Abstract
During reperfusion, the interplay between excess reactive oxygen species (ROS) production, mitochondrial Ca2+ overload, and mitochondrial permeability transition pore (mPTP) opening, as the crucial mechanism of cardiomyocyte injury, remains intriguing. Here, we investigated whether an induction of a partial decrease in mitochondrial membrane potential (ΔΨm) is an underlying mechanism of protection by anesthetic-induced preconditioning (APC) with isoflurane, specifically addressing the interplay between ROS, Ca2+, and mPTP opening. The magnitude of APC-induced decrease in ΔΨm was mimicked with the protonophore 2,4-dinitrophenol (DNP), and the addition of pyruvate was used to reverse APC- and DNP-induced decrease in ΔΨm. In cardiomyocytes, ΔΨm, ROS, mPTP opening, and cytosolic and mitochondrial Ca2+ were measured using confocal microscope, and cardiomyocyte survival was assessed by Trypan blue exclusion. In isolated cardiac mitochondria, antimycin A-induced ROS production and Ca2+ uptake were determined spectrofluorometrically. In cells exposed to oxidative stress, APC and DNP increased cell survival, delayed mPTP opening, and attenuated ROS production, which was reversed by mitochondrial repolarization with pyruvate. In isolated mitochondria, depolarization by APC and DNP attenuated ROS production, but not Ca2+ uptake. However, in stressed cardiomyocytes, a similar decrease in ΔΨm attenuated both cytosolic and mitochondrial Ca2+ accumulation. In conclusion, a partial decrease in ΔΨm underlies cardioprotective effects of APC by attenuating excess ROS production, resulting in a delay in mPTP opening and an increase in cell survival. Such decrease in ΔΨm primarily attenuates mitochondrial ROS production, with consequential decrease in mitochondrial Ca2+ uptake.
Keywords: cardioprotection, oxidative stress, mitochondria, reactive oxygen species
damage during ischemia and reperfusion (I/R) of the heart involves complex processes where the cellular machinery itself becomes a source of deleterious mediators of injury. Such processes include excessive production of mitochondrial reactive oxygen species (ROS) and cellular Ca2+ overload (6, 37), which trigger opening of the mitochondrial permeability transition pore (mPTP) (16, 19), a critical event in the transition towards cell death (18). The mPTP opening instantly dissipates mitochondrial membrane potential (ΔΨm), ceases mitochondrial ATP production, and initiates cell death pathways (6).
Studies suggest that most of the cell death occurs during reperfusion (51, 53), which can be attenuated with antioxidants (52), indicating an important role of ROS. In cardiomyocytes, ROS are primarily generated at complexes I and III of the mitochondrial electron transport chain (50). During I/R, accumulation of cytosolic Ca2+, which drives accumulation of mitochondrial Ca2+ (45), is primarily attributed to insufficient ATP production and derangement of intracellular ion homeostasis (37). It is suggested that excess ROS production and mitochondrial Ca2+ overload are mutually dependent processes, where ROS can stimulate Ca2+ release into the cytosol from the sarcoplasmic reticulum (12, 55) and decrease cytosolic Ca2+ clearance by inhibiting Ca2+ pumps (25, 35). In turn, Ca2+ can enhance mitochondrial ROS production (6). The vicious cycle is closed when ROS and Ca2+ trigger mPTP opening in individual mitochondria that by itself causes a burst of ROS and mitochondrial Ca2+ release, resulting in a self-sustained progression of mPTP opening throughout the mitochondrial population. These processes have been described as ROS-induced ROS release (3, 56) and Ca2+-induced Ca2+ release from mitochondria (22). Thus, the disruption of these interdependent processes could have important implications in attenuation of injury and increase in cardiomyocyte survival.
Anesthetic-induced preconditioning (APC) elicits endogenous defense mechanisms that increase resistance of cardiomyocytes to I/R injury (8). Multiple protective pathways have been implicated in APC, including activation of mitochondrial ATP-sensitive K+ channels, as one of the effectors of protection (34). Interestingly, cardioprotection can also be mediated by mitochondrial uncoupling proteins and agents known to depolarize mitochondria (2, 14), indicating the importance of ΔΨm regulation for the resistance to I/R injury. Although cardioprotective effects of volatile anesthetics have been demonstrated in randomized clinical trials (30) and recognized by the American College of Cardiology/American Heart Association Guidelines (13), the mechanisms of protection are not completely understood.
To further delineate the mechanism underlying cardioprotection from oxidative stress by preconditioning with isoflurane, we designed our study to investigate the role of subtle changes in ΔΨm as an effector of protection. We hypothesized that APC induces partial decrease in ΔΨm that attenuates mitochondrial ROS generation in stressed cardiomyocytes, which leads to the delay in mPTP opening and subsequent increase in cell survival. The magnitude of APC-induced decrease in ΔΨm was mimicked with the protonophore 2,4-dinitrophenol (DNP) and reversed by the addition of pyruvate, which allows for the separation of the effects of changing ΔΨm from other effectors of protection by APC. We also investigated the interdependence between ROS production and mitochondrial Ca2+ accumulation in relation to mild changes in ΔΨm.
METHODS
The Institutional Animal Care and Use Committee of the Medical College of Wisconsin (Milwaukee, WI) approved the animal use and experimental protocols of this study.
Isolation of ventricular myocytes.
Cardiomyocytes were isolated from ventricles of adult male Wistar rats (180–250 g) using collagenase and protease for enzymatic dissociation, as previously reported (46). Rats were anesthetized with sodium thiobutabarbital (Inactin, Sigma-Aldrich, St. Louis, MO, 150 mg/kg, administered intraperitoneally). After isolation, myocytes were stored in Tyrode solution (in mM: 132 NaCl, 10 HEPES, 5 glucose, 5 KCl, 1 CaCl2, 1.2 MgCl2; adjusted to pH 7.4). Experiments were conducted at room temperature within 5 h after isolation using Tyrode solution.
Isolation of cardiac mitochondria.
Cardiac mitochondria were isolated from Wistar rats as reported previously (42). Hearts were placed in cold isolation buffer [in mM: 50 sucrose, 200 mannitol, 5 KH2PO4, 1 EGTA, 5 3-(N-morpholino) propanesulfonic acid, and 0.1% bovine serum albumin; pH 7.3 adjusted with KOH] and homogenized with a T 25 disperser (IKA-Werke, Staufen, Germany). Mitochondria were isolated by differential centrifugation, and protein concentration was determined. Mitochondria were stored in isolation buffer on ice and used within 4 h after isolation. Experiments were conducted at room temperature. In the APC group, mitochondria were isolated from rats that were preconditioned in vivo with isoflurane.
Experimental procedures in cardiomyocytes and oxidative stress.
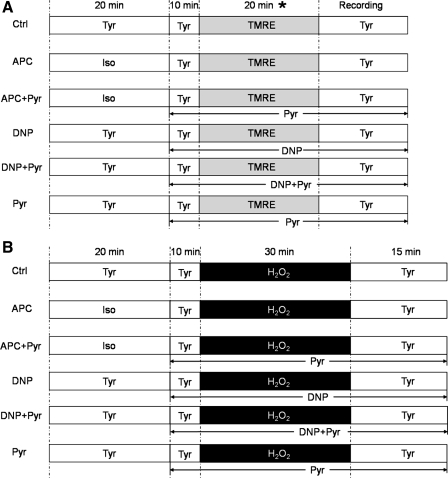
APC was induced by isoflurane (0.5 mM), which was dissolved in Tyrode solution by sonication and applied as depicted in Fig. 1. Isoflurane concentration was verified by gas chromatography (GC-8A, Shimadzu, Japan) and varied ± 10% of reported 0.5 mM. The protonophore DNP (200 nM, Sigma-Aldrich) was used to mimic the magnitude of APC-induced mitochondrial depolarization. Pyruvate, a respiration substrate that can increase ΔΨm, was titrated, and 2 μM was used to repolarize mitochondria following APC or DNP treatment. Hydrogen peroxide (H2O2, 250 μM) was used to induce oxidative stress in cell survival experiments, ROS production, and simultaneous cytosolic and mitochondrial Ca2+ measurements (33, 34). H2O2 was not used to trigger mPTP opening, since it caused cell hypercontracture, which hampered identification of pore opening. Instead, mPTP opening was instigated using a well-established approach, where oxidative stress was induced by laser-photoexcitation as described below and previously (42). H2O2 alone did not affect fluorescence probes [except 5-(and-6)-chloromethyl-2′,7′-dichlorofluorescein (CM-DCF)], as measured by spectrofluorometry.
Fig. 1.
Experimental procedures used for cardiomyocytes. A: protocols for mitochondrial membrane potential (ΔΨm) and mitochondrial permeability transition pore (mPTP) opening measurements. *Tetramethylrhodamine ethyl ester (TMRE) loading time in mPTP protocol was modified to ensure similar TMRE concentrations, despite differences in ΔΨm among experimental groups. B: protocols for cell survival experiments, reactive oxygen species (ROS) measurements, and simultaneous cytosolic and mitochondrial Ca2+ measurements. In ROS production measurements, H2O2 was discontinued after 20 min, followed by dye loading for 10 min to prevent oxidation of 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein (CM-H2DCF) by exogenous H2O2. Detailed explanation of protocols is found in appropriate sections in methods. Ctrl, control; APC, anesthetic-induced preconditioning; Pyr, pyruvate; DNP, 2,4-dinitrophenol; Iso, isoflurane; Tyr, Tyrode solution.
Laser-scanning confocal microscopy.
In cardiomyocytes, measurements of ΔΨm mitochondrial redox state, ROS production, cytosolic and mitochondrial Ca2+, and mPTP opening were conducted using a laser-scanning confocal microscope (Leica TCS SP5, Mannheim, Germany) with a ×63/1.4 numerical aperture Apo oil objective lens. Fluorescent indicators were excited with 488 nm line of an argon laser and 543 nm green HeNe laser, and images were acquired using LAS AF software (Leica).
Measurements of ΔΨm in cardiomyocytes.
The ΔΨm-sensitive fluorescent dye tetramethylrhodamine ethyl ester (TMRE) (Invitrogen, Carlsbad, CA) and autofluorescence of mitochondrial flavoproteins (FPs) were used for the assessment of ΔΨm in cardiomyocytes (Fig. 1A) as previously described (32). Excitation and emission wavelengths for TMRE were λex/λem = 543/560–610 nm and for FPs were λex/λem = 488/500–550 nm. For a better representation of the effect of pyruvate on ΔΨm, pyruvate was used initially at the higher concentrations (250 μM) than in the rest of our experiments. To reverse the APC-induced mitochondrial depolarization, 2 μM pyruvate was used. Fluorescence values were normalized to baseline expressed as 100%.
Cardiomyocyte survival experiments.
Cardiomyocytes were exposed to H2O2-induced oxidative stress as depicted in Fig. 1B. The number of live cells was counted at the beginning and at the end of the experiment. Rod-shaped cells without membrane blebs that excluded Trypan blue were considered alive (46). Cell death was normalized to control (Ctrl), expressed as 100%.
Analysis of mPTP opening in cardiomyocytes.
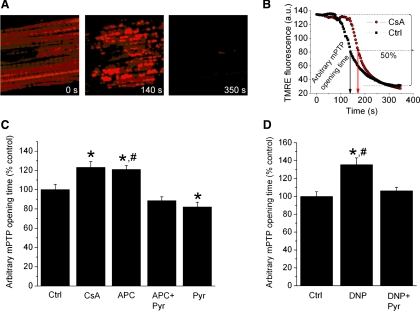
Photoexcitation-generated oxidative stress was used to induce opening of mPTP that was observed as a rapid and complete mitochondrial depolarization using ΔΨm-sensitive dye TMRE (21, 24, 42, 56). The TMRE (100 nM) incubation time was adjusted as needed to ensure equal TMRE concentrations in all experimental groups, despite differences in ΔΨm. After TMRE loading, the dye was washed out, and 30 × 30 μm recording region of cardiomyocytes was exposed to narrowly focused laser scanning. Only cells with equal initial TMRE fluorescence intensity were included in the study, and the settings of the confocal microscope were consistent to ensure equal delivery of oxidative stress. As we noted previously, the rapid release of TMRE coincides with the release of calcein, a 620-Da molecule, confirming opening of the mPTP (42, 56). Cyclosporine A (1 μM) was used to inhibit mPTP opening. Arbitrary mPTP opening time was determined as the time of loss of average TMRE fluorescence intensity from the recorded region (excluding nucleus) by half between initial and residual fluorescence intensity (Fig. 4B). It corresponded to complete depolarization of ∼50% of mitochondria in the recorded region.
Fig. 4.
Stress-induced opening of mPTP is delayed by APC and DNP. A: photoexcitation-generated oxidative stress induces mPTP opening as observed by the rapid dissipation of TMRE fluorescence. B: the time by which TMRE fluorescence intensity decreased by half between values at the beginning and after global mPTP opening was considered as the “arbitrary mPTP opening time” (arrows) and was compared among experimental groups. AU, arbitrary units. C: cyclosporine A (CsA), an inhibitor of mPTP, extended arbitrary mPTP opening time compared with control, indicating a delay in mPTP opening. APC also increased mPTP opening time, which was abrogated by 2 μM pyruvate (APC + Pyr). D: DNP (200 nM) increased mPTP opening time, and 2 μM pyruvate (DNP + Pyr) attenuated this effect. Data are means ± SE; n = 6–12/group. *P < 0.05 vs. Ctrl; #P < 0.05 APC or DNP vs. APC + Pyr or DNP + Pyr, respectively.
Measurements of ROS production in cardiomyocytes.
ROS production was monitored using 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) (Invitrogen) that, upon deesterification and oxidation by ROS, yields fluorescent CM-DCF. The fluorescence intensity of CM-DCF was acquired using 8,000-Hz resonant scanner of the confocal microscope with λex/λem = 488/500–550 nm. After exposure of cardiomyocytes to 250 μM H2O2 for 20 min, H2O2 was washed out twice before cells were loaded with 4 μM CM-H2DCFDA for 10 min. Data were normalized to initial value expressed as 100%.
Measurements of ROS production in isolated mitochondria.
Isolated mitochondria (0.5 mg/ml protein) were suspended in experimental buffer (in mM: 130 KCl, 5 K2HPO4, 20 MOPS, 2.5 EGTA, 5 pyruvate, 5 malate, 0.001 Na4P2O7, and 0.1% BSA; pH adjusted to 7.3 with KOH) containing the fluorescence probe Amplex Red (12.5 μM, Invitrogen) and 0.1 U/ml horseradish peroxidase to monitor the rate of H2O2 production. The fluorescence intensity of resorufin that reflects H2O2 release was measured with a spectrofluorometer (QM-8, Photon Technology International) (λex/λem = 530/583 nm). Excess ROS production was initiated by antimycin A (AA, 200 nM), a mitochondrial complex III inhibitor (9). In all experimental groups, the rates of increase in resorufin fluorescence intensity were analyzed after the addition of AA. To eliminate the contribution of changes in mitochondrial Ca2+ on ROS production, experiments were conducted in a Ca2+-free buffer and in the presence of EGTA.
Measurements of Ca2+ uptake in isolated mitochondria.
Mitochondrial matrix free Ca2+ was assessed after loading indo-1 AM (10 μM, Invitrogen) into mitochondria (10 mg/ml protein) (15). Residual dye was removed by centrifugation at 8,000 g, and the mitochondrial pellet was resuspended in experimental buffer without EGTA. Spectrofluorometry was used for the detection of indo-1 fluorescence intensity (λex/λem = 350/405–460 nm). CaCl2 (20 μM) pulses were added every 1 min. Mitochondrial autofluorescence was not affected by DNP. Changes in indo-1 fluorescence intensity are expressed as a percentage of baseline.
Simultaneous measurements of Ca2+ in cardiomyocyte cytosol and mitochondria.
Ca2+-sensitive dyes, rhod-2 AM (4 μM, Invitrogen) and fluo-4 AM (2 μM, Invitrogen), were used to simultaneously detect mitochondrial and cytosolic Ca2+, respectively, by confocal microscopy. The predominant localization of rhod-2 and fluo-4 in mitochondria and cytosol, respectively, was achieved following a two-step cold/warm loading protocol (28). Briefly, cells were incubated with rhod-2 AM at room temperature for 60 min, followed by 60-min incubation at 37°C, and 120 min of dye washout at 37°C. During the last 30 min of rhod-2 AM washout, cells were loaded with fluo-4 at 37°C, followed by 20-min dye washout at room temperature. The switch from room temperature to 37°C and back to room temperature was gradual. Excitation and emission wavelengths of rhod-2 and fluo-4 were λex/λem = 543/560–610 and λex/λem = 488/500–550 nm, respectively. Data were normalized to baseline expressed as 100%.
Statistical analysis.
Data are presented as means ± SE, with n indicating the number of independent experiments. Statistical comparisons were performed using one-way or repeated-measures analysis of variance with Tukey post hoc test where appropriate. Differences at P < 0.05 were considered significant.
RESULTS
APC-induced mitochondrial depolarization in cardiomyocytes was mimicked by DNP and reversed by pyruvate.
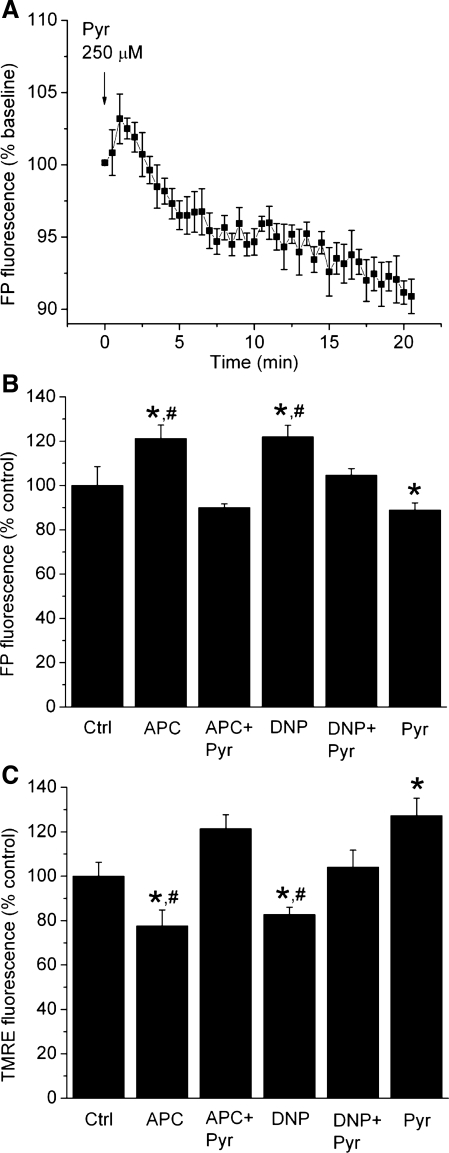
In isolated cardiomyocytes, ΔΨm was assessed using both ΔΨm-sensitive indicator TMRE and endogenous fluorescence of FPs. Figure 2A demonstrates the ability of pyruvate to induce sustained hyperpolarization of mitochondria (11), which will be used later to reverse drug-induced mitochondrial depolarization. Cardiomyocytes preconditioned with isoflurane (APC) exhibited partial mitochondrial depolarization, observed as increase in FP fluorescence intensity to 121 ± 6% of control (100%) (Fig. 2B) and decrease in TMRE fluorescence intensity to 78 ± 7% of control (Fig. 2C). At 200 nM, DNP had similar effects on FP and TMRE fluorescence intensities as APC, while 2 μM pyruvate reversed both APC- and DNP-induced changes in TMRE and FP fluorescence intensities (Fig. 2, B and C). This indicates that APC-induced mild mitochondrial depolarization can be mimicked with a low concentration of DNP and reversed by adding a low concentration of pyruvate.
Fig. 2.
APC- and DNP- induced mitochondrial depolarization in cardiomyocytes is reversed by pyruvate. Mitochondrial membrane potential was assessed by fluorescence of flavoproteins (FPs) and TMRE. A: the ability of pyruvate to increase the ΔΨm was demonstrated after the addition of 250 μM pyruvate. Following a transient FP oxidation and a decrease in ΔΨm, pyruvate induced a sustained FP reduction and mitochondrial hyperpolarization. B and C: APC and DNP (200 nM) increased FP and decreased TMRE fluorescence intensities compared with control. Addition of low concentration of pyruvate (2 μM) reversed the effects of APC and DNP, while pyruvate alone slightly hyperpolarized mitochondria. Data are means ± SE; n = 7–8/group. *P < 0.05 vs. Ctrl; #P < 0.05 APC or DNP vs. APC + Pyr or DNP + Pyr, respectively.
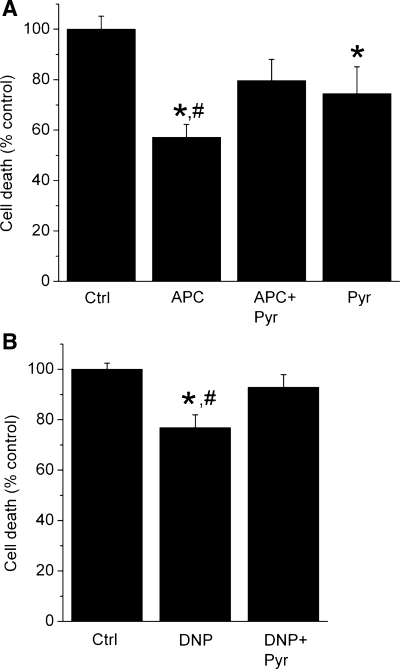
APC- and DNP-induced protection of cardiomyocytes is reversed by pyruvate.
Cell survival experiments were conducted to examine the cytoprotective effects of APC and the role of a partial decrease in ΔΨm. APC protected cardiomyocytes from oxidative stress, indicated by a decrease in cell death, 57 ± 5% of control (Fig. 3A). DNP, at the concentration that mimicked the magnitude of APC-induced decrease in ΔΨm, attenuated cell death, albeit less than APC (Fig. 3B). Addition of pyruvate at the concentration that repolarized mitochondria attenuated and abolished protection afforded by APC and DNP, respectively. This suggests that mitochondrial depolarization is, at least in part, a common mechanism of cytoprotection by APC and DNP.
Fig. 3.
Partial mitochondrial depolarization by APC and DNP increases survival of cardiomyocytes exposed to oxidative stress. Cell death after oxidative stress with H2O2 is expressed as percentage of dead cells in stress control (Ctrl). A: APC reduced cell death, and pyruvate (2 μM) (APC + Pyr) attenuated the cytoprotection. Pyruvate alone attenuated cell death. B: DNP (200 nM) also attenuated cell death, and coapplication of pyruvate and DNP attenuated DNP-induced cytoprotection (DNP + Pyr). Summary data from 8–9 experiments/group are means ± SE. *P < 0.05 vs. Ctrl; #P < 0.05 APC or DNP vs. APC + Pyr or DNP + Pyr, respectively.
Delay in mPTP opening by APC and DNP is reversed by pyruvate.
The mPTP opening, a trigger of cell death pathways, was assessed by following rapid decrease in TMRE fluorescence intensity, i.e., dissipation of ΔΨm (Fig. 4A). The arbitrary mPTP opening time was increased in the presence of cyclosporine A, an mPTP inhibitor, indicating a delay in mPTP opening (Fig. 4B). APC also increased arbitrary mPTP opening time (120 ± 4% of control), which was abolished in the presence of pyruvate (Fig. 4C). DNP also induced a delay in mPTP opening (136 ± 8% of control), an effect attenuated in the presence of pyruvate (Fig. 4D). The similar trend of a delay in mPTP opening by APC and DNP, which was in both groups reversed by pyruvate, indicated that a partial decrease in ΔΨm was an underlying protective mechanism in both groups.
Attenuation of ROS production by APC and DNP is reversed by pyruvate.
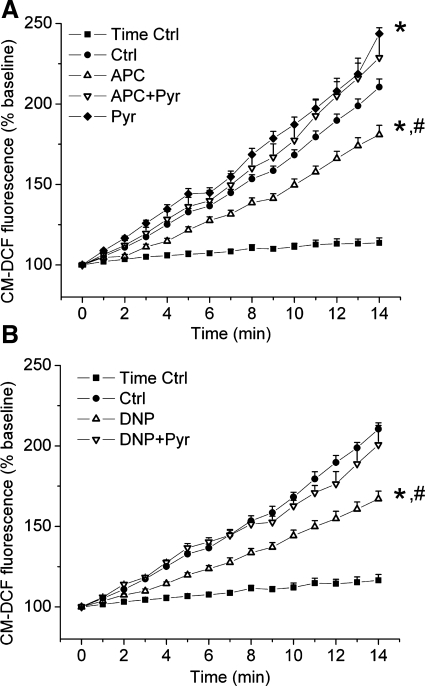
The H2O2 was used to induce oxidative stress. To assess endogenous ROS production, the CM-DCF fluorescence intensity was monitored after the washout of H2O2. Cells treated with H2O2 exhibited greater increase in CM-DCF fluorescence intensity than nontreated cells, indicating substantial increase in ROS production (Fig. 5, A and B). APC attenuated increase in CM-DCF fluorescence intensity in stressed cells (Ctrl vs. APC; 210.5 ± 5.0% vs. 181.0 ± 5.7%), indicating attenuation of ROS production (Fig. 5A). This effect of APC was abolished in the presence of pyruvate (APC + Pyr; 228.7 ± 14.0%). Consistent with the results in cell survival and mPTP opening experiments, DNP also attenuated ROS production, which was again reversed with pyruvate (Fig. 5B). These results suggest that a partial decrease in ΔΨm was also a common mechanism of attenuation of ROS production by APC and DNP.
Fig. 5.
APC and DNP attenuate ROS production in stressed cardiomyocytes. ROS production in cardiomyocytes preexposed to oxidative stress is measured by 5-(and-6)-chloromethyl-2′,7′-dichlorofluorescein (CM-DCF) fluorescence. A: compared with the nonstressed cells (time Ctrl), stressed cells (Ctrl) had a significantly greater increase in CM-DCF fluorescence, i.e., ROS production. APC decreased ROS production, and pyruvate (2 μM) reversed this effect (APC + Pyr). B: treatment of cells with DNP (200 nM) also attenuated ROS production compared with the stress control, which was again reversed by pyruvate (2 μM) (DNP + Pyr). Data are means ± SE; n = 7–12/group. *P < 0.05 vs. stress control; #P < 0.05 APC or DNP vs. APC + Pyr or DNP + Pyr, respectively.
In isolated mitochondria, partial decrease in ΔΨm attenuates excess ROS production, but not Ca2+ uptake.
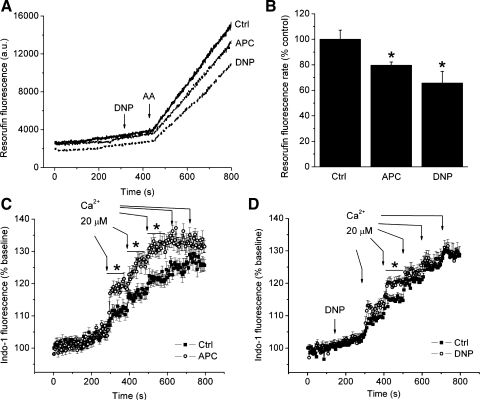
Isolated mitochondria were used to specifically examine whether a partial decrease in ΔΨm directly affects ROS production and Ca2+ uptake. Excess ROS production was induced with AA added to isolated mitochondria in a Ca2+-free buffer (Fig. 6A). AA caused a substantial burst of ROS in the control group, which was attenuated to 80 ± 3% of control in mitochondria isolated from APC-treated animals, and to 66 ± 9% of control in mitochondria treated with DNP (200 nM) (Fig. 6B). Addition of DNP alone did not significantly alter ROS production under basal conditions before treatment with AA. Figure 6, C and D, demonstrates Ca2+ uptake into mitochondria after the addition of cumulative pulses of 20 μM Ca2+. Compared with the control, APC or DNP treatment did not attenuate mitochondrial Ca2+ uptake despite the mitochondrial depolarization. On the contrary, Ca2+ uptake increased in both groups. These results indicate that in isolated mitochondria, a subtle depolarization by DNP significantly attenuates excess ROS, but not Ca2+ uptake.
Fig. 6.
Low concentrations of DNP and APC attenuate ROS production but not Ca2+ uptake in isolated mitochondria. A: representative signal traces of resorufin fluorescence intensity measurements as indication of ROS production. Application of antimycin A (AA; 200 nM), a complex III inhibitor, substantially enhanced ROS production (solid line). AA-induced ROS production was decreased in the presence of 200 nM DNP or in mitochondria isolated from preconditioned animals (dashed lines). B: summary data of rates of increase in resorufin fluorescence intensity during application of AA. APC and DNP significantly attenuated antimycin A-induced ROS production. C and D: measurements of mitochondrial Ca2+ using indo-1 AM. Addition of 20 μM Ca2+ pulses to the buffer resulted in mitochondrial Ca2+ uptake, which was not attenuated by DNP (200 nM) or APC, but it was increased. Data are means ± SE; n = 7–8/group in B and 8–11/group in C and D. *P < 0.05 vs. Ctrl.
In cardiomyocytes, partial decrease in ΔΨm attenuates cytosolic and mitochondrial Ca2+ accumulation.
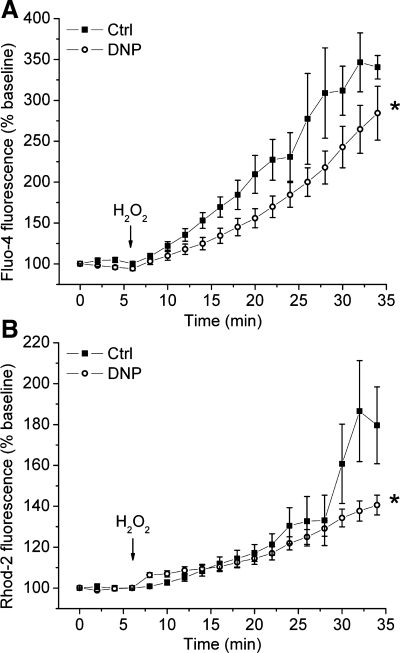
Our previous studies indicated that APC attenuates accumulation of cytosolic and mitochondrial Ca2+ (32, 48). Here we tested the effects of a partial decrease in ΔΨm on Ca2+ loading into cytosol (Fig. 7A) and mitochondria (Fig. 7B), measured simultaneously in cardiomyocytes exposed to oxidative stress. Application of H2O2 induced an increase in fluo-4 and rhod-2 fluorescence intensities, indicating increase in cytosolic and mitochondrial Ca2+, respectively, which is in agreement with previous studies showing ROS-induced Ca2+ release (55). A partial mitochondrial depolarization with DNP attenuated the increase in Ca2+-sensitive fluorescence in both compartments compared with control. This suggests that, unlike the results from the isolated mitochondria (Fig. 6D), a partial decrease in ΔΨm attenuates mitochondrial as well as cytosolic Ca2+ accumulation in cells exposed to oxidative stress.
Fig. 7.
Accumulation of cytosolic and mitochondrial Ca2+ in cardiomyocytes exposed to oxidative stress is attenuated by partial mitochondrial depolarization. Isolated cardiomyocytes were coloaded with cytosolic (A) and mitochondrial (B) Ca2+ indicators, fluo-4 AM and rhod-2 AM, respectively, and their fluorescence intensities were recorded simultaneously. Upon application of H2O2, fluo-4 and rhod-2 fluorescence intensities increased. In cells treated with DNP (200 nM), this effect was attenuated. Summarized data are means ± SE; n = 11–17/group. *P < 0.05 vs. Ctrl.
DISCUSSION
This study demonstrates the mechanisms by which a partial mitochondrial depolarization, elicited by preconditioning with isoflurane or by treatment with the uncoupling agent DNP, protected cardiomyocytes from oxidative stress. The similar trend in exerting cytoprotective effects when mitochondrial depolarization was induced by APC or DNP, and the reversal of these effects by repolarizing mitochondria with pyruvate, implies that a partial decrease in ΔΨm is a common mechanism of protection by both treatments. The ΔΨm-dependent attenuation of excess ROS production by mitochondria appears to play a central role in the adaptation to oxidative stress by APC, which induces a delay in oxidative stress-triggered mPTP opening, thereby increasing cell survival. Our results suggest that the attenuation of mitochondrial Ca2+ accumulation in cells with partly depolarized mitochondria following oxidative stress is not a direct result of mitochondrial depolarization, but an indirect effect of attenuated ROS production and oxidative stress-induced Ca2+ release into the cytosol and mitochondria.
Here, we describe a mechanism by which APC affords protection to cardiomyocytes against oxidative stress. Our data indicate that APC induced a mild decrease in ΔΨm and attenuated excess ROS production in cardiomyocytes exposed to oxidative stress. Comparable decrease in ΔΨm by the protonophore DNP also attenuated ROS production in stressed cells and in isolated mitochondria, as well. Moreover, a reversal of the ΔΨm by pyruvate attenuated these effects of APC and DNP, suggesting that a partial decrease in ΔΨm is a common effect of these treatments responsible for attenuation of ROS production. This is in agreement with previous studies that showed a strong correlation between ΔΨm and ROS production, where even a moderate decrease in ΔΨm of a few millivolts substantially decreased ROS production (29, 47). Oxidative stress, due to excess ROS production during early reperfusion, is suggested as one of the primary causes of cell injury, and its elimination by ROS scavengers can attenuate I/R injury (52). According to our results, it is possible that APC attenuates mitochondrial ROS production by slightly decreasing ΔΨm during reperfusion, when replenished oxygen allows ΔΨm buildup and the burst of ROS production. Indeed, it is suggested that transient inhibition of mitochondrial respiration during early reperfusion may exert cardioprotection (1, 7). However, it is unclear what mediates mitochondrial depolarization by APC. It is suggested that mitochondrial K+ channels, often identified as effectors of protection by preconditioning, could be these mediators (10, 27, 32, 34). However, their role in this mechanism is questionable since their activation has been correlated with both increase and decrease in ROS production (20, 49). These controversial findings may result from differential behavior of these channels depending on the metabolic state of the cell (44). Other interesting mediators of mitochondrial depolarization with strong cardioprotective effects are uncoupling proteins, which are in fact considered potential physiological regulators of mitochondrial ROS production (2, 38, 39). However, they have not been (yet) associated with APC.
APC and DNP also caused a delay in the opening of mPTP and improved cardiomyocyte survival during oxidative stress. Repolarization of mitochondria with pyruvate reversed these effects. This suggests that attenuation of ΔΨm-dependent endogenous excess ROS production, which is one of the major triggers of mPTP opening, delays pore opening and leads to increased survival of cardiomyocytes. Opening of mPTP is considered a critical step in the transition towards cell death during I/R, and many cardioprotective strategies, including APC, induce a delay in mPTP opening (16, 37, 42). While previous studies tested the role of mitochondrial KATP channels and protein kinase C as preconditioning signal mediators in APC-induced mPTP opening, here we describe the mechanism by which a partial decrease in ΔΨm, as an effector of protection by APC, leads to a delay of mPTP opening and subsequent increase in cardiomyocyte survival. The disparities between APC and DNP in the extent of cell protection may imply additional mechanism of protection by APC. For example, APC involves multiple protective pathways, such as activation of antiapoptotic proteins (43), sarcolemmal K+ channels (34), and protein kinase C (54), that may exert additional cardioprotective effects independent of changes in ΔΨm. Moreover, APC and the uncoupler DNP may not share the same mechanism to induce mitochondrial depolarization, as discussed above. However, the similar trend in attenuation of ROS production, delay in mPTP opening, and increase in cell survival between APC and DNP, and the reversibility of each by pyruvate, suggests that APC- and DNP-induced cytoprotection shares, at least in part, a common mechanism, a partial decrease in ΔΨm.
The mechanisms by which uncoupling agents induce cytoprotection are somewhat controversial. It has been suggested that another protonophore, carbonylcyanide p-trifluoromethoxyphenylhydrazone (FCCP), induces cardioprotection by generating a burst of ROS that triggers preconditioning, without detectable decrease in ΔΨm (4, 5). Contrary to this, we did not observe an increase in ROS production during DNP application, thus excluding the possibility of ROS-induced preconditioning by DNP in our model. It is possible that these differences are a result of variations in the approach to measure ΔΨm and ROS and/or the different nature of action and potency of DNP and FCCP, because it was shown that another uncoupling agent exhibited concentration-dependent, dual effect on mitochondrial ROS production (36). Consistent with our results, several groups have demonstrated attenuation of ROS production by DNP (40, 41).
A low concentration of pyruvate (2 μM) was used to reverse the mild mitochondrial depolarization by APC and DNP in cardiomyocytes. It was reported that the application of pyruvate or high concentrations of its metabolic precursor glucose to cells hyperpolarizes mitochondria (11). We observed a biphasic effect of pyruvate, a transient mitochondrial depolarization that is followed by sustained hyperpolarization, a so-called “pyruvate paradox” (11). That is, pyruvate initially depolarizes mitochondria due to pyruvate-proton symport into mitochondria (17, 23). As the pyruvate is being metabolized feeding electrons into the respiratory chain, a greater number of protons is extruded from mitochondria, increasing ΔΨm. In our cell survival experiments, application of pyruvate alone decreased cell death, which could be due to its effects independent of changes in ΔΨm. The ROS-scavenging properties of pyruvate have been reported, but at much higher concentrations (mM) than used in our study (2 μM) (33). Possibly, coapplication of pyruvate and H2O2 in cell survival experiments, even at this lower pyruvate concentration, scavenged H2O2 and increased cell survival. To verify that pyruvate antagonized the protective effects of APC by reversing ΔΨm, we also used oligomycin (25 ng/ml) to increase ΔΨm (data not shown). In this experiment, we observed, as we did for pyruvate, that oligomycin prevented APC-induced mitochondrial depolarization and abolished APC-induced attenuation of ROS production in stressed cells.
Attenuation of excess ROS production in cardiomyocytes and isolated mitochondria is caused by a decrease in ΔΨm since decrease in ΔΨm inhibits proton pumping by the respiratory chain and coupled electron transfer, which results in “electron leak” and ROS production (6). However, in isolated mitochondria, a similar mitochondrial depolarization was not sufficient to attenuate ΔΨm-driven Ca2+ uptake (Fig. 6, C and D), but it attenuated accumulation of cytosolic and mitochondrial Ca2+ in cardiomyocytes exposed to oxidative stress (Fig. 7). We showed that H2O2 increases cytosolic and mitochondrial Ca2+, which is in agreement with the well-established phenomenon of ROS-induced Ca2+ release (55), where oxidative stress/ROS can activate ryanodine receptor (12) and also inhibit Ca2+ pumps (26, 35). Therefore, it appears that attenuation of mitochondrial ROS by partial decrease in ΔΨm decreased ROS-induced Ca2+ release, which would explain attenuation of mitochondrial and cytosolic Ca2+ in stressed cells, but not in isolated mitochondria. This notion is supported by studies which show that Ca2+ overload occurs after ROS burst during reperfusion in cardiomyocytes (28). Interestingly, in experiments using isolated mitochondria, APC and DNP enhanced Ca2+ uptake, which may result from changes in the intramitochondrial H+ concentration that may also be affected by both treatments (31). Further detailed studies are needed to investigate the role of pH on mitochondrial Ca2+ handling.
The mechanisms of interplay between excess ROS production and accumulation of cytosolic and mitochondrial Ca2+ as mediators of cell injury during oxidative stress need further investigation. Nonetheless, there is the possibility of a self-sustained nature of mPTP opening caused either by bursts in mitochondrial ROS production or mitochondrial Ca2+ uptake (3, 22). It seems that excess ROS production, cytosolic, and mitochondrial Ca2+ accumulation, as mediators of I/R injury, are interdependent and part of a complex process where both ROS production and Ca2+ accumulation become amplified, leading to sustained mPTP opening and, eventually, cell death. Therefore, even a partial decrease in ΔΨm induced by APC or DNP may have dramatic effect on cell survival by attenuating excess ROS production and thereby interrupting the amplification process and the interactions between ROS production, Ca2+ accumulation, and mPTP opening induced during reperfusion.
A limitation of this study is that oxidative stress, such as H2O2, does not fully account for events that occur during I/R injury, but it has been used widely as an accepted method to mimic conditions during reperfusion injury. Another limitation of our study is that the experiments were performed at room temperature where reactions occur at a slower rate compared with living organisms. However, despite the difference in temperature, it is likely that similar (patho)physiological mechanisms would occur at 37°C as well. Experiments on isolated mitochondria in which APC was conducted in vivo should be interpreted with caution since potential stress that was inflicted during the isolation procedure may modify preconditioning-induced changes. Lastly, the study was conducted in isolated cardiomyocytes or mitochondria, which excludes other factors, for example blood flow, vascular elements, or paracrine and exocrine agents, that could modulate the anesthetic action during APC in vivo. Therefore, these findings should be also interpreted with caution with respect to the in vivo situation.
In conclusion, this study shows that cytoprotection by isoflurane-induced preconditioning could be in part mediated by a partial mitochondrial depolarization that primarily attenuates excess ROS production in cardiomyocytes exposed to oxidative stress. As a consequence, ROS-induced mPTP opening is delayed, which leads to an increase in cell survival. We also addressed the interplay between mild mitochondrial depolarization, ROS generation, and Ca2+ uptake and showed that attenuation of excess ROS production is the direct result of mild mitochondrial depolarization, while the attenuation of cytosolic and mitochondrial Ca2+ accumulation is likely a consequence of decreased ROS production.
GRANTS
This work was supported in part by National Institutes of Health Grants P01GM066730 and R01HL034708 (to Z. J. Bosnjak).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Aniko Szabo for the help with the statistical analysis and Mary Ziebell for the technical assistance.
REFERENCES
- 1.Abdel-Rahman U, Risteski P, Tizi K, Kerscher S, Behjati S, Zwicker K, Scholz M, Brandt U, Moritz A. Hypoxic reoxygenation during initial reperfusion attenuates cardiac dysfunction and limits ischemia-reperfusion injury after cardioplegic arrest in a porcine model. J Thorac Cardiovasc Surg 137: 978–982, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Bienengraeber M, Ozcan C, Terzic A. Stable transfection of UCP1 confers resistance to hypoxia/reoxygenation in a heart-derived cell line. J Mol Cell Cardiol 35: 861–865, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Brady NR, Hamacher-Brady A, Westerhoff HV, Gottlieb RA. A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxid Redox Signal 8: 1651–1665, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Brennan JP, Berry RG, Baghai M, Duchen MR, Shattock MJ. FCCP is cardioprotective at concentrations that cause mitochondrial oxidation without detectable depolarisation. Cardiovasc Res 72: 322–330, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Brennan JP, Southworth R, Medina RA, Davidson SM, Duchen MR, Shattock MJ. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc Res 72: 313–321, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287: C817–C833, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Burwell LS, Nadtochiy SM, Brookes PS. Cardioprotection by metabolic shut-down and gradual wake-up. J Mol Cell Cardiol 46: 804–810, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camara AK, Lesnefsky EJ, Stowe DF. Potential therapeutic benefits of strategies directed to mitochondria. Antioxid Redox Signal. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278: 36027–36031, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Costa AD, Quinlan CL, Andrukhiv A, West IC, Jaburek M, Garlid KD. The direct physiological effects of mitoKATP opening on heart mitochondria. Am J Physiol Heart Circ Physiol 290: H406–H415, 2006 [DOI] [PubMed] [Google Scholar]
- 11.de Andrade PB, Casimir M, Maechler P. Mitochondrial activation and the pyruvate paradox in a human cell line. FEBS Lett 578: 224–228, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Favero TG, Zable AC, Abramson JJ. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biol Chem 270: 25557–25563, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Ornato JP, Page RL, Tarkington LG, Yancy CW. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. J Am Coll Cardiol 50: e159–e241, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Ganote CE, Armstrong SC. Effects of CCCP-induced mitochondrial uncoupling and cyclosporin A on cell volume, cell injury and preconditioning protection of isolated rabbit cardiomyocytes. J Mol Cell Cardiol 35: 749–759, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Gursahani HI, Schaefer S. Acidification reduces mitochondrial calcium uptake in rat cardiac mitochondria. Am J Physiol Heart Circ Physiol 287: H2659–H2665, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Halestrap AP. Mitochondria and reperfusion injury of the heart–a holey death but not beyond salvation. J Bioenerg Biomembr 41: 113–121, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Halestrap AP. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. Biochem J 148: 85–96, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res 60: 617–625, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys 195: 460–467, 1979 [DOI] [PubMed] [Google Scholar]
- 20.Heinen A, Aldakkak M, Stowe DF, Rhodes SS, Riess ML, Varadarajan SG, Camara AK. Reverse electron flow-induced ROS production is attenuated by activation of mitochondrial Ca2+-sensitive K+ channels. Am J Physiol Heart Circ Physiol 293: H1400–H1407, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Huser J, Rechenmacher CE, Blatter LA. Imaging the permeability pore transition in single mitochondria. Biophys J 74: 2129–2137, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell 89: 1145–1153, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Jezek P, Borecky J. Mitochondrial uncoupling protein may participate in futile cycling of pyruvate and other monocarboxylates. Am J Physiol Cell Physiol 275: C496–C504, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 113: 1535–1549, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaneko M, Beamish RE, Dhalla NS. Depression of heart sarcolemmal Ca2+-pump activity by oxygen free radicals. Am J Physiol Heart Circ Physiol 256: H368–H374, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Kaneko M, Elimban V, Dhalla NS. Mechanism for depression of heart sarcolemmal Ca2+ pump by oxygen free radicals. Am J Physiol Heart Circ Physiol 257: H804–H811, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology 87: 361–370, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Kim JS, Jin Y, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am J Physiol Heart Circ Physiol 290: H2024–H2034, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Lambert AJ, Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J 382: 511–517, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landoni G, Fochi O, Tritapepe L, Guarracino F, Belloni I, Bignami E, Zangrillo A. Cardiac protection by volatile anesthetics. A review. Minerva Anestesiol 75: 269–273, 2009 [PubMed] [Google Scholar]
- 31.Liu T, O'Rourke B. Regulation of mitochondrial Ca2+ and its effects on energetics and redox balance in normal and failing heart. J Bioenerg Biomembr 41: 127–132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, Bienengraeber M. Isoflurane preconditioning uncouples mitochondria and protects against hypoxia-reoxygenation. Am J Physiol Cell Physiol 292: C1583–C1590, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Mallet RT, Squires JE, Bhatia S, Sun J. Pyruvate restores contractile function and antioxidant defenses of hydrogen peroxide-challenged myocardium. J Mol Cell Cardiol 34: 1173–1184, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Marinovic J, Bosnjak ZJ, Stadnicka A. Distinct roles for sarcolemmal and mitochondrial adenosine triphosphate-sensitive potassium channels in isoflurane-induced protection against oxidative stress. Anesthesiology 105: 98–104, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Morris TE, Sulakhe PV. Sarcoplasmic reticulum Ca(2+)-pump dysfunction in rat cardiomyocytes briefly exposed to hydroxyl radicals. Free Radic Biol Med 22: 37–47, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Mozo J, Ferry G, Studeny A, Pecqueur C, Rodriguez M, Boutin JA, Bouillaud F. Expression of UCP3 in CHO cells does not cause uncoupling, but controls mitochondrial activity in the presence of glucose. Biochem J 393: 431–439, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88: 581–609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadtochiy SM, Tompkins AJ, Brookes PS. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: implications for pathology and cardioprotection. Biochem J 395: 611–618, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Negre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, Salvayre R, Penicaud L, Casteilla L. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J 11: 809–815, 1997 [PubMed] [Google Scholar]
- 40.Okuda M, Lee HC, Kumar C, Chance B. Comparison of the effect of a mitochondrial uncoupler, 2,4-dinitrophenol and adrenaline on oxygen radical production in the isolated perfused rat liver. Acta Physiol Scand 145: 159–168, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Pelletier A, Coderre L. Ketone bodies alter dinitrophenol-induced glucose uptake through AMPK inhibition and oxidative stress generation in adult cardiomyocytes. Am J Physiol Endocrinol Metab 292: E1325–E1332, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Pravdic D, Sedlic F, Mio Y, Vladic N, Bienengraeber M, Bosnjak ZJ. Anesthetic-induced preconditioning delays opening of mitochondrial permeability transition pore via protein kinase C-epsilon-mediated pathway. Anesthesiology 111: 267–274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raphael J, Abedat S, Rivo J, Meir K, Beeri R, Pugatsch T, Zuo Z, Gozal Y. Volatile anesthetic preconditioning attenuates myocardial apoptosis in rabbits after regional ischemia and reperfusion via Akt signaling and modulation of Bcl-2 family proteins. J Pharmacol Exp Ther 318: 186–194, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Riess ML, Camara AK, Heinen A, Eells JT, Henry MM, Stowe DF. KATP channel openers have opposite effects on mitochondrial respiration under different energetic conditions. J Cardiovasc Pharmacol 51: 483–491, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saotome M, Katoh H, Satoh H, Nagasaka S, Yoshihara S, Terada H, Hayashi H. Mitochondrial membrane potential modulates regulation of mitochondrial Ca2+ in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 288: H1820–H1828, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Sedlic F, Pravdic D, Ljubkovic M, Marinovic J, Stadnicka A, Bosnjak ZJ. Differences in production of reactive oxygen species and mitochondrial uncoupling as events in the preconditioning signaling cascade between desflurane and sevoflurane. Anesth Analg 109: 405–411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem 86: 1101–1107, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Tampo A, Hogan CS, Sedlic F, Bosnjak ZJ, Kwok WM. Accelerated inactivation of cardiac L-type calcium channels triggered by anaesthetic-induced preconditioning. Br J Pharmacol 156: 432–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka K, Weihrauch D, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mitochondrial adenosine triphosphate-regulated potassium channel opening acts as a trigger for isoflurane-induced preconditioning by generating reactive oxygen species. Anesthesiology 98: 935–943, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanden Hoek TL, Qin Y, Wojcik K, Li CQ, Shao ZH, Anderson T, Becker LB, Hamann KJ. Reperfusion, not simulated ischemia, initiates intrinsic apoptosis injury in chick cardiomyocytes. Am J Physiol Heart Circ Physiol 284: H141–H150, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanden Hoek TL, Shao Z, Li C, Schumacker PT, Becker LB. Mitochondrial electron transport can become a significant source of oxidative injury in cardiomyocytes. J Mol Cell Cardiol 29: 2441–2450, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Vanden Hoek TL, Shao Z, Li C, Zak R, Schumacker PT, Becker LB. Reperfusion injury on cardiac myocytes after simulated ischemia. Am J Physiol Heart Circ Physiol 270: H1334–H1341, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Xu P, Wang J, Kodavatiganti R, Zeng Y, Kass IS. Activation of protein kinase C contributes to the isoflurane-induced improvement of functional and metabolic recovery in isolated ischemic rat hearts. Anesth Analg 99: 993–1000, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 71: 310–321, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.