Abstract
An important step in carcinoma progression is loss of cell-cell adhesion leading to increased invasion and metastasis. We show here that the protein tyrosine phosphatase, PTP-PEST, is a critical regulator of cell-cell junction integrity and epithelial cell motility. Using colon carcinoma cells, we show that the expression level of PTP-PEST regulates cell motility. Either transient small interfering RNA or stable short hairpin RNA knockdown of PTP-PEST enhances haptotactic and chemotactic migration of KM12C colon carcinoma cells. Furthermore, KM12C cells with stably knocked down PTP-PEST exhibit a mesenchymal-like phenotype with prominent membrane ruffles and lamellae. In contrast, ectopic expression of PTP-PEST in KM20 or DLD-1 cells, which lack detectable endogenous PTP-PEST expression, suppresses haptotactic migration. Importantly, we find that PTP-PEST localizes in adherens junctions. Concomitant with enhanced motility, stable knockdown of PTP-PEST causes a disruption of cell-cell junctions. These effects are due to a defect in junctional assembly and not to a loss of E-cadherin expression. Adherens junction assembly is impaired following calcium switch in KM12C cells with stably knocked down PTP-PEST and is accompanied by an increase in the activity of Rac1 and a suppression of RhoA activity in response to cadherin engagement. Taken together, these results suggest that PTP-PEST functions as a suppressor of epithelial cell motility by controlling Rho GTPase activity and the assembly of adherens junctions.
Keywords: colon carcinoma, E-cadherin, motility, phosphatase
invasion and metastasis are a major hallmark of cancer (17). One significant enabling factor in the development of an invasive phenotype is the dysregulation of E-cadherin. In a normal epithelium, E-cadherin mediates cell-cell adhesion and plays a structural role to limit motility through physical linkage to the actin cytoskeleton, via catenins (15). Cadherins also transmit signals to the actin cytoskeleton through regulation of the small GTPases, Rac1 and RhoA. Homophilic engagement of E-cadherin activates Rac1 to promote junctional assembly while RhoA activation promotes stable adhesion (6, 48). During carcinoma progression, loss of E-cadherin, which can occur through a variety of mechanisms, disrupts adherens junctions and is associated with the epithelial to mesenchymal transition and enhanced cell motility (43). E-cadherin expression can be suppressed by transcriptional repressors, such as snail, or by promoter hypermethylation (29, 33). Alternately, E-cadherin can be functionally downregulated at the protein level. For example, oncogenic tyrosine kinases (4, 7, 12, 18, 32, 35) or ras transformation (19) elevates tyrosine phosphorylation of catenins and other targets. These alterations can result in disassembly of cell-cell contacts through changes in E-cadherin trafficking or turnover, dissociation of catenins from E-cadherin, or loss of E-cadherin adhesive function (47).
An important consequence is dysregulation of Rho GTPases which destabilizes adherens junctions and contributes to increased cell motility. Indeed, perturbed activities of Rac1 and RhoA have been associated with loss of cell-cell junctions (21, 46) and increased invasion and metastasis (35). These effects are attributed to an interplay between tyrosine phosphorylation events and Rho GTPase activity at cell-cell contacts. Activation of c-src, c-abl/arg, or focal adhesion kinase (FAK) has been reported to directly phosphorylate catenins or upstream regulators of Rho GTPases to modulate their activity (5, 23, 44). However, the factors attenuating these signaling events are not well defined.
The protein tyrosine phosphatase, PTP-PEST, is a ubiquitously expressed protein tyrosine phosphatase that plays a role in cell motility, cytokinesis, and apoptosis (2, 11, 13, 16, 30, 38). In fibroblasts, PTP-PEST acts downstream of integrins and receptor tyrosine kinases (8, 10, 22) to regulate motility through its action on Rho GTPases (36, 37). Excess levels of PTP-PEST suppress Rac1 activity while decreased PTP-PEST levels elevate Rac1 and block RhoA activation (36, 37). Importantly, PTP-PEST acts, either directly or indirectly, on several tyrosine kinases including c-src, c-abl, and FAK, whose activities contribute to regulation of cell-cell junctions and Rho GTPases (3, 9, 31, 49, 50). Although the precise function of PTP-PEST in epithelial cells has not been determined, recent studies implicate PTP-PEST in the control of intestinal (42) and pancreatic cancer cell motility (39) through c-src or c-abl-dependent pathways, respectively. Further studies indicate that PTP-PEST expression or activity may be altered in carcinoma cells (24, 41). In DLD-1 colorectal carcinoma cells, point mutations in PTP-PEST result in a frameshift and loss of functional protein (24). Additional point mutations in PTP-PEST that alter its catalytic activity have been identified in breast, kidney, and squamous cancer cells (41). Taken together, these studies suggest that altered expression or activity of PTP-PEST may contribute to cell motility, tyrosine kinase signaling, and regulation of Rho GTPase activity in carcinoma cells.
In this study, using colon carcinoma cells as a model, we investigated the function of PTP-PEST in carcinoma cell motility. We utilized small interfering (si)RNA/short hairpin (sh)RNA ablation and gain of function experiments to demonstrate that PTP-PEST is a suppressor of colon carcinoma cell motility. Furthermore, we show that PTP-PEST localizes to adherens junctions. Decreased PTP-PEST expression results in a mesenchymal-like phenotype, disrupted adherens junctions, and enhanced motility. Finally, PTP-PEST ablation impairs adherens junction assembly and alters Rho GTPase activity in response to cadherin engagement. Together, these findings indicate that PTP-PEST expression is required to control adherens junction assembly and E-cadherin signaling to Rho GTPases, which, in turn, restricts motility of colon carcinoma cells.
MATERIALS AND METHODS
Cell culture, antibodies, and reagents.
IEC-6 rat intestinal epithelial cells were grown in DMEM + 5% FBS and 0.1 mg/ml insulin (Sigma) and maintained in 10% CO2. IEC-6 cells were a generous gift of Dr. Randy Mifflin [Dept. of Internal Medicine, Univ. of Texas Medical Branch (UTMB)]. KM12C, KML4A, and KM20 cells were grown in MEM, supplemented with 10% FBS, 1 mM sodium pyruvate, 1% nonessential amino acids, and 2% MEM essential vitamin mixture (Sigma) (42, 43). DLD-1 cells were maintained in DMEM + 5% FBS. Caco-2 cells were grown in MEM containing 10% FBS and 1% nonessential amino acids. Clone A cells were grown in RPMI + 10% FBS. Rat tail collagen I was purchased from BD Biosciences. Recombinant hepatocyte growth factor (HGF) was purchased from Peprotech. Lysophosphatidic acid (LPA) was purchased from Sigma. Anti-PTP-PEST monoclonal antibody AG-25 was purchased from Calbiochem or Cell Signaling. E-cadherin and β-catenin antibodies were purchased from Sigma. siRNA smart pools were purchased from Dharmacon. The pSUPER shRNA expression vector was a generous gift of Dr. Binhua Zhou (Dept. of Pharmacology, UTMB). The enhanced green fluorescent protein (EGFP)-N17Rac1 and EGFP-L63RhoA plasmids were a generous gift of Dr. R. Worthylake (Louisiana State University, New Orleans, LA).
Immunofluorescence.
For immunofluorescence staining, KM12C cells with or without shRNA treatment were plated on collagen-coated coverslips in growth medium. Cells were fixed in 3.7% formaldehyde, permeabilized in 0.5% Triton X-100, and then costained for E-cadherin (mouse anti-E-cadherin) and β-catenin [rabbit anti-β-catenin (C2206)] or costained for PTP-PEST (AG25 MAb) and β-catenin. Alexa-488-anti-mouse or Alexa-594-anti-rabbit was used as a secondary antibody (Molecular Probes). F-actin was stained with Alexa-594-phalloidin (Molecular Probes). Cells were viewed and imaged on a Nikon Eclipse TE-2000U inverted microscope equipped with a cooled charge-coupled device camera (Roper Scientific) and Metamorph imaging software (Universal Imaging) or on a Zeiss LSM510 confocal microscope at the Optical Imaging Laboratory at UTMB.
siRNA and transfection.
Smart pool RNA interference (RNAi) for human or rat PTP-PEST was purchased from Dharmacon. Smart pool siRNA (200 nM) was introduced into cells by electroporation; 4–6 × 106 cells were resuspended in 400 μl of serum-free medium. Smart pool siRNA (20 μM final concentration) was added and incubated for 10 min. A nontargeting siRNA was used as a negative control. Electroporation was carried out at 250 mV with a capacitance of 500 μF. Following a 10-min recovery, cells were allowed to recover for 48 h after electroporation in growth medium before lysis or functional analysis. All transfections of plasmid vectors were performed using Lipofectamine Plus as described previously (36). Cell motility and protein expression were assayed 24–48 h following transfection. For generation of stable shRNA-transfected KM12C cells, specific oligonucleotides 5′-GGCAATTCCTCAGATATCA-3′ and 5′-GTCCATTGCTAGACATAAT-3′ [Src homology 3 (SH3) or SH4] from the human PTP-PEST cDNA sequence were designed, annealed with the corresponding reverse oligos, and cloned into pSUPER.retro.puro vector using BglII and HindIII-specific enzyme sites. A nonsilencing sequence containing a single point mutation 5′-GGCAATTCCCCAGATATCA-3′ and 5′-GTCCATTGCCAGACATAAT-3′ was used as a control (C3 or C4). All constructs were verified by restriction analysis and DNA sequencing. The pSUPER.retro.puro plasmids were transfected into KM12C cells and selected in culture medium containing 1 μg/ml of puromycin. Cells were routinely maintained in growth medium supplemented with 1 μg/ml puromycin. After selection, total protein was extracted and lysates were submitted to Western blot analysis to determine extent of knockdown. The resulting cell lines are referred to as C3, C4, SH3, or SH4.
Cell motility assays.
Migration assays were carried out as described previously (36, 37). Briefly, haptotactic migration toward collagen I was assayed using a transwell insert with 8-μm pores (Corning). The bottom side of the filter was coated with collagen I (15 μg/ml in PBS). Serum-free medium containing 2% BSA was added to the lower chamber. For chemotaxis, both the upper and lower surface of the filter were coated with collagen I. Chemoattractant was diluted in serum-free medium containing 2% BSA and added to the lower chamber. Cells were labeled with Hoechst (1:10,000, Molecular Probes) for 30 min in growth medium, trypsinized, and washed in serum-free DMEM; 5 × 104 cells were added to the upper chamber. After 2 h in a 5% CO2 incubator at 37°C, nonmigrating cells were removed from the top of the filter with a cotton swab. Migration was quantified on an inverted microscope (Nikon Eclipse TE-2000U) with a UV filter to count the nuclei. Four fields were counted for each condition in triplicate using a ×10 objective. Data are expressed as number of cells migrated per field and are representative of four independent experiments. Error bars represent standard deviation of the mean.
Calcium switch assay and junctional assembly.
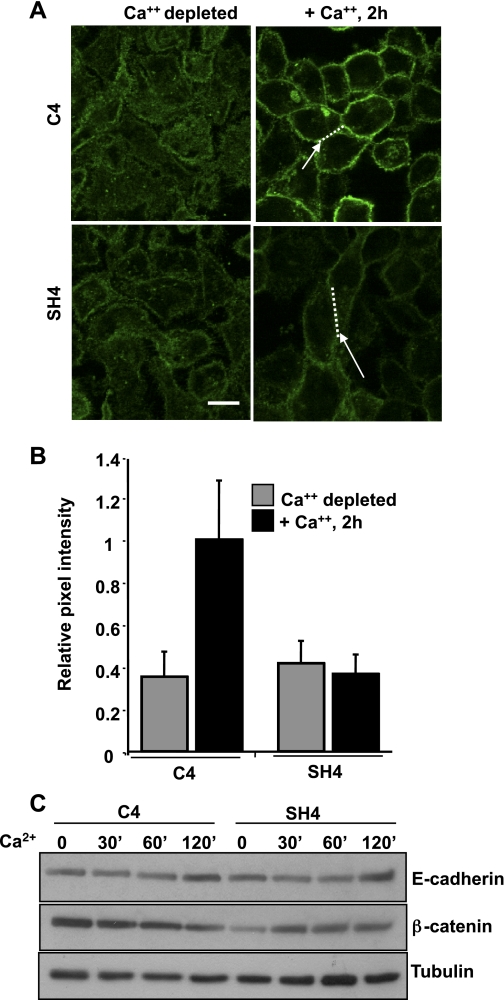
To disrupt adherens junctions, cells were starved for 4 h in Ca2+-free MEM containing 1% dialyzed FBS. To induce adherens junction assembly, DMEM with normal calcium levels (1.8 mM) was added back for various time points as indicated (27, 28). For assessment of junctional assembly, C4 or SH4 cells were plated on 12-mm glass coverslips for 48 h in growth medium. Following Ca2+ depletion/Ca2+ switch, assembly of junctions was assessed by immunofluorescence staining for E-cadherin and β-catenin. To quantify junctional intensity, confocal images were analyzed using Metamorph. For each image, a line was drawn along cell-cell boundaries using the line scan tool (represented by dotted lines in Fig. 5A, right). Line scans were performed for E-cadherin and β-catenin (not shown) staining separately. The junctional intensity is presented as the relative pixel intensity of the integrated intensity along each line divided by the length of the line. Twenty cell-cell boundaries were analyzed in three separate fields of view (60 total) for each condition or time point. The data are representative of three experiments, and error bars represent standard deviation of the mean. For Rac and Rho assays, cells were grown to confluency on 60-mm tissue culture plates in complete growth medium for 48 h before Ca2+ switch. Rac1 and RhoA activity were assayed as described below.
Fig. 5.
PTP-PEST is required for adherens junction assembly. A: Ca2+ switch assay in C4- and SH4-transfected KM12C cells shows that adherens junction assembly is impaired in the absence of PTP-PEST expression as assessed by immunofluorescence staining for E-cadherin. Representative areas are shown at right, with arrows showing regions where junctional assembly is decreased in SH4 relative to C4 cells 2 h following Ca2+ switch, and dotted lines indicating the cell-cell boundary region analyzed (n = 20). Scale bar = 20 μm. B: quantitation of junctional intensity using line scan as described in materials and methods. The data are an average of 20 junctional boundaries in three separate fields analyzed for each condition. Pixel intensity of E-cadherin is decreased by 2.5-fold in SH4 cells relative to C4 cells following Ca2+ switch, 2 h following Ca2+ recovery, indicating impaired adherens junction assembly. C: total expression of E-cadherin and β-catenin are unaffected by Ca2+ switch. Tubulin is shown as a loading control.
Surface biotinylation, Rho GTPase activity, and Western blots.
For cell surface biotinylation of E-cadherin, cells were incubated overnight in serum-free media, washed twice with ice-cold PBS, and treated with 0.1 mg/ml WZ-Link sulfo-NHS-SS-biotin (Pierce) for 30 min at 4°C. Unbound biotin was quenched with 50 mM glycine for 30 min at 4°C and cells were rinsed with PBS before lysis. Streptavidin-conjugated Sepharose beads were used to isolate biotinylated proteins. The relative levels of cell surface E-cadherin were detected by Western blot. Rac1 activity was assessed using the glutathione S-transferase (GST)-p21-activated kinase (PAK) pulldown assay and RhoA activity was measured using the GST-Rhotekin pulldown assay as described previously (14, 34). For examination of protein expression, whole cell lysates were prepared by scraping cells on ice into lysis buffer (50 mM Tris, pH 7.6, 150 mM NaCl, 1% Triton X-100, 0.25% deoxycholate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM PMSF). Lysates were cleared by centrifugation (13,000 rpm, 4°C, 20 min), and protein was determined using bicinchoninic acid assay (Pierce). SDS gels were prepared according to the protocol of Laemmli (20). Western Blot was performed according to the method of Towbin et al. (45). Following incubation of membranes with horseradish peroxidase-conjugated secondary antibodies (1:40,000), membranes were incubated with chemiluminescence reagent from Millipore and then exposed to X-ray film.
RESULTS
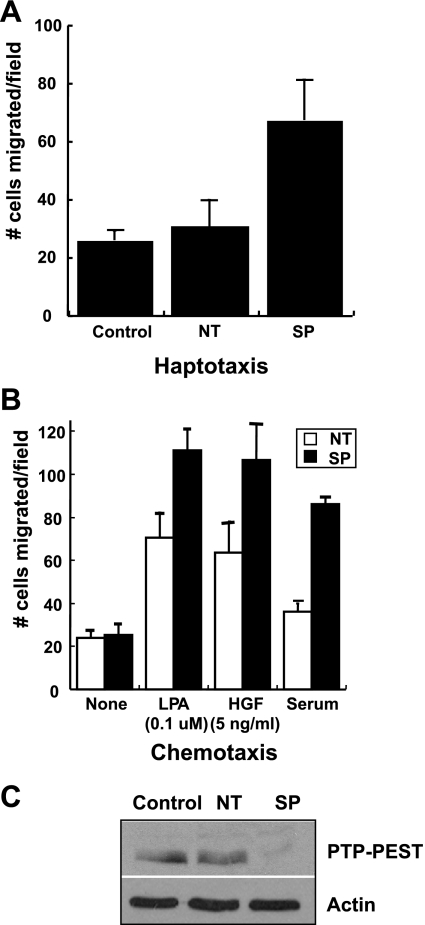
To determine the precise role of PTP-PEST in epithelial cell motility, we tested the function of PTP-PEST in colon carcinoma cells. We utilized KM12C colon carcinoma cells since they are tumorigenic, but not metastatic (25), and they express appreciable levels of endogenous PTP-PEST (Supplemental Figure S1; Supplemental Material for this article is available online at the Journal website). We used RNA interference to knock down endogenous PTP-PEST in KM12C cells and assessed motility toward collagen I as well as chemotaxis. Figure 1A shows that decreased PTP-PEST expression results in a nearly threefold increase in KM12C motility toward collagen I using a haptotaxis assay. SiRNA knockdown of PTP-PEST also led to a twofold increase in chemotaxis toward LPA, HGF, or serum as chemoattractants (Fig. 1B), while a nontargeting siRNA (NT) had no effect. In the absence of a chemoattractant (None), PTP-PEST siRNA did not affect cell motility. Western blot analysis confirms that PTP-PEST is efficiently knocked down by the Smart Pool siRNA treatment at 48 h (SP), whereas electroporation alone (control) or nontargeting siRNA (NT) has no effect on PTP-PEST expression (Fig. 1C). Similar results were obtained in HCT116 colon carcinoma cells (Supplemental Fig. S2).
Fig. 1.
Transient knockdown of protein tyrosine phosphatase, PTP-PEST, enhances colon carcinoma cell motility. A: Smart Pool small interfering (si)RNA knockdown of PTP-PEST (SP) in KM12C colon carcinoma cells leads to increased motility toward collagen I using a haptotaxis assay. Control refers to KM12C cells that have been electroporated only. NT, nontargeting siRNA control. B: siRNA knockdown of PTP-PEST in KM12C cells enhances chemotaxis toward lysophosphatidic acid (LPA), hepatocyte growth factor (HGF), or serum relative to the absence of a chemoattractant (None). C: PTP-PEST expression is efficiently decreased by the Smart Pool siRNA. Neither control nor nontargeting siRNA affects migration or PTP-PEST protein expression (n = 4). Actin was used as a loading control.
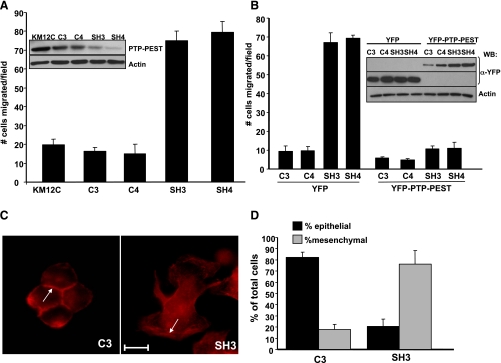
To confirm the effect of transient knockdown of PTP-PEST, we generated KM12C cell lines with stable knockdown of PTP-PEST. Two distinct shRNA constructs (SH3 or SH4) were transfected into KM12C cells and selected with puromycin. As controls, shRNA constructs (C3 or C4) containing a single nucleotide change were used. The SH3 and SH4 constructs effectively decreased PTP-PEST expression (Fig. 2A, inset), and moreover, these cell lines showed a fourfold increase in haptotactic motility toward collagen I (Fig. 2A). The C3 and C4 control cell lines resembled parental KM12C cells with respect to PTP-PEST expression and motility. To show that this phenotype was specific to PTP-PEST and is not an off-target effect, we transiently coexpressed a wild-type mouse PTP-PEST cDNA [yellow fluorescent protein (YFP)-PTP-PEST] in the SH3 and SH4 cell lines that is resistant to the human-specific shRNA. As shown in Fig. 2B, YFP-PTP-PEST expression blocked cell motility in the SH3 and SH4 cells while the expression of YFP alone had no effect. The level of YFP or YFP-PTP-PEST expression is shown in Fig. 2B (inset), where comparable amounts of transfected protein are present in the different cell lines. Thus, decreased levels of PTP-PEST coincide with increased colon carcinoma cell motility.
Fig. 2.
Stable knockdown of PTP-PEST enhances motility and induces a mesenchymal phenotype. A: haptotactic cell motility toward collagen I is enhanced 3- to 4-fold in KM12C cells in which PTP-PEST has been stably knocked down using Src homology 3 (SH3) or SH4 short hairpin (sh)RNA constructs relative to parental KM12C or cells transfected with control shRNA vectors (C3 and C4, which differ by one nucleotide). A, inset: Western blot analysis shows that SH3 and SH4 shRNA constructs reduce PTP-PEST expression while C3 and C4 constructs have no effect on PTP-PEST expression. B: C3, C4, SH3, and SH4 cell lines were transiently transfected with yellow fluorescent protein (YFP) plasmid or YFP-PTP-PEST plasmid that is resistant to the shRNA to “rescue” the shRNA phenotype. Expression of YFP-PTP-PEST in SH3 and SH4 cells reduces migration to basal levels. The decreased motility of SH3 and SH4 cells with reexpressed PTP-PEST shows that the effect on cell motility is specific and not an off-target effect. B, inset: Western blot (WB) analysis shows similar expression of YFP and YFP-PTP-PEST in all cell lines. A Western blot for actin is shown as a loading control. C: F-actin staining in SH3 cells shows a motile morphology with prominent membrane ruffles and lamellae (see arrows) while in C3 cells, F-actin is restricted to sites of cell-cell contact. Scale bar = 10 μm. D: quantitation of morphology in C. 80–90% of C3 cells grow in “epithelial clusters” whereas 85% of SH3-transfected cells are mesenchymal.
Since the SH3 and SH4 cells showed enhanced motility, we closely examined their morphology. Whereas KM12C cells and the C3 or C4 cells grow in tight clusters, the SH3 and SH4 cells showed a more dispersed and scattered distribution (not shown). In addition, the SH3 cells underwent a drastic change in morphology from an epithelial to a mesenchymal appearance (Fig. 2C). Staining for F-actin shows that the SH3 cells display a mesenchymal phenotype defined as highly spread, polarized, motile shape with prominent membrane ruffles and broad lamellae (Fig. 2C, see arrows) when compared with C3 controls. C3 cells retain an epithelial morphology, are more rounded, and F-actin is restricted at cell-cell contacts(Fig. 2C, see arrows). We determined that 85% of C3 cells grew in clusters of two or more cells (when cultured for 48 h or more in normal growth conditions). In contrast, 85–90% of SH3 cells showed a “mesenchymal-like” phenotype (Fig. 2D). It should be noted that similar results were observed for the C4/SH4 cells (data not shown). We further examined the expression of mesenchymal markers in C3 versus SH3 cells. Western blot analysis showed no change in the expression of vimentin, fibronectin, or N-cadherin in C3 and SH3 cells relative to parental KM12C cells (data not shown). Together, these results show that a sustained decrease in PTP-PEST expression leads to a highly motile and mesenchymal-like phenotype with a significant morphological change, but does not induce expression of mesenchymal markers.
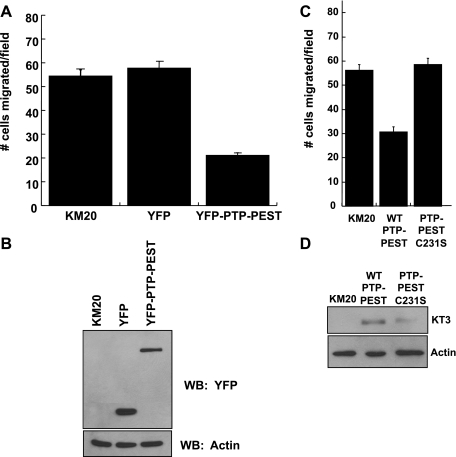
To further confirm the importance of PTP-PEST in epithelial cell motility, we performed overexpression studies using KM20 cells, a highly metastatic colon cancer cell line (25, 26) that expresses low levels of endogenous PTP-PEST (Supplemental Fig. S1). In contrast to our earlier siRNA knockdown effects, transient expression of YFP-tagged wild-type PTP-PEST impaired migration toward collagen I (Fig. 3A). Expression of a control vector encoding YFP alone did not affect motility. Western blot analysis for YFP expression confirmed that YFP-PTP-PEST and YFP were expressed at similar levels (Fig. 3B). Interestingly, a catalytically inactive mutant of PTP-PEST, C231S, when expressed in KM20 cells had no effect on cell motility (Fig. 3, C and D), indicating that the suppressive effect of PTP-PEST is due to its catalytic activity. Similar results were obtained with DLD-1 colon carcinoma cells (Supplemental Fig. S3). Thus, the expression of PTP-PEST correlates with the suppression of cell motility. These findings together demonstrate that the level of PTP-PEST expression markedly alters the motile behavior of colon carcinoma cells.
Fig. 3.
Ectopic expression of PTP-PEST suppresses colon carcinoma motility. A: transient reexpression of PTP-PEST in KM20 cells, which lack endogenous PTP-PEST, impairs motility toward collagen I. B: Western blot analysis shows similar expression of YFP and YFP-PTP-PEST. C: expression of catalytically inactive PTP-PEST does not block KM20 cell motility. D: Western blot analysis shows that KT3-tagged wild-type (WT) and C231S PTP-PEST are expressed 48 h following transient transfection into KM20 cells. Actin is shown as a loading control.
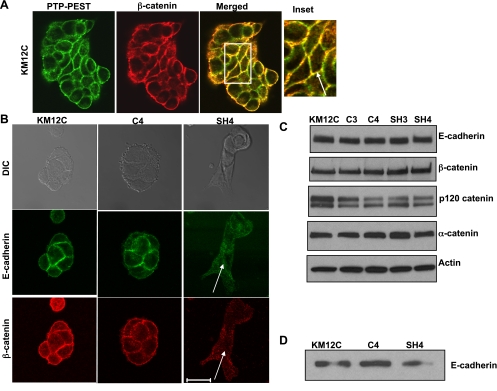
The above observations prompted us to investigate whether PTP-PEST plays a role in regulating cell-cell contacts. Therefore, we next examined the subcellular localization of endogenous PTP-PEST in parental KM12C cells. As seen in Fig. 4A, confocal imaging shows that PTP-PEST colocalized with β-catenin in adherens junctions. Examination of adherens junctions in the SH3 (data not shown) and SH4 cell lines that were able to form clusters revealed decreased staining of E-cadherin and β-catenin (Fig. 4B, see arrows) relative to wild-type KM12C cells or the respective shRNA controls. The Western blot in Fig. 4C shows that the expression of E-cadherin and β-catenin (and other junctional components including p120 catenin and α-catenin) are unchanged in the presence or absence of PTP-PEST expression. To rule out the possibility that cell surface expression of E-cadherin was affected by shRNA knockdown, we performed surface biotinylation of parental KM12C, C4, and SH4 cells. Streptavidin-Sepharose beads were used to isolate cell surface proteins and were analyzed by Western blot for E-cadherin. As seen in Fig. 4D, E-cadherin is present on the cell surface in all cell lines and is not internalized in response to knockdown of PTP-PEST per se. These results show that a decrease in PTP-PEST expression impairs adherens junction organization concomitant with an increase in motility.
Fig. 4.
PTP-PEST localizes in cell-cell junctions and affects junction integrity. A: localization of PTP-PEST in KM12C colon carcinoma cells. Confocal image of PTP-PEST (green) and β-catenin (red) staining shows that the two proteins colocalize in adherens junctions (merged) in KM12C cells. Scale bar = 20 μm. B: comparison of C4- and SH4-transfected KM12C cells shows that E-cadherin and β-catenin staining is not detectable in adherens junctions in the SH3 and SH4 knockdown cells (see arrows). Scale bar = 20 μm. C: total protein expression of E-cadherin, β-catenin, p120 catenin, and α-catenin is not altered by PTP-PEST stable knockdown. Actin is shown as a loading control. D: surface expression of E-cadherin is comparable in parental KM12C, C4, and SH4 cells.
We then determined the effect of PTP-PEST knockdown on junctional assembly using a calcium switch assay (27, 28). The C3, C4, SH3, or SH4 cell lines were Ca2+ depleted (4 h), stimulated with Ca2+-containing medium for the times indicated (Fig. 5), and then immunostained for E-cadherin (and β-catenin, not shown) to monitor junctional assembly. As seen in Fig. 5A, adherens junctions reform in the C4 cells within 60 min (data not shown) and show substantial recovery within 2 h after Ca2+ addition. In contrast, in SH4 cells depleted of PTP-PEST expression, E-cadherin is not well organized in adherens junctions (see arrows in Fig. 5A). Furthermore, E-cadherin shows a punctate cytoplasmic distribution as opposed to a continuous membrane distribution detected in C4 cells at the same time point (Fig. 5A). Figure 5B shows quantitative analysis of junctional intensity using a line scan to measure pixel density of E-cadherin in the different cell lines. A total of 20 adherens junctions (cell-cell boundaries; dotted line, Fig. 5A) were analyzed for each cell type and condition in three separate fields (60 cell-cell contacts total; see materials and methods). As shown in Fig. 5A, in the absence of Ca2+, all cells show reduced junctional staining of E-cadherin. Addition of Ca2+ lead to a 2.5-fold increase in E-cadherin staining intensity in C4 cells (Fig. 5B). However, the E-cadherin intensities in SH4 cells remained at basal levels at 2 h after Ca2+ addition, an effect that persisted in SH4 cells up to 8 h (Supplemental Fig. S4) and 24 h (data not shown) following Ca2+ recovery. Western blot analysis confirmed that total protein levels of E-cadherin and β-catenin were unaffected by Ca2+ switch treatment, indicating that protein degradation could not account for the assembly defect observed (Fig. 5C). Therefore, adherens junction assembly in the absence of PTP-PEST expression is substantially impaired.
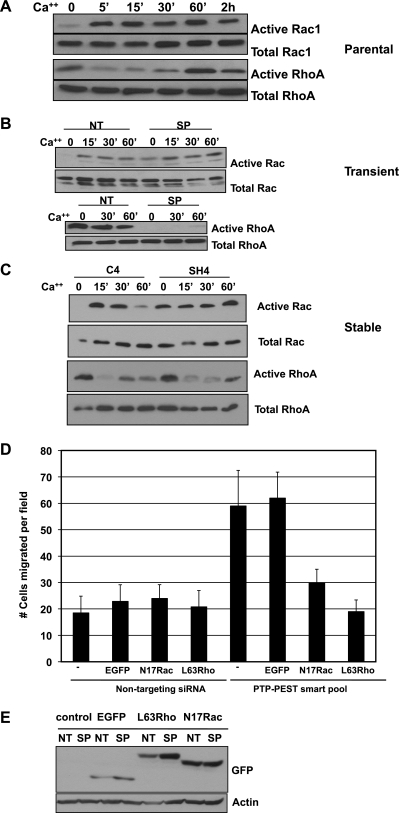
Adherens junction assembly relies on Rho GTPase activity (6, 27, 28, 48). Given that our previous studies demonstrate that PTP-PEST regulates Rac1 and RhoA activity in fibroblasts (36, 37), we next examined the effect of PTP-PEST on the regulation of Rho GTPases downstream of cadherin engagement. Calcium-depleted cells were stimulated with Ca2+-containing medium for the indicated times, and Rac1 and RhoA activities were assayed using GST-PAK or GST-Rhotekin pulldown assays, respectively (14, 34). Using parental KM12C cells, we first examined the time course of Rho GTPase activity following Ca2+ switch. As shown in Fig. 6A, Rac1 is activated within 5 min of cadherin engagement and persists up to 2 h, at which time Rac1 activity decreases. RhoA activity is suppressed following initial cadherin engagement and is subsequently activated after 60 min to 2 h. This result agrees with previously published studies showing similar activation in other epithelial cell types (6, 27, 28, 48). Both transient siRNA (Fig. 6B) or stable shRNA (Fig. 6C) knockdown of PTP-PEST in KM12C cells results in an increased basal level of Rac1 activity in the absence of Ca2+ as well as enhanced and sustained activation following addition of Ca2+ relative to control cells treated with a nontargeting siRNA or control shRNA vector. RhoA activity is suppressed in response to cadherin engagement in KM12C cells treated with siRNA or shRNA. We then tested the ability of Rac or Rho mutants to rescue the PTP-PEST knockdown phenotype. C4 and SH4 cell lines were transiently transfected with expression plasmids of dominant negative Rac1, N17Rac1-GFP, or activated RhoA, L63RhoA-GFP, or GFP alone and assessed for cell motility. As shown in Fig. 6D, either N17Rac1 or L63RhoA was able to reduce cell motility in SH4 cells to the level of control (C4 only) cells while GFP expression had no effect. The Western blot in Fig. 6E shows expression of the GFP-tagged constructs with actin as a loading control. Taken together, these results show that PTP-PEST affects Rho GTPase activity to control adherens junction assembly and cell motility.
Fig. 6.
PTP-PEST modulates Rac1 and RhoA activity by cadherins. A: time course of Rac1 and RhoA activity in parental KM12C cells in response to Ca2+ switch shows that Rac1 is activated and that RhoA is initially suppressed by cadherin engagement within 30–60 min. RhoA is subsequently activated by 60 min to 2 h. B: transient siRNA knockdown of PTP-PEST in KM12C cells enhances Rac1 (top) and suppresses RhoA activity (bottom) in response to cadherin engagement. C: stable shRNA knockdown of PTP-PEST elevates Rac1 and suppresses RhoA activity following Ca2+ switch. D: transient expression of green fluorescent protein (GFP)-tagged N17Rac1 or GFP-tagged L63RhoA reverses the effect of PTP-PEST knockdown on cell motility. EGFP, enhanced GFP. E: GFP, GFP-N17Rac1, and GFP L63RhoA expression is comparable between C4 and SH4 cells. Actin is shown as a loading control.
DISCUSSION
In this study, we demonstrate for the first time that the protein tyrosine phosphatase, PTP-PEST, plays a key role in regulating the integrity of cell-cell contacts, which, in turn, affects colon carcinoma cell motility. Importantly, we show that PTP-PEST is localized in adherens junctions, where it controls the activity of the Rho GTPases, Rac1 and RhoA, to modulate the motile versus nonmotile state. The altered activity of Rac1 and RhoA leads to a disruption or inability to assemble cell-cell junctions. Therefore, we conclude that PTP-PEST normally functions to maintain adherens junction integrity and to restrict cell motility. The data presented here are consistent with recent findings that implicate PTP-PEST in epithelial cell motility (39, 42). Our findings are novel in that they extend these reports by demonstrating a role for PTP-PEST in the regulation of adherens junctions. Our data would suggest that PTP-PEST affects the ability of E-cadherin to signal to Rac1 and RhoA, thereby causing impaired junctional assembly.
The primary functional consequence of decreased PTP-PEST expression is increased carcinoma cell motility. We showed that either transient or stable RNAi-mediated knockdown of PTP-PEST results in enhanced migration towards collagen I and enhanced chemotaxis in colon carcinoma cells. Interestingly, this effect does not appear to be limited to carcinoma cells. We also found that knockdown of PTP-PEST in normal epithelial cells, such as MDCK or IEC-6, caused increased migration (R. Espejo, unpublished observations). The effect of PTP-PEST knockdown on colon carcinoma cell motility is in direct contrast to what has previously been observed in fibroblasts. Decreased expression of PTP-PEST in fibroblasts blocks cell motility (2, 36). One possible explanation for this discrepancy is that PTP-PEST acts on distinct targets in epithelial cells versus fibroblasts. Our findings here suggest that the function of PTP-PEST could likely be to prevent aberrant epithelial cell motility. In the colon, this is particularly critical. Normal intestinal homeostasis relies on the ability of epithelial cells to migrate from the crypts to the surface of the lumen, where they differentiate and eventually undergo apoptosis. This is a tightly controlled process, requiring rapid and transient activation of migratory signals. This process further requires remodeling of cell-cell adhesive contacts. In this scenario, transient downregulation of PTP-PEST would be permissive for cell turnover, while a sustained decrease could promote aberrant motility and possibly invasion contributing to tumor progression. It is possible that PTP-PEST expression or activity is transiently downmodulated during normal events such as developmental epithelio-mesenchymal transition (EMT) or epithelial wound healing. It will be important to determine at which stage of tumor progression PTP-PEST expression is decreased and the factors controlling PTP-PEST expression and activity in normal tissues.
Although the upstream regulation of PTP-PEST expression is not known, our studies implicate PTP-PEST in regulating an “epithelial-mesenchymal-like” transition. The enhanced cell motility and drastic morphology change of KM12C cells with stable knockdown of PTP-PEST support this hypothesis. However, we do not observe a bona fide EMT, rather a partial EMT that affects cell morphology. A key requisite toward a full EMT is a loss of E-cadherin function. Although in many cases EMT is accompanied by a loss of E-cadherin expression (43), in our system, E-cadherin levels are unchanged. Our observation that adherens junction organization is impaired in the absence of PTP-PEST indicates that we are perturbing E-cadherin function as opposed to its expression. On the basis of our finding that PTP-PEST colocalizes with β-catenin at adherens junctions, we conclude that the recruitment of PTP-PEST to sites of cell-cell contact plays an essential role in controlling junctional assembly and stability. Several mechanisms for altered E-cadherin function exist including altered turnover/trafficking, disruption of the E-cadherin/catenin complex, loss of extracellular domain adhesive activity, and ectodomain shedding (15). Our results with the Ca2+ switch assembly assay support the idea that E-cadherin function is impaired in the absence of PTP-PEST. Our findings are in agreement with previous studies showing that hyperactivation of Rac1 or blocking of RhoA in epithelial cells disrupts adherens junctions (6). Our data would suggest that PTP-PEST normally serves to strengthen adherens junctions by limiting the activation of Rac1 and potentiating the activation of RhoA. In the absence of PTP-PEST, Rac1 activity is elevated while RhoA is suppressed, leading to a weakened adhesive interaction that may be more susceptible to motile signals. In addition, elevated levels of active Rac1 are associated with increased endocytosis of E-cadherin (1), a situation that would be favored in the absence of PTP-PEST. Studies remain to be done that aim at identifying junctional targets of PTP-PEST and how these interactions affect E-cadherin function and/or Rho GTPase activity. Several targets of PTP-PEST previously identified in the literature including FAK (50), c-abl (10), VAV2, and p190RhoGAP (37) have been associated with regulation of cell-cell junction assembly and Rho GTPase activity downstream of E-cadherin (28, 31, 49). Our preliminary evidence using substrate trapping in colon cancer cells indicates that PTP-PEST targets multiple of these substrates as well as novel substrates (R. Espejo and S. Sastry, unpublished observations). Thus, the precise mechanism by which PTP-PEST affects cell-cell junctions and Rho GTPase activity is complex, likely involving multiple pathways and targets, including either direct or indirect regulation of phosphorylation of GEFs or GAPs or phosphorylation of catenins (R. Espejo and S. Sastry, unpublished observations).
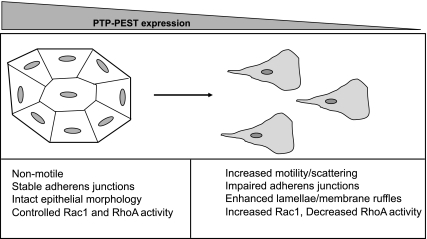
In summary, our findings strongly support a role for PTP-PEST as a negative regulator of colon carcinoma motility. Taken together, the data suggest a model in which PTP-PEST is recruited to adherens junctions where it acts on Rho family GTPases to control junctional assembly presumably to restrict cell motility. In the absence of PTP-PEST, the epithelial architecture and adherens junctions are disrupted and Rho GTPase activity is unregulated leading to a highly motile, mesenchymal phenotype (see model, Fig. 7). In the context of colon cancer progression, loss of PTP-PEST expression may enhance the invasive potential of carcinoma cells. Future experiments will focus on the effect of decreased PTP-PEST expression on colon carcinoma cell invasion and metastasis in vivo using mouse models.
Fig. 7.
Model for PTP-PEST regulation of colon carcinoma motility. PTP-PEST expression controls epithelial morphology in KM12C colon carcinoma cells. Through its localization in adherens junctions, PTP-PEST restricts epithelial cell motility. PTP-PEST controls the activity of Rac1 and RhoA at sites of cadherin engagement to promote junctional assembly and stability. In the absence of PTP-PEST, Rac1 and RhoA activity are misregulated, adherens junctions are impaired, and the cells acquire a highly motile and mesenchymal phenotype.
GRANTS
This work was supported by National Institutes of Health Grants CA-118405 (to S. K. Sastry) and CA-104748 (to B. M. Evers).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Dr. L. A. Elferink for critical reading of the manuscript. We also thank Tim Walker, Dr. Lindsay Jackson, and Daniel Olivares for technical assistance. We are grateful to Dr. Leoncio Vergara and Dr. Adriana Paulucci-Holthauzen of the Optical Imaging Laboratory at University of Texas Medical Branch for assistance with confocal microscopy and Metamorph image analysis.
REFERENCES
- 1. Akhtar N, Hotchin NA. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol Biol Cell 12: 847–862, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angers-Lousteau A, Cote J, Charest A, Dowbenko D, Spencer S, Lasky L, Tremblay M. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J Cell Biol 144: 1019–1031, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Avizienyte E, Wyke AW, Jones RL, McLean GW, Westhoff MA, Brunton VG, Frame MC. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signaling. Nat Cell Biol 4: 632–638, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Blume-Jensen P, Hunter T. Oncogenic kinase signaling. Nature 411: 355–365, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci 122: 3441–3454, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braga V, Yap AS. The challenges of abundance: epithelial junctions and small GTPase signaling. Curr Opin Cell Biol 17: 466–474, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Brunton V, Ozanne B, Paraskeva C, Frame M. A role for epidermal growth factor receptor, c-src and focal adhesion kinase in an in vitro model for the progression of colon cancer. Oncogene 14: 283–293, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Charest A, Wagner J, Kwan M, Tremblay ML. Coupling of the murine protein tyrosine phosphatase PEST to the epidermal growth factor (EGF) receptor through a Src homology 3 (SH3) domain-mediated association with Grb2. Oncogene 14: 1643–1651, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Chellaiah MA, Schaller MD. Activation of src kinase by protein-tyrosine phosphatase-PEST in osteoclasts: comparative analysis of the effects of bisphosphonate and protein-tyrosine phosphatase inhibitor on src activation in vitro. J Cell Physiol 220: 382–393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cong F, Spencer S, Cote JF, Wu Y, Tremblay ML, Lasky LA, Goff SP. Cytoskeletal protein PSTPIP1 directs the PEST-type protein tyrosine phosphatase to the c-Abl kinase to mediate Abl dephosphorylation. Molecular Cell 6: 1413–1423, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Cousin H, Alfandari D. A PTP-PEST-like protein affects α5β1-integrin-dependent matrix assembly, cell adhesion, and migration in Xenopus gastrula. Dev Biol 265: 416–432, 2004 [DOI] [PubMed] [Google Scholar]
- 12. DeSeau V, Rosen N, Bolen J. Analysis of pp60-Src tyrosine kinase activity and phosphotyrosyl phosphatase activity in human colon carcinoma and normal human colon mucosal cells. J Cell Biochem 35: 113–128, 1987 [DOI] [PubMed] [Google Scholar]
- 13. Garton A, Tonks N. Regulation of fibroblast motility by the protein tyrosine phosphatase PTP-PEST. J Biol Chem 274: 3811–3818, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Glaven J, Whitehead I, Bagrodia S, Kay R, Cerione R. The Dbl-related protein, Lfc, localizes to microtubules and mediates the activation of Rac signaling pathways in cells. J Biol Chem 274: 2279–2285, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 6: 622–634, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Halle M, Liu YC, Hardy S, Theberge JF, Blanchetot C, Bourdeau A, Meng TC, Tremblay ML. Caspase-3 regulates catalytic activity and scaffolding functions of the protein tyrosine phosphatase PEST, a novel modulator of the apoptotic response. Mol Cell Biol 27: 1172–1190, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 100: 57–70, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Irby R, Yeatman T. Role of Src expression and activation in human cancer. Oncogene 19: 5636–5642, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Kinch MS, Clark GJ, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J Cell Biol 130: 461–471, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Laemmli U. Cleavage of the structural proteins during assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 21. Lozano E, Frasa M, Smolarczyk K, Knaus UG, Braga VM. PAK is required for the disruption of E-cadherin adhesion by the small GTPase Rac. J Cell Sci 121: 933–938, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Lyons P, Dunty J, Schaefer E, Schaller M. Inhibition of the catalytic activity of cell adhesion kinase beta by protein tyrosine phosphatase-PEST-mediated dephosporylation. J Biol Chem 276: 24422–24431, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Mariner DJ, Anastasiadis P, Keilhack H, Bohmer F, Wang J, Reynolds AB. Identification of src phosphorylation sites in the catenin p120catenin. J Biol Chem 276: 28006–28013, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Mathew S, George SP, Wang Y, Siddiqui MR, Srinivasan K, Tan L, Khurana S. Potential molecular mechanism for c-Src kinase-mediated regulation of intestinal cell migration. J Biol Chem 283: 22709–22722, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morikawa K, Walker S, Jessup J, Fidler I. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res 48: 1943–1948, 1988 [PubMed] [Google Scholar]
- 26. Morikawa K, Walker S, Nakajima M, Pathak S, Jessup M, Fidler I. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res 48: 6863–6871, 1988 [PubMed] [Google Scholar]
- 27. Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J Cell Sci 114: 1829–1838, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem 276: 33305–33308, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Peinado H, Olmeda D, Cano A. Snail, zeb, and bHLH factors in tumor progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7: 415–428, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Playford MP, Lyons PD, Sastry SK, Schaller MD. Identification of a filamin docking site on PTP-PEST. J Biol Chem 281: 34104–34112, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Playford MP, Vadali K, Cai X, Burridge K, Schaller MD. Focal adhesion kinase regulates cell-cell contact formation in epithelial cells via modulation of Rho. Exp Cell Res 314: 3187–3197, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pouliot N, Nice E, Burgess A. Laminin-10 mediates basal and EGF-stimulated motility of human colon carcinoma cells via α3β1 and α6β4 integrins. Exp Cell Res 266: 1–10, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Reinhold WC, Reimers MA, Lorenzi P, Ho J, Shankavaram UT, Ziegler MS, Bussey KJ, Nishizuka S, Ikediobi O, Pommier YG, Weinstein JN. Multifactorial regulation of E-cadherin expression: an integrative study. Mol Cancer Ther 9: 1–16, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ren X, Kiosses W, Schwartz M. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J 18: 578–585, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rengifo-Cam W, Konishi A, Morishita N, Matsuoka H, Yamori T, Nada S, Okada M. Csk defines the ability of integrin-mediated cell adhesion and migration in human colon cancer cells: implication for a potential role in cancer metastasis. Oncogene 23: 289–297, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Sahai E, Marshall CJ. Rho-GTPases and cancer. Nat Rev Cancer 2: 133–142, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Sastry S, Lyons P, Schaller M, Burridge K. PTP-PEST controls motility through regulation of Rac1. J Cell Sci 115: 4305–4316, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Sastry SK, Rajfur Z, Liu BP, Cote JF, Tremblay M, Burridge K. PTP-PEST couples membrane protrusion and tail retraction via VAV2 and p190RhoGAP. J Biol Chem 281: 11627–11636, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sirois J, Cote JF, Charest A, Uetani N, Bourdeau A, Duncan SA, Daniels E, Tremblay ML. Essential function of PTP-PEST during mouse embryonic vascularization, mesenchyme formation, neurogenesis and early liver development. Mech Dev 123: 869–880, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Streit S, Ruhe JE, Knyazev P, Knyazeva T, Iacobelli S, Peter S, Hoefler H, Ullrich A. PTP-PEST phosphatase variations in human cancer. Cancer Genet Cytogenet 170: 48–53, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Taieb D, Roignot J, André F, Garcia S, Masson B, Pierres A, Iovanna JL, Soubeyran P. ArgBP2-dependent signaling regulates pancreatic cell migration, adhesion, and tumorigenicity. Cancer Res 68: 4588–4596, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Takekawa M, Itoh F, Hinoda Y, Adachi M, Ariyama T, Inazawa J, Imai K, Yachi A. Chromosomal localization of the protein tyrosine phosphatase G1 gene and characterization of the aberrant transcripts in human colon cancer cells. FEBS Lett 339: 222–228, 1994 [DOI] [PubMed] [Google Scholar]
- 43. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2: 442–454, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Tomar A, Schlaepfer DD. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr Opin Cell Biol 21: 676–683, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Towbin H, Staehlin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Warner SJ, Longmore GD. Distinct functions for Rho1 in maintaining adherens junctions and apical tension in remodeling epithelia. J Cell Biol 185: 1111–1125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol 178: 517–527, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yap AS, Kovacs EM. Direct cadherin-activated cell signaling: a view from the plasma membrane. J Cell Biol 160: 11–16, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zandy NL, Playford M, Pendergast AM. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc Natl Acad Sci 104: 17686–17691, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zheng Y, Xia Y, Hawke D, Halle M, Tremblay ML, Gao X, Zhou XZ, Aldape K, Cobb MH, Xie K, He J, Lu Z. FAK phosphorylation by erk primes ras-induced tyrosine dephosphorylation of FAK mediated by PIN1 and PTP-PEST. Mol Cell 35: 11–25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]