Abstract
Ezrin is an important membrane/actin cytoskeleton linker protein, especially in epithelia. Ezrin has two important binding domains: an NH2-terminal region that binds to plasma membrane and a COOH-terminal region that binds to F-actin only after a conformational activation by phosphorylation at Thr567 of ezrin. The present experiments were undertaken to investigate the detailed cellular changes in the time course of expression of ezrin-T567 mutants (nonphosphorylatable T567A and permanent phospho-mimic T567D) in parietal cells and to assess ezrin distribution and its influence on the elaborate membrane recruitment processes of these cells. T567A mutant and wild-type (WT) ezrin were consistently localized to the apical plasma membrane, even with overexpression. On the other hand, T567D went first to apical membrane at early times and low expression levels, then accumulated mainly at the basal surface after 24 h. Overexpression of WT or T567A led to incorporation of internal membranes to apical vacuoles, while overexpression of T567D led to large incorporation of apical and intracellular membranes (including H-K-ATPase) to the basal surface. Differences in polar distribution of ezrin suggest a role for the linker protein in promoting formation and plasticity of membrane surface projections, forming the basis for a novel theory for ezrin as an organizer and regulator of membrane recruitment. A model simulating the cellular distribution of ezrin and its associated membrane- and F-actin-binding forms is given to predict redistributions observed with phosphorylation and mutant overexpression, and it can easily be modified as more specific information regarding binding constants and specific sites becomes available.
Keywords: cytoskeleton, ezrin/radixin/moesin proteins, gastric, microvilli, parietal cell
the ezrin/radixin/moesin (ERM) family of proteins serves important functions as F-actin/membrane linker proteins, especially in epithelia (3, 7). The ERM proteins bind to F-actin via their COOH-terminal association domains (C-ERMAD), whereas the NH2-terminal domains (N-ERMAD) bind to plasma membranes through direct interaction with membrane lipids or proteins (13, 21), or indirectly through adaptor proteins such as PALS1 (4, 18, 20, 24). Both in vitro and in vivo evidence suggest that NH2- and COOH-terminal domains of ezrin also bind each other in a dormant state (9, 30), preventing the interaction of the C-ERMAD with F-actin; however, this “closed” conformation of ezrin still maintains interaction with the membrane although with reduced affinity compared with “open” ezrin (31). The intramolecular N-C binding of ezrin is primarily regulated by a phosphorylation event. Fluorescence resonance energy transfer (FRET) analysis with ezrin constructs carrying yellow (YFP) and cyan (CFP) fluorescent proteins at the respective NH2 and COOH termini indicated that phosphorylation on threonine 567 is sufficient to disrupt the N-C binding of ezrin (30), as predicted from in vitro studies on other ERM proteins and structural studies (19).
Ezrin is an abundant protein at the apical membrane of gastric parietal cells, and a large body of evidence suggests that ezrin plays an essential role in the secretory function of the cells, particularly in the apical membrane remodeling that accompanies physiological stimulation to secrete acid (25–28). Our previous studies on parietal cell cultures, using fluorescent tagged ezrin constructs with point mutations of the T567 site, provided results that were reminiscent of studies in many other systems, albeit with some important differences (6). The nonphosphorylatable T567A mutant (Ala substituted for Thr) was still targeted to the apical plasma membrane, and the cells were functionally competent to secrete acid and process H-K-ATPase recruitment (although the latter events may have been somewhat supported by the endogenous native ezrin). On the other hand, when the phosphorylation mimic, ezrin-T567D mutant (Asp for Thr) was expressed—the negatively charged Asp side chain mimicking permanent phosphorylation—there was a surprising reorientation of cell polarity, including the misdirected targeting of ezrin, as well as recruitment of H-K-ATPase-rich cytoplasmic vesicles, to the surrounding basal (basolateral) membrane, a totally unexpected and novel result (28).
The present experiments were undertaken to investigate the detailed cellular changes in the time course of expression of ezrin-T567-mutants in parietal cells, and to assess the ezrin distribution and its influence on membrane recruitment processes. A new paradigm for ezrin-mediated F-actin/membrane interaction is offered, in which ezrin is suggested to act as a director or codirector of membrane recruitment and cytoskeleton surface extensions.
MATERIALS AND METHODS
Parietal cell culture.
Parietal cells were isolated from New Zealand White rabbits following the method of Chew et al. (5). The procedures and treatments for handling animals were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, Berkeley, and the State University of New York, Buffalo. Briefly, the gastric vasculature of anesthetized animals was perfused with phosphate-buffered saline (PBS), via retrograde cannulation of the abdominal aorta, before the stomach was removed. The mucosa was then scraped off, minced, and digested with collagenase (Worthington type 4, 1 mg/ml in MEM, with 1 mg/ml BSA) for 30 min. The digestion was filtered through two layers of cheesecloth to remove undigested tissue. Gastric glands and large conglomerates of cells settled out in 10–15 min by gravity, leaving a dense suspension of individual cells, which were subjected to a 40-μm cell strainer. Intact cells were recovered by centrifuging the suspension three times at 100 g for 5 min, followed by resuspension in fresh medium A, which consisted of DMEM-F-12 (Gibco BRL), supplemented with 20 mM HEPES, 0.2% BSA, 10 mM glucose, 1× SITE medium (Sigma), 1 mM glutamine, 100 U/ml penicillin/streptomycin, 400 μg/ml gentamicin sulfate, and 15 μg/ml geneticin or 20 μg/ml novobiocin, pH 7.4. These procedures resulted in a cell suspension that was typically 70–75% parietal cells. Cells were plated onto Matrigel, on coverslips (for immunofluorescence) or on six-well plates (for Western blots), and kept at 37°C in a humidified incubator.
Recombinant adenoviruses.
Recombinant adenoviruses rAD-ezrin-wild-type (WT)-CFP, rAD-ezrin-T567A (TA)-CFP, and rAd-ezrin-T567D (TD)-CFP expressing CFP-tagged ezrin were described previously (5). The YFP-tagged ezrin construct used in this study, rAd-ezrin-WT-YFP, is indistinguishable from rAd-ezrin-WT-CFP regarding the localization of FP-tagged wild-type ezrin and its effect on parietal cell morphology and function.
To generate the wild-type ezrin construct with a YFP tag, a series of polymerase chain reactions (PCR) was performed. Ezrin sequence (aa 176–234) was PCR amplified from pDC311/WT-ezrin-CFP with primer 1 (5′-AGG TGT GGC ATG CGG AAC ACC GTG GGA TGC-3′) and primer 2 (5′-CTC GCC CTT GCT CAC CAT TAA CTT ATC ATC TTT CTC-3′). YFP sequence was PCR amplified from pEYFP-N1 (Clontech) with primer 3 (5′-GAG AAA GAT GAT AAG TTA ATG GTG AGC AAG GGC GAG-3′) and primer 4 (5′-GCC AAT CTT TGG GGT CTT GTA CAG CTC GTC-3′). Using the mixture of the above PCR products as template, a fusion gene of ezrin-YFP (920 bp) was PCR amplified with primer 1 and primer 4. Ezrin sequence (aa235–aa586) was amplified (1,100 bp) with primer 5 (5′-GAC GAG CTG TAC AAG ACC CCA AAG ATT GGC-3′) and primer 6 (5′-C CCG CGG TGC GGC CGC TTA CAG GGC CTC GAA CTC-3′). In the final PCR reaction, ezrin sequence aa176–aa586 fused with YFP was amplified with primer 1 and primer 6, with a mixture of 920-bp and 1,100-bp products serving as template. The final PCR product was then purified and digested with Sph I and Not I and ligated with similarly digested pDC311/WT-ezrin-CFP (provided DNA sequence for aa1–175 of ezrin), producing pDC311/WT-ezrin-YFP, the plasmid expressing full-length wild-type ezrin fused with a YFP tag. The open-reading frame of the construct was fully sequenced to ensure the accuracy of PCR and enzymatic operations.
Recombinant adenovirus rAd-ezrin-WT-YFP was generated by cotransfecting human embryonic kidney 293 cells with pDC311/WT-ezrin-YFP and pBHGloxΔE1,3Cre (Microbix Biosystems, containing modified adenovirus type-5 genome) using the CellPhect Transfection kit (Amersham Biosciences). A single viral colony was isolated, amplified, and titrated. Aliquots of virus were stored at −80°C.
Immunofluorescence.
Parietal cells grown on Matrigel-coated coverslips were infected with rAd-ezrin-WT-YFP, rAd-ezrin-TA-CFP mutant, or rAd-ezrin-TD-CFP mutant. Infected cells were fixed with 3.7% formaldehyde at the postplating time points specified in the figure legends. Fixed cells were permeabilized with 0.5% Triton X-100 in PBS. To stain exogenous ezrin and H-K-ATPase, cells were incubated with rabbit anti-green fluorescent protein (GFP) (which also recognizes GFP derivatives YFP and CFP, from Immunology Consultants Laboratory, Newberg, OR) and mouse monoclonal anti-H-K-ATPase (2G11, Affinity Bioreagents, Boulder, CO). To stain for F-actin, Alexa 546-Phalloidin (Invitrogen) was used. The secondary antibodies were Alexa 488-conjugated goat-anti-rabbit IgG and Alexa 555-conjugated goat-anti-mouse IgG from Invitrogen (Eugene, OR). In some instances the secondary antibody for H-K-ATPase staining was cy5-donkey anti-mouse (Jackson ImmunoResearch), excitation 633 nm and emission 640–704 nm. Images of Alexa 555 (excitation with 543-nm laser, emission from 590–655 nm) and Alexa 488 (excitation with 488-nm laser, emission from 505–580 nm) were collected 1) on a Zeiss LSM 510 meta confocal microscope at 1 airy unit pinhole with Plan-Neofluar ×40/1.3 oil differential interference contrast (DIC) objective, or 2) on a conventional fluorescence microscope, Nikon microphot-FXA, using a DIC ×40/0.7 air objective or a DIC ×60/0.17 oil objective.
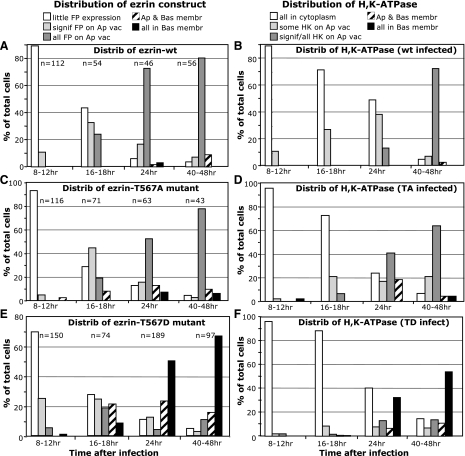
A semiquantitative analysis of the imaging data for time course experiments was carried out. All images collected for cells infected with the various ezrin constructs and at designated times were evaluated and scored according to several morphological criteria. Nonparietal cells [assessed by H-K-ATPase (HK) staining] and dead cells (cell shape) within an image were not scored. The total number of parietal cells (n in the summary Fig. 5) is indicated for each time point and ezrin construct. The total number of individual experiments (i.e., N = rabbits) is indicated in Fig. 5 legend. Images were collected by two investigators and were independently counted and scored by a different investigator. Cell phenotypic categories for expression of rAd-fluorescent protein (FP)-labeled ezrin were as follows: 1) little or no expression of the ezrin construct in the cell (little FP expression); 2) significant appearance of ezrin construct on apical vacuoles (sign FP on Ap vac); 3) all ezrin construct on apical vacuoles (all FP on Ap vac); 3) ezrin construct appearance on apical vacuoles and basal membrane (Ap and Bas membr); 4) all ezrin construct on basal membrane (all on Bas membr). For the distribution of H-K-ATPase, the categorical descriptions were slightly different in some cases, primarily because this is an endogenous protein: 1) all H-K-ATPase in cytoplasm (all in cytoplasm); 2) small amounts of H-K-ATPase on apical vacuoles but most in cytoplasm (some HK on Ap vac); 3) most or all H-K-ATPase on apical vacuoles (sign/all HK on Ap vac); 4) H-K-ATPase on apical vacuoles and basal membrane (Ap and Bas membr); 5) all on basal membrane (all on Bas membr).
Fig. 5.
Enhanced expression of ezrin-T567 phosphorylation mutants greatly influences the progressive targeting of ezrin and intrinsic membrane proteins. Several different cultures (N) were used for the time course of expression and localization of fluorescent protein (FP)-labeled ezrin mutants (A, C, and E) and the location and relocalization of parietal cell H-K-ATPase (HK) (B, D, and F). For 8–12 h, N = 2–3; for 16–18 h, N = 2–3; for 24 h, N = 2; and for 40–48 h, N = 5–9. As described previously, cells were infected at “zero” time with adenovirus containing designated constructs of ezrin: A and B, ezrin-WT-YFP; C and D, ezrin-TA-CFP; E and F, ezrin-TD-CFP. Large numbers of cells, as shown by “n” above each of the indicated time periods, were counted and given a categorical score based on appearance as described in materials and methods and indicated by the abbreviated description in the legend. All viable cells showing H-K-ATPase were included in the analysis. Actual numbers of cells were converted to percentage for normalization. Trends show the very low ezrin construct expression levels and general cytoplasmic distribution of H-K-ATPase at early times (8 h) for all constructs and the subsequent progressive distribution of ezrin-WT and ezrin-TA mutant, as well as H-K-ATPase, toward apical membrane vacuoles. However, there is a distinct difference in the time-dependent trends for the ezrin-TD mutant. At 16 h postinfection, the slight trend toward apical vacuole location for ezrin-TD mutant has totally shifted, so that by 24 and 40 h, ezrin-TD mutant as well as H-K-ATPase have heavily shifted toward a basal location.
In selected instances the parietal cell cultures at designated postplating times were treated for 20–30 min with secretagogues [100 μM histamine and 50 μM isobutylmethylxanthine (IBMX)] to effect an acid secretory response. These cells were then fixed, permeabilized, and stained as above. In a correlative set of experiments the cell cultures were treated with secretagogues, in the presence of 1 μM SCH28080, a well-known inhibitor of the H-K-ATPase proton pump (1, 28).
Western blot analysis.
Parietal cells infected with or without rAd were harvested at different time points as specified in figure legends. Harvested cells were resuspended in a small volume of PBS, mixed with one third volume of 4× SDS-PAGE loading buffer, and boiled for 5 min. Total protein concentration for each of the samples was determined by a modified Bradford method (16). Equal amounts of total protein were then loaded for SDS-PAGE electrophoresis, transferred onto nitrocellulose membrane, blocked and probed with mouse monoclonal anti-ezrin antibody (Covance), and subsequently stripped and reprobed for the β-subunit of H-K-ATPase (Affinity Bioreagents). Signals were revealed using horseradish peroxidase-conjugated goat-anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) with SuperSignal West Dura Extended Duration Substrate (Invitrogen). Images were directly recorded with an image reader LAS-1000 (Fujifilm).
Setting up a model of ezrin binding.
To simulate the nature and location of various bound forms of ezrin in the parietal cell as well as relative changes in the levels and cellular distribution of expressed ezrin-T567 mutant forms, we constructed a model of predicted change using Berkeley Madonna software (version 8.3.22; www.berkeleymadonna.com), which conveniently solves simple differential equations.
Definition of the phosphorylation state of ezrin at T567 is as follows. For ezrin phosphorylation (kinase) and dephosphorylation (phosphatase) reactions, E is unphosphorylated ezrin, EP is phosphorylated ezrin, the rate of phosphorylation = kp × E, and the rate of dephosphorylation = kdp × EP, where kp and kdp are rate constants for the kinase and phosphatase reactions at T567, applying equally to ezrin that is free or bound to membrane. (These constants are both set to zero for the accumulation of exogenous TA or TD, which are treated in the model as E that cannot be phosphorylated or EP that cannot be dephosphorylated.)
Reactions for binding of E and EP to cellular membranes and to F-actin were as follows:
Ezrin-membrane and ezrin-actin equilibrium binding reactions:
where AM is the number of apical membrane binding sites; BM is the number of basal membrane binding sites; AAct is the number of apical F-actin binding sites; BAct is the number of basal F-actin binding sites; and subscripts bnd and unb indicate ezrin binding and unbinding constants, respectively.
RESULTS
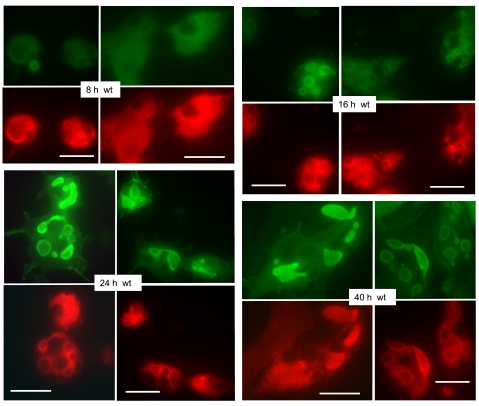
The time course of fluorescent protein-labeled ezrin expression in parietal cell cultures is shown in Figs. 1, 3, and 4 at selected intervals after infection with ezrin-expressing recombinant adenoviral constructs (rAd-ezrin). Figure 1 shows exemplary images for the cellular distribution of ezrin-WT-YFP and endogenous H-K-ATPase over a 40-h time course after infection with the rAd-ezrin-WT-YFP construct. At 8 h there was usually very little expression of the infected ezrin-WT-YFP, whereas the endogenous H-K-ATPase was prominently distributed throughout the cytoplasm in keeping with the hypothesis that in nonstimulated resting parietal cells the pump enzyme is broadly distributed as an intrinsic protein of the large compartment of cytoplasmic tubulovesicles. By 16 h, significant levels of ezrin-WT-YFP were seen on the large (2–5 μm) vacuolar membranes within the interior of most cells. These vacuoles are the apical canalicular membranes that have been internalized as parietal cells adapt to culture conditions (1, 28). With further times of expression, virtually all parietal cells expressed ezrin-WT-YFP prominently on the apical vacuoles as reported earlier (28). In addition, at the times after rAd-ezrin-WT infection (>24 h), more and more of the endogenous H-K-ATPase appears to be proximal to, or associated with, the apical membrane vacuoles, along with ezrin-WT-YFP, though a good deal of H-K-ATPase was also apparent in the cytoplasm. In very few cells was either H-K-ATPase or ezrin-WT-YFP found on the surrounding basal membrane. (Merged images of the ezrin and H-K-ATPase staining patterns for cells infected with all the rAd-ezrin constructs are included as Supplemental Material, available online at the Journal website.) A semiquantitative analysis from many images of ezrin-WT-YFP and H-K-ATPase time-dependent localization is presented in Fig. 5, A and B. For simplicity we established five categories of cellular appearance for each of the time points after infection: 1) very low expression (in the case of ezrin-WT-YFP) or all signal found in cytoplasm (in the case of H-K-ATPase); 2) significant amount of signal associated with apical vacuoles; 3) virtually all signal associated with apical vacuoles; 4) signal on apical and basal membranes; and 5) virtually all signal on basal membrane. The trend is obvious. After a period of 8–16 h the highly expressed ezrin-WT-YFP is clearly targeted to the apical membrane vacuoles, and in a parallel time course, significant amounts of H-K-ATPase also migrate to the same vacuoles.
Fig. 1.
The expression of ezrin-wild-type (WT)-yellow fluorescent protein (YFP) in parietal cells increases with time after infection and is distributed primarily to incorporated apical membrane vacuoles. Primary parietal cell cultures were infected with recombinant adenovirus (rAd)-ezrin-WT-YFP construct immediately after plating and subjected to fixation for immunocytochemistry at sequential times afterward, as indicated. Primary antibody against green fluorescent protein (GFP) was used to detect ezrin-YFP (green). Cells were also immunostained for H-K-ATPase (red), which shows a tendency to be redistributed from a primarily general cytoplasmic locale to one more associated with apical vacuoles at the later times of culture. Bar markers = 20 μm.
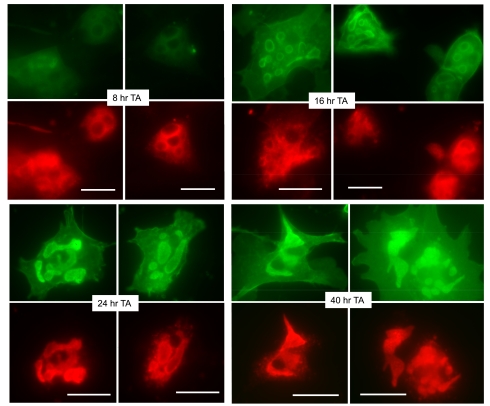
Fig. 3.
Parietal cells expressing ezrin-T567A (TA)-cyan fluorescent protein (CFP) have a similar time course of appearance and distribution of the ezrin-TA mutant as for the ezrin-WT. As in Fig. 1, cell cultures were infected with rAd-ezrin-TA-CFP construct and prepared for immunocytochemistry at indicated sequential times. Expression of ezrin-TA-CFP (green) and H-K-ATPase (red) is shown. Bar markers = 20 μm.
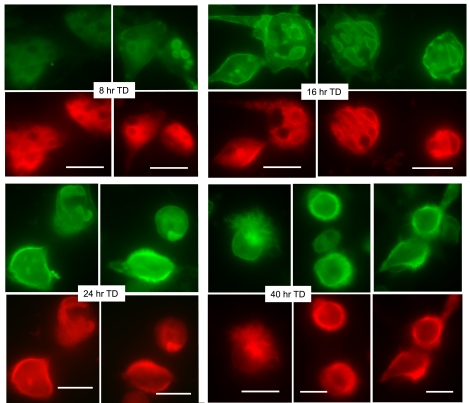
Fig. 4.
Parietal cells expressing ezrin-T567D (TD)-CFP have a distinctly different time course of appearance and distribution of the ezrin-TD mutant than either ezrin-WT or the ezrin-TA mutant. Similar to other experiments, cell cultures were infected with rAd-ezrin-TD-CFP construct and prepared for immunocytochemistry at sequential times. Early expression ezrin-TD mutant is observed primarily on apical vacuoles (green), and by 24 h there is distinct basal expression of ezrin-TD mutant; usually, in these cases, H-K-ATPase is also located to the same basal sites. Bar markers = 20 μm.
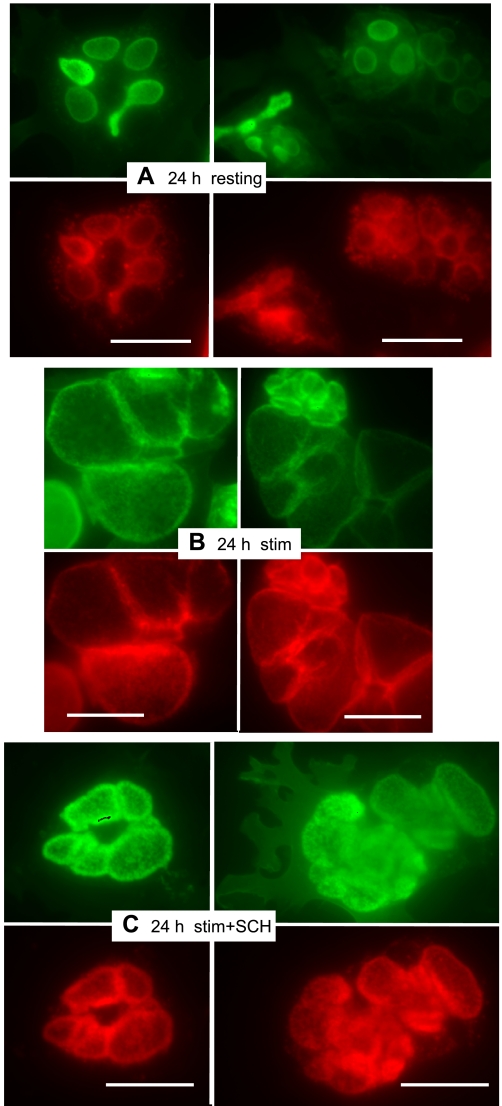
Thus far in the time course experiments none of the cells had been stimulated with secretagogues to secrete acid. As a comparison and contrast, parietal cell cultures infected for 24 h with rAd-ezrin-WT-YFP were examined in resting and stimulated conditions. The nonstimulated cells, immunostained for H-K-ATPase and GFP-ezrin (Fig. 2A), appeared much the same as for the 24-h infectants shown above, but there were remarkable and predictable morphological changes after treatment of cells with histamine-IBMX. When acid secretion occurred, the apical membrane vacuoles were greatly swollen and the H-K-ATPase and ezrin-WT-YFP signals colocalized to the membranes of the swollen vacuoles (Fig. 2B) in conformance with the results of several other studies (1, 5, 28). In the case of histamine-IBMX-treated cells also treated with the proton pump inhibitor, SCH28080, there was fairly tight colocalization of H-K-ATPase and ezrin-WT-YFP at the apical membrane vacuoles, but relatively little swelling as acid secretion was inhibited (Fig. 2C). Similar results were reported earlier for cells immunostained for H-K-ATPase and native ezrin (1), and they are shown in the present context to demonstrate full functional capability of acid secretion by cells infected with rAd-ezrin-WT-YFP and general similarities of morphological phenotype between the SCH28080-treated cells and cells infected for prolonged periods with rAd-ezrin-WT-YFP.
Fig. 2.
Cells expressing ezrin-WT-YFP for 24 h functionally respond to stimulants of acid secretion in a predictable manner. Antibody staining was the same as in Fig. 1 (ezrin-WT-YFP, green; H-K-ATPase, red). A: resting parietal cells show distribution similar to 24-h controls in Fig. 1. B: stimulation with histamine and IBMX effects a large swelling of vacuoles, indicative of HCl accumulation. C: in cells treated with 1 μM proton pump inhibitor SCH28080, as well as secretory stimulants, vacuolar swelling is very much reduced but ezrin-WT-YFP and H-K-ATPase almost entirely colocalize into the apparently thickened walls of apical membrane vacuoles. Bar markers = 20 μm.
The time course of expression and localization of the ezrin-T567A mutant in cells infected with rAd-ezrin-T567A-CFP (ezrin-TA-CFP) and endogenous H-K-ATPase is shown in Fig. 3, along with counterpart summary data in Fig. 5, C and D. The pattern of expression and marker distribution was generally similar to that observed for ezrin-WT-YFP: little expression of ezrin-TA-CFP at early times with strong localization to the apical membrane vacuoles at 24 h and thereafter. There was also a similar shift in H-K-ATPase staining from cytoplasm to apical membrane vacuoles at the 24- and 40-h time points. Again, very little ezrin-TA-CFP or H-K-ATPase appeared on the basal membranes. We also found that cells expressing the ezrin-TA-CFP mutant responded very well to stimulation by histamine-IBMX (data not shown).
The expression of the ezrin-T567D phospho-mimic mutant labeled with CFP (ezrin-TD-CFP) and the location of endogenous H-K-ATPase are shown in Fig. 4, along with summary data in Fig. 5, E and F. Time-dependent expression of the ezrin-TD-CFP produced a distinctly different pattern of localization than either the WT or the TA mutant. Within 16–18 h after infection, significant numbers of cells displayed apical vacuolar accumulation of ezrin-TD-CFP (27%), but a substantial number of cells also appeared with ezrin-TD-CFP localized to both apical vacuoles and basal membranes (21%), and the number with exclusive basal ezrin-TD-CFP became prominent and even dominant by 24 h (50% of cells). In the early times after infection, H-K-ATPase was broadly distributed to the cytoplasm, but by 16 and 24 h, some H-K-ATPase staining also appeared on apical membrane vacuoles. At postinfection times of 24 h and longer, H-K-ATPase staining was also commonly found on the surrounding basal membrane, almost always costaining structures along with ezrin-TD-CFP. As in our previous report (12), the coaccumulation of ezrin-TD-CFP and H-K-ATPase at the surrounding basal membrane was accompanied by the expansion of that surface in the form of long microvillar and filopodial membrane extensions. The membrane extensions were sometimes confined to one pole or surface of the cell, but they were also frequently distributed throughout the entire surrounding cell surface. It is of special interest that, as the basal extensions became more prominent in the TD-infected cells, there was a parallel diminution or disappearance of the apical membrane vacuoles as well as the obvious decrease in cytoplasmic H-K-ATPase staining.
Experiments to evaluate the effect of exogenously expressed ezrin on the cellular distribution F-actin are shown in Fig. 6. Cells infected for 48 h with rAd-ezrin-WT-YFP showed the typical expression of F-actin on apical vacuoles of parietal cells, colocalizing with H-K-ATPase and ezrin-YFP. In the case of ezrin-WT, F-actin staining was also seen on the basal membrane, but usually with less intensity than the apical vacuoles. For cells infected with the ezrin-TD mutant for 48 h, the pattern of distribution was quite different. Now, phalloidin (F-actin) was predominantly associated with the basal membrane where the ezrin-TD mutant and H-K-ATPase were also localized. Thus misdirection of all three proteins was mediated by the single T567D mutation.
Fig. 6.
Comparison of endogenous F-actin expression and distribution in parietal cells infected with ezrin constructs. Parietal cells were infected with rAd-WT-ezrin-YFP or rAd-TD-ezrin-CFP for 48 h, before fixation and immunostaining as described in materials and methods. Images were collected with a Zeiss confocal microscope and converted to pseudocolor as shown: ezrin, green; F-actin, red; H-K-ATPase, blue. The merge of all the images is also shown. Bar markers = 10 μm.
To more fully examine the results from the above described time course immunofluorescence experiments, the possible influence of endogenous ezrin and its relationship to rAd-expressed ezrin constructs need to be considered. Therefore, time course experiments were performed to examine the expression levels of endogenous and exogenously expressed ezrin. Parietal cells infected with rAd-ezrin constructs were harvested at various times, from 12 to 96 h, after infection. Noninfected cells harvested at 12 and 96 h postinfection were run in parallel as controls. The blots were probed with a monoclonal anti-ezrin antibody which detected both endogenous ezrin (∼80 kDa) and ezrin-CFP fusion proteins (∼106 kDa, ezrin + CFP), as indicated previously (28). As shown in Fig. 7A, expression of all three rAd-ezrin-constructs was very low at 12 h, became clearly apparent by 24 h, and continued to rise above the endogenous ezrin levels until a plateau at about 72 h. Endogenous ezrin was expressed at relatively constant levels throughout most of the time course except for some diminution toward the later times and the appearance of a 55-kDa band, probably the result of some calpain activity (27). The ezrin blots were stripped and reprobed for H-K-ATPase as shown in Fig. 7B. In all cases there was strong signal for the first 2 days, with a tendency to diminish at later time. This was also seen in blots of control noninfected cells. We have frequently observed the diminution of H-K-ATPase and loss of the parietal cell secretory phenotype over a period of 4 or 5 days in culture (unpublished results).
Fig. 7.
Expression of ezrin and H-K-ATPase as a function of time after infection with various rAd constructs of ezrin as determined by Western blot analysis. Similar to the immunocytochemistry, parietal cells were infected at zero time and taken at the indicated times for SDS-PAGE and Western blotting. A: blots were first probed for ezrin in which native ezrin (80 kDa) can be distinguished from ∼106 kDa fluorescent protein ezrin constructs. In all cases the expressed ezrin constructs increase dramatically over the first 2–3 days, while native ezrin remains relatively constant over a long time course. B: blots were then stripped and probed for expression of H-K-ATPase, which shows a decrease with time the cells are maintained in culture. Uninfected control cultures (non-inf) taken at 12 and 96 h of culture are shown to the left of the blots.
DISCUSSION
The data presented in this study are qualitatively similar to those previously published by our laboratory regarding the morphological distribution of ezrin T567-phosphorylation mutants in parietal cells (28). Those previous data along with studies in a gene knockdown mouse model (25) suggest that ezrin expression can be tightly coupled to polarity of the parietal cell. Yet specific mechanisms for these associations were not given or known. Recognizing the limitation, that these are in vitro experiments with expression and overexpression of ezrin constructs, they do offer results for primary parietal cell cultures without alterations induced via derivative cell lines. The more detailed time course analysis of the progression of morphologic change and distribution of ezrin mutants shown here provides some mechanistic insight as to how ERM proteins may operate to influence cell polarity.
First, the amount of H-K-ATPase translocation from the cytoplasmic vesicular pool is positively correlated to the expression levels and location of ezrin. Western blot results indicated that the expression levels of exogenous ezrin introduced by rAd constructs greatly exceeded that of endogenous ezrin at time points later than 24 h. The immunofluorescence experiments clearly demonstrate that the exogenously expressed ezrin-WT and ezrin-TA constructs accumulated on apical membrane, followed by the translocation of H-K-ATPase onto or in the immediate vicinity of the apical membrane, at later time points. The ezrin-TD construct is different in that ezrin first accumulated on apical membrane and then appeared and accumulated on the basal membrane at later time points. Although there is no absolute proof of the order of migration, the consensus is that H-K-ATPase still followed (at later time points) the footprints of ezrin: first appearing on apical membrane and then on basal membrane. With all these ezrin constructs, it is striking that wherever ezrin went, H-K-ATPase would faithfully relocalize to the same location, at a later time point. These time course experiments provided new evidence supporting the hypothesis that ezrin recruits new membrane onto the target membrane where it resides.
Second, the time course experiments also provide evidence that ezrin can stimulate recruitment of new membrane even when unphosphorylated. The T567A mutant and WT ezrin both increased tubulovesicle recruitment to apical vacuoles when overexpressed, yet the vacuoles never showed the gross expansion of stimulated cells (unless treated with secretagogues, e.g., Fig. 2). Normal levels of endogenous ezrin lead to this recruitment when ezrin is phosphorylated, but not when unphosphorylated, even though the latter form is still largely associated with apical membrane. Thus, both the amount of ezrin and its phosphorylation state seem to affect the degree of membrane recruitment, with the normal range of morphological change for the parietal cell dependent on both. Overexpression of the T567D “constitutively phosphorylated” mutant would bring both of these factors to bear simultaneously, driving the membrane recruitment phenomenon to an extreme.
These data lead to a consideration of the membrane and cytoskeleton binding activities of ezrin. Our view, similar to the general position in the field, is that the N-ERMAD binds to membranes via adaptor proteins and inositol lipids, and the C-ERMAD binds to F-actin when it is conformationally relaxed by phosphorylation at Thr567 (T567-P) (6, 9, 10, 17, 19, 20, 31). However, our lab has specifically emphasized the importance of the rapid turnover of T567-P to account for the dynamics of its function as well as apparently distinct roles of ezrin (and other ERMs) in different cell types (29, 31). Ezrin-actin interaction (as well as surface membrane interaction) is a direct function of the relative phosphorylation on ezrin Thr567, in both the steady-state level and the turnover. In microvilli of proximal tubules and intestinal cells, steady-state ezrin T567 is predominantly in the T567-P form (29). In parietal cells, steady-state phosphorylation of microvillar ezrin depends on functional activity: resting cells have low T567-P; stimulated cells have higher T567-P (31). All three cell types demonstrate high turnover of T567-P as measured by immunoblot or by incorporation of AT32P (26, 31). Thus this T567-P turnover hypothesis proposes that when kinase activity is consistently higher than phosphatase activity, the T567-P form predominates, with ezrin-cytoskeleton binding consistently firm, as in brush-border membranes. When kinase activity is quite variable relative to phosphatase activity, dephospho-T567 sometimes predominates and ezrin-cytoskeleton binding is more flexible, allowing for the greater surface plasticity and microvillar pliability that we see in the functioning parietal cell.
To account for the present data, we would propose that the N-ERMAD (similar for all of the expressed ezrin constructs) and a predominance of ezrin-binding membrane components in the apical membrane determine the targeting of ezrin to the apical membrane, which is supported by studies expressing only the N-ERMAD (4). Even with overexpression, the WT (without secretagogues) and T567A mutant, both with inactive C-ERMAD, remain largely at the apical domain. However, overexpressed T567D, with constitutively active C-ERMAD, could begin to saturate available apical F-actin sites and increase its binding to the smaller pool of basal F-actin. We propose that the ability of the T567D mutant to link both F-actin and the very few ezrin-binding basal membrane components leads to increased formation of basal microvilli and filopodia, initiating a repeating and self-reinforcing cycle.
In the first 16 h of expression, there is a measurable accumulation of WT and T567-mutant forms of ezrin on the putative apical vacuolar membrane binding sites, where there is also a predominance of native endogenous ezrin. In the case of the TD mutant one would predict that there may be some competition of endogenous F-actin pools for the C-ERMAD of the T567D mutant so that, with time, there might be a shift in the abundance of T567D toward the basal F-actin. This would be consistent with the shift in T567D mutant ezrin from apical vacuoles to basal membranes through the 40-h time course. What then accounts for the relocalization and basal recruitment of H-K-ATPase which follows a very similar pattern to the ezrin T567D mutant? We propose that the relatively tight association of the T567D C-ERMAD with basal F-actin and membrane provides a force or strain to ruffle or project the surface in space. Though this is an ad hoc postulate, it is not unreasonable to suspect that multiple and increasing sites of interaction between a planar membrane and a relatively stiff linear structure might impose some surface strain. Such forces may promote membrane recruitment, and in the parietal cell the most prominent membrane source is the tubulovesicular compartment. The elongated microvillar and filopodial forms at the surrounding basal membrane are thus enriched in both ezrin T567D and H-K-ATPase.
The parietal cell in primary culture, with its engulfed apical membrane, may present a special case in which membrane expansion is far less physically constrained at the basal than at the apical membrane, leading to the overwhelming recruitment of internal (including, in this special case, apical) membranes to the basal domain following overexpression of an F-actin-binding ezrin mutant. Certainly, ezrin is not the sole component involved in membrane recruitment. There are many additional critical factors, such as docking, fusing and accessory proteins (4, 8, 14, 22). It is important to note that the striking relocalizations of ezrin and H-K-ATPase reported here with T567D are triggered by a single mutation in ezrin, mimicking permanent phosphorylation, and that they absolutely require massive shifting of F-actin and membrane to the basal domain. The observed data and the proposed interpretation clearly suggest that ezrin and its membrane surface interaction can direct membrane recruitment.
Similar ezrin-related phenomena of recruitment and redirection of membrane surfaces have been seen in other systems. Overexpression of the ezrin-TD mutant in mouse early embryos induces formation of elongated microvilli at cortical surfaces and numerous ectopic microvilli at cell-cell contacts, disturbing normal compaction and cavitation (6). In a renal epithelial cell line, activation of the Rho kinase pathway by microinjecting constitutively active RhoA produced translocation of Na-K-ATPase from the basolateral surface (normal location) to the apical surface along with accumulation of phosphorylated ERM proteins at the apical surfaces (15).
How does the proposal for ezrin T567D fit with data for WT ezrin and the T567A mutant? Obviously, the T567A mutant cannot be phosphorylated at that site, and in the parietal cell the steady-state level of ezrin T567-P is relatively low, yet membrane binding capacity via the N-ERMAD still exists (29) and thus high level apical targeting is expected. In 24–40 h of high expression, virtually all of the exogenously expressed WT and T567A ezrin accumulate at the apical membrane vacuoles. We posit that similar forces of strain at the apical surface demand incorporation of new membrane which is derived from the abundant tubulovesicular pool, and thus there is concomitant recruitment of H-K-ATPase to the apical vacuoles.
Therefore, our data are consistent with a two-step mechanism for ezrin's function in establishing cell polarity. First, through tissue-specific mechanisms, ezrin determines the location for new membrane incorporation by its own specific subcellular localization. Second, through its manipulation of membrane tension, ezrin recruits new membrane, establishing/maintaining a cell-specific polarity.
A model for predicting the cellular distribution of ezrin and its binding to membranes and F-actin.
We have constructed a model to simulate the distribution of endogenous ezrin between apical and basal domains of the parietal cell as well as to account for the striking differences in ultimate distributions of the T567A and T567D ezrin mutants (TA and TD). The model with initial assumptions as put forth in materials and methods assumes simple equilibria for the phosphorylation state of ezrin, and for the interactions between ezrin and the membrane and F-actin binding sites at both apical and basal cellular domains. The model incorporates multiple pools of ezrin—phosphorylated or unphosphorylated at T567, bound or unbound to membrane or F-actin, and associated with the apical or basal domains—with assumed rates for conversion from one state to another. The model can be run to show the effects of parietal cell stimulation over relatively short periods (i.e., change in ezrin phosphorylation) and can also be run to simulate the appearance and distribution of exogenous TA or TD constructs over an extended time course. In addition to its application to the parietal cell and the present experimental results, the model is sufficiently general and may be modified for application to other cells and tissues, and other members of the ERM family. The Supplemental Material, which is linked to this article and is available on the Journal web site, includes additional descriptive details and the model files themselves (which can be run with a free trial version of Berkeley Madonna, available at www.berkeleymadonna.com).
On the basis of observations in many cell systems, with special emphasis on what is established for parietal cells (in vivo and primary cell culture), we use the following relationships: To account for observations in parietal cells 1) that ezrin is mostly unphosphorylated (E) at T567 in the resting state (31), 2) that this unphosphorylated ezrin is mostly bound to membrane (28), 3) that endogenous ezrin is localized mostly to the apical vacuolar membrane, with small amounts seen at the basal membrane (1, 11), and 4) that a roughly fourfold increase in total ezrin occurs over 40–50 h after infection with either WT or TA resulting in ezrin maintaining this same distribution (present study), we have modeled enough ezrin-binding membrane sites in the apical membrane to accommodate the increase in ezrin forms (TA) that can bind to membrane but not to F-actin, while placing 10-fold fewer such sites in the basal membrane [based on immunofluorescence distribution of native ezrin (1, 12)]. Most parietal cell actin has been shown to be in the F-actin form (2), and we have modeled the apical-to-basal distribution of F-actin to be 3:1 [based on apparent distribution of phalloidin staining and binding data from Zhu et al. (31)]. Rate constants for binding to membrane were set to favor binding 5:1 over unbinding for ezrin (E) and 8:1 for phosphorylated ezrin (EP), the somewhat higher affinity for EP over E reflecting reported data (7, 29). EP was set to favor binding to F-actin 5:1, whereas E was excluded from binding F-actin.
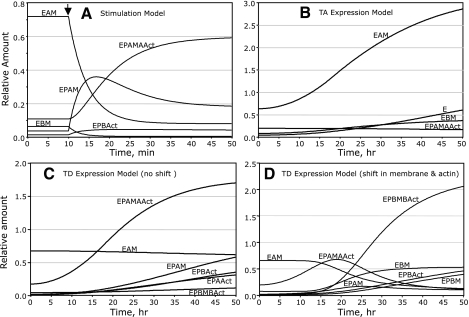
When the model is run over a 50-min period (Fig. 8A), the first 10 min representing the resting state (phosphatase rate favored 5:1 over the kinase rate), ezrin is mostly in the E form bound to apical membrane (EAM). After stimulation by secretagogues beginning at 10 min (kinase now favored 10:1 over phosphatase), the EP form rapidly increases, mostly bound to apical membrane (EPAM), followed by a slightly slower, but ultimately dominant, increase in EP bound to both apical membrane and apical F-actin (EPAMAAct). In effect, there is no polar shift in ezrin distribution, just a change in the predominant bound form of ezrin. Conditions allow for newly formed EP to bind F-actin and/or membrane in either the apical or basal domain; however, the model predicts nearly 90% of EP resides in the apical domain after 40 min of stimulation because of very few basal membrane sites for ezrin, along with binding constants that favor binding of EP to both F-actin and membrane. At 50 min, near stimulation equilibrium, the sum of all basally localized ezrin forms does not exceed 8% of total ezrin.
Fig. 8.
Modeling data predict changes in the equilibrium distribution of ezrin-associated forms (defined in text) consistent with experimental observations. In A, the total amount of endogenous ezrin is set at 1.0, so relative numerical amount indicates the fraction of ezrin in each form. In B–D, the expression of exogenous ezrin mutants (TA or TD) is input to the model as described in the text so that endogenous ezrin is maintained at 1.0 and mutant ezrin expression increases sigmoidally to 3.0 (total = 4.0) over a 50-h time course. A: the parietal cell transitions from the resting to secreting state after secretagogue stimulation at 10 min (arrow), where a great change of ezrin phosphorylation (E → EP) is input to the model (no change in total ezrin, see text). The major forms of ezrin are shown in the graph with phospho-ezrin bound to both apical membrane and apical actin (EPAMAAct) predominating near steady state. Ezrin forms that do not rise above 5% of total ezrin are not shown. B: prediction for changes in distribution of major ezrin-associated forms when model is input to overexpress ezrin-TA mutant over 50 h. Because there is no phosphorylation of expressed ezrin-TA, the predominant dephospho-ezrin form is bound to apical membrane sites (EAM). C: predictions for redistribution of ezrin-associated forms when model is input to overexpress ezrin-TD mutant. In this plot, no changes in apical and basal membrane and actin binding sites are assumed; endogenous ezrin and the newly expressed ezrin phospho-mimic simply distribute among available binding sites as with previous models. Here the distribution of ezrin to apical binding sites predominates, approaching steady state (EPAMAAct and EPAM). D: as in C, the model is input to overexpress ezrin-TD mutant of ezrin binding forms; however, a dynamic change in ezrin binding sites, from apical to basal domains, is input to the model, as described in the text. There is an initial transient increase in EPAMAAct, but as the system approaches steady state, the basal forms of bound ezrin predominate (EPBMBAct, EBM, EPBAct, and EPBM).
To model TA (or TD) infection and consequent expression over 2 days, we added to the initial pool of total E (or EP in the case of the TD mutant), with kinase and phosphatase rates set to zero (the mutants being unaffected by these enzymes) and with the newly expressed pool increasing total ezrin to four times the initial (endogenous E) amount after 2 days. In the Madonna modeling program, a “flow module” was used to increase these pools sigmoidally. A model run representing a 50-h period, with TA expression starting at time zero (Fig. 8B), shows apical membrane-bound E (EAM) increasing greatly and remaining the predominant form, consistent with immunocytochemical localization and the fact that TA cannot bind F-actin. Much smaller increases were seen in basal membrane-bound E (EBM) and free E. A model run to mimic WT-ezrin overexpression was done as with TA infection, but with kinase and phosphatase rates set as in the resting state of Fig. 8A. The results were so similar to Fig. 8B that they are not shown here but are included in the Supplemental Material.
The same flow module used to increase E for TA expression was used to increase EP for TD expression (TD treated as “permanently phosphorylated” EP). To test how the increased EP would interact with preexisting basal amounts of F-actin and membrane binding sites, we first ran the TD-expression model with no presumed shifts of F-actin or membrane to the basal domain. In the simulation shown in Fig. 8C, after 50 h, total ezrin has increased fourfold, with >86% of bound ezrin apically located (mainly EPAMAAct) and with basally associated ezrin comprising <14% of bound ezrin. However, due to the overexpression, this basal ezrin is more than sixfold greater than the basal ezrin predicted in the stimulation model of endogenous ezrin (amounts not shown in Fig. 8A).
Although the simple equilibrium model in Fig. 8C predicts some increase in basal ezrin association, it does not account for the striking experimental results seen during extended TD expression (Fig. 4), including 1) the shift of TD from apical to basal membrane, 2) the near disappearance of the apical vacuoles, 3) the shift of H-K-ATPase from diffuse cytoplasmic (tubulovesicular) to basal localization, and 4) the marked increase in basal membrane projections (microvilli and filopodia). To address this problem, we presumed a shift in equilibrium of F-actin, and ezrin-binding membrane sites, from apical to basal domains. Because tubulovesicles lack ezrin-binding components, the proposed membrane and actin recruitment processes were modeled as direct shifts from apical to basal domains, following sigmoidal time curves. On the basis of the observed large redistribution of ezrin-TD, the shifts are presumed to end with basal domain favored 8:1 over apical for both membrane and F-actin sites. When these shifts are incorporated into the TD-expression model as shown in Fig. 8D, TD (as EP) initially accumulates mainly in the apical domain, but basally localized EP begins to appear and dominates after 24 h. As the system reaches 50 h, >88% of bound ezrin resides at basal sites, with the predominant form being EPBMBAct, bound to both basal F-actin and basal membrane. A recent study using WIF-B cells, a liver cell line that maintains some phenotypic similarities to hepatocytes, reports an analogous pattern of distribution for expressed phosphorylation mutants of the ERM family member radixin. That is, WT-radixin and the T564A-radixin mutant were localized to internalized apical canalicular membranes, whereas the T564D-radixin mutant was primarily relocalized to long projections at the surrounding basal membrane, with concomitant disappearance of canalicular vacuoles (23), consistent with our data on parietal cells.
Summary.
The work presented here demonstrates the cellular distribution of endogenous parietal cell ezrin and its redistribution with functional stimulation. The time course for the expression of exogenous ezrin, as well as two T567 phosphorylation mutants, is carefully documented. Differences in polar distribution of ezrin suggest a role for the membrane-cytoskeletal linker protein in promoting formation and plasticity of membrane surface projections, forming the basis for a novel theory for ezrin as an organizer and regulator of membrane recruitment. A model simulating the cellular distribution of ezrin and its associated membrane and F-actin binding forms is given, which can be modified as more specific information regarding binding constants and sites becomes available.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant AM10141-34.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the advice and support of Prof. Robert Macey in coaching through the workings of the Berkeley Madonna Modeling Program.
REFERENCES
- 1.Agnew BJ, Duman JG, Watson CL, Coling DE, Forte JG. Cytological transformations associated with parietal cell stimulation: critical steps in the activation cascade. J Cell Sci 112: 2639–2646, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Ammar DA, Nguyen PN, Forte JG. Functionally distinct pools of actin in secretory cells. Am J Physiol Cell Physiol 281: C407–C417, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bretscher A. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol 108: 921–930, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao X, Ding X, Guo Z, Zhou R, Wang F, Long F, Wu F, Bi F, Wang Q, Fan D, Forte JG, Teng M, Yao X. PALS1 specifies the localization of ezrin to the apical membrane of gastric parietal cells. J Biol Chem 280: 13584–13592, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Chew CS, Ljungstrom M, Smolka A, Brown MR. Primary culture of secretagogue-responsive parietal cells from rabbit gastric mucosa. Am J Physiol Gastrointest Liver Physiol 256: G254–G263, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Dard N, Louvet-Vallee S, Santa-Maria A, Maro B. Phosphorylation of ezrin on threonine T567 plays a crucial role during compaction in the mouse early embryo. Dev Biol 271: 87–97, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, Arpin M. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol 164: 653–659, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forte JG, Zhu L. Apical recycling of the gastric parietal cell H,K-ATPase. Annu Rev Physiol 72: 273–296 [DOI] [PubMed] [Google Scholar]
- 9.Gary R, Bretscher A. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol Biol Cell 6: 1061–1075, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gautreau A, Louvard D, Arpin M. Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J Cell Biol 150: 193–203, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanzel D, Reggio H, Bretscher A, Forte JG, Mangeat P. The secretion-stimulated 80K phosphoprotein of parietal cells is ezrin, and has properties of a membrane cytoskeletal linker in the induced apical microvilli. EMBO J 10: 2363–2373, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanzel DK, Urushidani T, Usinger WR, Smolka A, Forte JG. Immunological localization of an 80-kDa phosphoprotein to the apical membrane of gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 256: G1082–G1089, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Heiska L, Alfthan K, Gronholm M, Vilja P, Vaheri A, Carpen O. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4,5-bisphosphate. J Biol Chem 273: 21893–21900, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Ding X, Wang D, Deng H, Feng M, Wang M, Yu X, Jiang K, Ward T, Aikhionbare F, Guo Z, Forte JG, Yao X. A mechanism of Munc18b-syntaxin 3-SNAP25 complex assembly in regulated epithelial secretion. FEBS Lett 581: 4318–4324, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda A, Amano M, Fukata Y, Kaibuchi K. Translocation of Na(+),K(+)-ATPase is induced by Rho small GTPase in renal epithelial cells. Biochem Biophys Res Commun 297: 1231–1237, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Minamide LS, Bamburg JR. A filter paper dye-binding assay for quantitative determination of protein without interference from reducing agents or detergents. Anal Biochem 190: 66–70, 1990 [DOI] [PubMed] [Google Scholar]
- 17.Nakamura K, Zhou CJ, Parente J, Chew CS. Parietal cell MAP kinases: multiple activation pathways. Am J Physiol Gastrointest Liver Physiol 271: G640–G649, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Niggli V, Andreoli C, Roy C, Mangeat P. Identification of a phosphatidylinositol-4,5-bisphosphate-binding domain in the N-terminal region of ezrin. FEBS Lett 376: 172–176, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Pearson MA, Reczek D, Bretscher A, Karplus PA. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101: 259–270, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Reczek D, Berryman M, Bretscher A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol 139: 169–179, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serrador JM, Vicente-Manzanares M, Calvo J, Barreiro O, Montoya MC, Schwartz-Albiez R, Furthmayr H, Lozano F, Sanchez-Madrid F. A novel serine-rich motif in the intercellular adhesion molecule 3 is critical for its ezrin/radixin/moesin-directed subcellular targeting. J Biol Chem 277: 10400–10409, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75: 409–418, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Suda J, Zhu L, Karvar S. Intracellular localization of radixin and NHERF-1 on regulation of MRP-2 trafficking in hepatocytes (Abstract). Hepatology 50: 659, 2010 [Google Scholar]
- 24.Sun F, Hug MJ, Lewarchik CM, Yun CH, Bradbury NA, Frizzell RA. E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J Biol Chem 275: 29539–29546, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Tamura A, Kikuchi S, Hata M, Katsuno T, Matsui T, Hayashi H, Suzuki Y, Noda T, Tsukita S. Achlorhydria by ezrin knockdown: defects in the formation/expansion of apical canaliculi in gastric parietal cells. J Cell Biol 169: 21–28, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urushidani T, Hanzel DK, Forte JG. Protein phosphorylation associated with stimulation of rabbit gastric glands. Biochim Biophys Acta 930: 209–219, 1987 [DOI] [PubMed] [Google Scholar]
- 27.Yao X, Thibodeau A, Forte JG. Ezrin-calpain I interactions in gastric parietal cells. Am J Physiol Cell Physiol 265: C36–C46, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Zhou R, Zhu L, Kodani A, Hauser P, Yao X, Forte JG. Phosphorylation of ezrin on threonine 567 produces a change in secretory phenotype and repolarizes the gastric parietal cell. J Cell Sci 118: 4381–4391, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Zhu L, Hatakeyama J, Chen C, Shastri A, Poon K, Forte JG. Comparative study of ezrin phosphorylation among different tissues: more is good; too much is bad. Am J Physiol Cell Physiol 295: C192–C202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu L, Liu Y, Forte JG. Ezrin oligomers are the membrane-bound dormant form in gastric parietal cells. Am J Physiol Cell Physiol 288: C1242–C1254, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Zhu L, Zhou R, Mettler S, Wu T, Abbas A, Delaney J, Forte JG. High turnover of ezrin T567 phosphorylation: conformation, activity, and cellular function. Am J Physiol Cell Physiol 293: C874–C884, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.