Abstract
Glucose is the preferred carbon source for most cell types and a major determinant of cell growth. In yeast and certain mammalian cells, glucose activates the cAMP-dependent protein kinase A (PKA), but the mechanisms of PKA activation remain unknown. Here, we identify cytosolic pH as a second messenger for glucose that mediates activation of the PKA pathway in yeast. We find that cytosolic pH is rapidly and reversibly regulated by glucose metabolism and identify the vacuolar ATPase (V-ATPase), a proton pump required for the acidification of vacuoles, as a sensor of cytosolic pH. V-ATPase assembly is regulated by cytosolic pH and is required for full activation of the PKA pathway in response to glucose, suggesting that it mediates, at least in part, the pH signal to PKA. Finally, V-ATPase is also regulated by glucose in the Min6 β-cell line and contributes to PKA activation and insulin secretion. Thus, these data suggest a novel and potentially conserved glucose-sensing pathway and identify a mechanism how cytosolic pH can act as a signal to promote cell growth.
Keywords: cytosolic pH, glucose sensing, metabolism, PKA, V-ATPase
Introduction
The vacuolar ATPase (V-ATPase) is a highly conserved proton pump, which resides at the membranes of organelles of biosynthetic and endocytic pathways and mediates intraluminal acidification in an ATP-dependent manner, thereby regulating vacuolar protein turnover, vesicular trafficking and vacuolar fusion (Kane, 2006; Forgac, 2007). However, increasing evidence suggests that V-ATPase is also required for faithful signal transduction processes regulating cell growth and survival (Li et al, 2006; Yan et al, 2009; Cruciat et al, 2010).
V-ATPase is a multi-subunit complex consisting of the membrane inserted V0 sector that comprises the proton pore and a peripheral V1 sector responsible for ATP hydrolysis. In budding yeast, V-ATPase activity is regulated by reversible assembly of the V0–V1 holo-complex. On glucose withdrawal, V-ATPase disassembles into the V0 and V1 sectors, leading to the dissociation of V1 from vacuolar membranes (Kane and Parra, 2000; Seol et al, 2001; Kane, 2006), thus providing an efficient mechanism to shut down V-ATPase activity, possibly to reduce energy consumption during starvation (Kane and Parra, 2000; Forgac, 2007). Yet, the mechanism of V-ATPase regulation remains poorly understood. Interestingly, V-ATPase regulation has been shown to be independent of known glucose-signalling pathways (Parra and Kane, 1998), although this remains a matter of debate in the literature (Bond and Forgac, 2008). Thus, V-ATPase might be part of an earlier unappreciated glucose-signalling pathway in yeast, which could act upstream or in parallel to known glucose-signalling pathways.
One of the major glucose-signalling pathways of budding yeast is the cAMP/protein kinase A (PKA) pathway, which is essential for progression through the G1-phase of the cell cycle (Santangelo, 2006; Dechant and Peter, 2008). PKA is activated through the heterotrimeric G-protein-coupled receptor Gpr1p and its associated Gα subunit Gpa2p, which stimulates cAMP production by adenylate cyclase in response to extracellular glucose (Kubler et al, 1997; Xue et al, 1998; Yun et al, 1998; Rolland et al, 2000). However, activation of the PKA pathway also depends on glucose transport and phosphorylation, suggesting that the accumulation of a glucose metabolite downstream of hexokinase is crucial for PKA activation (Rolland et al, 2001; Gorner et al, 2002). Although the identity of this intracellular signal remains to be identified, it is believed to activate PKA by the highly conserved small G-protein Ras, encoded by RAS1 and RAS2 (Toda et al, 1985; Mbonyi et al, 1988), which stimulates adenylate cyclase in parallel to the Gpr1p/Gpa2p system (Colombo et al, 1998). Glucose-dependent regulation of the Ras proteins might be mediated by the GTPase-activating proteins Ira1p and Ira2p (Colombo et al, 1998, 2004), or its nucleotide exchange factor, Cdc25p (van Aelst et al, 1991; Gross et al, 1992, 1999; Paiardi et al, 2007), but despite its important function in the regulation of cell growth, the molecular mechanisms of glucose-mediated Ras activation are poorly understood.
Interestingly, PKA is also regulated by glucose in certain mammalian cell types. In particular, glucose stimulates the activity of PKA in pancreatic β cells (Kasai et al, 2002; Costes et al, 2004; Hatakeyama et al, 2006). In this cell type, regulation of PKA contributes to the stimulation of insulin secretion (Nesher et al, 2002; MacDonald et al, 2005), which regulates glucose homeostasis and cell growth. Yet, the molecular mechanism of PKA activation remains unknown. As the basic requirements of PKA activation, namely glucose uptake and phosphorylation, are similar in yeast and human cells, it seems likely that the intracellular glucose-sensing mechanism is evolutionary conserved.
Here, we identify V-ATPase as a novel activator of PKA in response to glucose. We find that cytosolic pH is reduced in glucose-starved cells and reduction of cytosolic pH is necessary and sufficient for V-ATPase inactivation on starvation. Thus, cytosolic pH seems to act as a cellular signal for glucose sensing. Furthermore, we provide evidence that changes of the ATP levels directly impinge on cytosolic pH. This is reminiscent of the described glucose-sensing mechanism in pancreatic β-cells, in which increasing ATP concentration mediates glucose sensing through the inactivation of ATP-dependent K+ channels (MacDonald et al, 2005). Finally, we present evidence that V-ATPase activity is regulated by glucose levels in the pancreatic Min6 β-cell line and that V-ATPase regulates PKA activity and insulin secretion in this cell type. Thus, we suggest that intracellular glucose sensing, at least in part, is mediated by conserved pathways in yeast and certain mammalian cells.
Results
V-ATPase is regulated by glucose metabolism
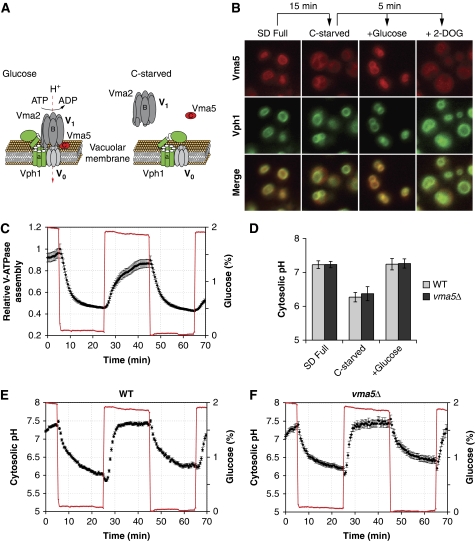
To study the regulation of V-ATPase by glucose, we developed an in vivo assay of V-ATPase assembly using fluorescently tagged subunits of different V-ATPase components. Under control conditions, Vph1p-GFP (a component of the V0 sector), Vma5p-RFP (associated with the V1 sector) and Vma2p-GFP (an integral component of the V1 sector) were all localized to the vacuolar membrane (Figure 1A and B; Supplementary Figure S1; Seol et al, 2001; Sambade et al, 2005; Smardon and Kane, 2007). However, glucose starvation led to a rapid redistribution of Vma5p-RFP and Vma2p-GFP to the cytoplasm, whereas Vph1p-GFP localization was unaffected (Figure 1B; Supplementary Figure S1), demonstrating V-ATPase disassembly under these conditions. Addition of glucose, but not 2-deoxy-glucose (2-DOG), rapidly restored V-ATPase assembly (Figure 1B and C; Parra and Kane, 1998). As 2-DOG is a substrate of hexose transporters and hexokinase, but cannot be metabolized further, these data indicate that V-ATPase assembly requires active glucose metabolism beyond the hexokinase reaction.
Figure 1.
V-ATPase and cytosolic pH are regulated by glucose. (A) Schematic representation of V-ATPase structure and its regulation by glucose. Subunits (a: Vph1p; B: Vma2p, C: Vma5p) used to follow V-ATPase assembly are indicated. (B) Regulation of V-ATPase requires glucose metabolism. Cells were starved for 15 min and imaged 5 min after addition of the indicated carbon source. 2-DOG, 2-deoxy-glucose. Localization of Vma5p (Vma5p-RFP, red) and Vph1p (Vph1p-GFP, green) and merged images are shown. (C) Time-lapse analysis of V-ATPase assembly during glucose starvation/readdition in a microfluidic chamber. V-ATPase assembly is quantified as the coefficient of variation of the intensity of the total cellular Vma5p-RFP signal and plotted as the mean±s.e.m. together with glucose concentration as a function of time (see Materials and methods and Supplementary data for details). (D–F) Cytosolic pH is regulated by glucose, but independent of V-ATPase activity. (D) Cytosolic pH was determined in cells expressing pHluorin grown in SD medium, 10 min after glucose starvation and 10 min after readdition of glucose to starved cells. Data are represented as mean±σ of at least three independent experiments. (E, F) Time-lapse analysis of cytosolic pH during glucose starvation/readdition. The mean±s.e.m. of cytosolic pH and glucose concentration in wild type (E) and vma5Δ (F) expressing pHluorin are plotted as a function of time.
Time-lapse analysis of V-ATPase assembly during repeated cycles of glucose starvation and readdition in a microfluidic chamber (Lee et al, 2007, 2008) confirmed the rapid and reversible regulation of V-ATPase assembly by glucose (Figure 1C). Owing to the fast kinetics observed for V-ATPase regulation, which is independent of known glucose-signalling pathways (Parra and Kane, 1998), we hypothesized that V-ATPase might itself participate in glucose signalling. For example, regulation of V-ATPase might contribute to glucose signalling through modulation of pH homeostasis of yeast cells.
Indeed, in vivo measurement of cytosolic pH using pH-sensitive fluorescent probes revealed a strong glucose dependence of cytosolic pH. Glucose starvation led to a significant decrease of cytosolic pH, which was restored on readdition of glucose to starved cells (Figure 1D; Martinez-Munoz and Kane, 2008; Orij et al, 2009). Similar to V-ATPase assembly, time-lapse analysis of cytosolic pH during repeated cycles of glucose starvation and readdition showed very fast kinetics of cytosolic pH regulation (Figure 1E). Surprisingly, however, deletion of V-ATPase subunits, or inactivation of V-ATPase using Concanamycin A, did not affect the dynamics of cytosolic pH (Figure 1D and F; Supplementary Figure S1). Thus, changes in cytosolic pH are not caused by glucose-dependent regulation of V-ATPase. Rather, cytosolic pH might be an immediate consequence of glucose metabolism acting as a signal in response to glucose.
V-ATPase regulation is mediated by cytosolic pH
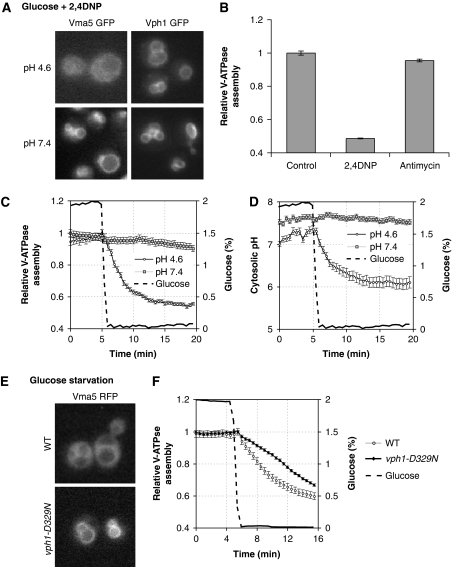
As V-ATPases have been earlier suggested to act as pH sensors (Marshansky, 2007), we directly tested whether cytosolic pH might regulate V-ATPase assembly. Addition of the protonophore 2,4-dinitrophenol (2,4-DNP) equilibrates pH gradients across biological membranes and caused rapid disassembly of V-ATPase in cells grown at low pH even in the presence of glucose (Figure 2A and B). Importantly, 2,4-DNP did not have an effect if the medium was buffered to higher pH (Figure 2A). Moreover, 2,4-DNP did not affect the localization of Vph1p-GFP, thus excluding indirect effects on vacuole integrity or GFP fluorescence at low cytosolic pH. This suggests that 2,4-DNP triggers V-ATPase disassembly through reduction of cytosolic pH. Alternatively, addition of 2,4-DNP might indirectly affect V-ATPase assembly through its effect on mitochondrial membrane potential. However, addition of Antimycin A, an inhibitor of mitochondrial activity, did not affect V-ATPase assembly (Figure 2B), thus effectively ruling out this possibility.
Figure 2.
V-ATPase is regulated by pH in vivo. (A, B) V-ATPase disassembles on intracellular acidification. Logarithmically grown cells were resuspended in fresh SD adjusted to pH 4.6 or 7.4. V-ATPase assembly was scored 2 min after addition of 2,4-DNP (2 mM) in cells expressing Vma5p-GFP and representative images are shown. Cells expressing Vph1p-GFP serve as control. (B) Cells expressing Vma5p-GFP were resuspended in fresh SD medium (pH 4.6) and scored for V-ATPase assembly as in Figure 1C 2 min after addition of 2,4-DNP (2 mM) or 15 min after addition of Antimycin A (1 μg/ml). Data are represented as mean±s.e.m. (C, D) V-ATPase disassembly is defective on starvation at high pH. Time-lapse analysis of V-ATPase assembly (C) and cytosolic pH (D) of cells expressing Vma5p-RFP and pHluorin on starvation with medium adjusted to pH 4.6 or 7.4. Data are represented as in Figure 1C and E. (E, F) Influence of the V-ATPase subunit ‘a' on V-ATPase regulation by glucose. (E) Representative images of cells after 15 min starvation. (F) Time-lapse analysis of V-ATPase assembly of cells expressing wild type or mutated Vph1p. Data are represented as described in Figure 1C.
To test whether reduction of cytosolic pH is necessary for V-ATPase disassembly, we followed V-ATPase assembly and cytosolic pH in cells starved for glucose in media adjusted to low (4.6) or high (7.4) pH. Interestingly, although starvation in low pH media led to rapid disassembly of V-ATPase and reduction of cytosolic pH, glucose starvation in high pH media completely alleviated cytosolic acidification (Figure 2C and D). Likewise, no V-ATPase disassembly was observed on starvation at high pH (Figure 2C). Taken together, these data strongly suggest that reduction of cytosolic pH is both necessary and sufficient for V-ATPase disassembly on glucose starvation. Moreover, this indicates that cytosolic acidification on starvation is, at least in part, caused by an influx of protons from the medium into the cytosol, suggesting that cells are unable to maintain a stable pH gradient across the plasma membrane on starvation.
To further analyse the regulation of V-ATPase by glucose, we aimed to identify point mutants in the vacuole-specific regulatory subunit ‘a', encoded by VPH1, which would abolish glucose-dependent regulation. This subunit has earlier been suggested to act as a pH sensor in mammals (Marshansky, 2007). We, therefore, mutated conserved histidine and aspartic acid residues in the cytoplasmic domain of Vph1p, as these residues might undergo protonation in the observed pH range (Jasti et al, 2007; Marshansky, 2007). Interestingly, although the mutation D329N did not affect V-ATPase assembly or function in the presence of glucose (Figure 2F; Supplementary Figure S2), this mutation significantly delayed V-ATPase disassembly on glucose starvation (Figure 2E and F). Although from the present data it is not possible to conclude that the identified residue is directly protonated on decreasing cytosolic pH in vivo, this mutation allows partial uncoupling of V-ATPase assembly from glucose levels and, therefore, allows testing the functional consequences of V-ATPase disassembly on starvation (see below).
Regulation of cytosolic pH is linked to energy metabolism
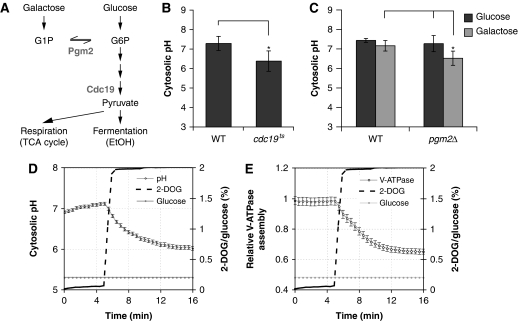
Initial analysis of V-ATPase regulation by glucose suggests that V-ATPase requires an intracellular, metabolic signal that is mediated by cytosolic pH (Figures 1B and 2; Parra and Kane, 1998). Yet, glucose promotes cellular signalling not only through metabolic signals, but also through glucose sensors at the plasma membrane (Santangelo, 2006), which might likewise contribute to the regulation of V-ATPase assembly. Thus, to better characterize the metabolic nature of this signal, we sought to specifically interfere with glucose metabolism, while leaving extracellular glucose sensors unaffected (Figure 3A). Interestingly, inactivation of pyruvate kinase (Cdc19p), a major regulatory enzyme of glycolysis, using a temperature-sensitive allele, cdc19-1, led to significantly reduced cytosolic pH compared with wild type on glucose-containing media (Figure 3B), suggesting that reduced glycolytic flux, rather than altered extracellular glucose signalling on starvation, leads to acidification of the cytosol.
Figure 3.
Regulation of cytosolic pH by glucose metabolism. (A) Important metabolic pathways for glucose and galactose. Genes encoding for enzymes important in this study are indicated. (B) Influence of pyruvate kinase (CDC19) on cytosolic pH. Logarithmically grown cells (25°C) were processed for SNARF-4 staining at 37°C. pH was determined 10 min after glucose addition at 37°C. The average±σ of four independent experiments is shown. Asterisks (*) indicate P-values obtained from a t-test of P<0.05. (C) V-ATPase assembly and cytosolic pH is reduced in pgm2Δ cells on galactose (gal), but not glucose (glc). Cells were grown in YPD or YPGal medium and processed for SNARF-4 staining. Cells were resuspended in SD or SGal and pH was determined after 10 min. Data from four independent experiments are represented as in (B) and asterisks (*) indicate P-values obtained from a t-test of P<0.05. (D, E) Excess 2-DOG leads to reduction of cytosolic pH in glucose-grown cells. Time-lapse analysis of cells expressing Vma5p-RFP and pHluorin grown in 0.2% glucose that were subjected to an excess of 2-DOG (2%). (D) cytosolic pH and (E) V-ATPase assembly were scored as in Figure 1C and E.
To test this hypothesis, we conditionally reduced glycolytic flux, making use of the differential entry of glucose and galactose into glycolysis (Figure 3A). Galactose is first converted to G1P before it enters glycolysis as G6P. Thus, inhibition of the conversion of G1P to G6P using a strain partially defective for phosphoglucomutase by deletion of one of its isoforms, PGM2, will affect glycolysis specifically on galactose, but not on glucose (Boles et al, 1994). Interestingly, cytosolic pH was significantly reduced in pgm2Δ cells on galactose as compared with glucose, whereas no significant difference was observed in wild type for the different carbon sources (Figure 3C). Thus, high cytosolic pH correlates with high glycolytic flux, but does not depend on the activity of a specific glycolytic enzyme.
In mammalian cells, glucose sensing is tightly linked to ATP levels, which increase on glucose stimulation and mediate, at least in part, the glucose signal (reviewed in MacDonald et al, 2005). We thus speculated that efficient glycolysis might promote high cytosolic pH by sustaining high levels of ATP. As 2-DOG cannot be metabolized beyond the hexokinase reaction and the accumulating 2-DOG-phosphate acts as a competitive inhibitor of phosphoglucoisomerase, 2-DOG depletes cells from ATP (Serrano, 1977). Interestingly, addition of an excess of 2-DOG to cells grown in the presence of glucose rapidly decreased cytosolic pH (Figure 3D) and caused disassembly of V-ATPase (Figure 3E). Taken together, these data show that cytosolic pH is tightly linked to glucose metabolism, most likely through ATP, and regulates V-ATPase assembly.
Effects of cytosolic pH on glucose signalling
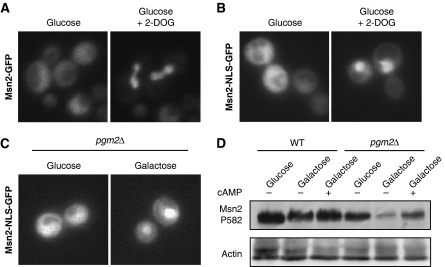
To test whether changes in cytosolic pH would also impinge on glucose signalling, we tested for the localization of the transcription factor Msn2p, which is targeted by multiple signalling cascades in response to environmental cues, including PKA, Snf1/AMPK and TOR, under conditions reducing cytosolic pH. As expected, Msn2p-GFP translocated to the nucleus on glucose starvation (Gorner et al, 1998, 2002; data not shown). Similarly, Msn2p-GFP also accumulated in the nucleus on reduction of cytosolic pH through addition of 2-DOG, suggesting that cytosolic pH also acts as a signal for a glucose-sensitive-signalling pathway (Figure 4A).
Figure 4.
Regulation of glucose signalling by glucose metabolism. (A) Reduction of cytosolic pH triggers nuclear accumulation of Msn2p-GFP. Cells expressing Msn2p-GFP were incubated in 0.2% glucose with or without 2-DOG for 5 min. Localization of Msn2p-GFP is shown. (B) Cells expressing Msn2p-NLS-GFP were treated as in panel A, and scored for Msn2p-NLS-GFP localization. (C) PGM2 is required for cytoplasmic localization of Msn2p-NLS-GFP on galactose, but not glucose. Cells were grown in medium containing 2% glucose or 2% galactose and scored for Msn2p-NLS-GFP localization. (D) Cells of the indicated genotypes were grown in SD (glc) or SGal (gal) medium and stimulated with 20 mM cAMP for 30 min. Msn2p phosphorylation was determined by western blot using a phospho-specific antibody against Msn2p-S582. Actin serves as a loading control.
Although osmotic stress and TOR activity affect Msn2p localization by specifically regulating its nuclear export, PKA and Snf1 target the nuclear import sequence of Msn2p (Gorner et al, 1998, 2002). We, therefore, used a fragment of Msn2p, Msn2p-NLS-GFP, whose localization is regulated by glucose, but is unaffected by TORC1 or osmotic stress (Supplementary Figure S3; Gorner et al, 2002), thus allowing to specifically monitor glucose-mediated regulation of Msn2p. Addition of excess 2-DOG to glucose-grown cells triggered rapid accumulation of Msn2p-NLS-GFP in the nucleus (Figure 4B). Msn2p-NLS-GFP was also found in the nucleus of pgm2Δ cells on galactose (Figure 4C), but not on glucose, which correlates with reduced phosphorylation of a PKA site of Msn2p (S582) on galactose, but not glucose. Moreover, addition of cAMP restored Msn2p phosphorylation, showing that the lack of phosphorylation is due to impaired PKA activation in these cells (Figure 4D). Thus, we conclude that cytosolic pH acts as a cellular signal regulating PKA, which might be mediated, at least in part, by V-ATPase.
V-ATPase is required for activation of PKA
To address whether V-ATPase participates in glucose signalling, we quantified nuclear accumulation of Msn2p-GFP in cells deleted for VMA5 on glucose starvation in a microfluidic chamber. Interestingly, vma5Δ cells displayed significantly faster nuclear accumulation of Msn2p-GFP on glucose starvation compared with wild type (Figure 5A). This suggests that vma5Δ cells are exquisitely sensitive to glucose starvation, possibly because of reduced PKA activity in these cells. To directly test whether V-ATPase is required for activation of the PKA pathway, we measured cAMP levels after glucose stimulation of derepressed cells. Although wild-type cells displayed a robust, transient accumulation of cAMP, cells deleted for V-ATPase activity displayed strongly attenuated cAMP accumulation (Figure 5B), showing that V-ATPase is required for full activation of the PKA pathway in response to glucose. Moreover, expression of a reporter construct assaying Msn2p-dependent transcription of HSP12 and thus indirectly reporting on PKA pathway activity was significantly derepressed in cells deleted for VMA5. However, HSP12 expression could be partially suppressed by addition of exogenous cAMP (Supplementary Figure S4), likely because of reduced PKA activity in vma5Δ cells.
Figure 5.
V-ATPase is required for activation of the Ras/PKA pathway. (A) V-ATPase is required for Msn2p regulation by glucose. Cells expressing Msn2p-GFP were scored for Msn2p localization on glucose withdrawal in a microfluidic chamber. Nuclear accumulation was measured as the coefficient of variation of a region containing the 500 brightest pixels of each cell and plotted as the mean±s.e.m. together with glucose concentration as a function of time. Asterisk denotes P<0.001 derived from a t-test for the indicated time points. (B) V-ATPase is required for efficient cAMP accumulation. cAMP levels in derepressed cells of the indicated genotypes was assayed over time after glucose addition. A representative result of three independent experiments is shown. (C) Ras2p-GTP loading is regulated by V-ATPase. Cells were grown under derepressed conditions, starved and stimulated with glucose at time 0 and the amount of Ras2p-GTP was determined by affinity purification using GST-RBD. The relative amount of Ras2p in the pull down and the input was determined by densitometry. (D) V-ATPase and Gpa2p cooperate in the activation of PKA. Cells of the indicated genotype expressing Msn2p-GFP were starved in a microfluidic chamber for 20 min and Msn2p-GFP localization was followed as in panel A over time after addition of glucose. (E) Disassembly of V-ATPase is required for timely nuclear accumulation of Msn2p-NLS-GFP. Cells expressing Msn2p-NLS-GFP were scored as in panel A for Msn2p-NLS localization on glucose withdrawal in a microfluidic chamber.
V-ATPase might activate PKA by either promoting Ras activity or by stimulating adenylate cyclase activity in a Ras-independent manner. To discriminate between these possibilities, we monitored activation of Ras2p by determination of the GTP loading of Ras2p (Colombo et al, 2004). In wild type, glucose stimulation of starved cells increased the levels of GTP-bound Ras2p. However, the response was only transient (Figure 5C), because of a PKA-dependent negative feedback loop (Colombo et al, 2004). Mutants defective for Ras activation display reduced GTP loading of Ras2p, whereas mutations in the catalytic subunits of PKA, which diminish PKA activity and, therefore, interfere with the negative feedback loop, increase GTP loading of Ras2p (Colombo et al, 2004). Similarly, Ras2p-GTP levels were increased and persisted for a prolonged time after glucose stimulation in cells deleted for VMA2 (Figure 5C), suggesting that V-ATPase promotes PKA pathway activity in parallel, or potentially downstream of Ras2p activity.
Adenylate cyclase is also activated by the plasma membrane receptor Gpr1p and its associated Gα subunit Gpa2p, and V-ATPase might regulate PKA activity through modulation of Gpa2p activity. To test this, we performed time-lapse analysis of Msn2p-GFP localization on glucose addition to starved cells (Figure 5D). Interestingly, although single deletions of either VMA5 or GPA2 only mildly affected nuclear export of Msn2p-GFP in this assay, nuclear export of Msn2p-GFP was strongly delayed in vma5Δ; gpa2Δ double mutants. Similarly, simultaneous deletion of genes encoding for V-ATPase subunits together with GPA2 led to decreased cAMP accumulation on glucose stimulation of starved cells compared with either single mutant (Figure 5B) and strong synthetic-growth defects (Supplementary Figure S5). Finally, although HSP12 was derepressed in either vma2Δ or gpa2Δ single mutants compared with wild type, the gpa2Δ; vma2Δ double mutant displayed even higher expression of HSP12 (Supplementary Figure S4). Thus, V-ATPase and Gpa2p act in parallel to activate the PKA pathway, most likely through adenylate cyclase.
We also sought to investigate whether V-ATPase disassembly contributes to glucose signalling on starvation. Indeed, nuclear accumulation of Msn2p-NLS-GFP was significantly delayed in cells expressing Vph1p-D329N (Figure 5E), showing that impaired disassembly of V-ATPase on starvation is sufficient to interfere with glucose signalling. Taken together, these data establish that activation of the PKA pathway by glucose requires a functional V-ATPase, and shows that, in yeast, cytosolic pH activates PKA, at least in part, through V-ATPase.
Conservation of V-ATPase function in glucose sensing/signalling
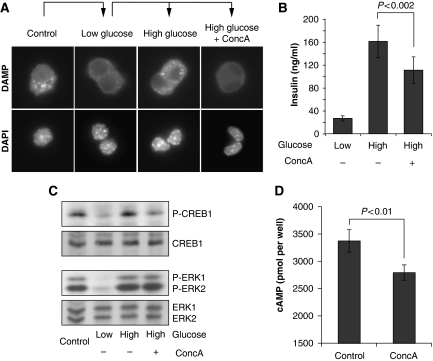
PKA is also regulated by glucose levels in pancreatic β-cells and contributes to the regulation of insulin secretion on glucose stimulation (Kasai et al, 2002; Costes et al, 2004; Hatakeyama et al, 2006). Similarly, cytosolic pH is also regulated by glucose in this cell type (Lindstrom and Sehlin, 1984; Juntti-Berggren et al, 1991; Martinez-Zaguilan et al, 1996; Gunawardana et al, 2004), suggesting that the mechanism of PKA activation might be conserved in yeast and certain mammalian cells. To assay V-ATPase activity, we stained Min6 cells with DAMP, which accumulates in acidified organelles and revealed a punctuated, glucose-dependent pattern. Addition of Concanamycin A before glucose stimulation of starved cells completely abolished the glucose-dependent DAMP staining (Figure 6A), confirming that V-ATPase is also regulated by glucose levels in this cell type.
Figure 6.
V-ATPase is required for insulin secretion and PKA activation in the pancreatic β-cell line Min6. (A) V-ATPase activity is regulated by glucose in Min6 cells. Min6 cells were incubated with low glucose (2.5 mM) medium for 4 h before incubation with KRBH buffer (2.5 mM glucose) for 30 min in the absence or presence of Concanamycin A. Cells were incubated with KRBH containing DAMP, glucose and drug as indicated for 30 min before cells were fixed and DAMP was visualized by immunofluorescence. For control, Min6 cells were grown in high glucose (25 mM) media and incubated with DAMP for 30 min before fixation. (B) V-ATPase inhibition decreases glucose-stimulated insulin secretion. Supernatants of cells in (A) were collected immediately before fixation and assayed for insulin content. A representative result of four independent experiments is shown. Error bars indicating s.d. (σ) and P-value obtained from a t-test are shown. (C, D) V-ATPase contributes to the activation of PKA in Min6 cells. (C) Cells were grown as in (A) and samples were taken for preparation of total lysates 10 min after glucose stimulation and blotted for phospho-S122 CREB1 and phospho-ERK1/2. Equal amounts of extract were blotted for CREB1 and ERK1/2 for control. (D) Cells were incubated with serum-free medium in the presence of the phosphodieasterase inhibitor IBMX and treated with Concanamycin A or drug vehicle for 30 min before determination of cAMP. A representative result of three independent experiments is presented as in (B).
Interestingly, inhibition of V-ATPase with Concanamycin A decreased glucose-stimulated insulin secretion (Figure 6B; Sun-Wada et al, 2006; Stiernet et al, 2007). This defect is unlikely because of impaired processing of the insulin precursor (Sun-Wada et al, 2006), yet could be caused by impaired vesicular fusion of secretory granules, or, potentially by altered glucose signalling. Therefore, we monitored PKA activity by performing immunoblotting experiments with an antibody specifically recognizing CREB1 phosphorylated at S122. As expected (Costes et al, 2004), CREB1 was phosphorylated in control cells in a glucose-dependent manner. However, treatment of cells with the V-ATPase inhibitor Concanamycin A strongly reduced CREB1 phosphorylation on glucose stimulation, whereas glucose-dependent regulation of ERK1 and ERK2 (Costes et al, 2004) was unaffected (Figure 6C). Moreover, acute inhibition of V-ATPase by Concanamycin A significantly reduced intracellular cAMP accumulation (Figure 6D). Thus, we conclude that V-ATPase is required for full activation of PKA in Min6 β-cells and suggest that V-ATPase might be part of a conserved glucose-sensing/signalling pathway in yeast and certain mammalian cells.
Discussion
Despite longstanding interest in the PKA pathway, the molecular mechanisms of its activation in response to glucose have remained elusive. Our findings that V-ATPase is a novel, conserved activator of this pathway shed new light on glucose sensing by PKA in yeast and mammals.
Cytosolic pH as a cellular signal mediating glucose sensing
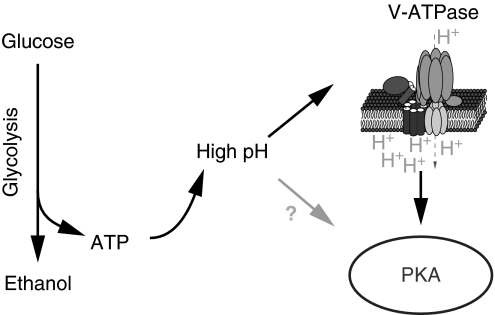
By analysing the regulation of cytosolic pH by glucose and its functional consequences, our studies begin to unravel a conserved signalling pathway. We propose that V-ATPase functions as an intracellular glucose sensor for the PKA pathway, which mediates activation of PKA by pH (Figure 7). Interestingly, we find that cytosolic pH and V-ATPase are regulated by glucose or other fermentable carbon sources with indistinguishable efficiency (Supplementary Table S3). Thus, rather than monitoring the abundance of a specific carbon source or metabolite thereof, yeast cells appear to use cytosolic pH as a second messenger that reports on the availability of a high-quality carbon source.
Figure 7.
Cytosolic pH may act as a second messenger for glucose. Glucose addition to starved cells activates glucose metabolism (glycolysis) and triggers an increase of cytosolic pH, possibly through increasing ATP levels. Increasing pH promotes V-ATPase assembly. Activation of V-ATPase is required for full activation of PKA on glucose stimulation, thereby transducing, at least in part, the pH signal to PKA. In addition, other mechanisms might exist that link cytosolic pH and PKA activation.
This finding identifies an important function of pH as a cellular signal. Despite the fact that changes in pH might be expected to modulate many different cellular activities, we identify V-ATPase as sensor for changes in pH, which in turn modulates PKA activity. Interestingly, various reports have earlier hinted to a link between cytosolic pH and cell proliferation. For example, intracellular pH rapidly increases on fertilization of oocytes, coinciding with proliferation. Similarly, the cytosolic pH of tumour cells was found to be higher than in untransformed controls (Busa and Nuccitelli, 1984; Kurkdjian and Guern, 1989; Casey et al, 2009) and increasing cytosolic pH was sufficient to confer tumourigenicity to cultured fibroblasts (Perona and Serrano, 1988; Perona et al, 1990), raising the possibility that a rather alkaline cytosolic pH is a prerequisite, if not a trigger, for cell proliferation.
Our analysis further suggests that the regulation of cytosolic pH is mediated by energy metabolism. Indeed, inactivation of pyruvate kinase, which is responsible for most of the ATP production of yeast on glucose media (Kuepfer et al, 2005), is sufficient to reduce cytosolic pH. Similarly, depletion of yeast cells from ATP by addition of excess of 2-DOG lowers cytosolic pH (Figure 3; Serrano, 1977). ATP hydrolysis to ADP and Pi generates protons, possibly contributing to cytosolic acidification (Supplementary Table S4). However, because of the rather high buffer capacity of the cytosol (Sigler et al, 1981; Kresnowati et al, 2008), compared with the ATP concentration, the direct effect of ATP hydrolysis probably has a minor function in the regulation of cytosolic pH. It has also been suggested that changes in metabolite concentrations contribute to changes of pH in yeast cells, yet were insufficient to quantitatively explain the observed changes in pH (Kresnowati et al, 2007). Most importantly, however, the plasma membrane ATPase (P-ATPase), which pumps protons from the cytosol into the medium, is highly sensitive to ATP levels (Goossens et al, 2000) and quantitatively contributes to pH homeostasis in yeast (Sigler et al, 1981; Kotyk and Lapathitis, 1998). Although the exact relative contributions of these processes remain to be characterized, this suggests that increasing ATP levels on glucose addition to starved cells raise cytosolic pH both directly and indirectly through P-ATPase (Supplementary Figure S7).
Our findings are reminiscent of the established glucose-sensing mechanism in pancreatic β-cells, in which increasing ATP concentration mediates glucose sensing through closure of ATP-dependent K+ channels in the plasma membrane (MacDonald et al, 2005) to regulate membrane potential. Similarly, glucose signalling in yeast cells appears to depend on their ability to establish a proton gradient across the plasma membrane using an ATP-sensitive proton pump in the plasma membrane, further suggesting that glucose sensing may underlie similar principles in yeast and mammalian cells.
V-ATPase as an activator of PKA
Several lines of evidence identify V-ATPase as a novel upstream regulator of PKA in both systems. In yeast, we show that regulation of V-ATPase assembly is required for faithful regulation of Msn2p localization. More importantly, V-ATPase is required for maximal stimulation of cAMP production on glucose addition and regulation of GTP loading of Ras2p, clearly showing a function of V-ATPase in the activation of the PKA pathway. Similarly, inhibition of V-ATPase activity using Concanamycin A impaired glucose-stimulated CREB1 phosphorylation at a PKA-specific site (S133) and reduced cAMP accumulation. In principle, V-ATPase might regulate cAMP levels through activation of adenylate cyclase or inhibition of phosphodiesterases, which degrade cAMP (Supplementary Figure S7). However, decreased cAMP accumulation on V-ATPase inhibition in Min6 cells was observed in the presence of the phosphodiesterase inhibitor IBMX, suggesting that V-ATPase activity stimulates cAMP production rather than inhibiting its turnover. Nevertheless, the specific mechanism, how V-ATPase promotes PKA pathway activity, remains to be identified.
It is important to note that PKA was recently suggested to regulate V-ATPase activity in yeast. Specifically, V-ATPase failed to disassemble on starvation using biochemical assays for V-ATPase assembly in strains with elevated PKA activity (Bond and Forgac, 2008), thereby contradicting earlier reports (Parra and Kane, 1998). Similarly, our in vivo data place V-ATPase upstream of the PKA pathway and failed to show an effect of PKA activity on V-ATPase disassembly (Supplementary Figure S6). Moreover, we found that, in vivo, V-ATPase assembly is exquisitely sensitive to pH, which cannot be appropriately accounted for in cell extracts prepared in buffer of a certain pH. Yet, a positive feedback loop might exist, which further increases V-ATPase activity on activation of PKA (Supplementary Figure S7). Therefore, hyperactivation of PKA might lead to delayed disassembly on glucose starvation under certain experimental settings. Although an influence of this feedback loop on V-ATPase disassembly can be readily detected cell extracts, when effects of pH on V-ATPase assembly are somewhat alleviated, this feedback loop is too weak to significantly influence V-ATPase disassembly under our experimental conditions in vivo.
Regulation of V-ATPase in higher eukaryotic cells
Regulation of V-ATPase assembly and activity is a well-known phenomenon that can be observed in many model systems. For example, glucose stimulation decreases luminal pH of secretory granules in β-cells through stimulation of V-ATPase activity (Stiernet et al, 2006). Likewise, V-ATPase assembly and localization are regulated by glucose in kidney cells, and require PI-3 kinase activity (Sautin et al, 2005). Similar to our data from yeast, V-ATPase activity has been shown to be pH sensitive in kidney epithelial cells (Hurtado-Lorenzo et al, 2006), yet it remains to be shown if changes in cytosolic pH on glucose stimulation are responsible for V-ATPase regulation in other cell types.
In addition, several kinases have been implicated in the regulation of V-ATPase in animals, including PKA and AMPK (Sautin et al, 2005; Dames et al, 2006; Rein et al, 2008; Hallows et al, 2009; Voss et al, 2009; Gong et al, 2010; Paunescu et al, 2010). In contrast to yeast cells, regulation of cell growth in animals is governed not only by metabolic signals, but also critically depends on growth factors, which coordinate cell growth in tissues and organisms. It will, therefore, be interesting to determine whether the regulation of V-ATPase by AMPK and PKA has evolved as an additional mechanism that integrates with metabolic regulation of V-ATPase or has evolved to coordinately regulate V-ATPase activity within an organism or tissue.
V-ATPase as a drug target
Defects in glucose signalling in β-cells are intimately linked with the development of metabolic disorders, such as diabetes (Bell and Polonsky, 2001; Muoio and Newgard, 2008), and are thus subject of increasing interest for therapeutic intervention. The identification of a novel glucose-sensing pathway might contribute to further understanding of the molecular defects leading to these pathologies. Interestingly, three loci linked with the development of diabetes map close to genes encoding for components of the V-ATPase (Online Mendelian Inheritance in Man, OMIM (TM)), consistent with a potential genetic contribution of alterations of V-ATPase to the development of the disease.
Interestingly, inhibitors of V-ATPase function have earlier been suggested to be potent anti-cancer drugs (Bowman and Bowman, 2005; Forgac, 2007) and overexpression of the V-ATPase subunit ‘B2' has been correlated with increased survival on application of apoptotic stimuli. This effect was alleviated by inhibition of the MEK1/ERK MAP kinase pathway (Li et al, 2006), suggesting that V-ATPase modulates cellular signalling also in this system. Thus, V-ATPases might have widespread functions in cellular signalling beyond their function in the intraluminal acidification of organelles, and studying the emerging function of V-ATPases in the regulation of cell growth and survival will contribute to our understanding of cellular signalling.
Materials and methods
Yeast strains (Supplementary Table S1) and plasmids (Supplementary Table S2) are listed in Supplementary data. Yeast cells were grown in synthetic medium (SD or SGal; 0.17% yeast nitrogen base, 0.5% (NH4)2SO4, 2% glucose or galactose and amino acids). Glucose starvation was performed in SC (SD w/o glucose). Unless stated otherwise, synthetic media were adjusted to pH 4.6 by addition of NaOH. YFP expression was analysed on an FACScalibur device (Becton & Dickinson). cAMP measurement of yeast cells (Rolland et al, 2000) and Quinacrine staining (Roberts et al, 1991) were performed as described. Chemicals were purchased from Sigma unless stated otherwise.
pH measurements
Cytosolic pH was measured in cells expressing a pH-sensitive GFP variant, pHluorin (Brett et al, 2005; Schulte et al, 2006), and imaged on excitation with CFP (436±20 nm) and YFP (500±20 nm) excitation filters. Emission was recorded with a YFP emission filter (550±30 nm) filter and the ratio of intensities in these channels was quantified using in-house MATLAB (The Mathworks)-based software. Alternatively, pH was measured using the pH-sensitive dye SNARF-4 (Molecular Probes) essentially as described (Valli et al, 2005). Cells were grown in YPD or YPGal, and incubated with the dye in McIlvaine buffer (pH 4.6) for 30 min at room temperature. Afterwards, cells were resuspended in SC and treated with or without the indicated carbon source for 10 min. Images were taken with filter sets appropriate for Rhodamine (excitation: 560±40 nm, emission: 630±75 nm) and GFP (470±40 nm, 525±50 nm). Calibration was performed by incubation of cells in McIlvaine buffer of different pH containing digitonin essentially as described (Orij et al, 2009).
Microfluidics experiments
Microfluidic experiments were performed in a polydimethylsiloxane microfluidic cell culture chamber and an automated pressure controller available from CellASIC Corp (San Leandro, CA) to control the exchange of media (Lee et al, 2007, 2008). Cells were loaded into the microfluidic chamber (height=4–5 μm) by pressurizing (Cookson et al, 2005). Cells were maintained in a fixed position and followed by fluorescence microscopy during repeated exchange of medium with and without glucose. Glucose concentration was monitored by addition of a fluorescent dye (Alexa Fluor 647-Dextran 10 000 M.W., Invitrogen) to glucose-containing media.
Automated image segmentation and quantification
Cells are segmented on the basis of a MATLAB (The Mathworks)-based in-house algorithm and the mean intensity and the s.d. of pixel intensities of each cell are calculated. From these values, the coefficient of variation (CV=s.d./mean) is calculated as a quantitative readout for V-ATPase assembly. Cells with assembled V-ATPase are characterized by bright fluorescence at the vacuolar rim, but low cytoplasmic fluorescence (high CV), whereas cells with disassembled V-ATPase display mostly uniform cytoplasmic staining (low CV). Note that this readout is insensitive to cell-to-cell variation of expression levels, yet dependent on vacuole morphology. To normalize for the influence of vacuole morphology, data are divided by the maximal CV observed throughout the experiment for a given strain (relative V-ATPase assembly). These values do not directly allow quantifying an absolute ratio of assembled V-ATPase/total V-ATPase, but allow for a relative assessment of V-ATPase assembly under changing environmental conditions. For quantification of Msn2p relocalization, the CV was calculated for the 500 brightest pixels in the GFP channel (a region approximately twice as big as the nucleus).
Ras2p-GTP loading
Determination of GTP loading of Ras2p has been performed essentially as described (Colombo et al, 2004). In brief, cells were starved for glucose and total cell lysates were prepared after glucose addition at the indicated time points. Lysates were subjected to affinity purification with GST-RBD (Ras-GTP-binding domain) and the ratio of Ras2p-GTP/total Ras2p was quantified after western blotting using densitometry.
Cell culture and DAMP staining, insulin and cAMP measurements
Min6 cells were grown in DMEM (Invitrogen) supplemented with 15% FCS, 2 μl/l β-mercaptoethanol and 100 mM Na-pyruvate. For detection of acidic compartments, cells were grown in low glucose media for 4 h, washed and incubated in Krebs–Ringer buffer (KRBH, 140 mM NaCl, 3.6 mM KCl, 1.5 mM CaCl2, 0.5 mM NaH2P04, 0.5 mM MgSO4, 10 mM Hepes, 2 mM NaHCO3, 0.1% BSA) containing 2.5 mM glucose and 50 μM DAMP (Molecular Probes) for 30 min. Then, cells were stimulated for 30 min with KRBH containing 25 mM glucose and 100 μM Concanamycin A or drug vehicle alone as indicated. Cells were stained for DAMP using anti-dinitrophenyl antibody (Molecular Probes) as described (Sautin et al, 2005). For insulin measurements, supernatants of cells processed for DAMP staining were collected before cell fixation and analysed for insulin content by ELISA assay (Linco). Three to four samples per experiment per condition were measured in duplicate in parallel. cAMP was measured essentially as described (Costes et al, 2004) using an ELISA-based kit (Amersham).
Western blotting and antibodies
Yeast extracts were prepared by the TCA method as described (Kraft et al, 2008). Whole cell lysates of Min6 cells were obtained by resuspension of cells in sample buffer on ice. Phospho-Msn2p (S582) antibody was a kind gift of Gustav Ammerer. Phospho-CREB1 (S133) and Phospho-p44/p42 MAPK (Erk1/Erk2) antibodies were purchased from Cell Signaling Technology.
Supplementary Material
Acknowledgments
We thank B Andrews for communicating unpublished results, G Ammerer, W Reiter, F Rudolf, R Rao and C Boone for yeast strains and reagents, J Rosseels and Ch Rupp for excellent technical assistance and F Rudolf and members of the Peter Laboratory and the Competence Center for Systems Physiology and Metabolic Disease (CC-SPMD) for helpful discussions. We are indebted to J Zehetner and C Danzer for help with Min6 experiments. RD was supported by a Schrödinger Fellowship by the Austrian FWF and the CC-SPMD. JW is supported by the FWO-Vlaanderen and KU Leuven. Work in the Peter Laboratory is supported by the EU projects QUASI and UNICELLSYS, the Swiss National Science Foundation (SNF), the Swiss Initiative in Systems Biology SystemsX (RTD projects YeastX and LiverX) and the ETH Zürich.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bell GI, Polonsky KS (2001) Diabetes mellitus and genetically programmed defects in beta-cell function. Nature 414: 788–791 [DOI] [PubMed] [Google Scholar]
- Boles E, Liebetrau W, Hofmann M, Zimmermann FK (1994) A family of hexosephosphate mutases in Saccharomyces cerevisiae. Eur J Biochem 220: 83–96 [DOI] [PubMed] [Google Scholar]
- Bond S, Forgac M (2008) The Ras/cAMP/protein kinase A pathway regulates glucose-dependent assembly of the vacuolar (H+)-ATPase in yeast. J Biol Chem 283: 36513–36521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman EJ, Bowman BJ (2005) V-ATPases as drug targets. J Bioenerg Biomembr 37: 431–435 [DOI] [PubMed] [Google Scholar]
- Brett CL, Tukaye DN, Mukherjee S, Rao R (2005) The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol Biol Cell 16: 1396–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busa WB, Nuccitelli R (1984) Metabolic regulation via intracellular pH. Am J Physiol 246(4 Part 2): R409–R438 [DOI] [PubMed] [Google Scholar]
- Casey JR, Grinstein S, Orlowski J (2009) Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11: 50–61 [DOI] [PubMed] [Google Scholar]
- Colombo S, Ma P, Cauwenberg L, Winderickx J, Crauwels M, Teunissen A, Nauwelaers D, de Winde JH, Gorwa MF, Colavizza D, Thevelein JM (1998) Involvement of distinct G-proteins, Gpa2 and Ras, in glucose- and intracellular acidification-induced cAMP signalling in the yeast Saccharomyces cerevisiae. EMBO J 17: 3326–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo S, Ronchetti D, Thevelein JM, Winderickx J, Martegani E (2004) Activation state of the Ras2 protein and glucose-induced signaling in Saccharomyces cerevisiae. J Biol Chem 279: 46715–46722 [DOI] [PubMed] [Google Scholar]
- Cookson S, Ostroff N, Pang WL, Volfson D, Hasty J (2005) Monitoring dynamics of single-cell gene expression over multiple cell cycles. Mol Syst Biol 1: 2005.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes S, Longuet C, Broca C, Faruque O, Hani EH, Bataille D, Dalle S (2004) Cooperative effects between protein kinase A and p44/p42 mitogen-activated protein kinase to promote cAMP-responsive element binding protein activation after beta cell stimulation by glucose and its alteration due to glucotoxicity. Ann NY Acad Sci 1030: 230–242 [DOI] [PubMed] [Google Scholar]
- Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C (2010) Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327: 459–463 [DOI] [PubMed] [Google Scholar]
- Dames P, Zimmermann B, Schmidt R, Rein J, Voss M, Schewe B, Walz B, Baumann O (2006) cAMP regulates plasma membrane vacuolar-type H+-ATPase assembly and activity in blowfly salivary glands. Proc Natl Acad Sci USA 103: 3926–3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechant R, Peter M (2008) Nutrient signals driving cell growth. Curr Opin Cell Biol 20: 678–687 [DOI] [PubMed] [Google Scholar]
- Forgac M (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8: 917–929 [DOI] [PubMed] [Google Scholar]
- Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, Hallows KR, Pastor-Soler NM (2010) Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol 298: F1162–F1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens A, de La Fuente N, Forment J, Serrano R, Portillo F (2000) Regulation of yeast H(+)-ATPase by protein kinases belonging to a family dedicated to activation of plasma membrane transporters. Mol Cell Biol 20: 7654–7661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorner W, Durchschlag E, Martinez-Pastor MT, Estruch F, Ammerer G, Hamilton B, Ruis H, Schuller C (1998) Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev 12: 586–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorner W, Durchschlag E, Wolf J, Brown EL, Ammerer G, Ruis H, Schuller C (2002) Acute glucose starvation activates the nuclear localization signal of a stress-specific yeast transcription factor. EMBO J 21: 135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, Winograd S, Marbach I, Levitzki A (1999) The N-terminal half of Cdc25 is essential for processing glucose signaling in Saccharomyces cerevisiae. Biochemistry 38: 13252–13262 [DOI] [PubMed] [Google Scholar]
- Gross E, Goldberg D, Levitzki A (1992) Phosphorylation of the S. cerevisiae Cdc25 in response to glucose results in its dissociation from Ras. Nature 360: 762–765 [DOI] [PubMed] [Google Scholar]
- Gunawardana SC, Rocheleau JV, Head WS, Piston DW (2004) Nutrient-stimulated insulin secretion in mouse islets is critically dependent on intracellular pH. BMC Endocr Disord 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows KR, Alzamora R, Li H, Gong F, Smolak C, Neumann D, Pastor-Soler NM (2009) AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am J Physiol Cell Physiol 296: C672–C681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama H, Kishimoto T, Nemoto T, Kasai H, Takahashi N (2006) Rapid glucose sensing by protein kinase A for insulin exocytosis in mouse pancreatic islets. J Physiol 570(Part 2): 271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, Brown D, Marshansky V (2006) V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 8: 124–136 [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E (2007) Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323 [DOI] [PubMed] [Google Scholar]
- Juntti-Berggren L, Arkhammar P, Nilsson T, Rorsman P, Berggren PO (1991) Glucose-induced increase in cytoplasmic pH in pancreatic beta-cells is mediated by Na+/H+ exchange, an effect not dependent on protein kinase C. J Biol Chem 266: 23537–23541 [PubMed] [Google Scholar]
- Kane PM (2006) The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol Mol Biol Rev 70: 177–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane PM, Parra KJ (2000) Assembly and regulation of the yeast vacuolar H(+)-ATPase. J Exp Biol 203(Part 1): 81–87 [DOI] [PubMed] [Google Scholar]
- Kasai H, Suzuki T, Liu TT, Kishimoto T, Takahashi N (2002) Fast and cAMP-sensitive mode of Ca(2+)-dependent exocytosis in pancreatic beta-cells. Diabetes 51(Suppl 1): S19–S24 [DOI] [PubMed] [Google Scholar]
- Kotyk A, Lapathitis G (1998) Extracellular acidification by Saccharomyces cerevisiae in normal and in heavy water. Folia Microbiol (Praha) 43: 623–625 [DOI] [PubMed] [Google Scholar]
- Kraft C, Deplazes A, Sohrmann M, Peter M (2008) Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat Cell Biol 10: 602–610 [DOI] [PubMed] [Google Scholar]
- Kresnowati MT, Suarez-Mendez C, Groothuizen MK, van Winden WA, Heijnen JJ (2007) Measurement of fast dynamic intracellular pH in Saccharomyces cerevisiae using benzoic acid pulse. Biotechnol Bioeng 97: 86–98 [DOI] [PubMed] [Google Scholar]
- Kresnowati MT, Suarez-Mendez CM, van Winden WA, van Gulik WM, Heijnen JJ (2008) Quantitative physiological study of the fast dynamics in the intracellular pH of Saccharomyces cerevisiae in response to glucose and ethanol pulses. Metab Eng 10: 39–54 [DOI] [PubMed] [Google Scholar]
- Kubler E, Mosch HU, Rupp S, Lisanti MP (1997) Gpa2p, a G-protein alpha-subunit, regulates growth and pseudohyphal development in Saccharomyces cerevisiae via a cAMP-dependent mechanism. J Biol Chem 272: 20321–20323 [DOI] [PubMed] [Google Scholar]
- Kuepfer L, Sauer U, Blank LM (2005) Metabolic functions of duplicate genes in Saccharomyces cerevisiae. Genome Res 15: 1421–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkdjian A, Guern J (1989) Intracellular pH: measurement and importance in cell activity. Annu Rev Plant Physiol Plant Mol Biol 40: 271–303 [Google Scholar]
- Lee PJ, Ghorashian N, Gaige TA, Hung PJ (2007) Microfluidic system for automated cell-based assays. JALA Charlottesv Va 12: 363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PJ, Helman NC, Lim WA, Hung PJ (2008) A microfluidic system for dynamic yeast cell imaging. Biotechniques 44: 91–95 [DOI] [PubMed] [Google Scholar]
- Li G, Yang Q, Krishnan S, Alexander EA, Borkan SC, Schwartz JH (2006) A novel cellular survival factor—the B2 subunit of vacuolar H+-ATPase inhibits apoptosis. Cell Death Differ 13: 2109–2117 [DOI] [PubMed] [Google Scholar]
- Lindstrom P, Sehlin J (1984) Effect of glucose on the intracellular pH of pancreatic islet cells. Biochem J 218: 887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PE, Joseph JW, Rorsman P (2005) Glucose-sensing mechanisms in pancreatic beta-cells. Philos Trans R Soc Lond B Biol Sci 360: 2211–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshansky V (2007) The V-ATPase a2-subunit as a putative endosomal pH-sensor. Biochem Soc Trans 35(Part 5): 1092–1099 [DOI] [PubMed] [Google Scholar]
- Martinez-Munoz GA, Kane PM (2008) Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. J Biol Chem 283: 20309–20319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Zaguilan R, Gurule MW, Lynch RM (1996) Simultaneous measurement of intracellular pH and Ca2+ in insulin-secreting cells by spectral imaging microscopy. Am J Physiol 270 (5 Part 1): C1438–C1446 [DOI] [PubMed] [Google Scholar]
- Mbonyi K, Beullens M, Detremerie K, Geerts L, Thevelein JM (1988) Requirement of one functional RAS gene and inability of an oncogenic ras variant to mediate the glucose-induced cyclic AMP signal in the yeast Saccharomyces cerevisiae. Mol Cell Biol 8: 3051–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, Newgard CB (2008) Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 9: 193–205 [DOI] [PubMed] [Google Scholar]
- Nesher R, Anteby E, Yedovizky M, Warwar N, Kaiser N, Cerasi E (2002) Beta-cell protein kinases and the dynamics of the insulin response to glucose. Diabetes 51(Suppl 1): S68–S73 [DOI] [PubMed] [Google Scholar]
- Online Mendelian Inheritance in Man (OMIM (TM)) McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University (Baltimore, MD) and National Center for Biotechnology Information, National Library of Medicine (Bethesda, MD). http://www.ncbi.nlm.nih.gov/omim/
- Orij R, Postmus J, Ter Beek A, Brul S, Smits GJ (2009) In vivo measurement of cytosolic and mitochondrial pH using a pH-sensitive GFP derivative in Saccharomyces cerevisiae reveals a relation between intracellular pH and growth. Microbiology 155 (Part 1): 268–278 [DOI] [PubMed] [Google Scholar]
- Paiardi C, Belotti F, Colombo S, Tisi R, Martegani E (2007) The large N-terminal domain of Cdc25 protein of the yeast Saccharomyces cerevisiae is required for glucose-induced Ras2 activation. FEMS Yeast Res 7: 1270–1275 [DOI] [PubMed] [Google Scholar]
- Parra KJ, Kane PM (1998) Reversible association between the V1 and V0 domains of yeast vacuolar H+-ATPase is an unconventional glucose-induced effect. Mol Cell Biol 18: 7064–7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D (2010) cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol 298: F643–F654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona R, Portillo F, Giraldez F, Serrano R (1990) Transformation and pH homeostasis of fibroblasts expressing yeast H(+)-ATPase containing site-directed mutations. Mol Cell Biol 10: 4110–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona R, Serrano R (1988) Increased pH and tumorigenicity of fibroblasts expressing a yeast proton pump. Nature 334: 438–440 [DOI] [PubMed] [Google Scholar]
- Rein J, Voss M, Blenau W, Walz B, Baumann O (2008) Hormone-induced assembly and activation of V-ATPase in blowfly salivary glands is mediated by protein kinase A. Am J Physiol Cell Physiol 294: C56–C65 [DOI] [PubMed] [Google Scholar]
- Roberts CJ, Raymond CK, Yamashiro CT, Stevens TH (1991) Methods for studying the yeast vacuole. Methods Enzymol 194: 644–661 [DOI] [PubMed] [Google Scholar]
- Rolland F, De Winde JH, Lemaire K, Boles E, Thevelein JM, Winderickx J (2000) Glucose-induced cAMP signalling in yeast requires both a G-protein coupled receptor system for extracellular glucose detection and a separable hexose kinase-dependent sensing process. Mol Microbiol 38: 348–358 [DOI] [PubMed] [Google Scholar]
- Rolland F, Wanke V, Cauwenberg L, Ma P, Boles E, Vanoni M, de Winde JH, Thevelein JM, Winderickx J (2001) The role of hexose transport and phosphorylation in cAMP signalling in the yeast Saccharomyces cerevisiae. FEMS Yeast Res 1: 33–45 [DOI] [PubMed] [Google Scholar]
- Sambade M, Alba M, Smardon AM, West RW, Kane PM (2005) A genomic screen for yeast vacuolar membrane ATPase mutants. Genetics 170: 1539–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo GM (2006) Glucose signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 70: 253–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautin YY, Lu M, Gaugler A, Zhang L, Gluck SL (2005) Phosphatidylinositol 3-kinase-mediated effects of glucose on vacuolar H+-ATPase assembly, translocation, and acidification of intracellular compartments in renal epithelial cells. Mol Cell Biol 25: 575–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte A, Lorenzen I, Bottcher M, Plieth C (2006) A novel fluorescent pH probe for expression in plants. Plant Methods 2: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol JH, Shevchenko A, Deshaies RJ (2001) Skp1 forms multiple protein complexes, including RAVE, a regulator of V-ATPase assembly. Nat Cell Biol 3: 384–391 [DOI] [PubMed] [Google Scholar]
- Serrano R (1977) Energy requirements for maltose transport in yeast. Eur J Biochem 80: 97–102 [DOI] [PubMed] [Google Scholar]
- Sigler K, Kotyk A, Knotkova A, Opekarova M (1981) Processes involved in the creation of buffering capacity and in substrate-induced proton extrusion in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 643: 583–592 [DOI] [PubMed] [Google Scholar]
- Smardon AM, Kane PM (2007) RAVE is essential for the efficient assembly of the C subunit with the vacuolar H(+)-ATPase. J Biol Chem 282: 26185–26194 [DOI] [PubMed] [Google Scholar]
- Stiernet P, Guiot Y, Gilon P, Henquin JC (2006) Glucose acutely decreases pH of secretory granules in mouse pancreatic islets. Mechanisms and influence on insulin secretion. J Biol Chem 281: 22142–22151 [DOI] [PubMed] [Google Scholar]
- Stiernet P, Nenquin M, Moulin P, Jonas JC, Henquin JC (2007) Glucose-induced cytosolic pH changes in beta-cells and insulin secretion are not causally related: studies in islets lacking the Na+/H+ exchangeR NHE1. J Biol Chem 282: 24538–24546 [DOI] [PubMed] [Google Scholar]
- Sun-Wada GH, Toyomura T, Murata Y, Yamamoto A, Futai M, Wada Y (2006) The a3 isoform of V-ATPase regulates insulin secretion from pancreatic beta-cells. J Cell Sci 119 (Part 21): 4531–4540 [DOI] [PubMed] [Google Scholar]
- Toda T, Uno I, Ishikawa T, Powers S, Kataoka T, Broek D, Cameron S, Broach J, Matsumoto K, Wigler M (1985) In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell 40: 27–36 [DOI] [PubMed] [Google Scholar]
- Valli M, Sauer M, Branduardi P, Borth N, Porro D, Mattanovich D (2005) Intracellular pH distribution in Saccharomyces cerevisiae cell populations, analyzed by flow cytometry. Appl Environ Microbiol 71: 1515–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Aelst L, Jans AW, Thevelein JM (1991) Involvement of the CDC25 gene product in the signal transmission pathway of the glucose-induced RAS-mediated cAMP signal in the yeast Saccharomyces cerevisiae. J Gen Microbiol 137: 341–349 [DOI] [PubMed] [Google Scholar]
- Voss M, Schmidt R, Walz B, Baumann O (2009) Stimulus-induced translocation of the protein kinase A catalytic subunit to the apical membrane in blowfly salivary glands. Cell Tissue Res 335: 657–662 [DOI] [PubMed] [Google Scholar]
- Xue Y, Batlle M, Hirsch JP (1998) GPR1 encodes a putative G protein-coupled receptor that associates with the Gpa2p Galpha subunit and functions in a Ras-independent pathway. EMBO J 17: 1996–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Denef N, Schupbach T (2009) The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev Cell 17: 387–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun CW, Tamaki H, Nakayama R, Yamamoto K, Kumagai H (1998) Gpr1p, a putative G-protein coupled receptor, regulates glucose-dependent cellular cAMP level in yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun 252: 29–33 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.