Abstract
The mammalian target of rapamycin (mTOR) assembles into two distinct multiprotein complexes known as mTORC1 and mTORC2. Of the two complexes, mTORC1 acts to integrate a variety of positive and negative signals to downstream targets that regulate cell growth. The lipid second messenger, phosphatidic acid (PA), represents one positive input to mTORC1, and it is thought to act by binding directly to mTOR, thereby enhancing the protein kinase activity of mTORC1. Support for this model includes findings that PA binds directly to mTOR and addition of PA to the medium of cells in culture results in activation of mTORC1. In contrast, the results of the present study do not support a model in which PA activates mTORC1 through direct interaction with the protein kinase but, instead, show that the lipid promotes mTORC1 signaling through activation of the ERK pathway. Moreover, rather than acting directly on mTORC1, the results suggest that exogenous PA must be metabolized to lysophosphatidic acid (LPA), which subsequently activates the LPA receptor endothelial differentiation gene (EDG-2). Finally, in contrast to previous studies, the results of the present study demonstrate that leucine does not act through phospholipase D and PA to activate mTORC1 and, instead, show that the two mediators act through parallel upstream signaling pathways to activate mTORC1. Overall, the results demonstrate that leucine and PA signal through parallel pathways to activate mTORC1 and that PA mediates its effect through the ERK pathway, rather than through direct binding to mTOR.
Keywords: leucine, phospholipase D, insulin, MEK, lysophosphatidic acid
the mammalian target of rapamycin (mTOR) is a serine-threonine protein kinase that functions as a nutrient sensor and acts to govern a wide range of cellular processes, including cell growth, proliferation, metabolism, autophagy, and survival (28, 34). It assembles into two multiprotein complexes, referred to as mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). The two complexes are similar, in that both contain mTOR and mLST8 (also known as GβL), but are distinguished by the presence of raptor (regulatory-associated protein of mTOR) in mTORC1 and rictor (rapamycin-insensitive companion of TOR) in mTORC2 (15, 24, 43). They also differ in their sensitivity to rapamycin, the activity of mTORC1 being largely inhibited, whereas the activity of mTORC2 is not affected acutely (44). Activation of mTORC1 stimulates protein synthesis and cell growth, in part through phosphorylation of downstream targets, such as eukaryotic initiation factor (eIF) 4E-binding protein 1 (4E-BP1), which enhances assembly of the active mRNA cap binding complex eIF4F, and the ribosomal protein S6 kinase (S6K), which in turn phosphorylates a number of proteins involved in mRNA translation (28, 34).
Activation of mTORC1 is mediated by a small GTPase referred to as Rheb (Ras homolog enriched in brain). When Rheb is in the GTP-bound state, it acts to activate mTORC1, but when it is associated with GDP, it instead functions as a repressor (32). The GTPase activity of Rheb is governed by the tumor suppressors tuberous sclerosis complex (TSC) 1 and 2 (TSC1/2), with TSC2 functioning as a GTPase-activating protein and, thus, as a negative regulator of mTORC1 signaling (21, 30, 50). TSC1/2 serves as a major hub for the integration of multiple signaling pathways upstream of mTORC1, including those mediating the actions of growth factors, cellular energy state, glucagon, glucocorticoids, and hypoxia (2, 6, 16, 25, 45).
Because of its central role in regulating various anabolic pathways, activation of mTORC1 is critical in mediating the anabolic response to various perturbations that promote tissue hypertrophy. For example, 4E-BP1 and S6K1 phosphorylation are increased in skeletal muscle in various animal models of resistance exercise (5). Additionally, S6K1 phosphorylation is increased in humans after exercise (51), showing that mTORC1 signaling is increased by exercise. Treatment with the selective mTORC1 inhibitor rapamycin dramatically attenuates exercise-induced mTORC1 activation and muscle hypertrophy (4, 10, 19). The mechanism through which exercise promotes activation of mTORC1 is incompletely defined and may be multifactorial. A recent study (38) shows that, in vivo, electrically induced muscle contraction resulted in activation of phospholipase D (PLD), leading to increased phosphatidic acid (PA) production. Moreover, inhibition of PLD, but not phosphoinositol-3 kinase, attenuated contraction-induced mTORC1 activation. On the basis of other studies (12, 54) showing that PA binds directly to mTOR, a model was proposed (38) in which muscle contraction results in activation of PLD and increased PA production, with subsequent binding of PA to mTOR leading to its activation. In support of such a model, addition of PA to cells in culture also resulted in activation of mTORC1 (12, 31, 38). In those studies, it was assumed that addition of PA to the culture medium led to its intracellular accumulation, thereby resulting in mTORC1 activation. A caveat to this assumption is that phospholipase A2, present in the cell culture medium due to secretion from cells and/or its presence in serum added to the culture medium, may lead to hydrolysis of PA to produce lysophosphatidic acid (LPA). LPA is known to bind to specific receptors on the plasma membrane, specifically endothelial differentiation gene (EDG)-2, -4, and -7, leading to activation of multiple intracellular signaling pathways, including PLD and ERK1/2 (56). Moreover, PLD and ERK1/2 have been shown to activate mTORC1 (11, 33). Thus an alternative explanation for the activation of mTORC1 by exogenous addition of PA is that the lipid is hydrolyzed to LPA, which subsequently binds to its receptor, leading to activation of one or more signaling pathways upstream of mTORC1.
Given the uncertainties noted above, the study described here was designed to assess the mechanism through which PA acts to govern the activation state of mTORC1. We show that although PA may associate directly with mTOR, it primarily signals through the ERK signaling pathway to activate mTORC1. Moreover, addition of PA to cells in culture leads to mTORC1 activation through an LPA receptor-based signaling pathway. Finally, we show that the PLD pathway and the amino acid-sensing pathway function in parallel, producing an additive effect on mTORC1 activation.
MATERIALS AND METHODS
Materials and reagents.
Cell culture medium lacking leucine, histidine, and pyruvate was a custom formulation purchased from Atlanta Biologicals; histidine was added to the medium prior to use. Leucine, tert-butanol (Reagent Plus), anti-actin antibody, ethyl acetate, and 2,2,4-trimethylpentane were obtained from Sigma-Aldrich. 18:1 LPA [1-oleoyl-2-hydroxy-sn-glycero-3-phosphate (sodium salt)], 16:0–18:1 PA [1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate (monosodium salt)], and phosphatidylbutanol [(PtdBuOH) 1,2-dioleoyl-sn-glycero-3-phosphobutanol (sodium salt)] were purchased from Avanti Polar Lipids. 1-Butanol and [9,10-3H(N)]palmitic acid were obtained from Fisher Scientific and PerkinElmer, respectively. Anti-mTOR, anti-S6K1, anti-4E-BP1, and anti-phospho-S6K1(T389) antibodies were purchased from Bethyl Laboratories, anti-phospho-ERK(T202/Y204) antibody was purchased from Cell Signaling, and anti-GAPDH antibody was purchased from Santa Cruz Biotechnology. TLC plates (catalog no. 4865-821) were purchased from Whatman Schleicher & Schuell. PD-98059, U-0126, and Ki16425 were purchased from Calbiochem, Promega, and Cayman Chemical, respectively. Rapamycin was obtained from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute.
Cell culture.
Rat2 fibroblasts, purchased from the American Type Culture Collection, were maintained in DMEM lacking sodium pyruvate and containing high glucose (GIBCO/Invitrogen Life Sciences), 10% FBS (Atlas Biologicals), and 1% penicillin-streptomycin (GIBCO). On the day of the study, the medium was replaced with medium lacking leucine and pyruvate. The medium also contained FBS as noted in figure legends. In studies involving inhibition of the ERK kinase (MEK), cells were incubated for 2.25 h in medium containing 0.5% FBS and lacking leucine. During the last 15 min, PD-98059 or U-0126 at a final concentration of 20 μM or 10 μM, respectively, was added to the medium. In studies involving inhibition of the EDG-2 receptor, cells were incubated for 2.5 h in medium containing 0.5% FBS and lacking leucine. During the last 30 min, Ki16425 at a final concentration of 10 μM was added to the medium. In other studies, cells were incubated for 2 h in medium lacking FBS and leucine, and during the last 15 min, rapamycin at a final concentration of 100 nM, was added to the medium. The cells were then treated with 22 μM LPA, 10 nM insulin (Novolin R), and/or 0.76 mM leucine for 2 h.
PA and LPA treatments.
PA, suspended in chloroform, was dried by vacuum centrifugation and then suspended at a final concentration of 10 mM in 150 mM NaCl and 10 mM Tris·HCl, pH 8. The solution was mixed vigorously for 2 min, incubated at 37°C for 10 min, and mixed again for 2 min. PA was promptly added to culture dishes at the concentrations noted in the figure legends. Because of its relatively short half-life in aqueous solution, fresh PA was added at 45-min intervals. LPA was prepared as suggested by the manufacturer. Specifically, the LPA powder was suspended in 1:1 water-100% ethanol, incubated at 37°C for 5 min, and then sonicated for 5 min using a water bath sonicator.
PLD activity assay.
Cells were grown to ∼60% confluence in six-well plates and then radiolabeled with [3H]palmitate (5 μCi/ml) for 16 h. The cells were then washed with PBS, and the medium was replaced with serum- and leucine-free medium or complete DMEM, and the cells were returned to the incubator. After 2 h, 50 mM 1-butanol was added to the medium, then 0.76 mM leucine or 22 μM LPA was added, and the plates were returned to the incubator for 30 min. Cells were collected in 1 ml of ice-cold methanol and transferred to disposable glass culture tubes. Chloroform (1 ml; EMD Chemicals) and 10 mg of PtdBuOH were added to each tube, and the solution was mixed thoroughly and then incubated at room temperature for 20 min. After the incubation, 0.9 ml of 0.88% KCl in H2O was added, and the solution was mixed and then centrifuged at 1,000 g for 5 min at room temperature. The organic phase was removed and dried via vacuum centrifugation. The sample was resuspended in 50 μl of chloroform, and an aliquot (10 μl) was analyzed by liquid scintillation spectrometry using Formula 989 scintillation cocktail (PerkinElmer). A solvent consisting of ethyl acetate, 2,2,4-trimethylpentane, acetic acid, and PicoPure Water (11:5:2:10 by volume) was used to spot the remainder of the sample (40 μl) on a TLC plate. After chromatography, PtdBuOH bands were detected by iodine staining.
Lipid extraction and mass spectrometry.
Cells were deprived of leucine and serum for 2 h and treated with leucine or LPA for 30 min, as described above. Cells were collected in 10 mM Tris-base, pH 7.2. Protein concentration was determined using a Bio-Rad DC protein assay kit. An aliquot of sample containing 1 mg of protein was added to 4 ml of a 1:1 chloroform-methanol solution, then 5% acetic acid (2 ml) and 500 pmol of di14:0 PA, which served as an internal standard, were added. The samples were mixed and then centrifuged at 2,000 rpm for 10 min. The organic layer was removed and placed in a disposable glass tube. The aqueous phase was reextracted with 2 ml of chloroform, and the organic phases were combined. The sample was then dried under a stream of nitrogen gas, resuspended using chloroform-methanol containing 5% acetic acid, and reextracted with chloroform. Samples were resuspended in chloroform and filtered using Whatman Puradisc syringe filters (catalog no. 6784-1302) into glass conical tubes, dried under nitrogen gas, and then resuspended in 1:1 chloroform-methanol. Samples were analyzed using an ABI 4000 Q Trap Mass Spectrometer (55) after dilution into 1:1 chloroform-methanol. Precursor ion scanning for 153 (glycerolphosphate) was utilized to identify PA (22).
Western blot analysis.
For Western blot analysis, cells were scraped into 1× Laemmli buffer. Hyperphosphorylation of S6K1 was assessed by gel-shift analysis, as previously described (26). All other analyses were performed as previously described (26), except samples were resolved on Bio-Rad Criterion Tris·HCl 4–15% gels. After development, blots probed with anti-phospho-S6K1(T389) or anti-phospho-ERK1/2(T202/Y204) antibody were stripped using buffer containing 62.5 mM Tris·HCl, 69.35 mM SDS, and 18.3 μM β-mercaptoethanol, pH 6.7, and then reprobed with polyclonal anti-actin or monoclonal anti-GAPDH antibody. Values for phospho-S6K1(T389) and phospho-ERK(T202/Y204) were normalized to actin or GAPDH.
Statistics.
Data were analyzed by one-way ANOVA using the Prism 5 software program (GraphPad). If a significant difference was detected, data were analyzed further by unpaired t-test. Mass spectrometry data were analyzed by one-sample t-test. P < 0.05 was considered statistically significant.
RESULTS
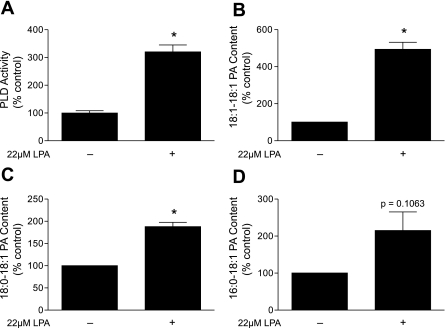
LPA is a potent activator of PLD in many cells (17, 23). Indeed, as shown in Fig. 1A, LPA caused a greater than threefold increase in PLD activity in serum- and leucine-deprived Rat2 cells. LPA also dramatically increased the amount of PA present as the 18:1-18:1 (Fig. 1B), 18:0-18:1 (Fig. 1C), and 16:0-18:1 (Fig. 1D) products. The products generated in response to LPA treatment indicated that it not only caused the expected increase in PLD activity but also may have led to an increase in its flux through LPA acyltransferase (LPAAT). That is, the fatty acid side chain of the exogenous LPA was the 18:1 moiety, and the major product measured was 18:1-18:1 PA.
Fig. 1.
Lysophosphatidic acid (LPA) treatment increases phospholipase D (PLD) activity and phosphatidic acid (PA) content. A: Rat2 cells were deprived of serum and leucine for 2 h before addition of 22 μM LPA, which approximated its concentration in serum (48). Values are means ± SE of 3 experiments; in each experiment, 3 samples per condition were independently analyzed. *P < 0.0001 vs. deprived (−) condition or leucine replacement. B–D: 18:1-18:1 PA, 18:0-18:1 PA, and 16:0-18:1 PA content was assessed by mass spectrometry. Values are means ± SE (n = 3). *P < 0.002 vs. deprived (−).
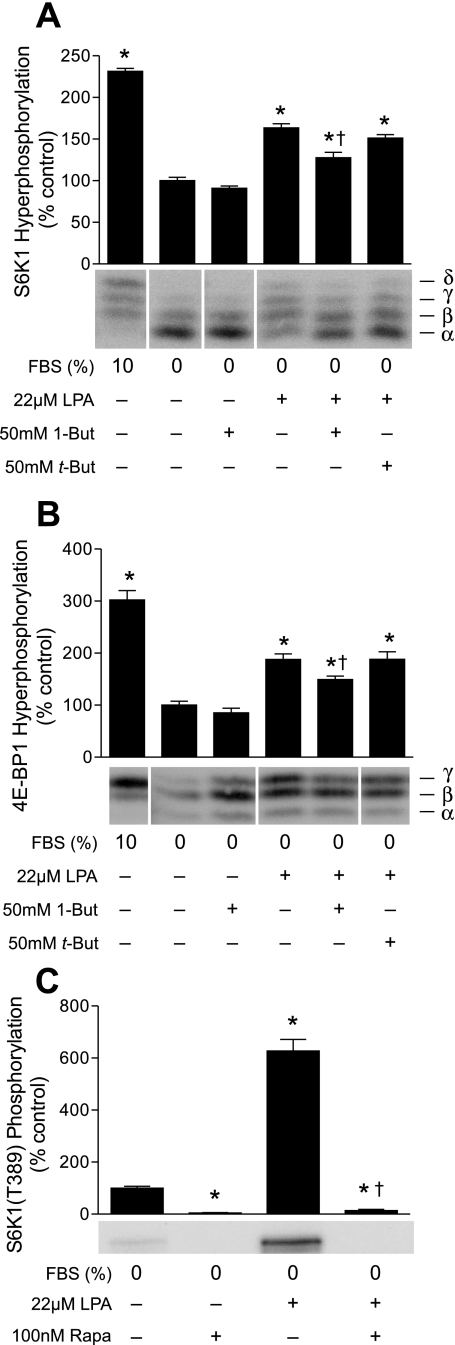
To assess the effect of LPA on activation of mTORC1, the phosphorylation state of the two best-characterized mTORC1 substrates, S6K1 and 4E-BP1, was assessed. LPA treatment led to a significant increase in phosphorylation of S6K1 (Fig. 2A) and 4E-BP1 (Fig. 2B), and this effect was attenuated by 1-butanol, but not by tert-butanol, suggesting that the lipid was acting to activate mTORC1, in part through PLD. In contrast, 1-butanol treatment was without effect in serum-deprived cells, demonstrating a maximal repression of mTORC1 signaling induced by a combination of serum and leucine deprivation. To provide further evidence that LPA was increasing S6K1 and 4E-BP1 phosphorylation by activating mTORC1, cells were treated with the selective inhibitor rapamycin prior to addition of LPA to the culture medium. As shown in Fig. 2C, rapamycin blocked the LPA-induced phosphorylation of S6K1. Rapamycin also blocked phosphorylation of 4E-BP1 (data not shown).
Fig. 2.
Rapamycin and 1-butanol, but not tert-butanol, attenuate LPA-mediated activation of mammalian target of rapamycin (mTOR) complex 1 (mTORC1). Cells were maintained in DMEM containing 10% FBS or deprived of serum and leucine for 2 h. 1-Butanol (1-But) or tert-butanol (t-But; A and B) or rapamycin (Rapa, C) was added to the medium 30 min prior to harvest. S6 protein kinase 1 (S6K1) hyperphosphorylation was assessed as proportion of the protein present in β-, γ-, and δ-forms relative to the total amount of the protein (α + β + γ + δ; A) or phosphorylation on Thr389 using an antibody specific for the phosphorylated form of the protein (C). Eukaryotic initiation factor 4E-binding protein (4E-BP1) hyperphosphorylation was assessed as proportion of the protein present in the γ-form relative to the total amount of the protein (α + β + γ; B). For representative blots, all samples were run on the same gel, but not in contiguous lanes. Noncontiguous lanes are separated by white lines. Values are means ± SE of 3 experiments; in each experiment, 3 samples per condition were independently analyzed. In A and B, *P < 0.003 vs. cells deprived of serum and leucine; †P < 0.008 vs. LPA. In C, *P < 0.0001 vs. cells deprived of serum and leucine; †P < 0.0001 vs. LPA.
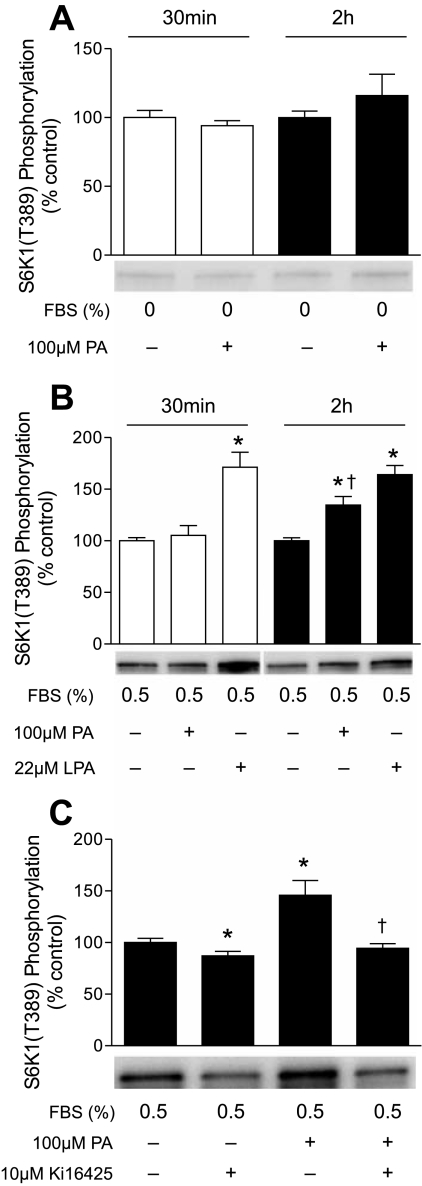
To further investigate the mechanism through which PA acts to increase mTORC1 signaling, cells were treated directly with PA. Surprisingly, in contrast to LPA, PA did not promote significant phosphorylation of S6K1 30 min or 2 h after addition to the culture medium (Fig. 3A). A previous study (53) demonstrating activation of mTORC1 by exogenously added PA used cells incubated in medium containing 0.5% serum, rather than no serum. Therefore, the study in Fig. 3A was repeated, except the cells were incubated in medium containing 0.5% serum. As observed in the absence of serum, in cells incubated in medium containing 0.5% serum, mTORC1 signaling was not significantly increased 30 min after addition of PA, even though LPA was effective at this time point (Fig. 3B). However, mTORC1 signaling was significantly increased 2 h after PA addition to cells incubated in 0.5% serum. A plausible explanation for the permissive effect of serum on PA-induced mTORC1 activation is that phospholipase A present in serum hydrolyzed PA to LPA and that LPA subsequently bound to its receptor, thereby leading to activation of mTORC1. This explanation would also account for the delayed response to PA compared with LPA. To assess this possibility, cells incubated in 0.5% serum were treated with a selective inhibitor of the LPA receptor, Ki16425 (37), prior to addition of PA. A shown in Fig. 3C, the inhibitor completely prevented PA-induced mTORC1 activation, demonstrating that activation of the LPA receptor accounted for the majority of the PA effect.
Fig. 3.
Extracellular PA acts through the LPA receptor to activate mTORC1. Cells were deprived of serum and leucine (A) or incubated in medium containing 0.5% serum without leucine (B and C) for 2 h before addition of PA or LPA. Cells were harvested 30 min (A and B) or 2 h (A–C) later. When present, Ki16425 was added to the medium 30 min prior to PA. S6K1 phosphorylation on Thr389 was assessed as described in Fig. 2 legend. Representative blots are shown. Values are means ± SE of 3 experiments; in each experiment, 3 samples per condition were independently analyzed. In B, *P < 0.002 vs. cells incubated in medium containing 0.5% serum without leucine at the corresponding time point; †P < 0.035 vs. PA at 30 min. In C, *P < 0.05 vs. cells deprived of serum and leucine; †P < 0.005 vs. PA.
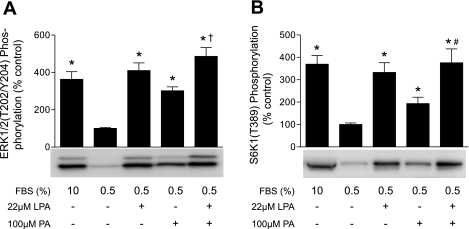
Stimulation of the LPA receptor leads to activation of the PLD and ERK1/2 signaling pathways (56). To assess the contribution of the two pathways to activation of mTORC1 signaling, we initially examined the individual and combined effects of LPA and PA at maximally effective concentrations (see Supplemental Material, Supplemental Fig. 1 at the AJP-Cell Physiol website) on ERK1/2 (Fig. 4A) and S6K1 (Fig. 4B) phosphorylation. In cells treated with LPA or PA, phosphorylation of both proteins was significantly increased compared with controls. In contrast, when cells were treated with a combination of LPA and PA at maximally effective concentrations, there was no additive effect on ERK1/2 or S6K1 phosphorylation, indicating that LPA and PA were acting through the same pathway to activate mTORC1.
Fig. 4.
LPA and PA act through the same signaling pathway to activate mTORC1. Cells were incubated in medium containing 0.5% serum without leucine for 2 h before addition of LPA and/or PA. A: ERK1/2 phosphorylation on Thr202/Tyr204 was measured by Western blot analysis using an antibody specific for the phosphorylated form of the protein. B: S6K1 phosphorylation was assessed as described in Fig. 3 legend. Values are means ± SE of 3 experiments; in each experiment, 3 samples per condition were independently analyzed. Representative blots are shown. *P < 0.0005 vs. cells incubated in medium containing 0.5% serum without leucine. †P < 0.02; #P < 0.04 vs. PA alone.
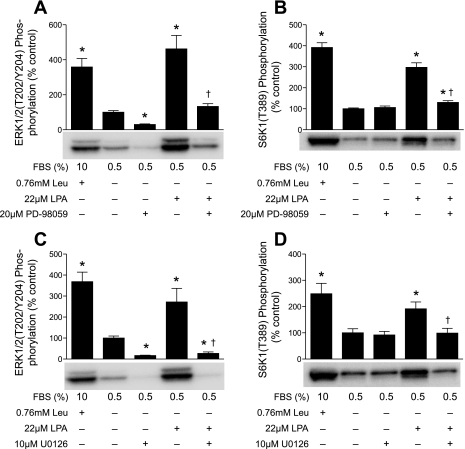
To determine whether ERK1/2-mediated signaling was required for LPA-induced activation of mTORC1 signaling, two structurally distinct MEK inhibitors, PD-98059 and U-0126, were used. Addition of PD-98059 (Fig. 5A) or U-0126 (Fig. 5C) 15 min before LPA significantly attenuated ERK phosphorylation. Moreover, addition of either inhibitor effectively blocked LPA-induced S6K1 phosphorylation (Fig. 5, B and D). Similarly, inhibition of MEK effectively blocked PA-induced phosphorylation of ERK and S6K1 (see supplemental Fig. 2). Thus, LPA- and PA-induced activation of mTORC1 signaling was primarily mediated by ERK1/2, rather than through the direct binding of PA to mTOR.
Fig. 5.
LPA, but not leucine, activates mTORC1 signaling through the MAP kinase pathway. Cells were incubated in medium containing 0.5% serum without leucine for 2 h. Leucine, LPA, PD-98059, and/or U-0126 was added to the medium, and S6K1 phosphorylation on Thr389 and ERK1/2 phosphorylation on Thr202/Tyr204 were assessed as described in Fig. 3 and 4 legends, respectively. A and B: means ± SE of 5 experiments. In each experiment, 2 samples per condition were independently analyzed. C and D: means ± SE of 3 experiments. In each experiment, 3 samples per condition were independently analyzed. Representative blots are shown. *P < 0.02 vs. cells treated in medium containing 0.5% serum without leucine. †P < 0.011 vs. cells treated with LPA alone.
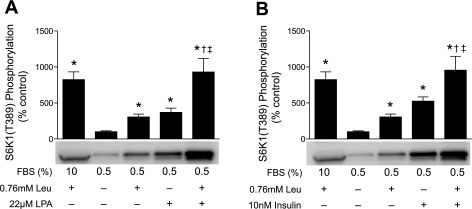
To further explore the role of ERK1/2 in mediating the effect of LPA and PA on the activation of mTORC1, the combined effect of LPA and leucine was assessed. Leucine was chosen for this study because it acts in parallel with TSC1/2 to regulate mTORC1 (47). Thus, if LPA were acting through ERK1/2 to repress TSC1/2 and activate mTORC1, then combined treatment with LPA and leucine should act in an additive manner to activate mTORC1. However, because a previous study (46) suggested that PLD might lie downstream of amino acids in the mTORC1 signaling pathway, we initially assessed the effect of leucine readdition to leucine-deprived cells on PLD activity and PA concentration. As shown in supplemental Fig. 3, leucine had no effect on PLD activity and did not increase PA concentration. In contrast, addition of leucine or LPA to deprived cells led to an approximately threefold increase in S6K1 phosphorylation (Fig. 6A). Moreover, an eightfold increase was observed when the two were added together. Similarly, PA acted in an additive manner with leucine to activate mTORC1, but not ERK, signaling (see supplemental Fig. 4). These findings suggest that leucine acts through a pathway parallel to that utilized by LPA and PA to activate mTORC1 signaling. Further evidence that leucine acts in a pathway parallel to TSC1/2 to activate mTORC1 is the additive effect of leucine and insulin on mTORC1 activity (Fig. 6B). Similar to LPA, insulin activates mTORC1 by repressing TSC1/2 function (13), and the additive effect of leucine and insulin suggests that leucine acts in parallel with TSC1/2.
Fig. 6.
Leucine acts in an additive manner with LPA or insulin to activate mTORC1 signaling. Cells were incubated in medium containing 0.5% serum without leucine for 2 h. Leucine and/or LPA (A) or leucine and/or insulin (B) were added, and, after 2 h, S6K1 phosphorylation was assessed as described in Fig. 3 legend. Values are means ± SE of 3 experiments; in each experiment, 3 samples per condition were independently analyzed. Representative blots are shown. *P < 0.0005 vs. cells incubated in medium containing 0.5% serum without leucine. †P < 0.005 vs. cells treated with leucine alone. ‡P < 0.05 vs. cells treated with LPA alone or insulin alone.
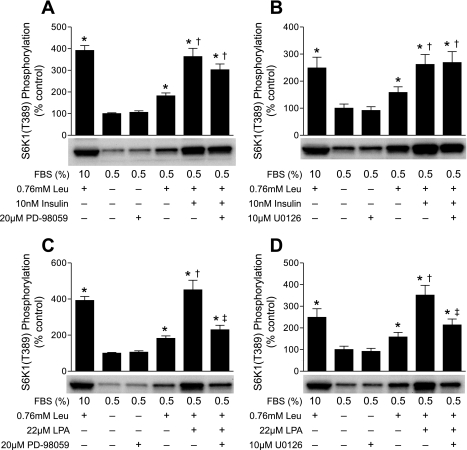
If LPA were signaling primarily through the ERK pathway, then inhibition of MEK would be expected to attenuate the additive effect of leucine and LPA on the activation of mTORC1 and return S6K1 phosphorylation to the value observed in cells treated with leucine alone. In contrast, because leucine and insulin had no effect on ERK phosphorylation at the time point used for these studies (see supplemental Fig. 5), inhibition of ERK should not prevent their additive effect on activation of mTORC1 signaling. To further address this possibility, cells were treated with PD-98059 (Fig. 7A) or U-0126 (Fig. 7B), and then leucine, insulin, or a combination of both was added to the culture medium. Under these conditions, U-0126 had no effect and PD-98059 had a small, but statistically insignificant, effect on leucine- and insulin-induced activation of mTORC1. Because U-0126 was a more effective inhibitor of ERK1 phosphorylation than PD-98059 (Fig. 5), this result suggests that neither leucine- nor insulin-induced activation of mTORC1 signaling required ERK activation. In contrast, PD-98059 (Fig. 7C) and U-0126 (Fig. 7D) were effective in preventing the additive effect of LPA and leucine and restored mTORC1 signaling to a value that was not significantly different from that in cells treated with leucine alone. This finding provides further support for the conclusion that LPA, but not leucine, signals primarily through the ERK1/2 pathway to modulate mTORC1.
Fig. 7.
Inhibition of MEK attenuates LPA-induced, but not leucine- or insulin-induced, activation of mTORC1 signaling. Cells were incubated in medium containing 0.5% serum without leucine for 2 h. Leucine, insulin, LPA, U-0126, and/or PD-98059 was added to the medium, and S6K1 phosphorylation was assessed as described in Fig. 3 legend. A and C: means ± SE of 5 experiments. In each experiment, 2 samples per condition were independently analyzed. B and D: means ± SE of 3 experiments. In each experiment, 3 samples per condition were independently analyzed. Representative blots are shown. *P < 0.045 vs. cells incubated in medium containing 0.5% serum without leucine. †P < 0.03 vs. cells treated with leucine alone. ‡P < 0.02 vs. cells treated with leucine and LPA.
DISCUSSION
Over the past decade, numerous studies have implied a role for an upstream lipid signaling pathway involving PLD and PA in the activation of mTORC1 under a variety of conditions. These conditions include cancer (8, 60, 61), cardiac hypertrophy (3, 57–59), systemic inflammatory responses (31), and mechanical stimulation of skeletal muscle (18). The results of those studies showed a positive correlation between changes in PLD activity and/or expression and mTORC1 signaling. Other evidence appearing to link PLD and PA to activation of mTORC1 include the findings that addition of PA to cells in culture led to increased phosphorylation and/or activation of S6K1 (12, 31, 38, 53) and that PA bound directly to the FKBP12·rapamycin binding (FRB) domain of mTOR (12). Moreover, in some (38, 53), but not all (12, 31), studies, addition of PA to cells in culture partially attenuated the rapamycin-induced repression of mTORC1. On the basis of such findings, a model was proposed in which increasing intracellular PA concentrations, by activation of PLD or by exogenous addition of PA to cells in culture, led to increased binding of the lipid to the FRB domain of mTOR, resulting in activation of mTORC1 (12, 54). This model has been widely accepted, even though PA was shown to have no direct effect on mTORC1 activity in vitro, and mutations in the FRB domain that disrupt PA binding have no effect on mTORC1 kinase activity (12). Moreover, the mechanism through which PA mediates activation of mTORC1 was made less clear by the observation that PA binds to and activates the mTORC1 substrate S6K1 independently of mTOR (29). Therefore, studies relying solely on changes in S6K1 activity may mistakenly assume that such changes reflect alterations in mTORC1 activity.
An alternative mechanism through which PA might act to promote activation of mTORC1 involves the MEK-ERK signaling pathway. A number of studies (14, 40, 41) have shown that PA binds to and activates Raf, an upstream component of the ERK signaling pathway, leading to increased ERK phosphorylation and activity. Other studies have shown that preferential activation of the ERK pathway, for example, using the phorbol ester PMA (1, 42, 49), leads to phosphorylation of TSC2 and, subsequently, dissociation of the active TSC1·TSC2 complex (33). Mutation of the residues phosphorylated in response to PMA treatment to residues that cannot be phosphorylated attenuates phorbol ester-induced mTORC1 activation. Moreover, treatment with U-0126 dramatically attenuated PMA-induced activation of mTORC1 (49). Interestingly, TSC2 is phosphorylated by ERK and by its downstream effector p90RSK (20). Consequently, increased intracellular PA concentrations, e.g., occurring as a result of activation of PLD, could lead to activation of mTORC1 through the ERK signaling pathway, rather than through direct binding of PA to mTOR. The results of the present study strongly support this idea. Thus, inhibition of the MEK-ERK pathway using either of two structurally distinct inhibitors blocked the PA- and LPA-induced activation of ERK and mTORC1. Moreover, although inhibition of MEK-ERK blocked the PA- and LPA-induced activation of mTORC1, it had no effect on the actions of leucine or insulin. Indeed, leucine acted in an additive manner with LPA to activate mTORC1.
The results of the present study also bring into question the assumption that addition of PA to cells in culture leads to mTORC1 activation through internalization of the lipid and its subsequent direct action on intracellular targets, e.g., Raf and/or mTOR. An alternative mechanism through which exogenous PA activates ERK and mTORC1 is through its hydrolysis to LPA by phospholipases (i.e., phospholipase A) present in the culture medium, followed by subsequent binding of LPA to endothelial differentiation gene (EDG) receptors. Activation of EDG receptors would then lead to increased signaling through the MEK-ERK pathway (52). Phospholipase A is present in serum (35) and is also secreted by cells in culture (27, 39). The findings in the present study that PA-induced activation of mTORC1 requires serum and that, in the presence of serum, PA-induced mTORC1 activation is delayed relative to the effect of LPA are consistent with a model in which PA must be hydrolyzed to LPA to activate mTORC1. More definitive evidence supporting this idea is provided by the finding that Ki16425, a specific inhibitor of EDG-2 (37), the predominant form of EDG receptor in Rat2 cells (56), blocks PA-induced activation of mTORC1. A caveat to this model is that, in some studies (12, 46), addition of PA to serum-starved cells leads to activation of mTORC1. In most of those studies, 16:0-18:1 PA, the same lipid used in the present study, was used. However, in those studies, cells were deprived of serum overnight prior to the introduction of exogenous PA, as opposed to the present study, in which cells were deprived of serum for only 2 h. Therefore, it is tempting to speculate that the longer-duration deprivation permitted accumulation of phospholipase A to levels sufficient to hydrolyze enough PA to LPA to activate EDG-2.
It has been suggested that PLD and PA constitute a signaling pathway upstream of mTORC1 that functions in parallel to an amino acid-signaling pathway (7, 11). However, more recently, the same authors concluded that amino acids activate mTORC1 through Rheb-mediated activation of PLD (46). The evidence supporting their conclusion includes the findings that short-term amino acid deprivation attenuates serum-induced activation of PLD and that amino acid replenishment restores PLD activation by serum. In addition, amino acid deprivation was found to block the increase in PLD activation associated with repression of TSC2 expression. Because TSC2 acts as an upstream repressor of Rheb function, the latter finding was taken as evidence that it acted through Rheb to promote PLD activation and, subsequently, to activate mTORC1. In contrast, the results presented here demonstrate that the readdition of the amino acid leucine to leucine-deprived cells has no effect on PLD activity. Additionally, 1-butanol has no effect on PA content in leucine-deprived cells (data not shown), suggesting that, in the absence of PLD activation, most of the PA present in the cell originates from an alternative source such as diacylglycerol kinase (36) or LPAAT (9). However, because leucine has no effect on PA content, it is unlikely that the amino acid activates diacylglycerol kinase or LPAAT.
The results of the present study strongly support the conclusion drawn from earlier studies (7, 12), i.e., that amino acids act through a pathway parallel to PLD to activate mTORC1. For example, activation of mTORC1 by a combination of the amino acid leucine and either LPA or PA was two- to threefold greater than the effect of either one alone, suggesting that the two mediators act through distinct pathways. This conclusion is in agreement with the results of a previous study showing that addition of PA, together with a complete mixture of amino acids, to amino acid-deprived cells led to a synergistic activation of mTORC1 (12). Further support for this conclusion is provided by the results presented here showing that inhibition of the ERK pathway reduced the combined effect of LPA and leucine to the level observed with leucine alone, suggesting that LPA, but not leucine, acts through the ERK pathway to promote activation of mTORC1. The finding that inhibition of the ERK pathway had no effect on the combined effect of insulin and leucine demonstrates that, like leucine, insulin acts through a pathway distinct from ERK to activate mTORC1. Together, the findings reported here provide convincing evidence that leucine and LPA act through distinct mechanisms to activate mTORC1.
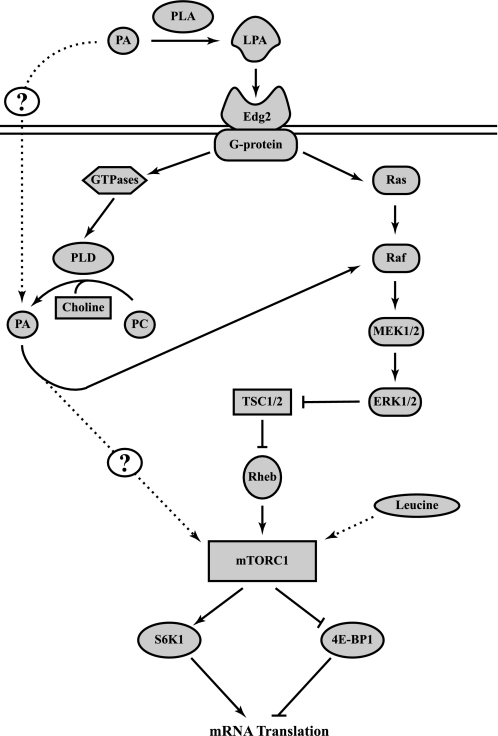
Overall, the results presented here support the model depicted in Fig. 8. In this model, exogenous PA is hydrolyzed to LPA, which binds EDG-2, resulting in activation of the PLD and ERK signaling pathways. The finding that 1-butanol attenuates, but does not prevent, LPA-induced mTORC1 activation suggests that activation of PLD and Ras contributes to activation of ERK and, subsequently, mTORC1. The results are also consistent with the conclusion that leucine acts through a pathway that is parallel to, and independent of, PLD. The components of the leucine signaling pathway and how they lead to mTORC1 activation remain poorly characterized and provide an area for future studies.
Fig. 8.
Model for the PA-mediated activation of mTORC1 signaling. PA is hydrolyzed by phospholipase A (PLA), and resulting LPA activates the endothelial differentiation gene (EDG-2) receptor. Activation of the EDG-2 receptor results in upregulated signaling through the MEK-ERK pathway via 2 distinct mechanisms. One mechanism involves a G protein-mediated increase in PLD activity, leading to hydrolysis of phosphatidylcholine (PC) to choline and PA. PA then binds to Raf, allowing for activation of the ERK cascade. The second mechanism involves G proteins acting through Ras to activate the MEK-ERK pathway. Subsequently, ERK acts to inhibit tuberous sclerosis complex (TSC1/2), thereby increasing GTP loading on Ras homolog enriched in brain (Rheb) and, thus, upregulating mTORC1 activation. mTORC1 then phosphorylates downstream targets, such as S6K1 and eukaryotic initiation factor 4E-binding protein (4E-BP1), ultimately leading to increased mRNA translation. Leucine also activates mTORC1, although the mechanism involved in the effect is not understood.
GRANTS
The studies described here were supported by funds from the Ajinomoto Amino Acid Research Program (to S. R. Kimball), National Institutes of Health Grants DK-13499 (to L. S. Jefferson), DK-15658 (to L. S. Jefferson), HL-076789 (to M. Kester), and EY-018336 (to M. Kester and T. E. Fox), and a grant from the American Diabetes Association (to M. Kester and T. E. Fox). The ABI 4000 QTrap, within the Pennsylvania State University Core Facilities, used in this project, is funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Lydia Kutzler for technical help.
REFERENCES
- 1.Ballif BA, Roux PP, Gerber SA, MacKeigan JP, Blenis J, Gygi SP. Quantitative phosphorylation profiling of the ERK/p90 ribosomal S6 kinase-signaling cassette and its targets, the tuberous sclerosis tumor suppressors. Proc Natl Acad Sci USA 102: 667–672, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum JI, Kimball SR, Jefferson LS. Glucagon acts in a dominant manner to repress insulin-induced mammalian target of rapamycin complex 1 signaling in perfused rat liver. Am J Physiol Endocrinol Metab 297: E410–E415, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bocckino SB, Wilson PB, Exton JH. Phosphatidate-dependent protein phosphorylation. Proc Natl Acad Sci USA 88: 6210–6213, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulous GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol 553: 213–220, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev 18: 2893–2904, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Fang Y. A novel pathway regulating the mammalian target of rapamycin (mTOR) signaling. Biochem Pharmacol 64: 1071–1077, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Zheng Y, Foster DA. Phospholipase D confers rapamycin resistance in human breast cancer cells. Oncogene 22: 3937–3942, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Coon M, Ball A, Pound J, Ap S, Hollenback D, White T, Tulinsky J, Bonham L, Morrison DK, Finney R, Singer JW. Inhibition of lysophosphatidic acid acyltransferase-β disrupts proliferative and survival signals in normal cells and induces apoptosis of tumor cells. Mol Cancer Ther 2: 1067–1078, 2003 [PubMed] [Google Scholar]
- 10.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587: 1535–1546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang Y, Park IH, Wu AL, Du G, Huang P, Frohman MA, Walker SJ, Brown HA, Chen J. PLD1 regulates mTOR signaling and mediates Cdc42 activation of S6K1. Curr Biol 13: 2037–2044, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 294: 1942–1945, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell 11: 1457–1466, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Ghosh S, Strum JC, Sciorra VA, Daniel L, Bell RM. Raf-1 kinase possesses distinct binding domains for phosphatidylserine and phosphatidic acid. Phosphatidic acid regulates the translocation of Raf-1 in 12-O-tetradecanoylphorbol-13-acetate-stimulated Madin-Darby canine kidney cells. J Biol Chem 271: 8472–8480, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177–189, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol 166: 213–223, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong JH, Oh SO, Lee M, Kim YR, Kim DU, Hur GM, Lee JH, Lim K, Hwang BD, Park SK. Enhancement of lysophosphatidic acid-induced ERK phosphorylation by phospholipase D1 via the formation of phosphatidic acid. Biochem Biophys Res Commun 281: 1337–1342, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci USA 103: 4741–4746, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornberger TA, McLoughlin TJ, Leszczynski JK, Armstrong DD, Jameson RR, Bowen PE, Hwang ES, Hou H, Moustafa ME, Carlson BA, Hatfield DL, Diamond AM, Esser KA. Selenoprotein-deficient transgenic mice exhibit enhanced exercise-induced muscle growth. J Nutr 133: 3091–3097, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 412: 179–190, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17: 1829–1834, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanova PT, Milne SB, Myers DS, Brown HA. Lipidomics: a mass spectrometry based systems level analysis of cellular lipids. Curr Opin Chem Biol 13: 526–531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kam Y, Exton JH. Role of phospholipase D1 in the regulation of mTOR activity by lysophosphatidic acid. FASEB J 18: 311–319, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, Tempst P, Sabatini DM. GβL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell 11: 895–904, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Kimball SR, Do AN, Kutzler L, Cavener DR, Jefferson LS. Rapid turnover of the mTOR complex 1 (mTORC1) repressor REDD1 and activation of mTORC1 signaling following inhibition of protein synthesis. J Biol Chem 283: 3465–3475, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimball SR, Siegfried BA, Jefferson LS. Glucagon represses signaling through the mammalian target of rapamycin in rat liver by activating AMP-activated protein kinase. J Biol Chem 279: 54103–54109, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Koduri RS, Baker SF, Snitko Y, Han SK, Cho W, Wilton DC, Gelb MH. Action of human group IIa secreted phospholipase A2 on cell membranes. Vesicle but not heparinoid binding determines rate of fatty acid release by exogenously added enzyme. J Biol Chem 273: 32142–32153, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci 122: 3589–3594, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehman N, Ledford B, Di Fulvio M, Frondorf K, McPhail LC, Gomez-Cambronero J. Phospholipase D2-derived phosphatidic acid binds to and activates ribosomal p70 S6 kinase independently of mTOR. FASEB J 21: 1075–1087, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Inoki K, Guan KL. Biochemical and functional characterization of small GTPase Rheb and TSC2 GAP activity. Mol Cell Biol 24: 7965–7975, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim HK, Choi YA, Park W, Lee T, Ryu SH, Kim SY, Kim JR, Kim JH, Baek SH. Phosphatidic acid regulates systemic inflammatory responses by modulating the Akt-mammalian target of rapamycin-p70 S6 kinase 1 pathway. J Biol Chem 278: 45117–45127, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol 15: 702–713, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121: 179–193, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Nevalainen TJ. Serum phospholipases A2 in inflammatory diseases. Clin Chem 39: 2453–2459, 1993 [PubMed] [Google Scholar]
- 36.Novotna R, De Vito P, Currado L, Luly P, Baldini PM. Involvement of phospholipids in the mechanism of insulin action in HEPG2 cells. Physiol Res 52: 447–454, 2003 [PubMed] [Google Scholar]
- 37.Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, Kimura T, Tobo M, Yamazaki Y, Watanabe T, Yagi M, Sato M, Suzuki R, Murooka H, Sakai T, Nishitoba T, Im DS, Nochi H, Tamoto K, Tomura H, Okajima F. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol 64: 994–1005, 2003 [DOI] [PubMed] [Google Scholar]
- 38.O'Neil TK, Duffy LR, Frey JW, Hornberger TA. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol 587: 3691–3701, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfeilschifter J, Schalkwijk C, Briner VA, van den Bosch H. Cytokine-stimulated secretion of group II phospholipase A2 by rat mesangial cells. Its contribution to arachidonic acid release and prostaglandin synthesis by cultured rat glomerular cells. J Clin Invest 92: 2516–2523, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizzo MA, Shome K, Vasudevan C, Stolz DB, Sung TC, Frohman MA, Watkins SC, Romero G. Phospholipase D and its product, phosphatidic acid, mediate agonist-dependent raf-1 translocation to the plasma membrane and the activation of the mitogen-activated protein kinase pathway. J Biol Chem 274: 1131–1139, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Rizzo MA, Shome K, Watkins SC, Romero G. The recruitment of Raf-1 to membranes is mediated by direct interaction with phosphatidic acid and is independent of association with Ras. J Biol Chem 275: 23911–23918, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci USA 101: 13489–13494, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 14: 1296–1302, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 22: 159–168, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Sofer A, Lei K, Johannessen CM, Ellisen LW. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol 25: 5834–5845, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun Y, Fang Y, Yoon MS, Zhang C, Roccio M, Zwartkruis FJ, Armstrong M, Brown HA, Chen J. Phospholipase D1 is an effector of Rheb in the mTOR pathway. Proc Natl Acad Sci USA 105: 8286–8291, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab 295: E868–E875, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swarthout JT, Walling HW. Lysophosphatidic acid: receptors, signaling and survival. Cell Mol Life Sci 57: 1978–1985, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tee AR, Anjum R, Blenis J. Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase/Akt-dependent and -independent phosphorylation of tuberin. J Biol Chem 278: 37288–37296, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA 99: 13571–13576, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 102: 145–152, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Torkhovskaya TI, Ipatova OM, Zakharova TS, Kochetova MM, Khalilov EM. Lysophospholipid receptors in cell signaling. Biochemistry (Mosc) 72: 125–131, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Toschi A, Lee E, Xu L, Garcia A, Gadir N, Foster DA. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol Cell Biol 29: 1411–1420, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veverka V, Crabbe T, Bird I, Lennie G, Muskett FW, Taylor RJ, Carr MD. Structural characterization of the interaction of mTOR with phosphatidic acid and a novel class of inhibitor: compelling evidence for a central role of the FRB domain in small molecule-mediated regulation of mTOR. Oncogene 27: 585–595, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Wijesinghe DS, Allegood JC, Gentile LB, Fox TE, Kester M, Chalfant CE. Use of high pressure liquid chromatography, electrospray ionization-tandem mass spectrometry for the analysis of ceramide-1-phosphate levels. J Lipid Res. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu J, Love LM, Singh I, Zhang QX, Dewald J, Wang DA, Fischer DJ, Tigyi G, Berthiaume LG, Waggoner DW, Brindley DN. Lipid phosphate phosphatase-1 and Ca2+ control lysophosphatidate signaling through EDG-2 receptors. J Biol Chem 275: 27520–27530, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Xu YJ, Botsford MW, Panagia V, Dhalla NS. Responses of heart function and intracellular free Ca2+ to phosphatidic acid in chronic diabetes. Can J Cardiol 12: 1092–1098, 1996 [PubMed] [Google Scholar]
- 58.Xu YJ, Panagia V, Shao Q, Wang X, Dhalla NS. Phosphatidic acid increases intracellular free Ca2+ and cardiac contractile force. Am J Physiol Heart Circ Physiol 271: H651–H659, 1996 [DOI] [PubMed] [Google Scholar]
- 59.Xu YJ, Yau L, Yu LP, Elimban V, Zahradka P, Dhalla NS. Stimulation of protein synthesis by phosphatidic acid in rat cardiomyocytes. Biochem Pharmacol 52: 1735–1740, 1996 [DOI] [PubMed] [Google Scholar]
- 60.Yamada Y, Hamajima N, Kato T, Iwata H, Yamamura Y, Shinoda M, Suyama M, Mitsudomi T, Tajima K, Kusakabe S, Yoshida H, Banno Y, Akao Y, Tanaka M, Nozawa Y. Association of a polymorphism of the phospholipase D2 gene with the prevalence of colorectal cancer. J Mol Med 81: 126–131, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Zhao Y, Ehara H, Akao Y, Shamoto M, Nakagawa Y, Banno Y, Deguchi T, Ohishi N, Yagi K, Nozawa Y. Increased activity and intranuclear expression of phospholipase D2 in human renal cancer. Biochem Biophys Res Commun 278: 140–143, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.