although the amino acid arginine is commonly associated with nitric oxide (NO) production via NO synthase (NOS), it also participates in the synthesis of urea, creatine, creatinine, agmatine, polyamines, as well as overall protein synthesis. Furthermore, it also influences hormone release (insulin, prolactin, and others) and synthesis of pyrimidine bases. Thus, physiologically arginine participates in disposal of protein metabolic waste, muscle metabolism, vascular regulation, immune system function, neurotransmission, RNA synthesis, and hormone-mediated signaling (4). More importantly, although all cells require arginine, not all cells possess the metabolic capacity to produce it and thus must obtain arginine via the circulation. In cells that must acquire l-arginine exogenously, it seems logical that there would be regulatory mechanisms in place to moderate the rate of l-arginine uptake via cationic amino acid transporters (CATs). Surprisingly, there are few reports that address CATs as possible metabolic sites of regulation.
In light of limited information, the report from Zhou et al. (28), the current article in focus (published in this issue of American Journal of Physiology-Cell Physiology), is particularly important. Their article describes findings from freshly isolated rat ventricular cardiomyocytes that endogenously produced NO negatively regulates l-arginine uptake (28). Functionally, this l-arginine transport appears to be mediated via the cationic amino acid transporters 1 and 2 (CAT-1 and CAT-2A; human genes SLC7A1 and SLC7A2, respectively) (25). This important observation extends previous work from the Peluffo laboratory functionally identifying CAT-1 and CAT-2A as equal contributors to l-arginine uptake in cardiac muscle (15, 18). These observations identify a critical regulatory role for CATs in NO signaling and thus contribute to cardiac muscle physiology and pathophysiology.
CAT structure-function.
The CAT proteins, a subgroup of the solute carrier family 7, function as the predominant entrance pathway for cationic amino acids in nonepithelial cells. Four members of the CAT family have been functionally characterized (CAT-1, CAT-2A, CAT-2B, and CAT-3), and all are glycosylated plasma membrane proteins that contain 14 putative transmembrane spans. (Recently, a fifth member, CAT-4, has been identified but remains poorly understood.) The four isoforms display similar substrate specificity for cationic amino acids. The transport process for all four members is driven by the electrochemical gradient of the transported amino acid and is Na+ independent.
CATs belong to the system y+ subgroup of the cationic amino acid transporters (4). The system y+ transporters are highly selective for cationic amino acids with a preference for a long carbon backbone, e.g., homoarginine > arginine ≈ lysine > ornithine > 2,4-diamino-n-butyric acid (2, 18). However, separate isoforms do display different apparent affinities and rates of transport for these substrates. For example, CAT-1 and CAT-2B transport l-arginine with relatively high apparent affinity (0.07–0.25 mM) and low capacity, whereas CAT-2A has a higher maximal velocity and lower apparent affinity (2–5 mM) for this amino acid (4). CAT-mediated transport is stereospecific; that is, d-isomers are not substrates for system y+ transporters. CAT-2A and CAT-2B are mutually exclusive splice variants of the SLC7A2 gene that only differ by a 42 amino acid stretch encompassing the putative TM8–TM9 hairpin, which includes the fourth intracellular loop (4). Subsequent studies identified two amino acids within this 42 amino acid segment in CAT-2A (i.e., Arg369 and Ser381) that contribute to the significantly lower apparent substrate affinities in this isoform (9).
Members of the system y+ transporter family can also recognize l-arginine analogs that are methylated at the guanido group such as NG-nitro-l-arginine methyl ester (l-NAME), an inhibitor of constitutive NOS (5). Also, system y+ CATs are sensitive to the sulfhydryl reagent N-ethylmaleimide (NEM), which inhibits members of system y+ at concentrations nearly tenfold lower than is required to block other proteins (3, 15, 18). In red blood cells, 200 μM NEM blocked system y+ transporters, whereas system y+L transporters also present in these cells were not NEM sensitive (19). System y+L transporters move cationic amino acids in an uncoupled manner, as well as neutral amino acids coupled with Na+.
NO regulates cardiac CATs.
The important role that constitutively produced NO plays in myocardial function has been reviewed recently by Seddon et al. (22). It has been three decades since Furchgott and Zawadzki (7) reported on endothelium-derived relaxation factor (subsequently identified as NO) (11, 17), and since then, NO signaling has been described in many tissues in addition to smooth muscle. Indeed, most cell types contain one or more NOS isoforms, with the predominant isoforms being NOS-1 (neuronal NOS) and NOS-3 (endothelial NOS), both of which are in cardiac muscle (1). The third isoform, NOS-2, is an inducible form (iNOS), so named for its increased synthesis during an inflammatory response. NO is a reactive free radical gas formed by oxidation of the guanidine group of l-arginine to produce NO and l-citrulline (8). Given that NO can readily diffuse through membranes and the highly reactive nature of free radicals, free cytosolic NO has an extremely short half-life. However, there is increasing support for the notion that nitrosothiols may serve as a NO reservoir for subsequent release and signaling (14).
In any case, initial NO production depends directly on the availability of l-arginine to NOS. The NOS enzymes are some of the most highly regulated enzymes known, and in this issue of the Journal, Peluffo and colleagues make a compelling argument that, at least in cardiac myocytes, regulation extends upstream to the control of substrate availability by decreasing l-arginine uptake via CAT-1 and CAT-2A (28) (Fig. 1). Indeed, the bioavailability of NO has been linked to a reduced rate of l-arginine uptake in congestive heart failure. This decreased l-arginine uptake was concomitantly associated with lower CAT-1 mRNA levels (12). Given that cardiac myocytes, like most cells, must import l-arginine from the circulation, NO production and CATs are intimately linked. Thus, the observation by Zhou and colleagues (28) that NO directly decreases l-arginine uptake by CATs is quite novel in that it demonstrates a direct link between NOS and CATs, as opposed to a signaling induced (e.g., cGMP-mediated phosphorylation) phenomenon.
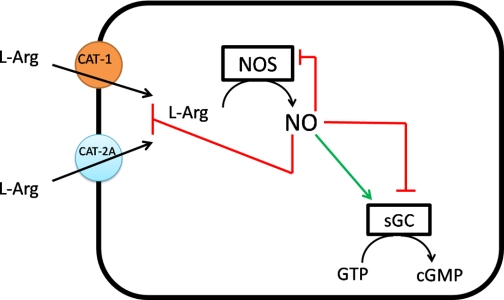
Fig. 1.
Schematic diagram of key nitric oxide (NO) autoregulatory sites. In cardiac myocytes, l-arginine (l-Arg) substrate is supplied to NO synthase (NOS) solely via cationic amino acid transporters 1 and 2A (CAT-1 and CAT-2A). Direct modulation of NO signaling via NO-mediated S-nitrosylation of NOS and soluble guanylate cyclase (sGC) to reduce their activities has been reported (Refs. 20 and 21, respectively). In the figure, NO binding and stimulation are depicted by a green arrow, whereas inhibition via NO S-nitrosylation is depicted by a red blunted line. The findings of Zhou et al. (28) now extend NO autoregulation upstream to CAT-1 and CAT-2A, the sources of l-arginine supply.
The next big question is how CAT-1 and CAT-2A are modulated by NO. Here again, the thorough experimental approach provides compelling, albeit circumstantial, evidence for direct NO-mediated S-nitrosylation of these CATs (28). That is, in general, the actions of NO are thought to be mediated through stimulation of soluble guanylate cyclase (sGC) and elevation of cGMP levels, which in turn activates cGMP-dependent protein kinases (6). Alternatively, many effects of NO are attributed to S-nitrosylation, i.e., the covalent modification of cysteine -SH groups by NO to generate S-nitrosothiol. For example, nitric oxide can increase the open probability of ryanodine receptor channels through this mechanism, contributing to positive inotropy (24, 27). Indeed, NO S-nitrosylation has been reported to modify several important proteins within the cardiovascular system: 1) The duration of β-adrenergic stimulation is reciprocally regulated in the endothelium and myocardium by S-nitrosylation of β-arrestin 2 which enhances β-adrenergic receptor downregulation (16) and G protein-coupled receptor kinase which prolongs β-adrenergic receptor desensitization (26). 2) S-nitrosylation has been shown to stimulate and inhibit the function of cardiac L-type Ca2+ channels, which can directly affect cardiac contractility (10, 23). 3) Significant reductions in myocardial injury following coronary artery occlusion and ischemia have also been attributed to S-nitrosylation. Specifically, S-nitrosylation of hypoxia-inducible factor-1α promotes myocardial capillary synthesis via increases in vascular endothelial growth factor (13). Many other proteins contributing to cardiac pathophysiology have also been reported to contain levels of S-nitrosylation (14).
In addition, NO has been reported to modulate its own signaling via S-nitrosylation of NOS itself (20), as well as downstream effectors of cGMP signaling, such as sGC (21). Therefore, it stands to reason that S-nitrosylation may also play a critical role in upstream regulation of NO signaling as well. Although one cannot rule out the involvement of additional protein mediators without direct experimental evidence, the simplest and most direct explanation for the observations of Zhou et al. (28) is consistent with NO-mediated S-nitrosylation of CAT-1 and CAT-2A. In particular: 1) Incubation with the exogenous NO producers sodium pentacyanonitrosyl ferrate(III) dehydrate (SNP) or S-nitroso-N-acetyl-dl-penicillamine (SNAP) decreased currents stimulated by 10 mM l-arginine (and l-lysine) in whole cell voltage-clamped myocytes. 2) Although application of an exogenous NO producer might inhibit CAT-mediated transport via cGMP production and subsequent G-kinase phosphorylation of the transporter, this does not appear to be the case because both SNP and SNAP decreased l-lysine fluxes in vesicle preparations, which do not contain cytosolic sGC and G-kinase. 3) They observed a biphasic behavior of l-arginine-induced currents, indicative of l-arginine entry and subsequent NOS conversion to NO and l-citrulline, with this endogenously produced NO then acting back on CAT to decrease l-arginine inward currents. This plausible mode of action was supported pharmacologically by inclusion of the ubiquitous NOS inhibitor l-NAME, which eliminated the inhibitory component of the l-arginine currents. In contrast, the sGC inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), was without effect. Taken together these data strongly support that there is a direct NO-mediated inhibition of CAT transport in cardiac myocytes.
In conclusion, the article by Zhou et al. (28) brings to light new relevant understanding of NO signaling, which now must include regulation of its own synthesis by downregulation of substrate delivery via CATs. The cationic amino acid transporters CAT-1 and CAT-2A are now tentative new participants in this scenario and if confirmed, these transporters could be targets for drug development for the treatment of some cardiac insufficiencies. We are all aware that cardiac excitation-contraction coupling involves a diverse team of players, which now appears to include the plasma membrane cationic amino acid transporters CAT-1 and CAT-2A.
GRANTS
The author's work is supported by National Institutes of Health Grants GM-061583 and DK-83859 and National Science Foundation Grant MCB 0347202.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
ACKNOWLEDGMENTS
I thank Drs. Pablo Artigas and Charles Costa for comments on the manuscript.
REFERENCES
- 1.Bredt DS. Nitric oxide signaling specificity - the heart of the problem. J Cell Sci 116: 9–15, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Closs EI, Boissel JP, Habermeier A, Rotmann A. Structure and function of cationic amino transporters (CATs). J Membr Biol 213: 67–77, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Deves R, Angelo S, Chavez P. N-ethylmaleimide discriminates between two lysine transport systems in human erythrocytes. J Physiol 468: 753–766, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deves R, Boyd CAR. Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev 78: 487–545, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Forray MI, Angelo S, Boyd CAR, Deves R. Transport of nitric oxide synthase inhibitors through cationic amino acid carriers in human erythrocytes. Biochem Pharmacol 50: 1963–1968, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Friebe A, Koesling D. The function of NO-sensitive guanylyl cyclase: what we can learn from genetic mouse models. Nitric Oxide 21: 149–56, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 8.Gross SS, Wolin MS. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol 57: 737–769, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Habermeier A, Wolf S, Martine U, Graf P, Closs EI. Two amino acid residues determine the low substrate affinity of human cationic amino acid transporter-2A. J Biol Chem 278: 19492–19499, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Hu H, Chiamvimonvat N, Yamagishi T, Marban E. Direct inhibition of expressed cardiac L-type Ca2+ channels by S-nitrosothiol nitric oxide donors. Circ Res 81: 742–52, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84: 9265–9269, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaye DM, Parnell MM, Ahlers BA. Reduced myocardial and systemic l-arginine uptake in heart failure. Circ Res 91: 1198–1203, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Lima B, Lam GK, Xie L, Diesen DL, Villamizar N, Nienaber J, Messina E, Bowles D, Kontos CD, Hare JM, Stamler JS, Rockman HA. Endogenous S-nitrosothiols protect against myocardial injury. Proc Natl Acad Sci USA 106: 6297–6302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res 106: 633–646, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu X, Zheng R, González J, Gaspers L, Kuzhikandathil E, Peluffo RD. l-Lysine uptake in giant vesicles from cardiac ventricular sarcolemma: two components of cationic amino acid transport. Biosci Rep 29: 271–281, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Ozawa K, Whalen EJ, Nelson CD, Mu Y, Hess DT, Lefkowitz RJ, Stamler JS. S-nitrosylation of β-arrestin regulates β-adrenergic receptor trafficking. Mol Cell 31: 395–405, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Peluffo RD. l-Arginine currents in rat cardiac ventricular myocytes. J Physiol 580: 925–936, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pola E, Bertran J, Roca A, Palacin M, Zorzano A, Testar X. Sensitivity of system A and ASC transport activities to thiol-group-modifying reagents in rat liver plasma membrane vesicles. Evidence for a direct binding of N-ethylmalemide and iodoacetamide on A and ASC carriers. Biochem J 271: 297–303, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci USA 101: 2619–2624, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci USA 104: 12312–12317, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seddon M, Shah AM, Casadei B. Cardiomyocytes as effectors of nitric oxide signaling. Cardiovasc Res 75: 315–326, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha-1 subunit and reduced ischemia/reperfusion injury. Circ Res 98: 403–411, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Sun J, Yamaguchi N, Xu L, Eu JP, Stamler JS, Meissner G. Regulation of the cardiac muscle ryanodine receptor by O2 tension and S-nitrosoglutathione. Biochemistry 47: 13985–13990, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflügers Arch 447: 532–42, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Whalen EJ, Foster MW, Matsumoto A, Ozawa K, Violin JD, Que LG, Nelson CD, Benhar M, Keys JR, Rockman HA, Koch WJ, Daaka Y, Lefkowitz RJ, Stamler JS. Regulation of β-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell 129: 511–522, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279: 234–237, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Kim DD, Peluffo RD. Nitric oxide can acutely modulate its biosynthesis through a negative feedback mechanism on l-arginine transport in cardiac myocytes. Am J Physiol Cell Physiol (May26, 2010). doi:10.1152/ajpcell.00077.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]