Abstract
Muscle wasting during sepsis is in part regulated by glucocorticoids. In recent studies, treatment of cultured muscle cells in vitro with dexamethasone upregulated expression and activity of p300, a histone acetyl transferase (HAT), and reduced expression and activity of the histone deacetylases-3 (HDAC3) and -6, changes that favor hyperacetylation. Here, we tested the hypothesis that sepsis and glucocorticoids regulate p300 and HDAC3 and -6 in skeletal muscle in vivo. Because sepsis-induced metabolic changes are particularly pronounced in white, fast-twitch skeletal muscle, most experiments were performed in extensor digitorum longus muscles. Sepsis in rats upregulated p300 mRNA and protein levels, stimulated HAT activity, and reduced HDAC6 expression and HDAC activity. The sepsis-induced changes in p300 and HDAC expression were prevented by the glucocorticoid receptor antagonist RU38486. Treatment of rats with dexamethasone increased expression of p300 and HAT activity, reduced expression of HDAC3 and -6, and inhibited HDAC activity. Finally, treatment with the HDAC inhibitor trichostatin A resulted in increased muscle proteolysis and expression of the ubiquitin ligase atrogin-1. Taken together, our results suggest for the first time that sepsis-induced muscle wasting may be regulated by glucocorticoid-dependent hyperacetylation caused by increased p300 and reduced HDAC expression and activity. The recent development of pharmacological HDAC activators may provide a novel avenue to prevent and treat muscle wasting in sepsis and other catabolic conditions.
Keywords: acetylation, muscle wasting
muscle wasting during sepsis is, at least in part, regulated by glucocorticoids (15, 50) and is mainly caused by increased degradation of myofibrillar proteins, although inhibited protein synthesis may contribute as well (16, 25). Gene transcription is altered in atrophying muscle, and in recent studies in experimental animals, a common set of genes, so called atrogenes, was upregulated in different catabolic conditions (27). Among the atrogenes, the genes for the ubiquitin ligases muscle atrophy F-box, also known as atrogin-1 (MAFbx/atrogin-1) and muscle ring finger 1 (MuRF1) are particularly important (3, 14). Because changes in gene transcription play an important role in loss of muscle mass it is likely that transcription factors are involved in muscle wasting. Indeed, recent reports from our and other laboratories suggest that the transcription factors NF-κB (4, 44, 59), CCAAT/enhancer-binding protein (C/EBP)β and -δ (43, 62), AP-1 (41, 44), and forkhead box (FOXO)1 and -3a (11, 12, 21, 22, 52) regulate muscle-wasting-related genes in sepsis and other catabolic conditions.
In addition to transcription factors, gene activation is also regulated by other factors, including nuclear cofactors that can act as activators or repressors (28). For example, in recent in vitro experiments we found evidence that the nuclear cofactor p300 may be involved in muscle wasting. Thus, treatment of cultured myotubes with dexamethasone, a commonly used in vitro model of muscle wasting (37), resulted in increased expression of p300 and p300-dependent muscle proteolysis (63, 64). The role of p300 in glucocorticoid-induced atrophy of cultured myotubes was confirmed in a recent report by Tobimatsu et al. (58). By its intrinsic histone acetyl transferase (HAT) activity, p300 influences gene transcription, but it can also regulate gene transcription by interacting with other nuclear proteins, including transcription factors and nuclear cofactors (40, 45). Importantly, treatment of myotubes with dexamethasone also decreased the expression of the histone deacetylases (HDAC)3 and -6 and reduced HDAC activity (64), further supporting the concept that muscle wasting may be associated with hyperacetylation.
Although our previous experiments in cultured myotubes provided in vitro evidence that increased p300/HAT and reduced HDAC3 and -6 expression and activity may be involved in muscle wasting, it is not known whether conditions characterized by loss of muscle mass, such as sepsis, influence p300 and HDACs in a similar manner in vivo. Here, we tested the hypotheses that sepsis in rats upregulates the expression and activity of p300/HAT and reduces the expression and activity of HDAC3 and -6. The potential role of glucocorticoids in the regulation of p300 and HDAC3 and -6 was examined by treating normal rats with dexamethasone or septic rats with the glucocorticoid receptor antagonist RU38486 (26). Our results suggest that sepsis upregulates p300 expression and HAT activity and downregulates HDAC6 expression and HDAC activity and that these changes are, at least in part, glucocorticoid dependent.
MATERIALS AND METHODS
Animals were treated and cared for in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. The experimental protocols were approved by the Institutional Animal Care and Use Committee at the Beth Israel Deaconess Medical Center (Boston, MA).
Animal experiments.
Four series of experiments were performed. In the first series of experiments, we tested the effect of sepsis on the expression and activity of p300 and HDAC3 and -6 in skeletal muscle. Sepsis was induced in male Sprague-Dawley rats (50–60 g body wt) by cecal ligation and puncture (CLP) as described in detail previously (16, 43, 44). In short, with rats under general anesthesia induced by pentobarbital (50 mg/kg administered ip) the abdomen was opened through a midline incision and the cecum was ligated below the ileocecal junction with a 3-0 silk ligature and punctured twice with an 18-gauge needle. Control rats underwent sham operation consisting of laparotomy and manipulation, but no ligation or puncture, of the cecum. Rats were resuscitated with 10 ml/100 g body wt of saline administered subcutaneously on the back at the time of sham operation or CLP to prevent hypovolemia and septic shock. Animals had free access to water, but food was withheld after the surgical procedures to avoid the influence of differences in food intake on metabolic changes between sham-operated and septic rats. In previous experiments, we used small-growing rats (50–60 g) because their lower extremity muscles are thin enough to allow for measurement of protein breakdown rates during in vitro incubation with maintained viability (16, 54). Rats of the same size were used in the present study to make possible comparisons with previous observations. The septic model used here is associated with a reproducible and substantial increase in muscle protein breakdown (16, 54, 55). The model resembles the situation in many surgical patients presenting with septic peritonitis caused by perforated bowel, such as perforated diverticulitis or ruptured appendicitis, and intra-abdominal abscesses. We reported previously that some of the metabolic and molecular changes seen in the CLP model in rats are present in muscle from patients with sepsis, suggesting that the model at least in part reflects the clinical situation in sepsis (56).
At different time points (4, 8, and 16 h) after sham operation or CLP, extensor digitorum longus (EDL) or soleus muscles were harvested, immediately frozen in liquid nitrogen, and stored at −80°C until used for determination of p300, HDAC3, HDAC6, MAFbx/atrogin-1, and MuRF1 expression. Other muscles were used for measurement of p300/HAT and HDAC activity. EDL muscles were studied in the majority of experiments because, in previous reports, we found that white, fast-twitch skeletal muscles are particularly sensitive to the effects of sepsis (16, 55, 57). In additional experiments, soleus muscles were examined to test whether sepsis-induced changes in the expression of p300 and HDACs are differentially regulated in fast- and slow-twitch muscle, similar to other catabolic responses (16, 55, 57).
In a second series of experiments, rats were treated with dexamethasone (10 mg/kg) or corresponding volume of vehicle administered intraperitoneally as described previously (54, 62). Rats had free access to water, but food was withheld after the injections. Muscles were harvested 8 and 16 h after administration of dexamethasone or vehicle for determination of p300, HDAC3, HDAC6, MAFbx/atrogin-1, and MuRF1 mRNA levels and p300/HAT and HDAC activity.
In a third series of experiments, sham-operated and septic rats were treated with 10 mg/kg of the glucocorticoid receptor antagonist RU38486 (26) or vehicle (0.1 ml of 25% ethanol) administered intraperitoneally 2 h before sham operation or CLP. EDL muscles were removed 8 or 16 h after sham operation or CLP for determination of p300 and HDAC3 and -6 mRNA levels. We found in previous studies that treatment of rats with RU38486 prevented sepsis-induced muscle proteolysis and activation of the ubiquitin-proteasome pathway (54, 61) but the effect of RU38486 on sepsis-induced changes in p300 and HDAC3 and -6 expression is not known. Because glucocorticoid receptors are expressed in skeletal muscle (67) and because RU38486 exerts a direct effect on cultured muscle cells (37), it is likely that the effects of RU38486 in the present experiments, at least in part, reflect a direct effect on muscle. It cannot be ruled out, however, that other mechanisms may also be involved, such as a systemic change in inflammation.
In a final series of experiments, the role of acetylation on muscle protein breakdown was tested by treating rats with trichostatin A (TSA; 10 mg/kg) or vehicle administered intraperitoneally. TSA increases acetylation by inhibiting HDAC activity (65). The dose of TSA used here increased histone acetylation in previous studies in mice (2). EDL muscles were harvested 2 and 4 h after administration of TSA for determination of HDAC activity, protein breakdown rates, and mRNA levels for atrogin-1 and MuRF1.
Muscle incubations.
Protein breakdown rates were measured in incubated EDL muscles 2 and 4 h after rats were treated with TSA (10 mg/kg) or vehicle. Muscles were gently dissected with intact tendons, mounted on stainless steel supports at resting length, and incubated for 2 h under physiological conditions in a shaking water bath at 37°C as described in detail previously (16). Protein breakdown rates were determined by measuring net release of free tyrosine into the incubation medium. Because tyrosine is not synthesized or degraded in muscle tissue and because reincorporation of tyrosine into protein was prevented by the presence of cycloheximide (0.5 mM) in the medium, net release of tyrosine provided a reliable measure of protein breakdown rates. Tyrosine was measured as described by Waalkes and Udenfriend (60).
Real-time PCR.
RNA was extracted from muscles by the acid guanidinium thiocyanate-phenol-chloroform method (8) using TRI Reagent (Molecular Research Center, Cincinnati, OH). Messenger RNA levels for rat p300, HDAC3, HDAC6, MAFbx/atrogin-1, and MuRF1 were determined by real-time PCR using TaqMan analysis. Multiplex real-time PCR was performed using the One-Step PCR Master Mix Reagents Kit for quantitation of mRNA expression with simultaneous amplification of 18S RNA as endogenous control to normalize the mRNA concentrations. TaqMan analysis and subsequent calculations were performed with an ABI Prism 7700 Sequence Detection System (Perkin-Elmer, Waltham, MA). For each sample, 100 ng of total RNA was subjected to real-time PCR (in duplicate) according to the protocol provided by the manufacturer. The sequences of the forward, reverse, and double-labeled oligonucleotides for rat atrogin-1 and MuRF1 used here were reported recently (10). The corresponding sequences for rat p300 were forward, 5′-GCC AAA CAT GCA GTA CCC AA -3′; reverse, 5′-CCC TGC TGT AGT GGC TCA GTC-3′; and double-labeled TaqMan oligonucleotide probe, 5′-AGG CAT GGG CAA TGC TGG CAG TT-3′. The corresponding sequences for rat HDAC3 were forward, 5′-TGT GTT TCC CGG GCT CTT C-3′; reverse, 5′-GTG TTG CCC CTT GCA GAG A-3′; and double-labeled TaqMan oligonucleotide probe, 5′-AGT TCT GCT CCC GCT ATA CAG GCG C-3′. The corresponding sequences for rat HDAC6 were forward, 5′-TTG CAT GTT CAA CCA CCT GG-3′; reverse, 5′-CCT CTG AAT GCG GTG CTT CT-3′; and double-labeled TaqMan oligonucleotide probe, 5′-TGT GGC TGC CCG CTA TGC ACA-3′. Amplification of 18S RNA was performed in the same reaction tubes as an internal standard with an alternatively labeled probe (VIC-labeled probe) to distinguish its product from those derived from MAFbx/atrogin-1, MuRF1, p300, HDAC3, and HDAC6 RNA. The mRNA concentrations were normalized to the 18S mRNA levels and were expressed as arbitrary units.
Western blot analysis.
Western blot analysis was performed to determine MAFbx/atrogin-1 and MuRF1 protein levels in total muscle extracts and HDAC3, HDAC6, and SIRT1 (a class III HDAC) protein levels in nuclear extracts. Total and nuclear muscle extracts were prepared as described in detail recently (52). Aliquots (50 μg protein) of total or nuclear extracts were subjected to SDS-PAGE using 10% gels, followed by transfer to PVDF membranes. The membranes were blocked with 5% nonfat milk in TTBS buffer (50 mM Tris·HCl, 150 mM NaCl, and 1% Tween-20, pH 7.4) and incubated with the following primary antibodies and the appropriate secondary antibodies: a rabbit polyclonal anti-rat HDAC3 antibody (1:1,000, Cell Signaling Technology, Danver, MA); a rabbit polyclonal anti-rat HDAC6 antibody (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA); a rabbit polyclonal anti-rat SIRT1 antibody (1:500, Abcam, Cambridge, MA); a rabbit polyclonal anti-mouse MAFbx/atrogin-1 antibody (1:1,000; kindly supplied by Dr. Stewart Lecker, Harvard Medical School); and a rabbit polyclonal anti-mouse MuRF1 antibody (kindly supplied by Regeneron Pharmaceuticals to Dr. Alfred Goldberg, Harvard Medical School, and used in the present experiments after permission from Regeneron Pharmaceuticals). An anti-rat lamin A/C antibody (1:1,000, Cell Signaling Technology) or an anti-rat OCT 1 antibody (1:1,000, Santa Cruz Biotechnology) was used for loading control when protein levels were determined in the nuclear fraction. A mouse monoclonal anti-rat α-tubulin antibody (1:2,000, Sigma-Aldrich, St. Louis, MO) was used for loading control when whole muscle extracts were studied. Immunoreactive protein bands were detected by using the Western Lightning kit for enhanced chemiluminescence detection (Perkin-Elmer) and analyzed using the public domain Image J program (http://rsb.info.nih.gov/ij/index.htm). The bands were quantified by densitometry and normalized to the appropriate loading controls.
Because p300 levels in skeletal muscle are low, p300 protein levels were determined by coimmunoprecipitation using a rabbit polyclonal anti-p300 antibody (N-15; Santa Cruz Biotechnology) for immunoprecipitation (pull down) and a mouse anti-human p300 monoclonal antibody (NM-11; Pharmingen, San Diego, CA) for immunoblotting as described in detail previously (63).
Measurement of p300/HAT activity.
The activity of p300/HAT was measured by using a commercially available p300 immunoprecipitation HAT activity assay kit and following the detailed instructions provided by the manufacturer (Upstate Cell Signaling Solutions, Lake Placid, NY). In short, nuclear p300 was immunoprecipitated, and its activity was determined by measuring the acetylation of histone H4 peptide in the presence of [3H]acetyl CoA. The amount of radioactivity incorporated into H4 peptide was determined in a TRI-CARB 1600 TR liquid scintillation counter (Packard, Meriden, CT). HAT activity was determined in nuclear extracts from EDL muscles of septic and dexamethasone-treated rats. Sham-operated or vehicle-treated rats served as controls, and results were expressed as a percentage of control.
Measurement of HDAC activity.
HDAC activity was measured by using a commercially available fluorometric HDAC activity assay kit and following the manufacturer's detailed instructions (BioVision, Mountain View, CA). An HDAC fluorometric substrate containing an acetylated lysine side chain [Boc-Lys(Ac)-AMC] was incubated with a sample (80 μg protein) of nuclear extract. Deacetylation of the substrate sensitizes it to treatment with a lysine developer producing a fluorophore. The fluorescence was measured by using a Victor 3 Fluorescence Plate Reader (Perkin-Elmer) and the results were expressed as relative fluorescence units per microgram of protein. Water, instead of nuclear extract, was added as a blank. HeLa cell nuclear extracts supplied in the assay kit were used for positive controls, and TSA was added to the reaction (2 μl of 1 mM TSA solution added to a final volume of 100 μl reaction mixture) for negative control. The positive and negative controls were included each time the assay was performed, thus validating the assay. HDAC activity was determined in nuclear extracts from EDL muscles of septic or dexamethasone-treated rats. Sham-operated or vehicle-treated rats served as controls, and results were expressed as a percentage of control.
Statistics.
Results are reported as means ± SE. Statistical analysis was performed by using Student's t-test or one-way ANOVA followed by Tukey's post hoc test as appropriate. P < 0.05 was considered statistically significant.
RESULTS
In initial experiments, we determined p300 mRNA levels in EDL muscles at different time points after sham operation or induction of sepsis by CLP in rats. Messenger RNA levels for p300 were increased by ∼50% 16 h after CLP but were not altered at earlier time points (Fig. 1A). The increase in p300 mRNA levels noticed here in septic muscle was quantitatively similar to the response observed in dexamethasone-treated myotubes but occurred later than in myotubes, p300 mRNA levels being increased in myotubes after 2–4 h of dexamethasone treatment (63).
Fig. 1.
Sepsis in rats increases p300 expression and histone acetyl transferase (HAT) activity in skeletal muscle. A: p300 mRNA levels were determined by real-time PCR in extensor digitorum longus (EDL) muscles from sham-operated rats and rats made septic by cecal ligation and puncture (CLP). AU, arbitrary units. Results are means ± SE with n = 8 per group. *P < 0.05 vs. sham by ANOVA. B: p300 protein levels were determined by coimmunoprecipitation in EDL muscles 16 h after sham operation or CLP. Top: representative immunoblots and graph shows quantification by densitometry of 6 blots in each group. Results are means ± SE with n = 6 in each group. *P < 0.05 vs. sham by Student's t-test. C: HAT activity was measured in EDL muscles from sham-operated rats and rats made septic by CLP. Results are means ± SE with n = 8 per group. *P < 0.05 vs. sham by ANOVA.
To examine whether the increased p300 mRNA levels observed in muscle from septic rats were accompanied by increased p300 protein expression, p300 protein levels were determined by coimmunoprecipitation. Similar to the findings in dexamethasone-treated myotubes (63), the increase in p300 mRNA levels in muscle from septic rats was accompanied by increased p300 protein levels (Fig. 1B). In addition, and importantly, muscle HAT activity was stimulated 16 h after CLP (Fig. 1C).
Increased p300/HAT expression and activity may favor a state of hyperacetylation in skeletal muscle during sepsis. An additional finding that can result in hyperacetylation may be reduced expression and activity of histone deacetylases as suggested by our previous experiments in dexamethasone-treated myotubes (64). To test whether inhibited deacetylation may be involved in sepsis-induced muscle wasting, we next determined mRNA levels for HDAC3 and -6 in EDL muscles at different time points after induction of sepsis by CLP. HDAC3 and -6 represent class I and II HDACs, respectively (36), and were studied here because they were expressed in cultured muscle cells in our previous experiments and were downregulated by dexamethasone treatment (64). Although there was a trend toward reduced mRNA levels for HDAC3 and -6 at 8 h after CLP, the differences between sham-operated and septic rats did not reach statistical significance (Fig. 2, A and B).
Fig. 2.
Histone deacetylases (HDAC)3 and -6 mRNA levels in skeletal muscle during sepsis in rats. HDAC3 (A) and HDAC6 (B) mRNA levels were determined by real-time PCR in EDL muscles at different time points after sham operation or induction of sepsis by CLP in rats. Results are means ± SE with n = 8 in each group. Statistical analysis was performed using ANOVA.
Whereas HDAC3 protein levels were not influenced by sepsis, HDAC6 protein levels were significantly reduced in EDL muscles from septic rats 8 h after CLP (Fig. 3, A and B). SIRT1 is a class III HDAC (36) that has been implicated in the regulation of myocyte differentiation and muscle mass (5), possibly secondary to interaction with and deacetylation of PGC-1α (13, 42). To test whether this deacetylase as well may be involved in sepsis-induced muscle wasting, we examined the expression of SIRT1 in muscle from sham-operated and septic rats. Similar to HDAC6, SIRT1 protein levels were reduced 8 h after CLP (Fig. 3C).
Fig. 3.
Sepsis in rats reduces protein levels of HDAC6 and SIRT1 (a class III HDAC) in skeletal muscle. Protein levels of HDAC3 (A), HDAC6 (B), and SIRT1 (C) were determined by Western blot analysis of nuclear proteins. OCT1 or lamin A/C levels were determined for loading control. Representative blots are shown. Bars represent densitometric quantifications of 7 or 8 blots. Results are means ± SE. *P < 0.05 vs. sham determined by Student's t-test.
Importantly, HDAC activity was significantly reduced (by ∼20%) in EDL muscles 8 h after induction of sepsis (Fig. 4). Taken together, the results shown in Figs. 2–4 suggest that sepsis results in reduced expression of some, but not all HDACs and that these changes are associated with reduced HDAC activity.
Fig. 4.
Sepsis in rats reduces HDAC activity in skeletal muscle. HDAC activity was measured as described in materials and methods in EDL muscles at different time points after sham operation or CLP. Results are means ± SE with n = 8 in each group. *P < 0.05 vs. sham by ANOVA.
MAFbx/atrogin-1 and MuRF1 mRNA levels are commonly used as molecular markers of muscle wasting (3, 14, 37, 61). Although we reported previously that sepsis results in increased expression of these ubiquitin ligases (10, 61), we wanted to confirm that the muscles studied in the present experiments displayed similar evidence of muscle wasting. Sepsis resulted in a robust increase in mRNA levels in EDL muscles for both MAFbx/atrogin-1 and MuRF1 with changes observed 8 and 16 h after CLP (Fig. 5, A and B). Although increased MAFbx/atrogin-1 and MuRF1 mRNA levels have been reported previously in various muscle-wasting conditions, including sepsis (10, 61), it is not known whether the changes in MAFbx/atrogin-1 and MuRF1 mRNA levels during sepsis are accompanied by corresponding changes in protein levels. Here we determined protein levels for the ubiquitin ligases 16 h after sham operation or CLP and found that MAFbx/atrogin-1 and MuRF1 protein levels were increased ∼4.5- and 3.5-fold, respectively (Fig. 5, C and D). Therefore, in addition to confirming previous results of increased MAFbx/atrogin-1 and MuRF1 mRNA levels in septic muscle, the experiments in Fig. 5 provided the novel observation of increased MAFbx/atrogin-1 and MuRF1 protein levels in the same muscles. Thus, the changes in HDAC and p300/HAT expression and activity were accompanied by molecular evidence of a catabolic response in skeletal muscle in the present experiments.
Fig. 5.
Sepsis in rats increases the expression of the ubiquitin ligases atrogin-1 and muscle ring finger 1 (MuRF1) in skeletal muscle. Atrogin-1 (A) and MuRF1 (B) mRNA levels were determined by real-time PCR in EDL muscles at different time points after sham operation or CLP. Results are means ± SE with n = 8 in each group. *P < 0.05 vs. sham at the corresponding time point by ANOVA. Atrogin-1 (C) and MuRF1 (D) protein levels were determined by Western blot analysis. Representative blots are shown. Bars represent densitometric quantifications of 8 blots in each group and results are means ± SE. *P < 0.05 vs. sham by Student's t-test.
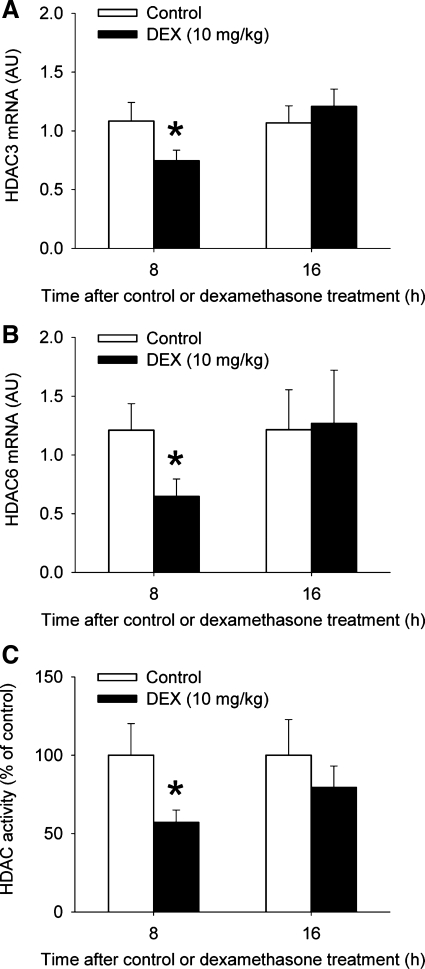
Dexamethasone-treated myotubes are commonly used as an in vitro model of muscle wasting because the catabolic response in muscle to sepsis and several other conditions is, at least in part, regulated by glucocorticoids (15, 37, 50). Although our previous reports suggest that glucocorticoids can upregulate p300/HAT and downregulate HDAC3 and -6 expression and activity in cultured myotubes (63, 64), it is not known whether glucocorticoids can exert the same effects in vivo. To address that question, we next treated rats with 10 mg/kg of dexamethasone or corresponding volumes of solvent. We found in previous studies that this treatment resulted in increased ubiquitin-proteasome-dependent protein breakdown and increased expression of C/EBPβ and -δ in skeletal muscle (54, 62). Here, we found that treatment of rats with dexamethasone resulted in upregulated p300/HAT expression and activity in EDL muscles after 16 h with no significant changes noted at 8 h (Fig. 6). The same treatment with dexamethasone resulted in reduced expression of HDAC3 and -6 and inhibited HDAC activity 8 h after injection of dexamethasone (Fig. 7). Of note, muscles were studied 8 and 16 h after dexamethasone treatment based on the results observed in the septic rats described above. Importantly, our results do not rule out the possibility that p300 and HDACs were affected by dexamethasone at earlier time points. Indeed, in a recent study we found evidence that the DNA binding activity of the transcription factors C/EBPβ and -δ was increased 4 h after treatment of rats with dexamethasone (62). Nevertheless, the present observations suggest that glucocorticoids may regulate p300 and HDAC3 and -6 expression and activity in vivo, similar to previous observations in cultured myotubes (63, 64) and that the sequence of changes (reduced HDAC expression and activity followed later by increased p300 expression and activity) was similar to that seen during sepsis.
Fig. 6.
Treatment of rats with dexamethasone increases the expression of p300 and stimulates HAT activity in skeletal muscle. Levels of p300 mRNA (A) and HAT activity (B) were determined in EDL muscles 8 and 16 h after treatment of rats with 10 mg/kg of dexamethasone or vehicle (control). Results are means ± SE with n = 8 in each group. *P < 0.05 vs. contol by ANOVA.
Fig. 7.
Treatment of rats with dexamethasone reduces the expression of HDAC3 and -6 and inhibits HDAC activity in skeletal muscle. HDAC3 (A) and HDAC6 (B) mRNA levels and HDAC activity (C) were determined in EDL muscles 8 and 16 h after treatment of rats with 10 mg/kg of dexamethasone or vehicle (control). Results are means ± SE with n = 8 in each group. *P < 0.05 vs. control at the corresponding time point by ANOVA.
Although we reported previously that treatment of rats with dexamethasone resulted in increased mRNA levels for ubiquitin (54), we wanted to test whether the changes in p300 and HDAC expression and activity induced by dexamethasone were accompanied by changes in MAFbx/atrogin-1 and MuRF1 expression. We therefore measured mRNA levels for MAFbx/atrogin-1 and MuRF1 in dexamethasone-treated rats and found that dexamethasone upregulated the expression of both ubiquitin ligases in EDL muscles after 8 and 16 h (Fig. 8). These results are important because they provide evidence at the molecular level that a catabolic response was present in the muscles in which the expression and activity of p300 were increased and the expression of HDAC3 and -6 and HDAC activity were reduced.
Fig. 8.
Treatment of rats with dexamethasone increases the expression of the ubiquitin ligases atrogin-1 and MuRF1 in skeletal muscle. Atrogin-1 (A) and MuRF1 (B) mRNA levels were determined by real-time PCR in EDL muscles 8 and 16 h after treatment of rats with 10 mg/kg of dexamethasone or vehicle (control). Results are means ± SE with n = 8 in each group. *P < 0.05 vs. control at the corresponding time point by ANOVA.
Although the results in dexamethasone-treated rats suggest that glucocorticoids can regulate the expression and activity of p300 and HDAC3 and -6 in vivo, they do not address the question whether glucocorticoids are involved in sepsis-induced changes in the expression of p300 and HDACs. To test the role of glucocorticoids in sepsis-induced changes, we next treated rats with the glucocorticoid receptor antagonist RU38486 (26). We found in previous reports that RU38486 prevented the sepsis-induced increase in muscle protein breakdown and expression of ubiquitin, MAFbx/atrogin-1, and MuRF1 (54, 61). Here, we found that treatment of rats with RU38486 prevented the sepsis-induced increase in p300 expression in EDL muscles (Fig. 9A). Although HDAC3 mRNA levels were not significantly reduced in septic rats, the trend toward decreased HDAC3 mRNA levels was reversed by RU38486 (Fig. 9B). In addition, the downregulation of HDAC6 expression was prevented by RU38486 (Fig. 9C). It should be noted that the significant decrease in HDAC6 mRNA levels observed in septic rats in this experiment differed from the results in Fig. 2B in which the changes in HDAC6 mRNA levels did not reach statistical significance. Although we do not have a definitive explanation for this apparent discrepancy, it is possible that the control injections of solvent for RU38486 accounted for the more robust decrease of HDAC6 mRNA levels in Fig. 9C than in Fig. 2B.
Fig. 9.
Treatment of rats with the glucocorticoid receptor antagonist RU38486 prevents sepsis-induced changes in p300 and HDAC6 expression in skeletal muscle. A: p300 mRNA levels were determined by real-time PCR in EDL muscles 16 h after sham operation or CLP. Rats were treated with 10 mg/kg of RU38486 or vehicle (control) by intraperitoneal injection 2 h before sham operation or CLP. HDAC3 (B) and HDAC6 (C) mRNA levels were determined in EDL muscles 8 h after sham operation or CLP. Rats were treated with 10 mg/kg of RU38486 or vehicle (control) by intraperitoneal injection 2 h before sham operation or CLP. Results are means ± SE with n = 8 in each group. *P < 0.05 vs. control by ANOVA.
Increased p300/HAT and reduced HDAC3 and -6 expression and activity, as observed here in septic and dexamethasone-treated rats, provide conditions consistent with hyperacetylation. To test whether hyperacetylation can induce a catabolic response in skeletal muscle, we next treated rats with the HDAC inhibitor TSA (64). In our recent experiments in cultured myotubes, TSA increased protein degradation to the same extent as dexamethasone treatment (64). The influence of TSA treatment in vivo on muscle protein degradation has not been reported. Here, we found that treatment of rats with 10 mg/kg of TSA resulted in a 50% inhibition of HDAC activity in EDL muscles (Fig. 10A) and a significant increase in protein breakdown rates noticed 4 h after injection of TSA (Fig. 10B). Interestingly, the same treatment resulted in an almost twofold increase in MAFbx/atrogin-1 mRNA levels, whereas MuRF1 mRNA levels were not affected (Fig. 10, C and D).
Fig. 10.
Treatment of rats with trichostatin A (TSA) reduces HDAC activity and increases protein degradation and atrogin-1 expression in skeletal muscle. A: HDAC activity was measured in EDL muscles 2 and 4 h after treatment of rats with 10 mg/kg of TSA or vehicle (control). B: protein breakdown rates were determined by measuring tyrosine release in incubated EDL muscles 2 and 4 h after treatment with TSA or vehicle. Atrogin-1 (C) and MuRF1 (D) mRNA levels were determined by real-time PCR in EDL muscles 2 and 4 h after treatment with TSA or vehicle. Results are means ± SE with n = 6 or 7 in each group. *P < 0.05 vs. control by ANOVA.
The experiments described above (Figs. 1–10) were performed in EDL muscles because previous reports suggest that the catabolic responses to sepsis are particularly pronounced in white, fast-twitch skeletal muscle (16, 57). To test whether sepsis-induced changes in p300 and HDAC expression are differentially regulated in fast- and slow-twitch muscle, we next determined the influence of sepsis on the expression of p300, HDAC3, and HDAC6 in the red, slow-twitch, soleus muscle. Interestingly, mRNA levels for p300 and HDAC6 were not altered in soleus muscles during sepsis, whereas HDAC3 mRNA levels were reduced (Fig. 11, A–C). Although we found in previous experiments that sepsis-induced changes in protein degradation and the expression of ubiquitin were less pronounced in red, slow-twitch than in white, fast-twitch, skeletal muscle (16, 57), it is not known whether the expression of MAFbx/atrogin-1 and MuRF1 is differentially regulated in the two types of muscle during sepsis. Here we found that although MAFbx/atrogin-1 and MuRF1 mRNA levels were substantially increased in soleus muscles of septic rats, the changes were less pronounced than observed in EDL muscles (Fig. 11, D and E, and compare with Fig. 5, A and B). Taken together, the results in Fig. 11 suggest that the expression of p300 and HDAC6 may be differentially regulated in red, slow-twitch and white, fast-twitch muscles, whereas reduced HDAC3 expression may be a more generalized consequence of sepsis.
Fig. 11.
Sepsis-induced changes in soleus muscles of p300, HDAC3, HDAC6, atrogin-1, and MuRF1 mRNA levels. The mRNA levels were determined by real-time PCR at different time points after sham operation or CLP. Results are means ± SE with n = 6–8 in each group. *P < 0.05 vs. sham by ANOVA.
DISCUSSION
In the present study, sepsis induced by CLP in rats, resulted in increased p300 expression and HAT activity and reduced HDAC6 and SIRT1 expression and HDAC activity in the white, fast-twitch EDL muscle. Treatment of rats with dexamethasone resulted in similar changes in p300 and HDAC expression and activity as those observed in septic rats and sepsis-induced changes in p300 and HDAC6 expression were prevented by RU38486. In addition, treatment of rats with the HDAC inhibitor TSA stimulated muscle protein breakdown and upregulated atrogin-1 expression. Taken together, our results suggest that sepsis-induced muscle wasting is associated with glucocorticoid-dependent hyperacetylation in skeletal muscle caused by increased p300 and reduced HDAC expression and activity.
Interestingly, whereas reduced HDAC3 expression was noted in both fast- and slow-twitch muscles, p300 and HDAC6 expression was altered only in white, fast-twitch muscle. This observation suggests that acetylation and deacetylation of cellular proteins may be differentially regulated in different types of skeletal muscle during sepsis. Whether this difference is related to the more pronounced catabolic response to sepsis typically seen in white, fast-twitch muscle remains to be determined. Because white, fast-twitch skeletal muscle is more sensitive to the catabolic effects of sepsis (and several other muscle-wasting conditions as well), the present experiments were focused mainly on changes in EDL muscles.
An interesting observation in the present study was the difference between p300 and HDAC expression and activity with regard to the temporal regulation during sepsis and after dexamethasone treatment. Although not fully understood at present, one reason why p300 expression and activity were upregulated later than the downregulation of HDAC expression and activity may be different sensitivity of p300 and HDACs to the effects of sepsis and glucocorticoids. It is also possible that different mechanisms were involved in the regulation of p300 and the HDACs. In addition, it may be speculated that the results reflect a regulatory effect of reduced HDAC activity on p300 expression and activity. Previous studies suggest that p300 and HDACs can interact and that reduced deacetylation may influence the expression and activity of p300 (24, 48).
Regulation of chromatin acetylation, in turn affecting the binding of transcription factors to DNA and influencing transcriptional activation, are important functions of p300 and HDACs (35). Studies performed both in vitro and in vivo in experimental animals suggest, however, that acetylation of other proteins involved in the regulation of gene transcription may also be influenced by p300 and HDACs, including transcription factors and nuclear cofactors, some of which may be involved in the regulation of muscle mass (6, 7, 9, 13, 23, 33, 42, 47, 63). Mechanisms by which p300 and HDACs can influence protein degradation were reviewed recently by Sadoul et al. (48) and include blocking the ubiquitination of lysine residues, changing the acetylation of chaperone proteins that regulate the stability of proteins, interacting with ubiquitin ligases, and even exerting intrinsic ubiquitin ligase activity.
It should be noted that although p300 and HDAC protein levels were measured in the nuclear fraction in the present study, acetyl transferases and deacetylases may be present in the cytoplasm as well (30, 34). Interestingly, a recent study in cultured kidney HEK-293 cells, HeLa cells, and neuro2a (N2Aa) cells suggests that cytoplasmic HDAC6 plays an important role in cellular protein degradation by regulating the trafficking of proteins to the autophagic/lysosomal machinery (19). It is intriguing that HDAC6 also possesses a domain that binds to polyubiquitin chains (51), raising the possibility that HDAC6 may influence both proteasomal and autophagic/lysosomal proteolysis. The presence of cytoplasmic acetylation and deacetylation supports the concept that the state of acetylation of nonhistone proteins is an important mechanism of regulation of protein degradation as well as other cellular functions.
Although the present results support the concept that hyperacetylation caused by reduced HDAC and increased p300/HAT activity may be involved in sepsis- and glucocorticoid-induced muscle wasting, apparently conflicting results have been reported with regard to the effects of acetylation in skeletal muscle. Thus, inhibition of class I and II HDACs in cultured C2C12 and human primary myocytes resulted in the formation of myotubes with increased cell size and abundance of muscle proteins (17). In a subsequent report from the same group, treatment of cultured C2C12 myotubes with TSA promoted recruitment of myoblasts and fusion into myotubes, at least in part, secondary to induction of the muscle sparing protein follistatin (18). In other studies, treatment with TSA resulted in functional and morphological recovery of muscles in mice with muscular dystrophy (39). In a recent report, HDAC6 activity was required for autophagic degradation of the Huntingtin protein (Htt), a protein that aggregates within intracellular inclusion bodies in neurons of patients with Huntington disease (19). The studies quoted here apparently contradict some of the results in the present report by suggesting that a state of hyperacetylation reduces the degradation of at least some proteins in different cell types and may also be associated with the formation of muscle cells with increased size and protein content.
In contrast, other studies support the conclusion of the current study that hyperacetylation may promote protein degradation and loss of muscle mass. For example, Jeong et al. (20) reported recently that acetylation caused by CREB-binding protein and specific inhibition of HDAC1 targeted mutant Htt and resulted in trafficking of acetylated Htt to autophagosomes and increased autophagic/lysosomal degradation. The recent report by Tobimatsu et al. (58) confirmed the role of p300 in glucocorticoid-induced atrophy of cultured muscle cells. In other studies, reduced HDAC activity resulted in negative effects in skeletal muscle, including inhibited muscle differentiation in cultured myoblasts and frog embryos (53).
There are several limitations of the present study that need to be taken into account when the results are interpreted. First, it is not known at present which cellular proteins that are hyperacetylated in skeletal muscle during sepsis or after treatment with dexamethasone. Second, it is not known which HDAC(s) accounted for the reduced HDAC activity noticed here in muscle from septic and dexamethasone-treated rats although our results suggest that both HDAC6 and SIRT1 may have been involved. Third, the present observations do not prove that there is a link between reduced HDAC and increased p300/HAT activities and muscle wasting, although the increased muscle protein degradation and MAFbx/atrogin-1 expression noticed after treatment of rats with TSA support a role of hyperacetylation in muscle wasting. Finally, it remains to be determined whether the expression and activity of p300 and HDACs are altered in skeletal muscle of septic patients as well. Despite the limitations, the present observations are important because they suggest for the first time that hyperacetylation caused by reduced HDAC activity and stimulated p300/HAT activity may be involved in sepsis- and glucocorticoid-induced muscle wasting.
Perspectives and Significance
The present report provides the first evidence that sepsis- and glucocorticoid-induced muscle wasting may be associated with hyperacetylation caused by increased p300/HAT and reduced HDAC expression and activity. Although the experiments do not prove that there is a link between hyperacetylation and the catabolic response in skeletal muscle during sepsis, it may be speculated that targeted inhibition of hyperacetylation, for example by stimulating HDAC activity (38), may be a method by which muscle wasting can be prevented in sepsis and other conditions characterized by glucocorticoid-regulated muscle wasting.
GRANTS
The study was supported in part by National Institutes of Health Grants DK-37908 and NR-08545 (to P. O. Hasselgren). Z. Aversa was supported by the Department of Clinical Medicine, “Sapienza,” University of Rome, Rome, Italy.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1β drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 5: 35–46, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Avila AM, Burnett BG, Taye AA, Gabanella F, Knight MA, Hartenstein P, Cizman Z, Di Prospero NA, Pellizoni L, Fischbeck KH, Sumner CJ. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J Clin Invest 117: 659–671, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodine SC, Latres E, Baumheuter S, Lai VK, Nunez L, Clarke BA, Poueymiron WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, Dechiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Cai D, Frantz JD, Tawa NE, Melandez PA, Oh BC, Lidov HGW, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKβ/NF-kB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliot PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458: 1056–1060, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cesena TI, Cardinaux JR, Kwok R, Schwartz J. CCAAT/enhancer-binding protein (C/EBP)β is acetylated at multiple lysines. Acetylation of C/EBPβ at lysine 39 modulates its ability to activate transcription. J Biol Chem 282: 956–967, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Chen LF, Greene WC. Shaping the nuclear action of NF-kB. Nat Rev Mol Cell Biol 5: 392–401, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 9.Czubryt MP, McAnally J, Fishman GI, Olson EN. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci USA 100: 1711–1716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fareed MU, Evenson AR, Wei W, Menconi M, Poylin V, Petkova V, Pignol B, Hasselgren PO. Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regul Integr Comp Physiol 290: R1589–R1597, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Furuyama T, Kitayama K, Yamashita H, Mori N. Forkhead transcription factor FOXO1 (FKHR)-dependent induction of PDK4 gene expression in skeletal muscle during energy deprivation. Biochem J 375: 365–371, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuyama T, Yamashita H, Kitayama K, Higami Y, Shimokawa I, Mori N. Effects of aging and caloric restriction on the gene expression of Foxo1, 3, and 4 (FKHR, FKHRL1, and AFX) in the rat skeletal muscles. Microsc Res Tech 59: 331–334, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty oxidation through SIRT1/PGC-1α. EMBO J 26: 1913–1923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98: 14440–14445, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasselgren PO. Glucocorticoids and muscle catabolism. Curr Opin Clin Nutr Metab Care 2: 201–205, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Hasselgren PO, James JH, Benson DW, Hall-Angeras M, Angeras U, Hiyama DT, Li S, Fischer JE. Total and myofibrillar protein breakdown in different types of skeletal muscle: effects of sepsis and regulation by insulin. Metabolism 38: 634–640, 1989 [DOI] [PubMed] [Google Scholar]
- 17.Iezzi S, Cossu G, Nervi C, Sartorelli V, Puri PL. Stage-specific modulation of skeletal myogenesis by inhibitors of nuclear deacetylases. Proc Natl Acad Sci USA 99: 7757–7762, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iezzi S, Di Padova M, Serra C, Caretti G, Simone C, Maklan E, Minetti G, Zhao P, Hoffman EP, Puri PL, Sartorelli V. Deacteylase inhibitors increase muscle cell size by promoting myoblast recruitment and fusion through induction of follistatin. Dev Cell 6: 673–684, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Iwata A, Riley BE, Johnston JA, Kopito RR. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J Biol Chem 280: 40282–40292, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Jeong H, Then F, Melia TJ, Mazzulli JR, Cui L, Savas JN, Voisine C, Paganetti P, Tomese N, Hart AC, Yamamoto A, Krainc D. Acetylation targets mutant huntingtin to autophagosomes for degradation. Cell 137: 60–72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, Nishino I, Ezaki O. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated type I (slow-twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem 279: 41114–41123, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Kamei Y, Mizukami J, Miura S, Suzuki M, Takahashi N, Kawada T, Taniguchi T, Ezaki O. A forkhead transcription factor FKHR up-regulates lipoprotein lipase expression in skeletal muscle. FEBS Lett 536: 232–236, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Kino T, Nordeen SK, Chrousos GP. Conditional modulation of glucocorticoid receptor activities by CREB-binding protein (CBP) and p300. J Steroid Biochem Mol Biol 70: 15–25, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Kuo MH, Allis CD. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioassays 20: 615–626, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab 293: E453–E459, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Lazar G, Agarwal MK. Physiological action and receptor binding of a newly synthesized and novel antiglucocorticoid. Biochem Biophys Res Commun 134: 44–50, 1986 [DOI] [PubMed] [Google Scholar]
- 27.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18: 39–51, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Lee KC, Lee Kraus W. Nuclear receptors coactivators, and chromatin: new approaches, new insights. Trends Endocrinol Metab 12: 191–197, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1: 361–370, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Lin Y, Randall WR, Schneider MF. Activity-dependent and independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J Cell Biol 168: 887–897, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. Foxo3 controls autophagy in skeletal muscle in vivo. Cell Metab 6: 458–471, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukumizu A. Acetylation of FOXO1 alters its DNA-binding activity and sensitivity to phosphorylation. Proc Natl Acad Sci USA 102: 11278–11283, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinsey TA, Zhang CL, Lu J, Olson EN. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408: 106–111, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKinsey TA, Zhang CL, Olson EN. Control of muscle development by dueling HATs and HDACs. Curr Opin Genet Dev 11: 497–504, 2001 [DOI] [PubMed] [Google Scholar]
- 36.McKinsey TA, Zhang CL, Olson EN. Signaling chromatin to make muscle. Curr Opin Cell Biol 14: 763–772, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Menconi M, Gonnella P, Petkova V, Lecker S, Hasselgren PO. Dexamethasone and corticosterone induce similar, but not identical, muscle wasting responses in cultured L6 and C2C12 myotubes. J Cell Biochem 105: 353–364, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450: 712–716, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minetti GC, Colussi C, Adami R, Serra C, Mozzetta C, Parente V, Fortuni S, Straino S, Sampaolesi M, Di Padova M, Illi B, Gallinari P, Steinkuhler C, Capogrossi MC, Sartorelli V, Bottinelli R, Gaetano C, Puri PL. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med 12: 1147–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Mink S, Haenig B, Klempnauer KH. Interaction and functional collaboration of p300 and C/EBPβ. Mol Cell Biol 17: 6609–6617, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore-Carrasco R, Garcia-Martinez C, Busquets S, Ametller E, Barreiro E, Lopez-Soriano FJ, Argiles JM. The AP-1/CJUN signaling cascade is involved in muscle differentiation: implications in muscle wasting during cancer cachexia. FEBS Lett 580: 691–696, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J Biol Chem 280: 16456–16460, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Penner CG, Gang G, Sun X, Wray C, Hasselgren PO. C/EBP DNA binding activity is upregulated by a glucocorticoid-dependent mechanism in septic muscle. Am J Physiol Regul Integr Comp Physiol 282: R439–R449, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Penner CG, Gang G, Wray C, Fischer JE, Hasselgren PO. The transcription factors NF-kB and AP-1 are differentially regulated in skeletal muscle during sepsis. Biochem Biophys Res Commun 281: 1331–13362001 [DOI] [PubMed] [Google Scholar]
- 45.Polesskaya A, Naguibneva I, Fritsch L, Duquet A, Ait-Si-Ali S, Robin P, Vervisch A, Pritchard LL, Cole P, Harel-Bellan A. CBP/p300 and muscle differentiation: No HAT, no muscle. EMBO J 20: 6816–6825, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Potthoff MJ, Wu H, Arnold MA, Shelton JM, Backs J, McAnally J, Richardson JA, Bassel-Duby R, Olson EN. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Invest 117: 2459–2467, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O'Malley B, Spiegelman BM. Activation of PPAR gamma coactivator-1 through transcription factor docking. Science 286: 1368–1371, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Sadoul K, Boyault C, Pabion M, Khochbin S. Regulation of protein turnover by acetyltransferases and deacetylases. Biochimie 90: 306–312, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1α protects skeletal muscle from atrophy by suppressing FoxO action and atrophy specific gene transcription. Proc Natl Acad Sci USA 103: 16260–16265, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol 197: 1–10, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Seigneurin-Berny D, Verdel A, Curtet S, Lemercier C, Garin J, Rousseaux S, Khochbin S. Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol Cell Biol 21: 8035–8044, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith IJ, Alamdari N, O'Neal P, Gonnella P, Aversa Z, Hasselgren PO. Sepsis increases the expression and activity of the transcription factor Forkhead Box O1 (FOXO1) in skeletal muscle by a glucocorticoid-dependent mechanism. Int J Biochem Cell Biol 42: 701–711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steinbac OC, Wolffe AP, Rupp RA. Histone deacteylase activity is required for the induction of the MyoD muscle cell lineage in Xenopus. Biol Chem 381: 1013–1016, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Tiao G, Fagan J, Roegner V, Lieberman M, Wang JJ, Fischer JE, Hasselgren PO. Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J Clin Invest 97: 339–348, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiao G, Fagan JM, Samuels N, James JH, Hudson K, Lieberman M, Fischer JE, Hasselgren PO. Sepsis stimulates nonlysosomal, energy-dependent proteolysis and increases ubiquitin mRNA levels in rat skeletal muscle. J Clin Invest 94: 2255–2264, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tiao G, Hobler S, Wang JJ, Meyer TA, Luchette FA, Fischer JE, Hasselgren PO. Sepsis is associated with increased mRNAs of the ubiquitin-proteasome proteolytic pathway in human skeletal muscle. J Clin Invest 99: 163–168, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tiao G, Lieberman MA, Fischer JE, Hasselgren PO. Intracellular regulation of protein degradation during sepsis is different in fast- and slow-twitch muscle. Am J Physiol Regul Integr Comp Physiol 272: R849–R856, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Tobimatsu K, Noguchi T, Hosooka T, Sakai M, Inagaki K, Matsuki Y, Hiramatsu R, Kasuga M. Overexpression of the transcriptional coregulator Cited2 protects against glucocorticoid-induced atrophy of C2C12 myotubes. Biochem Biophys Res Commun 378: 399–403, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Van Gummeren D, Damrauer JS, Jackman RW, Kandarian S. The IκB kinases IKKα and IKKβ are necessary and sufficient for skeletal muscle atrophy. FASEB J 23: 362–370, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waalkes TP, Udenfriend S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med 50: 733–736, 1957 [PubMed] [Google Scholar]
- 61.Wray CJ, Mammen JM, Hershko DD, Hasselgren PO. Sepsis upregulates the expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol 35: 698–705, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Yang H, Mammen J, Wei W, Menconi M, Evenson A, Fareed M, Petkova V, Hasselgren PO. Expression and activity of C/EBPβ and δ are upregulated by dexamethasone in skeletal muscle. J Cell Physiol 204: 219–226, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Yang H, Menconi MJ, Wei W, Petkova V, Hasselgren PO. Dexamethasone upregulates the expression of the nuclear cofactor p300 and its interaction with C/EBPβ in cultured myotubes. J Cell Biochem 94: 1058–1067, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Yang H, Wei W, Menconi M, Hasselgren PO. Dexamethasone-induced protein degradation in cultured myotubes is p300/HAT dependent. Am J Physiol Regul Integr Comp Physiol 292: R337–R344, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Yoshida M, Matsuyama A, Komatsu Y, Nishino N. From discovery to the coming generation of histone deacetylase inhibitors. Curr Med Chem 10: 2351–2358, 2003 [DOI] [PubMed] [Google Scholar]
- 66.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal pathways in atrophying muscle. Cell Metab 6: 472–483, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Zhao W, Qin W, Pan J, Wu Y, Bauman WA, Cardozo C. Dependence of dexamethasone-induced Akt/FOXO1 signaling, upregulation of MAFbx, and protein catabolism upon the glucocorticoid receptor. Biochem Biophys Res Commun 378: 668–672, 2009 [DOI] [PubMed] [Google Scholar]