Abstract
Ryanodine receptors (RyRs) regulate contractility in resistance-size cerebral artery smooth muscle, yet their molecular identity, subcellular location, and phenotype in this tissue remain unknown. Following rat resistance-size cerebral artery myocyte sarcoplasmic reticulum (SR) purification and incorporation into POPE-POPS-POPC (5:3:2; wt/wt) bilayers, unitary conductances of 110 ± 8, 334 ± 15, and 441 ± 27 pS in symmetric 300 mM Cs+ were usually detected. The most frequent (34/40 bilayers) conductance (334 pS) decreased to ≤100 pS when Cs+ was replaced with Ca2+. The predominant conductance displayed 66 bursts/min with at least three open and three closed states. The steady-state activity (NPo)-voltage curve was bell shaped, with NPo drastically decreasing when voltage was switched from −30 to −40 mV. NPo increased when intracellular calcium (Ca2+i) was raised within 0.1–100 μM to abruptly diminish with higher Ca2+i. Thus maximal activity occurred within the Ca2+i range found in rat cerebral artery myocytes under physiological conditions. NPo was reduced by ruthenium red (80 μM), increased monotonically by caffeine (0.1–5 mM) or ryanodine (0.05–5 μM), and unaffected by heparin (2 mg/ml). This phenotype resembles that of cardiac RyR and recombinant RyR2. RT-PCR detected RyR1, RyR2, and RyR3 transcripts in cerebral artery myocytes. However, real-time PCR indicated that RyR2 was 4 and 1.5 times more abundant than RyR1 and RyR3, respectively. Consistently, Western blotting showed that the RyR2 product was very abundant. Immunofluorescence showed that each RyR isoform distributed differentially among subcellular compartments. In particular, RyR2 was drastically stronger in the subplasmalemma than in other compartments, underscoring the predominance of RyR2 in a compartment where SR is abundant. Consistently, RyR from SR-enriched membranes displayed pharmacological specificity typical of RyR2, being activated by digoxin (1 μM), resistant to dantrolene (100 μM), and shifted to a subconductance by neomycin (100 nM). Therefore, RyR2 is the predominant molecular and functional RyR that is expressed in the SR membrane of rat resistance-size cerebral artery myocytes.
Keywords: calcium-release channels, vascular smooth muscle
ryanodine receptors (RyRs) are intracellular Ca2+ release channels found in the endoplasmic and sarcoplasmic reticulum (SR) membranes of most eukaryotes. Three receptor isoforms have been isolated and characterized from mammals, each encoded by a different gene: RyR1, which predominates in skeletal muscle; RyR2, which predominates in cardiac muscle, and RyR3, which is widely expressed in most tissues in miniscule amounts, yet relatively abundant in brain tissue (13, 23–25, 82). Each isoform consists of a homotetramer of one RyR gene product, although the native receptor is actually a heterooligomer with subunit composition of (RyRmonomer)4(FKBP)4 where FKBP refers to the FK506 binding protein, a polypeptide with a mass of ∼12 kDa. Thus native RyR complexes are the largest ionotropic receptor structures known, with a molecular weight of 2.2 million (24). Each RyR shares many similarities, including primary sequence and three-dimensional structure, with the others. However, RyR isoforms differ in key ion channel characteristics, which contributes to possible differential functional roles in the cell, some cells containing more than one RyR isoform (21, 22).
Together with InsP3-gated Ca2+ release channels, RyRs are important for mediating intracellular Ca2+ release and, thus, determine many critical cell functions (4, 22, 55, 62, 86). In small (resistance-size) cerebral arteries that control cerebral blood flow, intracellular Ca2+ release via RyRs regulates myogenic tone (29, 36, 57). RyRs, voltage-dependent Ca2+ (mainly CaV1.2) channels (VDCC), and large-conductance, Ca2+- and voltage-gated K+ (BK) channels operate in orchestration to control a variety of Ca2+ signal modalities in the arterial smooth muscle cell and, thus, control myogenic tone and vasomotion (6, 9, 29).
Ca2+ influx via CaV1.2 channels (20, 66) contributes to cytosolic Ca2+, which maintains SR Ca2+ load. In turn, activation of several sarcoplasmic RyRs generates a localized micromolar cytosolic Ca2+ elevation termed “Ca2+ spark.” A Ca2+ spark stimulates several nearby BK channels, leading to a transient BK current, and vasodilation (20, 29, 57). The local intracellular Ca2+ elevation resulting from RyR-generated sparks may feedback positively on SR RyRs causing further release of Ca2+ via Ca2+-induced, Ca2+-release (CICR). As a consequence of CICR, local Ca2+ close to the RyR and nearby the BK channel reaches ≥10 μM (60, 61), which activates the intracellular Ca2+-sensors of the BK channel (14) leading to channel activation. BK channel activation, in turn, generates outward K+ current that causes membrane re/hyperpolarization, leading to a reduction in CaV1.2 channel activity, a decrease in global intracellular calcium (Ca2+i) levels and thus promotes vasodilation (29). Therefore, RyRs in cerebral artery myocytes are key elements of a negative-feed back loop that limits Ca2+ influx and myogenic tone (29, 30, 36). Consistent with this role of RyR in vascular smooth muscle, several vasoconstrictors, including UTP and α1 adrenergic agonists, inhibit RyR-mediated Ca2+ sparks in arterial myocytes (28, 50). In addition, it has been recently reported that RyR inhibition and VDCC blockade synergistically inhibit basilar (but not femoral) artery constriction, raising speculation that pharmacological targeting of RyRs could be of therapeutic use in cerebral artery spasm (65). Despite the importance of RyRs in the physiology, pathophysiology, and pharmacology of cerebral artery smooth muscle, the molecular identity, subcellular location, and ion current phenotype of these native channels have not been determined.
Using PCR, real-time PCR, Western immunoblotting, confocal imaging, and single-channel electrophysiology after SR channel protein reconstitution into planar lipid bilayers, we have identified the predominant subtype, subcellular localization, and ion current biophysical and pharmacological properties of RyRs in rat cerebral artery smooth muscle cells. Our data reveal a distinct RyR isoform distribution across different domains of the cerebral artery myocyte, with RyR2 being prevalent in the subplasmalemma, a region enriched in SR membrane. Consistently, the most frequent conductance detected in a purified cerebral artery myocyte SR membrane preparation displayed electrophysiological and pharmacological features typical of homomeric RyR2. Our study also shows that these channels are ideally suited to operate within the Cai2+ range considered physiological in rat cerebral artery myocytes (75).
MATERIALS AND METHODS
Ethical approval.
The care of animals and protocols were performed in accordance to the guidelines of the Animal Care and Use Committee of the University of Tennessee Health Science Center, which is an Association for Assessment and Accreditation of Laboratory Animal Care-accredited institution.
Cerebral artery myocyte isolation.
Sprague-Dawley (∼250 g) rats of either sex were decapitated with a guillotine. The brain was removed in ice-cold physiological saline solution (42), and anterior, middle, and posterior cerebral arteries were dissected out under microscope (Nikon SMZ645, Nikon Instruments, Melville, NY). Myocytes were enzymatically dissociated in a HEPES-buffered solution (in mM): 55 NaCl, 80 Na+ glutamate, 5.6 KCl, 2 MgCl2, 10 HEPES, and 10 glucose (pH 7.3 with NaOH), which was supplemented with 0.7 mg/ml papain and 1.0 mg/ml collagenase. After a few centrifugations (84), the resuspended pellet was pipetted by using a series of borosilicate Pasteur pipettes having fire-polished, diminishing internal diameter tips. The procedure rendered a cell suspension containing relaxed, individual myocytes (≥5 myocytes/field using a ×40 objective) that could be identified under microscope (Olympus IX-70; Olympus America, Woodbury, NY).
Preparation of arterial myocyte SR membranes.
SR vesicles were obtained by following a procedure originally described for bovine coronary artery (39), with slight modifications. Briefly, 100–200 μm OD cerebral arteries (see Cerebral artery myocyte isolation) were deendothelized, cleaned of connective tissue under microscope (Nikon SMZ645), and cut into 2-mm-long segments (7, 73). Each segment was crushed in liquid N2 and homogenized with a Tenbroeck glass tissue grinder (Kimble Chase Life Science and Research Products, Vineland, NJ) in ice-cold MOPS buffer: 0.9% NaCl, 10 mM MOPS (pH 7.0), 2 μM leupeptin, and 0.8 μM benzamidine. The homogenate was centrifuged at 4,000 g and 4°C for 5 min. Next, the supernatant was centrifuged at 8,000 g and 4°C for 10 min. The resulting supernatant was centrifuged at 40,000 g and 4°C for 60 min. The pellet, which corresponded to a highly enriched SR membrane fraction (39), was resuspended in a “SR solution” (0.9% NaCl, 0.3 M sucrose, and 0.1 μM phenylmethylsulfonyl fluoride) to render a crude SR suspension. This suspension was fractionated on a discontinuous sucrose gradient as described (81). The following sucrose solutions (in %, wt/vol) containing 10 mM HEPES (pH 7.0) were layered sequentially in a centrifuge tube (Beckman SW55): 0.5 ml (45), 1 ml (40), 1.3 ml (35), 0.8 ml (30), and 0.5 ml (27). Crude SR (∼30 mg) resuspended in SR solution was deposited on top of the 25% sucrose layer by use of a micropipettor, and the centrifuge tube was then spun at 66,500 g at 4°C overnight. The sucrose fraction between the 35 and 40% layers contained the purified SR, which was collected and diluted in MOPS solution (composition above). The SR-containing preparation was further centrifuged at 40,000 g and 4°C for 90 min. The resulting pellet was resuspended in SR solution, aliquoted, frozen in liquid N2, and stored at −80°C until use.
Cell culture and transfection with RyR cDNAs.
HEK-293 cells were transfected with cDNAs encoding RyR1 inserted into pC1-neo (kindly provided by Paul Allen, Harvard University), RyR2, or RyR3 inserted into pcDNA3 (kindly provided by Dr. Wayne Chen; University of Calgary) by following procedures previously described (1, 10, 43). Cell transfection was carried out by use of Lipofectamine 2000 (Invitrogen, Grand Island, NY). Microsomal membranes from transfected and nontransfected HEK293 cells were prepared as previously described (10).
Reconstitution of SR ion channels into planar phospholipid bilayers, electrophysiology, and drug application.
SR membranes from rat cerebral artery myocytes or HEK293 cells were reconstituted into planar phospholipid bilayers following procedures described elsewhere (56), with slight modifications. Briefly, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine (POPS), and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) (5:3:2, wt/wt) were dissolved in decane (50 mg/ml) and used to form a vertical planar lipid bilayer across a 200-μm aperture between the cis and trans chambers. All phospholipid species and decane were purchased from Avanti Polar Lipids (Birmingham, AL).
The SR membrane preparation (50–100 μg) was added to the cis chamber solution, which bathed the cytosolic side of the RyR channel, with the trans chamber solution bathing the RyR luminal side. The trans chamber was held at ground and the cis was held at potentials relative to ground. Unless otherwise indicated, the recording solution in both cis and trans chambers contained (in mM): 300 Cs+ methanesulfonate and 10 MOPS, pH 7.2. Under these ionic conditions, Cs+ flows from the trans to the cis chamber when the electrical potential at the cis side of the bilayer is held at negative values. After addition of 5 μl of liposome preparation containing RyR to the cis chamber, channel incorporation onto the bilayer was facilitated via osmosis by substituting 50 for 300 mM Cs+ methanesulfonate in the trans chamber solution. Channel incorporation into the bilayer and mixing of modulators in solution were further facilitated by electronic stirring (SUNStir-3, Warner).
Cesium was chosen as charge carrier to determine channel slope conductance (γ) from unitary current-voltage plots because 1) γCs+/γCa2+ = ∼2 for RyR channels (11), increasing the signal-to-noise ratio, and 2) both presence of Cs+ and absence of Ca2+ avoid electrical interference from contaminant K+ channels (Ca2+-gated K+ channels in particular) that might be present in the SR membrane (39). The possibility of current contamination due to endogenous Cl− channels was minimized by replacing Cl− with methanesulfonate (39). In the experiments to study RyR channel Ca2+ sensitivity, CaCl2 and EGTA mixtures were added to the cis chamber to achieve a free Ca2+ final concentration ranging between 10−8 and 10−4 M. Nominal free Ca2+ was calculated with MaxChelator Sliders (C. Patton, Stanford University, Stanford, CA), and validated experimentally as described (16).
A 2 mM ryanodine stock solution was prepared by dissolving ryanodine (Biomol International, Plymouth Meeting, PA) in high-purity, deionized water containing <1% ethanol and then further diluted in cis solution to final concentration (0.05–100 μM). Caffeine was dissolved in chloroform, rendering a 2.54 M stock solution, and further diluted in cis solution to final concentration (0.1–5 mM). Ruthenium red, neomycin, and heparin were dissolved in high-purity, deionized water to make 12.7 mM, 0.17 M, and 50 mg/ml stock solutions, respectively. These solutions were diluted in cis solution to final concentration: 40–80 μM, 100 nM, and 2 mg/ml, respectively. Dantrolene was dissolved in methanol-dimethylformyde (1:1, vol/vol) to make a 148 mM stock solution, which was diluted in cis solution to final concentration (100 μM). Digoxin was dissolved in pure pyridine to 6.4 mM, which was pipetted into the cis chamber to achieve a final concentration of 1 μM. In all cases, drug-free solutions containing the appropriate solvent concentration were added to the cis chamber and used as control solutions.
Single-channel currents were acquired by using a BC-525C amplifier (Warner Instruments, Hamden, CT), low-pass filtered at 1 kHz with an eight-pole Bessel filter (Warner LPF-8), digitized at 5 kHz, and stored in a computer. Data acquisition and analysis were performed with Digidata 1440 and pClamp 9.2 (Molecular Devices, Sunnyvale, CA). All lipid bilayer experiments were performed at room temperature (∼20°C). The product of the number of channels in the bilayer (N) and the channel open probability (Po) was used as an index of channel steady-state activity (NPo). NPo and dwell-time distributions were determined from 3–5 min of gap-free recordings, as described (15). Unitary events were detected by using a 40–60% threshold detection. The minimum number of exponentials to adequately fit the dwell-time data was determined by using an F-table, setting statistical difference at P < 0.01 (17).
Reverse transcription and PCR.
Total RNA was isolated from rat deendothelized, resistance-size cerebral arteries (100–200 μm OD), and also from skeletal muscle, heart, and brain, message from these tissues serving as positive controls for RyR1, RyR2, and RyR3, respectively. In all cases, total RNA was isolated by using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Genomic DNA contamination was removed by DNA-free DNAse treatment and removal reagents (Ambion, Austin, TX). Briefly, 2 units of DNAse I were added to the isolated RNA, and the preparation was incubated at 37°C for 30 min. Then 2 μl of DNAse Inactivation Reagent were added, and the preparation was incubated at room temperature for 2 min. Samples were then centrifuged at 10,000 g for 1 min to pellet the DNAse inactivation reagent. Supernatants were collected, and RNA amounts were determined by optical density at 260 nm.
We used 1 μg of total RNA for first-strand cDNA synthesis with oligo(dT)30 primers (Invitrogen, Carlsbad, CA) and Superscript II RNAse H-Reverse Transcriptase. The resulting first-strand cDNAs were directly used as templates for PCR amplification, by use of sense and antisense primers specific for RyR1, RyR2, and RyR3 (Table 1). Using PCR SuperMix (GIBCO-BRL), 30 cycles of PCR were conducted, each including denaturation at 94°C for 30 s, annealing at 53–58°C for 30 s, and extension at 68°C for 45 s. Cycles were followed by a final extension step at 72°C for 10 min, and the PCR products were stored at 4°C. After thawing, the PCR products were analyzed by 1.2% agarose gel electrophoresis, visualized by staining with ethidium bromide, and sequenced at the University of Tennessee HSC Molecular Resource Center. The relative ratio between RyR isoforms was calculated by using XΔCt, where X is amplification efficiency and ΔCt is the difference in PCR cycle number.
Table 1.
PCR primers used for detection of RyR1, RyR2, and RyR3 transcripts in cerebral artery smooth muscle, brain, heart, and skeletal muscle
| Primer | Location on cDNA | Nucleotide Sequence 5′-3′ | Predicted PCR Fragments Length | Reference Sequences |
|---|---|---|---|---|
| RyR3RLTF | 13282–13303 | CAGACCAAGTTACTGCACTACC (22mer, Tm = 58.6) | 121 bp | XM_001080527 |
| RYR3RLTR | 13383–13403 | TCTAAAGGCTCTTCCGTGACC (21mer, Tm = 58.8) | ||
| RYRRLT2F | 13618–13619 | GCTGAACTATTTTGCTCGCAAC (22mer, Tm = 58.4) | 110 bp | EU346200 |
| RYRRLT2R | 13707–13727 | CCACAGAAGAAGTGGAGACCT (21mer, Tm = 58.8) | ||
| RYRRLT1F | 13641–13664 | CTTGAACTACTTGTCGAGGAACTT (24mer, Tm = 58.4) | 109 bp | XM_001078539 |
| RYRRLT1R | 13731–13749 | TGGCGGAGAGTCTGAAACC (19mer, Tm = 58.5) |
Real-time PCR.
Specific primers (Table 2) that spanned long introns to distinguish cDNA from genomic DNA were designed using universal probe library primer design software (www.roche-applied-science.com/sis/rtpcr/upl/index.jsp). We performed quantitative TaqMan PCR reactions on a LC480 light cycler (Roche Applied Science, Indianapolis, IN) including an initial activation step at 95°C for 5 min, followed by 40 cycles (95°C × 10 s, 60°C × 30 s, and 72°C × 10 s). Each PCR mixture contained (in μl): 5 Taqman LC480 master mix, 0.1 forward primer, 0.1 reverse primer, 0.1 hydrolysis probe, 2.7 RNAse-free water, and 2 cDNA template. We used platelet-endothelial cell adhesion molecule 1 (Pecam1), smooth muscle myosin heavy polypeptide 11 (Myh11), and aquaporin 4 (Aqp4) as markers of endothelial cells, smooth muscle cells and astrocytes, respectively. A negative control having smooth muscle cell RNA instead of cDNA was included in each experiment. Integrity of PCR products was verified by dissociation curve analysis. Relative RyR isoform RNA expression was calculated from the differences of fluorescence threshold values (ΔCt) between different RyR isoforms. Ct values for each RyR isoform were normalized to the Ct value of Myh11, and differences among the normalized Ct values of the different RyR isoforms let us establish the relative RyR isoform RNA expression (83). Standard curves for five 10-fold dilutions of cDNA samples were run for all primer pairs to determine the PCR efficiency. Amplification efficiencies for the three RyR subtypes were similar under our experimental conditions (for primers and probes, see Table 2), with standard curve slopes ranging between −1.85 and −2.25. All PCR reactions including the standard curves were performed in triplicates.
Table 2.
Primers and probes used in quantitative real-time PCR
| Gene | Accession No. | Primers | Primer sequence | UPL Probe No. | Amplicon Size, nt |
|---|---|---|---|---|---|
| RyR1 | XM_001078539.1 | Left | 5′-ACCAGGACTTCTTGCTGCAT-3′ | 42 | 96 |
| Right | 5′-TCTCCGTGGTGCTGAAAGT-3′ | ||||
| RyR2 | NM_032078.1 | Left | 5′-CAAACAGGGCAGAAGACACC-3′ | 115 | 103 |
| Right | 5′-CTCTGAGGGTGCTCCACCT-3′ | ||||
| RyR3 | XM_342491.3 | Left | 5′-TGGAACCTACCTCAGAAGCAA-3′ | 65 | 61 |
| Right | 5′-AGCACGAAATTGCAGACACA-3′ | ||||
| Pecam1 | NM_031591.1 | Left | 5′-CTCAGTCGGCTGACAAGATG-3′ | 21 | 61 |
| Right | 5′-AGGCTTGCATAGAGCAGCAT-3′ | ||||
| Myh11 | XM_573030.2 | Left | 5′-CCTGCTAGTCCACCCCAGTA-3′ | 119 | 68 |
| Right | 5′-ACTGAGCTGCCCTTTCTGTG-3′ | ||||
| Aqp4 | NM_012825.2 | Left | 5′-TGGGAGGATTGGGAGTCA-3′ | 22 | 93 |
| Right | 5′-TGAATACCAGCTGGAAAGTGATT-3′ |
SDS-PAGE and Western blot analysis.
The SR membrane protein (see above) was loaded onto 8–15% precast Tris-acetate gels (Pierce, Rockford, IL) at a concentration of 50 μg/lane by following the manufacturer's specifications. After electrophoretic separation, SR membrane protein was transferred to a 0.45-mm nitrocellulose membrane (Millipore, Billerica, MA) at 60 V and 4°C for 24 h. Upon a 1-h-long incubation at room temperature with phosphate-buffered saline (PBS) (in mM): 137 NaCl, 2.68 KCl, 10 Na2HPO4, 1.76 KH2PO4 (pH 7.4) and 3% nonfat dry milk (NFD) for RyR1, PBS+3% NFD+0.1% Tween-20 for RyR2, or 10% normal goat serum (NGS) in PBS for RyR3, membranes were probed with primary antibodies against RyR1 (monoclonal; catalog no. 05-269, Upstate, Lake Placid, NY), RyR2 (monoclonal; catalog no. R-128, Sigma, Saint Louis, MO), or RyR3 (polyclonal; catalog no. AB9082, Chemicon International, Temecula, CA). RyR1 and RyR2 antibodies were diluted in PBS containing 0.05% NFD at 1:2,000 and 1 μg/ml, respectively, and RyR3 antibodies were diluted in PBS containing 1% NGS at 1:2,000. We also followed a similar procedure using purified, high-affinity sequence-specific polyclonal antibodies against RyR1, RyR2, or RyR3 antibodies (31–33) at 1:500 dilutions in 3% (wt/vol) NFD in 0.02% PBS (containing 0.05% Tween-20) at 4°C for comparison. After wash with PBS containing 0.05% Tween-20 (PBS-T), membranes were incubated for 1 h at room temperature with horseradish peroxidase-coupled goat anti-mouse IgM or rabbit anti-mouse secondary antibodies (Pierce, Rockford, IL) diluted in 1% bovine serum albumin/PBS-Tween. After extensive wash in PBS-T, protein-antibody complexes were detected by chemiluminescence following the manufacturer's specifications (SuperSignal West Pico Chemiluminescent Substrate, Pierce, Rockford, IL). Image J (NIH; http://rsb.info.nih.gov/ij/download.html) was used to determine size and density of the bands. RyR isoform values were normalized to β-actin.
Immunofluorescence.
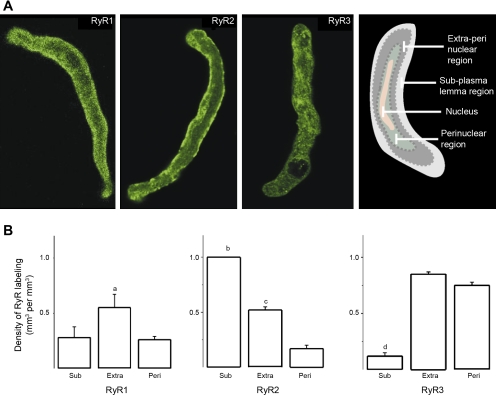
Isolated myocytes were allowed to settle onto poly-d-lysine-coated coverslips. Myocytes were fixed by a 15-min exposure to ice-cold methanol (−20°C), and permeabilized by a three 5-min-long washes in 0.6% Triton X-100 in PBS (pH 7.4). After three 5-min-long washes in “blocking solution,” namely, PBS containing 1% BSA, 4% goat serum, and 0.3% Triton X-100, myocytes were incubated overnight at 4°C with purified, high-affinity sequence-specific polyclonal antibodies (1:500 dilution): epitopes for anti-RyR1, anti-RyR2, and anti-RyR3 were channel peptide residues 4476–4486 (33), 1344–1365 (31), and 4236–4336 (32), respectively. Coverslips were subjected to four 5-min-long washes in blocking solution and then incubated at 22°C in the dark for 1 h with cyanin 3-conjugated donkey anti-rabbit antibody (1:200 dilution; Jackson Immuno-Research, West Grove, PA). After six 5-min-long washes in PBS, immunostained structures were mounted on slides using PBS:glycerol (1:1, vol/vol) for fluorescence imaging, which was conducted by using a Zeiss LSM 5 Pascal confocal microscope (Advanced Imaging Microscopy, Jena, Germany), with excitation and emission set to 550 and 570 nm. Controls to determine the threshold level for fluorescent imaging were made by omitting the primary antibody. Data were acquired by use of Zeiss LSM 5 Pascal Confocal Microscopy Software 3.2 (Advanced Imaging Microscopy). Images were stacked by using Image J software and processed with Adobe Photoshop (Adobe Systems, San Jose, CA). The myocyte fluorescence confocal image was subdivided into three distinct cellular regions (see cartoon on the far right of Fig. 9A): 1) perinuclear: within 1.5 μm of, and excluding any labeling within, the YOYO label of the nucleus; 2) subplasmalemmal: within 1.5 μm of the plasma membrane; and 3) extraperinuclear: corresponding to the remaining cytoplasmic volume of the cell. Data were analyzed as described in detail elsewhere (35).
Fig. 9.
Each RyR isoform is differentially distributed across subcellular regions in freshly isolated rat cerebral artery myocytes. A: fluorescent immunostaining for cerebral artery myocyte RyR isoforms visualized by confocal microcopy. The myocyte fluorescence confocal image was subdivided into 3 distinct cellular regions, as described in the main text. The cartoon at right shows this compartmentalization: perinuclear, extraperinuclear, and subplasmalemmal regions are shown in dark green, dark gray, and light gray, respectively, with the nucleus being pictured in pink. Confocal images indicate that the RyR2 label is highly concentrated in the subplasmalemma, with noticeable location in the extraperinuclear region. The RyR1 label, however, evenly distributes across subplasmalemmal and extraperinuclear regions. The RyR3 label follows a punctuate pattern across all cell regions. B: averaged label densities (in μm3 of label/μm3 of region) within each cellular region for RyR1 (n = 9), RyR2 (n = 12), and RyR3 (n = 10). Sub, subplasmalemma; Extra, extraperinuclear; Peri, perinuclear. aDifferent from Sub and Peri, P < 0.05; bdifferent from Peri, P < 0.001; cdifferent from Peri, P < 0.01; ddifferent from Extra and Peri, P < 0.001.
Chemicals.
Unless otherwise stated, chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Statistics.
Data are given as means ± SE. For multiple comparisons, data were analyzed with analysis of variance, followed by Bonferroni's multiple range test to determine the significance of the difference between individual means. A Student's t-test was used to determine statistical significance of differences between data. Unless otherwise stated, P < 0.05 was considered statistically significant.
RESULTS
Conduction and gating properties of rat cerebrovascular smooth muscle SR channels.
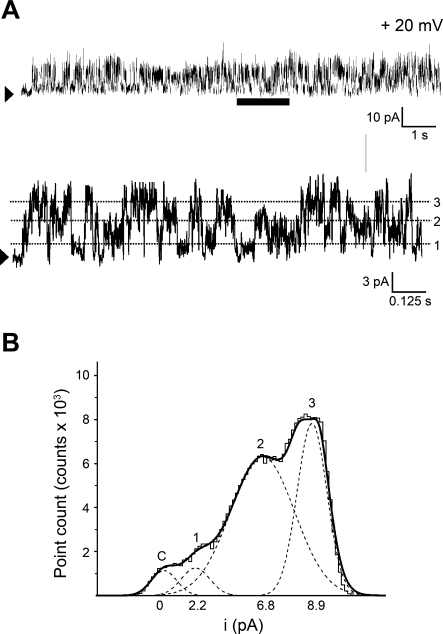
Ion channel protein reconstitution into planar phospholipid bilayers is a widely used approach that allows electrophysiological characterization of SR membrane ion channel phenotypes (13, 19, 39, 78, 85, 86). Immediately after incorporation of the SR membrane preparation into a POPE-POPS-POPC (5:3:2, wt/wt) planar bilayer, well-resolved single-channel events consisting of multiple current amplitudes were detected in symmetrical 300 mM Cs+ solution (Fig. 1A), provided that the cytosolic side of the channel was exposed to Ca2+ levels ≥1 μM. For instance, with the voltage set to +20 mV in the bilayer cis side (equivalent to the cytosolic side of the SR membrane), unitary events with single-channel amplitudes (i) of 2.2, 6.8, and 8.9 pA were frequently detected. These i values corresponded to unitary (i/V slope, where V is voltage) conductances of 110 ± 8, 334 ± 15, and 441 ± 27 pS, respectively.
Fig. 1.
Reconstitution of rat cerebral artery myocyte sarcoplasmic reticulum (SR) membrane protein into lipid bilayers renders unitary current events that correspond to at least 3 conductance phenotypes. A: gap-free channel current recording using Cs+ as permeant ion after SR protein reconstitution into 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine (POPS), and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) (5:3:2, wt/wt) planar bilayers. This bilayer reconstitution system was used in each of the studies shown in Figs. 2–7, 10, and 11 and in Supplemental Figs. S1 and S2. An arrowhead indicates the baseline (nonconducting channel level); voltage (V) = +20 mV. At the bottom of A, a time-expanded trace from the interval underscored with a horizontal bar is provided. The dotted lines highlight the 3 most frequent unitary current amplitudes (i). Data were low-passed at 1 and digitized at 10 kHz. B: all-points amplitude histogram obtained from the time-expanded interval. The 4 modes correspond to the channel closed state(s), and current levels 1, 2 and 3, which are defined by unitary conductances of 110 ± 8, 334 ± 15, and 441 ± 27 pS, respectively (n = 4–9).
The multiplicity of unitary current events can be clearly seen in the time-expanded current trace shown at the bottom of Fig. 1A. All points amplitude histograms fitted to Gaussian distributions are shown in Fig. 1B. Interestingly, open-in tandem conformations have been reported for both RyR1- and RyR2-type channels (48, 49). Thus the 334 pS level could be interpreted as the simultaneous opening and closing of three 110 pS channels. This interpretation requires that during all our recordings the channels existed only in closed, single open, and three-open-in tandem conformations, as we systematically failed to observe events of 220 pS, which would have reflected the two-open-in tandem conformation. Moreover, none of our recordings measured overlaps between three-open in tandem and single openings indicating that, if cerebral myocyte native SR channels opened in tandem, the single-channel open and the three-open-in tandem conformations would be mutually exclusive, which would be highly unusual. Thus “open-in tandem conformation” interpretation of the data shown in Fig. 1 appears highly unlikely. Rather, it is likely that different channel phenotypes contribute to the three conductances (110 ± 8, 334 ± 15, and 441 ± 27 pS) observed under fixed ionic and voltage conditions. These findings suggest that different RyR channel subtypes may exist in rat cerebral artery myocyte SR membranes. However, we also considered the possibility that SR channels other than RyRs might contribute to the multiplicity of conductances observed in our records. InsP3-gated Ca2+-release channels, in particular, deserve special consideration since they usually show lower conductances than RyRs and are also abundant in vascular smooth muscle SR (51, 74). Two lines of evidence indicate that the small conductance observed in our records does not correspond to InsP3-gated Ca2+-release channels: 1) in the absence of specific activators (particularly, InsP3 itself), InsP3-gated Ca2+-release channels remain nonconductive at μM Ca2+i used to record ion channel activity (47), and 2) none of the three conductances frequently observed in our preparation were blocked by heparin (2 mg/ml) in cis solution (n = 9), an InsP3- gated Ca2+-release channel blocker (2, 71).
The existence of different functional RyRs after protein reconstitution into a defined lipid environment may be explained by a variety of structural determinants, such as different homomeric RyRs, heteromeric RyRs, ion channel association with regulatory proteins, and/or current modulation by membrane-bound signals. The SR channel unitary amplitudes described in Fig. 1, however, are similar to those reported for RyR channels cloned from rabbit cardiac tissue, expressed in chinese hamster ovary (CHO) cells, and reconstituted into POPE/POPS/cholesterol bilayers (5). Plurality of unitary conductance values could also be explained by the existence of subconductance state(s) of a given, structurally defined RyR channel complex (40, 41). However, in absence of ryanodine (Figs. 1, 2A, 3A), we consistently failed to detect transitions from fully open to partially open events, making it unlikely that subconductance states of a structurally defined RyR are the main contributors to the multiplicity of single-channel events identified here.
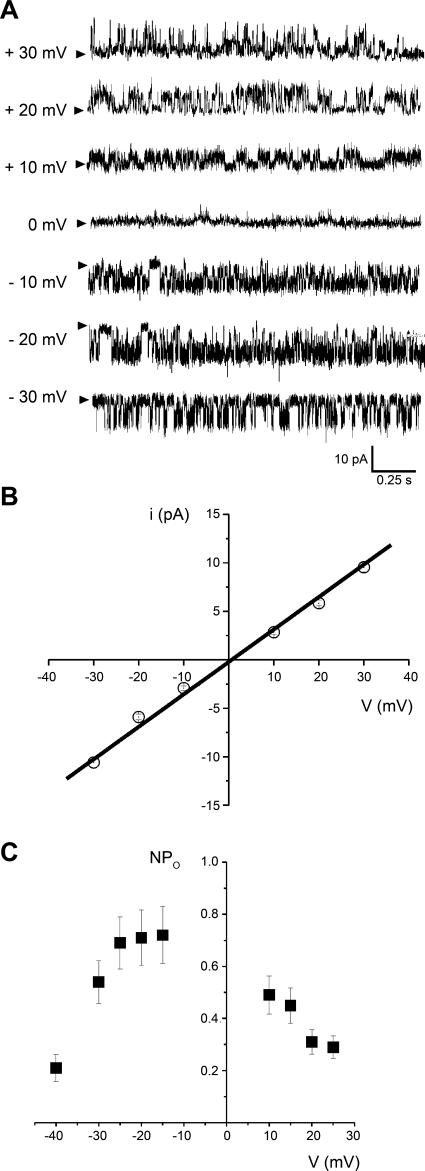
Fig. 2.
Basic ion conduction and voltage-gating properties of the ion conductance most frequently detected. A: traces of channel activity from the most common conductance observed after SR membrane channel reconstitution into planar phospholipid bilayers. This conductance was observed in 34 of 40 bilayers. Records were obtained in symmetric 300 mM Cs-methanesulfonate, with positive current flowing toward the cis and trans chambers, shown as positive and negative deflections, respectively. Arrowheads indicate the baseline. Data were low-passed at 1 and digitized at 10 kHz. B: i-V plot from the records shown in A; i values were obtained from all-points amplitude histograms (17). The plot shows no open channel rectification within ± 30 mV, with a slope conductance of 334 pS. C: channel steady-state activity (NPo)-V plot corresponding to the most frequent conductance; NPo values were obtained from the area under the curve (AUC) of all-points amplitude histograms (17). Openings could not be adequately resolved for fitting within −10 to +5 mV. The NPo-V curve is bell shaped, with NPo remaining fairly stable within −25 to −15 mV. For A–C, given voltages correspond to those at the bilayer cis side, i.e., the SR ion channel cytosolic side.
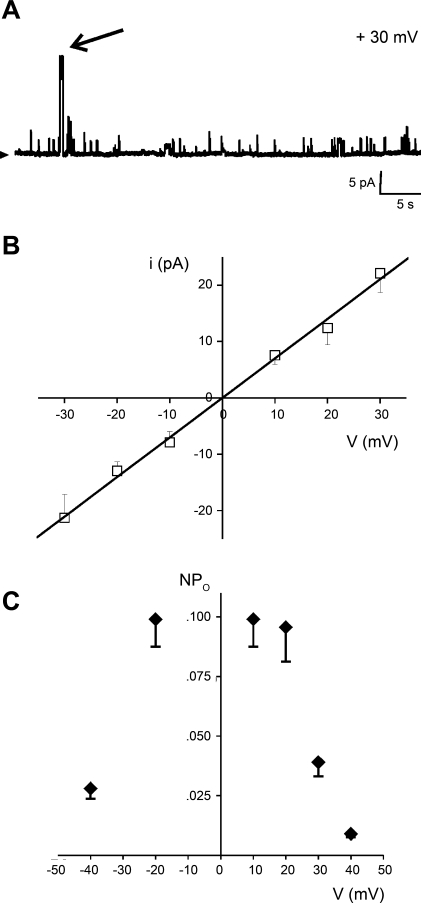
Fig. 3.
Basic ion conduction and voltage-gating properties of the SR channel phenotype with the largest unitary current amplitude. A: recordings of unitary current activity that include the channel phenotype with the largest current amplitude (arrow), obtained in symmetric 300 mM Cs-methanesulfonate. This conductance was observed only in 5 of 40 bilayers. Positive current flowing into the cis chamber is shown as upward deflections. An arrowhead indicates the baseline. Data were low-passed at 1 and digitized at 10 kHz. B: i-V plot corresponding to the records shown in A; i values were obtained from all-points amplitude histograms (17). The plot shows no open channel rectification within ±30 mV, with a slope conductance of 771 pS. C: NPo-V plot corresponding to the 771 pS conductance shows that this channel phenotype reaches maximal activity at ± 20 mV; NPo values were obtained from the AUC of all-points amplitude histograms (17). For A–C, given voltages correspond to those at the bilayer cis side.
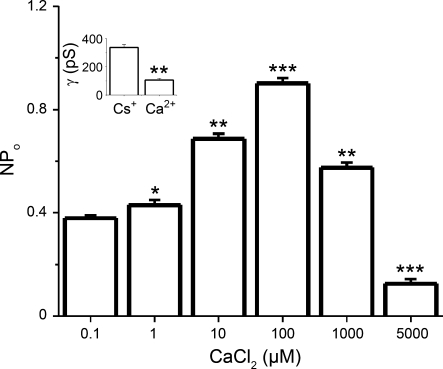
Among the several phenotypes identified, the channel with a unitary current amplitude of 6.8 pA (at 20 mV) was the most frequent, being detected in 34 of 40 (85%) bilayers. Because of its abundance, this conductance was studied in terms of ion permeability, gating, and pharmacological properties. i- V plots from recordings of the prevalent channel phenotype indicated current reversal at ∼0 mV in symmetrical 300 mM Cs+ solutions (Fig. 2, A and B). The i/V plot of this channel showed ohmic behavior from −30 to 30 mV, with a slope (open channel) conductance of 334 ± 15 pS (Fig. 2B). These conduction properties are similar to those reported for purified RyRs from sheep cardiac muscle (mainly RyR2) reconstituted into pure PE bilayers (40), and those of bovine coronary artery smooth muscle native RyRs reconstituted into PE/PS bilayers (39). Moreover, substituting Ca+ for Cs2+ as the ion carrier reduced the channel unitary conductance from >300 to ≤100 pS (see inset to Fig. 5). Collectively, ion conduction data indicate that the SR channel under study permeates both monovalent and divalent cations, with a conductance for Ca2+ that is typical of RyRs (82).
Fig. 5.
NPo of reconstituted SR channels is regulated by Ca2+ levels at the cytosolic side of the channel. Averaged NPo from the prevalent SR channel phenotype in response to changes in free Ca2+ in the solution bathing the bilayer cis side. Unitary currents were evoked at V = +15 mV and variant amounts of Ca2+ in the cis solution. NPo was obtained as described for Fig. 2. *Different from 0.1 μM Ca2+, P < 0.05; **different from 0.1 μM Ca2+, P < 0.01; ***different from 0.1 μM Ca2+, P < 0.01 (n = 8). Inset shows unitary conductance (γ) obtained in 10 μM Ca2+ vs. 300 mM Cs+ and indicates that Ca2+ modulation of NPo is accompanied by a decrease in conductance. **Different from in γ in Cs+, P < 0.01 (n = 8).
In addition to the three conductances described above, unitary currents of much larger amplitude were occasionally observed (time-expanded trace in Fig. 3A). This rare ion current phenotype was also characterized by a reversal potential of ∼0 mV in symmetrical 300 mM Cs+ (Fig. 3B), and ohmic behavior of the i/V relationship within ±30 mV. It had the largest slope conductance (740 ± 23 pS; Fig. 3B) of all events observed. However, it was seen in only 12% of the SR membrane preparations incorporated into the ternary phospholipid bilayer and thus was not characterized further.
The activity of the highly prevalent SR conductance (334 pS) was defined by bursting behavior, with ∼66 bursts/min (at −25 mV and a free Ca2+ ≤ 0.1 μM). The channel overall (intra- and interburst) NPo remained relatively constant within ± 25 mV (NPo ≈ 0.7; cis Ca2+ = 0.1 μM) (Fig. 2C). In bilayers where the signal-to-noise ratio was good enough to resolve channel openings in symmetric Cs+ conditions, NPo slightly decreased to 0.5–0.6 within ± 10 mV (not shown). Notably, voltages more negative than −30 mV or more positive than +25 mV significantly decreased NPo from its relatively steady level of 0.5–0.7, as shown in Fig. 2C. This figure appears to indicate that the rate of NPo drop is higher at very negative than at very positive voltages. This is consistent with data obtained with purified cardiac RyRs, showing that within a wide cis calcium concentration ([Ca2+]) range (low μM to 100 μM), Po is consistently lower at −40 than at +40 mV (68).
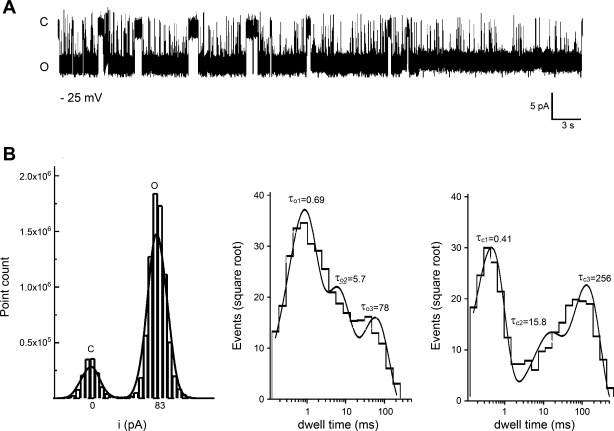
Although most of the SR membranes contained a variety of RyR-compatible conductances (Fig. 1), 5% of SR membrane incorporations contained solely the prevalent conductance described above. From these membranes, we obtained more detailed information regarding the gating behavior of the prevalent conductance by evaluating all-points amplitude histograms (Fig. 4B, left) and dwell-time distributions (Fig. 4B, middle and right). Typically, bursts of activity with a mean duration of 1,461 ± 594 ms and intraburst closures of 38 ± 18 ms were separated by interburst intervals of 761 ± 253 ms (−25 mV and free Ca2+ < 0.1 μM). Dwell-time analysis of overall (intra and interburst) channel activity further revealed that the distribution of open times could be well fit to a three-exponential function with time constants τo1 = 0.69 ms, τo2 = 5.7 ms, and τo3 = 78 ms, indicating the existence of at least three-channel-open states. The relative contribution of each lifetime to the total time spent in open states was 47, 28, and 25% for short, intermediate, and long openings, respectively, indicating that most of the open channel population dwells in short open state(s) (Fig. 4B, middle). The closed times distribution could also be well fit to a three-exponential function, indicating the existence of at least three closed states. Time constants τc1 = 0.41 ms, τc2 = 15.8 ms, and τc3 = 256 ms contributed 44, 16, and 40%, respectively, to the total time spent in closed states (Fig. 4B, right). Data indicate the existence of at least three closed states, with the vast majority of the closed channel population dwelling in short and long-lived closed state(s). Interestingly, cardiac RyR (considered RyR2) expressed in CHO cells also exhibit at least two open and two closed states (5). Collectively, ion conduction and dwell-time distribution data suggest that the most common native channel from rat cerebrovascular smooth muscle SR membranes exhibits a phenotype similar to that of homomeric RyR2 channels.
Fig. 4.
SR channel behavior of the most commonly observed conductance from bilayers containing a single-channel protein. A: representative current recordings from a bilayer containing a single ion channel defined by a unitary conductance similar to that of the channel shown in Fig. 2. C and O, channel closed and open states, respectively. Records were obtained in 300 mM Cs+ with V = −25 mV. Data were low-passed at 1 and digitized at 10 kHz. B: the all-points amplitude histogram from the records shown in A has 2 modes, which correspond to the close and open states (left). The difference between the modes renders a unitary amplitude of 8.3 pA. Open (middle) and closed (right) time distributions could be well fitted with 3 exponentials, with a solid line giving the composite fit. On top of each peak, the time constants corresponding to each component are given in ms. Number of events: 1,210 (openings) and 2,015 (closures).
A typical feature of all RyR subtypes is that their NPo depends heavily on cytosolic Ca2+ levels (27, 78, 86). Indeed, raising free Ca2+ concentration from 0.1 to 100 μM in the cis solution increased NPo of the SR prevalent channel from 0.37 ± 0.01 to 0.90 ± 0.02 (Vm = 30 mV). It is noteworthy that NPo values were high across the wide [Ca2+] range we used (Fig. 5). This is particularly noticeable at 0.1 μM when most RyR channels are expected to be closed (i.e., with low Po) (13, 38). Therefore, it is likely that these high NPo values reflect high N values, resulting from very efficient channel expression and functional channel reconstitution in our bilayer system. Remarkably, at 1 mM free Ca2+ NPo drastically decreased, with channel activity at 5 mM free Ca2+ reaching levels below those at free Ca2+ < 0.1 μM (Fig. 5). Although a bell-shaped Ca2+ dependence has been reported for all RyRs, the crossover from potentiation to inhibition of channel activity as a function of cytosolic Ca2+ varies among RyR subtypes: 1 mM cytosolic Ca2+ almost totally inhibits RyR1, whereas 2–10 mM cytosolic Ca2+ usually represents the IC50 for both RyR2 and RyR3 (21). Therefore, the Ca2+ dependence of rat cerebral artery smooth muscle SR channels also follows a pattern consistent with that of RyR2.
Basic pharmacological properties identify cerebral artery SR channels as RyR.
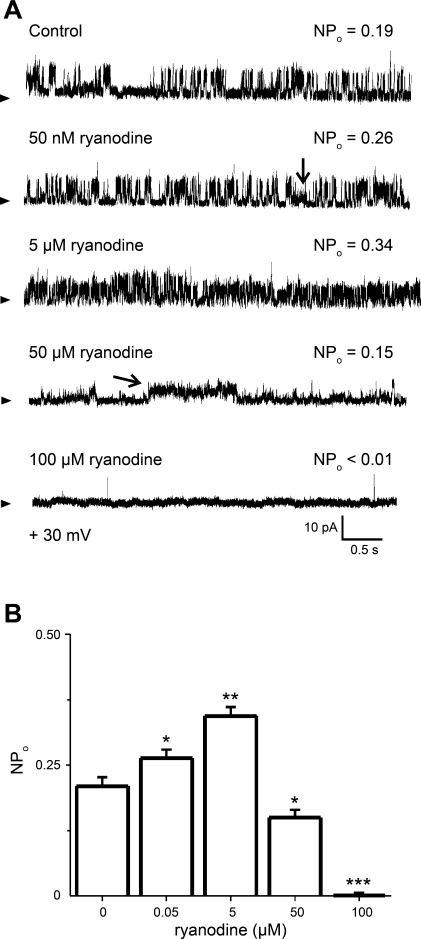
After examining the basic biophysical features of native rat cerebral artery smooth muscle SR channels, we studied the pharmacological properties of the most frequent channel phenotype. NPo was a monotonic function of ryanodine concentration (0.05–5 μM) applied to the cytosolic side of the channel, essentially identifying the SR channels under study as RyRs (Fig. 6). For example, 5 μM ryanodine increased NPo from 0.21 ± 0.02 to 0.34 ± 0.02 (V = +30 mV). However, at concentrations >5 μM, ryanodine reduced the frequency of appearance of the channel's main conductance level (Fig. 6, A and B). In addition, a subconductance defined by a unitary current amplitude that was 40% smaller than the main conductance could occasionally be observed at ryanodine concentrations as low as 50 nM (Fig. 6A, 2nd trace). However, higher ryanodine concentrations (e.g., ≥50 μM) “locked” the channel into this subconductance state (typically, several seconds long) (Fig. 6A, 4th trace). This switch from main to subconductance states at high ryanodine concentrations was observed in 13 of 15 bilayers. The presence of a long-lived subconductance state in ryanodine is similar to that reported for bovine coronary artery SR channels reconstituted into 5:3:2 PE-PS-PC bilayers (39) and supports a previous contention that ryanodine at tens of micromolar stabilizes the RyR channel into a different conformation with unique conduction properties (3). Finally, further increases in ryanodine (100 μM and above) caused the channel to switch from the ryanodine-induced subconductance to closed state(s). This behavior is similar to that reported with both skeletal and cardiac RyR reconstituted into bilayers and interpreted to reflect ryanodine interactions with high- and low-affinity sites in the RyR (37, 41, 54, 63).
Fig. 6.
Ryanodine has a dual effect on the NPo of reconstituted SR channels. A: recordings of prevalent SR channels in absence (top trace) and presence of increasing amounts of ryanodine (0.05–100 μM) added to solution bathing the bilayer cis side; V = 25 mV. NPo was obtained as described for Fig. 2. An oblique arrow (on 4th trace) points to prevalence of a ryanodine-induced subconductance state, which can coexist with the main conductance (vertical arrow on 2nd trace). B: a concentration response for ryanodine demonstrates a dual effect of the alkaloid on channel NPo (see main text). *Different from control, P < 0.05; **different from control, P < 0.01; ***different from control, P < 0.001 (n = 8).
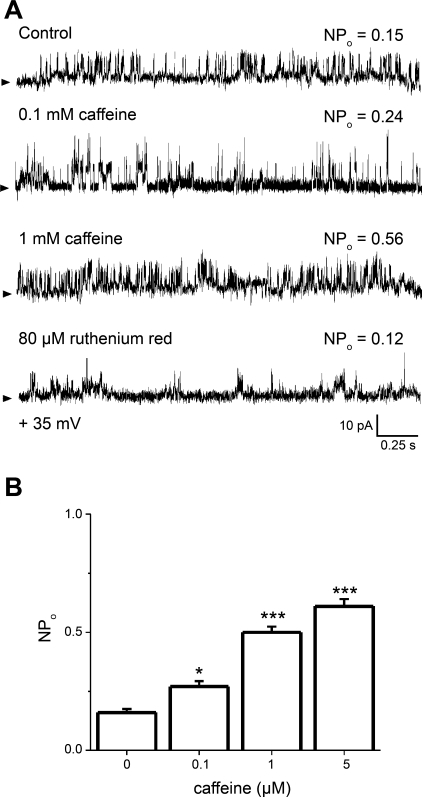
Steady-state channel activity was also a monotonic function of caffeine concentration. Caffeine at 0.1, 1, and 5 mM in the cis solution elevated NPo from a control value of 0.16 ± 0.02 to 0.27 ± 0.02, 0.52 ± 0.02, and 0.61 ± 0.29, respectively (Fig. 7, A and B). On the other hand, when applied in the cis solution with 1 mM caffeine, ruthenium red concentrations that block CICR (80 μM) (45, 86) reduced NPo by ∼80% (Fig. 7A, last trace).
Fig. 7.
Caffeine applied to the bilayer cis side increases the NPo of reconstituted SR ryanodine-sensitive channels. A: unitary current recordings of SR Ca2+- and ryanodine-sensitive channels in absence (top) and presence of 0.1 (2nd trace) or 1 mM (3rd trace) caffeine added to the cis solution. Further application of 80 μM ruthenium red (4th trace) drastically suppresses channel activity (see main text); V = +35 mV. NPo was obtained as described for Fig. 2. B: averaged NPo in response to caffeine added to the cis solution. *Different from control, P < 0.05; ***different from control, P < 0.001 (n = 8).
Identification of RyR subtypes.
The biophysical and pharmacological profiles of the SR membrane channels under study indicate that they possess all the basic features of functional RyRs. Furthermore, some channel functional properties of the most prevalent conductance (i.e., the Ca2+i-NPo relationship) strongly suggest that functional RyR2s (and perhaps also RyR3s), predominate in rat cerebral artery smooth muscle SR membranes. To identify the RyR subtypes that are expressed in cerebral artery smooth muscle cells, conventional RT-PCR was performed on rat endothelium-denuded, resistance-size, cerebral arteries (see materials and methods). Amplified transcripts exhibited predicted sizes for RyR1, RyR2, and RyR3, matching the positive controls obtained from skeletal muscle, heart, and brain, respectively (Fig. 8A). These results suggest that transcripts corresponding to all three RyR isoforms are present in rat cerebral artery smooth muscle, a result that mimics a previous finding in rat aortic smooth muscle (59).
Fig. 8.
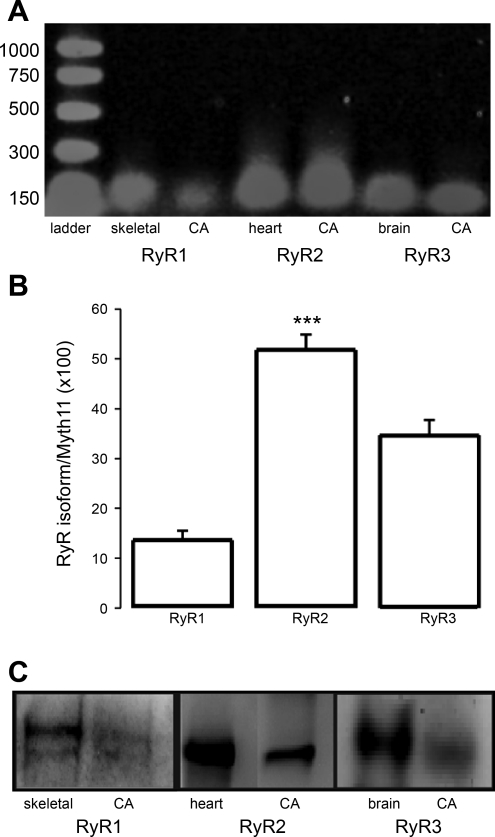
Ryanodine receptor (RyR)1, RyR2, and RyR3 transcripts are present in rat cerebral artery smooth muscle, yet RyR2 is the most abundant subtype at both message and protein levels. A: conventional RT-PCR performed on endothelium-free, resistance-size cerebral arteries (CA) shows that amplified transcripts exhibit the predicted sizes for RyR1, RyR2, and RyR3, respectively matching controls from skeletal muscle, heart, and brain. B: relative abundance of each RyR subtype transcript as determined using real-time PCR. C: total protein from rat cerebral smooth muscle SR, skeletal muscle, heart, and brain (each at 50 μg/lane) separated on SDS-PAGE. RyR immunoreactivity was determined with anti-RyR1, RyR2, and RyR3 polyclonal antibodies and enhanced chemiluminescence (materials and methods). Skeletal muscle, heart, and brain were chosen as positive controls for labeling with anti-RyR1, anti-RyR2, and anti-RyR3 antibodies, respectively. ***Different from RyR1 and RyR3, P < 0.001 (n = 10).
The relative abundance of each RyR subtype transcript in cerebral arteries was determined by quantitative real-time PCR (83). Following data normalization to myosin heavy chain 11 (MYH11), the relative amounts of RyR1, RyR2, and RyR3 transcripts were 13.6 ± 1.9, 51.8 ± 3.1, and 34.6 ± 3.2%, respectively. Levels of endothelial cell (Pecam1) and astrocyte (Aqp4) markers were negligible, strongly suggesting that endothelium-denuded cerebral arteries overwhelmingly consist of vascular smooth muscle. Thus RyR2 transcript is the most prevalent in rat cerebral artery smooth muscle cells, being ∼1.5 and 4 times more abundant than RyR3 and RyR1 (Fig. 8B).
Cerebral artery smooth muscle cell RyR protein product was measured by Western immunoblotting. Specific polyclonal antibodies directed against RyR1, RyR2, and RyR3 (31–33, 35) detected clear bands of >400 kDa from skeletal muscle, heart, and brain, respectively. Protein bands for all three subtypes were detected in lysate from deendothelized cerebral arteries (Fig. 8C). Consistent with data measuring RyR subtype message (Fig. 8B), we detected a very strong signal for the RyR2 protein label while signals for RyR1 and RyR3 proteins were rather weak (Fig. 8C). This Western immunoblotting result has to be interpreted with caution, however, considering that protein abundance labeling is dependent not only on the RyR subtype quantity in the sample but also on the affinity of each specific antibody to its antigen. Collectively, however, transcript and protein data indicate that RyR2 is the most prevalent RyR isoform in cerebral artery smooth muscle, matching electrophysiological characterization of the prevalent functional native channel in the SR membrane preparation.
Immunofluorescence using custom, highly purified polyclonal antibodies raised to each RyR isoform (31–33, 35) was next performed to examine the subcellular localization of each RyR isoform in cerebral artery myocytes (Fig. 9A) (35). The myocyte fluorescence confocal image was subdivided into three distinct cellular regions, as described in materials and methods (see also the far right of Fig. 9A). Data clearly show that in acutely isolated rat cerebral artery myocytes, RyR1, RyR2, and RyR3 are distinctly distributed within each subcellular region (Fig. 9A). Averaged, relative immunofluorescence signals for each isoform across the different subcellular regions are shown in Fig. 9B. RyR1 was similarly located in both subplasmalemma and perinuclear regions, with predominance in the extraperinuclear compartment. RyR3 was similarly distributed in extraperinuclear and perinuclear regions, being very scarce in the subplasmalemma. In contrast to RyR1 and Ry3, RyR2 was significantly more abundant in the subplasmalemma than in the extraperinuclear region and was rare in the perinuclear compartment. This preferential localization of RyR2 label in the subplasmalemma was observed in all cells examined (n = 12). Collectively, immunofluorescence confocal imaging data indicate that each RyR subtype exhibits differential subcellular localization in rat cerebral artery myocytes, with RyR2 residing predominantly near the plasmalemma, a subcellular domain abundant in SR.
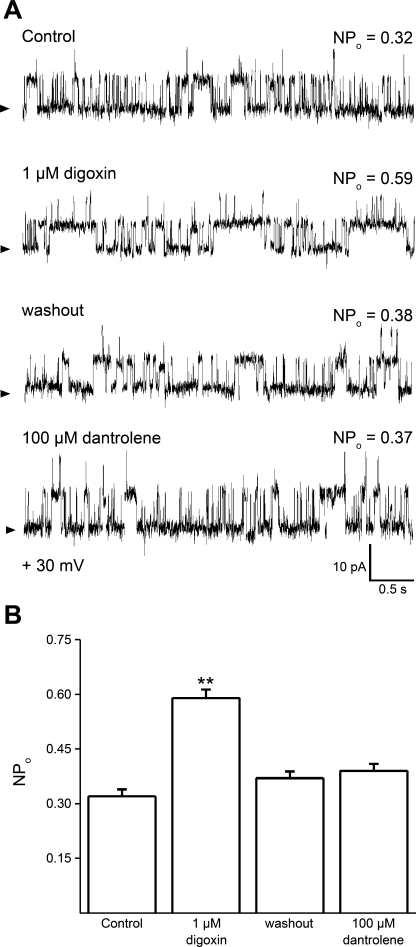
Selective pharmacological agents were used to identify the functional RyR subtype that predominates in cerebral artery myocyte SR membranes. Thus we evaluated the responses of native SR membrane RyR channels to digoxin, dantrolene, and neomycin. To determine the differential selectivity of these pharmacological agents on the different RyR subtypes, we first evaluated drug action on homomeric recombinant RyRs reconstituted into planar lipid bilayers. Digoxin (1 μM) readily increased homomeric RyR2 NPo while failing to alter RyR1 and RyR3 channel activity (Supplemental Fig. S1; the online version of this article contains supplemental data), confirming previous studies that documented the selectivity of micromolar digoxin on RyR2 over the other RyR subtypes (64). Remarkably, 1 μM digoxin reversibly increased the NPo of native cerebral artery SR RyR channel (Fig. 10A, traces 1–3), a result that was replicated in every single bilayer tested (Fig. 10B).
Fig. 10.
The RyR channel that is most frequently found in rat cerebral artery myocyte SR membranes shows a pharmacological profile that is characteristic of the RyR2 type. A: unitary current records of SR channels before (1st trace), during (2nd trace), and after washout (3rd trace) of 1 μM digoxin show a reversible increase in NPo in response to digoxin concentrations that activate homomeric RyR2 but not RyR1 or RyR3 (Supplemental Fig. S1). Application of dantrolene at concentrations that block homomeric RyR1 and RyR3 but not RyR2 (Supplemental Fig. S2) fails to reduce native SR RyR NPo (4th trace); V = +20 mV. B: averaged data from different bilayers (n = 10). **Different from control, P < 0.01.
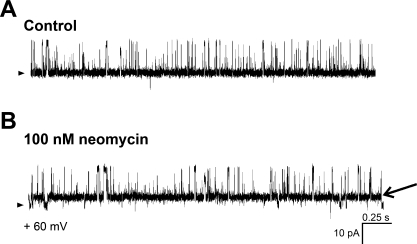
Next, we demonstrated that dantrolene (100 μM) blocked recombinant, homomeric RyR1 and RyR3 but not homomeric RyR2 channels (Supplemental Fig. S2), buttressing the idea that dantrolene is rather selective for RyR1 and RyR3 over RyR2 channels (58). Consistent with a RyR2-type pharmacological phenotype, dantrolene did not block the activity of cerebral artery myocyte SR channels (Fig. 10A, trace 4, and 10B). Finally, 100 nM neomycin (over a voltage range of +20 to +60 mV) shifted the native RyR channel from its primary conductance (fully resolved open state) to a long-lived subconductance (Fig. 11), this shift being reversed by potentials >+60 mV (not shown). This result is identical to neomycin findings reported with cardiac RyRs, which are predominantly homomeric RyR2 (53). Collectively, our profiling of native SR channels with selective agents indicates that the rat cerebral artery myocyte SR RyR has a pharmacological phenotype that is typical of homomeric RyR2.
Fig. 11.
Neomycin introduces a long-lived subconductance state in cerebral artery myocyte SR channels. Unitary current records of SR native channels after bilayer reconstitution before (A) and after (B) application of 100 nM neomycin to the cis side of the bilayer. The subconductance state introduced by neomycin may last several seconds, as previously reported with cardiac RyR (53). An arrowhead on the left of each panel highlights the baseline (nonconducting channel level). In B, an arrow on the right points to a transition from the neomycin-induced subconductance to the nonconducting channel level.
DISCUSSION
In the present study, we have identified the major electrophysiological and pharmacological properties, and the relative subtype expression and spatial distribution of RyRs in myocytes freshly isolated from rat resistance-size cerebral arteries. Our results demonstrate that 1) transcripts for all three RyR subtypes (RyR1–3) are detected in these cells, with RyR2 being the most abundant; 2) proteins corresponding to all RyR subtypes are expressed in cerebral artery smooth muscle, with RyR2 being particularly abundant; 3) RyR protein subtypes show differential cellular localization, with RyR2 being most abundant in the subplasmalemma, a cellular domain enriched in SR membrane; and 4) single-channel conductances from SR membranes are varied, yet the three distinct conductances that are usually detected in our records are compatible with RyR-mediated activity. Importantly, the most prevalent conductance (334 pS in symmetrical 300 mM Cs+) displays a channel phenotype defined by ion conduction, gating properties, and pharmacological responses that is characteristic of homomeric RyR2. Therefore, results at a variety of levels (message, protein, subcellular localization, ion conduction, gating, and selective pharmacology of ion channels) all point to homomeric RyR2 as the prevailing RyR channel subtype in SR membranes of rat, resistance-size cerebral artery smooth muscle cells.
Our documentation of the existence of mRNA coding for the three RyR isoforms in rat cerebral artery smooth muscle (Fig. 8A) is consistent with findings in other rat arterial smooth muscle preparations, including aorta, superior and inferior mesenteric (59), coronary (70), and main pulmonary arteries (80), but not basilar arteries (65). Our real-time PCR data, however, show that RyR2 is the most abundant transcript in rat cerebral artery myocytes, with RyR3 less highly expressed and RyR1 barely detected (Fig. 8B). Using PCR, it has recently been reported that only RyR3 RNA can be detected in rat basilar arteries (65). In both this study and ours, animals of same species, strain, and age were used. Therefore, it is possible to speculate that in rat brain arterial circulation RyR isoform transcription is region specific, with RyR2 predominating in anterior, middle, and posterior cerebral and RyR3 predominating in basilar arteries (65). Regional specificity, however, appears not to apply to RyR1, whose transcripts are negligible throughout the whole rat cerebral artery bed (Fig. 8, A and B; Ref. 65).
Several posttranscriptional processes that operate on mRNA, including translation, protein folding, subunit assembly into multimers, and targeting of the protein complex to specific organelles, are heavily regulated in cells of higher vertebrates. Some of these processes have been reported to control the RyR channel protein level and localization to the SR membrane (46, 67, 72, 82). However, Western blotting data (Fig. 8, C and D) are consistent with the transcript profile, underscoring the abundance of RyR2 protein in cerebral artery smooth muscle. Furthermore, immunofluorescence data from isolated cerebral artery myocytes demonstrate that RyR2 predominates in the subplasmalemma (Fig. 9) where SR membranes are abundant, and functional characterization of the most frequent conductance (Figs. 1, 2, 4–7) is consistent with a channel phenotype similar to that of homomeric RyR2. Thus the predominance of RyR2 over other RyR types in the cerebral artery myocyte at message, protein product, and functional levels in SR membranes suggest that such predominance is mainly determined at the genomic level. Comparison of real-time PCR and Western blotting data (Fig. 8) with immunoimaging results (Fig. 9), however, appears to indicate that posttranslational processes target RyR2 (over the other two isoforms) specifically to the subplasmalemmal region.
Our biochemical and immunostaining data cannot totally rule out the possibility that the native RyR channel in rat cerebral artery myocyte SR consists of the association between RyR2 and RyR1 or RyR3 subunits. However, it should be underscored that heterotetramers of RyR2 with RyR1 or RyR3 subunits could only be formed after subunit overexpression in heterologous systems, such as HEK cells (76). Notably, channel conduction and gating properties (Fig. 1–5) and pharmacological responses (Figs. 7, 8, 10, 11) indicate that the predominant RyR in the rat cerebral artery myocyte SR membrane displays a phenotype that is characteristic of cardiac myocyte RyRs (these native receptors being considered homomeric RyR2; cf. Ref. 40) and of homomeric RyR2 following heterologous expression (5).
It is widely recognized that homomeric RyR2 differs from RyR1 in several basic functional features that determine channel activity, including cytoplasmic Ca2+ sensitivity (8). In addition, it has been reported that RyR subtypes differ in their response to physiological modulators, including ATP and calmodulin, and in their association with regulatory FK506-binding proteins (21). Therefore, the differential subcellular distribution of RyR subtypes in cerebral artery myocytes may endow regional-specific regulation of each subtype. Within this context, it should be noted that the SR-prevalent RyR2 isoform displays a micromolar Ca2+ sensitivity (Fig. 5). In rat cerebral artery myocytes, micromolar Ca2+ concentrations can be reached near the SR membrane owing to plasmalemma Ca2+ influx via CaV1.2 (12, 20, 66), TRPV4 channels, and/or SR Ca2+ release (18, 29). Thus it is likely that the Ca2+ sensitivity of the RyR2 characterized here allows this channel to participate of some form of CICR and/or of a positive feedback mechanism that facilitates SR Ca2+ release via RyR.
In cerebral artery myocytes, a wide variety of Ca2+ signals occur, including “sparks” (29, 57), “sparklets” (66), and “waves” (30, 51). Determination of the importance of RyR2 in rat cerebral artery smooth muscle function requires future studies that will systematically explore the involvement of RyR2 in each of these signals, as well as determination of any possible cross talk between these and other ionotropic receptors that control Ca2+ signaling in these cells, such as voltage-dependent Ca2+ (20); BK (29, 60), TRPV4 (18), InsP3-gated Ca2+-release (51, 83), and, likely, RyR3 channels (44).
In particular, a major task accomplished by RyR in cerebral artery myocytes is the generation of Ca2+ sparks, a local vasodilatory Ca2+ signal (29, 57). Because RyR2 predominates in the cell domain where the SR is abundant (Fig. 9), a myocyte region in close association with plasmalemma regions where BK channels are clustered (75), it is highly likely that RyR2, via Ca2+ spark generation, functions as the main regulator of BK-mediated spontaneous transient outward currents (STOCs) in rat cerebral artery myocytes. This speculation is consistent with results from nonvascular smooth muscle where the presence of normal Ca2+-sparks in RyR3-null mouse detrusor smooth muscle has been interpreted as RyR2 contributing to spark and, thus, STOC generation (34). In addition, we show that RyR3 is scarce in the subplasmalemma (Fig. 9), a result that matches the RyR distribution observed in pulmonary artery myocytes (79). Thus in the SR/subplasmalemma region of both arterial preparations, the Ca2+ signal generated by RyR3 might fail to reach the micromolar range in the vicinity of the BK channel, a Ca2+ level that is needed to directly activate the BK channel (52). It has been reported that genetic ablation of RyR3 increases Ca2+ spark frequency in mouse cerebral artery smooth muscle cells. This finding has been interpreted to indicate that RyR3 is a negative modulator of RyR1 and/or RyR2 (44). Should a similar mechanism occur in rat cerebral artery myocytes, the differential location of RyR3 vs. RyR2 in the subplasmalemma (Fig. 9) would require that these two channels functionally cross talk at a distance via cell signaling and/or that RyR2 is regulated by a small subset of RyR3 that is specifically located in the subplasmalemma.
The voltage gating of RyR shown in Fig. 2C indicates that the cerebral artery myocyte SR receptor reaches maximal activity under voltage conditions that are physiological, since it is widely accepted that there is no significant potential gradient across SR membranes (69). Moreover, RyR NPo remained relatively constant (0.55–0.7) when studied in the absence of ryanodine and presence of μM cytosolic Ca2+ (Fig. 2C). This behavior is consistent with the poor voltage-sensitivity reported for other native RyR that are considered homomeric RyR2, such as purified channels from sheep (40), dog (77), and rabbit (80) cardiac muscle SR. Channel activity dropped at extreme voltages, in particular, more negative than −30 mV. Although the physiological role and kinetic bases of such voltage dependence of gating remain unknown, it is likely that inactivation processes play a role in the voltage dependence of gating of rat cerebral artery myocyte RyR at extreme (in particular, negative) voltages, as previously communicated for purified cardiac RyR (26).
In summary, we have identified functional RyRs in rat resistance-size cerebral artery myocytes. Biochemical, immunological, electrophysiological, and pharmacological studies indicate that the predominant SR RyR subtype is homomeric RyR2. We also show that the RyR2 channel exhibits ideal Ca2+-gating properties and subcellular localization to sense local Ca2+ under physiological conditions, raising the hypothesis that RyR2 contributes to Ca2+ spark generation in cerebral artery myocytes.
GRANTS
This study was supported by grants from the National Institutes of Health AA11560 and HL77424 to A. Dopico, and HL67061 and HL94378 to J. Jaggar.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Anna N. Bukiya for critical reading of the manuscript.
T. Vaithianathan is currently at the Burke-Cornell Research Institute in the Weill Medical College at Cornell University, New York, NY. J. Liu is currently at Purdue Pharma, Cranbury, NJ.
REFERENCES
- 1.Ahern CA, Sheridan DC, Cheng W, Mortenson L, Nataraj P, Allen P, De Waard M, Coronado R. Ca2+ current and charge movements in skeletal myotubes promoted by the beta-subunit of the dihydropyridine receptor in the absence of ryanodine receptor type 1. Biophys J 84: 942–959, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC). Br J Pharmacol 153: S133, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson K, Lai FA, Liu QY, Rousseau E, Erickson HP, Meissner G. Structural and functional characterization of the purified cardiac ryanodine receptor-Ca2+ release channel complex. J Biol Chem 264: 1329–1335, 1989 [PubMed] [Google Scholar]
- 4.Benkusky NA, Farrell EF, Valdivia HH. Ryanodine receptor channelopathies. Biochem Biophys Res Commun 322: 1280–1285, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Bhat MB, Hayek SM, Zhao J, Zang W, Takeshima H, Wier WG, Ma J. Expression and functional characterization of the cardiac muscle ryanodine receptor Ca2+ release channel in Chinese hamster ovary cells. Biophys J 77: 808–816, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolton TB. Calcium events in smooth muscles and their interstitial cells; physiological roles of sparks. J Physiol 570: 5–11, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukiya AN, Liu J, Toro L, Dopico AM. Beta1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Mol Pharmacol 72: 359–369, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Bull R, Marengo JJ. Sarcoplasmic reticulum release channels from frog skeletal muscle display two types of calcium dependence. FEBS Lett 331: 223–227, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Chalmers S, Olson ML, MacMillan D, Rainbow RD, McCarron JG. Ion channels in smooth muscle: regulation by the sarcoplasmic reticulum and mitochondria. Cell Calcium 42: 447–466, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Chen SR, Ebisawa K, Li X, Zhang L. Molecular identification of the ryanodine receptor Ca2+ sensor. J Biol Chem 273: 14675–14678, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Chen YF, Zhang AY, Zou AP, Campbell WB, Li PL. Protein methylation activates reconstituted ryanodine receptor-ca release channels from coronary artery myocytes. J Vasc Res 41: 229–240, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Collier ML, Ji G, Wang Y, Kotlikoff MI. Calcium-induced calcium release in smooth muscle: loose coupling between the action potential and calcium release. J Gen Physiol 115: 653–662, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coronado R, Morrissette J, Sukhareva M, Vaughan DM. Structure and function of ryanodine receptors. Am J Physiol Cell Physiol 266: C1485–C1504, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Cox DH. The BKCa channel's Ca2+-binding sites, multiple sites, multiple ions. J Gen Physiol 125: 253–255, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowley JJ, Treistman SN, Dopico AM. Cholesterol antagonizes ethanol potentiation of human brain BKCa channels reconstituted into phospholipid bilayers. Mol Pharmacol 64: 365–372, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Dopico AM. Ethanol sensitivity of BK(Ca) channels from arterial smooth muscle does not require the presence of the beta 1-subunit. Am J Physiol Cell Physiol 284: C1468–C1480, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Dopico AM, Lemos JR, Treistman SN. Ethanol activates large-conductance, Ca++-activated K+ channels in neurohypophysial terminals. Mol Pharmacol 49: 40–48, 1996 [PubMed] [Google Scholar]
- 18.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca2+-release channels. Trends Pharmacol Sci 15: 145–159, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Essin K, Welling A, Hofmann F, Luft FC, Gollasch M, Moosmang S. Indirect coupling between Cav1.2 channels and ryanodine receptors to generate Ca2+ sparks in murine arterial smooth muscle cells. J Physiol 584: 205–219, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev 82: 893–922, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Fill M, Coronado R. Ryanodine receptor channel of sarcoplasmic reticulum. Trends Neurosci 11: 453–457, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Fleischer S, Inui M. Biochemistry and biophysics of excitation-contraction coupling. Annu Rev Biophys Biophys Chem 18: 333–364, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Fleischer S. Personal recollections on the discovery of the ryanodine receptors of muscle. Biochem Biophys Res Commun 369: 195–207, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Hamilton SL. Ryanodine receptors. Cell Calcium 38: 253–260, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Hill AP, Sitsapesan R. DIDS modifies the conductance, gating, and inactivation mechanisms of the cardiac ryanodine receptor. Biophys J 82: 3037–3042, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hille B. Ion Channels of Excitable Membranes (3rd ed.). Sunderland, MA; Sinauer Associates, 2001 [Google Scholar]
- 28.Jaggar JH, Nelson MT. Differential regulation of Ca2+ sparks and Ca2+ waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 279: C1528–C1539, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol 278: C235–C256, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Jaggar JH, Wellman GC, Heppner TJ, Porter VA, Perez GJ, Gollasch M, Kleppisch T, Rubart M, Stevenson AS, Lederer WJ, Knot HJ, Bonev AD, Nelson MT. Ca2+ channels, ryanodine receptors and Ca2+-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol Scand 164: 577–587, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Jeyakumar LH, Ballester L, Cheng DS, McIntyre JO, Chang P, Olivey HE, Rollins-Smith L, Barnett JV, Murray K, Xin HB, Fleischer S. FKBP binding characteristics of cardiac microsomes from diverse vertebrates. Biochem Biophys Res Commun 281: 979–986, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Jeyakumar LH, Copello JA, O'Malley AM, Wu GM, Grassucci R, Wagenknecht T, Fleischer S. Purification and characterization of ryanodine receptor 3 from mammalian tissue. J Biol Chem 273: 16011–16020, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Jeyakumar LH, Gleaves LA, Ridley BD, Chang P, Atkinson J, Barnett JV, Fleischer S. The skeletal muscle ryanodine receptor isoform 1 is found at the intercalated discs in human and mouse hearts. J Muscle Res Cell Motil 23: 285–292, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Ji G, Feldman ME, Greene KS, Sorrentino V, Xin HB, Kotlikoff MI. RyR2 proteins contribute to the formation of Ca2+ sparks in smooth muscle. J Gen Physiol 123: 377–386, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinnear NP, Wyatt CN, Clark JH, Calcraft PJ, Fleischer S, Jeyakumar LH, Nixon GF, Evans AM. Lysosomes co-localize with ryanodine receptor subtype 3 to form a trigger zone for calcium signalling by NAADP in rat pulmonary arterial smooth muscle. Cell Calcium 44: 190–201, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knot HJ, Standen NB, Nelson MT. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol 508: 211–221, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai FA, Misra M, Xu L, Smith HA, Meissner G. The ryanodine receptor-Ca2+ release channel complex of skeletal muscle sarcoplasmic reticulum. J Biol Chem 264: 16776–16785, 1989 [PubMed] [Google Scholar]
- 38.Laver DR, Lamb GD. Inactivation of Ca2+-release channels (ryanodine receptors RyR1 and RyR2) with rapid steps in [Ca2+] and voltage. Biophys J 74: 2352–2364, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li PL, Tang WX, Valdivia HH, Zou AP, Campbell WB. cADP-ribose activates reconstituted ryanodine receptors from coronary arterial smooth muscle. Am J Physiol Heart Circ Physiol 280: H208–H215, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Lindsay AR, Manning SD, Williams AJ. Monovalent cation conductance in the ryanodine receptor-channel of sheep cardiac muscle sarcoplasmic reticulum. J Physiol 439: 463–480, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu QY, Lai FA, Rousseau E, Jones RV, Meissner G. Multiple conductance states of the purified calcium release channel complex from skeletal sarcoplasmic reticulum. Biophys J 55: 415–424, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu P, Xi Q, Ahmed A, Jaggar J, Dopico A. Essential role for smooth muscle BK channels in alcohol-induced cerebrovascular constriction. Proc Natl Acad Sci USA 101: 18217–18222, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Z, Zhang J, Wang R, Wayne Chen SR, Wagenknecht T. Location of divergent region 2 on the three-dimensional structure of cardiac muscle ryanodine receptor/calcium release channel. J Mol Biol 338: 533–545, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Löhn M, Jessner W, Fürstenau M, Wellner M, Sorrentino V, Haller H, Luft FC, Gollasch M. Regulation of calcium sparks and spontaneous transient outward currents by RyR3 in arterial vascular smooth muscle cells. Circ Res 89: 1051–1057, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Lynn S, Gillespie GA. Basic properties of a novel ryanodine-sensitive, caffeine-insensitive calcium-induced calcium release mechanism in permeabilised human vascular smooth muscle cells. FEBS Lett 367: 23–27, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Ma J, Hayek SM, Bhat MB. Membrane topology and membrane retention of the ryanodine receptor calcium release channel. Cell Biochem Biophys 40: 207–224, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Mak DO, McBride SM, Foskett JK. Spontaneous channel activity of the inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R). Application of allosteric modeling to calcium and InsP3 regulation of InsP3R single-channel gating. J Gen Physiol 122: 583–603, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marx SO, Gaburjakova J, Gaburjakova M, Henrikson C, Ondrias K, Marks AR. Coupled gating between cardiac calcium release channels (ryanodine receptors). Circ Res 88: 1151–1158, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Marx SO, Ondrias K, Marks AR. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors). Science 281: 818–821, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Mauban JR, Lamont C, Balke CW, Wier WG. Adrenergic stimulation of rat resistance arteries affects Ca2+ sparks, Ca2+ waves, and Ca2+ oscillations. Am J Physiol Heart Circ Physiol 280: H2399–H2405, 2001 [DOI] [PubMed] [Google Scholar]
- 51.McCarron JG, Bradley KN, MacMillan D, Chalmers S, Muir TC. The sarcoplasmic reticulum, Ca2+ trapping, and wave mechanisms in smooth muscle. News Physiol Sci 19: 138–147, 2004 [DOI] [PubMed] [Google Scholar]
- 52.McManus OB. Calcium-activated potassium channels: regulation by calcium. J Bioenerg Biomembr 23: 537–560, 1991 [DOI] [PubMed] [Google Scholar]
- 53.Mead FC, Williams AJ. Block of the ryanodine receptor channel by neomycin is relieved at high holding potentials. Biophys J 82: 1953–1963, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mead FC, Williams AJ. Electrostatic mechanisms underlie neomycin block of the cardiac ryanodine receptor channel (RyR2). Biophys J 87: 3814–3825, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meissner G. Regulation of mammalian ryanodine receptors. Front Biosci 7: d2072–d2080, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Meissner G, Rousseau E, Lai FA. Structural and functional correlation of the trypsin-digested Ca2+ release channel of skeletal muscle sarcoplasmic reticulum. J Biol Chem 264: 1715–1722, 1989 [PubMed] [Google Scholar]
- 57.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637, 1995 [DOI] [PubMed] [Google Scholar]
- 58.Nelson TE, Lin M, Zapata-Sudo G, Sudo RT. Dantrolene sodium can increase or attenuate activity of skeletal muscle ryanodine receptor calcium release channel. Clinical implications. Anesthesiology 84: 1368–1379, 1996 [DOI] [PubMed] [Google Scholar]
- 59.Neylon CB, Richards SM, Larsen MA, Agrotis A, Bobik A. Multiple types of ryanodine receptor/Ca2+ release channels are expressed in vascular smooth muscle. Biochem Biophys Res Commun 215: 814–821, 1995 [DOI] [PubMed] [Google Scholar]
- 60.Pérez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol 113: 229–238, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pérez GJ, Bonev AD, Nelson MT. Micromolar Ca2+ from sparks activates Ca2+-sensitive K+ channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol 281: C1769–C1775, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Ríos E, Brum G. Ca2+ release flux underlying Ca2+ transients and Ca2+ sparks in skeletal muscle. Front Biosci 7: d1195–d1211, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Rousseau E, Smith J, Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+-release channel. Am J Physiol Cell Physiol 253: C364–C368, 1987 [DOI] [PubMed] [Google Scholar]
- 64.Sagawa T, Nishio M, Sagawa K, Kelly JE, Lokuta AJ, Tsai J, Kan E, Wasserstrom JA. Activation of purified cardiac ryanodine receptors by dihydropyridine agonists. Am J Physiol Heart Circ Physiol 280: H1201–H1217, 2001 [DOI] [PubMed] [Google Scholar]
- 65.Salomone S, Soydan G, Moskowitz MA, Sims JR. Inhibition of cerebral vasoconstriction by dantrolene and nimodipine. Neurocrit Care 10: 93–102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santana LF, Navedo MF, Amberg GC, Nieves-Cintrón M, Votaw VS, Ufret-Vincenty CA. Calcium sparklets in arterial smooth muscle. Clin Exp Pharmacol Physiol 35: 1121–1126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shoshan-Barmatz V, Ashley RH. The structure, function, and cellular regulation of ryanodine-sensitive Ca2+ release channels. Int Rev Cytol 183: 185–270, 1998 [DOI] [PubMed] [Google Scholar]
- 68.Sitsapesan R, Williams AJ. Gating of the native and purified cardiac SR Ca2+-release channel with monovalent cations as permeant species. Biophys J 67: 1484–1494, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Somlyo AV, Gonzalez-Serratos HG, Shuman H, McClellan G, Somlyo AP. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probed study. J Cell Biol 90: 577–594, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thorne GD, Paul RJ. Effects of organ culture on arterial gene expression and hypoxic relaxation: role of the ryanodine receptor. Am J Physiol Cell Physiol 284: C999–C1005, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Thrower EC, Park HY, So SH, Yoo SH, Ehrlich BE. Activation of the inositol 1,4,5-trisphosphate receptor by the calcium storage protein chromogranin A. J Biol Chem 277: 15801–15806, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Tuvia S, Buhusi M, Davis L, Reedy M, Bennett V. Ankyrin-B is required for intracellular sorting of structurally diverse Ca2+ homeostasis proteins. J Cell Biol 147: 995–1008, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaithianathan T, Bukiya A, Liu J, Liu P, Asuncion-Chin M, Fan Z, Dopico A. Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway. J Gen Physiol 132: 13–28, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Breemen C, Skarsgard P, Laher I, McManus B, Wang X. Endothelial-smooth muscle interactions in blood vessels. Clin Exp Pharmacol Physiol 24: 989–992, 1997 [DOI] [PubMed] [Google Scholar]
- 75.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium 34: 211–229, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Xiao B, Masumiya H, Jiang D, Wang R, Sei Y, Zhang L, Murayama T, Ogawa Y, Lai FA, Wagenknecht T, Chen SR. Isoform-dependent formation of heteromeric Ca2+ release channels (ryanodine receptors). J Biol Chem 277: 41778–41785, 2002 [DOI] [PubMed] [Google Scholar]
- 77.Xu L, Meissner G. Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+. Biophys J 75: 2302–2312, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu L, Tripathy A, Pasek DA, Meissner G. Potential for pharmacology of ryanodine receptor/calcium release channels. Ann NY Acad Sci 853: 130–148, 1998 [DOI] [PubMed] [Google Scholar]
- 79.Yang XR, Lin MJ, Yip KP, Jeyakumar LH, Fleischer S, Leung GP, Sham JS. Multiple ryanodine receptor subtypes and heterogeneous ryanodine receptor-gated Ca2+ stores in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 289: L338–L348, 2005 [DOI] [PubMed] [Google Scholar]
- 80.Yasukochi M, Uehara A, Kobayashi S, Berlin JR. Ca2+ and voltage dependence of cardiac ryanodine receptor channel block by sphingosylphosphorylcholine. Pflügers Arch 445: 665–673, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Yi XY, Li VX, Zhang F, Yi F, Matson DR, Jiang MT, Li PL. Characteristics and actions of NAD(P)H oxidase on the sarcoplasmic reticulum of coronary artery smooth muscle. Am J Physiol Heart Circ Physiol 290: H1136–H1144, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem 76: 367–385, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Zhao G, Adebiyi A, Blaskova E, Xi Q, Jaggar JH. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Am J Physiol Cell Physiol 295: C1376–C1384, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao G, Adebiyi A, Xi Q, Jaggar JH. Hypoxia reduces KCa channel activity by inducing Ca2+ spark uncoupling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 292: C2122–C2128, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu X, Ghanta J, Walker JW, Allen PD, Valdivia HH. The calmodulin binding region of the skeletal ryanodine receptor acts as a self-modulatory domain. Cell Calcium 35: 165–77, 2004 [DOI] [PubMed] [Google Scholar]
- 86.Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol Rev 49: 1–51, 1997 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.