Abstract
Glutathione transport into mitochondria is mediated by oxoglutarate (OGC) and dicarboxylate carrier (DIC) in the kidney and liver. However, transport mechanisms in brain mitochondria are unknown. We found that both carriers were expressed in the brain. Using cortical mitochondria incubated with physiological levels of glutathione, we found that butylmalonate, a DIC inhibitor, reduced mitochondrial glutathione to levels similar to those seen in mitochondria incubated without extramitochondrial glutathione (59% of control). In contrast, phenylsuccinate, an OGC inhibitor, had no effect (97% of control). Additional experiments with DIC and OGC short hairpin RNA in neuronal-like PC12 cells resulted in similar findings. Significantly, DIC inhibition resulted in increased reactive oxygen species (ROS) content in and H2O2 release from mitochondria. It also led to decreased membrane potential, increased basal respiration rates, and decreased phosphorus-to-oxygen (P/O) ratios, especially when electron transport was initiated from complex I. Accordingly, we found that DIC inhibition impaired complex I activity, but not those for complexes II and III. This impairment was not associated with dislodgment of complex subunits. These results suggest that DIC is the main glutathione transporter in cortical mitochondria and that DIC-mediated glutathione transport is essential for these mitochondria to maintain ROS homeostasis and normal respiratory functions.
Keywords: oxidative stress, electron transport chain, complex I, oxoglutarate carrier
glutathione (GSH), the most abundant non-protein thiol in mammalian cells, is essential for reactive oxygen species (ROS) homeostasis and mitochondrial function. ROS are produced in mitochondria as obligatory by-products of cellular respiration. While electrons from energy substrates are passed along the respiratory chain to establish the proton gradient, which in turn drives the synthesis of ATP from ADP, leakage of electrons often occurs at complexes I and III, leading to the formation of superoxide (O2·−) through one-electron reduction of oxygen (18). O2·− is usually readily converted to hydrogen peroxide (H2O2) by manganese superoxide dismutase (MnSOD) in the matrix or copper/zinc SOD (Cu/ZnSOD) in the intermembrane space (18, 47). Although H2O2 is further reduced to water by peroxiredoxins, glutathione peroxidase, or, in some organs, catalase (2), it may also react with reduced transition metal ions, such as Fe2+, to form the hydroxyl radical (·OH), especially when there is an imbalance between its production and the capacity of antioxidants that reduce it as specified above. ·OH is a highly reactive radical that causes peroxidation of all cellular constituents, including lipids, proteins, and nucleic acids, and is believed to be mainly responsible for oxidative stress-induced cellular damage (38). Importantly, both peroxiredoxins and glutathione peroxidase, the main antioxidant mechanisms that reduce H2O2, depend on GSH for reducing equivalent and in turn their H2O2-reducing capabilities. In addition, GSH is also essential in glutathione S-transferase-mediated antioxidant effects (34). GSH is therefore a key element in the overall antioxidant defense (15).
Consequently, GSH depletion or oxidation is a major determinant of pathological cellular processes, such as excessive oxidative stress (19), mitochondrial transition pore opening (4), respiratory complex inhibition (21), and apoptosis (5, 15). Interestingly, the brain has been believed to be particularly vulnerable to oxidative stress-induced damage presumably because of the dependency of neurons on oxidative phosphorylation for energy supply, which is accompanied by production of large amounts of ROS (18). Perhaps more importantly, however, is the fact that the brain has considerably less catalase activity in comparison with other major organs, including the liver, the kidney, the lung, and the heart (20). Moreover, parts of the brain contain high levels of Fe2+, which favors the formation of ·OH through the Fenton reaction (18). Thus, it is possible that GSH plays an even more significant role in the maintenance of ROS homeostasis in the brain. Indeed, a deficit in cellular GSH has been implicated in the pathogenesis of several neurological disorders such as Parkinson's disease (10, 42), schizophrenia (13), stroke (18), and trauma (3).
Cellular GSH exists as two separate pools in the mitochondrial and the cytosolic compartments (24). Recent evidence suggests that the mitochondrial pool of GSH (mGSH) is more critical than cytosolic GSH for the survival of neuronal cells under various pathological conditions. For instance, selective depletion of mGSH has been observed during ischemia and reperfusion (1, 44), while ROS overload and neuronal degeneration occur only when mGSH levels fall below 60% of control levels in neurons subjected to GSH synthesis inhibition (51). Furthermore, mGSH, rather than cytosolic GSH, has been found to determine amyloid-β peptide susceptibility in human neuronal and glial cell lines (14) and a decrease in mGSH has been shown to result in selective inhibition of complex I that precedes dopaminergic cell death in Parkinson's disease (23).
Because GSH synthesis activities are not found in mitochondria (16), transport of GSH from the cytosol into the mitochondrial matrix is believed to be the sole mechanism that sustains the mGSH. This process was thought to be mediated by organic anion transporters located in the inner mitochondrial membrane (IMM) since GSH was negatively charged (8, 35). Recent studies have confirmed that out of the eight protein carriers in this category that are known to reside in the IMM, the dicarboxylate carrier (slc25a10, DIC) and the oxoglutarate carrier (slc25a11, OGC) possess GSH transport capabilities and act as main mitochondrial GSH transporters in renal and hepatic mitochondria (8, 9, 11). In particular, DIC and OGC together account for >80% and at least 50% of the total GSH transport across the mitochondrial membrane in rat renal and hepatic mitochondria, respectively (8, 54). Despite the expanding body of evidence that suggests an essential role of the mGSH in the brain, however, mechanisms underlying mitochondrial GSH transport in this important organ remain unclear. A recent study suggests that Bcl-2 may carry GSH to the mitochondrial membrane and the intermembrane space through direct binding of GSH to its BH3 groove (55). Whether Bcl-2 transports GSH into mitochondria is nevertheless uncertain, given the outer membrane localization of this protein (22, 37). Accordingly, the purpose of the current study was to investigate the mechanisms of GSH transport into mitochondria and their relevance to mitochondrial function in the rat brain. We hypothesized that DIC and/or OGC would be important to the integrity of the mGSH in the rat brain and that inhibition of their carrier activities would lead to functional impairment of brain mitochondria.
MATERIALS AND METHODS
Reagents.
Unless otherwise specified, all reagents were purchased from Sigma Chemical (St. Louis, MO).
Animals.
Male Sprague-Dawley rats (Charles River, Portage, MI) and C57BL/6 mice (Taconic Farms, Germantown, NY) were used in this study. All procedures were approved by the Animal Care and Use Committee of the University of Louisville.
Primary cell cultures.
Primary cultures of cortical neurons were obtained from 16- to 17-day-old fetal C57BL/6 mice following a previously described method (7). Primary glial cultures were prepared from the cortex of 12- to 24-h-old neonatal C57BL/6 mice as previously described (45).
Isolation of rat cortical mitochondria.
Rat cortical mitochondria were isolated using gradient and differential centrifugations, according to the method of Sims (43). The isolation buffer contained 10 mM HEPES (pH 7.5), 200 mM mannitol, 70 mM sucrose, and 1 mM EGTA. BSA (2 mg/ml) was added to this buffer prior to homogenization (isolation buffer A). Mitochondria isolated using this method had high purity, since >95% of particles were positively stained with MitoTracker Green (Molecular Probes, Eugene, OR).
Cell culture and gene silencing.
PC12 cells were grown in a humidified incubator (5% CO2, 37°C), in growth medium supplemented with l-glutamine, 25 mM HEPES, and 10% fetal bovine serum. They were transfected with SureSilencing short hairpin (sh)RNA plasmids (SABioscience, Frederick, MD) using the Amaxa Nucleofector (Amaxa, Cologne, Germany). These shRNAs were designed to target rat DIC (5′-CCACTTCCTCTCCAGTTTCAT-3′) and OGC (5′-GGAAGGGCTTCACACCATACT-3′), respectively, under the control of the U1 promoter. The shRNA plasmid with a scrambled sequence (5′-GGAATCTATTCGATGCATAC-3′) was used as a negative control.
Preparation of mitochondria from PC12 cells.
Cell pellet was collected 72 h after transfection, washed twice with cold PBS, and then spun down at 800 g (3 min, 4°C). The packed cell volume was determined and five volumes of the hypotonic buffer [10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, and 0.2 mM phenylmethylsulfonyl fluoride (PMSF)] were added. Cells were pelleted, incubated, and washed with the hypotonic buffer for several rounds and were then homogenized in 800 μl of isolation buffer A with 30 strokes of pestle B (Kontes Glass, Vineland, NJ). The homogenate was centrifuged at 1,000 g (4°C, 5 min). The mitochondrial pellet was obtained by spinning the supernatant at 10,000 g (4°C, 10 min), and then was washed twice with the isolation buffer.
Primary antibodies for immunoblotting.
The following antibodies were used: polyclonal rabbit anti-mouse DIC (Affinity Bioreagents, Golden, CO), polyclonal rabbit anti-mouse OGC (Abcam, Cambridge, MA), monoclonal mouse anti-bovine complexes I, II, and III (BD Biosciences, Franklin Parks, NJ), monoclonal mouse anti-pigeon cytochrome c (BD Biosciences), monoclonal mouse anti-mouse neuronal nuclear antigen (NeuN; Chemicon International, Temecula, CA) and polyclonal rabbit anti-bovine glial fibrillary acidic protein (GFAP; Dako, Carpinteria, CA).
Assessment of GSH transport.
Transport assays were carried out as previously reported (8, 54), with slight modifications. In brief, mitochondria from the cortex or from PC12 cells (0.5 mg/ml) were incubated in the base mitochondrial buffer (20 mM triethanolamine-HCl [pH 7.4], 225 mM sucrose, 3 mM potassium phosphate [pH 7.4], 5 mM MgCl2, 20 mM KCl, and 0.1 mM PMSF) with various additions (detailed in figure legends) in 1.5 ml microfuge tubes at 20°C. α-Glycerol phosphate (α-GP, 7 mM) was added to all tubes as the respiratory substrate. After incubation for 30 min or 1 h, microfuge tubes were centrifuged at 18,000 g for 30 s. The supernatant was removed and the pellet was washed twice with an equal volume of ice-cold phosphate-free buffer. Potential artifacts, including loss of GSH during sample processing and contamination of matrix GSH by inner membrane-bound GSH, were minimized by using the centrifugation-resuspension method.
GSH measurement.
This procedure takes advantage of the reaction of naphthalene-2,3-dicarboxaldehyde (NDA) with GSH (but not GSSG) that forms cyclized products that are highly fluorescent (39, 50). Mitochondrial samples from the GSH transport assay were resuspended in 5% sulfosalicylic acid, subjected to three freeze/thaw cycles, and then centrifuged at 10,000 g for 10 min. The supernatant was transferred to empty tubes, and 20-μl aliquots were transferred in triplicates to a 96-well plate. NDA derivatization solution (180 μl) [50 mM Tris (pH 10), 0.5 N NaOH, and 10 mM NDA in DMSO, vol/vol/vol = 1.4/0.2/0.2] was added to all wells. The plate was covered from lights and incubated at room temperature for 25 min. NDA-GSH fluorescence intensity was measured (472-nm excitation and 528-nm emission) using a Wallac Victor3 microplate reader (Perkin Elmer LAS, Waltham, MA). GSH levels were calculated using standard curves for GSH.
Mitochondrial ROS measurements.
Following the transport assays, mitochondrial suspensions were incubated at 30°C with 5 μM dihydrorhodamine-123 (DHR-123) (Molecular Probes) and either 20 mM succinate or 10/5 mM glutamate/malate. After 20 min, DHR-123 fluorescence intensity was evaluated with a FACSAria flow cytometer (BD Biosciences). Kinetic release of H2O2 in the assay medium was detected using the Amplex Red fluorescent dye (Molecular Probes). The assay medium contained 125 mM KCl, 20 mM HEPES, 2 mM K2HPO4, 1 mM MgCl2, 0.1 mM EGTA, pH 7.0 (KOH), 5 U/ml horseradish peroxidase, and 1 μm Amplex Red reagent. Mitochondrial samples were added to this solution in a 96-well plate, and H2O2 formation was initiated by adding either 20 mM succinate or 10/5 mM glutamate/malate. Fluorescence was detected at 30°C in the Wallac Victor3 microplate reader.
Detection of mitochondrial membrane potential.
After transport assays, mitochondrial samples were incubated with 100 nM tetramethylrhodamine methyl ester (TMRM, Molecular Probes) for 20 min at 30°C, and red fluorescence was measured by flow cytometry.
Measurement of mitochondrial respiration rates.
Oxygen consumption was measured with a Clark-type electrode (Strathkelvin Instruments, Glasgow, UK) at 30°C. State 4 respiration was determined in the presence of either 5/2.5 glutamate/malate or 10 mM succinate. State 3 was initiated by adding 18 nmol ADP.
Electron transport chain enzyme activities.
Complexes I (rotenone-sensitive NADH-ubiquinone oxidoreductase) and II (succinate ubiquinone oxidoreductase) activities were determined according to Birch-Machin and Turnbull (6). Complex III (antimycin A-sensitive ubiquinol-cytochrome c reductase) specific activity was measured by following the reduction of cytochrome c (III) at 550 nm (ϵ = 18.5 mM/cm) (6, 32). Decylubiquinol was freshly prepared by reduction of decylubiquinone with crystals of sodium borohydride. Mitochondria (20 μg) were preincubated (3 min at 30°C) in a reaction medium containing 0.25 M Tris·HCl (pH 7.4), 2 mM KCN, 0.01% BSA, 1 mM EDTA, 0.6 mM n-dodecyl β-d-maltoside, and 2 μg/ml rotenone; 15 μM cytochrome c was added and the baseline was recorded for 50 s. The reaction was initiated by adding decylubiquinol to a final concentration of 50 μM, and reduction of cytochrome c (III) was measured for 300 s with and without antimycin A.
Statistical analyses.
Comparisons between groups were performed using Student's t-tests with the SigmaStat software (Systat Software, San Jose, CA). P < 0.05 was considered statistically significant as indicated in individual figures.
RESULTS
Expression of DIC and OGC proteins in cortical mitochondria.
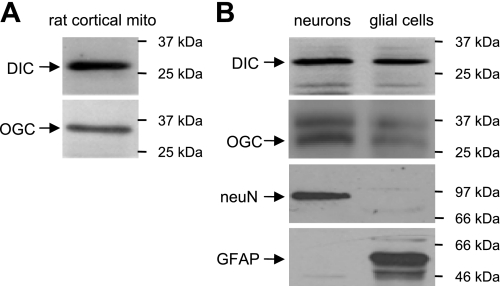
Using Western blot analysis, we found that both DIC and OGC were expressed in rat cortical mitochondria (Fig. 1A). These two transporters were also expressed at comparable levels in primary cultures of cortical neurons and cortical glial cells (Fig. 1B). The band at ≈37 kDa on the OGC blot in Fig. 1B was probably a nonspecific band derived from nonmitochondrial compartments, because it disappeared in blots using mitochondrial protein preparations, as shown in Fig. 1A.
Fig. 1.
Dicarboxylate carrier (DIC) and oxoglutarate carrier (OGC) protein expression patterns. Protein samples from rat cortical mitochondria (A) and mouse neurons and glial cells (B) were assayed for DIC and OGC expression. The neuronal nuclear antigen (NeuN) was used as a neuronal marker, and the glial fibrillary acidic protein (GFAP) was used as a glial marker. Shown are representative Western blots.
DIC inhibition depletes mGSH.
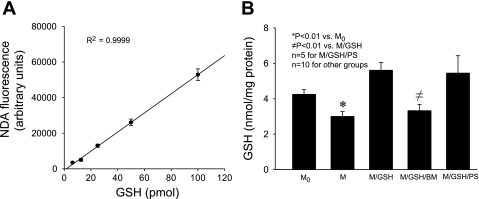
GSH transport assays were performed using high-quality mitochondria that had minimal contamination from synaptosomes and myelin and routinely exhibited respiratory control ratio (RCR) > 4. mGSH content was measured with the NDA method, which included an independent standard curve for each experiment and was shown to be linear and highly reproducible within a large range in previous publications (50, 52) and in this study (Fig. 2A). We found that a brief incubation of these mitochondria without extramitochondrial GSH (M) resulted in a considerable reduction in mGSH levels when compared with those freshly isolated (M0) (mGSH, 3.00 ± 0.42 and 4.25 ± 0.35 nmol/mg protein in M and M0, respectively) (Fig. 2B). Addition of GSH to the incubation buffer (2.5 mM, M/GSH), which mimicked the presence of a cytosolic GSH pool at physiological levels (12), maintained the normal level of mGSH after the same incubation through presumably a transport mechanism(s) across the IMM. Interestingly, this transport process was greatly diminished in the presence of butylmalonate (M/GSH/BM), a selective DIC inhibitor (mGSH, 3.33 ± 0.47 and 5.61 ± 0.54 nmol/mg protein in M/GSH/BM and M/GSH, respectively) (Fig. 2B). In contrast, phenylsuccinate (M/GSH/PS), an OGC inhibitor, at a molar ratio similar to what was shown to effectively inhibit OGC-mediated GSH transport in renal mitochondria (8), had no effect (mGSH, 5.45 ± 1.53 and 5.61 ± 0.54 nmol/mg protein in M/GSH/PS and M/GSH, respectively) (Fig. 2B). These results indicate that rat cortical mitochondria rely on GSH transport across the IMM to maintain their mGSH levels and that DIC appears to be the main GSH transporter in these mitochondria under normal conditions.
Fig. 2.
Glutathione (GSH) transport through DIC is essential for the maintenance of the mitochondrial (m)GSH pool. A: standard curve for naphthalene-2,3-dicarboxaldehyde (NDA)-GSH fluorescence assay, showing linearity and consistency. Data are means ± SE; n = 11. B: rat cortical mitochondria were incubated at 20°C for 30 min in 4 different conditions: M, buffer alone; M/GSH, buffer containing 2.5 mM GSH; M/GSH/BM, buffer containing 2.5 mM GSH and 7 mM butylmalonate; M/GSH/PS, buffer containing 2.5 mM GSH and 7 mM phenylsuccinate. M0 is freshly isolated mitochondria. mGSH content decreased in M in comparison with M0, indicating the importance of GSH transport across the inner mitochondrial membrane (IMM) even under totally unchallenged conditions. Furthermore, mGSH content decreased in M/GSH/BM but not in M/GSH/PS in comparison with M/GSH, indicating that DIC was responsible for GSH transport across the IMM in these mitochondria. Data are means ± SE.
Inhibition of GSH transport increases oxidative stress in rat cortical mitochondria.
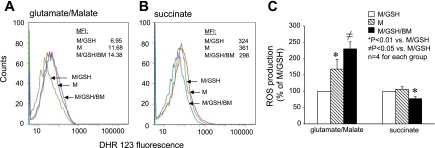
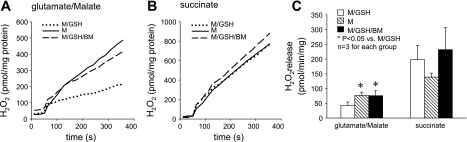
To assess the role of DIC-mediated GSH transport in maintaining mitochondrial ROS homeostasis, we examined intramitochondrial ROS levels under various conditions, using flow cytometry. We found that in comparison with those incubated with GSH (M/GSH), mitochondria incubated without GSH (M) or with both GSH and the DIC inhibitor (M/GSH/BM) had substantially higher levels of intramitochondrial ROS when complex I substrates (glutamate + malate) were used as the electron donor (≈ 168% and 229% of M/GSH, respectively) (Fig. 3, A and C). In contrast, no increase in ROS levels was detected when complex II substrate (succinate) was used (Fig. 3, B and C). We also examined kinetic release of H2O2 from these mitochondria. We found that, in line with intramitochondrial ROS levels, rates of H2O2 release from mitochondria incubated without GSH (M) and from those incubated with GSH and the DIC inhibitor (M/GSH/BM) were significantly higher than that from mitochondria incubated with GSH (M/GSH) when complex I substrates (glutamate + malate) were used (213.4 ± 68.3% and 192.2 ± 34.2% of M/GSH, respectively) (Fig. 4, A and C). Again, no increase in the rate of H2O2 release was detected when complex II substrate (succinate) was used (Fig. 4, B and C). These results indicate that DIC-mediated GSH transport and an intact mGSH pool are crucial for ROS homeostasis in rat cortical mitochondria. Inhibition of DIC-mediated GSH transport increases mitochondrial oxidative stress, especially when electron transport is initiated from complex I.
Fig. 3.
Inhibition of DIC-mediated GSH transport increases oxidative stress in rat cortical mitochondria. A: FACS analysis of reactive oxygen species (ROS) content in cortical mitochondria using glutamate/malate as the respiration substrate. DHR-123, dihydrorhodamine-123; MFI, median fluorescence intensity. B: FACS analysis of ROS content in cortical mitochondria using succinate as the respiration substrate. C: summary of intramitochondrial ROS levels in 4 individual experiments. Increased ROS levels were evident in the M and M/GSH/BM groups only when complex I substrates glutamate/malate were used to support respiration. Data are means ± SE.
Fig. 4.
Inhibition of DIC-mediated GSH transport increases H2O2 release by rat cortical mitochondria. A: kinetic release of H2O2 by cortical mitochondria using glutamate/malate as the respiration substrate. B: kinetic release of H2O2 by cortical mitochondria using succinate as the respiration substrate. C: summary of H2O2 release in 3 individual experiments. Rates of H2O2 release were significantly higher in the M and M/GSH/BM groups only when complex I substrates glutamate/malate were used to support respiration. With succinate, H2O2 release was similar in all groups. Data are means ± SE.
Inhibition of GSH transport damages IMM function.
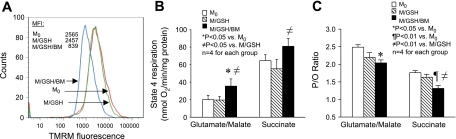
We further examined mitochondrial respiration under various conditions to determine the biological relevance of DIC-mediated GSH transport to fundamental mitochondrial functions. We found that mitochondria incubated with GSH (M/GSH) maintained their mitochondrial membrane potential (MMP) as compared with those freshly isolated; however, mitochondria incubated with GSH and the DIC inhibitor (M/GSH/BM) had drastically decreased MMP (Fig. 5A). Furthermore, we found that inhibition of DIC-mediated GSH transport with butylmalonate significantly increased the basal rate of mitochondrial oxygen consumption, as shown by the increase in state 4 respiration rate in the M/GSH/BM group (Fig. 5B). Similar to the increase in mitochondrial oxidative stress, state 4 respiration rate increased more prominently when complex I substrates were used as the electron donor (Fig. 5B). State 3 respiration rate also increased but to a lesser percentage, resulting in a slightly lower RCR in these mitochondria (data not shown). In line with these signs of IMM injury, inhibition of DIC-mediated GSH transport also decreased oxidative phosphorylation efficiency in rat cortical mitochondria, because the phosphorus-to-oxygen (P/O) ratio was significantly lower in the M/GSH/BM group in comparison with that of freshly isolated mitochondria (M0) regardless of the electron donors used (≈ 81% and 74% of M0 with complex I and II substrates, respectively) (Fig. 5C). These findings appear to indicate that mitochondrial respiration is critically dependent on the presence of a normal mGSH pool that is maintained through DIC-mediated GSH transport across the mitochondrial membrane.
Fig. 5.
Inhibition of DIC-mediated GSH transport alters IMM functions. A: membrane potential was drastically reduced in M/GSH/BM in comparison with either M0 or M/GSH, indicating severe impairment with DIC inhibition. Shown is a representative experiment. TMRM, tetramethylrhodamine methyl ester. B: state 4 respiration rates increased in the M/GSH/BM group, especially when complex I substrates were used, indicating increased oxygen consumption under basal conditions with DIC inhibition. Data are means ± SE. C: phosphorus-to-oxygen (P/O) ratio values decreased in the M/GSH/BM group regardless of the respiration substrate used, indicating a reduced efficiency in oxidative-phosphorylation in these mitochondria with DIC inhibition. Data are means ± SE.
Inhibition of GSH transport impairs complex I activity.
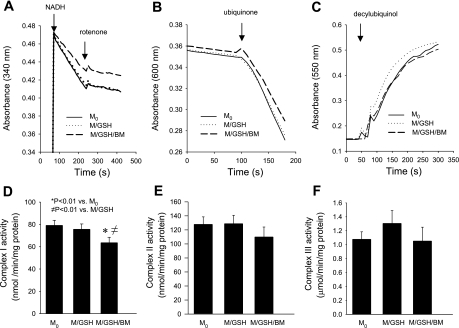
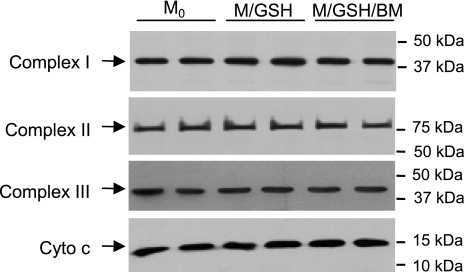
Because disturbance to mitochondrial ROS homeostasis and damage to IMM functions following inhibition of DIC-mediated GSH transport seemed to occur consistently when electron transfer was initiated from complex I, we wondered whether this important IMM component was particularly vulnerable to mGSH depletion. We found that the most basic complex I function, the NADH-ubiquinone oxidoreductase enzymatic activity, was intact in mitochondria incubated with GSH (M/GSH). However, it was significantly impaired when the DIC inhibitor butylmalonate was added to block GSH transport across the mitochondrial membrane, as shown by a ≈17% decrease in the rotenone-sensitive NADH-ubiquinone oxidoreductase activity in the M/GSH/BM group, in comparison with freshly isolated mitochondria (M0) (Fig. 6, A and D). On the contrary, neither complex II functionality, as reflected by succinate-ubiquinone oxidoreductase activity (Fig. 6, B and E), nor complex III functionality, as reflected by ubiquinol-cytochrome c reductase activity (Fig. 6, C and F), was significantly altered by inhibition of DIC-mediated GSH transport. Interestingly, DIC inhibition-induced impairment in complex I functionality did not appear to be associated with dislodgment of complex subunits from the IMM, as suggested by unaltered levels of the 39-kDa subunit protein in mitochondria incubated with various conditions (Fig. 7). Complexes II, III, and cytochrome c also appeared intact (Fig. 7). Thus, inhibition of GSH transport damages mitochondrial respiration through preferential impairment of complex I functionality, which involves a mechanism other than complex protein loss from the IMM.
Fig. 6.
Inhibition of DIC-mediated GSH transport impairs complex I activity A–C: representative activity assays for complexes I, II, and III, respectively. A: decreased consumption of NADH in M/GSH/BM, indicating a reduced complex I enzymatic activity with DIC inhibition. B: similar rates in 2,6-dichlorophenol indophenol (DCIP) consumption in all three samples, indicating no difference in complex II activity. C: similar rates in accumulation of reduced cytochrome c, indicating no difference in complex III activity. D–F: summaries of complexes I, II, and III activities in multiple experiments. Only complex I activity was significantly impaired in the M/GSH/BM group in which DIC was inhibited. Data are means ± SE; n = 8 for complexes I and II and n = 5 for complex III.
Fig. 7.
Inhibition of DIC-mediated GSH transport does not alter complexes I, II, and III integrity. Protein levels of representative subunits of complexes I, II, and III and cytochrome c (Cyto c) were similar in all three groups, indicating a lack of protein dislodgement from the IMM with DIC inhibition. Equal protein loading in each lane was verified by Ponceau staining. Shown are representative Western blots from 4 experiments.
RNA interference-mediated silencing of DIC decreases mGSH.
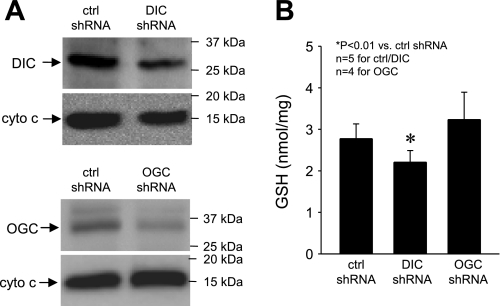
To confirm the specificity of the pharmacological approach, we studied cross-mitochondrial GSH transport in PC12 cells, a neuronal-like cell line, following DIC or OGC gene silencing using an shRNA approach. Expression of DIC and OGC proteins in these cells was significantly decreased 72 h after transfection with a plasmid encoding a DIC shRNA (40–60% of control DIC levels) or an OGC shRNA (20–50% of control OGC levels) (Fig. 8A). Mitochondria derived from transfected cells were then assayed for GSH transport the same way as described for cortical mitochondria. We found that silencing of DIC diminished the GSH transport process (mGSH, 2.21 ± 0.28 and 2.77 ± 0.36 nmol/mg protein in DIC and control shRNA, respectively; P < 0.01) (Fig. 8B). In contrast, silencing of OGC had no effect (mGSH, 3.23 ± 0.66 and 2.77 ± 0.36 nmol/mg protein in OGC and control shRNA, respectively) (Fig. 8B). These results seem to support the finding that DIC, but not OGC, is involved in GSH transport in mitochondria of neuronal cells under normal conditions.
Fig. 8.
DIC gene silencing decreases mGSH in PC12 cells. A: decreased DIC and OGC protein expression in PC12 cells following gene silencing with short hairpin (sh)RNA. Shown are representative Western blots from 4–5 experiments. Cytochrome c was used as a loading control. B: mitochondria from PC12 cells were incubated at 20°C for 30 min in buffer containing 2.5 mM GSH. mGSH content decreased in the DIC-shRNA group but was unaffected in the OGC-shRNA group, indicating that DIC was responsible for GSH transport in these mitochondria. These results are in line with those obtained from experiments using pharmacological agents. Data are means ± SE.
DISCUSSION
The present study demonstrates that GSH transport across the IMM is important in maintaining the mGSH pool in rat cortical mitochondria and that the mitochondrial dicarboxylate carrier (DIC) accounts for the majority of this transport activity under normal conditions, because inhibition of DIC reduces mGSH to a level similar to that seen in mitochondria incubated without extramitochondrial GSH. In contrast, the oxoglutarate carrier (OGC), which has been shown to be important for GSH transport in renal and hepatic mitochondria, does not play a significant role under the same conditions. This notion is further supported by the fact that silencing of DIC, but not OGC, results in an reduction of the mGSH pool in cultured PC12 cells. Involvement of other anion transporters, such as the tricarboxylate carrier (CIC), is unclear but seems doubtable. CIC was reported to be capable of transporting GSH across the IMM in rat brain mitochondria when facilitated by high levels of intramitochondrial malate (49), an exchange substrate for CIC (29). However, the actual amount of GSH transported into brain mitochondria through this carrier must be less significant under normal conditions, where intramitochondrial malate is at a much lower level (41). Additionally, the fact that CIC-mediated GSH transport activity was inhibited by citrate or isocitrate at a substrate-to-inhibitor concentration ratio of 5:1 (49), which was similar to the ratio of these substances in normal cells (38, 41), would suggest that CIC plays, at most, a minor role in transporting GSH in brain mitochondria. Of note, CIC was shown previously to play no role in GSH transport in renal cortical mitochondria (9).
Our finding that the dominant GSH transporter in cortical mitochondria is different from those in renal and hepatic mitochondria (8, 54) supports the assessment by Lash (30) that diverse mechanisms might be involved in GSH transport into mitochondria in various organs. While it is unknown at present whether other carriers are involved in this important function in other organs/cells and what determines the preferential usage of a particular carrier or carrier set in a particular organ/cell type, the concept of organ/cell-specific GSH transporter itself is significant in several aspects. For example, although the importance of mGSH in antioxidant defense is probably common in all organs/cells, mechanisms maintaining mGSH in different organs/cell types may need to be individually defined. Consequently, therapies targeting mGSH and GSH transport in a particular organ/cell type may need to be individually devised and tested.
Given the well-established role of mGSH in multiple aspects of mitochondrial antioxidant defense (27) and the lack of GSH-synthetic machinery inside mitochondria (16), it was surprising that the significance of GSH-transporting mechanisms and the consequence of their deficiencies in the brain had never been studied before. The current study therefore represents the first attempt in addressing these fundamental issues. It seems that the mGSH pool in cortical mitochondria is a small reserve in comparison with its turnover, since a significant portion (≈30–35%) of it was consumed within 30 min under an unchallenged condition as seen in mitochondria incubated without extramitochondrial GSH at a mild temperature (20°C). Accordingly, maintenance of mGSH in these mitochondria is critically dependent on active GSH transport across the IMM, because inhibition of DIC, the major GSH transporter in cortical mitochondria, for a short period of time resulted in depletion of the mGSH pool, disturbance of ROS homeostasis, and impairment of respiratory functions in these otherwise unchallenged mitochondria. The high sensitivity of cortical mitochondria to DIC inhibition suggests that DIC-mediated GSH transport is an essential part of and perhaps a major rate-limiting component in the entire mitochondrial antioxidant defense in the brain. Additional results from DIC silencing experiments using shRNA in PC12 cells are in line with this notion (Fig. 8).
There are a few interesting issues from this study that warrant additional discussion. The first is the location(s) in these cortical mitochondria where increased ROS production occurs after DIC inhibition. ROS are known to be produced at complex I and complex III through the forward electron flow when electron transport is initiated from complex I (17). It can also be produced at a separate location in complex I at the reverse electron flow site and at complex III through the forward electron flow when electron transport is initiated from complex II (31). The fact that increased ROS production after DIC inhibition occurred only when respiration was supported by complex I but not complex II substrate seemed to suggest that complex I, especially its iron-sulfur centers, might be damaged, which led to increased electron leakage. This notion would also be supported by additional results showing a more pronounced increase in state 4 oxygen consumption with complex I substrate (Fig. 5B) and significant impairment of complex I enzymatic activities (Fig. 6, A and D) in these mitochondria. However, potential damage in complex I at the reverse electron flow site cannot be ruled out at this time despite the fact that there lacks evidence showing increased ROS production through the reverse electron flow. Unlike ROS production through the forward electron flow that is for the most part independent of variations in membrane potential, ROS production at complex I through the reverse electron flow is highly sensitive to membrane potential (48). Even a small depolarization has been shown to significantly decrease ROS production in succinate-supported mitochondria (48). It is thus possible that significant damage exists at the reverse electron flow site in complex I, but production of ROS from this location is suppressed by the severe collapse of membrane potential in these mitochondria (Fig. 5A). Additional studies need to be performed to further clarify this interesting issue and to eventually map the sites of structural damage.
The second interesting issue that warrants additional discussion is the relationship between increased oxidative stress and impairment of respiratory functions after DIC inhibition. It seems only logical to postulate that increased mitochondrial oxidative stress associated with mGSH depletion leads to impairment of respiratory functions. However, the fact that the impairment of respiratory functions observed in our study was more severe than the increase in ROS production made one wonder whether this dogma was correct. Indeed, significant damage to respiratory functions occurred in cortical mitochondria following DIC inhibition. For instance, complex I enzymatic activities decreased by 17%. While such a number may appear small, it is important to note that complex I activities are reduced by 25–40% among the most severe Parkinson's disease cases in several postmortem studies (25, 53). Another example was MMP, which decreased by >65%. Intriguingly, both of these changes, which were not proportional to the increase in ROS production, could be ascribed to direct effects of intramitochondrial GSH redox status. In particular, recent studies have shown that thiol redox status of complex I is critical for its proper functioning and that depletion of mGSH results in selective inactivation of this large complex, likely through modifications of thiol residues of its subunit proteins (23, 33). Other studies have revealed that impaired GSH redox status is correlated with increased membrane permeability, leading to membrane potential collapse (36, 40). Thus, based on these considerations, we have formed a new paradigm that defines complex I and other protein components of the IMM as primary targets of altered GSH redox status following DIC inhibition. Modifications of thiol residues may reduce protein-protein and protein-substrate interactions and may cause disassembly and altered conformation of large complex proteins, but not severe enough to cause protein dislodgement from the IMM. These pathological changes of the IMM decrease its normal functionality and increase electron leakage that, combined with a compromised mitochondrial antioxidant defense secondary to mGSH depletion, lead to increased oxidative stress as seen in cortical mitochondria after DIC inhibition. It is expected that increased oxidative stress will in turn attack the IMM, accelerating its damage. Obviously, details of this paradigm need to be worked out in future studies.
The third issue that merits mentioning is the use of α-GP as the electron donor for mitochondria during GSH transport assays. To assess GSH transport in coupled mitochondria, it was necessary that a respiratory substrate be included in the assay buffer. However, commonly used substrates such as succinate and malate are dicarboxylates and their addition to the assay buffer would have interfered with the assessment of GSH transport. For this reason, we decided to use α-GP. This respiratory substrate does not require an IMM transporter, because it is oxidized to dihydroxyacetone phosphate by glycerol-3-phosphate dehydrogenase located on the outer surface of the IMM. FADH2 produced by this reaction donates its electrons to the respiratory chain through coenzyme Q (26). RCR values of brain and liver mitochondria with α-GP as the electron donor were relatively low (2.7–3.0) (28, 46). However, α-GP appeared to be sufficient as a respiratory substrate, because it maintained normal membrane potential and supported ROS production in these mitochondria (28, 46). Results from rat cortical mitochondria in the current study were similar to those reported in the literature.
In conclusion, DIC is the main GSH transporter in rat cortical mitochondria. Furthermore, DIC-mediated GSH transport is essential for these mitochondria to maintain ROS homeostasis and normal respiratory functions.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R01-HL-074369.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Anderson MF, Sims NR. The effects of focal ischemia and reperfusion on the glutathione content of mitochondria from rat brain subregions. J Neurochem 81: 541–549, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Andreyev A, Kushnareva Y, Starkov A. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70: 200–214, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med 45: 443–452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aon MA, Cortassa S, Maack C, O'Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem 282: 21889–21900, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong JS, Jones DP. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J 16: 1263–1265, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Birch-Machin MA, Turnbull DM. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol 65: 97–117, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J Neurosci Methods 71: 143–155, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Lash LH. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J Pharmacol Exp Ther 285: 608–618, 1998 [PubMed] [Google Scholar]
- 9.Chen Z, Putt DA, Lash LH. Enrichment and functional reconstitution of glutathione transport activity from rabbit kidney mitochondria: further evidence for the role of the dicarboxylate and 2-oxoglutarate carriers in mitochondrial glutathione transport. Arch Biochem Biophys 373: 193–202, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Chinta SJ, Andersen JK. Redox imbalance in Parkinson's disease. Biochim Biophys Acta 1780: 1362–1367, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coll O, Colell A, Garcia-Ruiz C, Kaplowitz N, Fernandez-Checa JC. Sensitivity of the 2-oxoglutarate carrier to alcohol intake contributes to mitochondrial glutathione depletion. Hepatology 38: 692–702, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Cooper AJL. Glutathione in the brain: disorders of glutathione metabolism. In: The Molecular and Genetic Basis of Neurological Disease, edited by Rosenberg RN, Prusiner SB, DiMauro S, Barchi RL, Kunk LM. Boston, MA: Butterworth-Heinemann, 1997, p. 1195–1230 [Google Scholar]
- 13.Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, schizophrenia. Curr Opin Neurobiol 19: 220–230, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Fernandez A, Llacuna L, Fernandez-Checa JC, Colell A. Mitochondrial cholesterol loading exacerbates amyloid beta peptide-induced inflammation and neurotoxicity. J Neurosci 29: 6394–6405, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franco R, Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ 16: 1303–1314, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Griffith OW, Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci USA 82: 4668–4672, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gyulkhandanyan AV, Pennefather PS. Shift in the localization of sites of hydrogen peroxide production in brain mitochondria by mitochondrial stress. J Neurochem 90: 405–421, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem 97: 1634–1658, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Han D, Canali R, Rettori D, Kaplowitz N. Effect of glutathione depletion on sites and topology of superoxide and hydrogen peroxide production in mitochondria. Mol Pharmacol 64: 1136–1144, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Ho YS, Xiong Y, Ma W, Spector A, Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem 279: 32804–32812, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Hsu M, Srinivas B, Kumar J, Subramanian R, Andersen J. Glutathione depletion resulting in selective mitochondrial complex I inhibition in dopaminergic cells is via an NO-mediated pathway not involving peroxynitrite: implications for Parkinson's disease. J Neurochem 92: 1091–1103, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Janiak F, Leber B, Andrews DW. Assembly of Bcl-2 into microsomal and outer mitochondrial membranes. J Biol Chem 269: 9842–9849, 1994 [PubMed] [Google Scholar]
- 23.Jha N, Jurma O, Lalli G, Liu Y, Pettus EH, Greenamyre JT, Liu RM, Forman HJ, Andersen JK. Glutathione depletion in PC12 results in selective inhibition of mitochondrial complex I activity. Implications for Parkinson's disease. J Biol Chem 275: 26096–26101, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Jocelyn PC, Kamminga A. The non-protein thiol of rat liver mitochondria. Biochim Biophys Acta 343: 356–362, 1974 [DOI] [PubMed] [Google Scholar]
- 25.Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr Parkinson's disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci 26: 5256–5264, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klingenberg M, Buecher T. [Glycerin-1-phosphate and flight muscle mitochondria]. Biochem Z 334: 1–17, 1961 [PubMed] [Google Scholar]
- 27.Kulinsky VI, Kolesnichenko LS. Mitochondrial glutathione. Biochemistry (Mosc) 72: 698–701, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Kunz W, Gellerich FN, Schild L. Contribution to control of mitochondrial oxidative phosphorylation by supplement of reducing equivalents. Biochem Med Metab Biol 52: 65–75, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Lash LH. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chem Biol Interact 163: 54–67, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lash LH. Renal glutathione transport: identification of carriers, physiological functions, and controversies. Biofactors 35: 500–508, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 80: 780–787, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Luo C, Long J, Liu J. An improved spectrophotometric method for a more specific and accurate assay of mitochondrial complex III activity. Clin Chim Acta 395: 38–41, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Marchetti P, Decaudin D, Macho A, Zamzami N, Hirsch T, Susin SA, Kroemer G. Redox regulation of apoptosis: impact of thiol oxidation status on mitochondrial function. Eur J Immunol 27: 289–296, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Mari M, Morales A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal 11: 2685–2700, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martensson J, Lai JC, Meister A. High-affinity transport of glutathione is part of a multicomponent system essential for mitochondrial function. Proc Natl Acad Sci USA 87: 7185–7189, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naoi M, Maruyama W, Yi H, Inaba K, Akao Y, Shamoto-Nagai M. Mitochondria in neurodegenerative disorders: regulation of the redox state and death signaling leading to neuronal death and survival. J Neural Transm 116: 1371–1381, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Nguyen M, Millar DG, Yong VW, Korsmeyer SJ, Shore GC. Targeting of Bcl-2 to the mitochondrial outer membrane by a COOH-terminal signal anchor sequence. J Biol Chem 268: 25265–25268, 1993 [PubMed] [Google Scholar]
- 38.Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, Floyd RA. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci USA 87: 5144–5147, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orwar O, Fishman HA, Ziv NE, Scheller RH, Zare RN. Use of 2,3-naphthalenedicarboxaldehyde derivatization for single-cell analysis of glutathione by capillary electrophoresis and histochemical localization by fluorescence microscopy. Anal Chem 67: 4261–4268, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Petronilli V, Costantini P, Scorrano L, Colonna R, Passamonti S, Bernardi P. The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J Biol Chem 269: 16638–16642, 1994 [PubMed] [Google Scholar]
- 41.Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J 9: 651–658, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol 36: 348–355, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem 55: 698–707, 1990 [DOI] [PubMed] [Google Scholar]
- 44.Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int 40: 511–526, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Swanson RA, Farrell K, Stein BA. Astrocyte energetics, function, and death under conditions of incomplete ischemia: a mechanism of glial death in the penumbra. Glia 21: 142–153, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Tretter L, Takacs K, Hegedus V, Adam-Vizi V. Characteristics of alpha-glycerophosphate-evoked H2O2 generation in brain mitochondria. J Neurochem 100: 650–663, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Votyakova TV, Reynolds IJ. DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem 79: 266–277, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Wadey AL, Muyderman H, Kwek PT, Sims NR. Mitochondrial glutathione uptake: characterization in isolated brain mitochondria and astrocytes in culture. J Neurochem 109, Suppl 1: 101–108, 2009 [DOI] [PubMed] [Google Scholar]
- 50.White CC, Viernes H, Krejsa CM, Botta D, Kavanagh TJ. Fluorescence-based microtiter plate assay for glutamate-cysteine ligase activity. Anal Biochem 318: 175–180, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Wullner U, Seyfried J, Groscurth P, Beinroth S, Winter S, Gleichmann M, Heneka M, Loschmann P, Schulz JB, Weller M, Klockgether T. Glutathione depletion and neuronal cell death: the role of reactive oxygen intermediates and mitochondrial function. Brain Res 826: 53–62, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Yan CC, Huxtable RJ. Fluorimetric determination of monobromobimane and o-phthalaldehyde adducts of gamma-glutamylcysteine and glutathione: application to assay of gamma-glutamylcysteinyl synthetase activity and glutathione concentration in liver. J Chromatogr B Biomed Appl 672: 217–224, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Yasutoshi Koga IN, Masanori Kobayashi Megumu Tojyo Kenji Nihei. Findings in muscle in complex I (NADH coenzyme Q reductase) deficiency. Ann Neurol 24: 749–756, 1988 [DOI] [PubMed] [Google Scholar]
- 54.Zhong Q, Putt DA, Xu F, Lash LH. Hepatic mitochondrial transport of glutathione: studies in isolated rat liver mitochondria and H4IIE rat hepatoma cells. Arch Biochem Biophys 474: 119–127, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmermann AK, Loucks FA, Schroeder EK, Bouchard RJ, Tyler KL, Linseman DA. Glutathione binding to the Bcl-2 homology-3 domain groove: a molecular basis for Bcl-2 antioxidant function at mitochondria. J Biol Chem 282: 29296–29304, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]