Abstract
Calcitriol, a regulator of calcium homeostasis with antitumor properties, is degraded by the product of the CYP24A1 gene which is downregulated in human prostate cancer by unknown mechanisms. We found that CYP24A1 expression is inversely correlated with promoter DNA methylation in prostate cancer cell lines. Treatment with the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine (DAC) activates CYP24A1 expression in prostate cancer cells. In vitro methylation of the CYP24A1 promoter represses its promoter activity. Furthermore, inhibition of histone deacetylases by trichostatin A (TSA) enhances the expression of CYP24A1 in prostate cancer cells. ChIP-qPCR reveals that specific histone modifications are associated with the CYP24A1 promoter region. Treatment with TSA increases H3K9ac and H3K4me2 and simultaneously decreases H3K9me2 at the CYP24A1 promoter. ChIP-qPCR assay reveals that treatment with DAC and TSA increases the recruitment of VDR to the CYP24A1 promoter. RT-PCR analysis of paired human prostate samples reveals that CYP24A1 expression is down-regulated in prostate malignant lesions compared to adjacent histologically benign lesions. Bisulfite pyrosequencing shows that CYP24A1 gene is hypermethylated in malignant lesions compared to matched benign lesions. Our findings indicate that repression of CYP24A1 gene expression in human prostate cancer cells is mediated in part by promoter DNA methylation and repressive histone modifications.
Keywords: CYP24A1 expression, DNA methylation, histone modification, prostate cancer
Introduction
1α,25-dihydroxycholecalciferol (calcitriol), the active form of vitamin D3, plays a major role in regulating calcium homeostasis and bone mineralization (1-3). Calcitriol also modulates cellular proliferation and differentiation in a variety of cell types(4). Calcitriol promotes cell cycle arrest and induces cell apoptosis (5). The cytochrome P450 enzyme 25-hydroxyvitamin D 24-hydroxylase (24-hydroxylase), encoded by the CYP24A1 gene, mediates 24-hydroxylation of 1α,25(OH)2D3 to the much less active vitamin D metabolites including 1α,24,25(OH)2D3 and its biliary excretory product, calcitroic acid (6, 7). The important role of CYP24A1 in maintaining vitamin D homeostasis and antitumor effects is supported by several lines of evidence: (i) CYP24A1 over-expression transgenic rats show low vitamin D3 levels (8, 9); (ii) rate of serum calcitriol clearance after calcitriol administration is delayed in CYP24A1 null mouse (10, 11) and (iii) pharmacologic inhibition of CYP24A1 in combination with calcitriol enhances antitumor effects in human prostate cancer and other tumor types (12-15).
Similar to CYP27B1, a vitamin D activating enzyme which is aberrantly expressed in tumors (16), CYP24A1 is dysregulated in wide range of tumors. Over-expression of CYP24A1 has been reported in several tumors including colon carcinomas (17, 18), ovarian cancer (17, 19), cervical carcinoma (19), lung cancer (17, 20), and cutaneous basal cell and squamous cell carcinoma (21, 22). The over-expression of CYP24A1 is associated with poor prognosis of some human cancers (17). Up-regulated CYP24A1 expression may counteract calcitriol antiproliferative activity, presumably by decreasing calcitriol levels. However, microarray expression studies published in ONCOMINE indicate that CYP24A1 is down-regulated in prostate cancer (23). The mechanism(s) underlying the dysregulation of CYP24A1 in tumors is not clear. Recently, Novakovic et al. demonstrated tissue-specific CYP24A1 promoter methylation in normal human tissues (24). The CYP24A1 gene is methylated in human placenta; no methylation was detected in somatic human tissues.(24). A lack of expression of CYP24A1 has also been directly associated with DNA methylation of the upstream promoter and the first exon of the CYP24A1 gene in tumor-derived endothelial cells (TDEC) in mice and in rat osteoblastic ROS17/2.8 cells (12, 25). Differential methylation of CYP24A1 gene contributed to selective anti-proliferative effects of calcitriol treatment on TDEC compared with Matrigel-derived endothelial cells (MDEC) (12).
The above data suggest that epigenetic mechanisms play a key role in regulating CYP24A1 expression in normal human tissues. However, little is known about the mechanism(s) underlying the regulation of CYP24A1 expression in human tumors. Given the importance of CYP24A1 in regulating 1α,25(OH)2D3 levels, we investigated the role of epigenetic regulation of CYP24A1 in human prostate cancer cell lines and primary prostate tumors.
Materials and methods
Human tissues and cells
Human prostate cancer cell lines DU145, LNCaP and PC3 were obtained from American Type Culture Collection and maintained in our laboratory. Human DNA and RNA from thirty paired human prostate benign and primary malignant lesions were obtained from Department of Pathology, Roswell Park Cancer Institute and approved by IRB.
Drug treatments
LNCaP, PC3 and DU145 cells were seeded at 1×105 cells in a 6-well plate overnight. For dose–response experiments, cells were treated with 5-aza-2′-deoxycytidine (DAC) at 0.1, 0.25, 0.5, 1.0 and 2.0 μM concentrations for 3 days followed by adding calcitriol (100 nM) for an additional 24 hours (h) and cells were harvested. Cells were treated with Trichostatin A (TSA) at 50, 100, 200, 300 and 400 nM for 8-h followed by adding calcitriol (100 nM) for additional 24-h and cells were harvested. For DAC and TSA combination treatments, cells were treated with 1 μM DAC for 3 days followed by adding TSA and calcitriol for additional 24-h and cells were harvested.
Quantitative reverse transcriptase PCR (qRT-PCR)
Expression of CYP24A1 mRNA was assessed by qRT-PCR using the CYP24A1 TaqMan® Gene Expression Assay (Hs00167999_m1, Applied Biosystems, Foster City, CA). The amplification conditions for both CYP24A1 and GAPDH were 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15s, 60°C for 30s on the 7300 real-time PCR system (Applied Biosystems). Gene expression of CYP24A1 was normalized to the GAPDH and all samples were analyzed in triplicate. Statistical analysis with Wilcoxon Signed-Rank test was performed to compare the expression levels of CYP24A1 in benign and malignant samples.
Bisulfite DNA sequencing
Genomic DNA was isolated using the genomic DNA isolation kit (Qiagen, Valencia, CA). DNA (1 μg) was converted with EZ DNA Methylation™ Kit (Zymo Research Corporation, Orange, CA). Primer design was accomplished using Methprimer (26). Bisulfite sequencing primers, region 1, (-500 to +6, 5′-AAAATTATTTTAGTTTAGGTTGGGG-3′ and 5′-TCTCCATATTCCTATACCCAAAAAC-3′); region 2 (-17 +609, 5′-TTTTTGGGTATAGGAATATGGAGAG-3′ and 5′-CCCAACAATAACCAACTAATAAAAC-3′) were used to amplify the CpG islands in the CYP24A1 promoter region. PCR included an initial incubation at 95 °C for 3 min, followed by 40 cycles of 95 °C for 30s, 56 °C for 30s, and 72 °C for 60s, followed by one cycle of 72 °C for 10 min. The PCR products were cloned into the pCR®4-TOPO vector using the TOPO TA cloning kit (Invitrogen, Carlsbad, CA) for sequencing. A total of 10 clones from each sample were sequenced at the Roswell Park Cancer Institute DNA sequencing core facility, and the methylation status for each CpG site was determined by assessing the presence of T (unmethylated) versus C (methylated) at each CpG site.
Bisulfite DNA pyrosequencing
The methylation statuses of two regions of the CYP24A1 promoter in human prostate tissue samples were analyzed by bisulfite pyrosequencing. For the first region, the primers used were: -499F (5′-AAAATTATTTTAGTTTGGTTGGGG-3′) and -244R (Biotin-5′–AAATAACCCCCAAAAAATCATAC-3′) for amplification and primer 5′-TTAGTAGGGGGAGGG-3′ for sequencing. For the second region, the primers used for amplification were +66F (5′-GTGGTTTTAGTTAGATTTTAGAGG - 3′ and +301R (Biotin-5′-CAAAAAAAACCAACTAATATAAT-3′) and the sequencing primer was 5′-TTAGTGTAAGGAGGTATTAA -3′. PCR cycling conditions were an initial incubation at 95 °C for 3 min, followed by 40 cycles of 95°C for 30s, 52°C (for region 1) and 51°C (for region 2) for 30s, and 72°C for 30s. Pyrosequencing was accomplished in duplicate using the PSQ 96 Pyrosequencing System (Biotage, Charlotte, NC) and were performed two times.
CYP24A1 activity assay
Cells were treated with DAC for 72-h and/or TSA for 8-h followed by 100 nM calcitriol for 48-h and were washed with 1% BSA-PBS twice. Then, cells (1×106) were incubated with 25(OH)D3 (1 μM) in 2ml of 1% BSA-medium for 12-h. 25(OH)D3 metabolites from cells and medium were extracted by liquid–liquid partition with 8 ml of chloroform/methanol (3:1). Prior to the extraction, 20 μl of EB1089 (10μg/ml) was added as an internal standard (IS). Dried sample extracts were dissolved in 100μl of high performance liquid chromatography (HPLC) mobile phase, hexane-isopropanol-methanol in 90:5:5 (v/v/v). Vitamin D3 metabolites and IS in 50μl of the extract were separated by HPLC on a 4.6 × 250-mm Zorbax SIL column (Agilent Technologies Inc, Palo Alto, CA) using HPLC mobile phase at a flow rate of 2ml/min. Metabolites were monitored at λ265nm and identified based on retention time and Diode array collected vitamin D3 chromophore spectral characteristics (UVmax= λ265, UVmin= λ228, UVmax/UVmin=1.75). CYP24A1 activity was calculated as the area ratio of 24, 25-(OH)2D3 : recovered 25-(OH)D3 + 24, 25-(OH)2D3/12hr/106 cells.
In vitro CYP24A1 promoter methylation assay
A 587 bp region of the human CYP24A1 promoter gene shown (27, 28) to confer vitamin D responsiveness was amplified using primers; Xho-CYP24–556F (5′-GCTAGCCGCAGAAAGCCAAACTTCCTC-3′) and NHeI-CYP24+40R (5′- -GCAGCTCGAGAGATGCTGCTGGCGCTGCGT-3′) with PfuUltra™ High-Fidelity DNA Polymerase DNA polymerase (Stratagene, La Jolla, CA). The Xho and NheI-digested amplicon was cloned into a promoterless luciferase expression vector pGL4.21 (Promega, Madison, WI) to produce the plasmid pGL4.21/547/+40. Methylation of the CYP24A1 promoter region was done as described (29). Briefly, the fragments were methylated in vitro with SssI, HpaII and HhaI (New England Biolabs, Ipswich, MA), or no enzyme (mock), according to the manufacturer's instructions. Methylation efficiency was confirmed using HhaI, HpaII and McrBC digestions (New England Biolabs). Methylated or mock-methylated fragments were ligated back into pGL4.21 vector (Promega). PC3 cells were transfected with 100 ng of the differentially methylated constructs along with 20 ng of a Renilla luciferase control construct (Promega). All transfections were carried out in triplicate wells of 96-well plates. Twenty-four hours post transfection, cells were treated with calcitriol (100 nM) for additional 24-h and harvested, and firefly and Renilla luciferase activity were measured using the Dual-Luciferase Reporter Assay System (Promega), according to the manufacturer's instructions. Statistical analysis with Student's t-test on luciferase values was performed to compare the CYP24A1 promoter activities in unmethylated or methylated CYP24A1 promoter constructs. The Experiment was repeated twice to confirm the reproducibility of results.
Quantitative chromatin immunoprecipitation-PCR (ChIP-qPCR)
ChIP-qPCR was performed essentially as described by Väisänen S et al (30). Briefly, cells were treated with DAC (1 μM), TSA (300 nM), or both agents. One hour after adding calcitriol, cells were fixed to cross-link nuclear protein to DNA by adding formaldehyde as described previously (30). Chromatin was sheared to an average length of 500 bp. The chromatin was precipitated with indicated antibodies. Antibodies against histone H3 dimethylated lysine 9 (H3K9me2), dimethylated lysine 4 (H3K4me2), and acetylated lysine 9 (H3K9ac) were purchased from Upstate Biotechnologies (Billerica, MA); an antibody against Vitamin D receptor (VDR) (sc-1008×) were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). ChIP-qPCR primers for the CYP24A1 promoter region 1 and region 2 were: -292F (5′-AGCACACCCGGTGAACTC-3′) and -152R: (5′-TGGAAGGAGGATGGAGTCAG-3′); +130F (5′-TTCAAGAGGTCCCCAGACAC-3′) and +333R (5′-AGTCGGGGCTTAACGATTCT-3′). ChIP-qPCR was performed using Power SYBR® Green PCR Master Mix (Applied Biosystems) with following cycling parameters: 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min. Copy number was determined using a standard curve containing serial dilutions (107–100 copies) of the CYP24A1 DNA amplicon. Samples were run in triplicate, and data were normalized to 5% input DNA amplifications, after subtraction of signals obtained from antibody isotype control. ChIP was repeated twice to confirm the reproducibility of results.
Results
Correlation of CYP24A1 expression in human prostate cell lines with promoter DNA methylation status
To investigate the mechanism(s) regulating CYP24A1 mRNA expression in prostate cancer, we analyzed LNCaP, PC3 and DU145 cells which express variable levels of CYP24A1. qRT–PCR showed that LNCaP and PC3 cells express CYP24A1 weakly in the presence of calcitriol, whereas DU145 cells express CYP24A1 at high baseline and show further induction following calcitriol treatment (Fig. 1A). We further tested whether CpG island methylation could account for differential CYP24A1 expression in the prostate cell lines. Analysis of the human CYP24A1 gene by Webgene indicated that it contains two CpG islands (580bp and 1232bp, respectively) that spans the transcriptional start site (Fig. 1B) (31). The 5′ CpG island of the CYP24A1 gene encompasses the promoter region and contains two known VDR response elements (VDREs), the proximal element VDRE, (-172/-143) and the distal element VDRE, (- 293/-273) (Fig. 1B) (27). To examine whether CYP24A1 is differentially methylated in these cell lines, we conducted bisulfite sequencing on 506 bp region 1 and 627 bp region 2 of the CYP24A1 5′-CpG island promoter (Fig. 1B). This assay revealed that the overall methylation of CYP24A1 promoter regions in the LNCaP and PC3 cells is 24% and 50%, respectively. The hypermethylation is particularly evident in region 2 of the CYP24A1 gene. In contrast, the CYP24A1 5′-CpG island was completely unmethylated in the DU145 cells, which is consistent with constitutive CYP24A1 expression in this cell line (Fig. 1C). These data suggest that promoter DNA methylation status may regulate differential CYP24A1 expression in prostate cancer cells.
Figure 1. CYP24A1 expression and promoter DNA methylation.
(A) qRT-PCR of CYP24A1 expression in human prostate cancer cells. (B) Diagram of the CYP24A1promoter CpG islands. The regions analyzed by bisulfite sequencing and pyrosequencing, along with the number of CpGs contained within each region, and vitamin D-responsive elements (VDR proximal element, VDREp and VDR distal element, VDREd), are shown. (C) Bisulfite sequencing of the CYP24A1 promoter CpG island methylation. The overall methylation percentage from ten individual clones shown indicates total proportion of methylated CpGs in the CYP24A1 promoter region in a given sample, taking into account all sequenced alleles.
Activation of CYP24A1 gene expression by epigenetic modulatory drugs in prostate cancer cells
To determine the role CYP24A1 gene methylation has on CYP24A1 expression, we treated LNCaP and PC3 cells with DAC, a DNA methyltransferase inhibitor. qRT-PCR revealed that DAC elicited a dose-dependent induction of CYP24A1 expression in LNCaP and PC3 cells that was dependent on calcitriol treatment (Fig. 2A). To confirm the demethylation effect of DAC, bisulfite sequencing was done on treated cell lines. Overall methylation of CYP24A1 promoter in LNCaP and PC3 cells was reduced from 24% to 5% and 50% to 13%, respectively (Figs 1C & 2B).
Figure 2. Activation of CYP24A1 by DAC treatment.
(A) qRT-PCR of CYP24A1 expression in cells treated with varying concentrations of DAC followed by calcitriol. Mean ± SD values of triplicate data points are shown. (B) Bisulfite sequencing of the CYP24A1 promoter CpG island methylation in DAC treated cells. The overall methylation percentage indicates total proportion of methylated CpGs in the CYP24A1 promoter region in a given sample, taking into account 10 sequenced alleles.
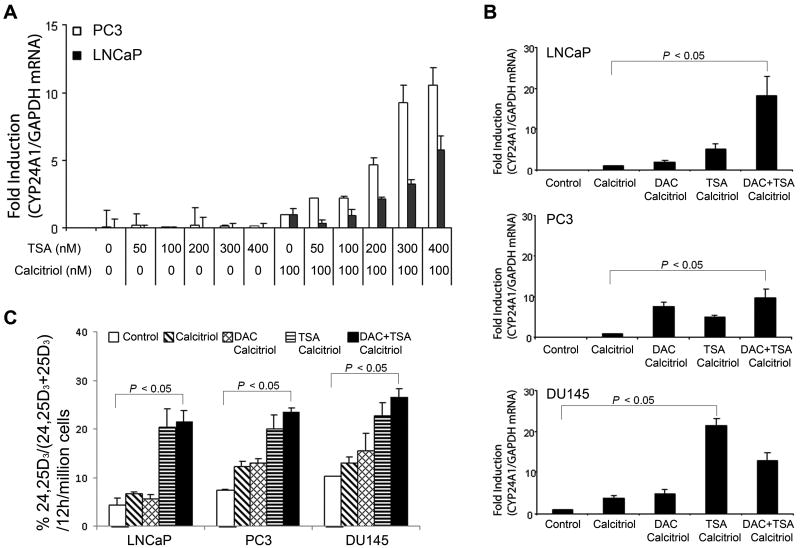
To determine whether histone deacetylation is also involved in repression of CYP24A1 expression, we treated LNCaP and PC3 cells with TSA, a histone deacetylase inhibitor. qRT-PCR revealed that TSA elicited a dose-dependent induction of CYP24A1 expression in LNCaP and PC3 cells that was dependent on calcitriol treatment (Fig. 3A). Combination treatment with DAC and TSA induced a significant increase in CYP24A1 mRNA and protein expression in LNCaP and PC3 cells (Fig. 3B, upper and middle panels and Supplemental Fig. 1). In contrast, DU145, which has an unmethylated promoter, showed no increase in CYP24A1 expression with DAC treatment but a robust effect with TSA treatment (Fig. 3B, bottom panel). The slightly less CYP24A1 expression in combination treatment of DAC and TSA compared to TSA alone could be due to the additive toxicity of this approach in DU145.
Figure 3. Activation of CYP24A1 gene expression and enzymatic activity by TSA alone and in combination with DAC.
(A) qRT-PCR of the CYP24A1 expression in cells treated with varying concentrations of TSA followed by calcitriol. Mean ± SD values of triplicate data points are shown. (B) qRT-PCR of CYP24A1 expression in cells treated with TSA in combination with DAC followed by calcitriol. Mean ± SD values of triplicate data points are shown. (C) CYP24A1 activity in cells treated with DAC and/or TSA.
To test whether the increased CYP24A1 expression by epigenetic modulators is associated with increased CYP24A1 activity, we performed enzyme activity assay by HPLC. Increased CYP24A1 enzyme activity was observed in LNCaP and PC3 cells treated with TSA (Fig.3C). In addition, there was no significant increase of enzyme activity in DU145 cells treated with DAC, which has hypomethylated CYP24A1 promoter (Fig. 3C). In all cell lines, combination treatment of DAC and TSA cause a significant increase in CYP24A1 activity (Fig. 3C). However, it is not clear why the difference of the enzyme activity between DU145 cells and other two cells lines are less than the relative differences at the mRNA expression level. We speculate that the enzyme activity may be affected by other endogenous factors, and that the mRNA and protein expression of CYP24A1 may closely, but not exactly, parallel.
Repression of human CYP24A1 gene promoter activity by DNA methylation
To examine whether DNA methylation directly represses CYP24A1 promoter activity, we cloned a 587 bp fragment of the 5′ end of CYP24A1, which contains 46 CpG sites, into a luciferase reporter construct. We methylated the cloned insert (29) using SssI methylase (methylation of 46 CpGs), HpaII methylases (methylation of 14 CpGs) and HhaI methylases (methylation of 4 CpGs). SssI methylase methylates all 5′-CpG-3′ sites, HpaII methylase methylates only the CpG within the sequence 5′-CCGG-3′ and HhaI methylase methylates only the CpG within the sequence 5′-GCGC-3′. Proper methylation of inserts was confirmed by digesting with the restriction enzymes McrBC, HpaII and HhaI (Fig. 4A). CYP24A1 promoter activity was tested by transfection of the luciferase construct containing methylated CYP24A1 promoter. Prior methylation with SssI, HpaII or HhaI methylases in PC3 cells repressed CYP24A1 promoter activity (Fig. 4B). Repression was methylation dose-dependent in that SssI, which methylates all CpG sites, showed the greatest repression and HhaI, which methylates 4 CpG sites, showed the least repression on CYP24A1 promoter activity (Fig. 4B).
Figure 4. Repression of CYP24A1 promoter activity by DNA methylation.
(A) Methylation of the CYP24A1 gene promoter region in vitro. Following in vitro methylation with SssI, HpaII, or HhaI methylases reactions, CYP24A1 promoter fragments were digested with McrBC (a methylation-specific restriction enzyme), HpaII or HhaI (a methylation-sensitive restriction enzyme) to confirm the methylation status of the CYP24A1 promoter construct. (B) Dual luciferase activity assay of differentially methylated CYP24A1 promoter constructs following transfection into PC3 cells. Luminescence values were normalized to Renilla luciferase and results are expressed as mean ± SD from triplicate data points relative to the mean promoter-less control vector. Results are representative of two independent experiments.
Histone modifications associated with the CYP24A1 promoter region
The association of CYP24A1 promoter DNA methylation and gene silencing in relation to histone modifications has not been investigated previously. To clarify the role of histone modifications in the regulation of the CYP24A1 gene, we examined active histone marks, H3K4me2 and H3K9ac, and the repressive histone mark, H3K9me2, in the chromatin associated with the CYP24A1 promoter region using ChIP-qPCR. The results showed similar patterns of histone acetylation and H3 methylation levels in LNCaP and PC3 cells with relative high H3K9me2 compared to DU145 cells (Fig. 5A). In contrast, the active histone markers (H3K4me2 and H3K9ac) were highest in DU145 cells, as expected (Fig. 5A). We also examined whether treatment with DAC and/or TSA changed the profile of histone modifications in the CYP24A1 promoter. The ChIP-qPCR assay revealed that TSA treatment induced increases of H3K9ac and H3K4me2, and a decrease of H3K9me2 in PC3 cells, giving rise to an active pattern of histone modifications following treatment (Fig. 5B). Similar histone modification changes were also observed in LNCaP cells after treatment (data not shown).
Figure 5. CYP24A1 promoter-associated histone modifications and VDR recruitment.
(A) qChIP-PCR of H3K9me2, H3K4me2 and H3K9ac at CYP24A1 promoter. The amplification value from immunoprecipitated DNA was normalized to 5% input and results were expressed as mean ± SD from triplicate data points. (B) qChIP-PCR of H3K9me2, H3K4me2 and H3K9ac at CYP24A1 promoter in PC3 cells treated with DAC and/or TSA. The amplification value from immunoprecipitated DNA was normalized to 5% DNA input and results were expressed as mean ± SD from triplicate data points. (C) qChIP-PCR analysis of VDR recruitment to CYP24A1 promoter in PC3 cells treated with DAC and/or TSA. The mean values from triplicate data points are plotted.
Increased recruitment of VDR to the CYP24A1 promoter in PC3 cells treated with epigenetic modulatory drugs
We next examined whether these epigenetic changes affect the binding of the VDR to the CYP24A1 promoter region. ChIP-qPCR analysis using a VDR-specific antibody revealed that there was significantly increased binding of VDR to the CYP24A1 promoter in PC3 cells upon treatment with DAC and TSA, in addition to calcitriol (Fig. 5C). These data provide functional evidence that decreased VDR binding to the CYP24A1 promoter in PC3 cells, due to hypermethylation and a repressive histone modifications, contributes to the suppression of CYP24A1 expression in prostate cancer cells.
Expression and methylation of CYP24A1 in primary normal and cancerous human prostate tissues
We analyzed CYP24A1 expression in human normal prostate, benign prostatic hyperplasia, prostate carcinoma, and metastatic prostate cancer using microarray expression studies published in ONCOMINE (23). CYP24A1 expression was significantly lower in prostate cancer compared with normal prostate in three out of four (P < 0.01) microarray expression studies (Fig. 6A). However, VDR expression was not down-regulated in studies published in ONCOMINE (data not shown). We obtained 30 matched human prostate benign and malignant prostate samples. Gleason Scores were 3+3 (n=6), 3+4 (n=15), 4+3 (n=7), 4+5 (n=1) and 5+4 (n=1). qRT-PCR revealed that CYP24A1 expression was significantly down-regulated in prostate malignant lesions compared to benign lesions (p=0.0336) (Fig. 6B & C). Bisulfite pyrosequencing assays for the CYP24A1 gene were designed to span VDREd and VDREp (21) and putative regulatory sequence located in exon 1 within the associated CpG island. Pyrosequencing revealed that the CYP24A1 gene was significantly hypermethylated in malignant lesions, as compared to matched benign lesions (Fig. 6D). There was no correlation of the level of CYP24A1 expression and methylation with Gleason Score. To determine whether malignant samples with decreased CYP24A1 expression correlated with CYP24A1 hypermethylation, we built a 2×2 contingency table by dividing the 30 samples based on the CYP24A1 expression change (≥ 1.5 fold down vs. other) and methylation change (≥ 2.0 fold up vs. other). Ten of the 15 samples with CYP24A1 down-regulation in malignant lesions have increased CYP24A1 DNA methylation, while only 4 of the other 15 samples have increased CYP24A1 DNA methylation. Fisher's exact test shows that decreased CYP24A1 expression is significantly associated with increased CYP24A1 DNA methylation (P< 0.05).
Figure 6. CYP24A1 expression and promoter DNA methylation in normal and cancerous prostate tissues.
(A) Analysis of ONCOMINE database for CYP24A1 expression in human prostate tumors (T) compared with normal prostate tissue (N). Dots represent maximum and minimum values; whiskers represent 90th and 10th percentile values and horizontal lines represent 75th, 50th, and 25th percentile values. (B) qRT-PCR analysis of the CYP24A1 expression in human matched prostate malignant and benign lesions. Mean ± SD values of triplicate data points are shown. (C) Comparison of CYP24A1 expression in human matched prostate malignant and benign lesions by Wilcoxon signed-rank test. (D) Pyrosequencing of the methylation status in human matched prostate malignant and benign lesions. Mean ± SD values of duplicate data points are indicated. Results are representative of two independent experiments.
Discussion
In this study, we showed that both DNA hypermethylation and repressive histone modifications at the CYP24A1 promoter were demonstrated to repress CYP24A1 gene expression. Epigenetic repression also impairs the recruitment of VDR to the CYP24A1 regulatory regions, providing a plausible mechanism for how the repressive epigenetic markers exert their effects on CYP24A1 expression.
This study indicates a direct molecular relationship between 5′CpG island methylation and down-regulation of CYP24A1 expression in human prostate cancer. This conclusion is supported by the following observations. First, the presence of a classical 5′CpG island in the CYP24A1 promoter suggests a role for DNA methylation-based regulation. Second, the CYP24A1 promoter is hypermethylated in human prostate cell lines that weakly express CYP24A1 (LNCaP and PC3), while the promoter is completely unmethylated in a human prostate cell line with high CYP24A1 expression (DU145). Third, treatment of low CYP24A1 expression prostate cell lines with the DNA methyltransferase inhibitor induces CYP24A1 expression in a dose-dependent manner, which coincides with DNA hypomethylation of the CYP24A1 promoter. However, no increased induction of CYP24A1 expression was observed in DAC treatment in DU145 which has hypomethylated CYP24A1. Furthermore, in vitro methylation of the CYP24A1 promoter directly represses its promoter activity. Finally, ChIP-qPCR shows that treatment of PC3 cells with DAC increases the recruitment of VDR to the CYP24A1 promoter. Data obtained from prostate cell lines were further supported by analysis of human matched prostate benign and malignant lesion samples. The expression of CYP24A1 was significantly down-regulated in malignant prostate tissues compared to benign prostate tissues. This finding was consistent with the data obtained from ONCOMINE (23). We further show that the decreased expression of CYP24A1 in malignant tissues was significantly associated with hypermethylation of the CYP24A1 promoter, demonstrating a relationship between 5′-CpG island methylation and reduced CYP24A1 expression in prostate tumor specimens. These observations suggest that DNA methylation may serve as a mechanism for controlling CYP24A1 expression in human cancers.
In the human CYP24A1 promoter, vitamin D response elements are located within the hypermethylated regions. Therefore, hypermethylation of these regions identified in this study could lead to a chromatin state in which VDR is prevented from binding to the VDREs that results in transcription silencing of CYP24A1. This model is supported by our observations that recruited VDR on CYP24A1 promoter is increased in PC3 cells treated with DAC or TSA. H3K9me2, a repressive histone modification, was high in LNCaP and PC3 cells, which have low CYP24A1 expression. In contrast, low H3K9me2, high H3K9ac and H3K4me2 in DU145 cells which has the highest CYP24A1 expression suggest an association of an open chromatin structure with active gene expression (32, 33). Notably, we found that treatment of CYP24A1 hypermethylated prostate cells with the histone deacetylase inhibitor and DNA methyltransferase inhibitor induces CYP24A1 expression in a dose-dependent manner, accompanied by an active pattern of histone modifications. These results are consistent with the hypothesis that aberrant DNA methylation and repressive histone modifications function in combination to silence important tumor suppressor genes in human cancers (34, 35). Studies have demonstrated that hypermethylated promoters often involve recruitment of methyl binding domain proteins and histone-modifying enzymes such as histone deacetylase (36-39). Binding of these proteins leads to a chromatin closed state and prevents binding of transcription factors like VDR to target promoters. Although evidence in the current study and previously reports (12, 24, 25, 40) supports promoter DNA hypermethylation-mediated CYP24A1 suppression, qRT-PCR analysis of human prostate tissues reveals that the level of CYP24A1 methylation does not entirely account for strong inverse correlation with the level of CYP24A1 expression in human prostate tissues (Figures 6C and 6D). Our results suggest that histone code modifications could provide an alternative regulatory mechanism which impacts CYP24A1 gene expression in vitro and in vivo (41-43).
Analysis of the CYP24A1 gene exon 1 region by NUBIScan (44) identified a putative VDRE. We found that treatment with DAC or TSA increases the recruitment of VDR to this putative VDRE region which is methylated in LNCaP and PC3 cells (Fig. 1C and 5C). These results suggest that the region of downstream of the transcriptional start site (TSS) may be involved in the recruitment of CYP24A1 regulators. Further studies are in progress to investigate this possibility.
Taken together, these data define promoter DNA methylation and altered histone codes as a key mechanism controlling CYP24A1 expression in human prostate cancers. Information obtained from this study has implications for developing strategies to optimize the vitamin D antitumor activity. Based on the apparent role of CYP24A1 as a key enzyme to degrade the activity of vitamin D, one could envision that direct inhibition of CYP24A1 function could enhance the antitumor activity of vitamin D (14, 45, 46). CYP24A1 expression is heterogeneous in prostate cancer (Figure 6B). Prostate cancer patients with high expression of CYP24A1 may not be as responsive to vitamin D analog therapy, presumably by the ability of CYP24A1 in prostate tumors to decrease 1, 25(OH)2D3 levels. Decreased expression of CYP24A1 in some malignant prostate tumor tissue, in part, is due to methylation and histone modification associated with the CYP24A1 promoter. Furthermore, Chung I et al. reported that induction of CYP24a1 by hypomethylating agents may attenuate responses to vitamin D, in mouse tumor derived endothelial cells that do not express CYP24 due to epigenetic silencing (12). Therefore, the use of DNA methyltransferase inhibitors and histone deacetylase inhibitors in vitamin D-based therapy for prostate cancer should be avoided due to the reactivation of CYP24A1 expression, potentially rendering the tumor less sensitive to vitamin D. In addition, decreased activity of extra-renal 1α-hydroxylase in prostate cancer may be also responsible for the increased proliferation of the cancer cells (47). In conclusion, the data obtained from this study contribute to our understanding of the mechanisms regulating CYP24A1 gene expression in malignant and normal human tissues, and facilitates the optimization of antitumor therapy of vitamin D in combination with repression of CYP24A1 in prostate cancer.
Supplementary Material
Acknowledgments
We thank Drs. Alan Hutson and Song Liu for statistical assistance, Dr. Moray J.Campbell for helpful discussions, Dr. Yingyu Ma for helping figure preparation, and Rui-Xian Kong, Smitha James and Petra Link for expert technical assistance.
This study was supported by NIH/NCI grants CA067267, CA085142 and CA095045
References
- 1.Haussler MR, Haussler CA, Jurutka PW, et al. The vitamin D hormone and its nuclear receptor: molecular actions and disease states. J Endocrinol. 1997;154(Suppl):S57–73. [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D and bone health. J Nutr. 1996;126:1159S–64S. doi: 10.1093/jn/126.suppl_4.1159S. [DOI] [PubMed] [Google Scholar]
- 3.DeLuca HF, Schnoes HK. Vitamin D: recent advances. Annu Rev Biochem. 1983;52:411–39. doi: 10.1146/annurev.bi.52.070183.002211. [DOI] [PubMed] [Google Scholar]
- 4.Pols HA, Birkenhager JC, Foekens JA, van Leeuwen JP. Vitamin D: a modulator of cell proliferation and differentiation. J Steroid Biochem Mol Biol. 1990;37:873–6. doi: 10.1016/0960-0760(90)90435-n. [DOI] [PubMed] [Google Scholar]
- 5.Chung I, Wong MK, Flynn G, Yu WD, Johnson CS, Trump DL. Differential antiproliferative effects of calcitriol on tumor-derived and matrigel-derived endothelial cells. Cancer Res. 2006;66:8565–73. doi: 10.1158/0008-5472.CAN-06-0905. [DOI] [PubMed] [Google Scholar]
- 6.Makin G, Lohnes D, Byford V, Ray R, Jones G. Target cell metabolism of 1,25-dihydroxyvitamin D3 to calcitroic acid. Evidence for a pathway in kidney and bone involving 24-oxidation. Biochem J. 1989;262:173–80. doi: 10.1042/bj2620173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckman MJ, Tadikonda P, Werner E, Prahl J, Yamada S, DeLuca HF. Human 25-hydroxyvitamin D3-24-hydroxylase, a multicatalytic enzyme. Biochemistry. 1996;35:8465–72. doi: 10.1021/bi960658i. [DOI] [PubMed] [Google Scholar]
- 8.Hosogane N, Shinki T, Kasuga H, Taketomi S, Toyama Y, Suda T. Mechanisms for the reduction of 24,25-dihydroxyvitamin D3 levels and bone mass in 24-hydroxylase transgenic rats. FASEB J. 2003;17:737–9. doi: 10.1096/fj.02-0965fje. [DOI] [PubMed] [Google Scholar]
- 9.Kasuga H, Hosogane N, Matsuoka K, et al. Characterization of transgenic rats constitutively expressing vitamin D-24-hydroxylase gene. Biochem Biophys Res Commun. 2002;297:1332–8. doi: 10.1016/s0006-291x(02)02254-4. [DOI] [PubMed] [Google Scholar]
- 10.Masuda S, Byford V, Arabian A, et al. Altered pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse. Endocrinology. 2005;146:825–34. doi: 10.1210/en.2004-1116. [DOI] [PubMed] [Google Scholar]
- 11.Masuda S, Strugnell SA, Knutson JC, St-Arnaud R, Jones G. Evidence for the activation of 1alpha-hydroxyvitamin D2 by 25-hydroxyvitamin D-24-hydroxylase: delineation of pathways involving 1alpha,24-dihydroxyvitamin D2 and 1alpha,25-dihydroxyvitamin D2. Biochim Biophys Acta. 2006;1761:221–34. doi: 10.1016/j.bbalip.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Chung I, Karpf AR, Muindi JR, et al. Epigenetic silencing of CYP24 in tumor-derived endothelial cells contributes to selective growth inhibition by calcitriol. J Biol Chem. 2007;282:8704–14. doi: 10.1074/jbc.M608894200. [DOI] [PubMed] [Google Scholar]
- 13.Swami S, Krishnan AV, Peehl DM, Feldman D. Genistein potentiates the growth inhibitory effects of 1,25-dihydroxyvitamin D3 in DU145 human prostate cancer cells: role of the direct inhibition of CYP24 enzyme activity. Mol Cell Endocrinol. 2005;241:49–61. doi: 10.1016/j.mce.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Yee SW, Campbell MJ, Simons C. Inhibition of Vitamin D3 metabolism enhances VDR signalling in androgen-independent prostate cancer cells. J Steroid Biochem Mol Biol. 2006;98:228–35. doi: 10.1016/j.jsbmb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Trump DL, Muindi J, Fakih M, Yu WD, Johnson CS. Vitamin D compounds: clinical development as cancer therapy and prevention agents. Anticancer Res. 2006;26:2551–6. [PubMed] [Google Scholar]
- 16.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 17.Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother Pharmacol. 2006;57:234–40. doi: 10.1007/s00280-005-0059-7. [DOI] [PubMed] [Google Scholar]
- 18.Bareis P, Bises G, Bischof MG, Cross HS, Peterlik M. 25-hydroxy-vitamin d metabolism in human colon cancer cells during tumor progression. Biochem Biophys Res Commun. 2001;285:1012–7. doi: 10.1006/bbrc.2001.5289. [DOI] [PubMed] [Google Scholar]
- 19.Friedrich M, Rafi L, Mitschele T, Tilgen W, Schmidt W, Reichrath J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent Results Cancer Res. 2003;164:239–46. doi: 10.1007/978-3-642-55580-0_17. [DOI] [PubMed] [Google Scholar]
- 20.Parise RA, Egorin MJ, Kanterewicz B, et al. CYP24, the enzyme that catabolizes the antiproliferative agent vitamin D, is increased in lung cancer. Int J Cancer. 2006;119:1819–28. doi: 10.1002/ijc.22058. [DOI] [PubMed] [Google Scholar]
- 21.Mitschele T, Diesel B, Friedrich M, et al. Analysis of the vitamin D system in basal cell carcinomas (BCCs) Lab Invest. 2004;84:693–702. doi: 10.1038/labinvest.3700096. [DOI] [PubMed] [Google Scholar]
- 22.Reichrath J, Rafi L, Rech M, et al. Analysis of the vitamin D system in cutaneous squamous cell carcinomas. J Cutan Pathol. 2004;31:224–31. doi: 10.1111/j.0303-6987.2003.00183.x. [DOI] [PubMed] [Google Scholar]
- 23.Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novakovic B, Sibson M, Ng HK, et al. Placenta-specific methylation of the vitamin D 24-hydroxylase gene: implications for feedback autoregulation of active vitamin D levels at the fetomaternal interface. J Biol Chem. 2009;284:14838–48. doi: 10.1074/jbc.M809542200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohyama Y, Kusada T, Yamasaki T, Ide H. Extensive methylation of CpG island of CYP24 gene in osteoblastic ROS17/2.8 cells. Nucleic Acids Res Suppl. 2002:249–50. doi: 10.1093/nass/2.1.249. [DOI] [PubMed] [Google Scholar]
- 26.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–31. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 27.Chen KS, DeLuca HF. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim Biophys Acta. 1995;1263:1–9. doi: 10.1016/0167-4781(95)00060-t. [DOI] [PubMed] [Google Scholar]
- 28.Tashiro K, Ishii C, Ryoji M. Role of distal upstream sequence in vitamin D-induced expression of human CYP24 gene. Biochem Biophys Res Commun. 2007;358:259–65. doi: 10.1016/j.bbrc.2007.04.103. [DOI] [PubMed] [Google Scholar]
- 29.Woloszynska-Read A, James SR, Link PA, Yu J, Odunsi K, Karpf AR. DNA methylation-dependent regulation of BORIS/CTCFL expression in ovarian cancer. Cancer Immun. 2007;7:21. [PMC free article] [PubMed] [Google Scholar]
- 30.Vaisanen S, Dunlop TW, Sinkkonen L, Frank C, Carlberg C. Spatio-temporal activation of chromatin on the human CYP24 gene promoter in the presence of 1alpha,25-Dihydroxyvitamin D3. J Mol Biol. 2005;350:65–77. doi: 10.1016/j.jmb.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 31.Milanesi L, D'Angelo D, Rogozin IB. GeneBuilder: interactive in silico prediction of gene structure. Bioinformatics. 1999;15:612–21. doi: 10.1093/bioinformatics/15.7.612. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15:2343–60. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- 33.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6:838–49. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 34.Kondo Y, Shen L, Issa JP. Critical role of histone methylation in tumor suppressor gene silencing in colorectal cancer. Mol Cell Biol. 2003;23:206–15. doi: 10.1128/MCB.23.1.206-215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawamoto K, Hirata H, Kikuno N, Tanaka Y, Nakagawa M, Dahiya R. DNA methylation and histone modifications cause silencing of Wnt antagonist gene in human renal cell carcinoma cell lines. Int J Cancer. 2008;123:535–42. doi: 10.1002/ijc.23514. [DOI] [PubMed] [Google Scholar]
- 36.Bakker J, Lin X, Nelson WG. Methyl-CpG binding domain protein 2 represses transcription from hypermethylated pi-class glutathione S-transferase gene promoters in hepatocellular carcinoma cells. J Biol Chem. 2002;277:22573–80. doi: 10.1074/jbc.M203009200. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Serra L, Ballestar E, Fraga MF, Alaminos M, Setien F, Esteller M. A profile of methyl-CpG binding domain protein occupancy of hypermethylated promoter CpG islands of tumor suppressor genes in human cancer. Cancer Res. 2006;66:8342–6. doi: 10.1158/0008-5472.CAN-06-1932. [DOI] [PubMed] [Google Scholar]
- 38.Nan X, Ng HH, Johnson CA, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–9. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 39.Jones PL, Veenstra GJ, Wade PA, et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–91. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 40.Khorchide M, Lechner D, Cross HS. Epigenetic regulation of vitamin D hydroxylase expression and activity in normal and malignant human prostate cells. J Steroid Biochem Mol Biol. 2005;93:167–72. doi: 10.1016/j.jsbmb.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 41.Bachman KE, Park BH, Rhee I, et al. Histone modifications and silencing prior to DNA methylation of a tumor suppressor gene. Cancer Cell. 2003;3:89–95. doi: 10.1016/s1535-6108(02)00234-9. [DOI] [PubMed] [Google Scholar]
- 42.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 43.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–80. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 44.Podvinec M, Kaufmann MR, Handschin C, Meyer UA. NUBIScan, an in silico approach for prediction of nuclear receptor response elements. Mol Endocrinol. 2002;16:1269–79. doi: 10.1210/mend.16.6.0851. [DOI] [PubMed] [Google Scholar]
- 45.Schuster I, Egger H, Herzig G, et al. Selective inhibitors of vitamin D metabolism--new concepts and perspectives. Anticancer Res. 2006;26:2653–68. [PubMed] [Google Scholar]
- 46.Schuster I, Egger H, Astecker N, Herzig G, Schussler M, Vorisek G. Selective inhibitors of CYP24: mechanistic tools to explore vitamin D metabolism in human keratinocytes. Steroids. 2001;66:451–62. doi: 10.1016/s0039-128x(00)00166-5. [DOI] [PubMed] [Google Scholar]
- 47.Chen TC. 25-Hydroxyvitamin D-1 alpha-hydroxylase (CYP27B1) is a new class of tumor suppressor in the prostate. Anticancer Res. 2008;28:2015–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.