Abstract
Hedgehog (Hh) pathway has a pivotal function in development and tumorigenesis, processes sustained by stem cells (SCs). The transcription factor Nanog controls stemness acting as a key determinant of both embryonic SC self-renewal and differentiated somatic cells reprogramming to pluripotency, in concert with the loss of the oncosuppressor p53. How Nanog is regulated by microenvironmental signals in postnatal SC niches has been poorly investigated. Here, we show that Nanog is highly expressed in SCs from postnatal cerebellum and medulloblastoma, and acts as a critical mediator of Hh-driven self-renewal. Indeed, the downstream effectors of Hh activity, Gli1 and Gli2, bind to Nanog-specific cis-regulatory sequences both in mouse and human SCs. Loss of p53, a key event promoting cell stemness, activates Hh signalling, thereby contributing to Nanog upregulation. Conversely, Hh downregulates p53 but does not require p53 to control Nanog. Our data reveal a mechanism for the function of Hh in the control of stemness that represents a crucial component of an integrated circuitry determining cell fate decision and involved in the maintenance of cancer SCs.
Keywords: Gli1, Hedgehog, medulloblastoma, Nanog, neural stem cells
Introduction
Nanog is a core intrinsic determinant of self-renewal and pluripotent cellular state of embryonic stem cells (ESCs) of the blastocyst inner cell mass, and its expression is downregulated on differentiation (Chambers et al, 2003; Mitsui et al, 2003; Boiani and Scholer, 2005; Pan and Thomson, 2007). Nanog is also required for early embryo development, as Nanog-deficient mice fail to develop beyond the blastocyst stage (Mitsui et al, 2003). Reprogramming experiments of differentiated somatic cells by expression of Oct4, Sox2, c-Myc and Klf4, reactivate the production of Nanog+ pluripotent stem cells (SCs) (Takahashi and Yamanaka, 2006; Brambrink et al, 2008), indicating that Nanog has an important function in determining stemness features. Consistently, overexpression of Nanog gives rise to pluripotent SCs (Yu et al, 2007; Hanna et al, 2009). Recently, Nanog has been described to be reactivated in cancer cells (e.g. glioma, pancreas, colorectal, endometrial) possibly as a reminiscence of stemness properties of cancer SCs (Clement et al, 2007; Hubbard et al, 2009; Jeter et al, 2009; Ji et al, 2009; Meng et al, 2010; Wen et al, 2010).
These observations have emphasized the notion that cancer cells and SCs might be governed by common mechanisms. Such conclusions are also supported by recent works demonstrating that loss of the tumor suppressor p53 is another key determinant of stemness and of transcriptional activation of Nanog (Lin et al, 2005; Hong et al, 2009; Kawamura et al, 2009). Thus, the elucidation of the poorly understood events that regulate Nanog in postnatal SCs represents a fundamental aspect to elucidate both SC and tumor biology.
Hedgehog (Hh) pathway has a pivotal function in cell development and tumorigenesis in a wide variety of tissues, both processes sustained by SCs. More specifically, Hh signalling sustains embryonic and postnatal NSCs of forebrain subventricular zone and hippocampus (Ahn and Joyner, 2005; Lai et al, 2003; Machold et al, 2003; Palma et al, 2005; Palma and Ruiz i Altaba, 2004), as well as cerebellar NSCs and glioma SCs overexpressing a stemness gene signature (e.g. Nanog, Oct4, Sox2, CD133) (Clement et al, 2007; Stecca and Ruiz i Altaba, 2009). A paradigmatic Hh-target organ is cerebellum, where Hh is critically required to keep transit-amplifying granule cell progenitors (GCPs) undifferentiated, to promote their proliferation (Dahmane and Ruiz i Altaba, 1999; Wallace, 1999; Wechsler-Reya and Scott, 1999) and to cause medulloblastoma (Mb) (Ruiz i Altaba, 2006). Aberrant Hh signalling activation occurs in Mb as a consequence of genetic or epigenetic changes affecting several components of the pathway (recently reviewed in Teglund and Toftgard, 2010). For instance, loss-of-function mutation of Patched1 (Ptc1) (the receptor of the Hh ligands) mimics the ligand-induced relief of the inhibition on the transducer Smoothened (Smo), leading to constitutive pathway activation. Downstream effectors of Smo activity are Gli transcription factors, which act on a set of target genes promoting cell proliferation and reducing cell differentiation. These target genes include Gli1 itself, thus autoreinforcing the signalling strength and representing a sensitive read out of the pathway.
The identity of Hh/Gli-target genes involved in the control of stemness in NSCs and cancer SCs is poorly understood. Here, we show that both Nanog and Gli1 are highly expressed in postnatal cerebellar NSCs and in Hh-dependent mouse and human Mb SCs. Nanog is required as a critical mediator of Hh-driven self-renewal of NSCs, as Hh acts through transcriptional activation of both mouse and human Nanog. Our data also suggest Nanog involvement in the Hh-dependent stemness properties of brain tumors.
Results
Cerebellar neurospheres coexpress Nanog and Gli1
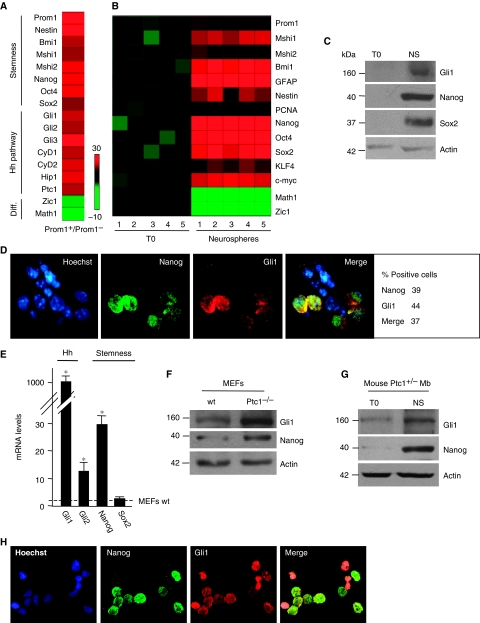
Earlier studies have shown the existence of a subpopulation of NSCs in mouse postnatal cerebellum (Klein et al, 2005; Lee et al, 2005). Thus, to investigate a functional connection between Hh and intrinsic stemness determinants, we sorted Prominin1+ cells, (Supplementary Figure S1A) from cerebella of 4-day-old mice. We found that these cells displayed enhanced expression of stemness markers (Nanog, Oct4, Sox2, Bmi1, Musashi1, Prominin1, Nestin) together with Hh pathway target genes (Ptc1, Hip1, Cyclin D1–2 and Gli1), compared with Prominin1-negative cells (Figure 1A). Conversely, differentiation markers (e.g. the granule cell lineage markers Math1 and Zic1) were significantly reduced (Figure 1A; Supplementary Figure S1B), suggesting that Hh activity preferentially associates to stemness features.
Figure 1.
Cerebellar neurospheres coexpress Nanog and Gli1. (A, B) Heat map of RT–qPCR gene expression (stemness, Hh pathway and differentiation (Diff) markers) of FACS-sorted Prominin1+ versus Prominin1− cells from eight cerebella of 4-day-old mice (three representative experiments) (A) or 24 h cultured cerebellar cells (T0) versus derived neurospheres (B). A red–green colour scale (−10 to +30) depicts markers expression normalized to three housekeeping genes. (C) Western blot analysis of endogenous Gli1 and stemness markers levels in cerebellar cells (T0) and derived neurospheres (NS). (D) Immunofluorescence or nuclear Hoechst (blue) staining with Nanog (green), Gli1 (red) or both antibodies in disgregated neurospheres from murine cerebellum. The relative percentage of either Nanog or Gli1 or double-positive (marked) cells is indicated on the right. (E) mRNA levels evaluated by RT–qPCR of Hh (Gli1, Gli2) or stemness (Nanog, Sox2) markers in mouse embryonic fibroblasts (MEFs) Ptc1−/− respect to wild-type MEFs (wt). *P<0.05 versus wild-type MEFs. (F) Western blot analysis of Gli1 and Nanog levels in Ptc1 wt versus Ptc1−/− MEFs. (G) Western blot analysis of endogenous Gli1 and Nanog in Mb cells (T0) and derived neurospheres (NS). (H) Immunofluorescence or nuclear Hoechst (blue) staining with Nanog (green) and Gli1 (red) antibodies in disgregated neurospheres from mouse Ptc1+/− Mb.
Cerebellar SCs formed primary neurospheres and were further propagated as secondary neurospheres from single-cell suspensions indicating that they were able to self-renew.
To characterize SCs, we analysed mRNA and protein expression of stemness markers, differentiation markers and Hh activity (Gli1 expression) in neurospheres with respect to pre-neurosphere cells. Although we found loss of differentiation markers Math1 and Zic1 (Figure 1B), we observed enhanced expression of stemness markers together with Hh pathway activation in neurospheres (Figure 1B and C).
Notably, we also found, by immunofluorescence, coexpression of Gli1 and Nanog in about 40% of cells within neurospheres (Figure 1D), whereas Nanog did not stain rare cells positive for neuronal differentiation marker MEF2D (Supplementary Figure S1C, upper panels). These results suggest us a possible functional connection between Hh signalling and Nanog.
To analyse this relationship, we studied Nanog expression ex vivo in conditions of Hh pathway constitutive activation using the Ptc1−/− mouse embryo fibroblasts (MEFs) model, in which the activation of Gli is a consequence of Ptc1 deletion (Goodrich et al, 1997). In this context, we observed increased Nanog mRNA and protein levels (Figure 1E and F). Accordingly, in neurospheres derived from murine Ptc1+/− Mbs, both Nanog and Gli1 levels were higher than in pre-neurosphere cells and mostly displayed cellular coexpression (Figure 1G and H). Collectively, these observations further support the notion that Nanog and Hh signalling are functionally connected.
Function of Hh–Gli and Nanog in cerebellar neurospheres
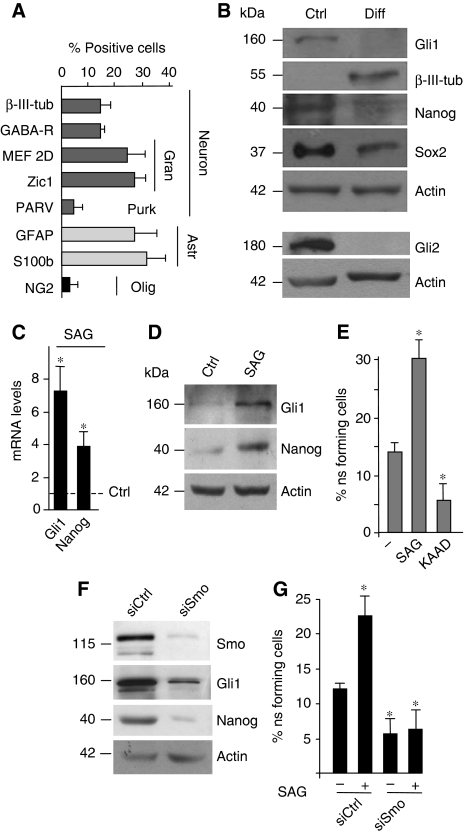
Cerebellar neurosphere multipotency is indicated by the ability to differentiate into distinct lineages (Figure 2A) when cultured under appropriate conditions (Lee et al, 2005), whereas the reduction of Gli1, Gli2, Nanog and Sox2 expression suggests that Hh activity and stemness features were coregulated during differentiation (Figure 2B). To understand the function of Hh in the control of cerebellar NSCs, we tested the effects of the activation of the pathway by the Smo-agonist SAG (Chen et al, 2002; Ruiz i Altaba, 2006). SAG enhanced Gli1, Nanog and Prominin1 mRNA and protein levels (Figure 2C and D; Supplementary Figure S2A) as well as the percentage of neurosphere-forming cells (Figure 2E). Conversely, treatment with the Hh inhibitor KAAD cyclopamine had a suppressive effect on NSCs self-renewal and on Gli1 and Nanog expression (Figure 2E; Supplementary Figure S2B). Importantly, SAG-induced Gli1 is coexpressed with Nanog (Supplementary Figure S2C), indicating that both are targets of Hh activity. Consistently, inactivation of Hh signalling by siRNA-mediated depletion of Smo significantly reduced the levels of Smo, Gli1 and Nanog (Figure 2F) as well as the self-renewal ability, both after SAG treatment and under basal conditions (Figure 2G).
Figure 2.
Function of Hh–Gli and Nanog in cerebellar neurospheres. (A, B) Percentage of positive cells detected with immunofluorescence staining (A) or western blot (B) with the indicated antibodies in neurospheres cultured in D-poly-ornithine-coated chamber slides with N2 medium plus PDGF (10 ng/ml) for 7 days (A, Gran, Granule cells; Purk, Purkinje cells; Astr, astrocytes; Olig, oligodendrocytes) (B, differentiated (Diff)). (C, D) mRNA (C) and protein levels (D) of Gli1 and Nanog evaluated by RT–qPCR or western blot relative to housekeeping controls, in neurospheres before (Ctrl) and after 48-h SAG treatment. (E) Neurosphere-forming assay of single cells derived from secondary neurospheres cultured in basal stem medium (−) or in the presence of SAG or KAAD cyclopamine for 10 days. (F, G) Western blot (F) or neurosphere-forming assay (G) after transfection with control (siCtrl) or Smo siRNA (siSmo) in the absence or in the presence of SAG for 10 days. *P<0.05 versus untreated cells.
As Hh enhances the proliferation of GCPs (Dahmane and Ruiz i Altaba, 1999; Wallace, 1999; Wechsler-Reya and Scott, 1999), we hypothesized that it could favour NSCs towards the expansion of the granule cell lineage. Treatment of neurospheres with SAG for 4 or 10 days followed by shift to differentiation medium did not influence the multipotency of cerebellar NSCs, as no significant change in the generation of the various cerebellar cell lineages was observed compared with untreated neurospheres (Supplementary Figure S2D). Overall, these findings show that Hh signalling is required to maintain both self-renewal and Nanog expression in cerebellar NSCs.
Nanog is required for Hh-induced NSCs self-renewal
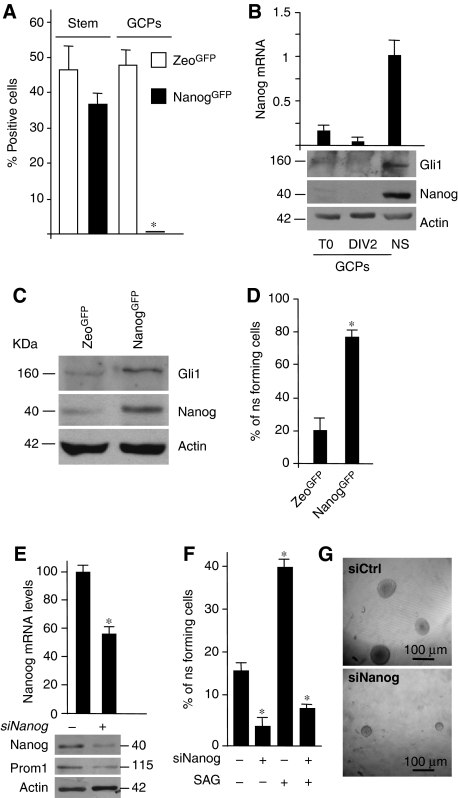
To understand the function of Nanog in postnatal NSCs and its connection with Hh signalling, we studied the self-renewal potential of Nanog-positive cells. To this purpose, we infected NSCs with a Nanog-GFP lentiviral vector expressing GFP protein under the control of Nanog promoter (Nanog-GFP). We observed a significant expression of GFP in NSCs, whereas we did not detect any expression in GCPs obtained from primary cerebellar cell cultures (as described in Di Marcotullio et al, 2006) (Figure 3A). GCPs are ‘already committed' precursor cells, which do not express stem markers but are characterized by neuronal markers (MEF2D) as shown in Supplementary Figure S1C.
Figure 3.
Nanog is required for Hh-induced NSC self-renewal. (A) Histograms showing the percentage of GFP+ cells in postnatal cerebellar secondary neurospheres (NS) and GCPs 48 h after infection with Nanog-GFP or Zeo-GFP control lentiviral vector. *P<0.05 versus Zeo-GFP-positive cells. (B) mRNA and protein levels of Nanog and Gli1 in neurospheres (NS) or GCPs before (T0) or after 2 days culture (DIV2). (C) Western blots of Gli1 and Nanog in Nanog-GFP versus Zeo-GFP-sorted neurosphere-derived cells. (D) Secondary neurosphere-forming assay of GFP-sorted cells as in panel (A). (E) mRNA (upper panel) and protein levels (bottom panel) of Nanog and Prominin1 in neurospheres after siRNA-mediated silencing of Nanog (siNanog +) versus control siRNA (−). (F) Secondary neurosphere-forming assay after control siRNA (siCtrl) or Nanog siRNA (siNanog), treated (+) or not (−) with SAG. *P<0.05 versus untreated or Zeo-GFP cells. (G) Representative bright field images of cerebellar neurosphere after transfection with control siRNA (siCtrl) or Nanog siRNA (siNanog).
As control, a CMV-driven-GFP lentivector (Zeo-GFP) was similarly expressed in both cell types. Of relevance, Nanog-GFP expression paralleled the one of endogenous Nanog in NSCs, whereas both were not expressed in differentiated GCPs (Figure 3B and C).
To show the function of Nanog in NSC self-renewal, we sorted NanogGFP+ cells and we found that they were able to form secondary neurospheres to a significantly higher extent compared with Zeo-GFP-positive cells (Figure 3D). Accordingly, silencing of Nanog reduced the expression of Prominin1 (Figure 3E) and significantly impaired the ability to form neurospheres compared with control (Figure 3F and G), elucidating its function in self-renewal. Moreover, Nanog depletion abrogated the enhancing activity of SAG on neurosphere-forming assay (Figure 3F), indicating that Nanog is required for Hh effects on NSCs.
Hh regulates SC self-renewal through a p53-independent pathway
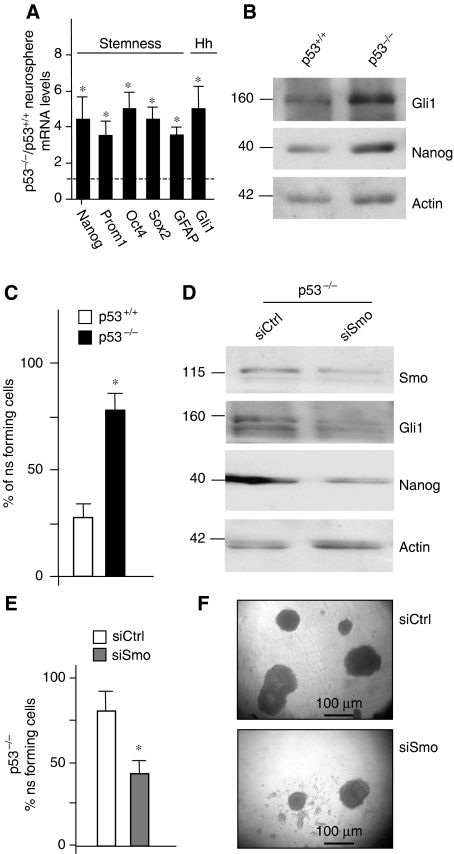
As p53 suppresses Nanog transcription and self-renewal ability in ESCs (Lin et al, 2005) and can be repressed by Hh signalling through Mdm2 (Abe et al, 2008; Stecca and Ruiz i Altaba, 2009), we addressed the function of p53 in Hh-induced regulation of Nanog and NSC self-renewal. To this end, cerebellar NSCs have been isolated from p53-deficient mice (Jacks et al, 1994). The derived neurospheres displayed a significantly enhanced expression of stemness markers including Nanog (Figure 4A and B) and, consistently, a higher clonogenic activity with respect to wild-type cells (Figure 4C). Interestingly, p53-deficient neurospheres also displayed higher levels of Gli1 (Figure 4A and B), suggesting that p53 downregulates Hh signalling and confirming recent observations (Stecca and Ruiz i Altaba, 2009). Importantly, abrogation of Hh signalling in p53-deficient neurospheres by means of siRNA-mediated depletion of Smo, still reduced Nanog levels and neurosphere-forming ability (Figure 4D–F), suggesting that p53 is at least in part dispensable in this context. Therefore, in addition to p53-mediated control, Hh regulates Nanog and NSCs self-renewal also in a p53-independent manner.
Figure 4.
Hh regulates stem cell self-renewal through a p53-independent pathway. (A, B) RT–qPCR (A) and western blot (B) analysis of stemness markers and Gli1 in neurospheres from p53-deficient mouse cerebella (p53−/−). (A) Results are expressed as mean values of five different neurosphere cultures from p53−/− mice with respect to p53+/+ wild type (dashed line). (C) Secondary neurosphere-forming assay of p53−/− cells with respect to cells from p53 wild-type mice. (D) Western blot analysis of Smo, Gli1 and Nanog in p53−/− neurospheres after transfection with siRNA against Smo (siSmo) compared with control siRNA (Ctrl). (E, F) Secondary neurosphere-forming assay (E) and representative bright field images (F) of cerebellar p53−/− cells after transfection with siRNA against Smo (siSmo) or control siRNA (siCtrl). *P<0.05 versus untreated p53+/+ cells (A, C) or siCtrl (E).
Hh/Gli activate Nanog transcription
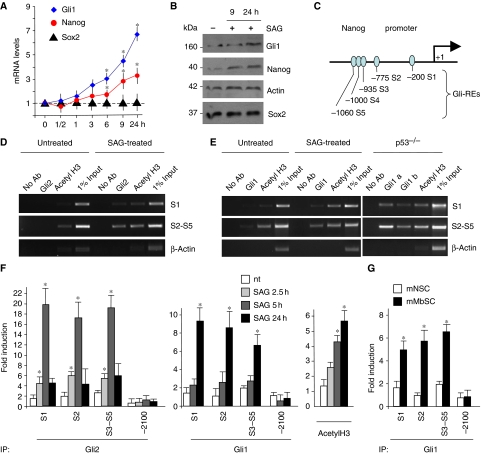
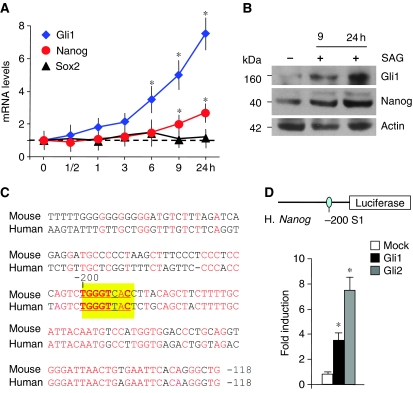
To address how Hh signalling affects Nanog expression, we performed a time course of SAG treatment. SAG increased Nanog mRNA (as early as 3–6 h) and protein levels (9 h) mimicking the induction of a direct Hh target, Gli1 (Figure 5A and B). Of note, other stemness markers, such as Sox2 (Figure 5A and B), were not affected, suggesting that Hh-mediated enhancement of Nanog could depend not only on expansion of NSC population, but also on specific transcriptional regulation. Indeed, a number of potential Gli-binding sequences were observed in a region spanning from −1060 to −200 bp of Nanog (Figure 5C; Supplementary Figure S3A). As Gli2 is an early transducer of SAG-dependent Smo activation leading to its accumulation into the nucleus (Supplementary Figure S3B) (Park et al, 2000; Ikram et al, 2004; Lipinski et al, 2006), we performed chromatin immunoprecipitation (ChIP) experiments to determine the in vivo occupancy of endogenous Gli2 onto the Nanog promoter in mouse NSCs. Gli2 was recruited to the S1 (−200 bp) and S2 to S5 (−775, −935, −1000 and −1060 bp) sites (but not to −2100 bp site devoid of Gli consensus sequence) following 2.5 and 5 h SAG treatment (Figure 5D and F). Gli2 recruitment was accompanied by an increase of acetylated histone H3, indicating that this promoter was transcriptionally activated by SAG (Figure 5D and F). As SAG-activated Gli2 enhances Gli1 transcription, representing the second wave of Hh activity, we determined Gli1 recruitment to Nanog promoter after SAG treatment. At later time, Gli1 substituted Gli2 on the S1 and S2–S5 sites, together with hyperacetylation of histone H3 (Figure 5E and F). According to p53-mediated regulation of Hh, a similar enhanced recruitment of Gli1 was observed in p53-deficient NSCs in the absence of SAG treatment (Figure 5E). A basal recruitment of Gli2 and Gli1 onto Nanog promoter was also detected, although lower than that found after SAG treatment with respect to an unrelated control gene (actin) or to non-specific −2100 bp Nanog site devoid of Gli consensus sequence. Consistently, the activation of Nanog and Gli1 expression observed in neurospheres derived from Ptc1+/− Mbs was associated with an enhanced Gli1 occupancy of Nanog promoter (Figure 5G).
Figure 5.
Hh/Gli activate Nanog transcription. (A, B) RT–qPCR (A) and western blot (B) analysis of Nanog, Sox2 and Gli1 levels in neurosphere cultures after SAG treatment up to 24 h (means±s.d. from four different experiments). *P<0.05 versus untreated cells. (C) Representation of Nanog promoter showing putative Gli-responsive elements (GliRE). (D–F) ChIP (D, E) and real-time qPCR-ChIP (F) assays from untreated (nt), 2.5 h (F), 5 h (D, F) and 24 h (E, F) SAG-treated neurospheres or p53−/− neurospheres (E), using anti-Gli2 (D, F) or anti-Gli1 (two different antibodies, see Materials and methods and panels (E, F)) and anti-acetyl-H3 antibodies. Eluted DNA was PCR amplified with primers shown in Supplementary Figure S4B. Real-time qPCR-ChIP results are expressed as fold induction versus endogenousβ-actin-amplified ChIP controls. Bars represent the mean of three independent experiments±s.d. (*P<0.05 versus nt). (G) RT–qPCR ChiP assay from untreated murine cerebellar neurospheres (mNSC) and murine medulloblastoma-derived neurospheres (mMbSC) using anti-Gli1 antibody. Eluted DNA was PCR amplified with primers shown in Supplementary Figure S4B. Bars represent the mean of three independent experiments±s.d. (*P<0.05 versus mNSC). A full-colour version of this figure is available at The EMBO Journal Online.
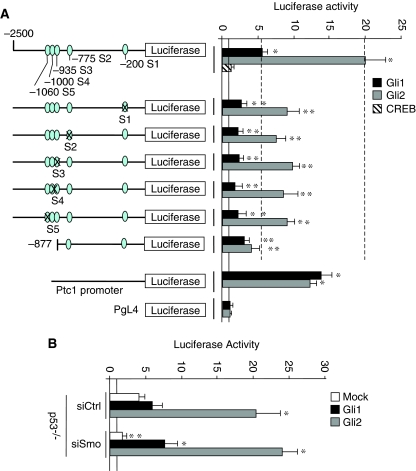
To verify whether these Gli-binding sites were responsible for the transcriptional activation of Nanog, we determined the effect of Gli transcription factors overexpression on mouse Nanog promoter-luciferase reporter. The luciferase activity was strongly induced by Gli1 and Gli2, whereas this induction was significantly reduced by deletion of the (−1060 to −935) Gli-binding sites or mutations of critical nucleotides in S1 to S5 sites (Figure 6A; Supplementary Figure S3A).
Figure 6.
Transcriptional activity of Gli-responsive sites of Nanog promoter. (A) Relative luciferase activity driven by either mouse Nanog (−2500/+1 bp) or (−877/+1 bp) region or (−2500/+1 bp) reporter constructs carrying either mutagenized S1 to S5 Gli consensus sequences (indicated in Supplementary Figure S3A) or Ptc1 promoter/reporter or PgL4 empty vector, transfected into mouse cerebellar neurospheres together with mock (continuous line), Gli1, Gli2 or CREB (as a negative control). Data are indicated as mean ratios with respect to pRL-CMV-Renilla Luciferase control (ctrl). *P<0.05 versus Ctrl. **P<0.05 versus wild-type constructs (dashed lines). (B) Relative luciferase activity driven by Nanog promoter in p53−/− compared with p53 wt neurospheres (continuous line). The day before Gli1 and Gli2 overexpression, cells were transfected with siRNA against Smo (siSmo) or control. *P<0.05 versus Mock-transfected cells; **P<0.05 versus siCtrl.
Moreover, to further strengthen the p53-independent and direct regulation of Nanog by Gli factors, we investigated the effect of the modulation of Hh signalling on Nanog promoter-luciferase reporter in a p53−/− neurospheres. We first observed that in this context, Nanog promoter has a basal higher activity when compared to wild type. The modulation of Hh signalling by Gli1 or Gli2 overexpression or by Smo siRNA increased or reduced, respectively, the luciferase activity, indicating that p53 is dispensable for Hh regulation (Figure 6B). Overall, these findings indicate the presence of discrete Gli-binding sites in the cis-regulatory sequences of Nanog that are responsible for its Hh-mediated transcriptional activation.
Conservation of human Nanog regulation by Hh
To investigate whether Hh-induced transcriptional regulation is also active on human Nanog, we treated human neurospheres (human neuronal precursor cells, HNPCs) with SAG. This resulted in enhanced transcription of Gli1 as well as of Nanog mRNA and protein (Figure 7A and B), suggesting that Hh signalling may enhance also human Nanog transcription. Interestingly, the −256 to −118 bp region of Nanog, including the S1 Gli site, is conserved between mouse and human (Figure 7C; Pan and Thomson, 2007). Accordingly, the activity of a human Nanog reporter (−2586/+1 bp), spanning from −2586 bp to the transcription start site, was upregulated by Gli1 and Gli2 overexpression in human cells (Figure 7D; Supplementary Figure S3C).
Figure 7.
Conservation of human Nanog regulation by Hh. (A, B) RT–qPCR (A) of Nanog, Sox2 and Gli1 levels and western blot analysis (B) of Nanog and Gli1 levels in HNPC after SAG treatment up to 24 h (means±s.d. from four different experiments). *P<0.05 versus untreated cells. (C) Alignment of mouse and human Nanog upstream cis-regulatory sequences, showing a good homology in the −256 to −118 bp region, encompassing a conserved Gli consensus site at −200 bp. (D) Relative luciferase activity driven by human Nanog (−2586/+1 bp) reporter transfected into human HNPC together with Mock, Gli1 and Gli2. Data are indicated as mean ratios with respect to pRL-CMV-Renilla Luciferase control (ctrl). *P<0.05 versus ctrl. A full-colour version of this figure is available at The EMBO Journal Online.
Hh-dependent activation of Nanog in human Mb SCs
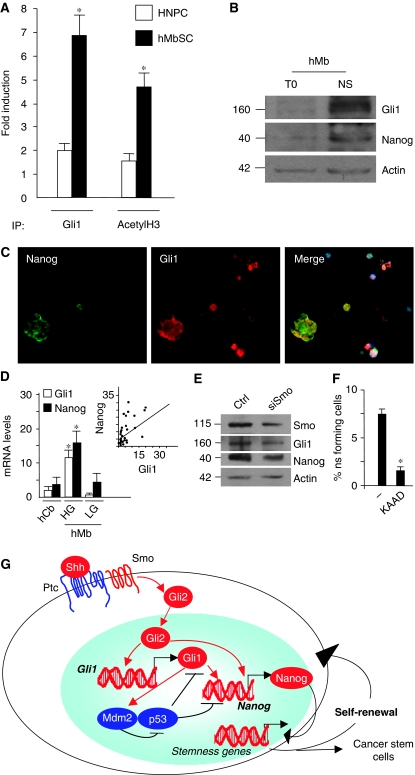
As Hh hyperactivation is responsible for cerebellar cell transformation into Mb and cancer SCs have a critical function in tumorigenesis (Singh et al, 2004; Morrison and Kimble, 2006; Ruiz i Altaba, 2006; Fan and Eberhart, 2008; Jiang and Hui, 2008), we investigated whether the possible connection between Nanog and Hh could also occur in cancer SCs. ChIP experiments show that Gli1 was recruited to the S1 site of the Nanog promoter in human Mb neurospheres, together with an increase of acetylated histone H3 (Figure 8A), indicating that this Gli1-bound promoter was transcriptionally active. Moreover, human Mb neurospheres display enhanced expression of both Nanog and Gli1 with respect to pre-neurosphere cells (Figure 8B), which were also coexpressed (Figure 8C), confirming the mouse Mb data (Figure 1G and H).
Figure 8.
Hh-dependent activation of Nanog in human Mb stem cells. (A) RT–qPCR ChiP assay from untreated HNPC and human Mb-derived neurospheres (hMbSCs) derived neurospheres with anti-Gli1 and anti-acetyl-H3 antibodies. Eluted DNA was PCR amplified with primers shown in Supplementary Figure S4B. Results are expressed as fold induction versus endogenous GAPDH-amplified ChIP controls. Bars represent the mean of three independent experiments±s.d. (*P<0.05 versus HNPC). (B) Protein levels of endogenous Nanog and Gli1 evaluated by western blot in cells derived from human Mb (T0) versus derived neurospheres (NS). (C) Immunofluorescence or nuclear Hoechst (blue) staining with Nanog (green) and Gli1 (red) antibodies in disgregated neurospheres from human Mb. (D) mRNA levels of Nanog and Gli1, evaluated by RT–qPCR relative to housekeeping controls, in human hMb samples (subsets expressing high (HG) or low levels (LG) of Gli1) and cerebellar controls (hCb). *P<0.05 in HG versus LG versus hCb. Inset: Regression analysis of Nanog versus Gli1 levels in 28 human Mb samples (P<0.01 by simple regression analysis). (E) Western blot analysis of endogenous Gli1, Smo and Nanog levels in human Mb neurospheres after siRNA-mediated silencing of Smo (siSmo) versus control siRNA (ctrl). (F) Neurosphere-forming assay of single cells derived from secondary human Mb neurospheres in basal stem medium (−), in the presence of Hh antagonist KAAD cyclopamine for 15 days. *P<0.05 versus untreated. (G) Schematic model of the regulatory loop between Hh and Nanog in cerebellar NSCs. Shh binds to Ptc1, releasing the inhibition on Smo, which in turn enhances Gli2 nuclear translocation and recruitment to promoters of target genes Gli1 and Nanog. Gli1 and p53 form a reciprocal inhibitory loop. Hh signal through Gli1 activates the E3 Ligase Mdm2, which degrades p53 protein, thus relieving its inhibitory activities on both Nanog promoter and Gli1 (see text). In turn, Gli1 and Gli2 bind to specific consensus cis-regulatory responsive elements on Nanog promoter enhancing its transcription. In this way, they mediate the Hh- and p53-dependent control of Nanog and downstream stemness genes, which promote self-renewal of NSCs and cancer SCs of Hh-induced tumors (e.g. Mb).
Furthermore, we analysed Nanog levels in human primary tumor samples (hMb) compared with normal cerebellum (controls, hCb). Notably, significantly higher levels of Nanog were detected in hMb with hyperactive Hh signalling (high Gli1 levels, HG) compared both to low Gli1 Mb (LG) and controls (Figure 8D).
In addition, we analysed the distribution of Nanog values as a function of Gli1 in all tumors. This revealed that Nanog positively correlated with Gli1 (P<0.01; Figure 8D). These observations suggest that Hh signalling is responsible for enhanced Nanog expression in Mb SCs. Therefore, to test whether Hh was required to maintain high Nanog expression in human Mb neurospheres, we abrogated Hh signalling by Smo siRNA and observed significantly reduced levels of Nanog (Figure 8E). Consistently, the Smo-antagonist KAAD cyclopamine also reduced the self-renewal of hMb neurospheres (Figure 8F). Overall, these findings support that enhanced expression of Nanog in Mb SCs is sustained by Hh signalling.
Discussion
We suggest here a model in which Nanog is a critical mediator of Hh effect on NSCs (Figure 8G). Indeed, both Nanog and Hh activity are specifically coexpressed in postnatal cerebellar NSCs and are essential to drive their self-renewal. We also show that Nanog is downstream to Hh, as its expression is required for Hh-induced self-renewal. Such stemness promoting activity is sustained by transcriptional activation of Nanog, which is conserved in mouse and human cells, through the in vivo binding of Gli1 and Gli2 to specific promoter cis-regulatory elements. These findings provide the key evidence that the Hh/Gli cascade activates a critical pluripotency gene in postnatal cerebellar NSCs.
Hh signalling is active in cerebellar NSCs
We show that the window of Hh activity also extends to cerebellar NSCs, besides the lineage committed GCPs, which are known to be a specific target of Hh (Dahmane and Ruiz i Altaba, 1999; Wallace, 1999; Wechsler-Reya and Scott, 1999). This is consistent with the increased expression of stemness genes, including Nanog, by Gli1 overexpressing prominin1-positive cells in mouse cerebellar tissue (Stecca and Ruiz i Altaba, 2009) and with the in vivo expansion of cerebellar NSCs observed in mice where the Hh pathway has been artificially activated (i.e. nestin-Gli1 transgenic mice or animals with hyperactive Hh signalling through Ptc1 deletion or Smo activation in GFAP-expressing NSCs) (Schuller et al, 2008; Yang et al, 2008; Stecca and Ruiz i Altaba, 2009). This raises the question of the possible relationships between the functions of Hh activity in either NSCs and committed GCPs. Indeed, although NSC and GCP lineage may be unrelated, at least some NSCs are able to generate differentiated GCPs (Klein et al, 2005; Lee et al, 2005 and this study). However, our data suggest that Hh is not involved in such a lineage commitment, as the GCP lineage choice or the expansion of GCP population are not influenced by Hh treatment of NSCs. These findings may be explained by the previously described ability of bFGF, which is required to maintain NSCs cultures, to inhibit the mitogenic effect of Shh on GCPs (Fogarty et al, 2007). Overall, these findings provide evidence that Hh signalling has a double activity: it maintains stemness properties of cerebellar NSCs and sustains proliferation of lineage committed GCPs. This could be explained by differential cooperation of the Hh pathway with other microenvironment-dependent signals involved in the control of NSCs or GCPs specific gene expression patterns.
Hh sustains NSCs through transcriptional activation of Nanog
To this regard, we identify here Nanog as one of the intrinsic determinants of the activity of Hh in NSCs, whereas Shh is not able to increase Nanog expression in GCPs (not shown). Although Nanog is known to be critical for the maintenance of pluripotency and self-renewal of ESC (Chambers et al, 2003; Mitsui et al, 2003), we describe here that it is also active in postnatal cerebellar NSCs where it acts as a stemness gene. Our findings are in keeping with the suggested function of Nanog to control somatic cell pluripotency in vivo in Huntington's disease Hdh-Q111 knock-in mice that display aberrant neurogenesis as well as NSCs characterized by impaired lineage restriction together with enhanced self-renewal and Nanog expression (Molero et al, 2009). In apparent contrast with this suggestion, genetic loss of Nanog in ESCs leads to the in vitro emergence of Nanog-negative ESCs that keep self-renewal and multipotency and do not display impaired integration into neuroepithelia of Nanog-positive/Nanog-negative chimeric mouse embryos (Chambers et al, 2007). The latter observations do not rule out a function of Nanog in the control of somatic NSCs in vivo, as it might be sustained by non-cell-autonomous paracrine Nanog-induced factors produced by Nanog-positive NSCs in the chimerical tissue, as recently described in primitive endoderm, possibly through FGF/Erk signalling (Messerschmidt and Kemler, 2010). To fully elucidate this issue, further studies using mice in which Nanog is conditionally targeted into NSCs need to be carried out.
Hh-dependent Nanog activation in Mb SCs
Consistent with the notion that Nanog may confer stemness features to somatic cells, we also show that Nanog is upregulated in Hh-promoted neuronal tumors, specifically featuring the cancer SC population. Notably, preferential upregulation of Nanog is observed in human Mbs with hyperactive Hh signalling. Additionally in Mb samples, Gli1 and Nanog levels positively correlate, suggesting their functional connection also in vivo in human tumors. Importantly, Nanog has been reported to be reactivated also in other tumors and suggested to have a function in tumor development (Clement et al, 2007; Jeter et al, 2009; Meng et al, 2010; Wen et al, 2010), possibly by featuring and controlling cancer SCs. The critical function of Hh pathway in promoting tumorigenesis has been recently highlighted by its ability to maintain cancer SCs in a number of tumor types (glioblastoma, breast cancer, myeloma, colon cancer and Mb) (Liu et al, 2006; Clement et al, 2007; Peacock et al, 2007; Varnat et al, 2009 and this study). Therefore, the Hh-dependent regulation of Nanog that we have described here in Mb SCs provides an explanation to the Hh-enhanced expression of Nanog in glioma (Clement et al, 2007) and Mb SCs (this study), as well as to the essential role of Nanog as a mediator of Hh-regulated glioma stemness properties and tumorigenicity (see accompanying paper by Zbinden et al, 2010). Overall, these observations suggest that targeting Nanog might be an approach to improve therapy of cancer SCs, as indicated by the ability of histone deacetylase inhibitors to suppress both the ESC signature (including Nanog) and growth inhibition of human embryonic carcinoma cells (You et al, 2009). To this regard, histone deacetylase inhibitors have been recently described to suppress Gli transcription function in Mb cells (Canettieri et al, 2010).
Hh and p53 cross-regulate Nanog
The Hh-dependent regulation of Nanog allows us to depict a model (Figure 8G) where a circuitry of Hh-intersecting signals converge on Nanog controlling stemness. To this regard, the oncosuppressor p53 has been recently described to have a critical function in reprogramming pluripotent and self-renewing SCs and to repress both Nanog (Lin et al, 2005; Hong et al, 2009; Kawamura et al, 2009) and neural cell stemness (Meletis et al, 2006). P53 also suppresses Hh pathway (Stecca and Ruiz i Altaba, 2009 and this study) that in turn represses p53 (Abe et al, 2008; Stecca and Ruiz i Altaba, 2009).
We have shown the presence of a p53-independent regulation of Nanog and stemness through Gli-mediated direct transcriptional control, which cross-talks with previously described p53- and Gli-mediated mechanisms.
Interestingly, p53 regulates polarity of self-renewing divisions of mammary SCs, where p53 loss causes enhanced symmetric cell divisions and clonogenic activity (Cicalese et al, 2009), similarly to the effects induced by Hh activation in NSCs (this study). Therefore, we speculate that p53 may restrain self-renewing NSC divisions by suppressing Nanog expression both directly (Xu, 2005) and through cross-inhibition of Hh signalling (Figure 4B). This model may also have implications for cancer SCs where Nanog is reactivated (Jeter et al, 2009; Li et al, 2009 and this study) and p53 cooperates with Hh pathway for Mb tumorigenesis (Romer et al, 2004).
In conclusion, Hh controls cell stemness through regulation of a pluripotency gene (Nanog), providing a crucial component of an integrated Hh- and p53-mediated circuitry determining cell fate decisions and involved in the maintenance of cancer SCs.
Materials and methods
Unless otherwise indicated, media and supplements for cell culture were purchased from Gibco-Invitrogen (Carlsbad, CA) and chemicals were purchased from Sigma-Aldrich (St Louis, MO).
Cell culture
Mouse cerebella were obtained from postnatal 4-day-old wild-type mice and p53−/− mice (The Jackson Laboratory, ME). Murine Mbs were isolated from Ptc1−/+ mice (Di Marcotullio et al, 2006) (EMMA, Monterotondo, Italy). Human Mb samples were collected during surgical resection with the approval of institutional review board as described earlier (Di Marcotullio et al, 2006).
Tissues were collected in HBSS supplemented with 0.5% glucose and penicillin-streptomycin, grossly triturated with serological pipette and treated with DNAse I to a final concentration of 0.04% for 20 min. Finally, cells aggregates were mechanically dissociated using pipettes of decreasing bore size to obtain a single-cell suspension. SCs were cultured as neurospheres in selective medium after centrifugation, DMEM/F12 supplemented with 0.6% glucose, 25 μg/ml insulin, 60 μg/ml N-acetyl-L-cystein, 2 μg/ml heparin, 20 ng/ml EGF, 20 ng/ml bFGF (Peprotech, Rocky Hill, NJ), 1X penicillin-streptomycin and B27 supplement without vitamin A. Cell isolation procedures did not influence Nanog or stress-induced proteins (i.e. p53), as their levels in pre-neuropshere cells both immediately after ex vivo isolation or 24 h in culture were not detectable (Supplementary Figure S4A).
Neurospheres were treated with the Smo-agonist SAG (200 nM, Alexis) and with the steroidal alkaloid Smo antagonist, cyclopamine KAAD (0.5 μM Calbiochem, Nottingham, UK). For the neurosphere-forming assay, cells were plated at clonal density (1–2 cells/mm2) into 96-well plates and cultured in selective medium as described above. For differentiation studies, neurospheres were mechanically dissociated, and the resulting cells were plated onto D-poly-ornithine-coated dishes in differentiation medium (DMEM/F12 with N2 supplement and 2 μg/ml heparin, 0.6% glucose, 60 μg/ml N-acetyl-L-cysteine, containing 1% Calf Serum and PDGF 10 ng/ml (P3076), for 7 days. Cerebellar GCPs (from 4-day-old mice) were isolated and cultured as described earlier (Di Marcotullio et al, 2006).
Human fetal neural stem/precursor cells (HNPC) (Lonza, Milan, Italy) were derived from whole brains of human fetus of 18 gestational weeks and were grown as neurospheres in Neural Progenitor basal medium containing bFGF and EGF (Lonza). Neurospheres were passaged every 2 weeks and were used at passage lower than 8.
Knockdown of mouse Nanog and Smo was performed with specific smart pool siRNAs (20 nM) (Dharmacon, Lafayette, CO) using INTERFERin siRNA Transfection Reagent (Polyplus Transfection, NY).
Neurospheres and GCPs were also transduced with pGreenZeo Lentiviral Reporter Vectors containing specific promoters for NANOG (Nanog-GFP) or CMV (Zeo-GFP) (Biocat, Heidelberg, Germany).
Fluorescence-activated cell sorting
Mechanically dissociated cerebellar cells were stained with anti-Prominin1 (Ab16518; Abcam) and with secondary antibody (Alexa Fluor 488; Molecular Probes, Invitrogen) or cells transduced with Nanog-GFP or Zeo-GFP were sorted by fluorescence-activated cell sorting (FACS) Aria (Becton Dickinson, NJ).
RNA isolation and real-time q-PCR
RNA isolation from cells and tissue samples was performed as described earlier (Di Marcotullio et al, 2006). cDNA synthesis was performed using High Capacity cDNA reverse transcription kit from Applied Biosystems (Foster City, CA). Quantitative reverse transcription (RT–PCR) analysis of Prominin1, Musashi1, Musashi2, BMI1, GFAP, Nestin, PCNA, Nanog, Oct-4, Sox2, KLF4, c-Myc, Math1, Zic1, Gli1, Gli2, Gli3, CyD1, CyD2, Ptch1, Hip1, Smo, β-actin, GAPDH and HPRT mRNA expression was analysed on cDNAs using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems) using TaqMan gene expression assay according to the manufacturer's instructions (Applied Biosystems). Each amplification reaction was performed in triplicate, and the average of the three threshold cycles was used to calculate the amount of transcripts in the sample (SDS software, AB). mRNA quantification was expressed, in arbitrary units, as the ratio of the sample quantity to the calibrator or to the mean values of control samples. All values were normalized to three endogenous controls, GAPDH, β-actin and HPRT.
Western blot assay
Cells were lysed in Tris–HCl pH 7.6, 50 mM, deoxycholic acid sodium salt 0.5%, NaCl 140 mM, NP40 1%, EDTA 5 mM, NaF 100 mM, Na pyrophosphate 2 mM and protease inhibitors. Lysates were separated on 8 or 6% acrylamide gel and immunoblotted using standard procedures. Anti-Mouse Nanog (Cosmo Bio Co, Japan), anti-Nanog (ab80892-Abcam, Cambridge, UK), anti-Gli1 H-300 (sc-20687; Santa Cruz Biotechnology, CA), anti-Smo N-19 (sc-6366; Santa Cruz Biotechnology), anti-Sox2 (MAB4343 Millipore Billerica, MA), anti-mouse ßIII Tubulin (MAB 1637 Millipore), anti-Gli2 sc-28674 H-300 Santa Cruz Biotechnology, CA), anti-CD133 (prominin1) Ab16518-Abcam, Cambridge, UK anti-actin I19 (sc-1616; Santa Cruz Biotechnology) and HRP-conjugated secondary antisera (Santa Cruz Biotechnology) were used followed by enhanced chemiluminescence (ECL Amersham, Amersham, UK).
Immunofluorescence
To detect Nanog and Gli1, cells were plated on poly-lysine-coated Lab-Tek chamber slides (cover slips) and allowed to adhere for 3 h. To detect differentiation markers, cells were plated on poly-ornithine-coated Lab-Tek chamber slides (cover slips) and cultured in N2 medium for 7 days. Cells were fixed with 4% paraformaldehyde for 20 min at room temperature, incubated in blocking solution (5% normal goat serum (NGS), 1% BSA, 0.1% Triton X-100) and stained overnight with primary antibodies diluted in blocking solution and for 2 h with secondary antibodies. Primary antibodies were rabbit anti-Nanog polyclonal, ab80892 (Abcam, Cambridge, UK) and mouse anti-Gli1, #2643 (Cell Signaling Technology Inc); mouse-anti-Oligodendrocyte marker O4 monoclonal antibody (Millipore) MAB 345; mouse anti-GFAP monoclonal antibody MAB360 (Millipore); mouse anti-MEF2D, 610774 (BD Bioscience); mouse anti-Parvalbumin P3088 (Sigma); rabbit anti-S100 S2644 (Sigma); mouse antiβ-III tubulin (MAB1637) (Millipore); rabbit anti-Zic1 polyclonal ab72694 (Abcam, Cambridge, UK); rabbit anti-GABA-R G4416 (Sigma); mouse anti-NG2 MAB5384 (Millipore). In all, 594- or 488-conjugated anti-mouse and anti-rabbit secondary antibodies were purchased from Molecular Probes (Invitrogen, Eugene, OR). Nuclei were counterstained with Hoechst reagent. Cover slips were mounted with fluorescence mounting medium (S3023) (Dako, Carpinteria, CA). Images were acquired with Carl Zeiss microscope (Axio Observer Z1) using Apotome technology and AxioVision Digital Image Processing Software.
Chromatin immunoprecipitation
ChIP was performed as described earlier (Canettieri et al, 2010). More in detail, cells were cross-linked 10 min with 1% formaldehyde, and the reaction was stopped with 0.125 M glycine for 5 min. Cells were washed and harvested, and cytoplasmic membranes were lysed with lysis buffer (5 mM Pipes, 85 mM KCl, and 0.5% Nonidet P-40). After centrifugation, nuclei were lysed with sonication buffer (1% SDS, 10 mM EDTA, and 50 mM Tris (pH 8) supplemented with protease inhibitors) and sonicated to obtain chromatin fragments of about 400–600 nucleotides. After sonication, the lysates were precleared for 1 h, diluted with nine volumes of dilution buffer (0.01% SDS, 1.2 mM EDTA, 16.7 mM Tris–HCl (pH 8), 1.1% Triton X-100 and 167 mM NaCl) and incubated with the specific antibodies overnight. The next day, salmon sperm-saturated protein A beads (Upstate) were added for 40 min, after which the lysates were washed five times with Buffer A (0.1% SDS, 2 mM EDTA, 20 mM Tris–HCl (pH 8), 1% Triton X-100 and 150 mM NaCl), four times with Buffer B (0.1% SDS, 2 mM EDTA, 20 mM Tris–HCl (pH 8), 1% Triton X-100 and 500 mM NaCl), and once with Buffer TE (10 mM Tris–HCl (pH 8) and 1 mM EDTA). After the final washing, the immunocomplexes were eluted with elution buffer (1% SDS and 100 mM NaHCO3) 30 min at room temperature, and after the addition of 200 mM NaCl, the cross-linking was reversed with an overnight incubation at 65°C. Subsequently, the samples were digested with proteinase K and RNase A for 2 h at 42°C, and the DNA was purified and precipitated. Eluted DNA was PCR amplified with primers encompassing the Gli-responsive sites (Supplementary Figure S4B) of murine and human Nanog promoter, the actin or GAPDH gene as controls. The following antibodies were used: rabbit polyclonal anti-Gli1 H-300X (Santa Cruz), rabbit polyclonal anti-Gli1 2553 (Cell Signaling), rabbit polyclonal anti-Gli2-H-300X (Santa Cruz), rabbit polyclonal anti-acetyl-histone3 (Cell Signaling). Eluted DNA has been analysed with standard PCR techniques or Q-PCR. Primers were designed with Primer Express software (Applied Biosystems).
Luciferase assays
Mouse Nanog promoter_luciferase vector (−877 bp) was PCR amplified from genomic DNA of SC using the following primers:
M_P_Nanog_Luc_F2: ACTGGTACCGCCAGTCTCGGGTACGTGA
Pro m_nanog com_Rv: ACTCTCGAGGCAGCCTTCCCACAGAAAG
and cloned in pGL4-vector (Promega, Madison, WI).
Mouse Nanog promoter_luciferase vector −2500 bp (Nanog5P reporter) was obtained from Addgene (Cambridge, MA). This construct was used to generate the mutant derivatives in at least two nucleotides of canonical Gli-binding sites (Pavletich and Pabo, 1993; Mizugishi et al, 2001).
Human Nanog promoter_luciferase vector −2586 bp (Human Nanog_Luciferase-T2A-ZeoR) was obtained from Biocat (Germany). NSCs were transfected in 24-well plates with 80 ng of Nanog promoter_luciferase vector (−2500 bp and −935 bp), 400 ng of Gli1, Gli2 or mock and 5 ng of or pRL-CMV-Renilla Luciferase control vector with Fugene6 Transfection Reagent (Roche). Cells were harvested and tested 24 h posttransfection with the dual-luciferase assay (Promega). Empty pGL4 vector was tested as negative ctrl with Gli1, Gli2 or mock. PtchP1A luciferase reporter (kindly donated by R Toftgärd) was used as positive control of response to Gli family elements, whereas myc-Gli2 plasmid was from A Dlugosz. Results are expressed as luciferase/renilla ratios and represent the average±s.d. of at least three experiments, each performed in triplicate.
Site-directed mutagenesis
Mutation of critical nucleotides in Gli-binding sites was designed according to Mizugishi et al (2001) and Pavletich and Pabo (1993). To mutagenize the promoter constructs, the QuickChange XL Site-Directed Mutagenesis Kit and the QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) were used. The reaction was carried out according to the manufacturers' protocol using the following primers:
S1 MUTfw: CCTCCCTCCCAGTCTGTGCCACCTTACAGCTTCTT
S1 MUTrv: AAGAAGCTGTAAGGTGGCACAGACTGGGAGGGAGG
S2 MUTfw: TTAGACGGCTGAGGCACTTAAGATTGCAGTAAGTCTGAAG
S2 MUTrv: CTTCAGACTTACTGCAATCTTAAGTGCCTCAGCCGTCTAA
And for multisite mutagenesis the following ones:
S3MUT: GATCGGCAAACTTTGAACTTGTGATTTGGAAATAGGAAGATCAGGA
S4MUT: CAGAAGCCGACTTAAGCTGTGCTAGAGTGCTTTCACTCACT
S5MUT: TCACTGTGGTAGAGTCTTCACATTGGATATGTTACGAGAGG
The mutations introduced in the Nanog sequence are underlined.
Statistical analysis
Statistical analysis was performed using StatView 4.1 software (Abacus Concepts, Berkeley, CA). Statistical differences were analysed by the Mann–Whitney U test for non-parametric values; MB markers were analysed by simple regression using the StatView 4.1 software (Abacus Concepts). The results are expressed as mean±s.d. from an appropriate number of experiments as indicated in the figure legends.
Supplementary Material
Acknowledgments
This work was supported by Associazione Italiana per la Ricerca sul Cancro, Telethon Grant GGP07118, Ministry of University and Research (FIRB and PRIN), Ministry of Health, Mariani Foundation, Fondazione Roma and Cenci-Bolognetti Foundation. AP was supported by a fellowship from FIRC.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abe Y, Oda-Sato E, Tobiume K, Kawauchi K, Taya Y, Okamoto K, Oren M, Tanaka N (2008) Hedgehog signaling overrides p53-mediated tumor suppression by activating Mdm2. Proc Natl Acad Sci USA 105: 4838–4843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Joyner AL (2005) In vivo analysis of quiescent adult neural stem cells responding to Sonic Hedgehog. Nature 437: 894–897 [DOI] [PubMed] [Google Scholar]
- Boiani M, Scholer HR (2005) Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol 6: 872–884 [DOI] [PubMed] [Google Scholar]
- Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, Jaenisch R (2008) Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell 2: 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canettieri G, Di Marcotullio L, Greco A, Coni S, Antonucci L, Infante P, Pietrosanti L, De Smaele E, Ferretti E, Miele E, Pelloni M, De Simone G, Pedone EM, Gallinari P, Giorgi A, Steinkuhler C, Vitagliano L, Pedone C, Schinin ME, Screpanti I et al. (2010) Histone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat Cell Biol 12: 132–142 [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A (2003) Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113: 643–655 [DOI] [PubMed] [Google Scholar]
- Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A (2007) Nanog safeguards pluripotency and mediates germline development. Nature 450: 1230–1234 [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Young KE, Maiti T, Beachy PA (2002) Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA 99: 14071–14076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicalese A, Bonizzi G, Pasi CE, Faretta M, Ronzoni S, Giulini B, Brisken C, Minucci S, Di Fiore PP, Pelicci PG (2009) The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell 138: 1083–1095 [DOI] [PubMed] [Google Scholar]
- Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A (2007) HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol 17: 165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmane N, Ruiz i Altaba A (1999) Sonic Hedgehog regulates the growth and patterning of the cerebellum. Development 126: 3089–3100 [DOI] [PubMed] [Google Scholar]
- Di Marcotullio L, Ferretti E, Greco A, De Smaele E, Po A, Sico MA, Alimandi M, Giannini G, Maroder M, Screpanti I, Gulino A (2006) Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nat Cell Biol 8: 1415–1423 [DOI] [PubMed] [Google Scholar]
- Fan X, Eberhart CG (2008) Medulloblastoma stem cells. J Clin Oncol 26: 2821–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MP, Emmenegger BA, Grasfeder LL, Oliver TG, Wechsler-Reya RJ (2007) Fibroblast growth factor blocks Sonic Hedgehog signaling in neuronal precursors and tumor cells. Proc Natl Acad Sci USA 104: 2973–2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP (1997) Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277: 1109–1113 [DOI] [PubMed] [Google Scholar]
- Hanna J, Saha K, Pando B, van Zon J, Lengner CJ, Creyghton MP, van Oudenaarden A, Jaenisch R (2009) Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 462: 595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S (2009) Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature 460: 1132–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard SA, Friel AM, Kumar B, Zhang L, Rueda BR, Gargett CE (2009) Evidence for cancer stem cells in human endometrial carcinoma. Cancer Res 69: 8241–8248 [DOI] [PubMed] [Google Scholar]
- Ikram MS, Neill GW, Regl G, Eichberger T, Frischauf AM, Aberger F, Quinn A, Philpott M (2004) GLI2 is expressed in normal human epidermis and BCC and induces GLI1 expression by binding to its promoter. J Invest Dermatol 122: 1503–1509 [DOI] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA (1994) Tumor spectrum analysis in p53-mutant mice. Curr Biol 4: 1–7 [DOI] [PubMed] [Google Scholar]
- Jeter CR, Badeaux M, Choy G, Chandra D, Patrawala L, Liu C, Calhoun-Davis T, Zaehres H, Daley GQ, Tang DG (2009) Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells 27: 993–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Werbowetski-Ogilvie TE, Zhong B, Hong SH, Bhatia M (2009) Pluripotent transcription factors possess distinct roles in normal versus transformed human stem cells. PLoS One 4: e8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Hui CC (2008) Hedgehog signaling in development and cancer. Dev Cell 15: 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Belmonte JC (2009) Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 460: 1140–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Butt SJ, Machold RP, Johnson JE, Fishell G (2005) Cerebellum- and forebrain-derived stem cells possess intrinsic regional character. Development 132: 4497–4508 [DOI] [PubMed] [Google Scholar]
- Lai K, Kaspar BK, Gage FH, Schaffer DV (2003) Sonic Hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci 6: 21–27 [DOI] [PubMed] [Google Scholar]
- Lee A, Kessler JD, Read TA, Kaiser C, Corbeil D, Huttner WB, Johnson JE, Wechsler-Reya RJ (2005) Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci 8: 723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Collado M, Villasante A, Strati K, Ortega S, Canamero M, Blasco MA, Serrano M (2009) The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature 460: 1136–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y (2005) p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol 7: 165–171 [DOI] [PubMed] [Google Scholar]
- Lipinski RJ, Gipp JJ, Zhang J, Doles JD, Bushman W (2006) Unique and complimentary activities of the Gli transcription factors in Hedgehog signaling. Exp Cell Res 312: 1925–1938 [DOI] [PubMed] [Google Scholar]
- Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS (2006) Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 66: 6063–6071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G (2003) Sonic Hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron 39: 937–950 [DOI] [PubMed] [Google Scholar]
- Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J (2006) p53 suppresses the self-renewal of adult neural stem cells. Development 133: 363–369 [DOI] [PubMed] [Google Scholar]
- Meng HM, Zheng P, Wang XY, Liu C, Sui HM, Wu SJ, Zhou J, Ding YQ, Li JM (2010) Overexpression of nanog predicts tumor progression and poor prognosis in colorectal cancer. Cancer Biol Ther 9: 295–302 [DOI] [PubMed] [Google Scholar]
- Messerschmidt DM, Kemler R (2010) Nanog is required for primitive endoderm formation through a non-cell autonomous mechanism. Dev Biol (doi:10.1016/j.ydbio.2010.04.020) [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S (2003) The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113: 631–642 [DOI] [PubMed] [Google Scholar]
- Mizugishi K, Aruga J, Nakata K, Mikoshiba K (2001) Molecular properties of Zic proteins as transcriptional regulators and their relationship to GLI proteins. J Biol Chem 276: 2180–2188 [DOI] [PubMed] [Google Scholar]
- Molero AE, Gokhan S, Gonzalez S, Feig JL, Alexandre LC, Mehler MF (2009) Impairment of developmental stem cell-mediated striatal neurogenesis and pluripotency genes in a knock-in model of Huntington′s disease. Proc Natl Acad Sci USA 106: 21900–21905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J (2006) Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441: 1068–1074 [DOI] [PubMed] [Google Scholar]
- Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A (2005) Sonic Hedgehog controls stem cell behavior in the postnatal and adult brain. Development 132: 335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma V, Ruiz i Altaba A (2004) Hedgehog-GLI signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Development 131: 337–345 [DOI] [PubMed] [Google Scholar]
- Pan G, Thomson JA (2007) Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res 17: 42–49 [DOI] [PubMed] [Google Scholar]
- Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL (2000) Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development 127: 1593–1605 [DOI] [PubMed] [Google Scholar]
- Pavletich NP, Pabo CO (1993) Crystal structure of a five-finger GLI-DNA complex: new perspectives on zinc fingers. Science 261: 1701–1707 [DOI] [PubMed] [Google Scholar]
- Peacock CD, Wang Q, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J, Devereux WL, Rhodes JT, Huff CA, Beachy PA, Watkins DN, Matsui W (2007) Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci USA 104: 4048–4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, Connelly M, Stewart CF, Gould S, Rubin LL, Curran T (2004) Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell 6: 229–240 [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A (2006) Hedgehog-Gli Signaling in Human Disease. Georgetown: Plenum Publisher [Google Scholar]
- Schuller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, Huillard E, Sun T, Ligon AH, Qian Y, Ma Q, Alvarez-Buylla A, McMahon AP, Rowitch DH, Ligon KL (2008) Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell 14: 123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432: 396–401 [DOI] [PubMed] [Google Scholar]
- Stecca B, Ruiz i Altaba A (2009) A GLI1-p53 inhibitory loop controls neural stem cell and tumour cell numbers. EMBO J 28: 663–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Teglund S, Toftgard R (2010) Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta 1805: 181–208 [DOI] [PubMed] [Google Scholar]
- Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, Ruiz i Altaba A (2009) Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med 1: 338–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VA (1999) Purkinje-cell-derived Sonic Hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol 9: 445–448 [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP (1999) Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 22: 103–114 [DOI] [PubMed] [Google Scholar]
- Wen J, Park JY, Park KH, Chung HW, Bang S, Park SW, Song SY (2010) Oct4 and Nanog expression is associated with early stages of pancreatic carcinogenesis. Pancreas (doi:10.1097/MPA.0b013e3181c75f5e) [DOI] [PubMed] [Google Scholar]
- Xu Y (2005) A new role for p53 in maintaining genetic stability in embryonic stem cells. Cell Cycle 4: 363–364 [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, Schuller U, Machold R, Fishell G, Rowitch DH, Wainwright BJ, Wechsler-Reya RJ (2008) Medulloblastoma can be initiated by deletion of patched in lineage-restricted progenitors or stem cells. Cancer Cell 14: 135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You JS, Kang JK, Seo DW, Park JH, Park JW, Lee JC, Jeon YJ, Cho EJ, Han JW (2009) Depletion of embryonic stem cell signature by histone deacetylase inhibitor in NCCIT cells: involvement of Nanog suppression. Cancer Res 69: 5716–5725 [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920 [DOI] [PubMed] [Google Scholar]
- Zbinden M, Duquet A, Lorente-Trigos A, Ngwabyt AN, Borges I, Ruiz i Altaba A (2010) NANOG is essential in human glioblastoma acting in a cross-functional network with Gli1 and p53. EMBO J 29: 2659–2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.