mmHg, +PVN muscimol; n = 5; P > 0.05). Experiments were performed in urethane-anesthetized male rats using previously published procedures (65, 161). *P < 0.05 compared with the other 2 treatments (ANOVA for repeated measures and the Newman Keuls post hoc test). Similar results have recently been found in female rats (34).
Neurosteroid Metabolite of Progesterone: a Contributor to Attenuated Baroreflex Sympathoexcitation in Pregnancy?
The studies discussed above indicate that decreased insulin in the forebrain of pregnant animals, possibly via the PVN, contributes to the decrease in maximum gain of the arterial baroreflex. However, the attenuated ability to increase heart rate or sympathetic nerve activity in response to a hypotensive challenge appears to be unrelated to changes in insulin levels and may instead involve augmented GABAergic influences in the RVLM. As described above, when arterial pressure was decreased to a level expected to produce maximum sympathoexcitation, activation of the RVLM (but not the NTS or CVLM) was attenuated in term pregnant rats (43), consistent with a role for the RVLM in the attenuated baroreflex sympathoexcitation in pregnancy (Fig. 1).
It is important to consider that arterial baroreflex sympathoinhibition during elevated pressure is mediated by increased inhibitory input and activation of GABAA receptors in the RVLM, whereas sympathoexcitation due to baroreceptor unloading (decreased arterial pressure) is mediated by withdrawal of GABAergic tone (disinhibition) (46, 80). Interestingly, arterial baroreflex sympathoinhibitory responses are well maintained or even potentiated in pregnancy (41, 93, 124) (Fig. 1), and the major effect of pregnancy on baroreflex range occurs at low arterial blood pressures, where baroreceptor discharge should be minimal. In this regard, the RVLM receives a substantial baroreflex independent GABAergic input, a large portion of which originates in the CVLM (42) (Fig. 2), although other GABAergic influences, including GABA interneurons within the RVLM, may contribute (81, 134, 170). Thus, although the effect of pregnancy is manifested as blunted baroreflex-mediated increases in heart rate and sympathetic nerve activity, it is possible that mechanisms unrelated to the arterial baroreflex contribute to the attenuated baroreflex sympathoexcitation.
The final sympathetic outflow emanating from the RVLM at any given time is determined by the balance of excitatory and inhibitory inputs to this region (46). Thus the observation of decreased activation of the RVLM during hypotension in pregnant rats (43) could be due to either decreased baseline excitatory drive to the RVLM or increased baseline baroreflex-independent inhibitory drive, or both, in pregnant compared with nonpregnant rats. To test the hypothesis that the RVLM of pregnant rats is under greater baroreflex-independent GABAergic inhibition, responses to bilateral blockade of GABAA receptors in the RVLM were tested in pregnant and nonpregnant rats in which the arterial baroreceptors had been surgically denervated. Disinhibition of the RVLM with the GABAA antagonist bicuculline produced greater increases in mean arterial pressure and renal sympathetic nerve activity in pregnant compared with nonpregnant rats (Fig. 5) (110). Thus it appears that pregnancy is associated with enhanced baseline GABAergic inhibition of the RVLM. Prior blockade of AT1 receptors in the RVLM decreased responses to disinhibition, but the difference in responses to bicuculline between pregnant and nonpregnant rats persisted. Thus, although ANG II likely contributes to elevated baseline sympathetic outflow in pregnancy (21, 149), within the RVLM other underlying excitatory influences must contribute to observed differences between pregnant and nonpregnant rats (110).
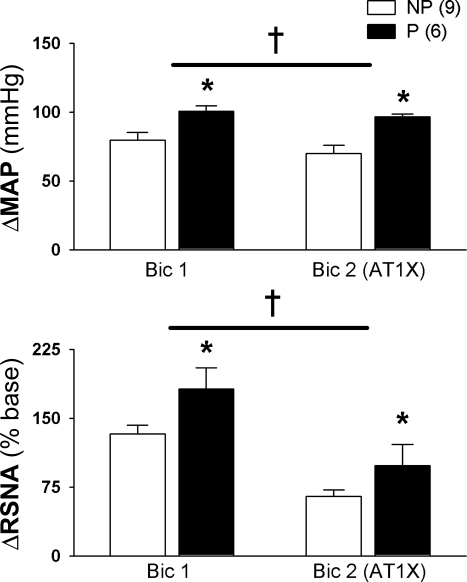
Fig. 5.
Increased baroreflex-independent GABAA inhibition of the RVLM in pregnant rats. In sinoaortic denervated rats, increases in mean arterial pressure (ΔMAP) and RSNA (ΔRSNA) in response to bilateral blockade of GABAA receptors (bicuculline, Bic) into the RVLM was greater in P compared with NP rats. Prior blockade of ANG II AT1 receptors (L158,809; AT1X) in the RVLM attenuated responses to Bic, but differences between NP and P rats persisted. *P ≤ 0.05, greater than in NP rats. †P ≤ 0.05, main effect of AT1X. [From Kvochina et al. (110)].
In regard to the mechanisms for increased GABAA influence in the RVLM, it is possible that, independent of arterial baroreflex inputs, GABA release in the RVLM is greater in pregnant animals. However, the source of drive to nonbaroreflex GABAergic inputs to the RVLM and the potential signal to increase that input during pregnancy is unclear. Another possibility, which would not require increased GABAergic input to the RVLM, would be an increase in positive modulation of GABAA receptors within the RVLM so that the effectiveness of existing GABAergic input would be amplified in pregnancy. It is known that through stereospecific binding to a unique site on the GABAA receptor complex, the major metabolite of progesterone, 3α-OH-DHP, which is elevated in pregnancy (38, 67, 68), is a potent positive modulator of GABAA receptor function (151). An autoradiographic study on brain sections from nonpregnant female rats indicated that in vitro exposure to 3α-OH-DHP increases binding of the GABAA ligand flunitrazepam in the brain stem (73). Thus GABAA receptors in brain regions involved in control of sympathetic nerve activity are susceptible to modulation by this neurosteroid metabolite of progesterone.
In support of this concept, acute systemic administration of the neurosteroid metabolite of progesterone, 3α-OH-DHP, to nonpregnant rats mimics the effects of pregnancy to suppress arterial baroreflex sympathoexcitation (89, 90, 124) while having no effect on afferent baroreceptor discharge (115). In all cases, the inactive isomer, 3β-OH-DHP, was without effect. Figure 6 shows effects of microinjection of 3α-OH-DHP into the RVLM, NTS, and CVLM of nonpregnant female rats on maximum sympathetic nerve activity achieved in response to baroreceptor unloading. Microinjection of 3α-OH-DHP, but not 3β-OH-DHP (not shown), into the RVLM resulted in a reversible decrease in maximum sympathetic nerve activity, whereas microinjection into the NTS and CVLM was without effect (63). Thus, similar to the effects of pregnancy on baroreflex-mediated activation of brain stem nuclei that are specific to the RVLM (43), effects of the neurosteroid metabolite of progesterone to suppress baroreflex-mediated increases in sympathetic nerve activity also appear to be specific to the RVLM. This result is consistent with the concept that 3α-OH-DHP, through positive modulation of GABAA receptors, may contribute to the increased baseline GABAergic inhibition of the RVLM that is seen in pregnancy (110).
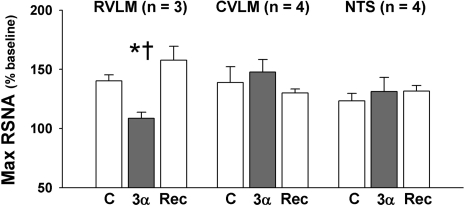
Fig. 6.
3α-OH-DHP in the RVLM of NP rats limits maximum baroreflex-mediated sympathoexcitation. Maximum (max) RSNA due to baroreflex unloading was reversibly attenuated following microinjection of 3α-OH-DHP (3α) into the RVLM but not into the CVLM or NTS. C, control; Rec, recovery. *P ≤ 0.05, less than control. †P ≤ 0.05, less than during recovery.
On the other hand, pregnancy is also characterized by well-maintained or potentiated baroreflex-mediated sympathoinhibition in response to a hypertensive challenge (41, 93, 124) (Fig. 1). Since baroreflex-mediated inhibition is due to incremental increases in GABAergic input to the RVLM, positive modulation of GABAA receptors by 3α-OH-DHP at the level of the RVLM would be consistent with increased responsiveness of RVLM neurons to increases in pressure. Indeed, intravenous administration of 3α-OH-DHP (but not 3β-OH-DHP) to nonpregnant female rats decreased the pressure threshold for baroreflex mediated inhibition of single-unit neuronal discharge in spinally projecting RVLM neurons (116). Likewise, local application of 3α-OH-DHP (<1 nl, 4 μM) directly onto spinally projecting, arterial pressure-sensitive neurons in the RVLM (n = 7) resulted in a decrease in pressure threshold for neuronal inhibition during a hypertensive stimulus. These results suggest that sensitivity of RVLM neurons to endogenously released GABA is increased by 3α-OH-DHP.
As discussed earlier, volume reflexes that are activated by atrial stretch are attenuated by pregnancy. Interestingly, exogenous administration of 3α-OH-DHP to virgin female rats mimics the effects of pregnancy to attenuate discharge of afferent fibers and to decrease activation of forebrain regions involved in control of blood volume (52, 183). Thus it appears that 3α-OH-DHP, a neurosteroid metabolite of progesterone, is a likely contributor to alterations that occur in both the arterial baroreflex and volume reflexes in pregnancy.
Sensitivity of GABAA receptors to modulation by neurosteroid compounds, such as 3α-OH-DHP, is partially dependent on subunit composition of GABAA receptors, which may change during pregnancy. Expression of different GABAA receptor subunits varies between brain regions and even cell types within a region (133) and can be differentially regulated by numerous factors, including neuronal activity (174), and the ovarian hormones estrogen (39, 172) and progesterone (60, 136, 140, 172, 174). It was previously reported that GABAA receptors with a higher proportion of α1- compared with α2-receptor subunits were most sensitive to neurosteroid modulation (153), and expression of the α1-subunit is increased in hypothalamic oxytocin neurons in late pregnancy (31, 59). In the RVLM, expression of the α1-subunit is several hundred times greater than expression of the α2-subunit, suggesting that GABAA receptors in this region should be sensitive to neurosteroid modulation (64). However, the α1:α2 ratio in the RVLM was not changed by pregnancy. Thus increased levels of 3α-OH-DHP in pregnant rats, rather than changes in α1-subunit, were proposed as a mechanism contributing to increased GABAergic tone in the RVLM of pregnant rats (64). More recently, it has been suggested that, rather than the GABAA receptor α1-subunit, the δ-subunit, which appears to be crucial for extrasynaptic tonic inhibition (15, 133), might be the most important receptor subunit in conferring high sensitivity to positive modulation by 3α-OH-DHP in native GABAA receptors (15, 133). Currently the effect of pregnancy on expression of the GABAA receptor δ-subunit in the RVLM is not known.
Although receptor subunit composition is one factor determining sensitivity to 3α-OH-DHP, other factors affecting efficacy of neurosteroids include regulation of local concentrations of 3α-OH-DHP (synthesis and degradation) and posttranslational changes such as phosphorylation, which can affect responses to GABAA receptor activation (58, 83, 107, 139). Enzymes responsible for synthesis of 3α-OH-DHP from progesterone, 5α-reductase and 3α-OH steroid oxidoreductase, are present in the medulla (105, 118, 168), and ovarian hormones can regulate 3α-OH-DHP synthesis in brain tissue (131). Importantly, differences in peripheral and CNS levels of 3α-OH-DHP during pregnancy suggest that local regulation determines the effective concentrations of 3α-OH-DHP in a brain region-specific manner (14, 38, 168, 173). Preliminary data suggest that messenger RNA for both 5α-reductase and 3α-OH steroid oxidoreductase is increased in the RVLM of term pregnant rats (87), and treatment of near-term pregnant rats with the 5α-reductase inhibitor Finasteride partially restores baroreflex-mediated sympathoexcitation (125). Together these studies in pregnant rats suggest that increased levels of the major metabolite of progesterone, 3α-OH-DHP, in the RVLM may contribute to attenuated baroreflex-mediated sympathoexcitation in pregnancy.
Conclusions and Future Directions
The function of the arterial baroreflex is in continuous flux. In some circumstances, such as stress or exercise, programmed efferent commands that issue from higher centers reset the baroreflex to a higher pressure, a teleologically appropriate response. In contrast, the often complex endocrine milieu associated with acute and chronic physiological and pathophysiological states influences multiple central sites to ultimately produce varied changes in the baroreflex.
During pregnancy, baroreflex gain is markedly attenuated, and at least two hormonal mechanisms have been shown to contribute, each at different brain sites: increased levels of 3α-OH-DHP acting in the RVLM and reduced actions of insulin in the forebrain. Maximal baroreflex gain depends on two parameters, the heart rate or sympathetic nerve activity range (difference between the maximum and minimum heart rate or level of sympathetic nerve activity) and the width (the arterial pressure range over which the reflex operates) (84). Alterations in both parameters are involved in the decreased gain induced by pregnancy (Fig. 1) (19, 24, 124). Interestingly, the decrease in brain insulin appears to primarily increase the range over which arterial pressure is regulated (X-axis, Fig. 1), whereas 3α-OH-DHP acts mainly to decrease the sympathetic nerve activity or heart rate range (Y-axis, Fig. 1), apparently by potentiating baroreflex-independent GABAergic inhibition of premotor neurons in the RVLM. This later effect is reminiscent of other situations in which control of sympathetic outflow is compromised. For example, heart rate range is decreased following pharmacological blockade of cardiac sympathetics (86), and splanchnic sympathetic nerve activity range is reduced following destruction of catecholaminergic RVLM premotor neurons (82). Where and how the forebrain action of insulin and the hindbrain effect of 3α-OH-DHP integrate in the brain to depress baroreflex function is not known, primarily because the neuronal pathways and mechanisms by which forebrain insulin influences brain stem circuitry have not been completely delineated. Nevertheless, the PVN is one site identified in the pathway (Fig. 4). Moreover, insulin has recently been shown to increase glutamatergic drive to the RVLM (11), and the vast majority of PVN projections to the RVLM are glutamatergic (182). Therefore, one potential scenario is that the RVLM is the site at which forebrain insulin-derived inputs and brain stem 3α-OH-DHP actions are integrated (Fig. 2).
As described earlier, undesirable effects of decreased baroreflex sensitivity in pregnancy include orthostatic hypotension and, more seriously, a decreased ability to compensate for blood loss. Nevertheless, the attenuated ability to rapidly increase sympathetic outflow through the arterial baroreflex could serve a protective role by limiting vasoconstriction in the face of substantially increased blood volume and cardiac output. Furthermore, this adaptation would minimize possible detrimental effects of excessive shunting of blood flow (due to constriction of other beds) to low-resistance beds, such as the uterine circulation in pregnancy.
Besides the baroreflex, pregnancy has been shown to suppress other homeostatic reflexes that utilize brain pathways, including fever and ACTH responses induced by systemic inflammation and ACTH released in response to behavioral stress, as well as the appetite-inhibiting actions of leptin (4, 28, 30, 179, 181). These changes are also likely beneficial to the mother and fetus, since they minimize the potentially negative consequences of elevated glucocorticoid concentrations or body temperature and favor increased energy consumption. The depression of parallel brain homeostatic mechanisms leads logically to the question as to whether similar mechanisms and brain pathways are involved. In support of this idea, 3α-OH-DHP has also been shown to be involved in the depressed responses of the hypothalamus-pituitary-adrenal axis to stress (30). Moreover, placental lactogen has been identified as one factor that reduces brain leptin sensitivity in the context of energy balance (4). Placental lactogen (40, 114) and other circulating factors emanating from the placenta and adipose tissue, such as progesterone, TNF-α, IL-6, resistin, and free fatty acids (9, 10, 32, 33, 57, 66, 75, 106), decrease insulin sensitivity during pregnancy. Therefore, these factors may also contribute to baroreflex impairment by decreasing brain levels or actions of insulin in brain to support baroreflex gain. Other parallels also may exist; for example, does the increased GABAergic tone in RVLM attenuate other sympathoexcitatory responses mediated by this brain region, such as the sympathoexcitation induced by stress? Future experimental work is required to further explore mechanisms and possible interactions between various neuromodulators that contribute to alterations in baroreflex function in pregnancy and other physiological and pathophysiological states.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL70962 (to V. L. Brooks), HL088552 (to V. L. Brooks), and HL 36245 (to C. M. Heesch), American Heart Association Grant 09GRNT2060630 (to V. L. Brooks), and the National Health and Medical Research Council and the National Heart Foundation of Australia (to R. A. L. Dampney).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We gratefully acknowledge the contributions of Korrina Freeman, Priscila Cassaglia, Shannon Burcks, and Charles Foley to the previously unpublished data contained in this review.
REFERENCES
- 1.Andresen MC, Doyle MW, Jin YH, Bailey TW. Cellular mechanisms of baroreceptor integration at the nucleus tractus solitarius. Ann NY Acad Sci 940: 132–141, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Anglin JC, Brooks VL. Tyrosine hydroxylase and norepinephrine transporter in sympathetic ganglia of female rats vary with reproductive state. Auton Neurosci 105: 8–15, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Anton L, Brosnihan KB. Systemic and uteroplacental renin–angiotensin system in normal and pre-eclamptic pregnancies. Ther Adv Cardiovasc Dis 2: 349–362, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augustine RA, Ladyman SR, Grattan DR. From feeding one to feeding many: hormone-induced changes in bodyweight homeostasis during pregnancy. J Physiol 586: 387–397, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avery ND, Wolfe LA, Amara CE, Davies GA, McGrath MJ. Effects of human pregnancy on cardiac autonomic function above and below the ventilatory threshold. J Appl Physiol 90: 321–328, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Azar AS, Brooks VL. Increases in brain insulin normalize baroreflex gain in conscious pregnant rats (Abstract). FASEB J 22: 1228.3, 2008 [Google Scholar]
- 7.Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci 26: 11893–11902, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banks WA. The source of cerebral insulin. Eur J Pharmacol 490: 5–12, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 30, Suppl 2: S112–S119, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Barbour LA, Shao J, Qiao L, Pulawa LK, Jensen DR, Bartke A, Garrity M, Draznin B, Friedman JE. Human placental growth hormone causes severe insulin resistance in transgenic mice. Am J Obstet Gynecol 186: 512–517, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension 55: 284–290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baskin DG, Porte D, Jr, Guest K, Dorsa DM. Regional concentrations of insulin in the rat brain. Endocrinology 112: 898–903, 1983 [DOI] [PubMed] [Google Scholar]
- 13.Baskin DG, Stein LJ, Ikeda H, Woods SC, Figlewicz DP, Porte D, Jr, Greenwood MR, Dorsa DM. Genetically obese Zucker rats have abnormally low brain insulin content. Life Sci 36: 627–633, 1985 [DOI] [PubMed] [Google Scholar]
- 14.Belelli D, Herd MB. The contraceptive agent Provera enhances GABAA receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci 23: 10013–10020, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ. Neuroactive steroids and inhibitory neurotransmission: mechanisms of action and physiological relevance. Neuroscience 138: 821–829, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Blake MJ, Martin A, Manktelow BN, Armstrong C, Halligan AW, Panerai RB, Potter JF. Changes in baroreceptor sensitivity for heart rate during normotensive pregnancy and the puerperium. Clin Sci (Lond) 98: 259–268, 2000 [PubMed] [Google Scholar]
- 17.Blessing WW, Hedger SC, Joh TH, Willoughby JO. Neurons in the area postrema are the only catecholamine- synthesizing cells in the medulla or pons with projections to the rostral ventrolateral medulla (C1-area) in the rabbit. Brain Res 419: 336–340, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Brooks VL. Vasopressin and ANG II in reflex regulation of heart rate: effect of water deprivation. Am J Physiol Regul Integr Comp Physiol 263: R756–R761, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Brooks VL, Clow KA, O'Hagan KP. Pregnancy and acute baroreflex resetting in conscious rabbits. Am J Physiol Regul Integr Comp Physiol 283: R429–R440, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Brooks VL, Clow KA, Welch LS, Giraud GD. Does nitric oxide contribute to the basal vasodilation of pregnancy in conscious rabbits? Am J Physiol Regul Integr Comp Physiol 281: R1624–R1632, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Brooks VL, Kane CM, Van Winkle DM. Altered heart rate baroreflex during pregnancy: role of sympathetic and parasympathetic nervous systems. Am J Physiol Regul Integr Comp Physiol 273: R960–R966, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Brooks VL, Kane CM, Welch LS. Regional conductance changes during hemorrhage in pregnant and nonpregnant conscious rabbits. Am J Physiol Regul Integr Comp Physiol 277: R675–R681, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Brooks VL, Keil LC. Changes in the baroreflex during pregnancy in conscious dogs: heart rate and hormonal responses. Endocrinology 135: 1894–1901, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Brooks VL, Mulvaney JM, Azar AS, Zhao D, Goldman RK. Pregnancy impairs baroreflex control of heart rate in rats: role of insulin sensitivity. Am J Physiol Regul Integr Comp Physiol 298: R419–R426, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooks VL, Pricher MP. Pregnancy decreases baroreflex gain: role of GABA in the paraventricular nucleus. Hypertension 52: e30, 2008 [Google Scholar]
- 26.Brooks VL, Quesnell RR, Cumbee SR, Bishop VS. Pregnancy attenuates activity of the baroreceptor reflex. Clin Exp Pharmacol Physiol 22: 152–156, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Brooks VL, Welch LS, Kane CM. Role of angiotensin II in altered baroreflex function of conscious rabbits during late pregnancy. Am J Obstet Gynecol 184: 476–482, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Brunton PJ, Russell JA. Attenuated hypothalamo-pituitary-adrenal axis responses to immune challenge during pregnancy: the neurosteroid opioid connection. J Physiol 586: 369–375, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci 9: 11–25, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Brussaard AB, Kits KS, Baker RE, Willems WPA, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABAA receptor subunit expression. Neuron 19: 1103–1114, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Caja S, Martinez I, Abelenda M, Puerta M. Resistin expression and plasma concentration peak at different times during pregnancy in rats. J Endocrinol 185: 551–559, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Caja S, Puerta M. White adipose tissue production and release of IL-6 and TNF-alpha do not parallel circulating and cerebrospinal fluid concentrations in pregnant rats. Horm Metab Res 40: 375–380, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Cassaglia PA, Brooks VL. The hypothalamic paraventricular nucleus is required for insulin's action to increase baroreflex gain of lumbar sympathetic nerve activity (Abstract). FASEB J 24: 1019.15, 2010 [Google Scholar]
- 35.Chen YL, Chan SH, Chan JY. Participation of galanin in baroreflex inhibition of heart rate by hypothalamic PVN in rat. Am J Physiol Heart Circ Physiol 271: H1823–H1828, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Clow KA, Giraud GD, Ogden BE, Brooks VL. Pregnancy alters hemodynamic responses to hemorrhage in conscious rabbits. Am J Physiol Heart Circ Physiol 284: H1110–H1118, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Cohen WR, Galen LH, Vega-Rich M, Young JB. Cardiac sympathetic activity during rat pregnancy. Metabolism 37: 771–777, 1988 [DOI] [PubMed] [Google Scholar]
- 38.Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RE, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA 95: 13284–13289, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa E. From GABAA receptor diversity emerges a unified vision of GABAergic inhibition. Annu Rev Pharmacol Toxicol 38: 321–350, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Cousins L. Insulin sensitivity in pregnancy. Diabetes 40, Suppl 2: 39–43, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Crandall ME, Heesch CM. Baroreflex control of sympathetic outflow in pregnant rats: effects of captopril. Am J Physiol Regul Integr Comp Physiol 258: R1417–R1423, 1990 [DOI] [PubMed] [Google Scholar]
- 42.Cravo SL, Morrison SF. The caudal ventrolateral medulla is a source of tonic sympathoinhibition. Brain Res 621: 133–136, 1993 [DOI] [PubMed] [Google Scholar]
- 43.Curtis KS, Cunningham JT, Heesch CM. Fos expression in brain stem nuclei of pregnant rats after hydralazine-induced hypotension. Am J Physiol Regul Integr Comp Physiol 277: R532–R540, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Dampney RA, Horiuchi J, McDowall LM. Hypothalamic mechanisms coordinating cardiorespiratory function during exercise and defensive behaviour. Auton Neurosci 142: 3–10, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Dampney RA, Tan PS, Sheriff MJ, Fontes MA, Horiuchi J. Cardiovascular effects of angiotensin II in the rostral ventrolateral medulla: the push-pull hypothesis. Curr Hypertens Rep 9: 222–227, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Daubert DL, Chung MY, Brooks VL. Insulin resistance and impaired baroreflex gain during pregnancy. Am J Physiol Regul Integr Comp Physiol 292: R2188–R2195, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Daubert DL, Liu D, Zucker IH, Brooks VL. Roles of nitric oxide and angiotensin II in the impaired baroreflex gain of pregnancy. Am J Physiol Regul Integr Comp Physiol 292: R2179–R2187, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Davern PJ, Head GA. Fos-related antigen immunoreactivity after acute and chronic angiotensin II-induced hypertension in the rabbit brain. Hypertension 49: 1170–1177, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Davidge ST, McLaughlin MK. Endogenous modulation of the blunted adrenergic response in resistance-sized mesenteric arteries from the pregnant rat. Am J Obstet Gynecol 167: 1691–1698, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Deng Y, Kaufman S. Effect of pregnancy on activation of central pathways following atrial distension. Am J Physiol Regul Integr Comp Physiol 269: R552–R556, 1995 [DOI] [PubMed] [Google Scholar]
- 52.Deng Y, Kaufman S. Pregnancy-induced changes in central response to atrial distension mimicked by progesterone metabolite. Am J Physiol Regul Integr Comp Physiol 275: R1875–R1877, 1998 [DOI] [PubMed] [Google Scholar]
- 53.DiBona GF, Jones SY, Brooks VL. ANG II receptor blockade and arterial baroreflex regulation of renal nerve activity in cardiac failure. Am J Physiol Regul Integr Comp Physiol 269: R1189–R1196, 1995 [DOI] [PubMed] [Google Scholar]
- 54.DiBona GF, Jones SY, Sawin LL. Effect of endogenous angiotensin II on renal nerve activity and its arterial baroreflex regulation. Am J Physiol Regul Integr Comp Physiol 271: R361–R367, 1996 [DOI] [PubMed] [Google Scholar]
- 55.Duan YF, Kopin IJ, Goldstein DS. Stimulation of the paraventricular nucleus modulates firing of neurons in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 277: R403–R411, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Easterling TR, Schmucker BC, Benedetti TJ. The hemodynamic effects of orthostatic stress during pregnancy. Obstet Gynecol 72: 550–552, 1988 [PubMed] [Google Scholar]
- 57.Einstein FH, Fishman S, Muzumdar RH, Yang XM, Atzmon G, Barzilai N. Accretion of visceral fat and hepatic insulin resistance in pregnant rats. Am J Physiol Endocrinol Metab 294: E451–E455, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Fancsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci 20: 3067–3075, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fenelon VS, Herbison AE. Plasticity in GABAA receptor subunit mRNA expression by hypothalamic magnocellular neurons in the adult rat. J Neurosci 16: 4872–4880, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fenelon VS, Herbison AE. Progesterone regulation of GABAA receptor plasticity in adult rat supraoptic nucleus. Eur J Neurosci 12: 1617–1623, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Ferguson AV, Washburn DL, Latchford KJ. Hormonal and neurotransmitter roles for angiotensin in the regulation of central autonomic function. Exp Biol Med (Maywood) 226: 85–96, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Figlewicz DP. Adiposity signals and food reward: expanding the CNS roles of insulin and leptin. Am J Physiol Regul Integr Comp Physiol 284: R882–R892, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Foley CM, Bruno SB, Kvochina L, Heesch CM. Alterations in baroreflex function by hindbrain microinjection of allopregnanolone in female rats (Abstract). FASEB J 19: A618, 2005 [Google Scholar]
- 64.Foley CM, Stanton JJ, Price EM, Cunningham JT, Hasser EM, Heesch CM. GABAA α and α2 receptor subunit expression in rostral ventrolateral medulla in nonpregnant and pregnant rats. Brain Res 975: 196–206, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Freeman KL, Brooks VL. AT1 and glutamatergic receptors in paraventricular nucleus support blood pressure during water deprivation. Am J Physiol Regul Integr Comp Physiol 292: R1675–R1682, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Freemark M. Regulation of maternal metabolism by pituitary and placental hormones: roles in fetal development and metabolic programming. Horm Res 65, Suppl 3: 41–49, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Frye CA, Bayon LE. Seizure activity is increased in endocrine states characterized by decline in endogenous levels of the neurosteroid 3α,5α-THP. Neuroendocrinology 68: 272–280, 1998 [DOI] [PubMed] [Google Scholar]
- 68.Frye CA, Walf AA. Hippocampal 3α,5α-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav 78: 531–540, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Fu Q, Levine BD. Autonomic circulatory control during pregnancy in humans. Semin Reprod Med 27: 330–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ganong WF. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clin Exp Pharmacol Physiol 27: 422–427, 2000 [DOI] [PubMed] [Google Scholar]
- 71.Gant NF, Whalley PJ, Everett RB, Worley RJ, MacDonald PC. Control of vascular reactivity in pregnancy. Am J Kidney Dis 9: 303–307, 1987 [DOI] [PubMed] [Google Scholar]
- 72.Ganten D, Hermann K, Bayer C, Unger T, Lang RE. Angiotensin synthesis in the brain and increased turnover in hypertensive rats. Science 221: 869–871, 1983 [DOI] [PubMed] [Google Scholar]
- 73.Garrett KM, Barron KW, Briscoe R, Heesch CM. Neurosteroid modulation of [3H]flunitrazepam binding in the medulla: an autoradiographic study. Brain Res 768: 301–309, 1997 [DOI] [PubMed] [Google Scholar]
- 74.Gerozissis K. Brain insulin: regulation, mechanisms of action and functions. Cell Mol Neurobiol 23: 1–25, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilbert M, Basile S, Baudelin A, Pere MC. Lowering plasma free fatty acid levels improves insulin action in conscious pregnant rabbits. Am J Physiol Endocrinol Metab 264: E576–E582, 1993 [DOI] [PubMed] [Google Scholar]
- 76.Gordon FJ. Aortic baroreceptor reflexes are mediated by NMDA receptors in caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 252: R628–R633, 1987 [DOI] [PubMed] [Google Scholar]
- 77.Gordon FJ, Leone C. Non-NMDA receptors in the nucleus of the tractus solitarius play the predominant role in mediating aortic baroreceptor reflexes. Brain Res 568: 319–322, 1991 [DOI] [PubMed] [Google Scholar]
- 78.Grassi G, Turri C, Dell'Oro R, Stella ML, Bolla GB, Mancia G. Effect of chronic angiotensin converting enzyme inhibition on sympathetic nerve traffic and baroreflex control of the circulation in essential hypertension. J Hypertens 16: 1789–1796, 1998 [DOI] [PubMed] [Google Scholar]
- 79.Greenwood JP, Scott EM, Stoker JB, Walker JJ, Mary DA. Sympathetic neural mechanisms in normal and hypertensive pregnancy in humans. Circulation 104: 2200–2204, 2001 [DOI] [PubMed] [Google Scholar]
- 80.Guyenet PG. Role of the ventral medulla oblongata in blood pressure regulation. In: Central Regulation of Autonomic Function, edited by Loewy AD, Spyer KM. New York: Oxford University Press, 1990, p. 145–167 [Google Scholar]
- 81.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Guyenet PG, Schreihofer AM, Stornetta RL. Regulation of sympathetic tone and arterial pressure by the rostral ventrolateral medulla after depletion of C1 cells in rats. Ann NY Acad Sci 940: 259–269, 2001 [DOI] [PubMed] [Google Scholar]
- 83.Harney SC, Frenguelli BG, Lambert JJ. Phosphorylation influences neurosteroid modulation of synaptic GABAA receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology 45: 873–883, 2003 [DOI] [PubMed] [Google Scholar]
- 84.Head GA. Cardiac baroreflexes and hypertension. Clin Exp Pharmacol Physiol 21: 791–802, 1994 [DOI] [PubMed] [Google Scholar]
- 85.Head GA, Mayorov DN. Central angiotensin and baroreceptor control of circulation. Ann NY Acad Sci 940: 361–379, 2001 [DOI] [PubMed] [Google Scholar]
- 86.Head GA, McCarty R. Vagal and sympathetic components of the heart rate range and gain of the baroreceptor-heart rate reflex in conscious rats. J Auton Nerv Syst 21: 203–213, 1987 [DOI] [PubMed] [Google Scholar]
- 87.Heesch CM, Burcks S. 5α-Reductase is increased in the rostral ventrolateral medulla (RVLM) of term pregnant rats (Abstract). FASEB J 23: 958, 2009 [Google Scholar]
- 88.Heesch CM, Crandall ME, Turbek JA. Converting enzyme inhibitors cause pressure-independent resetting of baroreflex control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol 270: R728–R737, 1996 [DOI] [PubMed] [Google Scholar]
- 89.Heesch CM, Foley CM. CNS effects of ovarian hormones and metabolites on neural control of circulation. Ann NY Acad Sci 940: 348–360, 2001 [DOI] [PubMed] [Google Scholar]
- 90.Heesch CM, Rogers RC. Effects of pregnancy and progesterone metabolites on regulation of sympathetic outflow. Clin Exp Pharmacol Physiol 22: 136–142, 1995 [DOI] [PubMed] [Google Scholar]
- 91.Heesch CM, Zheng H, Foley CM, Mueller PJ, Hasser EM, Patel KP. Nitric oxide synthase activity and expression is decreased in the paraventricular nucleus of pregnant rats. Brain Res 1251: 140–150, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hines T. Baroreceptor afferent discharge in the pregnant rat. Am J Physiol Regul Integr Comp Physiol 278: R1433–R1440, 2000 [DOI] [PubMed] [Google Scholar]
- 93.Hines T, Beauchamp D, Rice C. Baroreflex control of sympathetic nerve activity in hypertensive pregnant rats with reduced uterine perfusion. Hypertens Pregnancy 26: 303–314, 2007 [DOI] [PubMed] [Google Scholar]
- 94.Hines T, Hodgson TM. Pregnancy alters cardiac receptor afferent discharge in rats. Am J Physiol Regul Integr Comp Physiol 278: R149–R156, 2000 [DOI] [PubMed] [Google Scholar]
- 95.Hines T, Mifflin SW. Gestational effects on volume-sensitive cardiopulmonary receptor reflexes in the rat. Am J Physiol Regul Integr Comp Physiol 268: R736–R743, 1995 [DOI] [PubMed] [Google Scholar]
- 96.Huang C, Yoshimoto M, Miki K, Johns EJ. The contribution of brain angiotensin II to the baroreflex regulation of renal sympathetic nerve activity in conscious normotensive and hypertensive rats. J Physiol 574: 597–604, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Humphreys PW, Joels N. The carotid sinus baroreceptor reflex in the pregnant rabbit. J Physiol 239: 89–102, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Humphreys PW, Joels N. Changes in cardiac output and total peripheral resistance during the carotid sinus baroreceptor reflex in the pregnant rabbit. J Physiol 272: 45–55, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Humphreys PW, Joels N. Arterial pressure maintenance after hemorrhage in the pregnant rabbit. J Physiol 366: 17–25, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaaja RJ, Poyhonen-Alho MK. Insulin resistance and sympathetic overactivity in women. J Hypertens 24: 131–141, 2006 [DOI] [PubMed] [Google Scholar]
- 101.Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes 49: 1525–1533, 2000 [DOI] [PubMed] [Google Scholar]
- 102.Kanbar R, Orea V, Barres C, Julien C. Baroreflex control of renal sympathetic nerve activity during air-jet stress in rats. Am J Physiol Regul Integr Comp Physiol 292: R362–R367, 2007 [DOI] [PubMed] [Google Scholar]
- 103.Kaufman S, Deng Y. Renal response to atrial stretch during pregnancy in conscious rats. Am J Physiol Regul Integr Comp Physiol 265: R902–R906, 1993 [DOI] [PubMed] [Google Scholar]
- 104.Keller-Wood M. Reflex regulation of hormonal responses during pregnancy. Clin Exp Pharmacol Physiol 22: 143–151, 1995 [DOI] [PubMed] [Google Scholar]
- 105.Khanna M, Qin KN, Cheng KC. Distribution of 3 alpha-hydroxysteroid dehydrogenase in rat brain and molecular cloning of multiple cDNAs encoding structurally related proteins in humans. J Steroid Biochem Mol Biol 53: 41–46, 1995 [DOI] [PubMed] [Google Scholar]
- 106.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, Kalhan SC, Catalano PM. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes 51: 2207–2213, 2002 [DOI] [PubMed] [Google Scholar]
- 107.Koksma J, Van Kesteren RE, Rosahl TW, Zwart R, Smit AB, Luddens H, Brussaard AB. Oxytocin regulates neurosteroid modulation of GABAA receptors in supraoptic nucleus around parturition. J Neurosci 23: 788–797, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kumagai H, Averill DB, Ferrario CM. Renal nerve activity in rats with spontaneous hypertension: effect of converting enzyme inhibitor. Am J Physiol Regul Integr Comp Physiol 263: R109–R115, 1992 [DOI] [PubMed] [Google Scholar]
- 109.Kumagai H, Averill DB, Khosla MC, Ferrario CM. Role of nitric oxide and angiotensin II in the regulation of sympathetic nerve activity in spontaneously hypertensive rats. Hypertension 21: 476–484, 1993 [DOI] [PubMed] [Google Scholar]
- 110.Kvochina L, Hasser EM, Heesch CM. Pregnancy increases baroreflex independent GABAergic inhibition of the RVLM in rats. Am J Physiol Regul Integr Comp Physiol 293: R2295–R2305, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kvochina L, Hasser EM, Heesch CM. Pregnancy decreases GABAergic inhibition of the hypothalamic paraventricular nucleus. Physiol Behav 97: 171–179, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol 50: 938–948, 2007 [DOI] [PubMed] [Google Scholar]
- 115.Laiprasert JD, Hamlin R, Heesch CM. Afferent baroreceptor discharge in pregnant rats. Am J Physiol Heart Circ Physiol 281: H2456–H2462, 2001 [DOI] [PubMed] [Google Scholar]
- 116.Laiprasert JD, Rogers RC, Heesch CM. Neurosteroid modulation of arterial baroreflex-sensitive neurons in rat rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 274: R903–R911, 1998 [DOI] [PubMed] [Google Scholar]
- 117.Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why). J Hypertens 19: 523–528, 2001 [DOI] [PubMed] [Google Scholar]
- 118.Li X, Bertics PJ, Karavalas HJ. Regional distribution of cytosolic and particulate 5alpha-dihydroprogesterone 3alpha-hydroxysteroid oxidoreductases in female rat brain. J Steroid Biochem Mol Biol 60: 311–318, 1997 [DOI] [PubMed] [Google Scholar]
- 119.Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurons that innervate the medulla oblongata: role of GABA. Neuroscience 118: 585–601, 2003 [DOI] [PubMed] [Google Scholar]
- 120.Longo LD. Maternal blood volume and cardiac output during pregnancy: a hypothesis of endocrinologic control. Am J Physiol Regul Integr Comp Physiol 245: R720–R729, 1983 [DOI] [PubMed] [Google Scholar]
- 121.Lovick TA, Malpas S, Mahony MT. Renal vasodilatation in response to acute volume load is attenuated following lesions of parvocellular neurones in the paraventricular nucleus in rats. J Auton Nerv Syst 43: 247–255, 1993 [DOI] [PubMed] [Google Scholar]
- 122.Lucini D, Strappazzon P, Vecchia LD, Maggioni C, Pagani M. Cardiac autonomic adjustments to normal human pregnancy: insight from spectral analysis of R-R interval and systolic arterial pressure variability. J Hypertens 17: 1899–1904, 1999 [DOI] [PubMed] [Google Scholar]
- 123.Lumbers ER, Yu ZY. A method for determining baroreflex-mediated sympathetic and parasympathetic control of the heart in pregnant and non-pregnant sheep. J Physiol 515: 555–566, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Masilamani SME, Heesch CM. Effects of pregnancy and progesterone metabolites on baroreflex control of sympathetic outflow and heart rate in conscious rats. Am J Physiol Regul Integr Comp Physiol 272: R924–R934, 1997 [DOI] [PubMed] [Google Scholar]
- 125.Masilamani SME, Heesch CM. Finasteride (Fin) pretreatment reverses the effects of pregnancy on baroreflex control of renal sympathetic nerve activity (Abstract). FASEB J 11: A491997 [Google Scholar]
- 126.Matsumura K, Abe I, Tsuchihashi T, Fujishima M. Central nitric oxide attenuates the baroreceptor reflex in conscious rabbits. Am J Physiol Regul Integr Comp Physiol 274: R1142–R1149, 1998 [DOI] [PubMed] [Google Scholar]
- 127.McGuane JT, Debrah JE, Debrah DO, Rubin JP, Segal M, Shroff SG, Conrad KP. Role of relaxin in maternal systemic and renal vascular adaptations during gestation. Ann NY Acad Sci 1160: 304–312, 2009 [DOI] [PubMed] [Google Scholar]
- 128.McLaughlin MK, Conrad KP. Nitric oxide biosynthesis during pregnancy: Implications for circulatory changes. Clin Exp Pharmacol Physiol 22: 164–171, 1994 [DOI] [PubMed] [Google Scholar]
- 129.Mersich B, Rig OJ, Lenard Z, Studinger P, Visontai Z, Kollai M. Carotid artery stiffening does not explain baroreflex impairment in pre-eclampsia. Clin Sci (Lond) 107: 407–413, 2004 [DOI] [PubMed] [Google Scholar]
- 130.Miki K, Yoshimoto M, Tanimizu M. Acute shifts of baroreflex control of renal sympathetic nerve activity induced by treadmill exercise in rats. J Physiol 548: 313–322, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mitev YA, Darwish M, Wolf SS, Holsboer F, Almeida OFX, Patchev VK. Gender differences in the regulation of 3a-hydroxysteroid dehydrogenase in rat brain and sensitivity to neurosteroid-mediated stress protection. Neuroscience 120: 541–549, 2003 [DOI] [PubMed] [Google Scholar]
- 132.Miyake Y, Ohnishi M, Fujii TK, Yamamoto T, Yoneda C, Takahashi S, Ichimaru Y. The effects of postural changes of baroreflex gain in normal and hypertensive pregnancies. Clin Exp Hypertens 24: 23–31, 2002 [DOI] [PubMed] [Google Scholar]
- 133.Mody I. Aspects of the homeostatic plasticity of GABAA receptor-mediated inhibition. J Physiol 562: 37–46, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moffitt JA, Heesch CM, Hasser EM. Increased GABAA inhibition of the RVLM following hindlimb unloading in rats. Am J Physiol Regul Integr Comp Physiol 283: R604–R614, 2002 [DOI] [PubMed] [Google Scholar]
- 135.Mohamed MK, El Mas MM, Abdel-Rahman AA. Estrogen enhancement of baroreflex sensitivity is centrally mediated. Am J Physiol Regul Integr Comp Physiol 276: R1030–R1037, 1999 [DOI] [PubMed] [Google Scholar]
- 136.Mohler H, Fritschy JM, Luscher B, Rudolph U, Benson J, Benke D. The GABAA receptors from subunits to diverse functions. In: Ion Channels, edited by Narahashi T. New York: Plenum, 1996, p. 89–113 [PubMed] [Google Scholar]
- 137.Molino P, Veglio F, Genova GC, Melchio R, Benedetto C, Chiarolini L, Rabbia F, Grosso T, Mulatero P, Chiandussi L. Baroreflex control of heart rate is impaired in pre-eclampsia. J Hum Hypertens 13: 179–183, 1999 [DOI] [PubMed] [Google Scholar]
- 138.Morton MJ. Maternal hemodynamics in pregnancy. In: Exercise and Pregnancy, edited by Artal R, Wiswell RA. Baltimore, MD: Williams & Wilkins, 1990, p. 61–70 [Google Scholar]
- 139.Moss SJ, Smart TG. Constructing Inhibitory Synapses. Neuroscience 2: 240–250, 2001 [DOI] [PubMed] [Google Scholar]
- 140.Mostallino MC, Mura ML, Marico E, Murrow L, Sana E, Biggio G. Changes in expression of the δ subunit of the GABAA receptor and in receptor function induced by progesterone exposure and withdrawal. J Neurochem 99: 321–332, 2006 [DOI] [PubMed] [Google Scholar]
- 141.Munoz C, Lopez-Luna P, Herrera E. Glucose and insulin tolerance tests in the rat on different days of gestation. Biol Neonate 68: 282–291, 1995 [DOI] [PubMed] [Google Scholar]
- 142.Mantel MS, Anderson EA, Johnson AK, Mark AL. Mechanisms of insulin action on sympathetic nerve activity. Clin Exp Hypertens 17: 39–50, 1995 [DOI] [PubMed] [Google Scholar]
- 143.Murakami H, Liu JL, Onekama H, Nishida Y, Okada K, Osaka H, Morita H, Zucker IH. Blockade of neuronal nitric oxide synthase alters the baroreflex control of heart rate in the rabbit. Am J Physiol Regul Integr Comp Physiol 274: R181–R186, 1998 [DOI] [PubMed] [Google Scholar]
- 144.Murakami H, Liu JL, Zucker IH. Blockade of AT1 receptors enhances baroreflex control of heart rate in conscious rabbits with heart failure. Am J Physiol Regul Integr Comp Physiol 271: R303–R309, 1996 [DOI] [PubMed] [Google Scholar]
- 145.Murakami H, Liu JL, Zucker IH. Angiotensin II blockade [corrected] enhances baroreflex control of sympathetic outflow in heart failure. Hypertension 29: 564–569, 1997 [DOI] [PubMed] [Google Scholar]
- 146.Murakami H, Liu JL, Zucker IH. Angiotensin II enhances baroreflex control of sympathetic outflow in heart failure. Hypertension 29: 564–569, 1997 [DOI] [PubMed] [Google Scholar]
- 147.Unwashed B. Hemodynamic changes during pregnancy in the rabbit. Am J Obstet Gynecol 135: 590–596, 1999 [DOI] [PubMed] [Google Scholar]
- 148.O'Hagan KP, Casey SM. Arterial baroreflex during pregnancy and renal sympathetic activity during parturition in rabbits. Am J Physiol Heart Circ Physiol 274: H1635–H1642, 1998 [DOI] [PubMed] [Google Scholar]
- 149.O'Hagan KP, Slog KA, Stevenson JB. AT1 receptor block does not affect arterial baroreflex during pregnancy in rabbits. Am J Physiol Heart Circ Physiol 280: H1996–H2005, 2001 [DOI] [PubMed] [Google Scholar]
- 150.Okada M, Buna RD. Insulin acts centrally to enhance reflex tachycardia in conscious rats. Am J Physiol Regul Integr Comp Physiol 266: R481–R486, 1994 [DOI] [PubMed] [Google Scholar]
- 151.Olsen RW. Drug interactions at the GABA receptor-ionophore complex. Annu Rev Physiol 22: 245–277, 1982 [DOI] [PubMed] [Google Scholar]
- 152.Olsson K, Andean NE, Johansson K, Thornton U. Effects of acute haemorrhagic hypotension during pregnancy and lactation in conscious goats. Acta Physiol Scand 129: 479–487, 1987 [DOI] [PubMed] [Google Scholar]
- 153.Orchinik M, Weiland NG, McEwen BS. Chronic exposure to stress levels of corticosterone alters GABAA receptor subunit mRNA levels in rat hippocampus. Mol Brain Res 34: 29–37, 1995 [DOI] [PubMed] [Google Scholar]
- 154.Paller MS. Decreased pressor responsiveness in pregnancy: studies in experimental animals. Am J Kidney Dis 9: 308–311, 1987 [DOI] [PubMed] [Google Scholar]
- 155.Pamidimukkala J, Hay M. 17β-Estradiol inhibits angiotensin II activation of area postrema neurons. Am J Physiol Heart Circ Physiol 285: H1515–H1520, 2003 [DOI] [PubMed] [Google Scholar]
- 156.Panchal S, Arria AM, Labhsetwar SA. Maternal mortality during hospital admission for delivery: a retrospective analysis using a state-maintained database. Anesth Analg 93: 134–141, 2001 [DOI] [PubMed] [Google Scholar]
- 157.Patel KP, Li YF, Hirooka Y. Role of nitric oxide in central sympathetic outflow. Exp Biol Med (Maywood) 226: 814–824, 2001 [DOI] [PubMed] [Google Scholar]
- 158.Patel KP, Schmid PG. Role of paraventricular nucleus (PVH) in baroreflex-mediated changes in lumbar sympathetic nerve activity and heart rate. J Auton Nerv Syst 22: 211–219, 1988 [DOI] [PubMed] [Google Scholar]
- 159.Paton JF, Wang S, Polson JW, Kasparov S. Signalling across the blood brain barrier by angiotensin II: novel implications for neurogenic hypertension. J Mol Med 86: 705–710, 2008 [DOI] [PubMed] [Google Scholar]
- 160.Paul SM, Purdy RH. Neuroactive steroids. FASEB J 6: 2311–2322, 1992 [PubMed] [Google Scholar]
- 161.Pricher MP, Freeman KL, Brooks VL. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension 51: 514–520, 2008 [DOI] [PubMed] [Google Scholar]
- 162.Quesnell RR, Brooks VL. Alterations in the baroreflex occur late in pregnancy in conscious rabbits. Am J Obstet Gynecol 176: 692–694, 1997 [DOI] [PubMed] [Google Scholar]
- 163.Rang S, Wolf H, Montfrans GA, Karemaker JM. Non-invasive assessment of autonomic cardiovascular control in normal human pregnancy and pregnancy- associated hypertensive disorders: a review. J Hypertens 20: 2111–2119, 2002 [DOI] [PubMed] [Google Scholar]
- 164.Rang S, Wolf H, Van Montfrans GA, Karemaker JM. Serial assessment of cardiovascular control shows early signs of developing pre-eclampsia. J Hypertens 22: 369–376, 2004 [DOI] [PubMed] [Google Scholar]
- 165.Raven PB, Fadel PJ, Ogoh S. Arterial baroreflex resetting during exercise: a current perspective. Exp Physiol 91: 37–49, 2006 [DOI] [PubMed] [Google Scholar]
- 166.Rochat RW, Koonin LM, Atrash HK, Jewett JF. Maternal mortality in the United States: report from the Maternal Mortality Collaborative. Obstet Gynecol 72: 91–97, 1988 [PubMed] [Google Scholar]
- 167.Rossi G, Sherwin RS, Penzias AS, Lapaczewski P, Jacob RJ, Shulman GI, Diamond MP. Temporal changes in insulin resistance and secretion in 24-h-fasted conscious pregnant rats. Am J Physiol Endocrinol Metab 265: E845–E851, 1993 [DOI] [PubMed] [Google Scholar]
- 168.Russell JA, Brunton PJ. Neuroactive steroids attenuate oxytocin stress responses in late pregnancy. Neuroscience 138: 879–889, 2006 [DOI] [PubMed] [Google Scholar]
- 169.Saleh MC, Connell BJ, Saleh TM. Autonomic and cardiovascular reflex responses to central estrogen injection in ovariectomized female rats. Brain Res 879: 105–114, 2000 [DOI] [PubMed] [Google Scholar]
- 170.Schreihofer AM, Guyenet PG. The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29: 514–521, 2002 [DOI] [PubMed] [Google Scholar]
- 171.Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev 24: 855–872, 2000 [DOI] [PubMed] [Google Scholar]
- 172.Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases δ GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology 49: 573–586, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shimada M, Yoshinari K, Tanabe E, Shimakawa E, Kobashi M, Nagata K, Yamazoe Y. Identification of ST2A1 as a rat brain neurosteroid sulfotransferase mRNA. Brain Res 920: 222–225, 2001 [DOI] [PubMed] [Google Scholar]
- 174.Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev 47: 181–234, 1995 [PubMed] [Google Scholar]
- 175.Silver HM, Tahvanainen KU, Kuusela TA, Eckberg DL. Comparison of vagal baroreflex function in nonpregnant women and in women with normal pregnancy, preeclampsia, or gestational hypertension. Am J Obstet Gynecol 184: 1189–1195, 2001 [DOI] [PubMed] [Google Scholar]
- 176.Skinner SL, Lumbers ER, Symonds EM. Analysis of changes in the renin-angiotensin system during pregnancy. Clin Sci (Lond) 42: 479–488, 1972 [DOI] [PubMed] [Google Scholar]
- 177.Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol Regul Integr Comp Physiol 272: R441–R463, 1997 [DOI] [PubMed] [Google Scholar]
- 178.Slangen BF, Out IC, Verkeste CM, Peeters LL. Hemodynamic changes in early pregnancy in chronically instrumented, conscious rats. Am J Physiol Heart Circ Physiol 270: H1779–H1784, 1996 [DOI] [PubMed] [Google Scholar]
- 179.Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol 586: 377–385, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Solomon CG, Seely EW. Brief review: hypertension in pregnancy : a manifestation of the insulin resistance syndrome? Hypertension 37: 232–239, 2001 [DOI] [PubMed] [Google Scholar]
- 181.Spencer SJ, Mouihate A, Galic MA, Pittman QJ. Central and peripheral neuroimmune responses: hyporesponsiveness during pregnancy. J Physiol 586: 399–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol 494: 673–685, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Storey E, Kaufman S. Effect of pregnancy and 5α-pregnan-3α-ol-20-one on atrial receptor afferent discharge in rats. Am J Physiol Regul Integr Comp Physiol 287: R1427–R1433, 2004 [DOI] [PubMed] [Google Scholar]
- 184.Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. J Comp Neurol 444: 207–220, 2002 [DOI] [PubMed] [Google Scholar]
- 185.Struck J, Muck P, Trubger D, Handrock R, Weidinger G, Dendorfer A, Dodt C. Effects of selective angiotensin II receptor blockade on sympathetic nerve activity in primary hypertensive subjects. J Hypertens 20: 1143–1149, 2002 [DOI] [PubMed] [Google Scholar]
- 186.Tabsh K, Rudelstorfer R, Nuwayhid B, Assali NS. Circulatory responses to hypovolemia in the pregnant and nonpregnant sheep after pharmacological sympathectomy. Am J Obstet Gynecol 154: 411–419, 1986 [DOI] [PubMed] [Google Scholar]
- 187.Tam SL, Kaufman S. NOS inhibition restores renal responses to atrial distension during pregnancy. Am J Physiol Regul Integr Comp Physiol 282: R1364–R1367, 2002 [DOI] [PubMed] [Google Scholar]
- 188.Tam SL, Sims E, Kaufman S. Effect of NOS inhibition on central response to atrial distension during pregnancy. Am J Physiol Regul Integr Comp Physiol 283: R827–R831, 2002 [DOI] [PubMed] [Google Scholar]
- 189.Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Semin Perinatol 24: 11–14, 2000 [DOI] [PubMed] [Google Scholar]
- 190.Tsutsui K, Ukena K, Usui M, Sakamoto H, Takase M. Novel brain function: biosynthesis and actions of neurosteroids in neurons. Neurosci Res 36: 261–273, 2000 [DOI] [PubMed] [Google Scholar]
- 191.van den Berg MP, Verhoef JC, Romeijn SG, Merkus FW. Uptake of estradiol or progesterone into the CSF following intranasal and intravenous delivery in rats. Eur J Pharm Biopharm 58: 131–135, 2004 [DOI] [PubMed] [Google Scholar]
- 192.Van Houten M, Posner BI. Circumventricular organs: receptors and mediators of direct peptide hormone action on brain. Adv Metab Disord 10: 269–289, 1983 [DOI] [PubMed] [Google Scholar]
- 193.Voss A, Malberg H, Schumann A, Wessel N, Walther T, Stepan H, Faber R. Baroreflex sensitivity, heart rate, and blood pressure variability in normal pregnancy. Am J Hypertens 13: 1218–1225, 2000 [DOI] [PubMed] [Google Scholar]
- 194.Walther T, Wessel N, Malberg H, Voss A, Stepan H, Faber R. A combined technique for predicting pre-eclampsia: concurrent measurement of uterine perfusion and analysis of heart rate and blood pressure variability. J Hypertens 24: 747–750, 2006 [DOI] [PubMed] [Google Scholar]
- 195.Wasserstrum N, Kirshon B, Rossavik IK, Willis RS, Moise KJ, Cotton DB. Implications of sino-aortic baroreceptor reflex dysfunction in severe preeclampsia. Obstet Gynecol 74: 34–39, 1989 [PubMed] [Google Scholar]
- 196.Weiner CP, Thompson LP. Nitric oxide and pregnancy. Semin Perinatol 21: 367–380, 1997 [DOI] [PubMed] [Google Scholar]
- 197.Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FA. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology 121: 1562–1570, 1987 [DOI] [PubMed] [Google Scholar]
- 198.Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Curr Pharm Des 9: 795–800, 2003 [DOI] [PubMed] [Google Scholar]
- 199.Xu L, Brooks VL. Sodium intake, angiotensin II receptor blockade and baroreflex function in conscious rats. Hypertension 29: 450–457, 1997 [DOI] [PubMed] [Google Scholar]
- 200.Yang Z, Coote JH. Role of GABA and NO in the paraventricular nucleus-mediated reflex inhibition of renal sympathetic nerve activity following stimulation of right atrial receptors in the rat. Exp Physiol 88: 335–342, 2003 [DOI] [PubMed] [Google Scholar]