Abstract
Injury to epithelial cells results in the release of ATP and stimulation of purinergic receptors and is thought to alter cell migration and wound repair. Medium from the injured cells triggers Ca2+ mobilization and phosphorylation of ERK, both of which are inhibited if the medium is pretreated with apyrase. To understand the wound repair mechanism that occurs with injury, our goal was to determine which purinergic receptor(s) was the critical player in the wound response. We hypothesize that the P2Y2 receptor is the key player in the response of corneal epithelial cells to cell damage and subsequent repair events. Cells transfected with short interfering RNA to either P2Y2 or P2Y4 were stimulated either by injury or addition of UTP and imaged using fluo 3-AM to monitor changes in fluorescence. When cells with downregulated P2Y2 receptors were injured or stimulated with UTP, the intensity of the Ca2+ release was reduced significantly. However, when cells with downregulated P2Y4 receptors were stimulated, only the UTP-induced Ca2+ response was reduced significantly. In addition, downregulation of the P2Y2 receptor inhibited wound closure compared with unstimulated cells or cells transfected with nontargeting sequence. This downregulation resulted also in an attenuation in phosphorylation of Src and ERK. Together, these data indicate that the P2Y2 receptor plays a major biological role in the corneal injury response and repair mechanisms.
Keywords: wound healing, epithelium, P2Y receptors, imaging
epithelia serve a protective role as a barrier to outside factors, and injury disrupts this function. Reestablishment of the integrity of the tissue is one of the prototypical responses that occur; however, the mechanisms regulating initial and later responses, such as wound repair, are still not fully understood. The corneal epithelium is an excellent model system to examine pathways that lead to proper or impaired wound closure, as it is an avascular stratified squamous tissue that is readily accessible. Purines and pyrimidines (nucleotides) and growth factors (e.g., EGF) have been shown to stimulate events associated with epithelial wound repair. Nucleotides are released on injury and cause the propagation of a Ca2+ wave to neighboring cells (8, 12, 18, 34). When the wound medium collected from injured cells is added to independent cultures, a similar response is generated, which does not occur when the wound medium is pretreated with apyrase (19). In contrast, EGF is not released with injury (8). We hypothesize that the nucleotide-induced Ca2+ wave is one of the initial signals that induce cells to communicate that an injury has occurred and to initiate a signaling cascade.
Nucleotides are ligands for purinergic receptors, P2Y [G protein-coupled receptors (GPCR)] and P2X (ligand gated ion channels). The P2Y receptors couple primarily to Gαq and activate phospholipase C-β (PLC-β; resulting in an increase in intracellular Ca2+) (28). These receptors are known to mediate cell migration, proliferation, and inflammation (1, 9, 25). Our laboratory has previously demonstrated that adenosine triphosphate (ATP) and uridine triphosphate (UTP), ligands for P2Y2 and P2Y4 receptors, are essential components of an injury-induced wave for both primary corneal epithelial cells and corneal epithelial cell lines (19, 34). In this paper, we investigate the role of these P2Y receptors. Involvement of P2Y receptors in wound healing has been suggested both in activation of signaling pathways and in wound closure (1, 10, 19, 20, 24, 26, 27, 34).
The expression of P2Y receptors has been explored in many cell types. However, the members of the purinergic receptor family, which induce Ca2+ signaling in corneal epithelial cells, as well as the members involved in the injury-induced wave and cell migration, have yet to be elucidated. We hypothesize that the receptors mediate the short-term signaling cascades, resulting from injury, as well as long-term events, such as cell migration. Both the P2Y2 and P2Y4 receptors can activate downstream signaling pathways through both Ca2+- and non-Ca2+-mediated pathways. The P2Y2 receptor can interact with a number of proteins, including integrins, RGD peptides, filamin, and Src, all of which have been shown to be important for cell migration (14, 21, 34, 36). Furthermore, in PC12 cells, the P2Y2 receptor has been shown to activate Src, leading to the formation of a Grb/Shc complex and activation of ERK (30).
The goal of this paper is to determine which P2Y receptors are critical to epithelial wound repair. We demonstrate that the trinucleotide receptor, P2Y2, is the key receptor responsible in both the nucleotide and injury-induced propagation of Ca2+. Knockdown of the P2Y4 receptor did not significantly reduce the injury-induced response. While P2Y2 receptor downregulation decreased the rate and extent of cell migration in scratch wound assays, its downregulation inhibited the nucleotide-induced phosphorylation of Src and ERK. Together, these results indicate that the P2Y2 receptor is essential for the proper signaling and wound healing in corneal epithelial cells.
MATERIALS AND METHODS
Reagents.
Antibodies directed against total ERK1/2 and active MAPK were purchased from Promega (Madison, WI). Antibodies directed against p-Src 418 and c-Src were purchased from Upstate (Temecula, CA) and Santa Cruz (Santa Cruz, CA), respectively. Fluo 3-AM, and pluronic acid were purchased from Molecular Probes (Eugene, OR). BCA Protein Assay kit was purchased from Pierce (Rockford, IL). ATP, UTP, adenosine diphosphate (ADP), and other routine chemicals were obtained either from Sigma (St. Louis, MO) or from American Bioanalytical (Natick, MA). P2Y2 and P2Y4 short interfering RNA (siRNA) were purchased from Dharmacon (Lafayette, CO).
Cell culture.
A human corneal limbal epithelial cell (HCLE) line was used (15). Previous experiments compared a corneal epithelial cell line to primary epithelial cells, demonstrating the retention of primary cell characteristics in the assays presented here (34). Cells were grown in keratinocyte serum-free medium (K-SFM) (Ca2+ concentration = 0.09 mM), supplemented with 30 μg/ml bovine pituitary extract, 0.032 nM EGF (Invitrogen, Carlsbad, CA), 100 U/ml penicillin, 100 μg/ml streptomycin, (Mediatech, Manassas, VA), and an additional 0.3 mM CaCl2 (18). Twenty-four hours before the experiment, EGF and bovine pituitary extract were removed.
siRNA transfection.
Cells were transfected at 50–60% confluency. Media was replaced with K-SFM without antibiotics before transfection reagents were added to cells. In optimization experiments, multiple sequences were used individually or as part of a four-sequence pool, and the sequences resulting in the largest downregulation of transcripts as assayed by real-time PCR were used for further experimentation. All experiments were performed using the four-sequence pool or two individual sequences (D-003688-01 or -09 and D-005693-05 or -03), which showed responses equivalent to the pool (Table 1). Control nontargeting sequences were transfected as control in each experiment. Lipofectamine and siRNA sequences were incubated separately in K-SFM supplemented with 30 μg/ml bovine pituitary extract and 0.032 nM EGF without antibiotics, at room temperature, for 15 min. Mixtures were combined, incubated another 15 min, and added to cells at a final concentration of 2 μl/ml lipofectamine and 20 nM siRNA. Assays were performed a minimum of 72 h posttransfection, as determined by optimization experiments.
Table 1.
siRNA sequences
| Target | Dharmacon Product No. | Sequences | |
|---|---|---|---|
| P2Y2 | D-003688-01 | Sense | 5′-caacauggccuacaagguuuu-3′ |
| Antisense | 5′-aaccuuguaggccauguuguu-3′ | ||
| P2Y2 | D-003688-09 | Sense | 5′-gaacugacaugcagaggauuu-3′ |
| Antisense | 5′-auccucugcaugucaguucuu-3′ | ||
| P2Y2 | D-003688-02 | Sense | 5′-ggaaugcguccaccacauauu-3′ |
| Antisense | 5′-uaugugguggacgcauuccuu-3′ | ||
| P2Y2 | D-003688-04 | Sense | 5′-gcagaggcucguacgcuuuuu-3′ |
| Antisense | 5′-aaagcguacgagccucugcuu-3′ | ||
| P2Y4 | D-005693-05 | Sense | 5′-uagggcagauagauuguaauu-3′ |
| Antisense | 5′-uuacaaucuaucugcccuauu-3′ | ||
| P2Y4 | D-005693-03 | Sense | 5′-caacguggucuauaaaguguu-3′ |
| Antisense | 5′-cacuuuauagaccacguuguu-3′ | ||
| P2Y4 | D-005693-02 | Sense | 5′-gaagcugacugccgaguacuu-3′ |
| Antisense | 5′-guacucggcagucagcuucuu-3′ | ||
| P2Y4 | D-005693-04 | Sense | 5′-caacggccaccuacauguuuu-3′ |
| Antisense | 5′-aacauguagguggccguuguu-3′ |
siRNA, short interfering RNA.
RNA isolation and real-time PCR analysis.
RNA was extracted from cells using the TRIzol reagent, according to the manufacturer's protocol (Invitrogen). RNA concentrations were determined spectrophotometrically, and RNA was treated with DNase I (Invitrogen), along with an RNase inhibitor (Roche Applied Science, Indianapolis, IN). Reverse transcription was performed on the DNase-treated RNA using Moloney murine leukemia virus-reverse transcriptase. Negative controls were performed without the Moloney murine leukemia virus-reverse transcriptase. The cDNA template produced was treated with RNaseH (Invitrogen). The TaqMan Gene Expression Master Mix was used. Real-time PCR was performed using an ABI 7300 (Applied Biosystems, Foster City, CA). The following TaqMan probes were used: Hs00704965_s1 for P2Y1, Hs00602525_m1 for P2Y2, Hs00267404_s1 for P2Y4, and the eukaryotic 18S rRNA endogenous control for the 18S ribosomal subunit [(VIC/MGB Probe, Primer Limited) Applied Biosystems]. The cDNA template was incubated for an initial 2 min at 50°C and 10 min at 95°C, followed by 40–50 amplification cycles of 95°C for 15 s and 60°C for 1 min. Melting curve analysis and gel electrophoresis of PCR products verified that a single product of the expected size was generated with each primer set. ABI software was used to analyze the results, and the data were exported for further analysis. Data analysis used the ΔΔCt (cycle threshold) method. Ct was normalized to 18S ribosomal subunit. For Fig. 3, the controls for each were set to one. Values were given as the mean ± SE of the mean. Statistical comparisons were made using Student's t-test or ANOVA followed by Tukey's post hoc test.
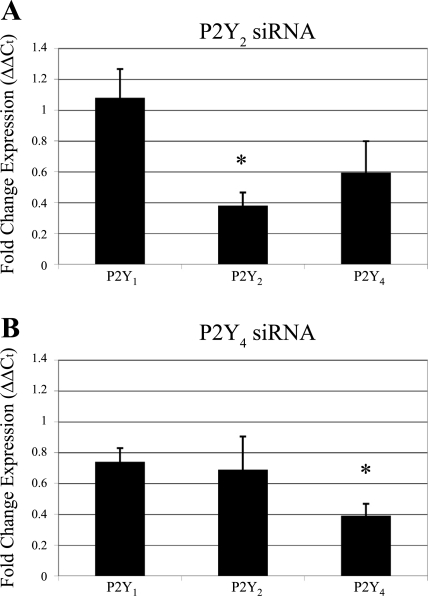
Fig. 3.
Expression of P2Y receptors after transfection. Real-time PCR was performed to assay for expression of P2Y receptor mRNA after transfection. The ΔΔCt method was used to determine fold change expression of P2Y1, P2Y2, and P2Y4 receptors in cells transfected with P2Y2 (A) and P2Y4 (B) siRNA and compared with control cells transfected with a nontargeting siRNA sequence. Results were normalized to the 18S ribosomal subunit and graphed as relative expression compared with nontargeting control (normalized to 1). Data are representative of a minimum of six independent experiments. Significance compared with the nontargeting control was determined by a one-way ANOVA followed by Tukey's post hoc test: *P < 0.05.
Calcium imaging.
Ca2+ imaging was performed on HCLEs, as previously described (18). Briefly, cells were incubated in an HEPES-buffered saline solution containing 137 mM NaCl, 5 mM KCl, 4 mM MgCl2, 3 mM CaCl2·2H2O, 25 mM glucose, and 10 mM HEPES (11) or a Ca2+-free HEPES-buffered saline solution containing 137 mM NaCl, 5 mM KCl, 4 mM MgCl2, 1 mM EGTA, 25 mM glucose, and 10 mM HEPES and loaded with the Ca2+ indicator dye fluo 3-AM (5 μM). The cells were imaged using a Zeiss Axiovert 100M LSM 510 equipped with an Argon and 2 HeNe lasers (Thornwood, NY). All perturbations were made while continuously scanning the cells every 789 ms. Cells were perfused with HEPES-buffered saline before stimulation or injury to establish a baseline fluorescence reading. Cells were stimulated with agonist prepared in HEPES-buffered saline and washed with HEPES-buffered saline. Ca2+ dynamics were evaluated as described (11, 18).
SDS-PAGE and Western blot analysis.
Lysates were collected and sheared, as described previously (35). The protein concentration of the supernatant was determined using the bicinchoninic acid assay. Equivalent amounts of protein from each lysate were subjected to SDS-PAGE and transferred to polyscreen polyvinylidene difluoride membrane (PerkinElmer, Boston, MA). Blots were blocked in a Tris buffer (10 mM Tris, 100 mM NaCl, 0.1% Tween-20) containing 0.2% I-block (Applied Biosystems), and membranes were incubated with appropriate primary antibodies, washed and incubated with appropriate secondary antibodies, and rinsed with Tris-buffered saline-Tween-20. Visualization was performed by enhanced chemiluminescence (Denville Scientific, Metuchen, NJ).
Scratch wound migration assay.
Cells were transfected and cultured for 72 h on eight-well glass-bottom chambers to test the role of P2Y2 in directed cell migration using scratch wounds. The media was replaced with unsupplemented media 18–24 h before experimentation. Cells were treated with either unsupplemented media or media containing UTP, and two linear wounds (200–300 μm in diameter) were made in each well. The culture chambers were placed on the stage of a Zeiss Axiovert 200M LSM 510 laser scanning confocal microscope (Zeiss, Thornwood, NY) in an environmental chamber maintained at 37°C and 5% CO2. Wounds were monitored using the multitime module in the LSM software allowing multiple locations to be observed over time. Tiled differential contrast images were taken at each location every 20 min for 20 h. Autofocus was used before the first image at each time point was acquired. LSM software was used to measure the wound area at various time points, and percentage and rate of closure were calculated. Values were given as means ± SE. Statistical comparisons were made using Student's t-test or ANOVA, followed by Tukey's post hoc test.
Transwell migration assay.
To determine the role of P2Y2 and Src in chemotactic migration to nucleotides, complete K-SFM was replaced with unsupplemented K-SFM 18–24 h before initiation of experiments. For Src inhibition assays, SU6656 or DMSO alone as a control was added 30 min before the assay (13). Cells were washed in phosphate-buffered saline (PBS) and trypsinized, and the reaction was stopped with filtered soybean trypsin inhibitor, as described (19). Cells were resuspended at a concentration of 125,000 cells/100 μl in binding buffer (unsupplemented K-SFM or DMEM, 0.05% gelatin, and 25 mM HEPES). Binding buffer (600 μl), with or without added stimuli and inhibitors, was placed in the wells of a 24-well plate. Costar Transwell inserts (6.5-mm-diameter polycarbonate membranes, 8-μm pore size) (Corning, Lowell, MA) were placed into the wells, and 100 μl of the cell suspension was added to the inserts. Cells were allowed to migrate for 8 h at 37°C, as determined by preliminary experiments.
To determine migration, upper and lower chambers were aspirated and rinsed with PBS. Cells were fixed at room temperature for 10 min and rinsed with PBS, and nonmigrated cells were removed. Migrated cells were stained with propidium iodide (5 μg/ml) (Invitrogen) for 5 min and rinsed with PBS. Membranes were mounted onto a slide with SlowFade Antifade (Invitrogen). For each membrane (33.2 mm2), six random ×10 fields (one field = 1.37 mm × 1.08 mm, or 1.48 mm2) were photographed. Cells were counted and averaged for each membrane, and experiments were performed in triplicate. Values were given as means ± SE. Statistical comparisons were made using Student's t-test or ANOVA, followed by Tukey's post hoc test.
RESULTS
P2Y2 receptor knockdown reduces the nucleotide-induced Ca2+ response.
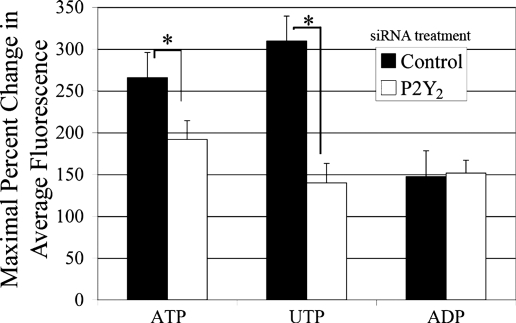
Previously, our laboratory showed that both primary corneal epithelial cells and corneal epithelial cell lines respond to ATP and UTP in an equivalent manner and desensitize the EGF receptor (EGFR)-induced response (19, 34). In the present experiments, a corneal cell line, HCLE, was transfected either with siRNA against P2Y2, the receptor that responds equally to ATP and UTP, or a nontargeting siRNA control to determine its role in nucleotide-induced calcium release. Cells were stimulated with agonists to P2Y receptors: ATP (100 μM), UTP (100 μM), or ADP (250 μM) (Fig. 1). A number of individual and pooled siRNA sequences against P2Y2 confirmed the specificity of the knockdown (data not shown). To detect responses to ADP, higher concentrations were used due to their higher EC50 (34). Cells stimulated with UTP or ATP showed a significant reduction in the intensity of the Ca2+ response on P2Y2 receptor knockdown (t-test; P < 0.01). The lesser inhibition of the ATP-induced response may be attributed to ATP being able to stimulate other receptors, including the P2X7, known to be present in corneal epithelium (22). Transfected cultures stimulated with ADP showed no reduction in Ca2+ response compared with control.
Fig. 1.
P2Y2-receptor downregulation inhibits nucleotide induced Ca2+ response. HCLE cells were incubated in 5 μM fluo 3-AM for 30 min and imaged in a flow-through apparatus on a Zeiss LSM 510 confocal microscope. Cells were washed in HEPES-buffered saline for at least 30 s to establish baseline fluorescence. P2Y2-receptor short interfering RNA (siRNA)-transfected cells were stimulated with ATP (100 μM), UTP (100 μM), or ADP (250 μM) and compared with cells transfected with a nontargeting control siRNA. Maximal percent change in average fluorescence and SE of the mean were calculated. Graphs represent a minimum of six independent experiments. Significance was determined by Student's t-test: *P < 0.01.
Endogenous expression of P2Y receptors.
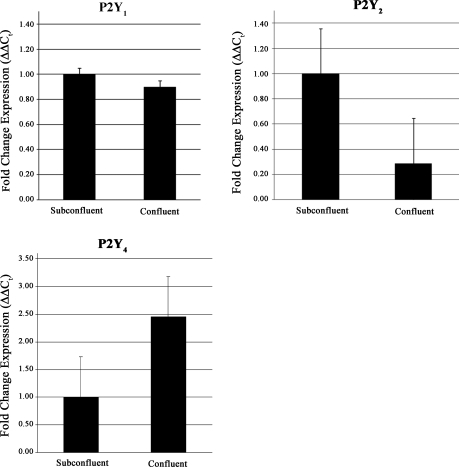
The ΔΔCt method was used to determine expression of P2Y1, P2Y2, and P2Y4 receptor mRNA from untransfected confluent and subconfluent cultures (30%). Values were normalized to 18S ribosomal subunit (Fig. 2). The subconfluent cultures were used to model the lower cell density that occurs after injury. The mean Ct for 18S for confluent and subconfluent cultures was 11.2 ± 0.4 and 11.5 ± 0.3 and for P2Y1 was 27.9 ± 0.3 and 27.8 ± 0.2, respectively. When the Ct values for the latter were normalized to 18S rRNA subunit and the ΔΔCt values calculated, there was no significant change between the two cell densities (t-test; P > 0.05) (Fig. 2). In contrast, the expression of P2Y2 receptor mRNA was significantly greater in the subconfluent cultures (t-test; P < 0.05). There was a decreased trend in the P2Y4 receptor in the subconfluent cultures, but it was not significant (t-test; P > 0.05) (Fig. 2).
Fig. 2.
Endogenous expression of P2Y receptors. Real-time PCR and the ΔΔCt (cycle threshold) method were used to determine expression of P2Y1, P2Y2, and P2Y4 receptors in epithelial cells. Data were normalized to the 18S ribosomal subunit and graphed as fold expression of confluency over subconfluency (30%) for each receptor. Each sample was run in triplicate on the same plate. Data are representative of a minimum of three independent experiments. Significance was determined by a Student's t-test.
Verification of knockdown in P2Y2- and P2Y4-receptor siRNA transfected cells was performed. The ΔΔCt method was used to determine relative expression of P2Y1-, P2Y2-, and P2Y4-receptor mRNA (Fig. 3). Ct was normalized to 18S rRNA, and data were presented as fold change expression compared with cells transfected with a nontargeting control siRNA (set to one; data not shown; see materials and methods). As expected, P2Y2- and P2Y4-receptor expression were decreased significantly (P < 0.05) on their respective siRNA transfection (Fig. 3, A and B). P2Y1 receptor expression was unaffected by transfection of either P2Y2 or P2Y4 siRNA oligonucleotides.
Role of P2Y2 and P2Y4 receptors in the Ca2+ response.
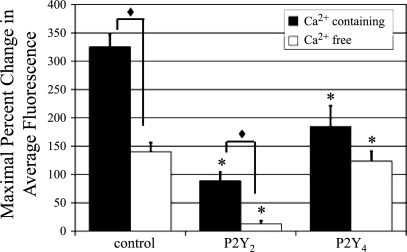
Since UTP can stimulate both P2Y4 and P2Y2 receptors, it was important to determine the contribution of the latter receptor to the Ca2+ response (Fig. 4, solid bars). Experiments were performed using siRNA to determine whether downregulating the P2Y4 receptor would reduce the UTP-induced Ca2+ mobilization (Fig. 4). As described above, UTP-stimulated cells transfected with P2Y2-receptor siRNA resulted in a significantly reduced response (73% less than control, *P < 0.01). In addition, cells transfected with P2Y4-receptor siRNA exhibited a significant reduction in Ca2+ release (fluorescence) compared with control cells (45% less than control, *P < 0.01).
Fig. 4.
P2Y2 and P2Y4 receptor downregulation decreases UTP-induced Ca2+ release. HCLE cells cultured on glass coverslips were incubated in 5 μM fluo 3-AM for 30 min and imaged in a flow-through apparatus on a Zeiss LSM 510 confocal microscope. Cells were washed in Ca2+-containing (solid bars) or Ca2+-free (open bars) HEPES-buffered saline for at least 30 s. P2Y2 and/or P2Y4 receptor siRNA transfected cells were stimulated with UTP (100 μM) and compared with cells transfected with a nontargeting control siRNA. Maximal percent change in average fluorescence and SE were calculated. Data are representative of at least three independent experiments. Significance of siRNA treated vs. control under the same Ca2+ condition was determined by a one-way ANOVA followed by Tukey's post hoc test: *P < 0.01. Significance of Ca2+ containing vs. Ca2+ free was determined by Student's t-test: ♦P < 0.001.
To remove the effects of extracellular Ca2+, a parallel set of experiments was performed in Ca2+-free HEPES buffer (Fig. 4, open bars). Under these conditions, the UTP response in P2Y2-receptor siRNA-transfected cells was reduced significantly by 90% compared with the control transfected cells (*P < 0.01, open bars). However, when cells transfected with P2Y4-receptor siRNA were incubated in Ca2+-free buffer and stimulated with UTP, the decrease was not significant (Fig. 4). To further explore the responses, comparisons between parallel transfections in Ca2+-containing and Ca2+-free media were made. Control and P2Y2-receptor siRNA-transfected cells elicited a significantly lower Ca2+ response to UTP in Ca2+-free media (solid diamond symbol, P < 0.001). The P2Y4 knockdown did not differ in Ca2+-containing compared with Ca2+-free conditions.
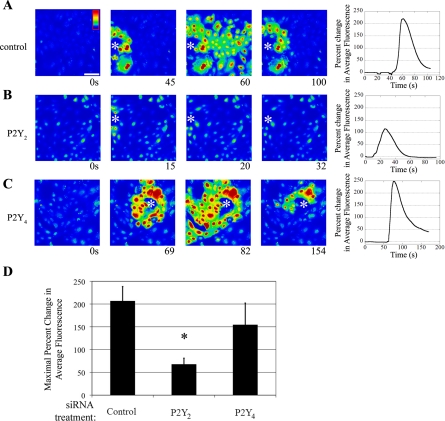
The injury-induced Ca2+ wave is dependent on P2Y2 activation.
While it is critical to examine the cellular response to specific agonists, we asked if the P2Y2 and P2Y4 receptors played a functional role in the injury response. To perform these experiments, cells were transfected, the cultures wounded, and the Ca2+ wave imaged. Representative time courses are shown (Fig. 5, A–C). In control cells, a typical Ca2+ response showed propagation away from the site of injury (Fig. 5A). Cells transfected with P2Y2-receptor siRNA (Fig. 5B) displayed a small increase in Ca2+, which was attributed to the cells adjacent to the wound. In addition, the distance that the wave propagated from the wound edge was negligible compared with the control (Fig. 5, A and B). Our laboratory has previously shown that the inflection by the cells at the wound edge (see asterisk; Fig. 5B) is due to an influx of Ca2+ from the extracellular space and not P2Y receptors (34). In contrast, cells transfected with P2Y4 receptor siRNA showed no reduction in the maximal percent change in fluorescence when experiments were performed in Ca2+-containing buffer (Fig. 5C). When the maximal percent change in fluorescence was calculated for multiple independent experiments, cells transfected with P2Y2-receptor siRNA showed a 45% reduction in fluorescence, which was significantly less than control [one-way ANOVA (P < 0.01), Fig. 5, A, B, and D], When cells were transfected with the P2Y4 receptor, the response is not attenuated. Together, the data indicate that communication to other cells after injury depended on the expression of the P2Y2 receptor.
Fig. 5.
P2Y2 receptor mediates in the injury-induced Ca2+ response in Ca2+-containing buffer. HCLE cells were incubated in 5 μM fluo 3-AM for 30 min and imaged in a flow-through apparatus on a Zeiss LSM 510 confocal microscope. Cells were washed in HEPES-buffered saline with Ca2+ for at least 30 s and stimulated by wounding. Intensity scale is shown in A, with red indicating highest Ca2+ levels and blue indicating lowest Ca2+ levels. The horizontal white bar in A represents 100 μm. A–C: cells were transfected with the indicated siRNA sequence. Cells were washed in HEPES buffer containing Ca2+ and wounded. A series of images taken from a time course of a representative experiment of a wound (shown at asterisk) is presented, and percent change in average fluorescence was graphed (right). D: maximal percent change in average fluorescence was calculated and averaged for multiple repeats of the experiment. Data are representative of at least nine independent experiments. Significance was determined by a one-way ANOVA: *P < 0.01.
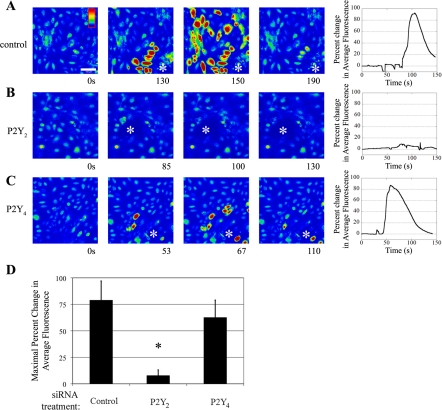
Parallel experiments were performed in Ca2+-free buffer to eliminate the inflection caused by the cells immediately adjacent to the wound edge, and representative time courses are shown (Fig. 6, A–C). In control cells, a typical Ca2+ wave moved away from the site of injury (Fig. 6A). However, in cells transfected with the P2Y2 receptor siRNA, there was no detectable response, as both the wave and the inflection at the wound edge were eliminated (Fig. 6B). In contrast, when cells were transfected with P2Y4 receptor siRNA, there was no reduction when experiments were performed in Ca2+-free buffer (Fig. 6C). When maximal percent change in fluorescence was calculated for multiple independent experiments, the injury-induced Ca2+ wave was reduced by 90% when P2Y2 receptor was downregulated, compared with control transfected cells. In comparison, when P2Y4 receptor was downregulated, the injury response is not attenuated, indicating that the expression of P2Y2 receptor is critical to an injury response (Fig. 6D).
Fig. 6.
P2Y2 receptor mediates the injury-induced Ca2+ response in Ca2+-free buffer. HCLE cells were incubated in 5 μM fluo 3-AM for 30 min and imaged in a flow-through apparatus on a Zeiss LSM 510 confocal microscope. Cells were washed in Ca2+-free HEPES-buffered saline for at least 30 s and stimulated by wounding. Intensity scale is shown in A, with red indicating highest Ca2+ levels and blue indicating lowest Ca2+ levels. The horizontal white bar in A represents 100 μm. A–C: cells were transfected with the indicated siRNA sequence. Cells were washed in Ca2+-free HEPES buffer and wounded. A series of images taken from a time course of a representative experiment of a wound (shown at asterisk) is presented, and percent change in average fluorescence was graphed (right). D: maximal percent change in average fluorescence was calculated and averaged for multiple repeats of the experiment. Data are representative of at least three independent experiments. Significance was determined by Student's t-test: *P < 0.05.
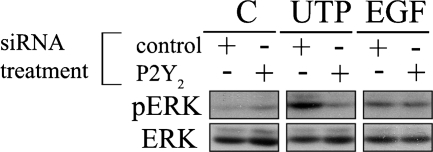
P2Y2-receptor signaling activates ERK.
Previously, our laboratory demonstrated that injury induced a rapid, yet transient, increase in phosphorylation of ERK that was inhibited by apyrase. In addition, we showed that phosphorylated ERK (pERK) immunolocalization was altered at the wound edge after injury and returned to baseline levels over time and distance from the wound (35). Furthermore, RB-2, a general purinergic inhibitor, inhibited phosphorylation of EGFR and ERK after injury or stimulation with nucleotides (8). In Fig. 7, cultures were exposed to one of the following: media change (C), 25 μM UTP, or 0.5 nM EGF. There was no difference in the cellular response to media change in either group. To determine the specific contribution of the P2Y2 receptor to ERK activation, cells were transfected with a siRNA sequence against the P2Y2 receptor or a nontargeting control (control) sequence, and real-time PCR verified downregulation. The P2Y2 receptor transcripts were reduced 80% in siRNA-transfected cells (data not shown). Cells transfected with a nontargeting control sequence and stimulated by UTP demonstrated an increase in ERK phosphorylation over the media change control. Phosphorylation of ERK was reduced when the P2Y2 receptor was downregulated compared with the control-transfected cells (Fig. 7). In contrast, the positive control for phosphorylation of ERK (EGF-stimulated cells) showed no change in phosphorylation when the P2Y2 receptor was knocked down. These indicate that nucleotide-induced phosphorylation of ERK depends on the expression of the P2Y2 receptor. In addition, when cells were preincubated with the MEK inhibitor (U0126), Src inhibitor (SU6656), or with the ERK inhibitor (PD98059), phosphorylation was not detected (data not shown).
Fig. 7.
Purinergic receptor activation induces ERK phosphorylation. Cells were cultured on p60 culture dishes and transfected with an siRNA sequence against P2Y2 or nontargeting control siRNA (control). Cells in both groups were stimulated for 5 min with a media change (C), UTP (25 μM), or EGF (0.5 nM). Lysates of equivalent protein concentration were resolved using SDS-PAGE and immunoblotted with antibodies directed against total and phosphorylated ERK (pERK). Blots are representative of three independent experiments. Real-time PCR was performed to verify knockdown of P2Y2 receptor mRNA (data not shown). Data are representative of a minimum of six independent experiments.
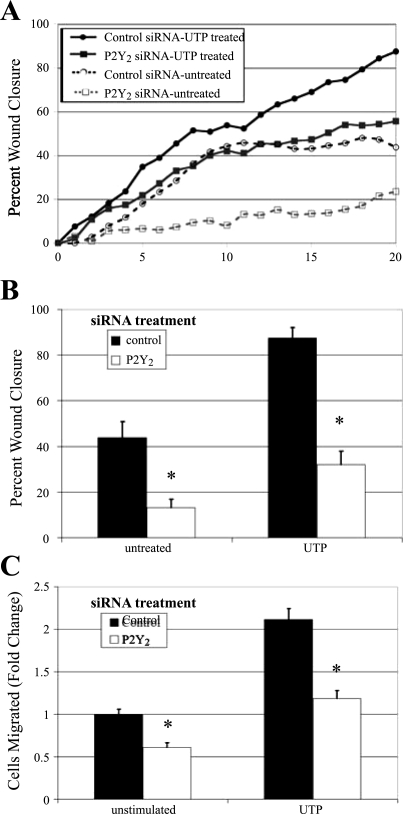
The P2Y2 receptor is necessary for cell migration.
Having demonstrated that the P2Y2 receptor plays a critical role in the early injury response, we asked if the receptor played an additional role in the long-term signals necessary for corneal epithelial wound repair. Previous work demonstrated that RB-2 inhibited cell migration in scratch wound assays (19). Thus cells were transfected with siRNA against P2Y2 receptor or a nontargeting control, and scratch wound assays were performed. To determine whether the receptor is required for directed cell migration, cells were stimulated with 100 μM UTP or control media, and wounds were made. Two contiguous images were taken along the wound margin to allow for analysis of a larger area. Images were collected every 20 min at each site for 20 h, and a representative time course for each condition is shown (Fig. 8A). Average percent wound closure was determined from multiple repeats of the experiment (Fig. 8B). Cells transfected with P2Y2 receptor siRNA and treated with media change showed significant reduction in migration rate and wound closure compared with those transfected with the nontargeting sequence and treated with media change (P < 0.01) (Fig. 8, A and B). When control cells were stimulated with UTP, the rate of migration was greater than all other conditions evaluated (Fig. 8A). Under these conditions, wound closure was enhanced significantly (87%, P < 0.01). In the UTP-treated P2Y2-transfected cells, the rate of migration was attenuated (Fig. 8A). Wound closure of the treated transfected cultures was significantly less than that of control (35%, P < 0.01).
Fig. 8.
P2Y2 receptor mediates cell migration. HCLE cells were transfected with siRNA against the P2Y2 receptor or a nontargeting control sequence. A: directed migration. Confluent cells in eight-well chamber slides were incubated overnight in media lacking growth factors. The media were replaced with either unsupplemented media or media containing 100 μM UTP immediately before wounding. Cells were placed on a heated microscope stage, wounded, and incubated in an environmental chamber at 37°C and 5% CO2 for 20 h. The wounds were demarcated, and contiguous regions were tiled and imaged every 20 min, and a representative run is graphed. B: percent wound closure from directed migration assays measured and averaged at 20 h. Data are representative of a minimum of three independent experiments. Significance was determined by a one-way ANOVA followed by Tukey's post hoc test: *P < 0.01. C: chemotactic migration. HCLEs were transfected with siRNA against the P2Y2 receptor or a nontargeting control sequence. Transwell migrations were performed for 8 h at 37°C with binding buffer alone (unstimulated) or binding buffer containing 1 μM UTP. Cells were stained with propidium iodide, counted in six randomly chosen fields (1.48 mm2), averaged, and normalized to unstimulated control, and SE of the mean was calculated. Data are representative of a minimum of three independent experiments. Significance was determined by Student's t-test: *P < 0.01.
Transwell migration assays were performed to determine whether the P2Y2 receptor was involved in chemotactic migration. Cells transfected with P2Y2-receptor siRNA were treated with binding buffer alone or 1 μM UTP prepared in binding buffer (Fig. 8C). P2Y2-receptor siRNA-transfected cells migrated significantly less (P < 0.01) in both treated and untreated conditions than control transfected cells. The migration assays indicate that the P2Y2 receptor plays a critical role in both directed and chemotactic migration.
Role of P2Y2 receptor on phosphorylation of Src and events following injury.
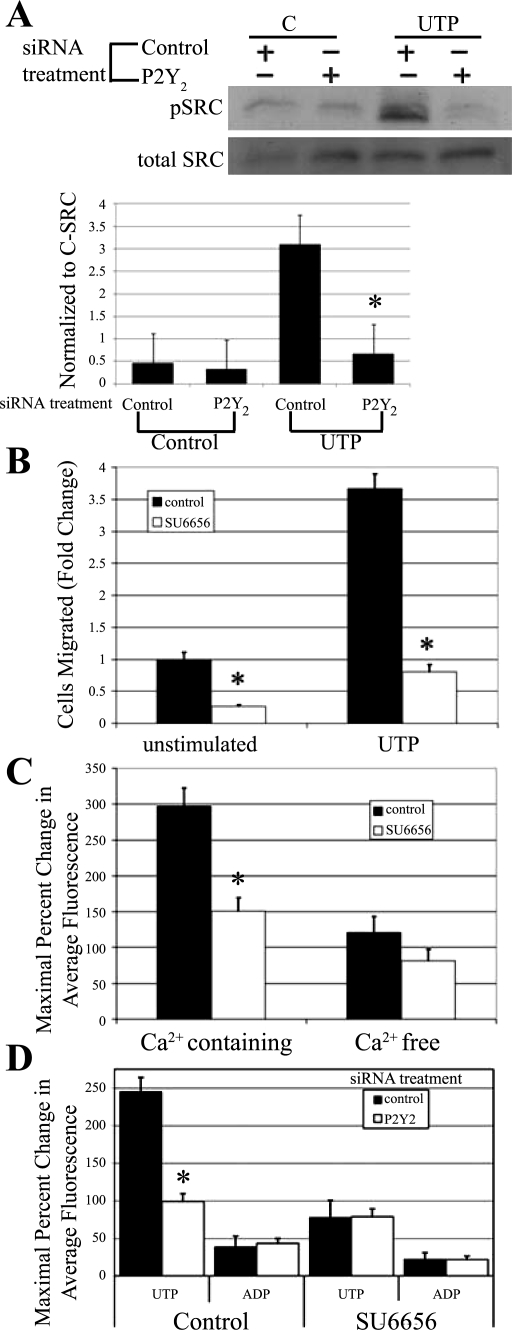
The role of Src was determined using a combination of approaches. Cells were transfected with siRNA to the P2Y2 receptor and incubated in the presence or absence of UTP or media change for 5 min and probed for phosphorylation of Src on Tyr418 and c-Src. There was a significant reduction in phosphorylation in cells transfected with siRNA to the P2Y2 receptor compared with cells that were transfected with siRNA to a nontargeting sequence (Fig. 9A). These indicate that the P2Y2 receptor can mediate partial activation of Src, as demonstrated by the phosphorylation at Tyr418. Experiments were performed to test the role of Src on migration using the inhibitor, SU6656 (13). Cells were preincubated for 30 min with SU6656 or DMSO, and, in addition, SU6656 or DMSO was present in the cell suspension before addition to the Transwell chamber. Cells were stimulated with 1 μM UTP prepared in binding buffer with inhibitor or binding buffer alone. Cells stimulated with UTP migrated more than those in binding buffer. When SU6656 was present in the cell suspension, cell migration was minimal in both unstimulated and UTP-stimulated conditions (P < 0.01) (Fig. 9B). Experiments with the inhibitor PP2 yielded similar results (data not shown).
Fig. 9.
Role of P2Y receptor regulation. A: HCLE cells were transfected with siRNA against the P2Y2 receptor or a nontargeting control sequence. Cells were incubated in the presence or absence of UTP for 5 min and immunoblotted with antibodies directed to pSrc and c-Src, and densitometric analysis was performed and normalized to c-Src. Control, nontargeting sequence; C, media change. Data are representative of a minimum of three independent experiments. Significance was determined by a Student's t-test: *P < 0.05. B: HCLE cells were incubated in the presence or absence of 10 μM Src kinase inhibitor SU6656 for the duration of the experiment. Transwell migrations were performed for 8 h at 37°C with binding buffer alone (unstimulated) or binding buffer containing 1 μM UTP. Cells were stained with propidium iodide, counted in six randomly chosen fields (1.46 mm2), averaged, and normalized to unstimulated control. Data are representative of a minimum of three independent experiments. Significance was determined by Student's t-test: *P < 0.01. C: HCLE cells were incubated in 5 μM fluo 3-AM in the presence or absence of 10 μM SU6656 for 30 min and imaged in a flow-through apparatus on a Zeiss LSM 510 confocal microscope. Cells were washed in either Ca2+-containing or Ca2+-free HEPES buffered saline ± 10 μM SU6656 for at least 30 s to obtain baseline. Cells were stimulated with UTP (100 μM), and maximal percent change in average fluorescence was calculated. Data are representative of a minimum of six independent experiments. Significance was determined by a Student's t-test: *P < 0.01. D: HCLE cells were transfected with siRNA against the P2Y2 receptor or a nontargeting control sequence. HCLE cells were incubated in 5 μM fluo 3-AM ± 10 μM SU6656 for 30 min and imaged in a flow-through apparatus on a Zeiss LSM 510 confocal microscope. Cells were washed in Ca2+-containing HEPES buffered saline ± 10 μM SU6656 for at least 30 s. Cells were stimulated with ADP (100 μM), washed, and stimulated with UTP (100 μM), and maximal percent change in response to each agonist in average fluorescence was calculated. Data are representative of a minimum of six independent experiments. Significance was determined by a Student's t-test: *P < 0.01.
Cells were then coincubated with SU6656 and fluo 3-AM for 30 min and stimulated with UTP prepared in Ca2+-containing HEPES-buffered saline. In the presence of SU6656, the maximal change in fluorescence on UTP stimulation was decreased by 65% compared with cells incubated with HEPES-buffered saline alone (P < 0.01) (Fig. 9C, solid bars). Parallel experiments were performed in Ca2+-free HEPES to remove the component contributed by the inflection immediately adjacent to the wound edge seen in Fig. 5 and described (34). When cells were stimulated with UTP in Ca2+-free HEPES buffer containing SU6656, there was a lesser reduction in the Ca2+ response (Fig. 9C).
To assess if the P2Y2 receptor played a role on the response, epithelial cells were transfected with siRNA against the P2Y2 receptor or a nontargeting siRNA sequence and incubated in fluo 3-AM. Cells transfected with the P2Y2 receptor and stimulated with 100 μM UTP showed a reduction in mobilization of Ca2+, as described previously. In contrast, when cells were transfected with siRNA to the P2Y2 receptor and incubated with SU6656 before stimulation with UTP, there was negligible additional reduction, indicating that the P2Y2 receptor-mediated response was no longer targeted by the inhibitor (Fig. 9D). We then demonstrated that responses induced by activation of other P2Y receptors did not alter the response. There was no significant difference in the ADP-induced Ca2+ increase with either P2Y2 receptor siRNA or with cells incubated with SU6656, indicating specificity of response (Fig. 9D).
DISCUSSION
The cornea is an excellent model system to examine receptors and signaling pathways that are required for proper wound closure. The cornea is avascular, and the epithelium is highly innervated and five to seven layers thick. We have shown that, when primary corneal epithelial cells are injured, ATP is released, and a Ca2+ wave propagates from the site of the wound to neighboring cells (18, 34). When wound media is added to cells, Ca2+ is released into the cytoplasm, which does not occur when the wound media is preincubated with apyrase (19, 35). Involvement of P2 receptors in wound healing has been suggested in both activation of signaling pathways and in wound closure (1, 19, 26, 35). The aim of this work was to determine which P2Y receptors were critical both for the injury-induced Ca2+ release and for downstream signals stimulated by P2Y receptor activation, such as cell migration and wound repair.
Previously, our laboratory showed that trinucleotide receptors were the main players in the injury-induced Ca2+ wave, and that only UTP and ATP resulted in desensitization of the EGF-induced Ca2+ wave (34). Furthermore, the P2Y-receptor-mediated transactivation of EGFR occurs in response to injury (8). Here we demonstrated that P2Y2 was the major receptor required for the propagation of the Ca2+ wave away from the site of injury. Downregulation of the P2Y2 receptor inhibited the UTP- and ATP-induced Ca2+ responses, but did not inhibit ADP-induced responses. In contrast, downregulation of the P2Y4 receptor inhibited the UTP-induced Ca2+ response, but did not reduce the maximum injury-induced Ca2+ increase. Neither did it alter the phosphorylation of EGFR (data not shown). These may reflect the greater concentration of ATP in the wound media and the agonist-receptor specificity profile (1).
Both ATP and UTP produce similar increases in intracellular Ca2+ in Ca2+-containing HEPES buffer (34). Upon P2Y2 receptor downregulation, the ATP- and UTP-induced Ca2+ responses were reduced significantly (Fig. 1). The difference in the ATP and UTP responses after transfection with P2Y2 receptor siRNA is likely due to the ability of ATP to activate a larger subset of receptors, including P2Y1, P2Y2, and P2X receptors, while UTP only activates P2Y2 and P2Y4 receptors (1). However, if ATP and UTP are activating different subsets of receptors, it is interesting that their dose-response curves, as well as their ability to desensitize the wound response, appear comparable (34).
Injury experiments indicate that the sources of Ca2+ utilized by receptors in the injury response may be influenced by the presence or absence of extracellular Ca2+ in the environment. The P2Y2 receptor knockdown eliminated the majority of the response; however, there was a slight inflection seen at the wound edge (Fig. 4). The cells closest to the site of injury appear to rely on extracellular sources of Ca2+ for the Ca2+ increase after wounding, as seen in Fig. 5. This supports our laboratory's previous data, where trinucleotides were unable to desensitize the Ca2+ response of cells closest to the wound (34). The cells closest to the wound margin may use P2X, stretch, or other receptors, or may be permeable to Ca2+ influx due to disruption of the membrane by injury. In addition, when cells were preincubated with BAPTA-AM, there was no detectable propagation of a Ca2+ wave (34). In other cell lines, there was a similar lack of detectable Ca2+ when cells were stimulated with thrombin in the presence of BAPTA-AM (7). Calcium has been shown to be required for formation of adhesion structures and motility. In epithelial cells, lamellipodial extensions were minimal in wounded cultures, and migration was not detected (data not shown). When adhesion assays were performed in the presence of BAPTA-AM, neither the release of Ca2+, nor the formation of lamellipodia was detected (32). The formation of adhesion structures, such as hemidesmosomes, depend on Ca2+ (31). Together, these indicate that both intracellular and extracellular Ca2+ stores are critical to immediate and long-term wound responses (37).
Our data indicate that the P2Y2 receptor is necessary for nucleotide-induced cell migration of the corneal epithelial cells. UTP has been shown to accelerate wound healing in epithelial debridment wounds, and the wound healing can be inhibited with purinergic antagonists, such as RB-2 (27). These confirmed the wound responses performed in vitro, demonstrating that RB-2 impaired migration in scratch wound assays (19). However, RB-2 is not specific for P2Y receptors, so the knockdown of P2Y2 receptor was necessary to demonstrate the specific role of P2Y2 receptor in migration (Fig. 8). Migration was also inhibited in astrocytes when they were transfected with P2Y2 receptor siRNA (33). Furthermore, when neutrophils were isolated from P2Y2 receptor knockout mice, there was minimal migration when cells were stimulated with the chemoattractant N-formyl-Met-Leu-Phe. This was in contrast to neutrophils isolated from wild-type mice, which, after stimulation, released ATP and migrated toward the stimulus (10). In addition, the receptor was shown to be pro-proliferative when neurons expressing reduced levels of P2Y2 receptor were less protected from nucleotide-induced apoptosis (3).
Previously, cholinergic agonists were shown to generate inositol 1,4,5-trisphosphate, which binds to receptors on the endoplasmic reticulum, releasing Ca2+ from intracellular stores (5). Others have shown in adhesion assays that inositol 1,4,5-trisphosphate enhanced release of Ca2+, and heparin inhibited the response (32). In yet another model system, carbachol was used to stimulate GPCRs, causing an increase in Ca2+ and MAPK through stimulation of Src, resulting in mucin secretion (17). Src family kinases have been shown to interact with the P2Y2 receptor (4). In addition, Src has been shown to bind the P2Y2 PXXP peptide (21). Interestingly, structure function studies have identified agonist-induced phosphorylation of COOH-terminus of the P2Y2 receptor (16). Increasing evidence indicates that GPCRs signal both dependently and independently of G proteins, and these data indicate that the concentration of agonist may provide a mechanism for this family of receptors to switch between dependent and independent signaling (23). In fact, the activation of Pyk2 through Ca2+ and formation of a Pyk2-Src complex has been considered an independent signaling module, linking increases in cytosolic Ca2+ to ERK (29). Our laboratory has shown that epithelial injury and/or trinucleotides cause an increase in Ca2+ and activation of pERK and migration (8, 19, 35), and we have now identified that the P2Y2 receptor played the critical role in these responses. We demonstrated that UTP caused the phosphorylation of Src on Tyr418, which decreased when cells are transfected with siRNA to the P2Y2 receptor. These indicate that the P2Y2 receptor can mediate partial activation of Src, as demonstrated by the phosphorylation at Tyr418. We also found that SU6656 reduced UTP-induced pERK (data not shown), cell migration, and changes in Ca2+. The latter results are surprising; however, when we transfected cells with siRNA to the P2Y2 receptor and then inhibited them with SU6656, there was no further inhibition, indicating that the response was specific to the receptor. SU6656 was used, as it is more specific and potent toward Src family kinases, unlike other Src inhibitors that inhibit other kinases, including PDGF receptor (6) and VEGFR2 (data not shown). These results have led us to question whether there are other potential interactions of Src and P2Y2 that need to be investigated in the future. In addition, there could be cell type-specific differences in response, as our results are complicated by the presence of the full repertoire of P2Y receptors in epithelial cells, while other elegant studies have been performed in cells lacking other P2Y receptors (21).
Our results demonstrate that epithelial cells respond rapidly to changes in the environment, and the P2Y receptors provide a coordinated response that mediates wound repair. The P2Y2 receptor has a critical role in the generation of the Ca2+ wave that occurs after injury, in the phosphorylation of Src and ERK, and in later events, such as cell migration that occurs to repair the wound. The P2Y4 receptor may have a modulatory role, which reflects the endogenous expression profile of the receptors. These mechanisms are thought to play a specific role in the maintenance of the avascular, but highly innervated, corneal epithelium and will hopefully lead to a further understanding of wound healing in the cornea and other epithelial tissues.
GRANTS
This work was supported by a grant from the National Institute of Health (EY06000, V. Trinkaus-Randall) and departmental grants from the Massachusetts Lions Eye Research Fund, and the New England Corneal Transplant Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Drs. Nader Rahimi, Darlene Dartt, and Matthew Nugent for suggestions, antibodies, and critical review of the manuscript.
Present addresses: I. Boucher, Renal Division, Department of Medicine, Brigham and Women's Hospital, Boston, MA 02115; M. Marcincin is a DO student at the University of New England.
REFERENCES
- 1.Abbracchio MP, Burnstock G. Purinergic signalling: pathophysiological roles. Jpn J Pharmacol 78: 113–145, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur DB, Georgi S, Akassoglou K, Insel PA. Inhibition of apoptosis by P2Y2 receptor activation: novel pathways for neuronal survival. J Neurosci 26: 3798–3804, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur DB, Akassoglou K, Insel PA. P2Y2 and TrkA receptors interact with Src family kinase for neuronal differentiation. Biochem Biophys Res Commun 347: 678–682, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Berridge MJ. Inositol trisphosphate and diacyglycerol: two interacting second messengers. Annu Rev Biochem 56: 159–193, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA. Su6656, a selective Src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol 23: 9018–9027, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobe R, Yin X, Roussanne MC, Stepien O, Polidano E, Faverdin C, Marche P. Evidence for ERK1/2 activation by thrombin that is independent of EGFR transactivation. Am J Physiol Heart Circ Physiol 285: H745–H754, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Boucher I, Yang L, Mayo C, Klepeis V, Trinkaus-Randall V. Injury and nucleotides induce phosphorylation of epidermal growth factor receptor: MMP and HB-EGF dependent pathway. Exp Eye Res 85: 130–141, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology 36: 1127–1139, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314: 1792–1795, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Cornell-Bell AH, Finkbeiner SH, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long range glial signaling. Science 247: 470–473, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Corriden R, Insel PA, Junger WG. A novel method using fluorescence microscopy for real-time assessment of ATP release from individual cells. Am J Physiol Cell Physiol 293: C1420–C1425, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Courtneidge SA. Role of Src in signal transduction pathways–the Jubilee Lecture. Biochem Soc Trans 2: 11–17, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Erb L, Liu J, Ockerhausen J, Kong Q, Garrad RC, Griffin K, Neal C, Krugh B, Santiago-Pérez LI, González FA, Gresham HD, Turner JT, Weisman GA. An RGD sequence in the P2Y2 receptor interacts with αVβ3 integrins and is required for Go-mediated signal transduction. J Cell Biol 153: 491–502, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gipson IK, Spurr-Michaud S, Argüeso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci 44: 2496–2506, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez FA, Weisman GA, Erb L, Seye CI, Sun GY, Velázquez B, Hernández-Pérez M, Chorna NE. Mechanisms for inhibition of P2 receptor signaling in neural cells. Mol Neurobiol 31: 65–79, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Kanno H, Hoikawa Y, Hodges RR, Zoukhri D, Shatos MA, Rios JD, Dartt DA. Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am J Physiol Cell Physiol 284: C988–C998, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Klepeis VE, Cornell-Bell A, Trinkaus-Randall V. Growth factors but not gap junctions play a role in injury-induced Ca2+ waves in epithelial cells. J Cell Sci 114: 4185–4195, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Klepeis VE, Weinger I, Kaczmarek E, Trinkaus-Randall V. P2Y receptors play a critical role in epithelial cell communication and migration. J Cell Biochem 93: 1115–1133, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Koizumi S, Fujishita K, Inoue K, Shigemoto-Mogami Y, Tsuda M, Inoue K. Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem J 380: 329–338, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Liao Z, Camden J, Griffin KD, Garrad RC, Santiago-Pérez LI, González FA, Seye CI, Weisman GA, Erb L. Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem 279: 8212–8218, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Mayo C, Ren R, Rich C, Stepp MA, Trinkaus-Randall V. Regulation by P2X7: epithelial migration and stromal organization in the cornea. Invest Ophthalmol Vis Sci 49: 4384–4391, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGarrigle D, Huang X-Y. GPCRs signaling directly through Src-family kinases. Sci Stke 392: 35, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Mediero A, Peral A, Pintor J. Dual roles of diadenosine polyphosphates in corneal epithelial cell migration. Invest Ophthalmol Vis Sci 10: 4500–4506, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Neary JT, Kang Y, Bu Y, Yu E, Akong K, Peters CM. Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway. J Neurosci 19: 4211–4220, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neary JT, Kang Y, Willoughby KA, Ellis EF. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci 23: 2348–2356, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pintor J, Bautista A, Carracedo G, Peral A. UTP and diadenosine tetraphosphate accelerate wound healing in the rabbit cornea. Ophthalmic Physiol Opt 24: 186–193, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 29.Schauwienold D, Perez Sastre A, Genzel N, Schaefer M, Reusch HP. The transactivated epidermal growth factor receptor recruits Pyk2 to regulate Src kinase activity. J Biol Chem 283: 27748–27756, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Soltoff SP, Avraham H, Avraham S, Cantley LC. Activation of P2Y2 receptors by UTP and ATP stimulates mitogen-activated kinase activity through a pathway that involves related adhesion focal tyrosine kinase and protein kinase C. J Biol Chem 273: 2653–2660, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Trinkaus-Randall V, Gipson IK. Role of calcium and calmodulin in hemidesmosome formation in vitro. J Cell Biol 98: 1565–1571, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trinkaus-Randall V, Kewalramani R, Payne J, Cornell-Bell A. Calcium signaling induced by adhesion mediates protein tyrosine phosphorylation and is independent of pHi. J Cell Physiol 184: 385–399, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Wang M, Kong Q, Gonzalez FA, Sun G, Erb L, Seye C, Weisman GA. P2Y nucleotide receptor interaction with alpha integrin mediates astrocyte migration. J Neurochem 95: 630–640, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Weinger I, Klepeis V, Trinkaus-Randall V. Tri-nucleotide receptors play a critical role in epithelial cell wound repair. Purinergic Signal 1: 281–292, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L, Cranson D, Trinkaus-Randall V. Cellular injury induces activation of MAPK via P2Y receptors. J Cell Biochem 91: 938–950, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Yu N, Erb L, Shivaji R, Weisman GA, Seye CI. Binding of the P2Y2 nucleotide receptor to filamin A regulates migration of vascular smooth muscle cells. Circ Res 102: 581–588, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Z, Walczysko P, Zhao M. Intracellular Ca2+ stores are essential for injury induced Ca2+ signaling and re-endothelialization. J Cell Physiol 214: 595–603, 2008 [DOI] [PubMed] [Google Scholar]