Abstract
A key brain site in the control of male sexual behavior is the medial preoptic area (mPOA) where dopamine stimulates both D1 and D2 receptor subtypes. Research completed to date in Japanese quail has only utilized systemic injections, so much is unknown about the specific role played by dopamine in the brain and mPOA in particular. The present study investigates the role of D1 and D2 receptors on male sexual behavior by examining how intracerebroventricular (ICV) injections and microinjections into the mPOA of D1 and D2 agonists and antagonists influence appetitive and consummatory aspects of sexual behavior in male quail. Experiments 1 and 2 investigate the effects of ICV injections at three doses of D1 or D2 agonists and antagonists. Results indicate that D1 receptors facilitate consummatory male sexual behavior while D2 receptors inhibit both appetitive and consummatory behaviors. Experiment 3 examines the effects of the same compounds specifically injected in the mPOA and shows that in this region, both receptors stimulate male sexual behaviors. Together, these data indicate that the stimulatory action of dopamine in the mPOA may require a combined activation of D1 and D2 receptors. Finally, the regulation of male sexual behavior by centrally infused dopaminergic compounds in a species lacking an intromittent organ suggests that dopamine action on male sexual behavior does not simply reflect the modulation of genital reflexes due to general arousal, but relates to the central control of sexual motivation. Together, these data support the claim that dopamine specifically regulates male sexual behavior.
Keywords: dopamine, dopamine receptors, birds, preoptic area, motivation
INTRODUCTION
One of the most robust observations about the catecholamine neurotransmitter dopamine is that it promotes the activation of male sexual behavior in mammals (Blackburn et al., 1992; Hull et al., 2002). Based on detailed studies in rats, dopamine action in the medial preoptic area (mPOA) in particular is hypothesized to focus the male’s motivation on sexually relevant stimuli, coordinate genital reflexes necessary for erection and ejaculation, and enhance species-typical motor patterns of copulation (Hull et al., 1999). In a critical review, Paredes and Ågmo (2004) questioned whether dopamine is specifically linked to the control of male sexual behavior rather than to the modulation of general arousal and motor ouput. Based on this view, dopamine in the mPOA would be related perhaps to ejaculation but not sexual motivation per se (see Hull et al., 2002; Hull et al., 2006 for an alternative perspective).
Studies in non-mammalian vertebrates could be useful in resolving this controversy as well as contributing to the development of a comprehensive theory of dopamine action. The general organization of the dopamine system (e.g. localization of cell bodies, projections, and receptor subtypes) appears to be highly conserved among diverse taxa (Smeets & Reiner, 1994). Japanese quail (Coturnix japonica) are a species of interest as they have been the subject of many studies on the neuroendocrine control of male-typical sexual behavior (for reviews see Balthazart & Ball, 1998; Ball & Balthazart, 2002; Ball & Balthazart, 2004). Furthermore, dopamine has been implicated in the control of sexual behavior in quail (Balthazart et al., 1997; Castagna et al., 1997). Specifically, peripheral injections of dopaminergic drugs suggest that activation of D1-like receptors enhances expression of male sexual behavior while the activation of D2-like receptors has the opposite effect (Castagna et al., 1997). However, data indicate that dopamine itself is inhibitory in this species unlike what is observed in mammals (Absil et al., 1994; Castagna et al., 1997; Cornil et al., 2005). It has been suggested that this difference in dopamine effects between birds and mammals may reflect the differential relative expression of D1- and D2- like receptors in the two taxa (more D2 in birds) so that inhibitory autoreceptors are more readily activated in birds than in mammals (Kleitz et al., 2009).
Behavioral measures are available to assess motivational and performance aspects of quail male sexual behavior (Adkins & Adler, 1972; Hutchison, 1978; Seiwert & Adkins-Regan, 1998). As is the case in most avian species, the males lack an intromittent organ and thus the nature of non-specific peripheral effects should be quite different and arguably less important than what has been hypothesized to be the case in mammals.
The current studies tested the effects of central injections of several dopaminergic drugs at various doses on both motivational and performance aspects of male sexual behavior in quail. We provide evidence consistent with the notion that dopamine indeed plays a specific role in the control of male sexual behavior and help clarify why dopamine appears to be inhibitory in some instances in quail.
MATERIALS AND METHODS
Animals and endocrine treatment
A total of 49 male Japanese quail (Coturnix japonica) were used as subjects in these experiments (Experiment 1, n =15; Experiment 2, n =14; and Experiment 3, n =20). Additionally, a total of 35 female Japanese quail were used as stimuli (Experiment 1, n=10; Experiment 2, n=10; and Experiment 3, n=15). Birds were experimentally and sexually naive prior to experimental procedures. They were obtained from a local breeder (CBT Farms, Chestertown, MD) at the age of 3–4 weeks and were first housed as a group in a large cage. Throughout their life at the breeding colony and in the laboratory, birds were exposed to a photoperiod simulating long days (14 h light and 10 h dark per day) and had food and water available ad libitum. One week after their arrival, all the males were castrated. They were deeply anesthetized with isoflurane gas anesthetic (IsoSol isoflurane from Vedco. Inc, St. Joseph, MO; Isotec 4 anesthesia machine from Surgivet, Inc., Waukesha, WI USA) and both testes removed through a unilateral incision below the last rib. After recovery from surgery, birds were brought back to the large cage.
Approximately 2 weeks later, subjects were implanted subcutaneously with one 20-mm long Silastic™ capsule (Silclear™ Tubing, Degania Silicone Ltd., Degania Bet, 15130, Israel; 1.57mm i.d.; 2.41mm o.d.) filled with crystalline testosterone (Sigma-Aldrich, St. Louis, MO, USA). They were then housed in individual cages. Throughout the experiment birds were periodically weighed to the nearest gram and the size of their cloacal gland, an androgen-dependent structure (Delville et al., 1985) was measured with calipers (cloacal gland area = largest length×largest width in mm2). These data confirmed the efficacy of the testosterone replacement, as well as the absence of adverse effects of the experimental treatments on the general health condition of the subjects. If at any point during the experiment, the subjects became ill or their cannulae became displaced, they were euthanized to avoid any pain or suffering and thus removed from the experiment and all analyses.
At the conclusion of the experiment, birds were anesthetized with isoflurane and euthanized by rapid decapitation. The completeness of castration and the presence of Silastic™ implants were checked and the brains were extracted and frozen at −70°C. Birds were housed, manipulated, and euthanized by using procedures approved by the IACUC at Johns Hopkins University.
Stereotaxic implantation of the injection cannula
Approximately 4 weeks after castration, birds were deeply anesthetized with isoflurane and placed in a stereotaxic apparatus (David Kopf instruments, Tujunga, CA, USA) with the pigeon-head holder placed at a 45° angle below the horizontal axis of the stereotaxic assembly. The skull was drilled at the level of the inter-parietal suture. A 22-gauge guide cannula (outside diameter = 750 µm) containing a 28-gauge dummy insert (Plastics One Inc., Roanoke, VA, USA) was inserted into the brain to reach the region of interest. The outside cannula was cut at a length of 7.8 mm and the dummy cut so that it protruded by about 300 µm from the guide cannula.
Implantation for Experiments 1 and 2 (ICV injections)
The guide cannulae for Experiments 1 and 2 were inserted into the brain to reach the third ventricle at the level of the mPOA (AP+1.8 mm, DV+2.8 mm). Because a wide blood vessel is present in the medial position at the surface of the brain, cannulae were implanted at an angle of 10° away from the vertical to enter the brain in a position just lateral to this blood vessel. Thus, the reference lateral coordinate was aimed at zero (midsagittal plane of the brain) and then moved laterally by 0.30 µm. The coordinates were obtained from previous studies in the lab (Cornil et al., 2005). The guide cannula was fixed to the skull with dental cement and the skin was sutured. The birds were kept in a warm environment until they fully recovered from anesthesia. Metacam® (meloxicam; Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO, USA) was administered for three days following the surgery to reduce pain and inflammation.
The accuracy of the cannula placement was initially verified in each bird by the fact that cerebrospinal fluid flows out the guide cannula when it has correctly reached the third ventricle. Once the subjects recovered from surgery, the cannula placement was further verified by assessing the effects of Angiotensin II (ANG II) infusion on drinking behavior. Birds received ANG II (Sigma-Aldrich, St. Louis, MO, USA) dissolved in 0.9% NaCl solution at a concentration of 1.0 mg/ml, corresponding to a dose of 1.0 µg/injection. Birds were kept in their home cages with only water available and their weight gain after 1 hour was assessed for a baseline measure and then an additional hour after ANG II infusion. It is presumed that when ANG II is injected centrally and fails to stimulate water intake, the cannula has not penetrated the ventricle (Richardson & Boswell, 1993). At the conclusion of the experiment, black ink was injected into the cannula and histology confirmed its placement by noting if the ink was present in the third ventricle.
Implantation for Experiment 3 (Tissue microinjection)
The target coordinates for Experiment 3 were determined with the stereotaxic quail brain atlas (Baylé et al., 1974) modified by our own trial placements. The guide cannulae were inserted unilaterally in the left hemisphere into the brain to reach the mPOA at 0° angle (AP+1.8 mm, ML+0.3 mm, DV+2.8 mm). The guide cannula was fixed to the skull with dental cement and the skin was sutured. The birds were kept in a warm environment until they fully recovered and Metacam® (meloxicam; Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO, USA) was administered for three days following the surgery to reduce pain and inflammation. Histology confirmed its placement in the mPOA.
Drug treatments
Injections of SKF-38393 (D1 agonist; D1+), SCH-23390 (D1 antagonist; D1−), Quinpirole (D2 agonist; D2+), and Raclopride (D2 antagonist; D2−), were performed with the use of a 5-µl Hamilton syringe (Hamilton Bonaduz AG, Bonaduz CH-7402, Switzerland) connected to a microinfusion pump. The dummy cannula was replaced by a 28-gauge internal cannula (Plastics One Inc., Roanoke, VA, USA) attached to the syringe by a cannula connector (Plastics One Inc., Roanoke, VA, USA). The liquid (1 µl for Experiments 1 and 2 and 0.5 µl for Experiment 3) was infused during a 90-s period. The needle remained in place for one additional minute before being removed to permit diffusion of the solution and avoid its leakage outside of the brain. The internal cannula was progressively lifted in successive steps and replaced by the dummy cannula. SCH-23390, SKF-38393, Raclopride, and Quinpirole were purchased from Sigma–Aldrich (St. Louis, MO, USA) and dissolved in 0.9% NaCl saline solution purchased from Henry Schein, Inc. For Experiments 1 and 2 concentrations of 0 µg/µl (Saline), 0.02 µg/µl (Low), 0.1 µg/µl (Medium), and 0.5 µg/µl (High) were used. The most effective dose for each compound based on both the appetitive and consummatory behavioral responses found in Experiments 1 and 2 were used in Experiment 3: 0 µg/µl (Saline), 0.02 µg/µl (SKF-38393), 0.1 µg/µl (SCH-23390), 0.1 µg/µl (Quinpirole), and 0.5 µg/µl (Raclopride).
Behavioral tests
Copulatory behavior was assessed in a large test arena measuring 60 cm×40 cm×50 cm (length×depth×height). The experimental bird was introduced into the test arena that contained a sexually mature female with which the male could freely interact during a 5-min period. During this period, the frequencies of the following behavior patterns were systematically noted: neck-grab (NG), mount attempt (MA), mount (M) and cloacal contact movements (CCM) (for a detailed description, see (Adkins & Adler, 1972).We also recorded the latency of the first occurrence of the observed behaviors. The data provide a measure of the consummatory sexual behavior (CSB) of the birds. Males were tested in a random order that was changed on each day with a female randomly selected from a large pool so that even if the behavior of females varied during and between the days, this effect was randomized in the experimental groups which are described in the next section.
Appetitive sexual behavior (ASB) was assessed by the measure of the rhythmic cloacal sphincter movements (RCSM). Gonadally intact males increase the rate of these movements when they are provided with only visual access to a female (Seiwert & Adkins-Regan, 1998). Thus, RCSM produced under these conditions in which a male is viewing a female in anticipation of copulation provides a measure of male ASB in quail. The RCSM tests took place in a small aquarium (20 cm×40 cm×25cm) located on a raised platform. A mirror was placed under the aquarium at a 45° angle and provided the observer with an unobstructed view of the male’s cloacal area. At the beginning of each behavioral test, the aquarium was divided into two chambers by an opaque sliding panel and a glass partition. One experimental male was placed in one of the chambers and a stimulus egg-laying female in the other chamber. The frequency of RCSM was directly counted for 2.5 min during which the male could not see the female (basal RCSM). The sliding opaque panel was then removed so that the male and female were only separated by the glass barrier; the male had visual access to the female although he was not able to physically interact with her. The frequency of female-elicited RCSM was quantified by direct observation for an additional 2.5 min under these conditions. Then, the glass panel was then removed and the birds were able to interact physically for an additional period of 2.5 min during which CSB frequency and latency were recorded for 2.5min. Again, the order in which males were tested was randomized. For Experiment 3, only the RCSM test followed by the copulatory test in the RCSM chamber was utilized.
Group assignment
Regardless of the experiment they were in, all birds received 5 pre-test trials for copulatory behavior at approximately 9 weeks of age (approximately 3 weeks after testosterone implantation and after recovery from cannula implantation) to let them acquire and mature the copulatory pattern and to ensure that all subjects were able to copulate. Within each experiment, each bird was then exposed once to each of the experimental treatments including the control condition (injection of Saline and of different doses of a given compound in Experiments 1 and 2 or injection of Saline and of different compounds in Experiment 3). The order in which each subject was exposed to these different conditions varied for different sub-groups of birds according to a Latin square design so that the sequence of conditions could not be confounded in the final analyses with the effects of conditions themselves. To determine the order of exposure to the different treatments (four different doses in Experiment 1 and 2 and five different compounds in Experiment 3, including in each case the Saline condition), subjects were assigned to four sub-groups of 2–4 birds (Experiments 1 and 2) or five sub-groups of 2–5 birds (Experiment 3). These sub-groups were matched based on the behavioral results of all past copulatory tests (mean frequencies of behavior displayed) and of the last copulatory test, as well as on results of the ANG II test for cannula placement (Experiments 1 and 2 only) to ensure that the sub-groups were not statistically different before the experimental manipulations. If at any point during the experiment, the subjects became ill or their cannulae became displaced, they were removed from the experiment resulting in a variable number of birds in each sub-group. Each bird thus served as its own control in a repeated measures design described below.
Experimental design
For Experiments 1 and 2, each subject was tested repeatedly in four conditions testing the effect of Saline, Low, Medium and High doses of D1 and D2 agonists and antagonists administered via ICV injections on ASB and CSB. Experiment 1 investigated the effects of ICV administration of the D1-like agonist SKF-38393 (D1+) and antagonist SCH-23390 (D1−). Experiment 2 tested the effects of ICV administration of the D2-like agonist Quinpirole (D2+) and antagonist Raclopride (D2−). For both experiments, each sub-group of subjects received the doses in different orders in a Latin square design such that a lower dose (Saline or Low) always followed/preceded a higher dose (Medium or High) with one day between tests to prevent any potential residual effects of drugs from previous tests. Fifteen minutes after the infusion of the drug, the behaviors were then quantified for each bird as previously described. Pre- and post-experiment behavioral baseline measures were conducted to evaluate any long-term effects of each compound, none of which were found significant (p>0.05). At the conclusion of each test, we waited seven days before testing the next compound.
Experiment 3 utilized the most effective doses from Experiments 1 and 2 to investigate the effects of both D1 and D2 receptor compounds after microinjection into the mPOA on male sexual behavior. Each of the 20 quail was tested repeatedly in five conditions testing the effects of the compounds on ASB and CSB in one trial. Each sub-group received the compounds in different orders in a Latin square design such that a compound which acts on the D1 receptors always followed/preceded a compound which acts on the D2 receptors with four days between tests to prevent any potential tissue damage and residual effects of drugs from previous tests. Immediately following the injection procedure, the behaviors were then quantified for each bird as previously described. Pre- and post-experiment behavioral baseline measures were conducted to evaluate any long-term effects of the drug. For all experiments (1, 2, and 3), all injections were administered and all tests took place between the hours of 0900–1500.
Data analysis
All data were analyzed by repeated measures analyses of the variance (ANOVA) with the dosage (Exp 1 and 2) or the treatment (Exp 3) as the repeated factor and the sub-groups defining the order of administration of the different treatments (test order) as the independent factor. Effects were considered significant for p<0.05. When appropriate, pairwise post-hoc comparisons were performed using Fisher’s least significant difference (LSD) test. All analyses were carried out with Windows version of the software SPSS, version 16.0 (Chicago, IL). All behavioral measures were analyzed and reported in table 1 through table 3, however data from only a few representative behaviors are included in the associated figures.
Table 1.
Effects of an ICV administered D1-like agonist and antagonist (a. D1+: SKF-38393; b. D1-: SCH-23390) on the frequency and latency of consummatory sexual behaviors in male Japanese quail (reported as mean ± standard error).
| SKF-38393 | D1+ | Saline | Low | Medium | High | F3,33= | p= | D1+ | Saline | Low | Medium | High | F3,12= | p= | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | Frequency | NG | 6.80 ±0.86 |
8.80 ±1.31 |
10.40 ±2.36 |
10.73 ±1.84 |
1.112 | 0.358 |
Post- RCSM Frequency |
NG | 5.75 ±1.00 |
9.50 ±1.50 |
10.25 ±2.34 |
10.25 ±2.29 |
3.373 | (0.053) |
| MA | 5.87 ±0.68 |
7.27 ±0.94 |
6.87 ±0.84 |
8.46 ±1.36 |
1.476 | 0.239 | MA | 4.50 ±0.71 |
13.50 a ±1.39 |
5.50 ±1.77 |
7.00 a ±1.32 |
4.343 | 0.027* | |||
| M | 5.13 ±0.55 |
5.53 ±0.61 |
5.40 ±0.71 |
7.27 ±1.19 |
2.369 | (0.088) | M | 4.38 ±0.65 |
8.50 a ±1.45 |
7.13 ±1.47 |
5.75 b ±0.92 |
5.712 | 0.012* | |||
| CCM | 4.40 ±0.53 |
4.07 ±0.58 |
4.40 ±0.59 |
5.73 ±0.73 |
2.743 | (0.059) | CCM | 3.75 ±0.73 |
6.13 a ±1.06 |
5.75 ±0.98 |
3.88 b ±0.64 |
3.671 | 0.044* | |||
| Latency | NG | 3.47 ±1.49 |
2.33 ±0.75 |
1.93 ±0.53 |
2.67 ±0.96 |
1.212 | 0.321 |
Post- RCSM Latency |
NG | 16.00 ±15.00 |
1.88 a ±0.64 |
1.25 a ±0.25 |
1.25 a ±0.25 |
1634.0 | <0.001* | |
| MA | 5.67 ±1.80 |
3.33 ±0.75 |
2.87 ±0.47 |
3.67 ±0.97 |
2.569 | (0.071) | MA | 18.50 ±14.86 |
3.13 a ±0.64 |
3.13 a±0.58 | 5.88 a,b,c ±2.16 |
309.04 | <0.001* | |||
| M | 5.40 ±1.50 |
4.20 ±0.77 |
7.93 ±2.70 |
4.40 ±1.01 |
2.519 | (0.075) | M | 19.38 ±14.88 |
3.75 a ±0.75 |
4.63 a±1.00 | 9.63 a ±3.55 |
51.206 | <0.001* | |||
| CCM | 7.27 ±1.75 |
5.40 ±0.79 |
25.93 ±19.63 |
6.20 ±1.40 |
1.766 | 0.173 | CCM | 21.13 ±14.83 |
5.50 a ±1.02 |
6.13 a ±1.01 |
23.63 b,c ±9.65 |
20.064 | <0.001* |
| SCH-23390 | D1- | Saline | Low | Medium | High | F3,15= | p= | D1- | Saline | Low | Medium | High | F3,12= | p= | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | Frequency | NG | 14.44 ±3.04 |
8.56 a ±1.89 |
9.67 a ±1.53 |
13.89 ±3.05 |
4.504 | 0.019* |
Post- RCSM Frequency |
NG | 10.25 ±1.44 |
5.63 a ±1.07 |
6.50 ±1.46 |
6.50 a ±1.13 |
3.487 | (0.050) |
| MA | 10.22 ±1.68 |
6.56 a ±1.16 |
7.78 a ±1.08 |
10.11 ±1.89 |
5.049 | 0.013* | MA | 8.88 ±1.32 |
4.88 ±1.01 |
5.89 ±1.34 |
6.00 ±1.10 |
2.310 | 0.128 | |||
| M | 8.11 ±1.24 |
5.67 ±0.91 |
6.89 ±1.03 |
8.44 ±1.49 |
2.287 | 0.120 | M | 7.50 ±1.22 |
3.88 ±0.81 |
5.13 ±1.40 |
5.38 ±0.89 |
2.227 | 0.138 | |||
| CCM | 6.22 ±0.81 |
4.78 ±0.86 |
5.44 ±1.13 |
5.56 ±0.92 |
0.725 | 0.553 | CCM | 6.75 ±1.31 |
3.00 a ±0.71 |
3.75 ±1.16 |
3.50 a ±0.40 |
4.47 | 0.025* | |||
| Latency | NG | 1.56 ±0.24 |
1.67 ±0.67 |
2.22 ±0.36 |
1.33 ±0.24 |
0.485 | 0.698 |
Post- RCSM Latency |
NG | 1.13 ±0.12 |
1.88 ±0.30 |
1.25 ±0.16 |
6.13 ±4.33 |
1.123 | 0.378 | |
| MA | 2.56 c ±0.24 |
2.67 ±0.67 |
3.67 ±0.44 |
2.33 c ±0.24 |
3.423 | 0.045* | MA | 3.63 ±0.89 |
4.63 ±1.36 |
4.88 ±1.74 |
7.25 ±4.31 |
0.368 | 0.777 | |||
| M | 3.89 c ±0.46 |
3.44 ±0.71 |
4.56 ±0.50 |
3.00 c ±0.33 |
3.45 | 0.044* | M | 5.75 ±1.68 |
5.75 ±1.36 |
5.75 ±1.77 |
8.13 ±4.34 |
0.220 | 0.881 | |||
| CCM | 4.89 ±0.46 |
4.44 ±0.71 |
6.78 ±1.09 |
4.00 ±0.33 |
3.207 | (0.053) | CCM | 6.88 ±1.78 |
11.25 ±4.32 |
23.88 ±18.05 |
11.25 ±4.88 |
0.625 | 0.612 |
Effects of an ICV administered D1-like agonist and antagonist (a. D1+: SKF-38393; b. D1-: SCH-23390) on the frequency and latency of consummatory sexual behaviors in male Japanese quail (reported as mean ± standard error). Behavior was tested during isolated tests for copulatory behavior (left part of each table) or during tests that immediately followed measures of RCSM (right part of the table). Results of the ANOVAs are listed in two separate columns and when appropriate, results of post hoc test are listed next to the corresponding means (a, b, c = p<0.05 vs. Saline, Low, and Medium, respectively).
Table 3.
Effects of mPOA microinjections of D1-like agonist and antagonist (D1+: SKF-38393; D1-: SCH-23390) and D2-like agonist and antagonist (D2+: Quinpirole; D2-: Raclopride) on the frequency (top) and latency (bottom) of consummatory sexual behaviors in male Japanese quail.
| Saline | SKF-38393 D1+ |
SCH-23390 D1− |
Quinpirole D2+ |
Raclopride D2− |
F4,60= | p= | ||
|---|---|---|---|---|---|---|---|---|
|
Post- RCSM Frequency |
NG | 6.50 ±1.28 |
5.15 ±1.29 |
4.35 ±0.99 |
5.25 ±0.71 |
4.35 ±1.20 |
1.549 | 0.200 |
| MA | 4.85 ±0.86 |
3.25 a ±0.52 |
2.65 a ±0.50 |
4.30 ±0.62 |
3.05 ±0.64 |
3.266 | 0.017* | |
| M | 2.95 ±0.59 |
2.05 ±0.32 |
1.55 a ±0.29 |
3.25 ±0.39 |
2.05 d ±0.44 |
3.512 | 0.012* | |
| CCM | 2.10 ±0.31 |
1.85 ±0.27 |
0.95 a,b ±0.23 |
2.00 ±0.30 |
1.60 c ±0.29 |
5.164 | 0.001* | |
|
Post- RCSM Latency |
NG | 8.60 ±2.09 |
15.25 ±5.81 |
39.50 a ±12.90 |
20.00 ±10.00 |
39.00 a ±13.07 |
2.818 | 0.033* |
| MA | 9.70 ±2.08 |
16.90 ±6.16 |
47.65 a,b ±13.75 |
23.80 ±10.03 |
40.50 a ±12.88 |
3.850 | 0.008* | |
| M | 13.65 ±3.01 |
27.45 ±9.03 |
48.50 a,b ±13.64 |
28.00 ±9.98 |
47.10 a ±12.53 |
3.092 | 0.022* | |
| CCM | 23.25 ±7.40 |
72.90 ±48.46 |
68.65 ±15.29 |
39.90 ±11.43 |
50.30 ±12.33 |
0.566 | 0.688 |
Effects of mPOA microinjections of D1-like agonist and antagonist (D1+: SKF-38393; D1-: SCH-23390) and D2-like agonist and antagonist (D2+: Quinpirole; D2-: Raclopride) on the frequency (top) and latency (bottom) of consummatory sexual behaviors in male Japanese quail. Behavior was tested during tests that immediately followed measures of RCSM. Behaviors are reported as mean ± standard error. Results of the ANOVAs are listed in a separate column and when appropriate, results of post-hoc tests are listed next to the corresponding means (a, b, c, d = p<0.05 versus Saline, SKF-38393, SCH-23390, and Quinpirole, respectively).
RESULTS
Unless otherwise stated, there was no main effect of the order of treatments of the sub-groups and no interaction between this order and the effects of the experimental treatments themselves (p>0.05 for all).
Experiment 1 – Dose-response analysis of ICV-administered D1 drugs
Table 1 reports all the behavioral frequencies and latencies observed after the ICV administration of the D1 agonist SKF-38393 during the tests for CSB performed alone as well as following the RCSM test. When copulatory behavior was tested alone, there was a consistent trend for all frequencies of CSB to increase in parallel with the dose of the D1 agonist, however this trend was never significant (p>0.05 in all cases; statistical trends (p<0.10) for M and CCM). There was also no effect of dose of SKF-38393 on the latency to sexual behavior onset (p>0.05 in all cases; statistical trends for MA and M), however there was a dose by order of administration interaction for M latency (F9,33=2.814, p=0.014) such that the quail who had received the Medium dose first had a longer latency of M at this dose.
Following ICV administration of the D1 antagonist SCH-23390, we observed a significant U-shaped dose effect on the frequency of both NG and MA (NG: F3,15=4.504, p=0.019; MA: F3,15=5.049, p=0.013) such that significantly lower behavior frequencies were detected after administration of the Low and Medium dose of the antagonist. For both NG and MA there was also a dose by order interaction such that the behavior frequency at the High dose was higher in the birds who received this dose on the last day of testing (NG: F9,15=3.636, p=0.013; MA: F9,15=3.654, p=0.013). Furthermore, there was a significant inverted-U shaped dose response in latency of MA and M (MA: F3,15=3.423, p=0.045; M: F3,15=3.45, p=0.044) such that the latency of these behaviors was the longest at the Medium dose.
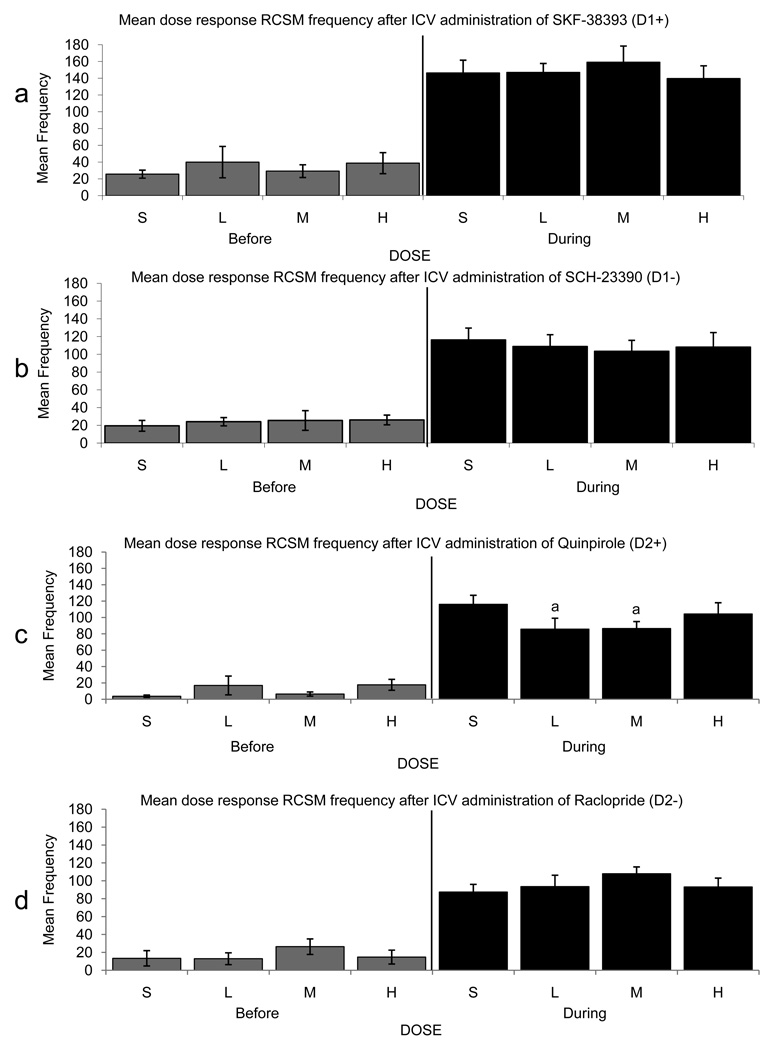
Upon completion of testing the effects of ICV administration of SKF-38393 and SCH-23390 on CSB, the effect of the same doses of these two drugs were tested on the ASB (RCSM test) immediately followed by another copulatory test performed directly in the RCSM arena. These tests revealed no significant effect on basal or female-elicited RCSM frequency after ICV administration of either the D1 agonist or antagonist (p>0.05 for all cases; figure 1a,b).
Figure 1.
Mean (± SEM) RCSM frequency observed before and during visual access to a female in male quail that had been injected ICV with Saline (S) or with a Low (L), Medium (M) or High (H) dose of the D1 agonist SKF-38393 (a), the D1 antagonist SCH-23390 (b), the D2 agonist Quinpirole (c) or the D2 antagonist Raclopride (d). (a)= p<0.05 for comparison versus Saline.
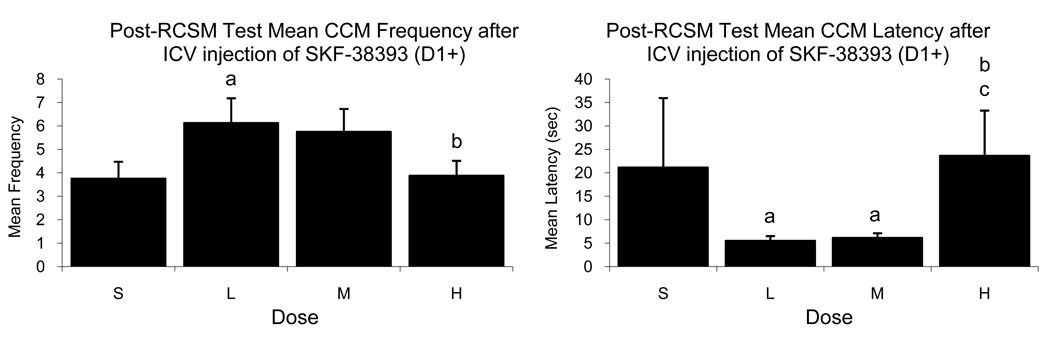
In contrast to the data just presented, SKF-38393 significantly affected the frequency of most copulatory behaviors during tests performed immediately after the assessment of RCSM (NG: F3,12=3.373, p=0.053; MA: F3,12=4.343, p=0.027; M: F3,12=5.712, p=0.012, CCM: F3,12=3.671, p=0.044). The significant dose-response curves had an inverted U-shape so that the highest frequencies were detected after injection of the Low dose (figure 2). Furthermore, this D1 agonist also significantly affected the onset latency of all behaviors (p<0.001 in each case) in a U-shaped function (figure 2). For all the latency measures following SKF-38393 administration after the RCSM test, there was a significant main effect of order as well as a dose by order interaction (p<0.05 for all), but this can be explained by the fact that at the end of the experiment only one bird remained in one of the groups, thereby affecting the between subjects analysis.
Figure 2.
Mean (± SEM) frequency (left) and latency (right) of CCM observed during tests performed immediately after the measure of RCSM in male quail that had been injected ICV with Saline (S) or with a Low (L), Medium (M) or High (H) dose of the D1 agonist SKF-38393. (a), (b), (c) = p<0.05 for comparison versus Saline (a), Low (b), and Medium (c) doses.
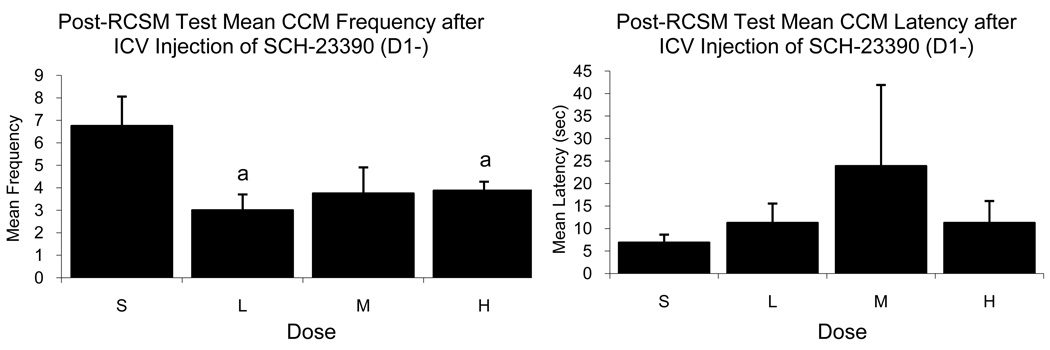
Statistical analyses revealed a marginally significant effect of the various doses of SCH-23390 on NG (F3,12=3.487, p=0.05) and a significant effect on CCM (F3,12=4.47, p=0.025) frequencies (figure 3) observed during the test that followed the quantification of RCSM. Post-hoc analysis revealed a significantly lower frequency following the Low dose and the High dose as compared to Saline for both behaviors. Further, there was an effect of order of dose administration on M and CCM (F3,4=9.252, p=0.028 and F3,4=18.222, p=0.009, respectively), but this could be a result of a low number of birds (n=2) in each group for this test due to the attrition of subjects. No significant effect of dose of SCH-23390 was detected on the latency for any behavior measured after the RCSM test (p>0.05 for all cases).
Figure 3.
Mean (± SEM) frequency (left) and latency (right) of CCM observed during tests performed immediately after the measure of RCSM in male quail that had been injected ICV with Saline (S) or with a Low (L), Medium (M) or High (H) dose of the D1 antagonist SCH-23390. (a) = p<0.05 for comparison versus Saline.
Together, these results indicate that throughout the brain, D1-like receptors appear to play a stimulatory role on CSB in quail. This is supported by the inhibitory effects of SCH-23390 (D1−) on CSB as shown by a decrease in behavioral frequency as well as the overall facilitative effect of SKF-38393 (D1+) on post-RCSM copulatory behavior as demonstrated by an increase in frequency and decrease in latency.
Experiment 2 – Dose-response analysis of ICV-administered D2 drugs
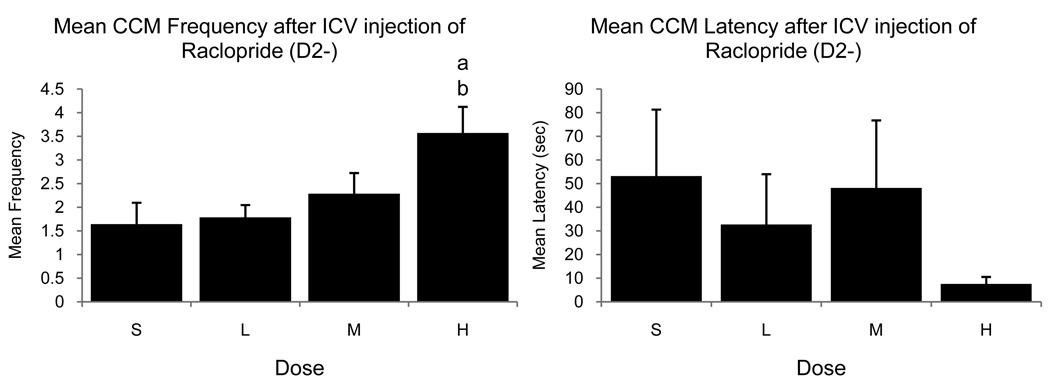
ICV administration of the D2 agonist Quinpirole yielded non-significant effects on both frequency and latency of CSB (p>0.05 in all cases; table 2). As illustrated in figure 4 and table 2, ICV administration of the D2 antagonist, Raclopride, resulted in a significant dose-dependent effect on CCM frequency (F3,33=5.242, p=0.005) such that the behavior frequency at the High dose was significantly greater than both in the Saline and Low dose conditions. A similar trend was observed for M frequencies (F3,33=3.365, p=0.094). Conversely, latencies of initiation of CSB tended to decrease with increasing doses of Raclopride. This trend approached significance for NG (F3,33=2.361, p=0.089) and MA (F3,33=2.876, p=0.051) but was far from significant for the two other behavior patterns.
Table 2.
Effects of an ICV administered D2-like agonist and antagonist (a. D2+: Quinpirole; b. D2-: Raclopride) on the frequency and latency of consummatory sexual behaviors in male Japanese quail (reported as mean ± standard error).
| Quinpirole | D2+ | Saline | Low | Medium | High | F3,30= | p= | D2+ | Saline | Low | Medium | High | F3,21= | p= | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | Frequency | NG | 10.77 ±3.10 |
7.46 ±1.46 |
12.69 ±3.23 |
7.30 ±1.51 |
1.788 | 0.171 |
Post- RCSM Frequency |
NG | 6.09 ±1.76 |
9.64 ±2.72 |
4.00 ±1.26 |
9.64 c ±1.86 |
3.210 | 0.044* |
| MA | 7.23 ±1.49 |
5.23 ±0.96 |
7.23 ±1.51 |
5.54 ±0.93 |
0.978 | 0.416 | MA | 4.82 ±1.21 |
6.18 ±1.66 |
2.82 ±0.77 |
6.91 ±1.23 |
2.377 | (0.099) | |||
| M | 4.54 ±0.87 |
3.23 ±0.50 |
3.54 ±0.87 |
4.08 ±0.90 |
0.610 | 0.614 | M | 4.00 ±1.03 |
3.36 ±0.77 |
1.64 ±0.47 |
4.45 ±1.05 |
2.441 | (0.093) | |||
| CCM | 2.54 ±0.49 |
2.31 ±0.35 |
1.85 ±0.50 |
2.69 ±0.67 |
0.517 | 0.674 | CCM | 2.64 ±0.65 |
1.91 ±0.41 |
1.36 ±0.34 |
2.27 ±0.63 |
1.400 | 0.271 | |||
| Latency | NG | 4.77 ±3.13 |
25.23 ±22.91 |
2.38 ±0.62 |
2.15 ±0.61 |
1.129 | 0.353 |
Post- RCSM Latency |
NG | 37.73 ±17.53 |
34.73 ±17.65 |
47.18 ±20.09 |
13.91 ±5.56 |
2.316 | 0.105 | |
| MA | 25.69 ±22.86 |
26.15 ±22.83 |
3.62 ±0.62 |
3.15 ±0.61 |
0.790 | 0.509 | MA | 39.18 ±17.36 |
36.18 ±17.44 |
48.09 ±19.91 |
16.27 ±5.38 |
2.128 | 0.127 | |||
| M | 49.23 ±30.87 |
26.85 ±22.77 | 28.15 ±22.70 |
28.92 ±22.62 |
0.262 | 0.853 | M | 40.00 ±17.24 |
41.36 ±16.99 |
60.73 ±20.58 |
30.36 ±13.04 |
0.569 | 0.641 | |||
| CCM | 53.92 ±30.41 |
29.23 ±22.61 |
41.00 ±24.02 |
77.77 ±35.28 |
0.494 | 0.689 | CCM | 40.91 ±17.18 |
43.64 ±16.61 |
62.09 ±20.30 |
51.73 ±19.15 |
0.256 | 0.856 |
| Raclopride | D2- | Saline | Low | Medium | High | F3,33= | p= | D2- | Saline | Low | Medium | High | F3,21= | p= | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | Frequency | NG | 7.64 ±2.55 |
7.35 ±2.20 |
7.86 ±2.74 |
12.36 ±3.94 |
0.671 | 0.576 |
Post- RCSM Frequency |
NG | 10.27 ±2.46 |
7.64 ±1.52 |
11.09 ±2.79 |
7.64 ±1.44 |
0.671 | 0.580 |
| MA | 5.43 ±1.38 |
4.93 ±1.35 |
5.43 ±1.19 |
6.21 ±1.24 |
0.260 | 0.854 | MA | 7.18 ±1.57 |
5.55 ±0.99 |
6.36 ±1.35 |
6.18 ±1.08 |
0.235 | 0.871 | |||
| M | 2.71 ±0.45 |
2.71 ±0.58 |
3.07 ±0.50 |
4.43 ±0.78 |
3.365 | (0.094) | M | 5.09 ±1.24 |
4.27 ±0.93 |
3.82 ±0.75 |
4.73 ±0.80 |
0.355 | 0.786 | |||
| CCM | 1.64 ±0.45 |
1.79 ±0.26 |
2.29 ±0.44 |
3.57a,b ±0.55 |
5.242 | 0.005* | CCM | 3.18 ±0.96 |
3.09 ±0.71 |
2.82 ±0.70 |
3.27 ±0.47 |
0.108 | 0.955 | |||
| Latency | NG | 4.00 ±1.59 |
8.00 ±5.40 |
1.57 ±0.37 |
1.50 ±0.31 |
2.361 | (0.089) |
Post- RCSM Latency |
NG | 3.09 ±1.81 |
2.91 ±1.52 |
1.55 ±0.39 |
18.55 ±12.53 |
1.238 | 0.321 | |
| MA | 5.50 ±1.59 |
9.71 ±5.36 |
2.57 ±0.37 |
2.57 ±0.34 |
2.876 | (0.051) | MA | 4.18 ±1.81 |
4.00 ±1.38 |
3.18 ±0.46 |
19.91 ±12.48 |
1.257 | 0.314 | |||
| M | 11.57 ±4.22 |
10.93 ±5.36 |
26.00 ±21.18 |
6.57 ±2.93 |
0.443 | 0.724 | M | 8.73 ±2.51 |
6.45 ±1.94 |
3.91 ±0.61 |
23.73 ±12.38 |
1.383 | 0.275 | |||
| CCM | 53.21 ±28.08 |
32.71 ±21.25 |
48.14 ±28.59 |
7.57 ±2.93 |
0.894 | 0.454 | CCM | 13.64 ±5.11 |
10.27 ±2.46 |
18.82 ±13.16 |
26.45 ±12.47 |
0.501 | 0.686 |
Effects of an ICV administered D2-like agonist and antagonist (a. D2+: Quinpirole; b. D2-: Raclopride) on the frequency and latency of consummatory sexual behaviors in male Japanese quail (reported as mean ± standard error). Behavior was tested during isolated tests for copulatory behavior (left part of each table) or during tests that immediately followed measures of RCSM (right part of the table). Results of the ANOVAs are listed in two separate columns and when appropriate, results of post hoc test are listed next to the corresponding means (a, b, c = p<0.05 vs. Saline, Low, and Medium, respectively).
Figure 4.
Mean (± SEM) frequency (left) and latency (right) of CCM observed during tests in the large arena (no RCSM test before) in male quail that had been injected ICV with Saline (S) or with a Low (L), Medium (M) or High (H) dose of the D2 antagonist Raclopride. (a), (b) = p<0.05 for comparison versus Saline (a) and Low (b) dose.
As was done in Experiment 1, after the tests involving the ICV administration of Quinpirole and Raclopride on CSB were completed, the effect of the same doses of these two drugs were tested on the frequency of RCSM. This test was immediately followed by another copulatory test performed in the same arena as was used for the RCSM test. As illustrated in figure 1c, we identified a significant dose effect of ICV Quinpirole administration on RCSM frequency only while viewing the female (F3,21=4.47, p=0.014). Post-hoc analysis revealed that the Low and Medium doses produced significantly fewer RCSM than Saline. In contrast, the RCSM frequency tended to increase after ICV administration of the Low and Medium dose of the D2 antagonist Raclopride so that there was an inverted U-shaped relation between dose and effect on RCSM while viewing the female. These effects were, however, non-significant (p>0.05 for both cases; figure 1d).
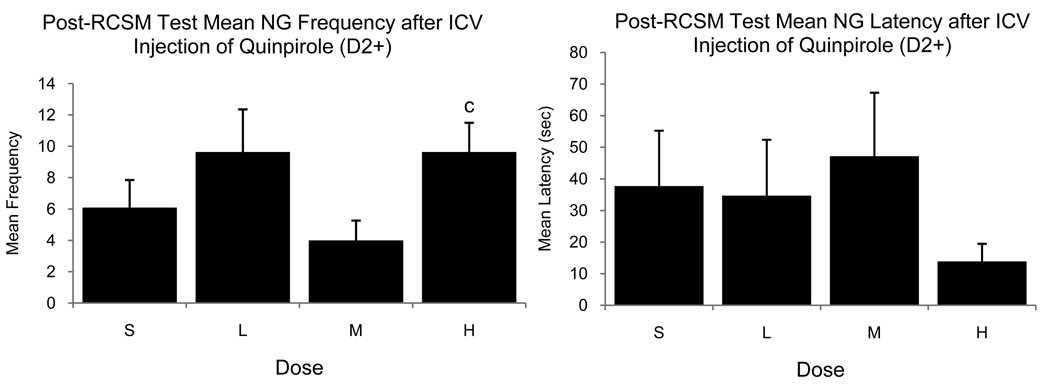
Furthermore, there was a significant dose effect of ICV Quinpirole administration on NG frequencies measured immediately after the RCSM tests (F3,21=3.210, p=0.044) and post-hoc analysis revealed that the Medium dose produced fewer NG frequencies than was observed after administration of the High dose (figure 5). Besides this change in NG frequency, there was no significant effect of Quinpirole or Raclopride at the three doses that were tested on the frequencies or latencies of all CSBs during post-RCSM test (p>0.05 for all cases). For all behaviors and both D2 receptor compounds, there was no main effect of dose order and no dose by order interaction (p>0.05 for all).
Figure 5.
Mean (± SEM) frequency (left) and latency (right) of NG observed during tests performed immediately after the measure of RCSM in male quail that had been injected ICV with Saline (S) or with a Low (L), Medium (M) or High (H) dose of the D2 agonist Quinpirole. (c) = p<0.05 for comparison versus the Medium dose.
The facilitative effect of Raclopride on copulatory frequency and the inhibitory effects of Quinpirole on both RCSM and copulatory frequencies post-RCSM indicate that throughout the brain, D2-like receptors appear to play an inhibitory role on male sexual behavior in quail. Taken together, the results of Experiments 1 and 2 based on the ICV administration of D1-like and D2-like receptor agonists and antagonists suggest that D1-like receptors facilitate CSB in quail while D2-like receptors inhibit both ASB and CSB.
Experiment 3 – Behavioral effects of D1 and D2 drugs microinjected into the mPOA
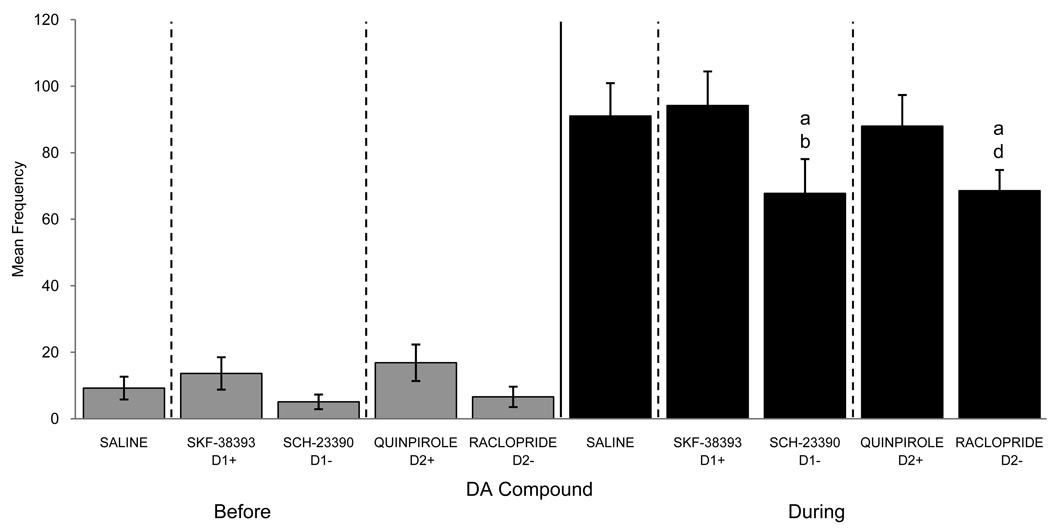
Utilizing the most efficient doses from Experiments 1 and 2, we then tested the effect of the injection of these compounds directly into the mPOA of quail. In order to focus our reported results on meaningful comparisons, only the following post-hoc analyses are presently reported: all compounds vs. Saline, D1+ vs. D1−, D2+ vs. D2−, D1+ vs. D2+, and D1− vs. D2−. As illustrated in figure 6, there was a significant treatment effect on RCSM frequency only while viewing the female (F4,60=5.864, p<0.001) and post-hoc analysis revealed that this effect resulted from an inhibitory effect of Raclopride as compared to Saline and Quinpirole, as well as of SCH-23390 as compared to Saline and SKF-38393. There was no main effect of the order of drug administration (p>0.05), but there was a significant drug by order interaction such that the animals who received the Saline injection first had a higher RCSM frequency while viewing the female after receiving this Saline injection on the first day of testing only (F16,60=2.316, p=0.01).
Figure 6.
Mean (± SEM) RCSM frequency observed before and during visual access to a female in male quail that had been microinjected in the mPOA with Saline, the D1 agonist SKF-38393, the D1 antagonist SCH-23390, the D2 agonist Quinpirole or the D2 antagonist Raclopride. (a), (b), (d) = p<0.05 for comparison versus Saline (a), SKF-38393 (b), and Quinpirole (d).
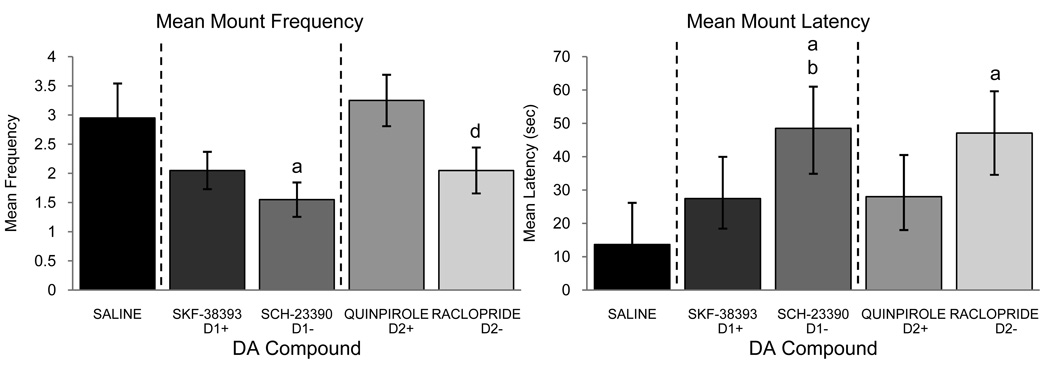
Refer to table 3 and figure 7 to see the significant effects of the injections on the copulatory frequencies of MA, M, and CCM (MA: F4,60=3.266, p=0.017; M: F4,60=3.512, p=0.012; CCM: F4,60=5.164, p=0.001). Post-hoc analysis revealed for MA that SCH-23390 treated animals expressed significantly fewer behaviors than those who received Saline, but surprisingly SKF-39393 treated animals also exhibited a decreased frequency of MA as compared to Saline treated. As illustrated in figure 7, post-hoc analysis showed that SCH-23390 treated quail displayed significantly fewer M than when treated with Saline and further, the frequency of M was significantly smaller after Raclopride injection than after Quinpirole. Lastly, for CCM, SCH-23390 injections led to a significantly reduced CCM frequency as compared to birds when they were injected with Saline, SKF-38393, and Raclopride. Furthermore, as also illustrated in table 3 and figure 7, there was a significant effect of the injections on the latency to the onset of NG, MA, and M (NG: F4,60=2.818, p=0.033; MA: F4,60=3.850, p=0.008; M: F4,60=3.092, p=0.022). Post-hoc analysis revealed that for all three behaviors, injections of either antagonist (SCH-23390 and Raclopride) significantly increased the latency to behavioral onset as compared to Saline injections. Further, SCH-23390 injections also significantly increased the latency to behavioral onset of MA and M as compared to SKF-38393 injections. For all CSB, there was no main effect of drug administration order or any drug by order interactions for either frequency or latency (p>0.05 for all cases).
Figure 7.
Mean (± SEM) frequency (left) and latency (right) of M observed in male quail that had been microinjected in the mPOA with Saline, the D1 agonist SKF-38393, the D1 antagonist SCH-23390, the D2 agonist Quinpirole or the D2 antagonist Raclopride. (a), (b), (d) = p<0.05 for comparison versus Saline (a), SKF-38393 (b), and Quinpirole (d).
Together, the decreased frequency and increased latency of CSB following SCH-23390 injection as well as the inhibition of the ASB frequency by SCH-23390 suggest that when bound by an antagonist, D1-like receptors in the mPOA of quail inhibit male sexual behavior. Similarly, however, the inhibitory effects of Raclopride on RCSM frequency and the increased latencies for showing NG, MA and M after injection of this D2 antagonist also suggest that when bound by an antagonist, the D2-like receptors in the mPOA also play an inhibitory role in male sexual behavior in quail.
DISCUSSION
In this series of experiments, we employed a central injection strategy to investigate the possible effects of D1 and D2 selective compounds on appetitive and consummatory measures of male-typical sexual behavior (ASB and CSB, respectively) in Japanese quail. Previous work addressing this question had employed a peripheral injection strategy (Balthazart et al., 1997; Castagna et al., 1997). In the first two experiments, we centrally injected three different doses of D1 and D2 selective compounds via ICV infusions that we compared to the infusion of Saline. Using the most effective doses based on the behavioral data from these first two experiments, in Experiment 3 we infused the same compounds directly in the mPOA, a key site for the action of dopamine in relation to sexual behavior.
Based on previous studies employing injections in rats (Hull et al., 1986; Pehek et al., 1988; Warner et al., 1991; Hull et al., 1992) and in quail (Balthazart et al., 1997; Castagna et al., 1997), we predicted that stimulation of D1 receptors would increase male-typical sexual behavior and stimulation of D2 receptors would decrease this behavior, while antagonists would have the opposite effect. As will be discussed in the subsequent sections of this discussion, the results for Experiments 1 and 2 are in broad agreement with this notion.
The paradoxical effects that dopamine and the non-selective agonist apomorphine (APO) in quail are inhibitory rather than stimulating (Castagna et al., 1997; Cornil et al., 2005), suggested to us that investigating the effects of compounds centrally and in a tissue specific manner (Experiment 3) was important to try to clarify how dopamine is acting in the brain to modulate male-typical sexual behavior in quail. Given the multiple doses we employed and the variety of dependent measures we used, our findings are understandably somewhat complex. Therefore, we focus our discussion on the overall patterns of the results that were identified, even though effects of some of the agonists or antagonists did not reach statistical significance for all behaviors. Indeed, when a significant difference was detected in one behavior of the copulatory sequence, consistent trends generally existed in the other behaviors of this sequence. Consequently, a careful examination of the data as a whole does indicate that certain themes have emerged (see table 4) and will be discussed further.
Table 4.
Summary of the effects on male sexual behavior in Japanese quail after ICV administration of D1 and D2 agonists and antagonists.
| D1+ SKF-38393 |
D1 SCH-23390 |
D2+ Quinpirole |
D2 Raclopride |
|
|---|---|---|---|---|
|
Appetitive Sex Behavior (RCSM) |
n.s. | n.s. | ↓ | n.s. |
|
Consummatory Sex Behavior (NG, MA, M, CCM) |
↑ | ↓ | ↓ | ↑ |
↑,↓, n.s. Represents a facilitation, inhibition, and non-significant effects on sexual behavior, respectively.
Effects of ICV Injections on Sexual Behavior
In Experiment 1, ICV administration of the D1 agonist SKF-38393 facilitated male CSB measured after RCSM by increasing the frequency of M and CCM at the Low dose and MA at both the Low and High doses. SKF-38393 administration also reduced the latency to initiate all behaviors at most doses (tests after RCSM). In contrast, the ICV administration of the D1 antagonist SCH-23390 inhibited the frequency of CSB, while increasing the latency of behavioral onset at select doses. These results derived from Experiment 1 thus suggest that overall the activation of D1 receptors throughout the brain tends to facilitate the expression of CSB.
Furthermore, Experiment 2 suggests that the activation of D2 receptors within the brain inhibits the expression of both ASB as well as CSB. Indeed, the ICV administration of the D2 agonist Quinpirole inhibited RCSM behavior at the Low and Medium doses and inhibited the frequency of NG at the Medium as compared to the High dose (tests after RCSM). In contrast, ICV administration of the D2 antagonist Raclopride increased the frequency of CCM at the High dose and decreased the latency of MA. In general, the results from Experiments 1 and 2 are thus consistent with past studies using systemic injections in quail (Balthazart et al., 1997) suggesting that throughout the brain in this species, D1 receptors primarily facilitate while D2 receptors primarily inhibit male sexual behavior.
Comparison of Copulatory Tests Conducted Alone and Following the RCSM Test
Another pattern that has emerged is that different types of results were observed depending on whether the copulatory tests occurred in the small arena immediately after the RCSM test or in the larger chambers alone (i.e., not following the RCSM test). One notable difference in the data is that it appears as though the administration of either the D1 or D2 agonists results in more significant behavioral effects when the copulatory tests follow the RCSM test. In contrast, the effectiveness of the antagonists’ decreases following the RCSM test as compared to the behavioral effects observed during the copulatory tests alone. A possible explanation for these differences could be that the female had less room in this smaller arena to remove herself from the vicinity of the male and thus could not pace or modulate his behavior as easily. Alternatively, these discrepancies could stem from the fact that when the copulatory test follows the RCSM test, the male views the female for 2.5 min prior to the onset of the copulatory test. This time in visual contact followed by time in an arena where the male is in close proximity with the female may result in an altered neurochemical state in the male. Based on previous studies in rats, this change in state could include, for example, an increased dopamine concentration in the mPOA resulting from the dopamine release that should take place following exposure to a female (Hull et al., 1995). With this higher level of endogenous dopamine, the effects of the agonists could be amplified whereas the effects of the antagonists would be diminished. This interpretation could be tested in future experiments.
Effects of Tissue Specific Injections on Male Sexual Behavior
We decided that for Experiment 3 we would test both ASB and CSB simultaneously in the same smaller chamber (RCSM test chamber) because (1) the observed behavioral trends were, in general, similar in tests performed in the larger arena alone and in the smaller chamber following the RCSM test and (2) these trends had in general a larger magnitude in the latter case. We utilized the most effective doses from Experiments 1 and 2 to investigate the effects of both D1 and D2 receptor compounds on male sexual behavior after microinjection into the mPOA.
As illustrated in table 5, Experiment 3 revealed that the injection in the mPOA of a D1 or a D2 antagonist decreased the frequency of both ASB and CSB and increased the latency to initiate CSB. Specifically, both SCH-23390 and Raclopride administration decreased RCSM frequency as compared to animals treated with Saline. SCH-23390 administration also decreased the frequencies of MA, M and CCM as compared to Saline-treated birds while increasing the latency of the first NG, MA and M. Raclopride decreased the frequency of M (when compared to Quinpirole) and increased the latency of NG, MA and M when compared to Saline.
Table 5.
Summary of the effects on male sexual behavior in Japanese quail after mPOA microinjections of D1 and D2 agonists and antagonists.
| D1+ SKF-38393 |
D1 SCH-23390 |
D2+ Quinpirole |
D2 Raclopride |
|
|---|---|---|---|---|
|
Appetitive Sex Behavior (RCSM) |
n.s. | ↓ | n.s. | ↓ |
|
Consummatory Sex Behavior (NG, MA, M, CCM) |
n.s. | ↓ | n.s. | ↓ |
↓, n.s. Represents an inhibition and non-significant effects on sexual behavior, respectively.
Similar to results from the first experiment, the behavioral inhibition resulting from the administration of the D1 antagonist in the mPOA suggests that the activation of preoptic D1 receptors facilitates male sexual behavior in quail. One single result seems to contradict this trend: there was a decrease in MA frequency following the injection of SKF-38393. It is possible that the dose of this compound that we selected for the third experiment may have been too high for the tissue-selective injection. In the tests after RCSM during Experiment 1, there was indeed a decrease in the stimulatory effects of this compound on MA at higher doses as compared to the Low dose. Even though Experiment 3 used the lowest of the SKF-38393 doses tested in Experiment 1, it may have produced a very high non-physiological concentration when administered directly into the tissue resulting in the activation of heterologous receptor sub-types and consequently an inhibition of behavior frequency. This interpretation can only be definitively evaluated by a future dose-response study in which this compound would be injected directly into the mPOA.
Contrary to the results from Experiment 2, the microinjection in the mPOA of the D2 antagonist indicated that the activation of preoptic D2 receptors might also facilitate male sexual behavior in quail. These findings suggest that the specific role played by dopamine action in the mPOA in the control of male-typical sexual behavior might be distinct from its actions elsewhere in the brain and periphery. There are several possible reasons for these differences between the two studies. The first obvious difference is that, in the case of the tissue-specific injections, the compounds administered are modulating only a relatively small portion of the entire population of brain dopamine receptors, i.e. those specifically located in the preoptic region in contrast to the ICV injections where a much more global population is affected. This fact alone could be responsible for the difference in effects.
However, it is also important to note that it has clearly been established based on studies in mammals that D2 autoreceptors are substantially more sensitive to dopamine than D2 post-synaptic receptors (Elsworth & Roth, 1997; Cooper et al., 2003). Based on a variety of studies completed to date there is every reason to expect that the pharmacology of the two types of dopamine receptors in quail and other birds is very similar to what has been described in mammals. As noted previously, the overall anatomical organization of the dopamine system is quite similar in birds and mammals (Smeets & Reiner, 1994). The pharmacological characterization of dopamine receptors in quail and other birds as well as their distribution based on the autoradiographic localization of binding sites support the claim that these receptors are homologous in birds and mammals (Dietl & Palacios, 1988; Casto & Ball, 1994; Ball et al., 1995; Levens et al., 2000; Kleitz et al., 2009; Kubikova et al., 2009). Recent studies involving the molecular cloning of dopamine receptors in finches and their localization via in situ hybridization studies are also consistent with this notion (Kubikova et al., 2010). Based on this similarity, one scenario that could explain the difference between the studies we conducted is that, when administered ICV and through peripheral injections, the D2 antagonist primarily blocked pre-synaptic autoreceptors and therefore enhanced the release of endogenous dopamine (since autoreceptor stimulation blocks dopamine release). In contrast, when we administered our selected dose of the D2 antagonist in a tissue specific manner, we may have produced a higher local concentration of the antagonist and blocked post-synaptic receptors. The differential blockade of D2 pre- or post-synaptic receptors could then result in opposite effects on behavior for a variety of reasons including: modulation or not of endogenous dopamine release (see above), differential pharmacological specificity of these two populations of receptors (Elsworth & Roth, 1997; Cooper et al., 2003), or even differential interaction of the endogenous dopamine with adrenergic receptors as has been described occurring in the quail POA (Cornil et al., 2002; Cornil & Ball, 2008). It is also worth noting that the recent observation that the ratio of D2 to D1 receptors is higher in quail than in rats (Kleitz et al., 2009) supports this scenario. A D2 antagonist at a lower-level dose would be even more likely to block D2 receptors in quail as compared to rats. Given the differential sensitivity of pre- and post-synaptic receptors, it is likely that such a lower dose of a D2 antagonist would preferentially affect pre-synaptic receptors and thus dopamine release. The data we have obtained in this study will obviously require additional studies to clarify fully their significance but they do raise interesting questions about the dopaminergic control of sexual behavior in a comparative perspective.
Comparison of the Present Report with Male Sexual Behavior in Rats
In agreement with microinjection studies to the mPOA in rats and past studies using systemic injections in quail (Rats: Hull et al., 1986; Pehek et al., 1988; Warner et al., 1991; Hull et al., 1992; Quail: Balthazart et al., 1997; Castagna et al., 1997), the results from Experiments 1 and 2 suggest that D1 receptors tend to facilitate while D2 receptors tend to inhibit male sexual behavior in quail. These data also support the notion that indeed dopamine acts centrally to modulate this behavior. However, the results from Experiment 3 suggest that the story may not be so simplistic.
In male rats, it has been suggested that D1 and D2 receptors in the mPOA differentially influence different aspects of copulatory behavior (Hull et al., 1992). Indeed, a D1 agonist injected into the mPOA increased erections and decreased seminal emissions while a D1 antagonist and/or D2 agonist injected into the mPOA decreased erections and increased seminal emissions. In addition, a D2 antagonist blocked the increased seminal emissions induced by high doses of the non-selective agonist APO (Hull et al., 1992). Thus, together the results from rats and quail (ICV data in current report and systemic injections in Balthazart et al., 1997) suggest that the mechanism of control of male sexual behavior by dopamine seems to be conserved across species.
However, contradictory to rat studies, systemic injections of APO inhibits both ASB and CSB in a dose-dependent manner in quail (Castagna et al., 1997) and ICV injections of APO or dopamine itself have also been shown to inhibit male sexual behavior in quail (Cornil et al., 2005). In order to provide a possible explanation for this difference between species, we recently assessed by in vitro quantitative receptor autoradiography the relative D1-like and D2-like binding in the quail and rat brain. As previously described, we discovered a greater ratio of D2 to D1 receptor binding in quail as compared to rat throughout the brain, including areas important in the control of male sexual behavior (Kleitz et al., 2009). Thus, the resulting inhibition of male sexual behavior following an injection (either systemic or ICV) of a non-selective dopamine agonist could be a result of the more pronounced activation of D2 as compared to D1 receptors in quail as compared to rats. We show here in Experiment 2 that activation of D2 receptors by ICV injection of a D2 agonist inhibits sexual behavior, thus providing one possible explanation as to how the administration of APO or dopamine inhibits male sexual behavior in quail but facilitates this behavior in rats.
In contrast to these results, Experiment 3 suggests that, specifically in the mPOA, the activation of both receptor sub-types facilitates male sexual behavior because the administration of either D1 or D2 selective antagonists directly into the mPOA results in the inhibition of male-typical sexual behavior. Our previous in vitro quantitative autoradiography experiment (Kleitz et al., 2009) reported a higher ratio of D2 to D1 receptors in the mPOA of both quail and rat when compared to other brain regions. It is therefore conceivable that there is a fundamental difference in the mechanisms of dopamine action in the mPOA as compared to other brain regions with D2-dependent mechanisms being more prominent in this region than in the rest of the brain. This theoretical overview was described in more detail in the previous section.
Additionally, the antagonist data from Experiment 3 suggests that in the mPOA there is an endogenous in vivo dopamine activity since, in its absence, there would be no neurochemical activity to be blocked by the two receptor-selective antagonists. One critical question that remains to be answered thus concerns the temporal pattern of dopamine release in the mPOA during behavioral interactions with a female. In rats, it has been shown that the presence of a female enhances dopamine release in the mPOA in a testosterone-dependent fashion (Hull et al., 1995) and this release is critical for the activation of copulatory behavior. In the absence of this precopulatory rise in dopamine, males failed to copulate when the barrier separating them from a female was removed. In contrast, males that showed a substantial increase in extracellular dopamine in the mPOA during pre-copulatory interactions behind the barrier, copulated with females, regardless of testosterone concentrations (Hull et al., 1995). Similarly, recent studies in quail identified a significant increase in dopamine release in the mPOA of Japanese quail during the expression of male sexual behavior (Kleitz-Nelson et al., 2010). The temporal pattern of this release in relation to the first visual exposure to the female and to the expression of copulation should now be determined.
Specificity of dopamine action on sexual behavior (central vs. peripheral, general vs. specific)
Some effects of dopamine in mammals appear to be related directly to the facilitation of penile erections, whereas other actions influence sexual motivation (for reviews, see Dominguez & Hull, 2005; Hull et al., 2006). The Japanese quail used in the present experiments exhibit a robust pattern of testosterone-dependent sexual motivation, but like most birds, lack an intromittent organ (Adkins-Regan, 1996; Seiwert & Adkins-Regan, 1998). Gamete transfer occurs via the male mounting the female and contacting his cloaca to hers. This cloacal apposition presumably does not require a complex male-typical neuromuscular control, as is the case in mammals (Seiwert & Adkins-Regan, 1998). Quail thus have the potential to provide important information about the central versus peripheral actions of dopamine in stimulating male sexual behavior. The similarity of D1 and D2 actions on quail copulatory behavior as compared to rats clearly suggests that dopamine action on sexual behavior in rats does not simply reflect a modulation of penile reflexes but most likely relates to central actions of this catecholamine that are common to both rats and quail. In this context, experiments investigating extracellular release of dopamine in the mPOA of quail remain essential to understand the function of this neurotransmitter in sexual motivation and performance, in general. One important piece of information provided by the recent microdialysis experiment mentioned above (Kleitz-Nelson et al., 2010) is that it supports the idea that preoptic dopamine indeed plays an important role in the control of male-typical sexual behavior independently of any action on a peripheral erectile organ. We are currently investigating the temporal release of dopamine in the mPOA in relation to behavioral expression in order to determine whether different dopamine concentrations are present during precopulatory interactions and during the expression of ASB and CSB. The knowledge gained from these future studies in conjunction with the results from the present experiments should help us theorize how different receptors may be activated at different concentrations and during different components of male-typical sexual behavior in quail.
Recently, questions have also been raised about the specificity of dopamine effects on sexual behavior (Paredes & Ågmo, 2004). These authors argued that pharmacological data support the notion that dopamine is important for motor functions and arousal and that these general effects explain most of the effects observed on sexual behavior without the need to invoke any specific action on sexual motivation or reward per se. Accordingly, dopamine would affect sexual behavior exclusively by modulating autonomic responses linked with erection and motor control rather than by a specific action on behavioral functions such as copulation, motivation, or reward. Furthermore, Paredes and Ågmo (2004) declare that basic principles of dose-response effects have been overlooked in past studies such that any drug will affect behavior when the dose is sufficiently large. Lastly, they state that systemic injections of dopamine agonists or antagonists may act at sites normally not involved in sexual behavior and that conclusions from studies utilizing these methods should be taken with a grain of salt since the precise sites of dopamine action are not known. The experiments on quail described in this paper allow us to address some of these concerns.
As already discussed, the similarity of behavioral responses in rats and quail in the absence of an intromittent organ in quail strongly argues against the notion that dopamine action on sexual behavior stems only as the result of the modulation of a penile response. Specifically, the results described in the present report show a lack of effect of dopaminergic compounds on RCSM frequency when the male is isolated, while significant changes in RCSM frequency were detected during Experiments 2 and 3 only when the male was viewing the female. Further, there was no observed difference in the general activity of the birds between compounds (no change in general locomotor behavior during testing and no change in feeding behavior in their home cage). It can thus be argued that the effects of these compounds did not affect the general arousal of the animal; the dopamine-induced modification in arousal was specific to the sexual context and only observed in the presence of a female.
Finally, it is important to note that somewhat different behavioral results were observed when the same drugs were injected ICV as opposed to directly into the mPOA. In some cases, this discrepancy could result from the production of different concentrations of a specific drug in the mPOA. It is indeed difficult, if not impossible to determine, what the relative local concentrations were in the mPOA after injection of a same dose in the third ventricle and directly into the tissue. The two sets of observations could thus relate to effects on behavior at a different point in the dose-response curve. However, these data also point to the potential specificity of dopaminergic effects in different brain areas. The ICV injections obviously target the brain in a more specific manner than peripheral treatments. They are, however, likely to affect multiple brain sites besides the mPOA adjacent to the third ventricle. This notion is supported by the great similarity of results obtained during experiments in which dopamine drugs were injected intraperitoneally (Balthazart et al., 1997) or in the third ventricle (present study). Both procedures presumably affect all dopaminoceptive brain sites that potentially play a role in the control of male sexual behavior. In contrast, tissue injections target a specific area and thus allow for a more precise identification of the site of action. This approach revealed here unexpected effects of the D2 antagonist, Raclopride, suggesting therefore the presence of some tissue-specificity in the actions of dopamine on behavior, potentially related to the higher relative density of D2- versus D1-like receptors (Kleitz et al., 2009). Given the central role of the mPOA in the activation of male sexual behavior and the existence in rats as well as in quail of a specific release of dopamine triggered by the interaction with a female, it appears quite likely that dopamine action in this brain area affects in a specific manner the expression of copulatory behavior. Future research should determine whether and to what extent, this local action differs from behavioral effects that were previously described based on peripheral injections.
Conclusions
In order to provide a comprehensive investigation of the central effects of dopamine on appetitive and consummatory aspects of male sexual behavior, we utilized multiple dopaminergic agonists and antagonists tested at several doses, two different central injection procedures, and the Japanese quail, an avian species that lacks an intromittent organ. This allowed us to address several concerns that were previously raised regarding the site and specificity of dopamine action on sexual behavior (Paredes & Ågmo, 2004). Overall, the ICV results support the notion that the activation of D1 receptors tends to facilitate the expression of these behaviors while the activation of D2 receptors has opposite effects. Specifically in the mPOA, as previously suggested in mammals, we also propose a stimulatory action of dopamine on male-typical sexual behavior in quail which appears to require a combined activation of D1 and D2 receptors. Finally, the regulation of male sexual behavior by centrally infused dopaminergic compounds in a species lacking an intromittent organ suggests that dopamine action on male sexual behavior does not simply reflect some sort of modulation of genital reflexes due to general arousal, rather it relates to the central control of sexual motivation. Together, these data support the claim that dopamine does indeed play a specific positive role in the control of male sexual behavior.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 NIH/MH50388) to G.F.B. and J.B. and the Belgian FRFC (2.4537.09) to J.B. H.K.K-N. is supported by NIH T32 HD007276 and C.A.C. is a F.R.S.-FNRS Research associate.
Abbreviations
- APO
apomorphine
- ASB
appetitive sexual behavior
- CCM
cloacal contact movement
- CSB
consummatory sexual behavior
- ICV
intracerebroventricular
- M
mount
- MA
mount attempt
- mPOA
medial preoptic area
- NG
neck grab
- RCSM
rhythmic cloacal sphincter movement
REFERENCES
- Absil P, Das S, Balthazart J. Effects of apomorphine on sexual behavior in male quail. Pharmacol Biochem Behav. 1994;47:77–88. doi: 10.1016/0091-3057(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E. Neuroanatomy of sexual behavior in the male Japanese quail from top to bottom. Poultry and Avian Biology Reviews. 1996;7:193–204. [Google Scholar]
- Adkins EK, Adler NT. Hormonal control of behavior in the Japanese quail. J Comp Physiol Psychol. 1972;81:27–36. doi: 10.1037/h0033315. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Neuroendocrine Mechanisms Regulating Reproductive Cycles and Reproductive Behavior in Birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrback SE, Rubin RT, editors. Hormones, Brain, Behavior. San Diego, California: Academic Press; 2002. pp. 649–798. [Google Scholar]
- Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Ball GF, Casto JM, Balthazart J. Autoradiographic localization of D1-like dopamine receptors in the forebrain of male and female Japanese quail and their relationship with immunoreactive tyrosine hydroxylase. J Chem Neuroanat. 1995;9:121–133. doi: 10.1016/0891-0618(95)00075-i. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. The Japanese quail as a model system for the investigation of steroid-catecholamine interactions mediating appetitive and consummatory aspects of male sexual behavior. Annu Rev Sex Res. 1998;9:96–176. [PubMed] [Google Scholar]
- Balthazart J, Castagna C, Ball GF. Differential effects of D1 and D2 dopamine-receptor agonists and antagonists on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Physiol Behav. 1997;62:571–580. doi: 10.1016/s0031-9384(97)00163-7. [DOI] [PubMed] [Google Scholar]
- Baylé JD, Ramade F, Oliver J. Stereotaxic topography of the brain of the quail (Coturnix coturnix japonica) J Physiol (Paris) 1974;68:219–241. [PubMed] [Google Scholar]
- Blackburn JR, Pfaus JG, Phillips AG. Dopamine functions in appetitive and defensive behaviours. Prog Neurobiol. 1992;39:247–279. doi: 10.1016/0301-0082(92)90018-a. [DOI] [PubMed] [Google Scholar]
- Castagna C, Ball GF, Balthazart J. Effects of dopamine agonists on appetitive and consummatory male sexual behavior in Japanese quail. Pharmacol Biochem Behav. 1997;58:403–414. doi: 10.1016/s0091-3057(97)00243-8. [DOI] [PubMed] [Google Scholar]
- Casto JM, Ball GF. Characterization and localization of D1 dopamine receptors in the sexually dimorphic vocal control nucleus, area X, and the basal ganglia of European starlings. J Neurobiol. 1994;25:767–780. doi: 10.1002/neu.480250703. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. New York: Oxford University Press; 2003. [Google Scholar]
- Cornil CA, Ball GF. Interplay among catecholamine systems: dopamine binds to alpha2-adrenergic receptors in birds and mammals. J Comp Neurol. 2008;511:610–627. doi: 10.1002/cne.21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Balthazart J, Motte P, Massotte L, Seutin V. Dopamine activates noradrenergic receptors in the preoptic area. J Neurosci. 2002;22:9320–9330. doi: 10.1523/JNEUROSCI.22-21-09320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Dejace C, Ball GF, Balthazart J. Dopamine modulates male sexual behavior in Japanese quail in part via actions on noradrenergic receptors. Behav Brain Res. 2005;163:42–57. doi: 10.1016/j.bbr.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Delville Y, Sulon J, Balthazart J. Hormonal correlates of gonadal regression and spontaneous recovery in Japanese quail exposed to short day-lengths. Arch Int Physiol Biochim. 1985;93:123–133. doi: 10.3109/13813458509079598. [DOI] [PubMed] [Google Scholar]
- Dietl M, Palacios JM. Receptor autoradiography as a tool for the study of the phylogeny of the basal ganglia. J Recept Res. 1988;8:521–532. doi: 10.3109/10799898809049009. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav. 2005;86:356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Roth RH. Dopamine synthesis, uptake, metabolism, and receptors: relevance to gene therapy of Parkinson's disease. Exp Neurol. 1997;144:4–9. doi: 10.1006/exnr.1996.6379. [DOI] [PubMed] [Google Scholar]
- Hull EM, Bitran D, Pehek EA, Warner RK, Band LC, Holmes GM. Dopaminergic control of male sex behavior in rats : effects of an intracerebrally-infused agonist. Brain Research. 1986;370:73–81. doi: 10.1016/0006-8993(86)91106-6. [DOI] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Eaton RC, Markowski VP, Moses J, Lumley LA, Loucks JA. Opposite influence of medial preoptic D1 and D2 receptors on genital reflexes: implications for copulation. Life Sci. 1992;51:1705–1713. doi: 10.1016/0024-3205(92)90299-5. [DOI] [PubMed] [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, Moses J. Hormone-neurotransmitter interactions in the control of sexual behavior. Behav Brain Res. 1999;105:105–116. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Hull EM, Meisel RL, Sachs BD. Male Sexual Behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrback SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego, California: Academic Press; 2002. [Google Scholar]
- Hull EM, Wood RI, McKenna KE. Neurobiology of Male Sexual Behavior. In: Neill JD, editor. Knobil and Neill’s Physiology of Reproduction. Elsevier: 2006. pp. 1729–1824. [Google Scholar]
- Hutchison RE. Hormonal differentiation of sexual behavior in Japanese quail. Horm Behav. 1978;11:363–387. doi: 10.1016/0018-506x(78)90038-7. [DOI] [PubMed] [Google Scholar]
- Kleitz HK, Cornil CA, Balthazart J, Ball GF. Species differences in the relative densities of D1- and D2-like dopamine receptor subtypes in the Japanese quail and rats: An in vitro quantitative receptor autoradiography study. Brain Behav Evol. 2009;73:81–90. doi: 10.1159/000209864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitz-Nelson HK, Dominguez JM, Cornil CA, Ball GF. Is sexual motivational state linked to dopamine release in the medial preoptic area? Behav. Neurosci. 2010;124:300–304. doi: 10.1037/a0018767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubikova L, Vyboh P, Kostal L. Kinetics and pharmacology of the D1- and D2-like dopamine receptors in Japanese quail brain. Cell Mol Neurobiol. 2009;29:961–970. doi: 10.1007/s10571-009-9382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubikova L, Wada K, Jarvis ED. Dopamine receptors in a songbird brain. J Comp Neurol. 2010;518:741–769. doi: 10.1002/cne.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens N, Green TA, Akins CK, Bardo MT. Dopamine D(2)-like receptor binding in the brain of male Japanese quail (Coturnix japonica) Neurosci Lett. 2000;296:77–80. doi: 10.1016/s0304-3940(00)01651-7. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Ågmo A. Has dopamine a physiological role in the control of sexual behavior? A critical review of the evidence. Prog Neurobiol. 2004;73:179–226. doi: 10.1016/j.pneurobio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Warner RK, Bazzett TJ, Bitran D, Band LC, Eaton RC, Hull EM. Microinjection of cis-flupenthixol, a dopamine antagonist, into the medial preoptic area impairs sexual behavior of male rats. Brain Research. 1988;443:70–76. doi: 10.1016/0006-8993(88)91599-5. [DOI] [PubMed] [Google Scholar]
- Richardson RD, Boswell T. A method for third ventricular cannulation of small passerine birds. Physiol Behav. 1993;53:209–213. doi: 10.1016/0031-9384(93)90033-c. [DOI] [PubMed] [Google Scholar]
- Seiwert CM, Adkins-Regan E. The foam production system of the male Japanese quail: characterization of structure and function. Brain Behav Evol. 1998;52:61–80. doi: 10.1159/000006553. [DOI] [PubMed] [Google Scholar]
- Smeets WJAJ, Reiner AE. Phylogeny and Development of Catecholamine Systems in the CNS of Vertebrates. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Warner RK, Thompson JT, Markowski VP, Loucks JA, Bazzett TJ, Eaton RC, Hull EM. Microinjection of the dopamine antagonist cis-flupenthixol into the MPOA impairs copulation, penile reflexes and sexual motivation in male rats. Brain Research. 1991;540:177–182. doi: 10.1016/0006-8993(91)90505-p. [DOI] [PubMed] [Google Scholar]