Abstract
Objective
Systemic corticosteroids are known to induce osteoporosis and increase the risk of fractures in adults and children. Inhaled corticosteroids have been shown to increase the risk of osteoporosis and fractures in adults at risk. However, long-term prospective studies in children to assess risks of multiple short courses of oral corticosteroids and chronic inhaled corticosteroids have not been done. Thus, we assessed the effects of multiple short courses of oral corticosteroids and long-term inhaled corticosteroids on bone mineral accretion over a period of years.
Patients and Methods
This was a cohort followup study for a median of 7 years of children with mild to moderate asthma initially randomized into the Childhood Asthma Management Program (CAMP) trial. Serial dual-energy x-ray absorptiometry (DEXA) scans of the lumbar spine for bone mineral density (BMD) were performed in all patients. Annual bone mineral accretion was calculated in 531 boys and 346 girls with asthma aged 5–12 years at baseline (84% of the initial cohort).
Results
Oral corticosteroid bursts produced a dose-dependent reduction in bone mineral accretion (0.052, 0.049, and 0.046 gm/cm2/year, p=0.0002) and an increase in risk of osteopenia (10%, 14% and 21%, p=0.02) for 0, 1–4, and 5+ courses, respectively, in males but not females. Cumulative inhaled corticosteroid use was associated with a small decrease in bone mineral accretion in males (p=0.05) but not females, but no increased risk of osteopenia.
Conclusion
Multiple oral corticosteroid bursts over a period of years can produce a dose-dependent reduction in bone mineral accretion and increased risk of osteopenia in children with asthma. Inhaled corticosteroid use has the potential for reducing bone mineral accretion in male children progressing through puberty but this risk is likely to be outweighed by the ability to reduce the amount of oral corticosteroids used in these children.
Keywords: Cohort study, bone mineral density, corticosteroids, asthma, children, osteopenia
Introduction
Oral corticosteroids (OCSs) can reduce bone mineral density (BMD) and produce osteoporosis in adults.1 Both current daily dose and cumulative dose have been shown to correlate with bone loss and increased fracture risk.2,3 Frequent short courses (bursts) of OCSs (> 2.5 courses per year) have been associated with decreased BMD in adults with asthma.4 However, cross-sectional studies in children on the effect of OCSs on BMD or fracture risk have been inconsistent.5–8 No prospective studies of the effect of multiple bursts of OCSs on bone mineral accretion in children have been published.
Studies in adults and children on the effects of inhaled corticosteroids (ICSs) on BMD and fracture risk are also conflicting. 6, 16–20 9–15 The prospective trials evaluating the effect of ICSs on BMD in children with asthma have been limited by small sample sizes and short durations.21–26.
CAMP was a long-term, randomized, placebo-controlled, prospective clinical trial comparing budesonide 200 μg bid or nedocromil 4mg bid to placebo in 1041 children with mild to moderate asthma for 4 to 6 years, followed by a 4-year post-trial follow-up study.27 Lumbar spine BMD was assessed serially by dual-energy x-ray absorptiometry (DEXA) scans to determine the safety of chronic corticosteroid use. Lumbar spine was selected because corticosteroids preferentially affect trabecular bone.1,2
We previously reported no effect of asthma severity or prior corticosteroid use on BMD in this cohort at the time of randomization into the CAMP trial.28 We also found no difference in final BMDs between treatments at the end of the CAMP treatment phase.27 However, this was an intention-to-treat analysis that would not have identified outliers with low rates of bone accretion. In addition, the protocol allowed use of prednisone “bursts” for exacerbation, the introduction of open-label treatment with beclomethasone or other ICSs as indicated for worsening symptoms over time, and weaning of study medication for well-controlled status. Open-label ICS and prednisone use were seen disproportionately in the non budesonide groups. Lastly, most of the patients may not have been at an age of maturity that was sensitive to possible intervention effects on bone mineral accretion. Following the CAMP trial the study drugs were discontinued and treatment was directed by primary care physicians with advice from CAMP physicians. Participants were followed for an additional 4.5 years in a post-trial follow-up study. The purpose of the current analysis is to further assess the potential effects of both bursts of OCSs and long-term use of ICSs on bone mineral accretion in the CAMP cohort followed prospectively.
Methods
Patient population
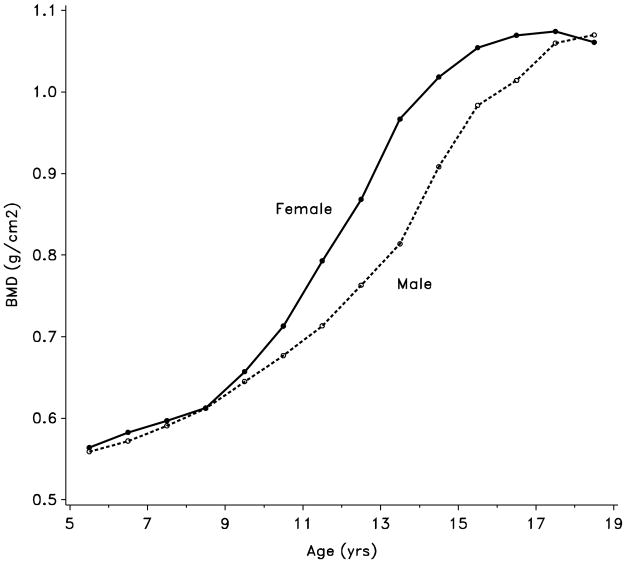
The demographics of the patients initially enrolled into CAMP have been extensively described elsewhere.27–29 All patients who were randomized into the CAMP treatment phase were eligible for the follow-up study. The follow-up study was approved by each of the local institutional review boards, and appropriate signed consent and assent forms were obtained from patients and their parents/guardians. Of the initial 1041 patients enrolled into CAMP, 941 elected to continue into the follow-up study. Unable to standardize the initial DEXA values obtained in CAMP at one center, we excluded those patients. All other patients who had a baseline BMD determination and at least one follow-up BMD (N = 877) were included. The children ranged in age from 5–12 years on entry into CAMP. Tanner Staging for sexual maturity was assessed annually until full maturity. Height by stadiometry and weight were measured every 6 months, and body mass index (BMI) was estimated by standard equations. As accrual of bone mass differs by age in males and females as a result of different ages for pubertal maturation, males and females are presented separately.30–32
Corticosteroid dosages
During the CAMP trial phase, patients received prednisone bursts per protocol with a standard regimen 2 mg/kg/day up to 60 mg prednisone for 2 days followed by 1 mg/kg/day up to 30 mg for 2 days, with an option to continue dosing if there was insufficient improvement in peak flows. Duration and dosage during the trial phase were recorded on daily diaries as well as reports of bursts by interview (date started, duration, and daily dose) at 3 per year follow-up visits (in-person) and during interim telephone contacts. During the follow-up study patients received OCS bursts of variable duration and dosage as determined by their asthma care providers, with the number of bursts since the last contact obtained by interview at semiannual follow-up visits and interim telephone contacts. Bursts during the follow-up study were assumed to conform to the CAMP trial regimen and were assumed to be equally spaced through the interval since the last contact. For analysis purposes, the total dose (mg) received during the two phases was divided by 180 to re-express the total dose received by each patient as the number of equivalent 4-day bursts (60/60/30/30 mg per day over 4 days) for a patient weighing at least 30 kg. Cumulative number of bursts was divided into three categories (0, 1–4, and 5+). The 5+ category represented a median (quartiles) of 8.9 (6.5, 12.3) bursts over the study period.
The ICSs included the blinded budesonide and unblinded ICSs during the CAMP treatment phase and then any ICS that the patients’ primary physicians prescribed during the follow-up study. Dosage form, dose and frequency were recorded at each visit and total dose for the duration was calculated. The ICS dose was divided into three categories (0, 1–437 mg, and 438+ mg). The cut point of 438 mg was somewhat arbitrary and represents 3 years of full dose (400 μg/day) budesonide. The median cumulative dose in the 1–437 mg category was 153 mg whereas patients in the 438+ category had a median dose of 690 mg. Thus, the 438+ category represents those patients receiving almost continuous ICSs through the trial and follow-up study phases whereas the 1–437 mg category represents a periodic or intermittent use.
BMD and fracture measurements
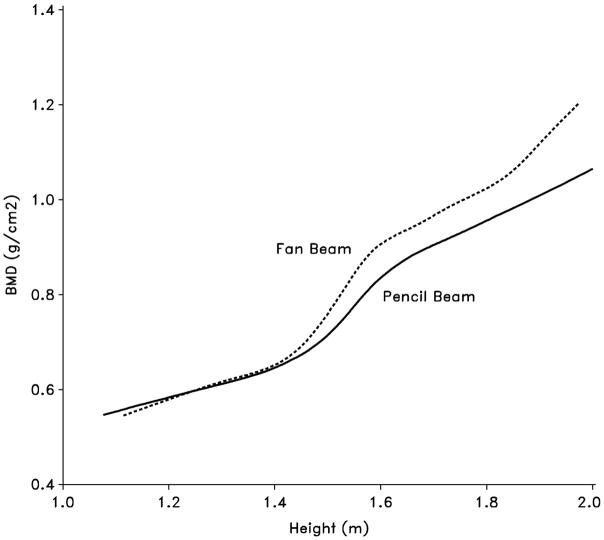
The BMD measurements were made at baseline, yearly during the treatment phase, and then twice during the follow-up study, at 7 and 9 years post randomization. Lumbar spine (L1–L4) BMD was measured by DEXA using either the Hologic QDR-1500 (six centers) or the Lunar DPX at two centers initially. However, the DEXA machines were not purchased by the study, so upgrades and changes were made at a number of the clinical centers (i.e. changing from Lunar to Hologic and vice versa, and changing within Hologic from pencil beam to fan beam). Density measures on an anthropomorphic spine Phantom (Hologic model DPA/QDR-1; S/N:1550) were obtained annually at each clinical center; these density measures were used to derive equations for converting density measurements to an Hologic fan beam equivalent. Lunar measures were converted to Hologic by the following: Hologic BMD = 0.885*Lunar BMD.33 As patients became larger the pencil beam and fan beam values deviate requiring further correction.33 This occurred at the height of 1.40 m in our patients so all values were standardized to Hologic fan beam by the following: fan beam BMD = pencil beam BMD + 0.0549 if height ≥1.40 m. The final corrected BMD measures are reported as total BMD (g/cm2). The BMD Z-scores were calculated using CAMP internal normals, mean and standard deviation of patients in the lowest to medium category for cumulative oral and inhaled corticosteroid doses (N= 398 or 45% of the population). Because children with asthma might not be considered as normals, we also analyzed our patients based upon normals provided by both Boot and colleagues34 and van der Sluis and colleagues.35 Fractures were prospectively collected on the data history forms.
Data analysis
The primary outcome was yearly bone mineral accretion (g/cm2/year) over the duration of both phases of CAMP defined as: last visit BMD – baseline BMD/years since baseline. The independent variables of interest included cumulative bursts of OCSs, cumulative ICS dose in milligrams, BMI, and active and passive smoking. Both simple and stepwise multiple linear regressions were used to assess the total and direct effects bone mineral accretion.36 Analyses were stratified by gender and predictors were tested for effect modification by gender. Multiple linear regressions were adjusted for race and BMD at baseline, OCS and ICS use, active and passive smoking, race, age, Tanner stage, machine type, beam type, BMI for age percentile and height evaluated at the last visit. The interaction between OCSs and ICSs was assessed by Chi square analysis. Assessment of risk factors for causing fracture utilized the Cox model with time to first fracture as the outcome. P-values are nominal and two-sided. All analyses were performed using SAS/STAT®software (SAS/STAT® User’s Guide, Version 8, Cary NC: SAS Institute., 1999).
Results
Patient demographics
We obtained baseline BMD and at least one follow-up BMD at a median duration of 7 years from 877 (93%) of the children from the eligible 7 centers involved in the treatment and observational phases, providing a total of 1709 female and 2708 male BMD measurements. The majority of patients (85% of males and 75% of females) were at Tanner Stage 1 at the beginning of CAMP, and the vast majority of patients progressed through the peak period of bone mineral accretion (Tanner Stages 2 and 4) during the follow-up study Table 1.
Table 1.
Characteristics of the population
| Males (n= 531) | Females (n=346) | ||
|---|---|---|---|
| Bone mineral density (g/cm2) | |||
| Baseline visit | Median (Quartiles) | 0.63 (0.57, 0.69) | 0.63 (0.58, 0.71) |
| Last followup visit | Median (Quartiles) | 0.99 (0.90, 1.09) | 1.02 (0.93, 1.13) |
| Age (yrs) | |||
| Baseline visit | Median (Quartiles) | 8.8 (7.1, 10.6) | 8.9 (7.3, 10.6) |
| Last followup visit | Median (Quartiles) | 16.6 (15.1, 17.7) | 16.6 (15.0, 17.7) |
| Race/ethnicity | % White/Other | 75.5 | 78.3 |
| % Black | 13.2 | 14.4 | |
| % Hispanic | 11.3 | 7.2 | |
| Tanner stage | |||
| Baseline visit | % Stage 1 | 71.3 | 70.5 |
| % Stage 2 | 22.6 | 17.0 | |
| % Stage 3 | 4.4 | 8.1 | |
| % Stage 4 | 1.7 | 3.5 | |
| % Stage 5 | 0.0 | 0.9 | |
| Last followup visit | % Stage 1 | 5.6 | 2.6 |
| % Stage 2 | 4.3 | 4.3 | |
| % Stage 3 | 7.3 | 10.1 | |
| % Stage 4 | 17.1 | 20.2 | |
| % Stage 5 | 65.6 | 62.8 | |
| Height (m) | |||
| Baseline visit | Median (Quartiles) | 1.33 (1.23, 1.43) | 1.32 (1.23, 1.43) |
| Last followup visit | Median (Quartiles) | 1.74 (1.68, 1.79) | 1.62 (1.58, 1.66) |
| Body mass index (kg/m2) | |||
| Baseline visit | Median (Quartiles) | 17.2 (15.8, 19.9) | 17.3 (15.7, 20.4) |
| Last followup visit | Median (Quartiles) | 22.6 (20.3, 26.1) | 22.7 (20.3, 26.6) |
| Body mass index for age (percentile) | |||
| Baseline visit | Median (Quartiles) | 70.6 (44.0, 90.2) | 70.5 (41.6, 89.7) |
| Last followup visit | Median (Quartiles) | 72.6 (46.2, 90.7) | 74.1 (51.2, 90.9) |
Factors affecting BMD
Initial regression analyses showed that age, gender, and Tanner Stage, and race had significant effects on BMD with blacks having significantly greater (p = 0.04) BMD (0.81 g/cm2) than Hispanics (0.80 g/cm2) and whites (0.79 g/cm2). Therefore all multiple regression models for bone mineral accretion were controlled for these variables, as well as BMD at baseline, DEXA machine and beam type, BMI for age percentile, height, and smoking history.
Factors affecting bone mineral accretion
There was no effect of initial CAMP treatment randomization using intent-to-treat analysis on bone mineral accretion (budesonide versus placebo, p = 0.31 for females and p = 0.84 for males; nedocromil versus placebo, p = 0.15 for females and p = 0.82 for males), or any effect of either passive or active smoking on bone mineral accretion but only 6% of patients smoked actively by the last visit Table 2. Bone mineral accretion was lowest in both boys and girls in the lowest BMI quartile (p < 0.0001)
Table 2.
Bone mineral density annual growth rate (g/cm2/yr) by corticosteroid use and smoking status stratified by gender
| Variable at last followup visit | Males | Females | Gender × Variable Interaction | ||||
|---|---|---|---|---|---|---|---|
| N | Rate*† | P | N | Rate*† | P | P | |
| Cumulative oral corticosteroids-courses | |||||||
| 0 | 108 | .052 | ref | 66 | .052 | ref | ref |
| > 0 and < 5 | 268 | .049 | 0.12 | 168 | .053 | 0.73 | 0.08 |
| 5+ | 155 | .046 | 0.0009 | 112 | .049 | 0.29 | 0.08 |
| Test for trend | 0.0002 | 0.23 | 0.11 | ||||
| Cumulative inhaled corticosteroids - mg | |||||||
| 0 | 189 | .051 | ref | 118 | .052 | ref | ref |
| 1–437 | 126 | .046 | 0.004 | 84 | .052 | 0.94 | 0.04 |
| 438+‡ | 216 | .048 | 0.03 | 144 | .051 | 0.54 | 0.58 |
| Test for trend | 0.05 | 0.45 | 0.64 | ||||
| Passive smoking | 0.91 | 0.35 | 0.75 | ||||
| No | 318 | .049 | 178 | .051 | |||
| Yes | 213 | .049 | 168 | .052 | |||
| Active smoking | 0.81 | 0.42 | 0.89 | ||||
| No | 503 | .049 | 320 | .051 | |||
| Yes | 28 | .050 | 26 | .054 | |||
Adjusted for all variables in table; race and bone mineral density at baseline; and the following variables with value at last followup visit: age, Tanner stage (genital stage for males and breast stage for females), machine type, fan beam type, body mass index for age percentile and height
438 mg is amount of full dose (400 μg/day) budesonide in CAMP trial for 3 years; Patients in 1–437 mg category had a median cumulative inhaled corticosteroid amount of 153 mg whereas patients in 438+ category had a median of 690 mg.
Effects of corticosteroids on bone mineral accretion
The effect of both OCSs and ICSs on bone mineral accretion was gender-specific. A cumulative OCS use of 5 bursts or greater resulted in a decreased bone mineral accretion in males (p = 0.0009) but not in females (p = 0.29) (Table 2). Test for trend indicated that the effect in males was dose-dependent (p = 0.0002). Males who received ICSs had a significantly decreased bone mineral accretion that did not appear to be dose-dependent. There was no effect of ICSs on bone mineral accretion in females. No significant interaction between OCS and ICS on bone mineral accretion was found (p = 0.97 for females and p = 0.23 for males).
Using the World Health Organizations definition of osteopenia (BMD between 1 and 2.5 standard deviations below age and sex adjusted means), 80 (15%) males and 77 (22%) females were classified as having osteopenia at their last BMD determination.37 Only 4 patients (2 males and 2 females) were classified as having osteoporosis (Z-score < −2.5) and so were included with the subjects with osteopenia for analysis. Similar to the effects on bone mineral accretion, only cumulative dose of OCSs in males demonstrated a dose-dependent risk for developing osteopenia (10.2%, 13.8%, and 20.6%) for 0, 1–4, and ≥5 courses respectively, p = 0.02 for trend (Table 3). Chi square analysis showed a significantly greater proportion of children classified with osteopenia were in the lowest quartile of bone mineral accretion (72% for males, p < 0.0001; 58% for females, p < 0.0001). Using the other published normal values,34, 35 the percent of patients classified as having osteopenia varied at most by 4% and the OCS dose-dependent increase in percent of male patients classified as having osteopenia was not significantly changed.
Table 3.
Prevalence of osteopenia by corticosteroid use and smoking status stratified by gender
| Variable at last followup visit | Males | Females | Gender × Variable Interaction | ||||
|---|---|---|---|---|---|---|---|
| N | % Osteo- penic† | P* | N | % Osteo- penic† | P* | P* | |
| Cumulative oral corticosteroids - courses | |||||||
| 0 | 108 | 10.2 | ref | 66 | 24.2 | ref | ref |
| > 0 and < 5 | 268 | 13.8 | 0.26 | 168 | 21.4 | 0.46 | 0.28 |
| 5+ | 155 | 20.6 | 0.02 | 112 | 22.3 | 0.31 | 0.05 |
| Test for trend | 0.02 | 0.33 | 0.05 | ||||
| Cumulative inhaled corticosteroids - mg | |||||||
| 0 | 189 | 14.3 | ref | 118 | 19.5 | ref | ref |
| 1–437 | 126 | 17.5 | 0.50 | 84 | 20.2 | 0.54 | 0.99 |
| 438+‡ | 216 | 14.4 | 0.23 | 144 | 25.7 | 0.24 | 0.33 |
| Test for trend | 0.23 | 0.23 | 0.31 | ||||
| Passive smoking | 0.90 | 0.36 | 0.70 | ||||
| No | 318 | 14.2 | 178 | 21.4 | |||
| Yes | 213 | 16.4 | 168 | 23.2 | |||
| Active smoking | 0.22 | 0.26 | 0.87 | ||||
| No | 503 | 14.9 | 320 | 21.2 | |||
| Yes | 28 | 17.9 | 26 | 34.6 | |||
Adjusted for all variables in table; race and bone mineral density at baseline; and the following variables with value at last followup visit: age, Tanner stage (genital stage for males and breast stage for females), machine type, fan beam type, body mass index for age percentile and height
Osteopenia defined as z-score less than −1 at last followup visit. Z-scores based on age and gender from longitudinal measurements of bone mineral density from patients with less than 438 mg cumulative inhaled corticosteroids and less than 5 cumulative courses of oral corticosteroids
438 mg is amount of full dose (400 μg/day) budesonide in CAMP trial for 3 years; Patients in 1–437 mg category had a median cumulative inhaled corticosteroid amount of 153 mg whereas patients in 438+ category had a median of 690 mg.
Fractures
There were only 27 fractures in the females and 40 fractures in the males out of 6,339 person-years of follow up. The overall fracture rate for boys and girls was 11 and 12 per 1000 person-years, respectively. Neither OCS nor ICS use was related to time of first fracture in males or females (adjusted p-values for trend all greater than 0.5).
Discussion
We found a significant dose-dependent effect of OCS bursts on bone mineral accretion and an associated increased risk of osteopenia in the lumbar spine of males but not females over a median time of 7 years in the CAMP children. We also found a small reduction of bone mineral accretion from ICS use in males that was not accompanied by an increased risk of osteopenia.
Our findings are consistent with a recent 4-year longitudinal study in adults with asthma that reported a significantly greater BMD loss in those patients receiving > 2.5 courses/year compared to ≤ 2.5 courses/year.4 Our study suggests a lower number of courses of OCSs (median of 8.9 over median of 7 years) can produce diminished bone mineralization in children. In contrast, Ducharme and colleagues reported no difference in mean lumbar spine BMD Z-score in 48 children with asthma exposed to a median of 4 OCS bursts (range 3–11) in the preceding year versus 35 unexposed children with asthma. However, both groups had lower than normal mean Z-scores (exposed −0.61 ± 1.0 versus unexposed −0.67 ± 0.9). Harris and colleagues reported a significant reduction in lumbar spine BMD Z-score in patients 4–12 years old receiving both high-dose ICSs and at least 1 OCS burst in the previous six months compared to patients receiving medium dose ICSs.6 Those patients receiving only high-dose ICSs did not have a decreased Z-scores. We did not find an interaction between OCSs and ICSs on bone mineral accretion.
Most cross-sectional6–7, 18–20 and even prospective longitudinal21, 23, 26, 27 studies in children with asthma report no negative effect of ICSs on BMD. Allen and colleagues compared 48 prepubertal children receiving budesonide dry-powder inhaler or beclomethasone dipropionate MDI (400–2000 μg/day) to 9 aged matched controls with asthma not receiving ICSs over 9–20 months.25 However, patients were only excluded if the number of OCS bursts exceeded 3 during the study period. Roux and colleagues assessed lumbar spine and femoral neck BMD in 174 children 6–14 years old receiving fluticasone propionate 200 μg/day by dry-powder inhaler or nedocromil sodium by MDI.26. Their patients were also allowed OCSs for asthma exacerbations, and significantly more patients in the nedocromil group required OCSs (43% versus 26%, p < 0.001 and 16% versus 3% for 3 or more courses). While they reported no difference in bone mineral accretion between treatments nor a significant effect of OCSs on treatment effect, they did report a slightly greater percentage increase (1.2%) in BMD in the fluticasone propionate group.
The relevant concern for inadequate bone mineral accretion relates to potential for fracture risk and suboptimal attainment of peak bone mass. We found no increase in risk of fracture from either OCSs or ICSs but likely had an insufficient number of fractures in our population to determine risk of this outcome. A large nested case-control study in 22,846 children 4–17 years old with fractures reported a significant increase in the risk of fractures in children taking ≥ 4 OCS bursts over a mean followup of 2.7 years (median 2.3 years).5 However, they were unable to adequately discern whether the increased risk was due to the OCSs or severity of underlying disease. Our data demonstrating an increased risk of osteopenia in children receiving ≥ 5 bursts over 7 years suggest a role for OCSs as a contributing cause. An epidemiologic study of 97,387 children 4–17 years old receiving ICSs reported an increased crude risk of fracture in those receiving greater than 200 μg/day beclomethasone equivalent, but when adjusted for measures of asthma severity the increased risk disappeared.16 A nested case-control analysis also failed to demonstrate a significant increase in fracture risk from current ICS use or long term exposure (≥ 20 prescriptions).38 Thus, the literature concerning risks of potential adverse effects of ICS on bone mineralization is reassuringly negative.
Why we saw the decreased accretion in males only is unclear. There were fewer females than males and a slightly higher percentage of females above Tanner Stage 2 upon enrollment (Table 1). More females may have reached the flat portion of their bone mineral accretion curves and therefore less susceptible to an intervention.39–42 Estrogen surge during pubertal development in females may overcome the small effect associated with the relatively low dose corticosteroids seen in the CAMP cohort as exogenous estrogen replacement therapy has been shown to be protective against corticosteroid-induced bone loss.43–44
A weakness of our study is that during the 4.5-year follow-up study therapy was controlled by the primary care physician and data was gathered over 4 visits/year (2 in clinic visits and 2 phone visits). On the other hand, this could be considered a strength of the study in that it is likely to better reflect the use of both OCSs and ICSs in the general population of children with mild to moderate asthma who are cared for by community physicians. While we did not control for asthma severity, this cohort had mild to moderate asthma upon entry into the study and initial measures of asthma severity were not associated with baseline height or BMD.28 The lack of accepted normal values for BMD in children to establish Z-scores for defining osteopenia and osteoporosis is problematic, but the fact that the number of patients we defined as osteopenic was not significantly altered by using two published external populations validates our use of internal normals.
In conclusion, our study demonstrates a significant dose-dependent reduction in lumbar spine bone mineral accretion and increased risk of osteopenia from OCS bursts in males. We found a lesser effect from ICSs in males but not females. The demonstrated ability of ICSs to significantly reduce the use of OCSs in children with persistent asthma is likely to outweigh the potential small decrement in bone mineral accretion seen with ICSs.27 Long-term ICS therapy appears to be safer than frequent OCS bursts on bone mineral accretion in children.
Figure 1.
Bone mineral density vs. height by beam type of densitometer
Figure 2.
Age-specific bone mineral density by gender
Abbreviations
- BMD
bone mineral density
- BMI
Body mass index
- CAMP
Childhood Asthma Management Program
- DEXA
dual-energy x-ray absorptiometry
- OCS
oral corticosteroid
- ICS
inhaled corticosteroid
CAMP Credit Roster
Source of funding
The Childhood Asthma Management Program is supported by contracts NO1-HR-16044, 16045, 16046, 16047, 16048, 16049, 16050, 16051, and 16052 with the National Heart, Lung, and Blood Institute and General Clinical Research Center grants M01RR00051, M01RR0099718–24, M01RR02719–14, and RR00036 from the National Center for Research Resources.
Members of the CAMP Research Group:
Clinical centers
ASTHMA, Inc, Seattle, WA
Gail G. Shapiro, MD (Director); Thomas R. DuHamel, PhD (Co-Director); Mary V. Lasley, MD (Co-Director); Tamara Chinn, MSN, ARNP (Coordinator). Michele Hinatsu, MSN, ARNP; Clifton T. Furukawa, MD; Leonard C. Altman, MD; Frank S. Virant, MD; Paul V. Williams, MD; Michael S. Kennedy, MD; Jonathan W. Becker, MD; Grace White. C. Warren Bierman, MD (1992–1997); Dan Crawford, RN (1996–2002); Heather Eliassen, BA (1996–1999); Babi Hammond (1996–1999); Dominick A. Minotti, MD (1992–2003); Chris Reagan (1992–2003); Marian Sharpe, RN (1992–1994); Timothy G. Wighton, PhD (1994–1998).
Brigham & Women’s Hospital, Boston, MA
Scott Weiss, MD, MS (Director); Anne Fuhlbrigge, MD (Principal Investigator); Anne Plunkett, NP, MS (Coordinator). Nancy Madden, RN, BSN; Peter Barrant, MD; Christine Darcy; Kelly Thompson, MD. Walter Torda, MD (Co-Investigator Director, 1993–2003); Martha Tata, RN (1993–2002); Sally Babigian, RN (1997–1999); Linda Benson (1998–2004); Jose Caicedo (1998–1999); Tatum Calder (1998–2001); Anthony DeFilippo (1994–2000); Cindy Dorsainvil (1998–2001); Julie Erickson (1998–1999); Phoebe Fulton (1997); Mary Grace, RN (1994–1996); Jennifer Gilbert (1997–1998); Dirk Greineder, MD (1993–2000); Stephanie Haynes (1993–1998); Margaret Higham, MD (1996–1998); Deborah Jakubowski (1999); Susan Kelleher (1993–1997); Jay Koslof, PhD (1993–1995); Dana Mandel (1996–1998); Patricia Martin (2001–2003); Agnes Martinez (1994–1997); Jean McAuliffe (1994–1995); Erika Nakamoto (2002–2004); Paola Pacella (1993–1998); Paula Parks (1993–1995); Johanna Sagarin (1998–1999); Kay Seligsohn, PhD (1995–2004); Susan Swords (2003–2005); Meghan Syring (1998–2001); June Traylor, MSN, RN (1996–1998); Melissa Van Horn, PhD (1996–1999); Carolyn Wells, RN (1993–1995); Ann Whitman, RN (1994–1996).
The Hospital for Sick Children, Toronto, Ontario, Canada
Ian MacLusky, MD, FRCP(C) (Director); Joe Reisman, MD, FRCP(C), MBA (Director, 1996–1999); Henry Levison, MD, FRCP(C) (Director, 1992–1996); Anita Hall, RN (Coordinator). Jennifer Chay; Melody Miki, RN, BScN; Reneé Sananes, PhD. Yola Benedet (1994–1999); Susan Carpenter, RN (1998–2001); Michelle Collinson, RN (1994–1998); Jane Finlayson-Kulchin, RN (1994–1998); Kenneth Gore, MA (1993–1999); Noreen Holmes, RRT (1998–1999); Sharon Klassen, MA(1999–2000); Joseé Quenneville, MSc (1993–1995); Christine Wasson, PhD (1999).
Johns Hopkins Asthma & Allergy Center, Baltimore, MD
N. Franklin Adkinson, Jr, MD (Director); Peyton Eggleston, MD (Co-Director); Elizabeth H. Aylward, PhD; Karen Huss, DNSc (Co-Investigator); Leslie Plotnick, MD (Co-Investigator); Margaret Pulsifer, PhD (Co-Investigator); Cynthia Rand, PhD (Co-Investigator); Nancy Bollers, RN (Coordinator). Deborah Bull, LPN; Robert Hamilton, PhD; Kimberly Hyatt; Susan Limb, MD; Mildred Pessaro; Stephanie Philips, RN; Barbara Wheeler, RN, BSN.
National Jewish Medical and Research Center, Denver, CO
Stanley Szefler, MD (Director); Harold S. Nelson, MD (Co-Director); Bruce Bender, PhD (Co-Investigator); Ronina Covar, MD (Co-Investigator); Andrew Liu, MD (Co-Investigator); Joseph Spahn, MD (Co-Investigator); D Sundström (Coordinator). Melanie Phillips; Michael P. White. Kristin Brelsford (1997–1999); Jessyca Bridges (1995–1997); Jody Ciacco (1993–1996); Michael Eltz (1994–1995); Jeryl Feeley, MA (Coordinator, 1992–1995); Michael Flynn (1995–1996); Melanie Gleason, PA-C (1992–1999); Tara Junk-Blanchard (1997–2000); Joseph Hassell (1992–1998); Marcia Hefner (1992–1994); Caroline Hendrickson, RN (1995–1998; Coordinator, 1995–1997); Daniel Hettleman, MA (1995–1996); Charles G. Irvin, PhD (1992–1998); Jeffrey Jacobs, MD (1996–1997); Alan Kamada, PharmD (1994–1997); Sai Nimmagadda, MD (1993–1996); Kendra Sandoval (1995–1997); Jessica Sheridan (1994–1995); Trella Washington (1993–1997); Eric Willcutt, MA (1996–1997). We also thank the pediatric allergy and immunology fellows for their participation (Kirstin Carel, MD; Neal Jain, MD; Harvey Leo, MD; Beth Macomber, MD; Chris Mjaanes, MD; Lora Stewart, MD; Ben Song, MD).
University of California, San Diego and Kaiser Permanente Southern California Region, San Diego, CA
Robert S. Zeiger, MD, PhD (Director); Noah Friedman, MD (Co-Investigator); Michael H. Mellon, MD (Co-Investigator); Michael Schatz, MD (Co-Investigator); Kathleen Harden, RN (Coordinator). Elaine M. Jenson; Serena Panzlau; Eva Rodriguez, RRT. James G. Easton, MD (Co-Director, 1993–1994); M. Feinberg (1997–1998); Linda L. Galbreath (1991–2002); Jennifer Gulczynski (1998–1999); Ellen Hansen (1995–1997); Al Jalowayski, PhD (Co-Investigator, 1991–2005); Alan Lincoln, PhD (Co-Investigator, 1991–2003); Jennie Kaufman (1994); Shirley King, MSW (1992–1999); Brian Lopez (1997–1998); Michaela Magiari-Ene, MA (1994–1998); Kathleen Mostafa, RN (1994–1995); Avraham Moscona (1994–1996); Catherine A. Nelle, RN (1991–2005); Jennifer Powers (2001–2003); Karen Sandoval (1995–1996); Nevin W. Wilson, MD (Co-Director, 1991–1993.
University of New Mexico, Albuquerque, NM
H. William Kelly, PharmD (Director); Aaron Jacobs (Co-Investigator); Mary Spicher, RN (Coordinator). Hengameh H. Raissy. Robert Annett, PhD (Co-Investigator, 1993–2004); Teresa Archibeque (1994–1999); Naim Bashir, MD (Co-Investigator, 1998–2005); H. Selda Bereket (1995–1998); Marisa Braun (1996–1999); Shannon Bush (2002–2006); Michael Clayton, MD (Co-Investigator, 1999–2001); Angel Colon-Semidey, MD (Co-Investigator, 1997–2000); Sara Devault (1993–1997); Roni Grad, MD (Co-Investigator, 1993–1995); David Hunt, RRT (1995–2004); Jeanne Larsson, RN (1995–1996); Sandra McClelland, RN (Coordinator, 1993–1995); Bennie McWilliams, MD (Co-Investigator, Director, 1992–1998); Elisha Montoya (1997–2000); Margaret Moreshead (1996–1999); Shirley Murphy, MD (Co-Investigator, 1992–1994); Barbara Ortega, RRT (1993–1999); David Weers (1997–1998); Jose Zayas (1995–1996).
Washington University, St. Louis, MO
Robert C. Strunk, MD (Director); Leonard Bacharier, MD (Co-Investigator); Gordon R. Bloomberg, MD (Co-Investigator); James M. Corry, MD (Co-Investigator); Denise Rodgers, RFPT (Coordinator). Lila Kertz, MSN, RN, CPNP; Valerie Morgan, RRT; Tina Oliver-Welker, CRTT; Deborah K. White, RPFT, RRT.
Resource centers
Chair’s Office, National Jewish Medical and Research Center, Denver, CO
Reuben Cherniack, MD (Study Chair).
Coordinating Center, The Johns Hopkins University, Baltimore, MD
James Tonascia, PhD (Director); Curtis Meinert, PhD (Co-Director). Patricia Belt; Karen Collins; Betty Collison; Ryan Colvin, MPH; John Dodge; Michele Donithan, MHS; Judith Harle; Rosetta Jackson; Hope Livingston; Jill Meinert; Kapreena Owens; Michael Smith; Alice Sternberg, ScM; Mark Van Natta, MHS; Margaret Wild; Laura Wilson, ScM; Robert Wise, MD; Katherine Yates, ScM.
Project Office, National Heart, Lung, and Blood Institute, Bethesda, MD
Virginia Taggart, MPH (Project Officer); Lois Eggers; James Kiley, PhD; Gang Zheng, PhD. Paul Albert, PhD (1991–1999); Suzanne Hurd, PhD (1991–1999); Sydney Parker, PhD (1991–1994); Pamela Randall (1992–2003); Margaret Wu, PhD (1991–2001).
Committees
Data and Safety Monitoring Board
Howard Eigen, MD (Chair); Michelle Cloutier, MD; John Connett, PhD; Leona Cuttler, MD; David Evans, PhD; Meyer Kattan, MD; Rogelio Menendez, MD; F. Estelle R. Simons, MD. Clarence E. Davis, PhD (1993–2003); Sanford Leikin, MD (1993–1999).
Executive Committee
Reuben Cherniack, MD (Chair);Robert Strunk, MD; Stanley Szefler, MD; Virginia Taggart, MPH; James Tonascia, PhD. Curtis Meinert, PhD (1992–2003).
Steering Committee
Reuben Cherniack, MD (Chair); Robert Strunk, MD (Vice-Chair); N. Franklin Adkinson, MD; Robert Annett, PhD (1992–1995, 1997–1999); Bruce Bender, PhD (1992–1994, 1997–1999); Mary Caesar, MHS (1994–1996); Thomas R. DuHamel, PhD (1992–1994, 1996–1999); H. William Kelly, PharmD; Henry Levison, MD (1992–1996); Alan Lincoln, PhD (1994–1995); Ian MacLusky, MD; Bennie McWilliams, MD (1992–1998); Curtis L. Meinert, PhD; Sydney Parker, PhD (1991–1994); Joe Reisman, MD, FRCP(C), MBA (1991–1999); Denise Rodgers (2003–2005); Kay Seligsohn, PhD (1996–1997); Gail G. Shapiro, MD; Marian Sharpe (1993–1994); D Sundström (1998–1999); Stanley Szefler, MD; Virginia Taggart, MPH; Martha Tata, RN (1996–1998); James Tonascia, PhD; Scott Weiss, MD, MS; Barbara Wheeler, RN, BSN (1993–1994); Robert Wise, MD; Robert Zeiger, MD, PhD.
Role of Authors
All authors participated in discussion of initial data analysis. All data analysis was performed by Mark van Natta (mvnatta@jhsph.edu). After initial data analysis and consensus of the writing group, H. William Kelly (hwkelly@salud.unm.edu) wrote initial draft that was reviewed and edited by all authors. Rebecca Green (green_b@kids.wustl.edu), an endocrinologist, then identified the issue of standardization of DEXA measures and with Mark van Natta developed our procedure for standardization of measures. Jim Tonascia (jtonasci@jhsph.edu) as director of the CAMP Coordinating Center oversaw all data analysis and assisted in writing the data analysis section of a number of the drafts of the manuscript. Drs. Strunk (Strunk@kids.wustl.edu) and Covar (CovarR@NJC.ORG) participated in all writing committee conference calls and provided significant editing and preparations of drafts 1–4 of the manuscript. Drs Kelly and Tonascia prepared the final draft of the manuscript.
Previous publication
Part of this data was presented at the American Thoracic Society International Conference, San Francisco, CA, May 23, 2007.
Footnotes
Conflicts of Interest: Dr. Kelly has participated on ad hoc scientific advisory boards for AstraZeneca, GlaxoSmithKline, Merck, Novartis, Schering, Genentech, MAP Pharmaceuticals, and Sepracor; has received honorarium for speaking from GlaxoSmithKline and AstraZeneca; and has received research funding from AstraZeneca, GlaxoSmithKline, and Sepracor. Dr. Covar has participated on an ad hoc scientific advisory board for Merck and received a research grant from AstraZeneca. Mark van Natta, Drs. Tonascia, Green, and Strunk have no conflicts to declare.
References
- 1.Saag KG. Glucocorticoid-induced osteoporosis. Endocrinol Metab Clin N Am. 2003;32:135–157. doi: 10.1016/s0889-8529(02)00064-6. [DOI] [PubMed] [Google Scholar]
- 2.Lane NE, Lukert B. The science and therapy of glucocorticoid-induced bone loss. Endocrinol Metab Clin N Am. 1998;27:465–483. doi: 10.1016/s0889-8529(05)70017-7. [DOI] [PubMed] [Google Scholar]
- 3.Walsh LJ, Lewis SA, Wong CA, et al. The impact of oral corticosteroid use on bone mineral density and vertebral fracture. Am J Respir Crit Care Med. 2002;166:691–695. doi: 10.1164/rccm.2110047. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto H, Ishihara K, Hasegawa T, Umeda B, Niimi A, Hino M. Effects of inhaled corticosteroid and short courses or oral corticosteroids on bone mineral density in asthmatic patients: a 4 year longitudinal study. Chest. 2001;120:1468–1473. doi: 10.1378/chest.120.5.1468. [DOI] [PubMed] [Google Scholar]
- 5.van Staa TP, Cooper C, Leufkens HG, Bishop N. Children and the risk of fractures caused by oral corticosteroids. J Bone Miner Res. 2003;18:913–918. doi: 10.1359/jbmr.2003.18.5.913. [DOI] [PubMed] [Google Scholar]
- 6.Harris M, Hauser S, Nguyen TV, et al. Bone mineral density in prepubertal asthmatics receiving corticosteroid treatment. J Paediatr Child Health. 2001;37:67–71. doi: 10.1046/j.1440-1754.2001.00628.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones G, Ponsonby AL, Smith BJ, Carmichael A. Asthma, inhaled corticosteroid use, and bone mass in prepubertal children. J Asthma. 2000;37:603–611. doi: 10.3109/02770900009090816. [DOI] [PubMed] [Google Scholar]
- 8.Ducharme FM, Chabot G, Polychronakos C, Glorieux F, Mazer B. Safety profile of frequent short courses of oral glucocorticoids in acute pediatric asthma: impact on bone metabolism, bone density, and adrenal function. Pediatrics. 2003;111:376–383. doi: 10.1542/peds.111.2.376. [DOI] [PubMed] [Google Scholar]
- 9.Wong CA, Walsh LJ, Smith CJP, et al. Inhaled corticosteroid use and bone-mineral density in patients with asthma. Lancet. 2000;355:1399–1403. doi: 10.1016/S0140-6736(00)02138-3. [DOI] [PubMed] [Google Scholar]
- 10.Hubbard RB, Smith CJP, Smeeth L, Harrison TW, Tattersfield AE. Inhaled corticosteroids and hip fracture: a population-based case-control study. Am J Respir Crit Care Med. 2002;166:1563–1566. doi: 10.1164/rccm.200206-606OC. [DOI] [PubMed] [Google Scholar]
- 11.Israel E, Banerjee TR, Fitzmaurice GM, Kotlov TV, LaHive K, LeBoff MS. Effects of inhaled glucocorticoids on bone density in premenopausal women. N Engl J Med. 2001;345:941–947. doi: 10.1056/NEJMoa002304. [DOI] [PubMed] [Google Scholar]
- 12.Hubbard R, Tattersfield A, Smith C, West J, Smeeth L, Fletcher A. Use of inhaled corticosteroids and the risk of fracture. Chest. 2006;130:1082–1088. doi: 10.1378/chest.130.4.1082. [DOI] [PubMed] [Google Scholar]
- 13.Hughes JA, Conry BG, Male SM, Eastell R. One year prospective open study of the effect of high dose inhaled steroids, fluticasone propionate, and budesonide on bone markers and bone mineral density. Thorax. 1999;54:223–229. doi: 10.1136/thx.54.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma PK, Malhotra S, Pandhi P, Kumar N. Effect of inhaled steroids on bone mineral density: a meta-analysis. J Clin Pharmacol. 2003;43:193–197. doi: 10.1177/0091270002239829. [DOI] [PubMed] [Google Scholar]
- 15.Kemp JP, Osur S, Shrewsbury SB, et al. Potential effects of fluticasone propionate on bone mineral density in patients with asthma: a 2 year randomized, double-blind, placebo-controlled trial. Mayo Clinic Proc. 2004;79:458–466. doi: 10.4065/79.4.458. [DOI] [PubMed] [Google Scholar]
- 16.van Staa TP, Bishop N, Leufkens HG, Cooper C. Are inhaled corticosteroids associated with an increased risk of fracture in children? Osteoporos Int. 2004;15:785–791. doi: 10.1007/s00198-004-1606-5. [DOI] [PubMed] [Google Scholar]
- 17.Boot AM, de Jongste JC, Verberne AAPH, Pols HAP, de Muinck Keizer-Schrama SMPF. Bone mineral density and bone metabolism of prepubertal children with asthma after long-term treatment with inhaled corticosteroids. Pediatr Pulmonol. 1997;24:379–384. doi: 10.1002/(sici)1099-0496(199712)24:6<379::aid-ppul1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Agertoft L, Pedersen S. Bone mineral density in children with asthma receiving long-term treatment with inhaled budesonide. Am J Respir Crit Care Med. 1998;157:178–183. doi: 10.1164/ajrccm.157.1.9707072. [DOI] [PubMed] [Google Scholar]
- 19.Bahceciler NN, Sezgin G, Nursoy MA, Barlan IB, Basaran MM. Inhaled corticosteroids and bone density of children with asthma. J Asthma. 2002;39:151–157. doi: 10.1081/jas-120002196. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths AL, Sim D, Strauss B, Rodda C, Armstrong D, Freezer N. Effect of high-dose fluticasone propionate on bone density and metabolism in children with asthma. Pediatr Pulmonol. 2004;37:116–121. doi: 10.1002/ppul.10396. [DOI] [PubMed] [Google Scholar]
- 21.Baraldi E, Bollini MC, de Marchi A, Zacchello F. Effect of beclomethasone dipropionate on bone mineral content assessed by X-ray densitometry in asthmatic children: a longitudinal evaluation. Eur Respir J. 1994;7:710–714. doi: 10.1183/09031936.94.07040710. [DOI] [PubMed] [Google Scholar]
- 22.Gregson RK, Rao R, Murrills AJ, Taylor PA, Warner JO. Effect of inhaled corticosteroids on bone mineral density in childhood asthma: comparison of fluticasone propionate with beclomethasone dipropionate. Osteoporos Int. 1998;8:418–422. doi: 10.1007/s001980050085. [DOI] [PubMed] [Google Scholar]
- 23.Martinati LC, Bertoldo F, Gasperi E, Fortunati P, Lo Cascio V, Boner A. Longitudinal evaluation of bone mass in asthmatic children treated with inhaled beclomethasone dipropionate or cromolyn sodium. Allergy. 1998;53:705–708. doi: 10.1111/j.1398-9995.1998.tb03957.x. [DOI] [PubMed] [Google Scholar]
- 24.Rao R, Gregson RK, Jones AC, Miles EA, Campbell MJ, Warner JO. Systemic effects of inhaled corticosteroids on growth and bone turnover in childhood asthma; a comparison of fluticasone with beclomethasone. Eur Respir J. 1999;13:87–94. doi: 10.1183/09031936.99.13108799. [DOI] [PubMed] [Google Scholar]
- 25.Allen HDW, Thong IG, Clifton-Bligh P, Holmes S, Nery L, Wilson KB. Effects of high-dose inhaled corticosteroids on bone metabolism in prepubertal children with asthma. Pediatr Pulmonol. 2000;29:188–193. doi: 10.1002/(sici)1099-0496(200003)29:3<188::aid-ppul6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 26.Roux C, Kolta S, Desfougeres J-L, Minini P, Bidat E. Long-term safety of fluticasone propionate and nedocromil sodium on bone in children with asthma. Pediatrics. 2003;111:e706–e713. doi: 10.1542/peds.111.6.e706. [DOI] [PubMed] [Google Scholar]
- 27.The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 28.Kelly HW, Strunk RC, Donithan M, Bloomberg GR, McWilliams BC, Szefler S Childhood Asthma Management Program Research Group. Growth and bone density in children with mild-moderate asthma: a cross-sectional study in children entering the Childhood Asthma Management Program (CAMP) J Pediatr. 2003;142:281–291. doi: 10.1067/mpd.2003.86. [DOI] [PubMed] [Google Scholar]
- 29.Zeiger RS, Dawson C, Weiss S Childhood Asthma Management Program (CAMP) Research Group. Relationships between duration of asthma and asthma severity among children in the Childhood Asthma Management Program (CAMP) J Allergy Clin Immunol. 1999;103:376–387. doi: 10.1016/s0091-6749(99)70460-4. [DOI] [PubMed] [Google Scholar]
- 30.Sabatier JP, Guaydier-Souquieres G, Benmalek A, Marcelli C. Evolution of lumbar bone mineral content during adolescence and adulthood: a longitudinal study in 395 healthy females 10–24 years of age and 206 premenopausal women. Osteoporos Int. 1999;9:476–482. doi: 10.1007/s001980050173. [DOI] [PubMed] [Google Scholar]
- 31.Whiting SJ, Vatanparast H, Baxter-Jones A, Faulkner RA, Mirwald R, Bailey DA. Factors that affect bone mineral accrual in the adolescent growth spurt. J Nutr. 2004;134:696S–700S. doi: 10.1093/jn/134.3.696S. [DOI] [PubMed] [Google Scholar]
- 32.Bonjour JP, Theintz G, Buchs B, Slosman D, Rizzoli R. Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metab. 1991;73:555–563. doi: 10.1210/jcem-73-3-555. [DOI] [PubMed] [Google Scholar]
- 33.Genant HK, Grampp S, Glüer CC, et al. Universal standardization for dual x-ray absorptiometry: patient and phantom cross-calibration results. J Bone Mineral Research. 1994;9:1503–1514. doi: 10.1002/jbmr.5650091002. [DOI] [PubMed] [Google Scholar]
- 34.Boot AM, de Ridder MAJ, Pols HAP, Krenning EP, de Muinck Keizer-Schrama SMPF. Bone mineral density in children and adolescents: relation to puberty, calcium intake, and physical activity. J Clin Endocrinol Metab. 1997;82:57–62. doi: 10.1210/jcem.82.1.3665. [DOI] [PubMed] [Google Scholar]
- 35.van der Sluis IM, de Ridder MAJ, Boot AM, Krenning EP, de Muinck Keizer-Schrama SMPF, Mughal Z. Reference data for bone density and body composition measured with dual energy × ray absorptiometry in white children and young adults. Arch Dis Child. 2002;87(4):341–7. doi: 10.1136/adc.87.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 37.National Institutes of Health. Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consensus Statement. Online 2000 March 27–29;17:1–36. Accessed at http://consensus.nih.gov/2000/2000Osteoporosis111html.htm. [PubMed]
- 38.Schlienger RG, Jick SS, Meier CR. Inhaled corticosteroids and the risk of fractures in children and adolescents. Pediatrics. 2004;114:469–473. doi: 10.1542/peds.114.2.469. [DOI] [PubMed] [Google Scholar]
- 39.Bailey D, McKay HA, Mirwald RL, Crocker PRE, Faulkner RA. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: the University of Saskatchewan Bone Mineral Accrual Study. J Bone Miner Res. 1999;14:1672–1679. doi: 10.1359/jbmr.1999.14.10.1672. [DOI] [PubMed] [Google Scholar]
- 40.Yilmaz D, Ersoy B, Bilgin E, Gumuser G, Onur E, Pinar ED. Bone mineral density in girls and boys at different pubertal stages: relation with gonadal steroids, bone formation markers, and growth parameters. J Bone Miner Metab. 2005;23:476–482. doi: 10.1007/s00774-005-0631-6. [DOI] [PubMed] [Google Scholar]
- 41.Baxter-Jones ADG, Mirwald RL, McKay HA, Bailey DA. A longitudinal analysis of sex differences in bone mineral accrual in healthy 8–19-year-old boys and girls. Ann Hum Biol. 2003;30:160–175. doi: 10.1080/0301446021000034642. [DOI] [PubMed] [Google Scholar]
- 42.Magarey AM, Boulton TJC, Chatterton BE, Schultz C, Nordin BEC, Cockington RA. Bone growth from 11–17 years: relationship to growth, gender and changes with pubertal status including timing of menarche. Acta Paediatr. 1999;88:139–146. doi: 10.1080/08035259950170286. [DOI] [PubMed] [Google Scholar]
- 43.Laatikainen AK, Kroger HPJ, Tukiainen HO, Honkanen RJ, Saarikoski SV. Bone mineral density in perimenopausal women with asthma: a population-based cross-sectional study. Am J Respir Crit Care Med. 1999;159:1179–1185. doi: 10.1164/ajrccm.159.4.9804084. [DOI] [PubMed] [Google Scholar]
- 44.American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 Update. Arthritis Rheum. 2001;44:1496–1503. doi: 10.1002/1529-0131(200107)44:7<1496::AID-ART271>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]