Abstract
Purpose
To evaluate CD4+Foxp3+ regulatory T cell populations in uveitis patients and to determine if T-regulatory cell populations are associated with disease features.
Methods
Uveitis patients were evaluated for CD4+Foxp3+ T-regulatory cells by flow cytometry. Systemic and ocular diagnoses, disease activity, and the presence of cystoid macular edema were reviewed. CD4+Foxp3+ lymphocyte percentages were compared for patients with inactive versus active disease, systemic versus ocular diagnoses, and the presence or absence of cystoid macular edema. RT-PCR testing was performed on two patients with extremely low CD4+Foxp3+ cell populations to assess Foxp3 mRNA.
Results
20 patients with intermediate, posterior and panuveitis were evaluated. Mean age was 40.6 years and mean visual acuity was 20/57. CD4+Foxp3+ cell percentages were lower in patients with active uveitis compared to inactive disease (p<0.05). No differences in T-regulatory cells were observed between the other subgroups. Two patients with recalcitrant uveitis who demonstrated <1% CD4+Foxp3+ lymphocytes showed extremely low or absent Foxp3 mRNA.
Conclusion
T-regulatory cells were reduced in patients with active disease compared to inactive patients. Severe depletion of CD4+Foxp3+ T-cells and Foxp3 mRNA in two patients with severe uveitis suggests that loss of T-regulatory cells of uveitis may be a salient feature in certain patients.
Keywords: Uveitis, Foxp3, regulatory T-cell, cystoid macular edema, intermediate uveitis, posterior uveitis, panuveitis, Wegener’s granulomatosis, sarcoidosis, birdshot retinochoroidopathy
Introduction
Uveitis has been reported to contribute to up to 10% of causes of blindness in developed nations and may result in significant visual handicap in untreated patients.(1) A recent report suggested that uveitis occurs with an incidence of 52/100,000 person-years with a prevalence of 115/100,000 persons.(2) Because of the significant impact of uveitis on visual disability and blindness, investigation of the basic mechanisms underlying ocular inflammation is warranted.
Evaluation of peripheral blood and aqueous fluid from patients and studies in experimental autoimmune uveitis (EAU) have supported the role of CD4+ T-helper type 1-mediated processes in the perpetuation of intraocular inflammation.(3–6) The roles of CD8+ cells, B-cells, and natural killer cells are thought to be important, but are less clearly defined.
The role of regulatory T-cells in modulating autoreactive T-cells in uveitis and other autoimmune diseases including rheumatoid arthritis(7, 8), systemic lupus erythematosus(8), sarcoidosis(9–11), and multiple sclerosis(12, 13) has recently gained attention. Several studies have demonstrated alterations in immunoregulatory T-cell populations in patients with uveitis.(14, 15) In EAU, the adoptive transfer of T-regulatory cells has been shown to confer protection against intraocular inflammation. (16, 17)
Several regulatory T-cell populations have been identified and are defined by cell surface markers, mechanism of action, and tissue of origin. The characterization of the transcription factor forkhead box P3 (Foxp3) as a key regulator of CD4+CD25+ T-cells has been valuable in identifying a subset of T-regulatory cells.(18, 19) CD4+CD25+Foxp3+ T-cells develop in the thymus and are termed “natural” T-regulatory cells (nTregs). This cell population is distinguished from several other classes of T-regulatory cells, which develop in peripheral lymphoid organs. These cell populations, which originate in peripheral lymphoid tissues, may be Foxp3-negative and have been termed “induced” T-regulatory cells (iTregs) because they can be generated by specific modes of antigen presentation (e.g. dendritic cells treated with the immunoregulatory cytokines interleukin-10 and transforming growth factor-β).(11)
We wished to assess whether any relationships existed between CD4+Foxp3+ T-regulatory cell populations and clinical disease characteristics in a group of uveitis patients. We evaluated the percentages of CD4+Foxp3+ lymphocytes using a 3-color intracellular flow cytometry protocol in a group of patients from a tertiary uveitis referral center. We then examined whether differences existed between CD4+Foxp3+ levels in various subgroups of uveitis patients (systemic versus isolated ophthalmic diagnoses, presence or absence of cystoid macular edema, active or inactive uveitis).
In this series of patients, we observed two patients with extremely low intracellular CD4+Foxp3+ expression who demonstrated severe, active uveitis and were recalcitrant to a number of immunosuppressive therapies. Evaluation of Foxp3 mRNA confirmed extremely low or undetectable levels of Foxp3 transcript in peripheral blood lymphocytes in these two patients, and their clinical features are summarized herein.
Methods
Patients were evaluated at the National Eye Institute at the Clinical Center, National Institutes of Health under an Institutional Review Board-approved protocol. Informed consent was obtained from all patients for ophthalmic examination and peripheral blood draws for research purposes. All research conformed to the ARVO statement on human and animal research and the Declaration of Helsinki.
Baseline ophthalmic examination and data collection
Medical record data gathered from each patient evaluated included demographic information (age, race, gender), diagnosis classified by anatomic location according to the Standardization of Uveitis Nomenclature (SUN) criteria,(20) laterality of disease, systemic versus isolated ocular inflammatory disease, visual acuity (i.e. best-corrected Snellen visual acuity or corrected Snellen visual acuity with an ETDRS chart at 4 meters), the presence or absence of disease activity, the presence or absence of cystoid macular edema by optical coherence tomography (OCT) and/or fluorescein angiography (FA), and immunosuppressive medications and dosages. The SUN classification was used to determine the degree of anterior chamber activity (i.e. anterior chamber grade 0), whereas vitreous inflammatory activity was evaluated according by the degree of vitreous haze.(21) In patients with multifocal choroiditis/panuveitis, activity was assessed by clinical examination and/or interpretation of fluorescein angiography by the treating physician.
Lymphocyte immunophenotyping and intracellular staining for Foxp3
Patients evaluated in this study underwent peripheral blood isolation for lymphocyte immunophenotyping and intracellular staining for Foxp3+ cells. Lymphocyte phenotyping was performed using previously described methods.(22) Briefly, peripheral blood mononuclear cells (PBMCs) were purified by Ficoll gradient centrifugation from 10 mL of peripheral blood drawn from patients and normal controls. Normal controls consisted of healthy volunteers aged 17 years and older. The majority of donors ranged between 30 and 55 years of age (Information obtained from National Institutes of Health Blood Bank).
Intracellular staining for Foxp3 staining and surface staining for phenotyping were performed using fixation and permeabilization buffers provided by a Foxp3 phenotyping kit according to the manufacturer’s instructions (eBioscience, San Diego, CA). Alexa Fluor labeled anti-human FoxP3 (clone PCH 1010) was obtained from eBioscience (San Diego, CA). Patients and normal controls were paired for the same day Foxp3 staining to minimize staining variations. Either staining with an isotype-matched Ig control or non-staining control was used to define negative staining and to establish a gate for Foxp3 staining. The compensation procedure was done following a standard flow cytometry multi-color staining protocol.
The following antibodies for cell surface staining were utilized - PE-Cy7 labeled CD3 (clone SK7), APC labeled anti-human CD8 (clone RPA-T8), and PE-labeled anti-human CD4 (clone RPA T4). All of these were obtained from BD Pharmingen (San Diego, CA).
Flow cytometry data were analyzed by FlowJo software (Treestar, San Jose, CA, USA). Briefly, lymphocytes were gated based on cell optic characteristics (FSC vs. SSC). CD3+, CD4+, CD3-, CD4-, CD8+, and CD8- as well as FoxP3+ and FoxP3- populations were gated based on antibody staining. A subpopulation was derived from its parent population. The gate for the positive staining of FoxP3 was set based on a negative control (above). The percentage of FoxP3+ cells in a defined subpopulation was calculated as the total number of FoxP3 positive cells in the subpopulation divided by the number of cells of the parent cell population.
Real-time PCR analysis for FoxP3
Two patients (Patients #1 and #2) with low CD4+Foxp3+ levels were assessed for Foxp3 mRNA levels with real-time PCR analysis using SYBR-Green based quantitative PCR technique (SuperArray Inc., Gaithursburg, MD). Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood as previously described.(23) Total RNA samples were purified from PBMCs using RNeasy kit (Qiagen, CA). For real-time PCR analysis, 1 μg of each of the RNA samples from the uveitis patients or normal controls were reverse transcribed into cDNAs using a first strand cDNA RT kit (SuperArray, MD). Real-time PCR was performed using an ABI 7500 real-time PCR unit (ABI, CA). Assay controls included a no template control and beta-actin house-keeping gene control for normalization purposes. Primers for FoxP3 and beta-actin genes were purchased from SA Biosciences (Gaithersburg, MD) and both primers had been pre-tested and confirmed by the manufacturer. Analysis of real-time PCR results strictly followed the manufacturer’s instructions (SuperArray Inc., MD). Data analysis is based on the ΔΔCt method with normalization of the raw data to housekeeping genes as described in the manufacturer’s manual.
Statistical analysis
The main outcome measure in this study was the percentage of CD4+Foxp3+ lymphocytes relative to the total number of lymphocytes for each patient. This percentage was used as a continuous variable in analyses. Wilcoxon 2-sample rank sum tests (Mann-Whitney) were performed to determine whether statistical differences existed between the following subgroups of uveitis patients: active versus inactive disease, systemic autoimmune disease versus isolated ocular diagnosis, presence versus absence of cystoid macular edema. Due to the small sample size, the t-approximation of the Wilcoxon two-sample test statistic was used and p-values < 0.05 were considered statistically significant. Linear regression was used to determine whether visual acuity was associated with the percentage of CD4+Foxp3+ regulatory T-cells.
Results
A total of 20 patients were enrolled; 15 (75%) were female and the mean age was 40.6 years (range 15-61 years). Mean visual acuity of patients evaluated was 20/57. Demographic characteristics and anatomic sites of ocular inflammation are summarized in Table 1. Anatomic diagnoses included intermediate uveitis (9 patients, 45%), posterior uveitis (4 patients, 20%), and panuveitis (7 patients, 35%). The majority of patients (80%) were on immunomodulatory therapy at the time of their evaluation and nearly all patients (90%) demonstrated bilateral disease. The clinical characteristics and CD4+Foxp3+ T-regulatory cell populations of patients evaluated are summarized in Table 2.
Table 1.
Baseline characteristics and anatomic diagnoses of patients
| Total patients enrolled | 20 |
| Male | 5 (25%) |
| Female | 15 (75%) |
| Mean age | 40.6 years (Range 15–61) |
| Anatomic location of disease | |
| Intermediate | 9 (45%) |
| Posterior uveitis | 4 (20%) |
| Panuveitis | 7 (35%)* |
| IMT | |
| Number of patients on IMT | 16 (80%) |
| Number of patients off IMT | 4 (20%) |
| Laterality of disease | |
| Bilateral | 18 (90%) |
| Unilateral | 2 (10%) |
| Mean visual acuity of enrolled patients | |
| All eyes | 20/57 |
| Better eyes | 20/41 |
| Worse eyes | 20/80 |
IMT Immunomodulatory therapy
One patient with panuveitis (Patient 1) also had active scleritis and a choroidal inflammatory lesion at the time of evaluation
Table 2.
Clinical characteristics and CD4+Foxp3+ lymphocytes of uveitis patients
| Patient No |
Age | Gen der |
Diagnosis | Laterality | VA OD |
VA OS |
Systemic vs. ocular |
Active vs. inactive |
CME | CD4+Foxp3+ lymphocytes (cells/uL) |
%CD4+Foxp3 + lymphocytes |
Medications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | M | Scleritis, panuveitis, Wegener's granulomatosis | U/L | 20/160 | 20/20 | Systemic | Active | Y | 93 | 0.95% | CSA 125 mg bid, INFLIX 8 mg/kg, Pred 40 mg/day |
| 2 | 22 | F | Panuveitis, sarcoidosis | B/L | 20/40 | 20/100 | Systemic | Active | Y | 75 | 0.56% | Pred 20 mg/day, MTX 20 mg/wk |
| 3 | 20 | F | Idiopathic granulomatous panuveitis | B/L | 20/500 | 20/640 | Ocular | Active | Y | 110 | 5.20% | MMF 1000 bid, Pred 20 mg/day, Dac 2 mg/kg/mo |
| 4 | 48 | F | Panuveitis, sarcoidosis | B/L | 20/63 | 20/50 | Systemic | Active | N | 178 | 3.66% | MMF 1000 bid, MTX 15 mg/wk |
| 5 | 43 | F | Intermediate uveitis | U/L | 20/32 | 20/12. 5 | Ocular | Active OD | Y | 308 | 5.96% | MTX 22.5 mg/wk |
| 6 | 58 | F | Intermediate uveitis | B/L | 20/125 | 20/125 | Ocular | Active OD | N | 419 | 3.39% | None |
| 7 | 22 | F | Intermediate uveitis, non- specific CTD, Pulmonary fibrosis, pericardial effusion | B/L | 20/40 | 20/125 | Systemic | Active | Y | 820 | 7.62% | Pred 20 mg/day |
| 8 | 61 | F | Intermediate uveitis, MS- associated | B/L | 20/200 | 20/800 | Systemic | Active | Y | 526 | 4.90% | Pred 17.5 mg/day, Dac 1 mg/kg/mo |
| 9 | 35 | F | Multifocal choroiditis/Punctate inner choroidopathy | B/L | 20/12.5 | 20/80 | Ocular | Inactive | N | 487 | 9.52% | SIR 2 MG qod |
| 10 | 31 | F | Multifocal choroiditis/Punctate inner choroidopathy | B/L | 20/20 | 20/20 | Ocular | Inactive | N | 645 | 5.40% | None |
| 11 | 45 | M | Multifocal choroiditis | B/L | 20/63 | 20/50 | Ocular | Inactive | N | 790 | 15.10% | None |
| 12 | 49 | F | Panuveitis, sarcoidosis | B/L | 20/100 | 20/20 | Systemic | Inactive | N | 147 | 4.33% | Dac 1 mg/kg |
| 13 | 51 | M | Panuveitis, Vogt-Koyanagi- Harada's disease | B/L | 20/25 | 20/40 | Systemic | Inactive | N | 120 | 2.93% | MMF 1000 bid, Pred 11 mg/day, Dac 2 mg/kg/mo |
| 14 | 46 | F | Intermediate uveitis, sarcoidosis | B/L | 20/20 | 20/20 | Systemic | Inactive | N | 471 | 6.19% | Prednisone 5 mg/day |
| 15 | 26 | M | Panuveitis with vasculitis, Behcet's disease | B/L | 20/400 | 20/320 | Systemic | Inactive | Y | 162 | 6.80% | MMF 750 bid, CSA 200 bid, Pred 16 mg/day |
| 16 | 46 | F | Intermediate uveitis, sarcoidosis | B/L | 20/20 | 20/20 | Systemic | Inactive | N | 471 | 6.19% | Pred 5 mg/day |
| 17 | 56 | F | Birdshot retinochoroidopathy | B/L | 20/32 | 20/20 | Ocular | Inactive | Y | 568 | 5.62% | MMF 1000 bid, CSA 100 bid, Pred 15 mg/day, Dac 2 mg/kg/mo |
| 18 | 52 | F | Intermediate uveitis | B/L | 20/100 | 20/63 | Ocular | Inactive | N | 512 | 6.82% | None |
| 19 | 52 | F | Intermediate uveitis | B/L | 20/32 | 20/20 | Ocular | Inactive | N | 300 | 18.00% | MTX 7.5 mg/wk |
| 20 | 15 | M | Intermediate uveitis | B/L | 20/60 | 20/40 | Ocular | Inactive | N | 182 | 5.40% | CSA 225 bid |
Abbreviations: CSA Cyclosporin, MMF Mycophenolate mofetil, MTX Methotrexate, SIR Sirolimus, INFLIX Infliximab, Pred Prednisone, Dac Daclizumab, CTD Connective tissue disease, MS Multiple sclerosis
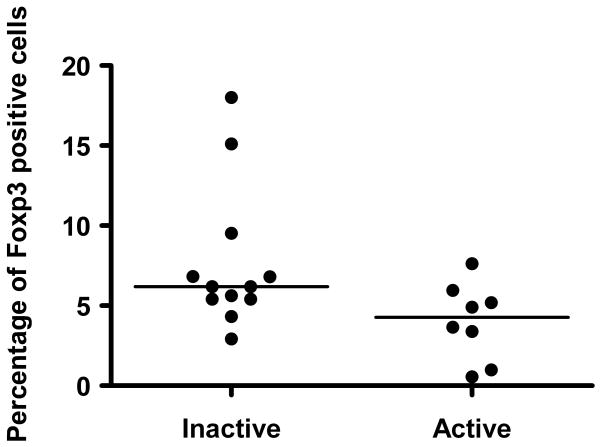
The percentage of CD4+Foxp3+ lymphocytes in patients with active uveitis (median 4.3%; range, 0.56%–7.62%) was lower than in patients with inactive disease (p = 0.047, Median 6.2%; range, 2.93–18%, Table 3 and Figure 1). No statistically significant differences in percentage of CD4+Foxp3+ lymphocytes were observed between patients with known systemic autoimmune diseases versus isolated ocular diagnoses (medians, 4.6% and 5.8%; p=0.12) or when comparing patients with cystoid macular edema versus no evidence of cystoid macular edema (medians, 5.8% and 5.4%; p=0.43).
Table 3.
Percentage of CD4+Foxp3 cells from total lymphocytes
| Clinical variable assessed (No. of patients) | Median percentage of CD4+Foxp3+ cells | p-value** |
|---|---|---|
| Disease activity* | ||
| Active (n=8) | 4.3 | 0.047** |
| Inactive (n=12) | 6.2 | |
| Systemic diagnoses vs. isolated uveitis | ||
| Systemic (n=10) | 4.6 | 0.12 |
| Ocular (n=10) | 5.8 | |
| Cystoid macular edema | ||
| Present (n=8) | 5.8 | 0.43 |
| Absent (n=12) | 5.4 | |
Presence of disease activity as defined by the SUN criteria(20),
Wilcoxon rank-sum test
Figure 1.
Comparison of median CD4+Foxp3+ percentages demonstrated a lower median of CD4+Foxp3+ T-regulatory cells in active versus inactive uveitis (p < 0.05).
No linear association between the percentage of CD4+Foxp3+ lymphocytes and logMAR visual acuity of the worse eye was observed. For each unit change in logMAR visual acuity (i.e. a decrease in Snellen visual acuity from 20/20 to 20/200), the percentage of CD4+Foxp3+ lymphocytes decreased on average by 0.018 (p =0.37).
Of patients who were assessed for CD4+Foxp3+ staining, two patients were observed to have extremely slow levels of CD4+Foxp3+ expression (Patient 1, 0.95%; patient 2, 0.56% CD4+Foxp3+ regulatory T cells). Foxp3 mRNA expression was performed to determine if this phenomena could be verified at the transcript level as well. Expression levels of Foxp3 mRNA transcript in both patients were either undetectable or exceedingly low by RT-PCR and this was repeated on two occasions per patient. In patient 1, Foxp3 expression was 13-fold lower (ΔΔCt 3.76) when compared to a normal control patient (standardized with house-keeping gene beta-actin) and in patient 2, Foxp3 expression was undetectable. Both patients displayed a panuveitis and required multiple immunosuppressive agents. However, their clinical phenotypes differed in several respects, and are herein described in detail.
Patient 1
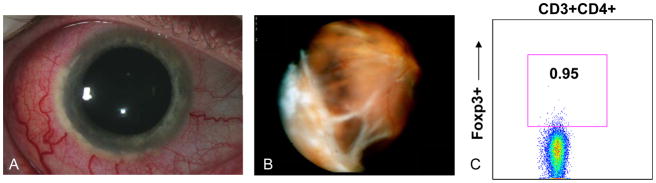
Patient 1 is a 35 year-old Caucasian male with a history of Wegener’s granulomatosis-associated scleritis, panuveitis, cystoid macular edema, and a choroidal inflammatory lesion involving the right eye (Figure 2). He had a history of episcleritis in the left eye, but this eye had been quiescent for 2–3 years. His medical history was notable for glomerulonephritis and chronic sinusitis. Immunosuppressive medications at the time of his evaluation for CD4+Foxp3+ lymphocyte populations included cyclosporine 125 mg bid (~2.5 mg/kg), infliximab 8 mg/kg/month and prednisone 40 mg/day. Prior immunosuppressive therapy had included Etanercept (Enbrel) and methotrexate 20 mg/week. Ophthalmic examination revealed visual acuity of 20/160 OD and 20/20 OS. Slit lamp examination was significant for diffuse 2–3+ scleral inflammation, peripheral keratitis, 1–2+ anterior chamber cell and flare, 1+ vitritis, and a large, elevated peripheral choroidal inflammatory lesion extending approximately 4 clock hours with a surrounding exudative retinal detachment (Figure 2A-B). Cystoid macular edema was also present.
Figure 2.
Slit lamp photograph shows severe diffuse scleritis and peripheral corneal limbal infiltrates (A). Wide angle view of fundus shows large, inferotemporal choroidal mass lesion (B). CD4+Foxp3+ staining shows 0.95% of total lymphocytes are CD4+Foxp3+ (C).
Flow cytometry showed an extremely low level of CD4+Foxp3+ lymphocytes of 93 cells/μL, and the percentage of CD4+Foxp3+ lymphocytes was 0.95% (Figure 2C). RT-PCR for Foxp3 mRNA transcript revealed a 13-fold decrease in Foxp3 mRNA expression compared to a normal control patient (Supplemental Figure 1).
The patient was subsequently treated with pulse intravenous solumedrol (1 gram IV × 3 days) and cyclophosphamide (2 mg/kg/day) was initiated; despite these measures, the diffuse, severe ocular inflammatory activity persisted and the patient’s visual acuity continued to decline. A scleral perforation and a total retinal detachment eventually developed and an enucleation procedure was required for a blind, painful eye. Pathologic evaluation revealed an occlusive retinal vasculitis with granulomatous infiltration of the ciliary body, choroid, and sclera (G. Levy-Clarke et al, manuscript in preparation).
Patient 2
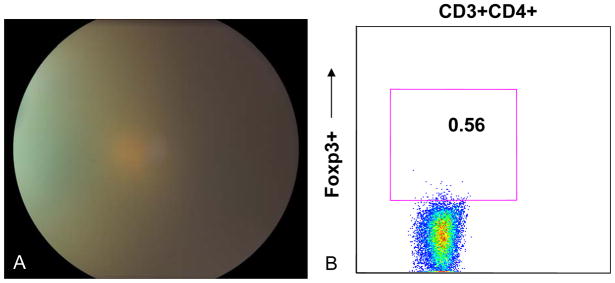
Patient 2 is a 25 year-old African American female who was referred for sarcoidosis-associated panuveitis. She was treated with topical prednisolone acetate 1% initially, but was subsequently started on prednisone 80 mg/day. Methotrexate was subsequently initiated at a dose of 15 mg/week, as the patient was unable to taper her prednisone. She had been maintained on prednisone 20 mg/day with recurrent vitritis and required intravitreal triamcinolone injections (40 mg) twice in the right eye and a periocular triamcinolone injection (40 mg) for the left eye. While on prednisone therapy, the patient reported a 100 pound weight gain. Visual acuity at the time of evaluation was 20/40 in the right eye and 20/100 in the left eye. Ophthalmic exam showed 2+ anterior chamber cell and flare in the right eye and 1–2+ cell and flare in the left eye. Numerous peripheral anterior synechiae were observed in both eyes. Dilated funduscopic exam showed 2+ vitritis and 2–3+ haze bilaterally with cystoid macular edema (Figure 3A).
Figure 3.
Fundus photograph showed severe vitritis and haze OS with obscuration of optic nerve and retinal vessels (A). CD4+Foxp3+ staining shows that 0.56% of total lymphocytes are CD4+Foxp3+ (B).
Flow cytometry showed an extremely low level of CD4+Foxp3+ lymphocytes at 75 cells/μL, which constituted 0.56% of total lymphocytes (Figure 3B). RT-PCR for Foxp3 transcription revealed undetectable Foxp3 mRNA expression (Supplemental Figure 1).
Cyclosporine therapy was recommended at a dose of 125 mg bid (2–3 mg/kg) with a subsequent decrease in anterior chamber and vitritis bilaterally at 3 months follow-up.
Discussion
In this study, we evaluated the CD4+Foxp3+ lymphocyte population in a group of uveitis patients with intermediate, posterior, and panuveitis and found that patients with active uveitis demonstrated a lower percentage of CD4+Foxp3+ lymphocytes when compared to patients with inactive disease. We were unable to identify any differences between the percentage of CD4+Foxp3+ lymphocytes in uveitis subgroups with systemic autoimmune disease versus isolated ophthalmic diagnoses or by the presence versus absence of cystoid macular edema. We have previously observed that the percentages of CD4+CD25+Foxp3+ lymphocytes do not appear to differ between uveitis patients and normal control subjects (unpublished data); however we did not evaluate whether differences existed between uveitis patients with active and inactive disease in our prior studies.
The role of T-regulatory cells in the control of inflammatory disease has generated considerable interest recently in uveitis studies, as well as in reports describing their role in systemic autoimmune conditions.(11, 24) Several reports have investigated the potential role of regulatory T-cells in Vogt-Koyanagi-Harada syndrome. Chen et al found that CD4+CD25high regulatory T-cells were reduced in frequency and showed diminished T-cell suppressive function in patients with Vogt-Koyanagi-Harada syndrome.(14) However, Commodaro et al recently reported no difference in percentages of CD4+Foxp3+ and CD4+CD25+ T-cells in VKH patients when compared to healthy controls.(25) Interestingly, Hamzaoui et al reported an increase in CD4+CD25high regulatory T-cells in patients with active Behcet’s disease when compared to patients with inactive Behcet’s disease and normal control subjects.(15)
In animal models of experimental autoimmune uveoretinitis, the adoptive transfer of CD4+CD25high regulatory T-cells expressing Foxp3 appears to confer protection from uveitis induced by the uveitogenic retinal antigen interphotoreceptor binding protein (IRBP). (16, 17, 26)However, CD8+ T-cells may also be stimulated by IRBP and transforming growth factor-β1 to express Foxp3 in high levels and may have functionally suppressive activity. (27) These studies suggest that both in rodent models and in patients, there are likely a number of cellular regulatory mechanisms that may be involved in the prevention of ocular autoimmunity.
Foxp3 currently is the most specific nuclear transcription factor for T-regulatory cells and has been found to be enriched in CD4+CD25hi T-cells. However, Foxp3 expression has also been observed in CD25−, CD25intermediate, and some CD8+ T-cell subsets.(28) Because the CD4+CD25hi population does not necessarily capture all Foxp3 cells (i.e. CD25-Foxp3+, CD25intermediateFoxp3+) cells, we chose to define CD4+Foxp3+ as T-regulatory cells in this study.
Several patients in our series demonstrated evidence of systemic autoimmune diseases including sarcoidosis, multiple sclerosis, Wegener’s granulomatosis, Behcet’s disease, Vogt-Koyanagi-Harada syndrome, and mixed connective tissue disease. Each of these conditions has been associated with abnormal populations of T-regulatory cells or deficits in T-regulatory suppressive function(8, 11, 12, 14, 15, 29); however we were unable to detect a difference in CD4+Foxp3+ regulatory T cell populations between patients with known systemic autoimmune diseases and isolated ocular diagnoses. However, the numbers of patients with each specific systemic autoimmune diagnosis were limited and their heterogeneous nature may have contributed to this lack of a difference. Furthermore, our sample size was small so our power to detect a difference was limited.
Patient 1, who was previously diagnosed with Wegener’s granulomatosis demonstrated a particularly severe disease phenotype, which featured scleritis, panuveitis, an inflammatory choroidal lesion, chronic sinusitis and glomerulonephritis requiring multiple immunosuppressive medications. Prior studies have shown that functionally defective CD4+CD25highFoxp3+ regulatory T cells are expanded in the peripheral blood of patients with Wegener’s granulomatosis. (30) However, it is possible that depletion of regulatory T cells as well as functionally defective regulatory T cells could be involved in conferring the severe disease phenotypes observed in Wegener’s granulomatosis.
Cystoid macular edema, which is associated with an elevation of pro-inflammatory cytokines(31) and vascular endothelial growth factor (32), was also evaluated in our group of uveitis patients. Our understanding of the pathogenic mechanisms underlying CME remains incomplete at this time; however, ischemic, inflammatory, and vascular factors likely play key roles. While it is likely that T cell-mediated mechanisms are involved with the inflammatory processes underlying uveitic macular edema, the role of defective regulatory T-cell populations in mediating CME was not evident from this study.
RT-PCR evaluation of the two patients (Patients 1 and 2) with extremely low levels of CD4+Foxp3+ cells showed low Foxp3 mRNA transcript levels. This decrease in Foxp3 mRNA transcript levels may be due to increased mRNA degradation or altered gene expression from the population of cells analyzed. Prior studies have suggested that in rheumatoid arthritis and sarcoidosis, a reduction in peripheral blood regulatory T cell populations are mediated via a shift of these cells to sites of active inflammation. Studies of patients with active sarcoidosis have not concurred on the nature of the change in CD4+CD25brightFoxp3+ cell populations in the peripheral blood and at local sites of inflammation (i.e. bronchoalveolar lavage fluid)(9, 10). However, regulatory T cells were found to accumulate in the periphery of sarcoid granulomas in one report.(10) In rheumatoid arthritis, CD4+CD25+ regulatory T-cells appear to be enriched in the synovial fluid at sites of active inflammation and were decreased in the peripheral blood compared to normal controls.(7, 33) Jiao et al recently observed that CD4+CD25+Foxp3+ cells in the synovial fluid of patients with rheumatoid arthritis expressed high levels of inflammatory chemokine receptors, which could participate in regulatory T cell recruitment to regions of active inflammation. Although levels of regulatory T cells in ocular tissue have not been evaluated, mechanisms of ocular inflammation similar to those found in rheumatoid arthritis could result in regulatory T cell trafficking to sites of local inflammation and a subsequent decrease in peripheral blood regulatory T cells. Our finding of decreased Foxp3 mRNA levels in patients 1 and 2 suggests that other alternative hypotheses (i.e. mRNA degradation, altered gene expression) for decreased CD4+Foxp3+ populations of T-regulatory cells may warrant further study.
Limitations of this study include its small sample size, the heterogeneous nature of disease entities evaluated, lack of age-matched controls, and the retrospective nature of the medical record review. However, despite these limitations, the differences observed in CD4+Foxp3+ lymphocyte percentages between patients with active and inactive disease suggests that a deficiency in regulatory T-cell populations may play a role in the pathogenesis of ocular autoimmunity. Decreased CD4+Foxp3+ lymphocyte populations were not universally demonstrated in this group of patients and while low levels of T-regulatory CD4+Foxp3+ cells may be salient features of some uveitic entities, this phenomena may not be generalizable to all uveitis patients. Whether Foxp3+ regulatory T-cell populations are depleted or functionally defective (i.e., lacking suppressive T-cell function) in specific types of uveitis requires further study and may provide further insight into the pathogenesis of ocular inflammatory disease.
Supplementary Material
Amplification plots of RT-PCR assay for Foxp3 mRNA expression. House-keeping gene expression of beta-actin mRNA shows normal amplification curves (yellow, pink and magenta lines). Normal donor control Foxp3 mRNA curve (cyan). Patient 1 shows a 13-fold decrease in Foxp3 mRNA expression compared to normal donor using ΔΔCt calculation method (dark blue). Patient 2 shows undetectable Foxp3 mRNA expression (asterisk, orange flat line).
Acknowledgments
This research is supported by the Intramural Research Program of the National Eye Institute, National Institutes of Health. Dr. Yeh has received support from the Heed Ophthalmic Foundation. Dr. Yeh and the principal investigator Dr. Nussenblatt are independent of any commercial funding sources and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–308. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- 2.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. discussion 500. [DOI] [PubMed] [Google Scholar]
- 3.Imai Y, Sugita M, Nakamura S, Toriyama S, Ohno S. Cytokine production and helper T cell subsets in Vogt-Koyanagi-Harada's disease. Curr Eye Res. 2001;22:312–318. doi: 10.1076/ceyr.22.4.312.5510. [DOI] [PubMed] [Google Scholar]
- 4.Caspi RR, Roberge FG, McAllister CG, et al. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986;136:928–933. [PubMed] [Google Scholar]
- 5.Takase H, Sugita S, Taguchi C, Imai Y, Mochizuki M. Capacity of ocular infiltrating T helper type 1 cells of patients with non-infectious uveitis to produce chemokines. Br J Ophthalmol. 2006;90:765–768. doi: 10.1136/bjo.2005.087353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takase H, Yu CR, Ham DI, et al. Inflammatory processes triggered by TCR engagement or by local cytokine expression: differences in profiles of gene expression and infiltrating cell populations. J Leukoc Biol. 2006;80:538–545. doi: 10.1189/jlb.1205719. [DOI] [PubMed] [Google Scholar]
- 7.Jiao Z, Wang W, Jia R, et al. Accumulation of FoxP3-expressing CD4+CD25+ T cells with distinct chemokine receptors in synovial fluid of patients with active rheumatoid arthritis. Scand J Rheumatol. 2007;36:428–433. doi: 10.1080/03009740701482800. [DOI] [PubMed] [Google Scholar]
- 8.Alvarado-Sanchez B, Hernandez-Castro B, Portales-Perez D, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun. 2006;27:110–118. doi: 10.1016/j.jaut.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Idali F, Wahlstrom J, Muller-Suur C, Eklund A, Grunewald J. Analysis of regulatory T cell associated forkhead box P3 expression in the lungs of patients with sarcoidosis. Clin Exp Immunol. 2008;152:127–137. doi: 10.1111/j.1365-2249.2008.03609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyara M, Amoura Z, Parizot C, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006;203:359–370. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valencia X, Lipsky PE. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol. 2007;3:619–626. doi: 10.1038/ncprheum0624. [DOI] [PubMed] [Google Scholar]
- 12.Kumar M, Putzki N, Limmroth V, et al. CD4+CD25+FoxP3+ T lymphocytes fail to suppress myelin basic protein-induced proliferation in patients with multiple sclerosis. J Neuroimmunol. 2006;180:178–184. doi: 10.1016/j.jneuroim.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Huan J, Culbertson N, Spencer L, et al. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Yang P, Zhou H, et al. Decreased frequency and diminished function of CD4+CD25high regulatory T cells are associated with active uveitis in patients with Vogt-Koyanagi-Harada syndrome. Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.08-1793. [DOI] [PubMed] [Google Scholar]
- 15.Hamzaoui K, Hamzaoui A, Houman H. CD4+CD25+ regulatory T cells in patients with Behcet's disease. Clin Exp Rheumatol. 2006;24:S71–78. [PubMed] [Google Scholar]
- 16.Silver PB, Agarwal RK, Su SB, et al. Hydrodynamic vaccination with DNA encoding an immunologically privileged retinal antigen protects from autoimmunity through induction of regulatory T cells. J Immunol. 2007;179:5146–5158. doi: 10.4049/jimmunol.179.8.5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keino H, Takeuchi M, Usui Y, et al. Supplementation of CD4+CD25+ regulatory T cells suppresses experimental autoimmune uveoretinitis. Br J Ophthalmol. 2007;91:105–110. doi: 10.1136/bjo.2006.099192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 19.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 20.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92:467–471. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Mahesh SP, Kim BJ, Buggage RR, Nussenblatt RB. Expression of glucocorticoid induced TNF receptor family related protein (GITR) on peripheral T cells from normal human donors and patients with non-infectious uveitis. J Autoimmun. 2003;21:83–92. doi: 10.1016/s0896-8411(03)00085-4. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Lim WK, Mahesh SP, Liu B, Nussenblatt RB. Cutting edge: in vivo blockade of human IL-2 receptor induces expansion of CD56(bright) regulatory NK cells in patients with active uveitis. J Immunol. 2005;174:5187–5191. doi: 10.4049/jimmunol.174.9.5187. [DOI] [PubMed] [Google Scholar]
- 24.Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 25.Commodaro A, Peron JPS, Macedo A, Arslanian C, Muccioli C, Rizzo LV, Belfort R., Jr Characterization of T regulatory cells and cytokine profile in peripheral blood of patients with Vogt-Koyanagi-Harada disease (VKH) and autoimmune uveitis. ARVO. 2008 E-abstracts 3232. [Google Scholar]
- 26.Siepmann K, Biester S, Plskova J, Muckersie E, Duncan L, Forrester JV. CD4+CD25+ T regulatory cells induced by LPS-activated bone marrow dendritic cells suppress experimental autoimmune uveoretinitis in vivo. Graefes Arch Clin Exp Ophthalmol. 2007;245:221–229. doi: 10.1007/s00417-006-0356-9. [DOI] [PubMed] [Google Scholar]
- 27.Peng Y, Shao H, Ke Y, et al. Minimally activated CD8 autoreactive T cells specific for IRBP express a high level of Foxp3 and are functionally suppressive. Invest Ophthalmol Vis Sci. 2007;48:2178–2184. doi: 10.1167/iovs.06-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costantino CM, Baecher-Allan CM, Hafler DA. Human regulatory T cells and autoimmunity. Eur J Immunol. 2008;38:921–924. doi: 10.1002/eji.200738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barath S, Sipka S, Aleksza M, et al. Regulatory T cells in peripheral blood of patients with mixed connective tissue disease. Scand J Rheumatol. 2006;35:300–304. doi: 10.1080/03009740600709790. [DOI] [PubMed] [Google Scholar]
- 30.Abdulahad WH, Stegeman CA, van der Geld YM, Doornbos-van der Meer B, Limburg PC, Kallenberg CG. Functional defect of circulating regulatory CD4+ T cells in patients with Wegener's granulomatosis in remission. Arthritis Rheum. 2007;56:2080–2091. doi: 10.1002/art.22692. [DOI] [PubMed] [Google Scholar]
- 31.Rothova A. Inflammatory cystoid macular edema. Curr Opin Ophthalmol. 2007;18:487–492. doi: 10.1097/ICU.0b013e3282f03d2e. [DOI] [PubMed] [Google Scholar]
- 32.Fine HF, Baffi J, Reed GF, Csaky KG, Nussenblatt RB. Aqueous humor and plasma vascular endothelial growth factor in uveitis-associated cystoid macular edema. Am J Ophthalmol. 2001;132:794–796. doi: 10.1016/s0002-9394(01)01103-5. [DOI] [PubMed] [Google Scholar]
- 33.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–2785. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amplification plots of RT-PCR assay for Foxp3 mRNA expression. House-keeping gene expression of beta-actin mRNA shows normal amplification curves (yellow, pink and magenta lines). Normal donor control Foxp3 mRNA curve (cyan). Patient 1 shows a 13-fold decrease in Foxp3 mRNA expression compared to normal donor using ΔΔCt calculation method (dark blue). Patient 2 shows undetectable Foxp3 mRNA expression (asterisk, orange flat line).