Abstract
Worldwide spreading of drug-resistant pathogens makes mechanistic understanding of antibiotic action an urgent task. The macrocyclic antibiotic lipiarmycin (Lpm), which is under development for clinical use, inhibits bacterial RNA polymerase (RNAP) by an unknown mechanism. Using genetic and biochemical approaches, we show that Lpm targets the σ70 subunit region 3.2 and the RNAP β′ subunit switch-2 element, which controls the clamping of promoter DNA in the RNAP active-site cleft. Lpm abolishes isomerization of the ‘closed'-promoter complex to the transcriptionally competent ‘open' complex and blocks σ70-stimulated RNA synthesis on promoter-less DNA templates. Lpm activity decreases when the template DNA strand is stabilized at the active site through the interaction of RNAP with the nascent RNA chain. Template DNA-strand fitting into the RNAP active-site cleft directed by the β′ subunit switch-2 element and the σ70 subunit region 3.2 is essential for promoter melting and for de novo initiation of RNA synthesis, and our results suggest that Lpm impedes this process.
Keywords: antibiotic resistance, lipiarmycin, RNA polymerase, sigma region 3.2, switch region
Introduction
DNA-dependent RNA polymerase (RNAP) is an essential enzyme for gene expression in living organisms from all kingdoms of life. The bacterial RNAP holoenzyme consists of a catalytic core (composed of subunits 2α, β, β′ and ω) and a σ subunit required for promoter recognition, promoter opening and transcription initiation (Gross et al, 1998; Helmann and deHaseth, 1999). Structural studies have revealed that the β′ and β subunits of the RNAP core form the pincers (or jaws) of a crab claw-like structure in which the active site is buried deeply in the cleft between the jaws (Zhang et al, 1999). The mobile clamp domain, formed mainly by the β′ subunit pincer, serves as a lock of the active-site cleft and a docking site for the σ subunit. In the elongation complex, the 8–9 bp DNA–RNA hybrid occupies the RNAP active-site cleft, whereas the ∼15 bp DNA duplex downstream of the start site (downstream DNA) is fixed by the pincers in the downstream channel (Vassylyev et al, 2007). The nascent RNA chain exits the active-site cleft through the RNA exit channel formed by the elements of the β′ clamp and β subunit. The σ subunit region 3.2 forms a linker between the σ regions 2 and 4 that fill the RNA exit channel in the holoenzyme (Vassylyev et al, 2002; Murakami et al, 2002b). The linker is ejected out of the channel as soon as 10–11 nucleotides (nt) of RNA are formed.
In Escherichia coli, the σ70 subunit is responsible for transcription initiation for most promoters during exponential growth. During promoter recognition, evolutionarily conserved regions 2 and 4 of σ70 bind the −10 and −35 elements of the promoter, respectively; this interaction leads to the formation of the initial ‘closed'-promoter complex. Initiation of RNA synthesis requires the formation of the ‘open'-promoter complex, in which ∼13 bp of promoter DNA around the transcription start site are melted to form a transcription bubble. The transition from the ‘closed'- to ‘open'-promoter complex includes several intermediates and proceeds through nucleation of melting in the −10 element, followed by the downstream propagation of the transcription bubble towards and beyond the transcription start site (Buc and McClure, 1985; Spassky et al, 1985; Sclavi et al, 2005; Rogozina et al, 2009; Schroeder et al, 2009).
Structural and biochemical data suggest that full open-complex formation requires clamping of the downstream part of the promoter DNA duplex (positions −5 to + 20) by the RNAP β′ clamp and β pincer (Craig et al, 1998; Murakami et al, 2002a; Sclavi et al, 2005). The β′ switch-2 element, which links the clamp domain to the RNAP body, likely has an important function in the positioning of template DNA in the RNAP active site (Cramer et al, 2001; Naji et al, 2008). Recently, the β′ switch-2 element was shown to be a target for the antibiotic myxopyronin, which prevents melting of the promoter transcription start site and consequently inhibits transcription (Mukhopadhyay et al, 2008; Belogurov et al, 2009).
Transcription inhibitors are of great interest as tools to dissect the molecular mechanisms of transcription initiation and elongation. In addition, RNAP, a target for a large body of antibiotics, remains an attractive target for antibacterial drug discovery (Villain-Guillot et al, 2007). Currently, rifamycins (rifampicin and its analogues) remain a first line of antibiotic treatment for tuberculosis and are the only clinical inhibitors targeting RNAP. However, use of rifampicin is strongly compromised now because of the spread of resistant forms of bacteria. For this reason, discovery of new inhibitors and their targets has become an urgent goal in medical research.
After rifampicin, the second inhibitor of RNAP proposed for use in the clinic is lipiarmycin (Lpm) (Figure 1A). This drug is a narrow-spectrum, macrocyclic antibiotic produced by Actinoplanes deccanensis (Coronelli et al, 1975). Lpm (also known as fidaxomicin tiacumicin B, OPT-80 or PAR-101) is currently in phase III clinical trials against Clostridium difficile infections and has been shown to be effective against rifampicin-resistant forms of Mycobacterium tuberculosis (Kurabachew et al, 2008). The mechanism of Lpm action is not understood. It was suggested that Lpm does not affect promoter binding, but instead blocks the formation of the first phosphodiester bond during the initiation of RNA synthesis (Sonenshein and Alexander, 1979). In addition, it was shown that the σ-like factors of SPO1 bacteriophage make Bacillus subtilis RNAP resistant to Lpm (Osburne and Sonenshein, 1980).
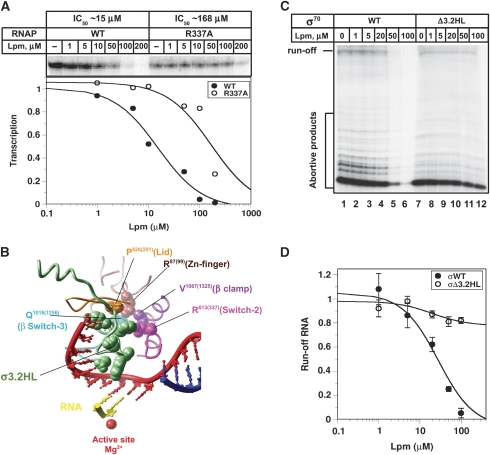
Figure 1.
Modelling of the mutations conferring resistance to Lpm in the structure of the T. thermophilus RNAP. (A) Chemical structure of Lpm. (B) Structure of the core RNAP in complex with scaffold DNA (Vassylyev et al, 2007) with the amino acids substitutions conferring resistance to Lpm shown as CPK. Numbering corresponds to T. thermophilus and E. coli (in brackets) enzymes. The β′ switch-2 (magenta), β switch-3 (cyan), β′ Lid (orange), β′ Zn-finger (brown) and β clamp (purple) elements of the clamp are shown as ribbons. DNA is shown in red (template strand) and dark blue (non-template strand), and RNA is indicated in yellow. Position of the active site is marked by magnesium ion (red sphere). RNAP subunits: β′—khaki, β—blue, α—gold, ω—grey. Molecular graphics images were produced using the UCSF Chimera package (Pettersen et al, 2004).
The probable-binding site for Lpm on the RNAP surface can be defined by the location of amino-acid changes that confer resistance in B. subtilis (Sonenshein et al, 1977; Gualtieri et al, 2006), M. tuberculosis (Kurabachew et al, 2008) and Enterococcus faecalis (Gualtieri et al, 2009). The LpmR substitutions, which mapped exclusively in the β and β′ subunits, are clustered at the N-terminal region of the β′ subunit (comprising evolutionarily conserved regions A, B and C) and the C-terminal region of the β subunit in proximity to region I (Supplementary Figure S1). Positioning of the LpmR mutations on the Thermus thermophilus RNAP structure revealed that the antibiotic targets several functional elements of the RNAP mobile clamp domain: the β′ subunit Zn-finger (E. coli R99 → P,G), β′ Lid (E. coli P251 → R), β′ switch-2 (E. coli R337 → L,Q) and β switch-3 (E. coli Q1256 → K,H,E) (Figure 1B). The β′ subunit Zn-finger and the β′ Lid are located in the ‘clamp core' close to the RNA exit channel, whereas the β′ switch-2 and β switch-3 connect the clamp to the RNAP body (Cramer et al, 2001; Murakami et al, 2002a, 2002b). Potentially, Lpm could affect the function of any of these elements during transcription initiation or could interfere with the growing RNA chain. Characterizing the structure of the RNAP-Lpm complex is necessary to fully understand the mechanism of inhibition. However, transcription experiments have shown that Thermus RNAPs, the only existing models suitable for structural studies, are resistant to Lpm (Supplementary Figure S2). This fact precludes us from performing crystallography experiments. To shed light on the mechanism of Lpm action, we used biochemical and genetic approaches in combination with structural modelling using the available structure of T. thermophilus RNAP (Vassylyev et al, 2002). We analysed the effect of Lpm on transcription initiation at the lacUV5 promoter and on promoter-independent initiation on short synthetic DNA templates (scaffold). Our experiments reveal a new mechanism of transcription inhibitor action that differs from any mechanism described before. We show that Lpm blocks promoter melting and likely traps RNAP at one of the closed intermediates on the pathway to the transcriptionally competent open-promoter complex. To our knowledge, Lpm is the first example of an antibiotic whose activity strongly depends on both the σ subunit structure and the β′ switch-2 element implicated in positioning of the template DNA strand at the RNAP catalytic-site cleft.
Results
Lpm blocks de novo and primed RNA synthesis at the lacUV5 promoter
Earlier, it has been suggested that Lpm targets formation of the first phosphodiester bond during transcription initiation (Sonenshein and Alexander, 1979). To test this possibility, we analysed the effect of Lpm addition on the [32P]-UTP- and ATP-initiated synthesis of the dinucleotide pppApU at the T7A1 promoter and the tetranucleotide pppApApUpU at the lacUV5 promoter (Figure 2A). Indeed, high-resolution gel analysis showed that synthesis of the dinucleotide and the tetranucleotide was inhibited by Lpm. If Lpm functions by blocking the first phosphodiester bond formation, then addition of a dinucleotide RNA primer, which bypasses the first catalytic step, should abrogate the inhibition. To test this assumption, abortive transcription at the lacUV5 promoter was initiated by the addition of three NTPs (UTP, ATP and GTP) either with or without the 100 μM ApA primer complementary to the positions +1 and +2 of the template (Figure 2B). Under these conditions, RNAP synthesizes abortive RNA products up to 9 nt and forms the promoter-proximal elongation complex containing a 16mer RNA (Brodolin et al, 2004). No difference in inhibition of abortive-products synthesis or elongation-complex formation was observed between de novo initiation (IC50∼9.2±2.6 μM) and initiation in the presence of ApA (IC50∼12.5±3 μM). Thus, we concluded that Lpm does not affect the first catalytic step per se. The inhibition concentration (IC50) values obtained for the lacUV5 promoter correspond well to the earlier reported IC50 for E. coli RNAP of 8 μg/ml (Sergio et al, 1975) and the equilibrium dissociation constant value for Lpm-RNAP complex (Kd∼12–23 μM; Talpaert et al, 1975). As earlier reported, the inhibition of RNA synthesis by Lpm exhibited an ‘order-of-addition' effect: it was observed if the drug was added to RNAP only before, not after, the formation of an open-promoter complex (Supplementary Figure S3A) (Sergio et al, 1975; Sonenshein and Alexander, 1979; Sarubbi et al, 2004).
Figure 2.
Lpm inhibits abortive RNA synthesis at the T7A1 and lacUV5 promoters. (A) Inhibition of dinucleotide (AU) and tetranucleotide (AAUU) synthesis by 100 μM of Lpm. Transcription was initiated at the T7A1 and lacUV5 promoters. The [32P]-RNA products separated in a 30% PAGE-7MUrea gel are shown. (B) Effect of ApA primer on the inhibition by Lpm. Transcription initiated at the lacUV5 promoter in the presence of U, G, and A or U, G, A and ApA in the presence of increasing concentrations of Lpm. The [32P]-RNA products separated in a 24% PAGE-7MUrea gel are shown.
Lpm targets the β′ switch-2 element of the E. coli RNAP
Mutations conferring Lpm resistance in E. coli have not been characterized biochemically yet. To explore the mechanism of Lpm action, we focused our study on the β′ switch-2 element, in which one of the spontaneous mutations conferring the LpmR phenotype to E. faecalis, B. subtilis (Gualtieri et al, 2006, 2009) and M. tuberculosis (Kurabachew et al, 2008) was localized. We proposed that Lpm also targets the E. coli RNAP β′ switch-2 element function. To test this possibility, we compared the ability of Lpm to inhibit transcription initiated at the lacUV5 promoter by wild-type E. coli RNAP or mutant RNAPR337A with a substitution of the conserved arginine residue in the β′ switch-2 element (Belogurov et al, 2009). Single-round transcription was initiated by the addition of four NTPs and heparin (Figure 3A). The experiment showed that Lpm effectively inhibited synthesis of full-length RNA transcripts by wild-type RNAP (IC50∼15 μM), whereas transcription initiation by the mutant RNAPR337A was much less sensitive to Lpm (IC50∼168 μM). Thus, we conclude that Lpm may hinder the β′ switch-2 element function, whereas the substitution R337A decreases the affinity of Lpm to its binding site.
Figure 3.
Influence of the mutation in the β′ switch-2 element and the σ70 region 3.2HL deletion on the inhibition of transcription at the lacUV5 promoter. (A) Inhibition of transcription initiated at the lacUV5 promoter by wild-type RNAP (WT) and mutant RNAPR337A (R337A) in the presence of increasing concentrations of Lpm. The run-off [32P]-RNA products separated in a 24% PAGE-7MUrea gel and quantification of the experiment are shown. The quantity of run-off RNA synthesized by wild-type (black circles) or mutant (open circles) RNAPs is plotted as a function of inhibitor concentration. (B) Modelling of the σA subunit region 3.2 from the T. thermophilus holoenzyme (Vassylyev et al, 2002) on the structure of scaffold-RNAP complex (Vassylyev et al, 2007). The σ subunit is shown as green ribbons with the deletion of amino acids 513–519 in the region 3.2 as CPK. The elements of the RNAP clamp domain-bearing LpmR mutations are shown as ribbons, and the mutated residues as spheres. Colour codes are as described in Figure 1B. Dinucleotide RNA primer is shown in yellow. (C) Inhibition of transcription initiated by RNAP carrying either wild-type σ70 (WT) or mutant σΔ3.2HL (Δ3.2HL) in the presence of increasing concentrations of Lpm. [32P]-RNA products separated in a 24% PAGE-7MUrea gel are shown. Transcription was performed in the presence of ApA primer. (D) Quantification of the experiment shown in (C). The quantity of run-off RNA synthesized by wild-type (black circles) or mutant (open circles) RNAPs is plotted as the function of the inhibitor concentration. Mean values and s.d. from two independent experiments are presented.
Deletion of residues 513–519 in the σ70 region 3.2 makes RNAP resistant to Lpm
Analysis of the Thermus RNAP holoenzyme structure showed that the σ region 3.2 filling the RNA exit channel is located close to the Lpm-binding determinants (Figure 3B). Furthermore, the region 3.2 hairpin loop (σ3.2HL) contacts the β′ switch-2 element and the DNA template strand. Thus, Lpm may affect σ-core RNAP interactions, or σ may affect the Lpm-binding site formed by the residues of core RNAP. Indeed, core RNAP-driven transcription was less sensitive to Lpm than holo RNAP-driven transcription (see below and Sonenshein et al (1977)). However, no difference in inhibition efficiency was observed if the drug was added to core RNAP before or after the σ70 subunit, and no change in affinity of the σ70 subunit to core RNAP was observed in the presence of 100 μM of Lpm (Supplementary Figure S3B).
To explore the influence of the σ structure on inhibition, we compared RNA synthesis initiated at the lacUV5 promoter by RNAP containing either wild-type σ70 or σ70 with a deletion of the residues 513–519 (σΔ3.2HL) (Figure 3B). The RNAP-bearing mutant σΔ3.2HL is deficient in de novo initiation on promoters, but is fully active when a dinucleotide primer is used for initiation (Kulbachinskiy and Mustaev, 2006). Transcription initiation at the lacUV5 promoter in the presence of an ApA primer and increasing concentrations of Lpm was analysed. The result of this experiment showed that, when RNAP with the σΔ3.2HL was used, the synthesis of run-off and abortive RNAs was resistant to 100 μM of Lpm, whereas the wild-type enzyme was completely inhibited by Lpm. We propose that region 3.2, because of its proximity to the Lpm-binding determinants, can either contribute to the formation of the binding site and, thus, increase its affinity to Lpm or can modulate the function of the β′ switch-2 in the positioning of template DNA at the active site. The mutant σΔ3.2HL in combination with an RNA primer used for initiation makes transcription resistant to Lpm.
Lpm abolishes lacUV5-promoter melting
If Lpm blocks transcription by hindering the β′ switch-2 function, then it should affect DNA–protein interactions in the open-promoter complex. To test this assumption, we explored whether Lpm blocks the formation of the heparin-resistant open-promoter complex, the step preceding the initiation of RNA synthesis (Figure 4A). First, RNAP binding to the fluorescein-labelled lacUV5-promoter fragments in the presence of 100 μM Lpm and heparin was analysed by the electrophoretic mobility shift assay (EMSA) (Figure 4B). The assay allows us to detect a mixed population of stable, heparin-resistant open (RPo) or intermediate complexes (RPi) formed on the lacUV5 promoter (Straney and Crothers, 1985). The results showed that formation of the heparin-resistant RPo or RPi was strongly abolished by Lpm, whereas closed complex (RPc) formation was not affected. Little inhibition of the RPo/RPi formation was observed when Lpm-resistant RNAPR337A was used in the experiment (Figure 4B). To test further whether the open-complex formation is the target of Lpm, we tested whether RNAP can melt promoter DNA in the presence of the inhibitor. Complexes of wild-type or mutant RNAP and lacUV5 promoter labelled at the template DNA strand were probed by KMnO4, which reacts with the thymines at the open (melted) DNA regions (Figure 4C). The result of the experiment performed with wild-type enzyme shows that the thymines at positions −11, −9, −8 and +1 of the promoter DNA were not accessible to KMnO4 in the presence of Lpm; therefore, the transcription bubble is closed. Little inhibition of promoter melting was observed for the mutant RNAPR337A (Figure 4C, lanes 4 and 5), or if Lpm was added to RNAP after the RPo formation (Figure 4C, lanes 3 and 6). As Lpm fully abolishes the formation of the transcription bubble at the lacUV5 promoter, we conclude that Lpm blocks isomerization of the closed-intermediate complex (RPi) to the open complex.
Figure 4.
Lpm blocks formation of the lacUV5-promoter-open complex. (A) Kinetic steps in transcription initiation at the lacUV5 promoter (Buc and McClure, 1985). P—promoter, R—RNAP, RPc—closed complex, RPi—intermediate complex, RPo—open complex. (B) EMSA of the complexes of RNAP (WT—wild-type, R337A—mutant RNAPR337A) and fluorescein-labelled lacUV5-promoter fragment formed either in the presence of Lpm or heparin or both. Complexes were resolved in native 5% PAGE. The position of the open complex (RPo), closed complex (RPc) and non-bound DNA (Free DNA) are shown. (C) KMnO4 probing of the lacUV5-promoter complexes formed by the wild-type RNAP (WT) and mutant RNAPR337A (R337A) in the presence of 50 μM of Lpm (lanes 2, 3, 5 and 6). Star indicates Lpm added after the RPo formation (lanes 3 and 6). DNA was labelled by fluorescein at the 5′ end of template strand. The positions of thymines reactive to KMnO4 in the open complex are indicated. M—A+G sequencing marker. Profiles on the right show the scan of the lanes for RPo (red) and RPo+Lpm (green). (D, E) DNAse I footprinting of the lacUV5-promoter complexes in the presence of 100 μM of Lpm. DNA labelled either at non-template (D) or template (E) strands. Profiles on the right show the scan of the lanes for free DNA (blue), RPo (red) and RPo+Lpm (green). (F) The bar graph shows quantification of the DNAse I footprinting result for template DNA strand. The ratio of peak area values for indicated bands within the promoter region protected by RNAP in RPo in the presence or without Lpm were normalized to the peak area values for corresponding bands of free DNA. [T-45] and [T-35] designate the sum of signals in the positions −45 to −39 and the positions −34 to −30 correspondingly. The peak areas values of the bands within the each lane were normalized to the area values of the bands not protected by RNAP within the same lane (positions −48, −50). An average and standard errors of two experiments are shown. The quantified bands are indicated by the red stars in panel E. The RNAP holoenzyme domains interacting with the DNA bases at the indicated positions of promoter in RPo are shown schematically beneath the bar graph.
Taking into account that lacUV5 is a mutant promoter with non-optimal 18 bp spacing between the −10 and −35 consensus elements, we explored if Lpm inhibits melting of other promoters as well. Similar to the lacUV5, complete inhibition of promoter melting was observed with two natural promoters: the −10/−35 consensus λPR promoter and the ‘extended –10' consensus galP2 promoter (Supplementary Figure S4A and B) suggesting that mechanism of inhibition is general.
Lpm does not prevent binding of promoter DNA to the RNAP β′ and β pincers
Two possible mechanisms of inhibition by Lpm can be inferred from the above experiments: (1) the downstream segment of promoter DNA (positions −5 to +20) cannot enter the RNAP active-site cleft, as was suggested in the case of myxopyronin (Mukhopadhyay et al, 2008), and, therefore, the promoter melting was abolished; (2) the contacts with downstream DNA were formed, but the melting was abolished, as was observed in the case of the RNAP-promoter complexes formed at low temperature (Spassky et al, 1985; Craig et al, 1998). To discriminate between the two mechanisms proposed above, we probed DNA–protein interactions between RNAP and lacUV5 promoter using DNAse I footprinting (Figure 4D and E). The experiment revealed no significant change in the upstream protection region (non-template positions −45 to −27 and template positions −50 to −28) in the presence of Lpm. Therefore, the RNAP α subunit C-terminal domain and the σ70 subunit region 4.2 remain bound to the promoter DNA in the presence of the drug. In addition, DNAse I hypersensitive sites (non-template positions −24 and −23 and template positions −25, −35 and −36) reflecting bending of DNA in the open complex remained unchanged. The most dramatic changes were observed downstream the −35 element, at the promoter DNA segment that enters into the active-site cleft and the channel formed by the β′, β pincers. Quantification of the template DNA-strand bands intensity showed only 20–40% of protection from DNAse I in the presence of Lpm at the positions −14 and −10 (contacted by the σ70 region 3) and positions −3 and +15 (contacted by the β′, β pincers) (Figure 4F). However, ∼50–60% of protection of the DNA bases at positions +1 and +8 persist in the presence of Lpm. This result suggests that interactions between RNAP and downstream DNA were destabilized, but not inhibited completely. Consistent with this conclusion, no effect of Lpm on the downstream DNAse I protection border was observed for the strong λPR promoter (Supplementary Figure S4C), whereas promoter melting was fully blocked (Supplementary Figure S4A). In addition, some enhancement in the accessibility of DNAse I was observed in the region between positions −20 and +10 of the λPR. Stronger inhibition by Lpm of the lacUV5 downstream DNAse I protection compared with that of λPR likely reflects a difference in the structure and stability of the kinetically significant intermediates formed on these two promoters (Saecker et al, 2002).
To provide additional proof that RNAP remains bound to promoter DNA in the presence of Lpm, we performed Exonuclease III (ExoIII) digestion of promoter complexes (Supplementary Figure S5). The results of the experiment showed that the upstream and downstream borders of protection (ExoIII stops around the position −45 and the position +18) remained unchanged. A slight variation in downstream protection likely reflects the destabilization of the downstream DNA binding and correlated with the DNAse I footprinting results. Finally, the results of the EMSA and footprinting experiments suggest that Lpm does not affect the interaction with the upstream part of the lacUV5 promoter (including the −35 element), but destabilizes binding of downstream DNA to the RNAP clamp as reflected by higher accessibility to endonucleolytic digestion by DNAse I in the presence of Lpm. On the basis of the above results, we propose that promoter melting is blocked by Lpm because of the inability of RNAP to bind the single-stranded DNA template (ssDNA) at the RNAP active-site cleft.
Lpm inhibits σ70-dependent RNA synthesis initiated at minimal DNA templates
Lpm can function either by occluding the downstream DNA from binding to the channel formed by the RNAP pincers or simply by preventing the ssDNA fitting (entry) at the active-site cleft. To explore further the mechanism of inhibition, we used a promoter-less synthetic DNA scaffold (Figure 5A) comprising a 10 nt single-stranded tail and a 9 nt DNA duplex, analogous to the one used for crystallization (Campbell et al, 2001; Vassylyev et al, 2007). The scaffold mimics the downstream segment of promoter DNA (template DNA positions −8 to +11; non-template DNA positions +3 to +11) that enters the RNAP channel formed by the β and β′ pincers in the open-promoter complex. Promoter-independent transcription initiation at the scaffold is strongly stimulated by the σ70 subunit (Figure 5B) in the same manner as the stimulation observed with the M13 origin DNA (Zenkin and Severinov, 2004). The 13mer RNA was the major product synthesized during initiation on the scaffold in the presence of all NTPs and the CpGpC RNA primer (pRNA3). This finding suggests that transcription starts from the one preferable site. Lpm efficiently inhibited de novo RNA synthesis and pRNA3 extension on the assembled scaffold or ssDNA (Figure 5B; Supplementary Figure S6). Furthermore, stronger inhibition was observed on ssDNA (IC50∼16 μM) than on the scaffold (IC50∼35 μM). This result indicates that interactions between the downstream DNA duplex and RNAP antagonized the inhibition.
Figure 5.
Lpm inhibits σ70-dependent RNA synthesis from promoter-less templates. (A) The sequences of the minimal DNA scaffold and the annealed 3 nt RNA primer (pRNA3) are shown. Nucleotides are numbered with respect to the site of the first nucleotide addition (+1). (B) Synthesis of RNA initiated from pRNA3 by core RNAP (lanes 1–10) or holoenzyme (lanes 11–20) in the presence of increasing concentrations of Lpm. Transcription was initiated from either single-stranded template (ssDNA, lanes 1–5, 11–15) or assembled scaffold (scaffold, lanes 6–10, 16–20). The insert on the right shows the low exposure of the gel for lanes 16–20. The lengths of the RNA chains are indicated on the right. (C) Synthesis of RNA initiated from pRNA3 at the scaffold by the mutant holoenzyme or core RNAPR337A in the presence of increasing concentrations of Lpm. (D) Quantification of the experiments shown in (B) and (C). The quantity of 9–12 nt RNA was plotted as a function of the inhibitor concentration. (E) The RNA primer length affects the sensitivity of RNA synthesis to Lpm. A scheme of the assembled scaffold and the 7 nt RNA primer (pRNA7) is shown. The panel on the right shows the RNA products produced after the addition of [32P]-UTP to the RNA primers (pRNA3 and pRNA7) in the presence of Lpm. The length of the synthesized RNA is indicated.
We also observe that transcription initiated by core RNAP at the scaffold or ssDNA is less sensitive to Lpm than transcription initiated by the holoenzyme (compare core RNAP IC50∼70 μM with holo RNAP IC50∼34 μM) (Figure 5B and D). This finding supports the idea that the σ70 subunit contributes to the formation of the Lpm-binding site. Primed RNA synthesis on ssDNA showed a similar sensitivity to Lpm as the sensitivity observed for lacUV5-promoter-dependent transcription (see Figure 2). Thus, the inhibition on the scaffold and on the promoter likely involves the same mechanism. To prove that inhibition of RNA synthesis at the scaffold also occurs by targeting the β′ switch-2, we performed a pRNA3 primer extension assay by mutant holo and core RNAPR337A at increasing Lpm concentrations (Figure 5C and D). The synthesis of RNA was not affected by Lpm in the case of the mutant RNAP. This result indicates that Lpm inhibited transcription on the scaffold through the same mechanism as it did on promoter DNA, likely by targeting switch-2-mediated loading of the ssDNA (positions −8 to +1) to the active-site cleft.
Long RNA–DNA hybrid suppresses Lpm activity
According to the structure of the RNAP-scaffold complex, the interactions with the first 6 nt of the RNA–DNA hybrid comprise most of the contacts formed by the RNAP active-site cleft in the elongation complex (Vassylyev et al, 2007). Indeed, formation of a 7-nt RNA–DNA hybrid leads to significant stabilization of the scaffold-RNAP complex (Korzheva et al, 1998; Sidorenkov et al, 1998). If Lpm impedes template DNA positioning at the RNAP active-site cleft, then stabilization of the template by a long DNA–RNA hybrid should suppress the inhibition. To explore this possibility, we compared the efficiency of inhibition of UTP addition to RNA primers of 2 (pRNA2), 3 (pRNA3), 7 (pRNA7) and 13 (pRNA13) nt in length (Figure 5E; Supplementary Figure S6). Addition of [32P]-UTP to RNA primers was performed in the presence of 100 μM Lpm (Figure 5E) or with increasing concentrations of Lpm (Supplementary Figure S6). The experiments showed that Lpm strongly inhibited nucleotide addition to the pRNA2 and pRNA3, whereas no (or weak) inhibition was observed for pRNA7 and pRNA13. Furthermore, the sensitivity to Lpm was stronger in the case of de novo initiation (IC50∼6 μM) than in the case of initiation from pRNA2 or pRNA3 (IC50∼15 and 35 μM, correspondingly). Thus, Lpm targets RNAP determinants that are required mainly for de novo initiation of RNA synthesis, but are dispensable after the transition to the elongation mode when a stable RNA–DNA hybrid is formed. We propose that long RNA primers confer resistance to Lpm on the scaffold because of stabilization of ssDNA in the active site.
Lpm disturbs contact between RNAP and ssDNA
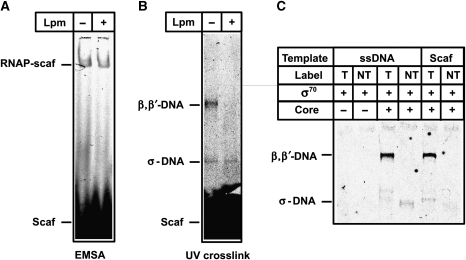
The results obtained on the lacUV5 promoter and on the scaffold suggest that Lpm affects interactions of RNAP with the downstream part of the promoter (positions −11 to +15). To test whether Lpm directly blocks binding of the scaffold to RNAP, EMSA and UV cross-linking experiments were performed in parallel (Figure 6). The EMSA experiment showed that formation of the RNAP-scaffold complex was not significantly affected by 100 μM Lpm at conditions in which transcription is inhibited completely (Supplementary Figure S5A). UV cross-linking was used to test the close interactions between scaffold DNA and RNAP at equilibrium conditions in solution. The cross-links formed between the scaffold (or ssDNA) and RNAP were to core RNAP (β′ or β subunit) and the σ70 subunit. Control experiments showed that cross-links were specific to the template DNA strand (labelled by fluorescein at the 5′ end), and no cross-link to the non-template strand was formed (Figure 6C). Lpm abolished core-specific cross-links (but not the σ70 cross-link) (Figure 6B), but did not prevent RNAP-scaffold complex formation in the EMSA experiment (Figure 6A). These results suggest that Lpm blocks the formation of the contacts between the template DNA strand and core RNAP, or changes the conformation of the DNA-binding site without significantly affecting the overall stability of the complex.
Figure 6.
UV cross-linking and EMSA to probe the DNA–protein interactions in the RNAP-scaffold complex in the presence of Lpm (A, B). The UV cross-linked RNAP-scaffold complexes formed without or in the presence of 100 μM of Lpm were resolved by EMSA (A) or SDS–PAGE (B). (C) UV cross-linking of the oligonucleotides used in scaffold assembly (ssDNA) and assembled scaffold to σ70 and holoenzyme. The position of the label either on template (T) or non-template (NT) DNA strands is indicated. Cross-linked complexes resolved on SDS–PAGE are shown.
Discussion
Lpm blocks promoter melting driven by the RNAP β′ clamp modules
There are two principal findings of this study: (1) the antibiotic Lpm inhibits transcription by blocking formation of a transcriptionally competent open-promoter complex, and (2) the inhibition requires the integrity of the σ70 region 3.2. Structure analysis has shown that Lpm targets several important regulatory elements of the RNAP (the base of β′ Zn-finger, the β′ Lid, the β′ switch-2 and β switch-3) located in the mobile clamp domain. We suggest that the inhibitory effect of Lpm is mainly because of targeting of the β′ switch-2 element and the σ70 region 3.2, because the antibiotic inhibits RNA synthesis started at the synthetic DNA scaffold, which is independent from other elements of the clamp (Naryshkina et al, 2006; Toulokhonov and Landick, 2006; Kent et al, 2009). Indeed, it was shown that the β′ switch-2 controls fitting (or loading) of template DNA into the active-site cleft. This process is required for melting of the promoter transcription start site during open-complex formation (Naji et al, 2008; Belogurov et al, 2009). According to the RNAP structure, σ70 region 3.2 contacts both the β′ switch-2 and template DNA; therefore, it can also participate in template fitting at the active-site cleft (Murakami et al, 2002a; Toulokhonov and Landick, 2006). Our data suggest that Lpm does not block the entry of downstream DNA duplex into the channel formed by the RNAP β′ and β pincers, but it completely blocks the formation of the transcription bubble. Furthermore, using a synthetic DNA scaffold, we show that Lpm impedes the interaction between the RNAP core and the template DNA strand. Thus, we suggest that Lpm abolishes β′ switch-2-dependent fitting of template DNA (positions −10 to +1) to the active-site cleft. This fitting is required for open-complex formation and initiation of RNA synthesis.
Two models based on different sets of data have been proposed for the mechanism of promoter melting during open-complex formation. An early model for open-complex formation suggests that RNAP forms contacts with the downstream part of the promoter (between promoter positions −5 and +25) before the formation of the open transcription bubble (promoter positions from −11 to +2) (Buc and McClure, 1985; Spassky et al, 1985; Craig et al, 1998; Davis et al, 2007). According to the new model based on real-time kinetics analysis (Rogozina et al, 2009; Schroeder et al, 2009), promoter melting can occur before the formation of RNAP contacts with the downstream part of the promoter DNA. Thus, melting and downstream DNA binding by the RNAP pincers can be uncoupled. Our data fit to the model in which binding of downstream DNA to the RNAP pincers are not sufficient for promoter melting, and active participation of the core RNAP clamp modules is required. Lpm hinders the function of the clamp and blocks the isomerization of the closed intermediate (RPi) to the open-promoter complex.
Lpm acts before myxopyronin in the pathway to the open complex
Recently, new antibiotics (myxopyronin, corallopyronin and ripostatin) targeting the β′ switch-2 region have been characterized (Mukhopadhyay et al, 2008; Belogurov et al, 2009). Two models of transcription inhibition for the drugs targeting β′ switch-2 have been proposed: one model suggested that the inhibitor precludes loading of the template DNA strand into the RNAP active-site cleft (Belogurov et al, 2009); another suggested that myxopyronin blocks the RNAP clamp in a partially closed conformation that prevents entry of downstream DNA to the channel formed by the β, β′ pincers (Mukhopadhyay et al, 2008). According to the ‘clamp-closure' model, the inhibitor should not work on ssDNAs. Our results show that Lpm inhibits transcription from an ssDNA more efficiently than from a scaffold DNA with a double-stranded DNA duplex. Hence, Lpm directly targets the ssDNA loading at the RNAP active-site cleft. This finding excludes the myxopyronin-like model of ‘clamp closure' and favours the ‘template loading block' model. Furthermore, we propose that Lpm can lock the clamp in an open or partially open conformation (or it can prevent clamp closure) because the downstream part of the promoter DNA becomes partially protected from DNAse I in the presence of the antibiotic. This result may reflect the weak binding of DNA at the RNAP active-site cleft and subsequent destabilization of the promoter complex.
Therefore, despite the closely positioned binding sites of Lpm and myxopyronin, there are striking differences in the mechanisms of inhibition. Both molecules act at the promoter-melting step; however, the effect of Lpm is much stronger because it completely abolished the transcription bubble formation, whereas myxopyronin only prevented melting of the promoter transcription start site. Therefore, Lpm blocks the transition from closed to open complex, whereas myxopyronin targets the open complex at the final step of promoter melting.
The stronger inhibition of melting induced by Lpm than by myxopyronin is likely due to the concerted targeting of several elements of the RNAP clamp and the σ70 subunit region 3.2. Indeed, blocking only the β′ switch-2 function prevents melting of the start site, but not the upstream part of the transcription bubble (Belogurov et al, 2009). Among the elements targeted by Lpm, only the β′ Lid was shown to be required for stable open-promoter-complex formation (Toulokhonov and Landick, 2006). However, deletion of the β′ Lid has no effect on promoter melting (Naryshkina et al, 2006). Deletion of the β′ Zn-finger has no effect on promoter-complex stability (King et al, 2004), whereas the β switch-3 is implicated in the maintenance of the RNA–DNA hybrid in elongation complexes (Kent et al, 2009). Future studies should explore the effect of LpmR substitutions in the Lid, switch-3 and Zn-finger on the promoter melting and transcription initiation.
Template strand fitting links open-complex formation and de novo initiation of RNA synthesis
We have shown that Lpm can efficiently inhibit σ3.2HL-stimulated (Brodolin K, unpublished) transcription initiation on the synthetic scaffold templates and ssDNA in a β′ switch-2-dependent manner. Thus, template fitting is not only required for promoter melting, but also for efficient binding of NTPs at the active site. Indeed, it was shown that three elements targeted by Lpm, β′ switch-2 (Naji et al, 2008), σ70 region 3.2 (Zenkin and Severinov, 2004; Kulbachinskiy and Mustaev, 2006) and the β′ Lid (Naryshkina et al, 2006; Toulokhonov and Landick, 2006), are required for de novo initiation of RNA synthesis. As these elements do not make direct contact with the RNAP NTP-binding site, whereas they interact with the template DNA, we suggest that the stabilization of the template stimulates the initiation of the first phosphodiester bond synthesis or NTP binding. These regions become dispensable as soon as a sufficiently long RNA is synthesized on a promoter or long RNA primers are used for initiation of transcription on promoters or promoter-less templates (Zenkin and Severinov, 2004; Naryshkina et al, 2006). Such RNA primers likely contribute to the stabilization of the template at the active site leading to promoter melting. Indeed, as was shown in the case of mutant (βΔ186–433) RNAP, lacking one of the pincers (β lobe1), the melting of the lacUV5-promoter transcription start site can be detected during the synthesis of long (>6 nt), but not short (<4 nt), RNAs (Brodolin et al, 2005).
Taken together, these results suggest that formation of the open-promoter complex and de novo initiation of RNA synthesis are tightly linked events subjected to regulation through the template DNA and the β′ switch-2. The best known examples are the ribosomal RNA promoters for which stable open-complex formation is stimulated by the first initiating nucleotides (Gourse, 1988; Ohlsen and Gralla, 1992; Gaal et al, 1997). Indeed, it was shown that the β′ switch-2 element is implicated in regulation of transcription of ribosomal promoters by DksA/ppGpp (Rutherford et al, 2009).
Lpm senses the structure of the σ factor region 3.2
We have shown that transcription inhibition by Lpm depends on the σ3.2HL, which is implicated in the stabilization of incoming NTP at the active site. Two possible mechanisms can be deduced from this finding: (1) σΔ3.2HL contributes to the formation of the Lpm-binding site by interacting with the RNAP clamp domain and (2) σΔ3.2HL regulates the β′ switch-2 function targeted by Lpm. Indeed, transcription initiation on scaffold DNA showed that core RNAP is less sensitive to Lpm (and less active in transcription) than holoenzyme. Hence, σ70 modulates Lpm activity, but it is not mandatory for Lpm binding.
It is tempting to speculate that Lpm abolishes function of the σ3.2HL by preventing the subunit's communication with the β′ switch-2 element or its interaction with the template DNA. Indeed, if Lpm targets the σ3.2HL, then transcription that starts from a dinucleotide or a longer RNA primer (when σ3.2HL is dispensable) should be resistant to Lpm. We observed such a resistance for transcription initiation at the lacUV5 promoter carried by RNAP with the mutant σΔ3.2HL. This finding suggests that the σ3.2HL either affects the conformation of the Lpm-binding site or stabilizes the active conformation of the β′ switch-2 or both. Interestingly, a similar effect of the deletion in the σ3.2HL (residues 507–519, overlapping with the deletion studied in our work) on sensitivity of transcription to rifamycins was earlier reported, and it was suggested that σ3.2HL acts allosterically through the template DNA strand (Artsimovitch et al, 2005). Thus, the drugs targeting different non-overlapping binding sites may inhibit transcription through a similar mechanism. Meanwhile, an alternative ‘steric-occlusion' model of rifampicin action was also proposed (Feklistov et al, 2008).
As the binding site of Lpm is close to the σ subunit region 3.2 that is highly variable between the alternative σ factors, we propose that Lpm inhibition activity should vary between RNAPs bearing alternative σ factors. In support of this proposal, it was shown that transcription of the phage SPO1 later genes driven by the B. subtilis RNAP and the phage σ-like factors is resistant to Lpm (Osburne and Sonenshein, 1980). These findings open the way to design σ-specific drugs targeting specific transcription pathways (e.g. activation of virulence genes). Finally, Lpm is the first example of a transcription inhibitor blocking the early step of open-promoter-complex formation and provides an excellent tool to study this complex process. Clarifying the mechanism of inhibition by Lpm is an essential step in future drug design and awaits new models suitable for crystallographic studies.
Materials and methods
Proteins, DNA templates and RNA
E. coli RNAP core enzyme was purchased from Epicentre. Mutant E. coli core RNAP-bearing substitution R337A was a generous gift from Dr Irina Artsimovitch. The wild-type σ70 subunit cloned in the pET21 vector was expressed in BL21(DE3) strain and purified by affinity chromatography according to Qiagen protocols. The σ70-bearing deletion of amino acids 513–519 was purified as described in Kulbachinskiy and Mustaev (2006). The 116 bp lacUV5-promoter fragment (promoter positions from −59 to +58) was produced by PCR with the primers 5′-CTCACTCATTAGGCACCCCAGGC-3′ and 5′-CCAGGCGGTGAAGGGCAATCAGC-3′ (Sigma). To label DNA either at the template or non-template strand, fluorescein was introduced at the 5′-end of the primer. DNA scaffolds were assembled from two oligonucleotides: 5′-ACAATGCA-3′ and 5′-TGCAATTGTCAGCGATCTA-3′ or 5′-TGCAATTGTCATCCGCTCA-3′ in the transcription buffer (TB) containing 40 mM HEPES pH 8.0, 50 mM NaCl, 5 mM MgCl2 and 5% glycerol. Oligonucleotides were heated at 65°C for 5 min and then annealed by lowering the temperature to 16°C for 30 min. The RNA primers pRNA2 (5′-CG-3′), pRNA3 (5′-CGC-3′), pRNA7 (5′-GAGCGGA-3′) and pRNA13 (5′-CGCUAGAUGUUAA-3′) were purchased from Eurogentec.
Transcription at the lacUV5 and scaffold DNA
Transcription at the lacUV5 promoter was performed in 10 μl TB. RNAP holoenzyme was reconstituted by the mixing of σ70 subunit (0.5 μM final) and 80 nM core RNAP and then incubating for 5 min at 24°C. Next, 1/10 reaction volume of Lpm solution in 50% methanol was added and incubated with RNAP at 37°C for 10 min. The mixture was incubated with lacUV5 DNA fragment (13 nM) at 37°C for 5 min to form an open complex. Transcription initiated by addition of ATP, GTP and CTP (50 μM each) and 3 μCi [α-32P] UTP was performed for 5 min at 37°C in the presence of 0.1 mg/ml heparin. Transcription at the scaffolds was performed as follows: 80 nM RNAP, 200 nM scaffold and 1 μM RNA primer were incubated at 16°C for 5 min then at 24°C for 5 min and supplemented with either [α-32P] UTP or 4 nt as above. Transcription was carried out for 5 min at 24°C. The reactions were stopped by adding 90% formamide. RNA was analysed on 24% PAGE-7MUrea denaturing gel.
DNA–protein cross-linking and EMSA
UV cross-linking of the scaffold-RNAP complexes was performed by 2-min irradiation of the complexes in open Eppendorf tubes placed under the UV-lamp (BLX-E254) at 254 nm. Afterwards, cross-linked complexes were resolved by 6% SDS–PAGE. For EMSA experiments, the RNAP-lacUV5-promoter complexes, formed as described above, were incubated with 100 μg/ml of heparin for 2 min at 37°C and loaded on a 5% native 0.5 × TBE–PAGE. RNAP-scaffold complexes assembled at 16°C were incubated at room temperature for 5 min and loaded on 6% native 0.5 × TBE–PAGE.
KMnO4 probing and DNAse I footprinting
For KMnO4 probing, reactions were performed in 10 μl TB. RNAP (400 nM) was incubated with 100 μM of Lpm for 10 min at 37°C. Fluorescein-labelled lacUV5 promoter (75 nM final) was added to the reaction, which was incubated for another 10 min at 37°C. The samples were treated with 5 mM KMnO4 for 30 s at 37°C. The reaction was stopped with the addition of a ½ volume of 1.5 M potassium acetate and 1 M β-mercaptoethanol. DNA was precipitated by ethanol and incubated with 100 μl 0.5 M piperidine at 90°C for 15 min. Then DNA was precipitated with n-butanol and washed with 70% ethanol. Dried samples were dissolved in 90% formamide and analysed on 10% sequencing gel. For DNAse I footprining experiments, lacUV5-promoter complexes were formed as described for permangante probing. The samples were treated with 5 U/ml DNAse I (Promega) for 1 min. The reactions were stopped by addition of 5 mM EDTA pH 8 and 200 ng poly(dA-dT). Samples were ethanol precipitated, dried and dissolved in 90% formamide. DNA fragments were analysed on 10% sequencing gel. Gels were scanned with Typhoon 9400 Imager (GE Healthcare) and quantified using ImageQuant software (Molecular Dynamics). Peak quantification and data processing were performed as described in Sclavi et al (2005).
Supplementary Material
Acknowledgments
We thank Dr Irina Artsimovitch for providing R337A RNAP, Dr Andrey Kulbachinskiy for the σ70 region 3.2 mutant and critical reading of the paper and Dr Bianca Sclavi and Dr Daria Mamaeva for critical reading and discussion of the paper. KB was supported by RTRS Infectiopole Sud and INSERM. AT was supported by the CNRS and the region Languedoc-Roussillon.
Footnotes
The authors declare that they have no conflict of interest.
References
- Artsimovitch I, Vassylyeva MN, Svetlov D, Svetlov V, Perederina A, Igarashi N, Matsugaki N, Wakatsuki S, Tahirov TH, Vassylyev DG (2005) Allosteric modulation of the RNA polymerase catalytic reaction is an essential component of transcription control by rifamycins. Cell 122: 351–363 [DOI] [PubMed] [Google Scholar]
- Belogurov GA, Vassylyeva MN, Sevostyanova A, Appleman JR, Xiang AX, Lira R, Webber SE, Klyuyev S, Nudler E, Artsimovitch I, Vassylyev DG (2009) Transcription inactivation through local refolding of the RNA polymerase structure. Nature 457: 332–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodolin K, Zenkin N, Mustaev A, Mamaeva D, Heumann H (2004) The sigma 70 subunit of RNA polymerase induces lacUV5 promoter-proximal pausing of transcription. Nat Struct Mol Biol 11: 551–557 [DOI] [PubMed] [Google Scholar]
- Brodolin K, Zenkin N, Severinov K (2005) Remodeling of the sigma70 subunit non-template DNA strand contacts during the final step of transcription initiation. J Mol Biol 350: 930–937 [DOI] [PubMed] [Google Scholar]
- Buc H, McClure WR (1985) Kinetics of open complex formation between Escherichia coli RNA polymerase and the lac UV5 promoter. Evidence for a sequential mechanism involving three steps. Biochemistry 24: 2712–2723 [DOI] [PubMed] [Google Scholar]
- Campbell EA, Korzheva N, Mustaev A, Murakami K, Nair S, Goldfarb A, Darst SA (2001) Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell 104: 901–912 [DOI] [PubMed] [Google Scholar]
- Coronelli C, White RJ, Lancini GC, Parenti F (1975) Lipiarmycin, a new antibiotic from Actinoplanes. II. Isolation, chemical, biological and biochemical characterization. J Antibiot (Tokyo) 28: 253–259 [DOI] [PubMed] [Google Scholar]
- Craig ML, Tsodikov OV, McQuade KL, Schlax PEJ, Capp MW, Saecker RM, Record MTJ (1998) DNA footprints of the two kinetically significant intermediates in formation of an RNA polymerase-promoter open complex: evidence that interactions with start site and downstream DNA induce sequential conformational changes in polymerase and DNA. J Mol Biol 283: 741–756 [DOI] [PubMed] [Google Scholar]
- Cramer P, Bushnell DA, Kornberg RD (2001) Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science 292: 1863–1876 [DOI] [PubMed] [Google Scholar]
- Davis CA, Bingman CA, Landick R, Record MTJ, Saecker RM (2007) Real-time footprinting of DNA in the first kinetically significant intermediate in open complex formation by Escherichia coli RNA polymerase. Proc Natl Acad Sci USA 104: 7833–7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feklistov A, Mekler V, Jiang Q, Westblade LF, Irschik H, Jansen R, Mustaev A, Darst SA, Ebright RH (2008) Rifamycins do not function by allosteric modulation of binding of Mg2+ to the RNA polymerase active center. Proc Natl Acad Sci USA 105: 14820–14825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal T, Bartlett MS, Ross W, Turnbough CLJ, Gourse RL (1997) Transcription regulation by initiating NTP concentration: rRNA synthesis in bacteria. Science 278: 2092–2097 [DOI] [PubMed] [Google Scholar]
- Gourse R (1988) Visualization and quantitative analysis of complex formation between E. coli RNA polymerase and an rRNA promoter in vitro. Nucleic Acids Res 16: 9789–9809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CA, Chan C, Dombroski A, Gruber T, Sharp M, Tupy J, Young B (1998) The functional and regulatory roles of sigma factors in transcription. Cold Spring Harb Symp Quant Biol 63: 141–155 [DOI] [PubMed] [Google Scholar]
- Gualtieri M, Tupin A, Brodolin K, Leonetti J (2009) Frequency and characterisation of spontaneous lipiarmycin-resistant Enterococcus faecalis mutants selected in vitro. Int J Antimicrob Agents 34: 605–606 [DOI] [PubMed] [Google Scholar]
- Gualtieri M, Villain-Guillot P, Latouche J, Leonetti J, Bastide L (2006) Mutation in the Bacillus subtilis RNA polymerase beta' subunit confers resistance to lipiarmycin. Antimicrob Agents Chemother 50: 401–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann JD, deHaseth PL (1999) Protein-nucleic acid interactions during open complex formation investigated by systematic alteration of the protein and DNA binding partners. Biochemistry 38: 5959–5967 [DOI] [PubMed] [Google Scholar]
- Kent T, Kashkina E, Anikin M, Temiakov D (2009) Maintenance of RNA-DNA hybrid length in bacterial RNA polymerases. J Biol Chem 284: 13497–13504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RA, Markov D, Sen R, Severinov K, Weisberg RA (2004) A conserved zinc binding domain in the largest subunit of DNA-dependent RNA polymerase modulates intrinsic transcription termination and antitermination but does not stabilize the elongation complex. J Mol Biol 342: 1143–1154 [DOI] [PubMed] [Google Scholar]
- Korzheva N, Mustaev A, Nudler E, Nikiforov V, Goldfarb A (1998) Mechanistic model of the elongation complex of Escherichia coli RNA polymerase. Cold Spring Harb Symp Quant Biol LXIII: 337–345 [DOI] [PubMed] [Google Scholar]
- Kulbachinskiy A, Mustaev A (2006) Region 3.2 of the sigma subunit contributes to the binding of the 3′-initiating nucleotide in the RNA polymerase active center and facilitates promoter clearance during initiation. J Biol Chem 281: 18273–18276 [DOI] [PubMed] [Google Scholar]
- Kurabachew M, Lu SHJ, Krastel P, Schmitt EK, Suresh BL, Goh A, Knox JE, Ma NL, Jiricek J, Beer D, Cynamon M, Petersen F, Dartois V, Keller T, Dick T, Sambandamurthy VK (2008) Lipiarmycin targets RNA polymerase and has good activity against multidrug-resistant strains of Mycobacterium tuberculosis. J Antimicrob Chemother 62: 713–719 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay J, Das K, Ismail S, Koppstein D, Jang M, Hudson B, Sarafianos S, Tuske S, Patel J, Jansen R, Irschik H, Arnold E, Ebright RH (2008) The RNA polymerase ‘switch region' is a target for inhibitors. Cell 135: 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami KS, Masuda S, Campbell EA, Muzzin O, Darst SA (2002a) Structural basis of transcription initiation: an RNA polymerase holoenzyme-DNA complex. Science 296: 1285–1290 [DOI] [PubMed] [Google Scholar]
- Murakami KS, Masuda S, Darst SA (2002b) Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 A resolution. Science 296: 1280–1284 [DOI] [PubMed] [Google Scholar]
- Naji S, Bertero MG, Spitalny P, Cramer P, Thomm M (2008) Structure-function analysis of the RNA polymerase cleft loops elucidates initial transcription, DNA unwinding and RNA displacement. Nucleic Acids Res 36: 676–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naryshkina T, Kuznedelov K, Severinov K (2006) The role of the largest RNA polymerase subunit lid element in preventing the formation of extended RNA-DNA hybrid. J Mol Biol 361: 634–643 [DOI] [PubMed] [Google Scholar]
- Ohlsen KL, Gralla JD (1992) DNA melting within stable closed complexes at the Escherichia coli rrnB P1 promoter. J Biol Chem 267: 19813–19818 [PubMed] [Google Scholar]
- Osburne MS, Sonenshein AL (1980) Inhibition by lipiarmycin of bacteriophage growth in Bacillus subtilis. J Virol 33: 945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612 [DOI] [PubMed] [Google Scholar]
- Rogozina A, Zaychikov E, Buckle M, Heumann H, Sclavi B (2009) DNA melting by RNA polymerase at the T7A1 promoter precedes the rate-limiting st. Nucleic Acids Res 37: 5390–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford ST, Villers CL, Lee J, Ross W, Gourse RL (2009) Allosteric control of Escherichia coli rRNA promoter complexes by DksA. Genes Dev 23: 236–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saecker RM, Tsodikov OV, McQuade KL, Schlax PE Jr, Capp MW, Record MT Jr (2002) Kinetic studies and structural models of the association of E. coli sigma(70) RNA polymerase with the lambdaP(R) promoter: large scale conformational changes in forming the kinetically significant intermediates. J Mol Biol 319: 649–671 [DOI] [PubMed] [Google Scholar]
- Sarubbi E, Monti F, Corti E, Miele A, Selva E (2004) Mode of action of the microbial metabolite GE23077, a novel potent and selective inhibitor of bacterial RNA polymerase. Eur J Biochem 271: 3146–3154 [DOI] [PubMed] [Google Scholar]
- Schroeder LA, Gries TJ, Saecker RM, Record MTJ, Harris ME, DeHaseths PL (2009) Evidence for a tyrosine-adenine stacking interaction and for a short-lived open intermediate subsequent to initial binding of Escherichia coli RNA polymerase to promoter DNA. J Mol Biol 385: 339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclavi B, Zaychikov E, Rogozina A, Walther F, Buckle M, Heumann H (2005) Real-time characterization of intermediates in the pathway to open complex formation by Escherichia coli RNA polymerase at the T7A1 promoter. Proc Natl Acad Sci USA 102: 4706–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergio S, Pirali G, White R, Parenti F (1975) Lipiarmycin, a new antibiotic from Actinoplanes III. Mechanism of action. J Antibiot (Tokyo) 28: 543–549 [DOI] [PubMed] [Google Scholar]
- Sidorenkov I, Komissarova N, Kashlev M (1998) Crucial role of the RNA:DNA hybrid in the processivity of transcription. Mol Cell 2: 55–64 [DOI] [PubMed] [Google Scholar]
- Sonenshein AL, Alexander HB (1979) Initiation of transcription in vitro inhibited by lipiarmycin. J Mol Biol 127: 55–72 [DOI] [PubMed] [Google Scholar]
- Sonenshein AL, Alexander HB, Rothstein DM, Fisher SH (1977) Lipiarmycin-resistant ribonucleic acid polymerase mutants of Bacillus subtilis. J Bacteriol 132: 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky A, Kirkegaard K, Buc H (1985) Changes in the DNA structure of the lac UV5 promoter during formation of an open complex with Escherichia coli RNA polymerase. Biochemistry 24: 2723–2731 [DOI] [PubMed] [Google Scholar]
- Straney DC, Crothers DM (1985) Intermediates in transcription initiation from the E. coli lac UV5 promoter. Cell 43: 449–459 [DOI] [PubMed] [Google Scholar]
- Talpaert M, Campagnari F, Clerici L (1975) Lipiarmycin: an antibiotic inhibiting nucleic acid polymerases. Biochem Biophys Res Commun 63: 328–334 [DOI] [PubMed] [Google Scholar]
- Toulokhonov I, Landick R (2006) The role of the lid element in transcription by E. coli RNA polymerase. J Mol Biol 361: 644–658 [DOI] [PubMed] [Google Scholar]
- Vassylyev DG, Sekine S, Laptenko O, Lee J, Vassylyeva MN, Borukhov S, Yokoyama S (2002) Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 A resolution. Nature 417: 712–719 [DOI] [PubMed] [Google Scholar]
- Vassylyev DG, Vassylyeva MN, Perederina A, Tahirov TH, Artsimovitch I (2007) Structural basis for transcription elongation by bacterial RNA polymerase. Nature 448: 157–162 [DOI] [PubMed] [Google Scholar]
- Villain-Guillot P, Bastide L, Gualtieri M, Leonetti J (2007) Progress in targeting bacterial transcription. Drug Discov Today 12: 200–208 [DOI] [PubMed] [Google Scholar]
- Zenkin N, Severinov K (2004) The role of RNA polymerase sigma subunit in promoter-independent initiation of transcription. Proc Natl Acad Sci USA 101: 4396–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Campbell EA, Minakhin L, Richter C, Severinov K, Darst SA (1999) Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 A resolution. Cell 98: 811–824 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.