EMBO J 29 15, 2515–2526 (2010); published online June252010
Eukaryotes harbour multiple signalling pathways to detect and respond to changes in glucose availability. Some of these pathways use plasma membrane receptors that sense extracellular glucose. Others are responsive to glycolytic flux and are activated by an elusive internal glucose signal. Many have proposed that a glycolytic intermediate serves dual functions as a metabolite and as a glucose signal. This issue of The EMBO Journal presents a study from Dechant et al (2010), which proposes that the glucose signal is elevated intracellular pH. An important player in the pH signalling pathway is the vacuolar ATPase, which reversibly assembles in response to elevated pH and activates the protein kinase A (PKA) pathway. This model is attractive in its simplicity. Could the elusive glucose signal be as simple as the concentration of protons?
Yeast uses three signalling pathways that respond to changes in glucose availability. The Restores Glucose Transport (RGT) pathway controls the expression of hexose transporters using two divergent transporters, Snf3 and Rgt2, that no longer transport glucose themselves, but bind extracellular glucose as a ligand (Ozcan et al, 1998). The cyclic AMP-dependent PKA pathway is activated by both external and internal glucose-sensing systems. The G protein-coupled receptor Gpr1 appears to bind external glucose and sucrose (Lemaire et al, 2004); this interaction is transduced to the activation of adenylate cyclase and, subsequently, PKA. PKA activation is also mediated by the yeast ras proteins whose activation requires internal glucose phosphorylation (Gorner et al, 2002). Finally, the Snf1 kinase pathway, the yeast version of the AMP-activated protein kinase (AMPK) pathway, is regulated in response to internal energy stores. The active state of the mammalian enzyme is stabilized by binding AMP (Sanders et al, 2007). The yeast AMPK also senses internal energy status, although nucleotide binding has yet to be shown. Thus, these three glucose signalling systems in yeast respond to external glucose as a ligand, some internal mediator of glucose metabolism or both.
The idea that glycolytic flux is sensed internally by cells is widespread. Glycolytic flux in pancreatic β cells is directly proportional to external glucose abundance and controls internal calcium ion concentration and ultimately secretion of insulin-loaded vesicles. Glycolytic flux is also sensed in yeast cells. Even when grown in glucose-rich conditions, the metabolism and gene expression patterns of yeast strains with artificially limited glucose transport capacity mimic those of glucose-starved cells, suggesting that glycolytic flux is a dominant signal over the information relayed by plasma membrane sensors saturated with external glucose (Otterstedt et al, 2004). Although candidate metabolites such as glucose-6-phosphase and fructose-2,6-bisphosphate have been proposed to serve as a glucose signal (Muller et al, 1995, 1997), the identity of the internal glucose signal has remained a mystery.
Recent studies using a pH-sensitive GFP derivative have allowed the accurate measurement of the intracellular pH of living yeast cells (Martinez-Munoz and Kane, 2008; Orij et al, 2009). Cells vigorously fermenting glucose have a mildly alkaline cytoplasmic pH of 7.2, whereas cells starved for glucose experience a more acidic intracellular pH of 6.0. The change in pH is an extremely sensitive readout of glycolytic flux. Dechant et al (2010) used microfluidic chips to conduct live cell microscopy during rapid and precise changes in glucose concentrations. Their results strongly suggest that intracellular pH regulates the assembly of the vacuolar ATPase, which then contributes to PKA pathway activation (Figure 1).
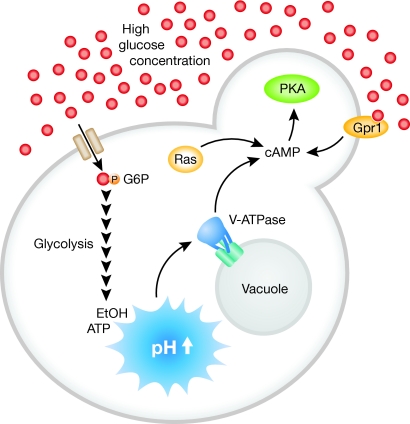
Figure 1.
pH as the glucose signal. In the presence of abundant glucose (red dots), high internal glycolytic flux leads to an increase of intracellular pH. Elevated internal pH promotes assembly of the V-ATPase, which promotes synthesis of cyclic AMP and subsequent activation of the PKA pathway.
The vacuolar ATPase is a multi-protein complex that uses energy from ATP to transport protons from the cytoplasm into the vacuolar compartment. The V-ATPase is composed of two subcomplexes, the membrane inserted V0 proton pore and the distinct ATP-hydrolysing V1, which reversibly associates with V0 in response to changes in glucose availability. By monitoring subcellular localization of GFP-tagged subunits, Dechant et al assessed V-ATPase assembly in live cells exposed to rapid shifts in glucose concentrations. During conditions of high glycolytic flux, the V1 subcomplex rapidly relocalizes to the vacuolar surface. Upon a shift to low glucose, the V1 subcomplex disassociates, limiting ATP consumption. The data presented by Dechant et al suggest that the V-ATPase additionally functions as an activator of PKA in response to elevated pH caused by glycolytic flux.
The idea that high intracellular pH is a secondary signal of glucose metabolism is intriguing. Evidence to support this idea comes from several lines of investigation. First, artificial manipulation of the intracellular pH with a protonophore mimics glycolytic flux in its effect on V-ATPase assembly. Second, a functional V-ATPase is needed for the full activation of the PKA pathway. Third, pyruvate kinase mutants grown in high glucose have reduced glycolytic flux and retain an acidic cytoplasm despite the presence of normal extracellular glucose sensors. Thus, the increase of the intracellular pH is a rapid and faithful reporter of glycolytic flux and is independent of extracellular glucose concentrations. Interestingly, the V-ATPase is not required for neutralization of the cytoplasm as mutants with a defective V-ATPase display intracellular pH profiles similar to wild-type cells. Rather, the V-ATPase assembly responds to intracellular pH and acts as a sensor. Cells lacking a functional V-ATPase have reduced synthesis of cyclic AMP and exhibit defects in activation of the PKA pathway. The molecular mechanism by which the V-ATPase regulates the PKA pathway remains to be elucidated, although it seems likely to act upstream of adenylate cyclase and may involve the ras proteins. Finally, Dechant et al show that the PKA pathway in mammalian cells is also responsive to glucose and depends on a functional V-ATPase. Taken together, these data suggest that elevated intracellular pH acts as a secondary signal of glucose metabolism in eukaryotes and may in fact be the elusive glucose signal.
Acknowledgments
We thank the National Institutes of Health for research support. EMR was supported in part by a Ruth Kirschstein post-doctoral fellowship. MCS was supported by a grant (GM46443) from the National Institute of General Medical Sciences.
Footnotes
The authors declare that they have no conflict of interest.
References
- Dechant R, Binda M, Lee S, Pelet S, Winderickx J, Peter M (2010) Cytosolic pH is a second messenger for glucose and regulates the PKA pathway via V-ATPase. EMBO J 29: 2515–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorner W, Durchschlag E, Wolf J, Brown EL, Ammerer G, Ruis H, Schuller C (2002) Acute glucose starvation activates the nuclear localization signal of a stress-specific yeast transcription factor. EMBO J 21: 135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire K, Van de Velde S, Van Dijck P, Thevelein JM (2004) Glucose and sucrose act as agonist and mannose as antagonist ligands of the G protein-coupled receptor Gpr1 in the yeast Saccharomyces cerevisiae. Mol Cell 16: 293–299 [DOI] [PubMed] [Google Scholar]
- Martinez-Munoz GA, Kane P (2008) Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. J Biol Chem 283: 20309–20319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S, Boles E, May M, Zimmermann FK (1995) Different internal metabolites trigger the induction of glycolytic gene expression in Saccharomyces cerevisiae. J Bacteriol 177: 4517–4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S, Zimmermann FK, Boles E (1997) Mutant studies of phosphofructo-2-kinases do not reveal an essential role of fructose-2,6-bisphosphate in the regulation of carbon fluxes in yeast cells. Microbiology 143: 3055–3061 [DOI] [PubMed] [Google Scholar]
- Orij R, Postmus J, Ter Beek A, Brul S, Smits GJ (2009) In vivo measurement of cytosolic and mitochondrial pH using a pH-sensitive GFP derivative in Saccharomyces cerevisiae reveals a relation between intracellular pH and growth. Microbiology 155(Part 1): 268–278 [DOI] [PubMed] [Google Scholar]
- Otterstedt K, Larsson C, Bill RM, Stahlberg A, Boles E, Hohmann S, Gustafsson L (2004) Switching the mode of metabolism in the yeast Saccharomyces cerevisiae. EMBO Rep 5: 532–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan S, Dover J, Johnston M (1998) Glucose sensing and signaling by two glucose receptors in the yeast Saccharomyces cerevisiae. EMBO J 17: 2566–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D (2007) Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J 403: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]