Abstract
The extracellular signal-regulated kinase (ERK) pathway is an important signalling pathway that regulates a large number of cellular processes, including proliferation, differentiation and gene expression. Hyperosmotic stress activates the ERK pathway, whereas little is known about the regulatory mechanisms and physiological functions of ERK activation in hyperosmotic response. Here, we show that MAPK/ERK kinase kinase 2 (MEKK2), a member of the MAPKKK family, mediated the specific and transient activation of ERK, which was required for the induction of aquaporin 1 (AQP1) and AQP5 gene expression in response to hyperosmotic stress. Moreover, we identified the E3 ubiquitin ligase carboxyl terminus of Hsc70-interacting protein (CHIP) as a binding partner of MEKK2. Depletion of CHIP by small-interference RNA or gene targeting attenuated the degradation of MEKK2 and prolonged the ERK activity. Interestingly, hyperosmolality-induced gene expression of AQP1 and AQP5 was suppressed by CHIP depletion and was reversed by inhibition of the prolonged phase of ERK activity. These findings show that transient activation of the ERK pathway, which depends not only on MEKK2 activation, but also on CHIP-dependent MEKK2 degradation, is crucial for proper gene expression in hyperosmotic stress response.
Keywords: CHIP, hyperosmotic stress, MEKK2, ubiquitin-proteasomal degradation
Introduction
Mitogen-activated protein kinase (MAPK) pathways are composed of three sequentially activating kinases, MAPK, MAPK kinase (MAP2K) and MAP2K kinase (MAP3K), that are highly conserved from yeast to human beings. They coordinate diverse cellular reactions, such as gene expression, migration, survival, differentiation and apoptosis, in response to a wide variety of stimuli. Hyperosmotic stress activates the three principal MAPKs: extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38. In the budding yeast Saccharomyces cerevisiae, high osmolarity glycerol 1, the yeast homologue of mammalian p38, is an essential gene for hyperosmotic stress response (Brewster et al, 1993; Hohmann, 2002). Mammalian cells also possess osmo-responsive mechanisms, such as cytoskeletal remodelling and gene expression, that are regulated by MAPK pathways (Burg et al, 2007). However, the functions of ERK, JNK and p38 in hyperosmotic response remain elusive.
MAPK/ERK kinase kinase 2 (MEKK2) is an MAP3K family member that is closely related to MEKK3. MEKK2 and MEKK3 are widely expressed and are potential activators of the ERK, JNK, p38 and ERK5 pathways (Blank et al, 1996; Schaefer et al, 1999; Sun et al, 2003). The function of MEKK3 in hyperosmotic stress response has been characterized in some detail. MEKK3 is recruited to GTPase Rac-containing membrane ruffles and activates p38 in response to sorbitol, an inducer of hyperosmotic stress (Uhlik et al, 2003). Sorbitol-induced activation of p38, but not of ERK and JNK, is abrogated by MEKK3 depletion (Uhlik et al, 2003), suggesting that the Rac-MEKK3 axis has a crucial function in hyperosmotic stress-induced p38 activation. Hyperosmotic stress also activates MEKK2 (Nakamura and Johnson, 2007). MEKK2 knockout mouse studies have revealed that MEKK2 has important functions in the T-cell receptor, EGF and FGF-2 signalling pathways (Schaefer et al, 1999; Su et al, 2001; Guo et al, 2002; Sun et al, 2003; Kesavan et al, 2004) as well as in rheumatoid arthritis (Hammaker et al, 2004), whereas the physiological function of MEKK2 in hyperosmotic stress response is largely unknown. Several reports have shown the regulatory mechanisms underlying the MEKK2 pathway. Dimerization of MEKK2 in its kinase domain is a critical step for MEKK2 activation through trans-autophosphorylation of a serine residue in the activation loop of the kinase domain (Cheng et al, 2005a; Zhang et al, 2006). MEKK2-interacting protein (Mip-1) was identified as the negative regulator of MEKK2. Under non-stress conditions, Mip-1 associates with the MEKK2-dimerization domain and prevents MEKK2 from being activated. When cells are exposed to EGF, Mip-1 dissociates from MEKK2, which then undergoes autophosphorylation-dependent activation and thereby phosphorylates and activates the downstream kinases (Cheng et al, 2005b). In another activation mechanism of the MEKK2 pathway, the phox and Bem1p domain of MEKK2 binds both MEK5 and MKK7, allowing MEKK2 to coordinately activate the ERK5 and JNK pathways (Nakamura and Johnson, 2003, 2007; Nakamura et al, 2006). However, the regulatory mechanisms of MEKK2 activity in hyperosmotic response have not been elucidated.
The carboxyl terminus of Hsc70-interacting protein (CHIP) is a chaperone-dependent E3 ubiquitin ligase and possesses a tetratricopeptide repeat (TPR) domain and a U-box-dependent E3 ubiquitin ligase domain (Ballinger et al, 1999; Connell et al, 2001; Murata et al, 2001). The TPR domain of CHIP interacts with heat-shock proteins, such as Hsp70 and Hsp90 (Hsp70/90), and thus is required for the association with Hsp70/90 client proteins (Wu et al, 2001). The U-box domain of CHIP has a tertiary structure highly similar to the RING finger domain and selectively polyubiquitinates unfolded or misfolded proteins (Murata et al, 2003). Various molecules have been identified as CHIP substrates, including glucocorticoid receptors (Connell et al, 2001), misfolded cystic fibrosis transmembrane-conductance regulator (Meacham et al, 2001), heat-denatured luciferase (Murata et al, 2001), transmembrane receptor tyrosine kinase ErbB2 (Xu et al, 2002), androgen receptor (Cardozo et al, 2003), hyperphosphorylated Tau (Shimura et al, 2004) and mutant SOD1 (Urushitani et al, 2004). However, little is known about the physiological functions of CHIP in MAPK pathways or in hyperosmotic response.
Here, we report that CHIP is a negative regulator of the MEKK2-ERK pathway in hyperosmotic stress response. MEKK2 was activated by treating cells with sorbitol (hyperosmotic stress) and was required for sorbitol-induced activation of ERK, but not of JNK or p38. Moreover, CHIP bound, polyubiquitinated and thereby degraded MEKK2 in response to sorbitol, eventually leading to the inhibition of the sustained activation of the MEKK2-ERK pathway. Sorbitol-induced expression of water channel proteins, aquaporin 1 (AQP1) and AQP5, was found to depend on the transient activation of the MEKK2-ERK pathway. These results show that the termination of ERK activity by CHIP-dependent degradation of MEKK2 is a novel mechanism for proper gene expression in response to hyperosmotic stress.
Results
MEKK2 is required for hyperosmotic stress-induced ERK activation
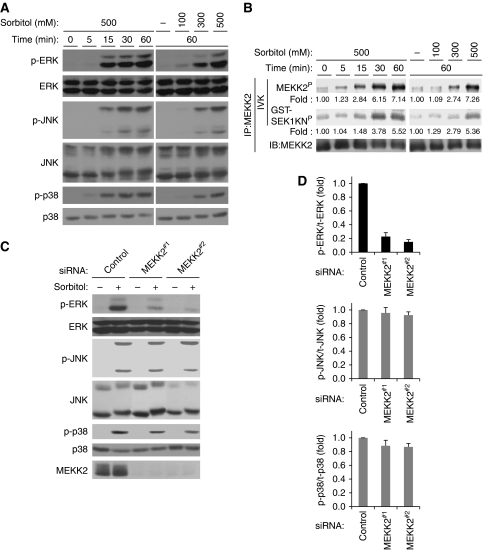
To explore the functions of MAPK pathways in hyperosmotic stress response, we first analysed the effects of hyperosmotic stress on the activities of ERK, JNK and p38. On treatment of HEK293 cells with sorbitol, endogenous ERK, JNK and p38 were strongly activated in time- and dose-dependent manners (Figure 1A). MEKK2, a potential activator of these MAPKs, has been reported to be activated by treating CCL64 mink lung epithelial cells and mouse embryonic fibroblasts (MEFs) with sorbitol (Nakamura and Johnson, 2007). We thus examined whether sorbitol induces the activation of MEKK2 in HEK293 cells. The activities of endogenous MEKK2 were analysed by an in vitro kinase (IVK) assay using recombinant GST-SEK1KN (kinase-inactive mutant form of SEK1) as a substrate. Sorbitol-induced activation of MEKK2 was observed in time- and dose-dependent manners (Figure 1B) and correlated well with the activation status of MAPKs (Figure 1A). It has been reported that sorbitol-induced p38 activation was abrogated in MEKK3 knockout MEFs, whereas other MAPK pathways were largely unaffected (Uhlik et al, 2003). We thus examined whether MEKK2 is required for sorbitol-induced activation of MAPK pathways using small-interference RNA (siRNA) against MEKK2 in HEK293 cells. MEKK2 siRNA effectively suppressed the expression of MEKK2 without affecting that of ERK, JNK or p38 (Figure 1C). MEKK2 knockdown strongly and significantly inhibited sorbitol-induced activation of ERK, but not JNK or p38 (Figure 1C and D; Supplementary Figure S1A and B). In contrast, MEKK3 knockdown exhibited no effect, if any, on sorbitol-induced ERK activation at various time points tested (Supplementary Figure S1A, B, E and F). These results suggest that MEKK2 is specifically required for the activation of the ERK pathway in response to hyperosmotic stress.
Figure 1.
MEKK2 is required for sorbitol-induced ERK activation. (A) Sorbitol-induced activation of ERK, JNK and p38. HEK293 cells were treated for indicated time periods with indicated concentrations of sorbitol. Cell lysates were subjected to immunoblotting with anti-phospho-ERK (p-ERK), anti-ERK, anti-phospho-JNK (p-JNK), anti-JNK, anti-phospho-p38 (p-p38) and anti-p38 antibodies. (B) Sorbitol-induced activation of MEKK2. HEK293 cells were stimulated as described in (A). Activation of MEKK2 was analysed by in vitro kinase assay (IVK) using GST-SEK1 kinase-inactive (SEK1KN) as a substrate. The amount of immunoprecipitated MEKK2 was confirmed by immunoblotting (IB) with anti-MEKK2 antibody. (MEKK2P) Autophosphorylated MEKK2. (GST-SEK1KNP) Phosphorylated GST-SEK1KN. Intensities of MEKK2P and GST-SEK1KNP relative to the amount of MEKK2 protein were calculated and shown as fold intensities. (C, D) Requirement of MEKK2 for sorbitol-induced ERK activation. Control, MEKK2#1 or MEKK2#2 siRNAs were transfected into HEK293 cells. After 48 h, cells were treated with 500 mM sorbitol for 1 h, and cell lysates were subjected to immunoblotting with indicated antibodies (C). Sorbitol-induced ERK, JNK and p38 phosphorylation levels relative to the amount of total proteins are shown as fold increase compared with control cells (n=3) (D).
CHIP interacts with MEKK2
A yeast two-hybrid screening for MEKK2-binding proteins was used and we identified E3 ubiquitin ligase CHIP, which is known to promote the ubiquitination and degradation of substrate proteins (Murata et al, 2003). We thus examined whether MEKK2 interacts with CHIP in mammalian cells by a co-immunoprecipitation analysis. When Flag-tagged MEKK2 (Flag-MEKK2) was co-expressed with haemagglutinin (HA)-tagged CHIP (HA-CHIP) in HEK293 cells, HA-CHIP was co-immunoprecipitated with Flag-MEKK2 (Figure 2A). MEKK1 and MEKK3, which possess kinase domains closely related to MEKK2, were not co-immunoprecipitated with CHIP (Figure 2A). Furthermore, no interaction was observed between CHIP and MEKK2KN (a kinase-inactive mutant form of MEKK2 in which Lys 385 is substituted by Met) (Figure 2B and C). These results suggest that CHIP specifically interacts with MEKK2 in a kinase activity-dependent manner.
Figure 2.
MEKK2 interacts with CHIP. (A) Specific interaction of CHIP with MEKK2. HEK293 cells were transfected with the indicated combination of Flag-MEKK1, Flag-MEKK2, Flag-MEKK3 and HA-CHIP. After 48 h, cell lysates were immunoprecipitated with anti-Flag antibody followed by immunoblotting with indicated antibodies. The expression level of HA-CHIP was confirmed by immunoblotting with anti-HA antibody. (B) Schematic representation of various mutant forms of MEKK2 and CHIP. (C) Requirement of kinase activity for the interaction of CHIP with MEKK2. HEK293 cells were transfected with the indicated combination of Flag-MEKK2WT, Flag-MEKK2KN and HA-CHIP. Cell lysates were analysed as described in (A). (D) Interaction of CHIP with MEKK2-CT. HEK293 cells were transfected with the indicated combination of Flag-MEKK2WT, Flag-MEKK2-NT, Flag-MEKK2-CTWT and HA-CHIP. Cell lysates were analysed as described in (A). (E) Interaction of MEKK2 with CHIPΔU. HEK293 cells were transfected with the indicated combination of Myc-CHIPΔU, Myc-CHIPΔTPR and Flag-MEKK2. Cell lysates were analysed as described in (A). The expression levels of Myc-CHIPWT and Myc-CHIPΔU were confirmed by immunoblotting with anti-Myc antibody.
To determine the regions of interaction, we constructed expression plasmids for truncated forms of MEKK2 and CHIP (Figure 2B). Similar to the case with wild-type MEKK2 (MEKK2WT), a C-terminal fragment of MEKK2 lacking the regulatory domain (MEKK2-CTWT), but not an N-terminal fragment of MEKK2 lacking the kinase domain (MEKK2-NT), was co-immunoprecipitated with CHIP (Figure 2D), indicating that CHIP is recruited to the kinase domain of MEKK2. CHIP possesses the C-terminal U-box domain (Jiang et al, 2001) and the N-terminal TPR domain (Wu et al, 2001), the latter of which is implicated in protein–protein interactions (Blatch and Lassle, 1999) (Figure 2B). The interaction of a truncated form of CHIP lacking the U-box (CHIPΔU) with MEKK2 was readily detectable (Figure 2E), and a truncated form of CHIP lacking the TPR domain (CHIPΔTPR) showed weaker interaction with MEKK2 compared with CHIPΔU (Figure 2E). As CHIP forms dimers through its U-box domain (Zhang et al, 2005), this weak co-immunoprecipitation of CHIPΔTPR with MEKK2 may be caused by the interaction of endogenous CHIP with CHIPΔTPR. These results suggest that the TPR domain of CHIP interacts with the MEKK2 kinase domain in a kinase activity-dependent manner.
CHIP promotes the degradation of MEKK2 in response to hyperosmotic stress
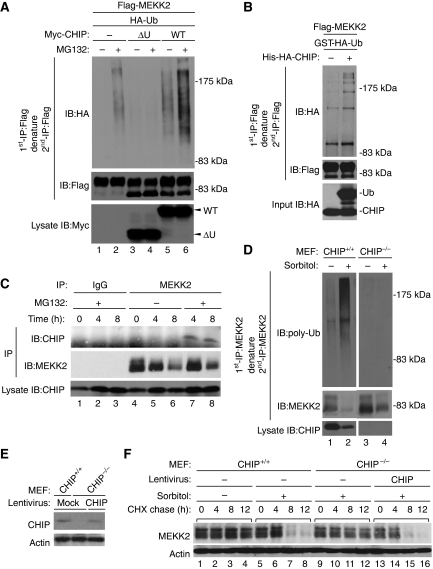
As CHIP promotes the ubiquitination and proteasome-dependent degradation of several substrates (Wiederkehr et al, 2002), we examined whether CHIP ubiquitinates MEKK2. Flag-MEKK2 and HA-Ub were co-transfected with Myc-CHIPWT or Myc-CHIPΔU into HEK293 cells. Ubiquitinated MEKK2 was observed after treatment with MG132 (Figure 3A, lane 2). Overexpression of CHIP increased the amount of ubiquitinated MEKK2 (Figure 3A, lanes 5 and 6) and CHIPΔU, which can interact with MEKK2, but lacks E3 ubiquitin ligase activity, clearly decreased the amount of ubiquitinated MEKK2 (Figure 3A, lane 4). We also found that MEKK2KN was not ubiquitinated (Supplementary Figure S2A). This was consistent with the lack of interaction with CHIP (Figure 2C). We examined whether CHIP ubiquitinated MEKK2 by an in vitro ubiquitination assay. Recombinant CHIP markedly enhanced the ubiquitination of MEKK2 in vitro (Figure 3B), suggesting that CHIP directly ubiquitinates MEKK2.
Figure 3.
CHIP is required for the proteasomal degradation of MEKK2 in response to sorbitol. (A) CHIP-dependent ubiquitination of MEKK2. HEK293 cells were transfected with the indicated combination of Flag-MEKK2WT, Myc-CHIPWT, Myc-CHIPΔU and HA-Ubiquitin (HA-Ub). After 24 h, cells were incubated with or without 100 nM MG132 for 18 h. Flag-MEKK2 was immunoprecipitated with anti-Flag antibody and re-immunoprecipitated with anti-Flag antibody after denaturation with 1% SDS followed by immunoblotting with indicated antibodies. The expression levels of Myc-CHIPWT and Myc-CHIPΔU were confirmed by immunoblotting with anti-Myc antibody. (B) In vitro ubiquitination of MEKK2 by CHIP. Flag-MEKK2WT transfected into HEK293 cells was purified with anti-Flag antibody followed by in vitro ubiquitination assay using ATP and recombinant proteins of E1, E2, GST-HA-Ub, His-HA-CHIP and Hsp70. Samples were analysed as described in (A). The protein amount of GST-HA-Ub and His-HA-CHIP was confirmed by immunoblotting with anti-HA antibody (Input). (C) Sorbitol-induced endogenous interaction of MEKK2 with CHIP. Cell lysates from MEFs treated with 300 mM sorbitol with or without 100 nM MG132 were immunoprecipitated with anti-MEKK2 antibody or control rabbit IgG followed by immunoblotting with indicated antibodies. (D) Sorbitol-induced endogenous ubiquitination of MEKK2 by CHIP. CHIP+/+ or CHIP−/− MEFs were pre-cultured in medium containing 0.1% FBS for 24 h and treated with or without 300 mM sorbitol in the presence of 100 nM MG132 for 12 h. Endogenous MEKK2 was immunoprecipitated with anti-MEKK2 antibody and re-immunoprecipitated with anti-MEKK2 antibody after denaturation with 1% SDS. Ubiquitinated MEKK2 was detected by anti-polyubiquitin antibody. The protein amount of MEKK2 and CHIP was confirmed by immunoblotting with anti-MEKK2 and CHIP antibodies. (E, F) Requirement of CHIP for sorbitol-induced MEKK2 degradation. CHIP+/+ or CHIP−/− MEFs were infected with lentivirus encoding CHIP or mock virus. After 48 h, MEFs pre-cultured in DMEM containing 0.1% FBS for 24 h were treated with or without 300 mM sorbitol in the presence of 100 μg/ml cycloheximide (CHX). The protein amount of MEKK2 and actin was confirmed by immunoblotting with anti-MEKK2 and actin antibodies. Endogenous and lentivirus-mediated expression of CHIP was detected by immunoblotting with anti-CHIP antibody (E).
To confirm these results under more physiological conditions, we investigated the interaction, ubiquitination and degradation of endogenous MEKK2 in response to sorbitol. We first examined the association of endogenous MEKK2 with CHIP in MEFs. Anti-MEKK2 antibody, but not control IgG, specifically recognized endogenous MEKK2 (Figure 3C, middle panel). When cells were treated with sorbitol in the presence of MG132, CHIP was found to associate with MEKK2 (Figure 3C, lanes 7 and 8). As this interaction was not observed in the absence of MG132 (Figure 3C, lanes 5 and 6), the sorbitol-induced MEKK2–CHIP complex seems to be susceptible to proteasome-dependent degradation. We next examined the ubiquitination and degradation of endogenous MEKK2 in CHIP−/− MEFs. The amount of ubiquitinated MEKK2 was strongly increased by treatment with sorbitol in CHIP+/+ MEFs (Figure 3D, lane 2), whereas the ubiquitination of MEKK2 was not observed in CHIP−/− MEFs (Figure 3D, lanes 3 and 4). CHIP-dependent ubiquitination of MEKK2 was also confirmed by the treatment with 300 mM NaCl (Supplementary Figure S2B). These observations suggest that CHIP is required for the ubiquitination of MEKK2 in response to hyperosmotic stress. We next examined the requirement of CHIP for the degradation of MEKK2. A cycloheximide (CHX)-based protein-chase assay revealed that treatment with sorbitol for 8 h induced the degradation of MEKK2 in CHIP+/+ MEFs (Figure 3F, lanes 7 and 8), but not in CHIP−/− MEFs (Figure 3F, lanes 9–12). To further confirm CHIP-dependent degradation of MEKK2, CHIP was exogenously re-expressed by lentivirus encoding CHIP in CHIP−/− MEFs (Figure 3E). The inhibition of MEKK2 degradation in CHIP−/− MEFs was completely recovered by the lentivirus-mediated expression of CHIP (Figure 3F, lanes 15 and 16; compare with lanes 11 and 12). These observations indicate that CHIP is essential for the proteasomal degradation of MEKK2 in response to sorbitol.
CHIP interacts with dephosphorylated MEKK2
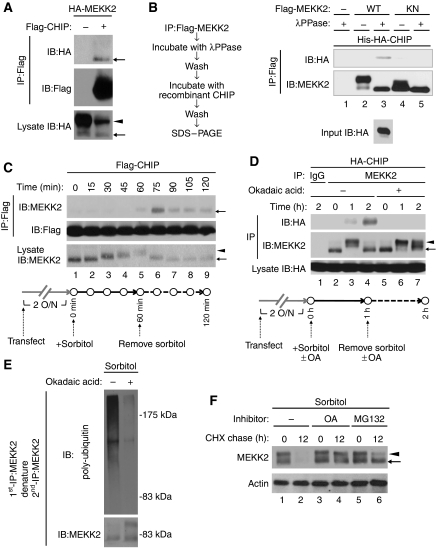
We next addressed the mechanism of interaction between CHIP and MEKK2. Exogenously expressed MEKK2WT, but not MEKK2KN, was detected as a doublet of bands on SDS–PAGE (Figures 2A, C, D, and 4A). MEKK2WT incubated with recombinant λphosphatase (λPPase) was found to migrate faster on SDS–PAGE and settle down to the lower molecular size in the manner of MEKK2KN (Supplementary Figure S3A), suggesting that the upper and lower bands of MEKK2WT are phosphorylated and non-phosphorylated forms of MEKK2, respectively. To examine whether CHIP interacts with phosphorylated MEKK2 or non-phosphorylated MEKK2, HA-MEKK2 co-immunoprecipitated with Flag-CHIP was immunoblotted with anti-HA antibody (Figure 4A). Considering that the MEKK2–CHIP interaction required the kinase activity of MEKK2 (Figure 2C), we hypothesized that activated (phosphorylated) MEKK2 may be able to interact with CHIP. Surprisingly, non-phosphorylated MEKK2, but not phosphorylated MEKK2, was mainly co-immunoprecipitated with Flag-CHIP (Figure 4A, top panel, arrow) despite the fact that phosphorylated MEKK2 was more abundant than non-phosphorylated MEKK2 in whole cell lysates (Figure 4A, bottom panel, arrow and arrowhead). Considering that MEKK2KN failed to interact with CHIP (Figure 2C), co-immunoprecipitated MEKK2 may be the dephosphorylated form of activated MEKK2. We thus examined whether CHIP interacts with dephosphorylated MEKK2 in vitro. Immunoprecipitated Flag-MEKK2WT and Flag-MEKK2KN were dephosphorylated by λPPase and incubated with recombinant HA-CHIP. MEKK2–CHIP interaction was then detected by immunoblotting with anti-HA antibody (Figure 4B). The treatment with λPPase dephosphorylated MEKK2WT, which was represented by its faster mobility on SDS–PAGE (Figure 4B, middle panel). Interestingly, we found that only dephosphorylated MEKK2WT (Figure 4B, lane 3), but not phosphorylated MEKK2WT (lane 2) or MEKK2KN (lanes 4 and 5), interacted with CHIP in vitro, suggesting that the dephosphorylation of activated MEKK2 may allow MEKK2 to interact with CHIP. Instead of λPPase, the treatment of MEKK2WT with CHIP−/− MEF lysates also induced the interaction of MEKK2 with CHIP (Supplementary Figure S3B, lane 3). These results suggest that an unidentified endogenous PPase activity may be involved in recruiting CHIP to MEKK2.
Figure 4.
Dephosphorylation of MEKK2 is required for CHIP-dependent proteasomal degradation of MEKK2. (A) Interaction of CHIP with non-phosphorylated MEKK2. HEK293 cells were transfected with the indicated combination of HA-MEKK2 and Flag-CHIP. Cell lysates were analysed as described in Figure 2A. Arrows and arrowhead denote non-phosphorylated and phosphorylated MEKK2, respectively. (B) Interaction of CHIP with dephosphorylated MEKK2 in vitro. Flag-MEKK2WT and Flag-MEKK2KN overexpressed in HEK293 cells were immunoprecipitated with anti-Flag antibody and were incubated with or without λPPase. After washing the immunocomplex beads, MEKK2 proteins were incubated with recombinant His-HA-CHIP (recombinant CHIP) followed by immunoblotting with indicated antibodies. Amount of the input CHIP proteins was detected by immunoblotting with anti-HA antibody. The left schema represents the experimental protocol. (C) Sorbitol-induced interaction of CHIP with MEKK2. HEK293 cells transfected with Flag-CHIP were treated with 500 mM sorbitol for 1 h, and medium was changed to new medium without sorbitol. After 1 h, cell lysates were immunoprecipitated with anti-Flag antibody followed by immunoblotting with indicated antibodies. The expression level of MEKK2 was confirmed by immunoblotting with anti-MEKK2 antibody. The bottom schema represents the experimental time schedules. (D) Inhibition of MEKK2–CHIP interaction by okadaic acid. HEK293 cells transfected with HA-CHIP were treated with 500 mM sorbitol in the presence of 100 nM okadaic acid (OA) or DMSO for 1 h, and medium was changed to new medium without sorbitol. After 1 h, cell lysates were immunoprecipitated with anti-MEKK2 antibody followed by immunoblotting with indicated antibodies. The expression level of HA-CHIP was confirmed by immunoblotting with anti-HA antibody. The bottom schema represents the experimental time schedules. (E) Inhibition of MEKK2 ubiquitination by okadaic acid. MEFs were incubated with medium containing 0.1% FBS. After 24 h, MEFs were pre-treated with or without 100 nM okadaic acid. Then, cells were continuously treated with 300 mM sorbitol and 100 nM MG132 for 12 h. Ubiquitination of endogenous MEKK2 was analysed as described in Figure 3D. (F) Inhibition of MEKK2 degradation by okadaic acid. MEFs were pre-cultured in medium containing 0.1% FBS for 24 h and treated with 300 mM sorbitol in the presence of 100 μg/ml CHX. The protein amount of MEKK2 and actin was detected as described in Figure 3E.
Dephosphorylation is required for the ubiquitination and degradation of MEKK2
We next examined the association of CHIP with dephosphorylated MEKK2 under more physiological conditions. HEK293 cells transfected with Flag-CHIP were transiently treated with 500 mM sorbitol, which strongly activates MEKK2 (Figure 1B) for 60 min, and sorbitol was removed from the culture medium to prevent the further activation-dependent phosphorylation of MEKK2 (Figure 4C, bottom). On sorbitol stimulation, endogenous MEKK2 was phosphorylated and migrated slowly on SDS–PAGE in a time-dependent manner (Figure 4C, bottom panel, lanes 1–5), which correlated well with the time course of sorbitol-induced MEKK2 activation (Figure 1B). The interaction of phosphorylated endogenous MEKK2 with CHIP was undetectable in the presence of sorbitol for 60 min (Figure 4C, top panel, lanes 1–5). The removal of sorbitol induced the dephosphorylation of MEKK2, which is represented by the faster migration of MEKK2 (Figure 4C, bottom panel, lanes 6–9). In parallel, the removal of sorbitol induced the interaction between MEKK2 and CHIP, which peaked at 15 min after the removal of sorbitol [top panel, lane 6 (time point; 75 min)] and decreased thereafter (top panel, lanes 6–9). Moreover, removal of sorbitol after 1 h treatment of cells even with 100 and 300 mM sorbitol, which induced slight and moderate activations of ERK, respectively (Figure 1A), also induced the interaction between MEKK2 and CHIP (Supplementary Figure S4A, see long exposure panel, lanes 5, 6, 8 and 9), suggesting that MEKK2 dephosphorylation-dependent recruitment of CHIP may occur in physiological conditions.
These results also suggest that an unidentified cellular PPase may contribute to MEKK2 dephosphorylation before the interaction with CHIP and the degradation of MEKK2. We thus examined the requirement of PPase activity for the MEKK2–CHIP interaction using okadaic acid (OA), a Ser/Thr phosphatase inhibitor with a wide spectrum. HEK293 cells transfected with HA-CHIP were treated with sorbitol in the presence or absence of OA for 60 min, and sorbitol was removed from the culture medium (Figure 4D, top). OA treatment abrogated sorbitol deprivation-induced MEKK2 dephosphorylation and MEKK2–CHIP interaction (Figure 4D, lanes 4 and 7), suggesting that dephosphorylation of MEKK2 is required for interaction with CHIP in vivo. Consistent with these observations, OA treatment also inhibited sorbitol-induced ubiquitination (Figure 4E) and degradation (Figure 4F, lanes 2 and 4) of MEKK2. Moreover, treatment with MG132 resulted in a greater accumulation of dephosphorylated MEKK2 compared with phosphorylated MEKK2 (Figure 4F, lane 6). These observations suggest that the dephosphorylation of activated MEKK2 is a prerequisite for CHIP-mediated proteasomal degradation of MEKK2.
As MEKK2-CT, which mainly consists of the kinase domain, is the region of interaction with CHIP (Figure 2D), we further examined whether the phosphorylation status of MEKK2-CT contributes to the interaction with CHIP using several mutant forms of MEKK2-CT (Supplementary Figure S4B). Consistent with the data of MEKK2WT (Figure 4A), MEKK2-CTWT was detected as a doublet of bands on SDS–PAGE, and the lower band of MEKK2-CTWT, but not the upper band of MEKK2-CTWT, was specifically co-immunoprecipitated with HA-CHIP (Supplementary Figure S4C, arrow). Contrary to the results from the full-length MEKK2KN (Figure 2C), we found that MEKK2-CTKN clearly interacted with CHIP (Supplementary Figure S4C), suggesting that unphosphorylated C-terminal kinase domain of MEKK2 can interact with CHIP in the absence of N-terminal-regulatory domain of MEKK2. As two phosphorylation sites, Ser 519 and Ser 523, in MEKK2 have been reported to be required for MEKK2 activation (Zhang et al, 2006), we examined whether the phosphorylation status of them is involved in the interaction with CHIP. The non-phosphorylation mimetic mutant form of MEKK2 (MEKK2-CTAA), in which Ser 513 and Ser 523 are substituted by Ala, exhibited the lower migration on SDS–PAGE and interacted with CHIP (Supplementary Figure S4C, arrow). On the other hand, the phosphorylation mimetic mutation of Ser 519 and Ser 523 to Glu (MEKK2-CTEE) abolished the interaction with CHIP (Supplementary Figure S4D). Although we cannot rule out the potential involvement of other unknown phosphorylation sites in MEKK2–CHIP interaction, the phosphorylation of Ser 519 and Ser 523 in MEKK2 is likely to prevent the interaction with CHIP.
CHIP attenuates hyperosmotic stress-induced sustained activation of ERK
On the basis of these findings, which indicate that CHIP induces MEKK2 degradation in hyperosmotic stress response, we hypothesized that CHIP downregulates the MEKK2-ERK pathway. We thus examined the function of CHIP in the sorbitol-induced ERK activation. As continual treatment with sorbitol for >2 h killed HEK293 cells, the cells transfected with MEKK2 siRNA and/or CHIP siRNA were transiently treated with sorbitol for 1 h. At 7 h after stimulation, the ERK activities were determined by an Elk1-promoter luciferase assay as a read-out of ERK activation. Without sorbitol treatment, the knockdown of MEKK2 and/or CHIP had only a marginal effect on the basal activity of the reporter gene (Figure 5A, columns 1–4). However, sorbitol-induced ERK activation (column 5) was significantly reduced and increased by MEKK2 siRNA (column 6) and CHIP siRNA (column 7), respectively. Moreover, co-transfection with MEKK2 siRNA abrogated the CHIP siRNA-induced increase in ERK activity (Figure 5A, columns 7 and 8). These results suggest that CHIP downregulates sorbitol-induced ERK activation by targeting MEKK2.
Figure 5.
CHIP is required for the inhibition of sorbitol-induced sustained activation of ERK. (A) Function of MEKK2 and CHIP in sorbitol-induced activation of ERK pathway. After transfection with the indicated combination of control, MEKK2 and CHIP siRNAs for 24 h, HEK293 cells were transfected with pFA-Elk1, pFR-Luc and pSV7d-βGal for 24 h. Cells were transiently treated with 500 mM sorbitol for 1 h. After 7 h, the Elk1-promoter luciferase activities were determined as described in Materials and methods. The luciferase activities relative to the β-galactosidase activities are shown as fold increase compared with control cells (n=3, mean±s.e., *P<0.05, **P<0.01). (B, C) Requirement of CHIP for the inhibition of sorbitol-induced ERK activation at a late phase. HEK293 cells transfected with siRNAs against control, CHIP#1 or CHIP#2 for 48 h were treated with 500 mM sorbitol for 1 h. Cell lysates were subjected to immunoblotting with indicated antibodies. The bottom schema represents the experimental time schedules (B). Sorbitol-induced ERK, JNK and p38 phosphorylation levels relative to the amount of total proteins are shown as fold increase compared with non-treated cells (0 h) (n=3, mean±s.e., **P<0.01) (C).
We next examined the effects of siRNAs for MEKK2 and CHIP on the time-dependent activation of the MAPK pathway in response to sorbitol. HEK293 cells transfected with siRNAs for 48 h were transiently treated with sorbitol for 60 min, and sorbitol was removed from the culture medium (Supplementary Figure S1C). MEKK2, but not MEKK3, siRNA clearly inhibited the prolonged ERK activation after sorbitol removal (Supplementary Figure S1D, E and F). CHIP siRNA exhibited no effect on the activation of ERK, JNK or p38 until 4 h after sorbitol treatment [Supplementary Figure S5A (until 60 min); Figure 5B, lanes 2–4, 8–10, 14–16 (at 1, 2 and 4 h)]. In contrast, sorbitol-induced activation of ERK, but not of JNK or p38, was maintained until 12 h only in CHIP knockdown cells (Figure 5B, lanes 5, 6, 11, 12, 17 and 18; Figure 5C). Consistent with these results, the prolonged activation of ERK, but not of JNK or p38, was observed until 12 h after continuous treatment with sorbitol in CHIP−/− MEFs (Supplementary Figure S5B). The time course of inactivation of ERK in CHIP+/+ MEFs (Supplementary Figure S5B, lanes 4 and 5) correlated well with that of sorbitol-induced MEKK2 degradation (Figure 3F, lanes 7 and 8). These observations strongly suggest that CHIP selectively downregulates the prolonged ERK activation by the degradation of MEKK2, resulting in the transient activation of ERK.
MEKK2- and CHIP-dependent transient ERK activation is required for the induction of AQP1 and AQP5 in hyperosmotic response
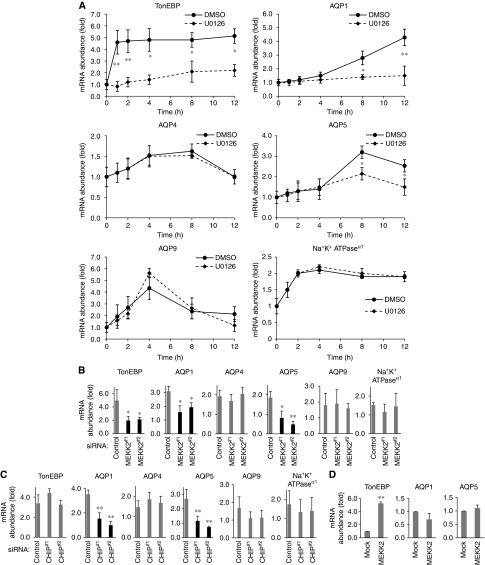
Finally, we assessed the physiological functions of MEKK2 and CHIP in sorbitol-induced gene expression. The expression of various genes is transcriptionally induced in response to hyperosmotic stress (Burg et al, 2007). Earlier studies have reported that hyperosmolarity-induced expression of tonicity enhancer-binding protein (TonEBP; osmoprotective transcription factor), AQP1, AQP4, AQP5 and AQP9 depends on the activities of MAPK pathways (Umenishi et al, 1996; Hoffert et al, 2000; Tsai et al, 2007). We first examined the requirement of ERK activation for the sorbitol-induced expression of these genes. The expression of TonEBP, AQP1 and AQP5, but not AQP4 and AQP9, was significantly reduced by the treatment with an MEK1/2 inhibitor, U0126 (Figure 6A). Expression of Na+K+ATPaseα1, which is induced by an MAPK-independent pathway (Burg et al, 2007), was neither affected by U0126 (Figure 6A). These findings are consistent with the earlier observations that the ERK pathway is required for hyperosmolarity-induced expression of TonEBP, AQP1 and AQP5, but not of AQP4 and AQP9 (Umenishi et al, 1996; Hoffert et al, 2000; Arima et al, 2003; Tsai et al, 2007). Furthermore, the sorbitol-induced expression of TonEBP, AQP1 and AQP5, but not AQP4 and AQP9, was significantly reduced in MEKK2 knockdown cells (Figure 6B). Collectively, these results suggest that MEKK2-MEK-ERK activity is required for the inductions of mRNAs of TonEBP, AQP1 and AQP5 in response to sorbitol.
Figure 6a-d.
MEKK2- and CHIP-dependent transient ERK activation is required for sorbitol-induced expression of AQP1 and AQP5. (A) Requirement of ERK activity for sorbitol-induced expression of TonEBP, AQP1 and AQP5. HEK293 cells pre-treated with 20 μM U0126 or DMSO for 1 h were treated with 500 mM sorbitol for 1 h, and medium was changed to new medium without sorbitol (time point 1 h). Then, expression levels of TonEBP, AQP1, AQP4, AQP5, AQP9, Na+K+-ATPaseα1 and 18S-rRNA were measured by the real-time PCR at the indicated time points. The values of genes expression were normalized to the value of 18S-rRNA expression and sorbitol-induced expression of genes are shown as fold increase compared with non-treated cells (n=5, mean±s.e., *P<0.05, **P<0.01). (B) Requirement of MEKK2 for sorbitol-induced expression TonEBP, AQP1 and AQP5. siRNA against control, MEKK2#1 or MEKK2#2 was transfected into HEK293 cells for 48 h. Cells were treated with 500 mM sorbitol for 1 h and sorbitol was removed from medium for 7 h. Expression levels of TonEBP, AQP1, AQP4, AQP5, AQP9, Na+K+-ATPaseα1 and 18S-rRNA were measured by the real-time PCR. The values of genes expression were normalized to the value of 18S-rRNA expression and sorbitol-induced expression of genes are shown as fold increase compared with non-treated cells (n=5, mean±s.e., *P<0.05, **P<0.01). (C) Requirement of CHIP for sorbitol-induced expression AQP1 and AQP5. HEK293 cells were transfected with siRNAs against control, CHIP#1 or CHIP#2 for 48 h. Sorbitol-induced expression of indicated genes were detected as described in (A) (n=5, mean±s.e., **P<0.01). (D) Insufficiency of MEKK2 overexpression for the induction of AQP1 and AQP5. HEK293 cells were transfected with mock or Flag-MEKK2 for 48 h. Sorbitol-induced expression of indicated genes were detected as described in (A) (n=5, mean±s.e., **P<0.01).
Although CHIP siRNA enhanced sorbitol-induced ERK activation (Figure 5A, column 7), the expression of AQP1 and AQP5, but not of TonEBP, was significantly decreased by CHIP siRNA (Figure 6C), suggesting that not only MEKK2 but also CHIP is required for sorbitol-induced expression of AQP1 and AQP5. We confirmed the requirement of CHIP for induction of TonEBP and AQP1 in MEFs, in which the expression of AQP4 and AQP5 was undetectable (data not shown). In CHIP−/− MEFs, sorbitol-induced expression of AQP1, but not of TonEBP, was clearly inhibited relative to that in CHIP+/+ MEFs (Supplementary Figure S6). These results suggest that different mechanisms may be involved in the inductions of mRNAs of AQP1/AQP5 and TonEBP, which are both MEKK2-MEK-ERK-responsive genes. Interestingly, whereas TonEBP mRNA was rapidly induced within 1 h after stimulation with sorbitol, AQP1, AQP5 and AQP9 were relatively slowly induced after 4 h (Figure 6A). The reason why CHIP knockdown-dependent prolonged ERK (Figure 5B) activity did not affect the induction of TonEBP (Figure 6C) may be because the ERK activation at an early phase may not only be necessary, but also be sufficient for TonEBP induction. On the other hand, overexpression of MEKK2, which can constitutively activate the ERK pathway (data not shown), induces the expression of TonEBP, but not that of AQP1 or AQP5 (Figure 6D), suggesting that not only the ERK activation but also CHIP-dependent termination of MEKK2-ERK activity may be required for the induction of AQP1 and AQP5 in hyperosmotic response. Since the time course of CHIP-dependent ERK downregulation, which starts after 4 h (Figure 5B and C), correlated well with the onset of induction of AQP1 and AQP5 (Figure 6A). AQP1 and AQP5 may not be simply induced by the activation of ERK, and AQP1 and AQP5 may require CHIP-dependent downregulation of ERK activity at the prolonged phase.
To test this hypothesis, we examined the requirement of CHIP-dependent inhibition of the prolonged phase of ERK activity for sorbitol-induced expression of TonEBP, AQP1 and AQP5 by using U0126. HEK293 cells transfected with control siRNA or CHIP siRNA were transiently treated with sorbitol for 1 h. Then the cells were treated with U0126 after removal of sorbitol for 2 h (Figure 6E). Treatment with U0126 artificially inhibited sorbitol-induced prolonged ERK activity in CHIP knockdown cells (Figure 6F, lanes 17 and 18), and thereby resulted in the transient ERK activation, which mimicked the profile of ERK activation in the control siRNA-transfected cells (lanes 1–6 and 13–18). We examined sorbitol-induced expression of TonEBP, AQP1 and AQP5 in U0126-treated cells. On expression of TonEBP, no significant difference was found between U0126-treated cells and DMSO-treated cells (Figure 6G). Interestingly, U0126 treatment significantly restored inhibition of sorbitol-induced expression of AQP1 and AQP5 in CHIP knockdown cells (Figure 6G). Together, these results strongly suggest that transient activation of ERK fine-tuned by not only MEKK2-dependent activation but also CHIP-dependent inhibition of ERK has a crucial function in the induction of AQP1 and AQP5 in hyperosmotic response.
Figure 6e-g.
(E, F) Artificial inhibition of sustained ERK activation by U0126 in CHIP knockdown cells. HEK293 cells were transfected with siRNAs against control, CHIP#1 or CHIP#2. After 48 h, cells were treated with 500 mM sorbitol for 1 h and medium was changed to new medium without sorbitol at the indicated time point (1 h). Then, 20 μM U0126 or DMSO was added to the medium at an indicated time point (3 h). (E) Represents the experimental time schedules. At the indicated time points, cell lysates were subjected to immunoblotting with indicated antibodies (F). (G) Expression of AQP1 and AQP5 by artificial transient ERK activation. After 5 h from the treatment with U0126 (time point; 8 h), sorbitol-induced expression of indicated genes were detected as described in (A) (n=5, mean±s.e., *P<0.05).
Discussion
The MAPK family proteins coordinate diverse cellular reactions, such as cytoskeletal remodelling and gene expression, in response to hyperosmotic stress (Burg et al, 2007). MEKK3 is known to be required for sorbitol-induced p38 activation (Uhlik et al, 2003), whereas the function of MEKK2 in hyperosmotic stress response has not been elucidated. We found that MEKK2 is required for the specific activation of ERK, but not of JNK or p38 in response to sorbitol. It is still unclear how MEKK2 specifically activates the ERK pathway in response to hyperosmotic stress. A recent study showed that sorbitol induces the formation of the Rac-osmosensing scaffold for the MEKK3 (OSM)–MEKK3–MKK3 complex on the membrane ruffles, which is required for p38 activation (Uhlik et al, 2003). Thus, a scaffold protein-dependent complex including MEKK2 and ERK may specifically determine hyperosmotic stress-induced activation of the ERK pathway.
We also found that hyperosmotic stress induced CHIP-dependent degradation of MEKK2. Many protein kinases are known to be subjected to ubiquitin-dependent degradation in response to activation, particularly when the kinase activation is sustained (Hunter, 2007). In the case of receptor tyrosine kinases and soluble tyrosine kinases (e.g. EGF, PDGF and Met/HGF receptors, c-Src and Chk1), the recognition of these kinases by E3 depends on activation-dependent phosphorylation (Kuivinen et al, 1993; Huang et al, 2006). MEKK2 also interacts with CHIP in a kinase activity-dependent manner (Figure 2C). However, dephosphorylated MEKK2, but not phosphorylated MEKK2, associated with CHIP in vitro and in vivo (Figure 4A and B). Moreover, the MEKK2–CHIP interaction correlated well with the dephosphorylation status of MEKK2 (Figure 4C). Consistent with these results, the treatment of cells with PPase inhibitor (OA) attenuated not only the MEKK2–CHIP interaction, but also sorbitol-induced ubiquitination and degradation of MEKK2 (Figure 4D–F). These results strongly show a novel mechanism that regulates MEKK2 activity by dephosphorylation-dependent proteasomal degradation. As OA preferentially inhibits phosphatase-2A (PP2A), we examined whether PP2A is involved in the interaction between MEKK2 and CHIP. Although co-expression of PP2A-A, which is one of the PP2A subunits, had no effect on the binding of CHIP with MEKK2 and on phosphorylation level of MEKK2 (Supplementary Figure S7), we cannot rule out the possibility that PP2A-induced dephosphorylation of MEKK2 contributes to the interaction with CHIP.
Smad ubiquitin-regulatory factor (Smurf) 1 has been identified as another HECT-type E3 ligase of MEKK2, which leads to the inhibition of the JNK pathway in osteoclasts (Yamashita et al, 2005). As Smurf1 promotes the ubiquitination of phosphorylated MEKK2, the recognition mechanism by Smurf1 seems to differ from that by CHIP.
It is still unclear why dual negative-regulation systems, which are composed of dephosphorylation and ubiquitination, are required for hyperosmotic response. As MEKK2 activation is mediated by its homo-dimerization through its kinase domain (Cheng et al, 2005a), PPase-induced dephosphorylation of MEKK2 may not be sufficient to inhibit re-trans-autophosphorylation and attenuate the MEKK2 signalling pathway. This hypothesis is supported by the result that MEKK2 dephosphorylated by cell lysate was re-autophosphorylated and re-activated by incubation with ATP in vitro (Supplementary Figure S8A and B). On the other hand, if the dephosphorylation step is unnecessary for the recruitment of CHIP, activated and phosphorylated MEKK2 might be immediately degraded, resulting in the shortage of duration of ERK activation. Thus, PPase-dependent dephosphorylation may determine the timing of MEKK2 degradation and enable the cell to adjust the duration of MEKK2-ERK activity in response to hyperosmotic stress. To address this issue, further studies will be needed to identify the PPase responsible for CHIP-dependent degradation of MEKK2.
There is currently no satisfactory explanation of why the inhibition of sustained ERK activation is required for the proper expression of some genes, such as AQP1 and AQP5. Distinct modes of MAPK activation, transient or sustained, are important for the cell fate decision (Chang and Karin, 2001). For instance, transient ERK activation induces proliferation in MEFs and PC12 cells (Marshall, 1995; Sasagawa et al, 2005), whereas sustained ERK activation induces differentiation in PC12 cells (Qui and Green, 1992) and T-cell receptor-mediated positive selection (Werlen et al, 2000). As sustained ERK activation may allow various biological responses (e.g. migration and differentiation) other than the adaptive responses to hyperosmotic stress (e.g. cell volume regulation), the temporal modulation of ERK activity may be an important mechanism underlying the appropriate response to hyperosmotic stress.
Why is the transiency or temporariness of ERK activation specifically required for the induction of AQP1 and AQP5? It is theoretically possible that the sustained activation of ERK may actively inhibit the induction of AQP1 and AQP5. When ERK activation is sustained, immediate early genes (IEGs) (e.g. c-Fos) are stabilized after phosphorylation by ERK and/or 90K-ribosomal S6 kinase, allowing cells to generate the appropriate responses (Murphy et al, 2002). Thus, one hypothesis to explain how transient ERK activation specifically induces AQP1 and AQP5 is that sustained ERK-dependent phosphorylation of IEG and/or IEG-target gene products may negatively regulate the expression of AQP1 and AQP5 (Supplementary Figure S9).
MEKK1 has been shown to mediate the degradation of ERK1/2 by its E3 ubiquitin ligase activity in response to sorbitol (Lu et al, 2002). The concentration of sorbitol used in the experiments was 500 mM, which eventually induced cell death after the prolonged treatment of NIH3T3 cells for 10 h because of the downregulation of MEK1-ERK1/2 survival signals. In this study, however, the treatment of MEFs with 300 mM sorbitol for 8 h or the transient treatment of HEK293 cells with 500 mM sorbitol for 1 h induced certain genes expression, but not cell death. Although it is not easy to compare the relative contributions of MEKK1 and MEKK2 to hyperosmolarity-induced ERK activation, further studies on these MEKK1 and MEKK2 may clarify the mechanism on how the downregulation of ERK pathway link to the determination of cell fate (gene expression or cell death) in response to hyperosmotic stress.
In conclusion, our findings showed a novel mechanism by which hyperosmotic stress induces the expression of proper genes (AQP1 and AQP5) through CHIP-dependent transient activation of the MEKK2-ERK pathway. Although further studies are needed to clarify the mechanisms of CHIP-dependent MEKK2 degradation and transient ERK-dependent proper gene expression, such a study may disclose the physiological function of the MEKK2-ERK pathway in hyperosmotic response.
Materials and methods
Cell culture and transfection
HEK293 cells and MEFs were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 4.5 mg/ml glucose, 10% foetal bovine serum and 100 units/ml penicillin G in a 5% CO2 atmosphere at 37°C. Transfection was performed using FuGENE6 (Roche Diagnostics) according to the manufacturer's instructions.
Plasmids, lentiviral vector and antibodies
A Flag tag was inserted at N-terminus of human MEKK1 (Flag-MEKK1), human MEKK3 (Flag-MEKK3), human MEKK2 (Flag-MEKK2 or Flag-MEKK2WT), MEKK2KN (Flag-MEKK2KN), MEKK2-NT (Flag-MEKK2-NT), MEKK2-CT (Flag-MEKK2-CT), MEKK2KN-CT (Flag-MEKK2KN-CT), human CHIP (HA-CHIP) and rat PP2A subunit A (PP2A-A) in pcDNA3.0 (Invitrogen). An HA tag was inserted at the N-terminus of MEKK2 (HA-MEKK2 or HA-MEKK2WT) and CHIP (HA-CHIP) in pcDNA3.0. Six copies of Myc tag were inserted at MEKK2-NT (Myc-MEKK2-NT), CHIP (Myc-CHIPWT), CHIPΔU (Myc-CHIPΔU) and CHIPTPR (Myc-CHIPTPR) in pcDNA3.0. HA-Ub has been described (Nishitoh et al, 2008). Lentivirus encoding CHIP was constructed as described (Nishitoh et al, 2008). Monoclonal antibodies to HA (clone 3F10), Flag (clone M2) and MEKK3 were purchased from Roche Diagnostics, Sigma-Aldrich and BD, respectively. Polyclonal antibodies to MEKK2 (Santa cruz), CHIP (Abcam), actin (Sigma-Aldrich) and polyubiquitin (MBL) were purchased. A control rabbit IgG was purchased from Santa Cruz.
Yeast two-hybrid screening
A mouse adult brain cDNA library (Invitrogen) was screened for proteins that interact with MEKK2KN as described (Saitoh et al, 1998) using the AH109 yeast reporter strain. The bait plasmid expressing MEKK2KN protein was constructed in-frame with the LexA DNA-binding domain of the pGBKT5 reporter vector (Clontech) bait plasmid. Plasmids of positive clones were recovered, and the cDNA inserts were sequenced.
Immunoprecipitation and immunoblotting
Cells were lysed in a lysis buffer containing 1% Triton X-100, 12 mM β-glycerophosphate, 150 mM NaCl, 20 mM Tris–HCl pH7.5, 5 mM EGTA, 1 mM NaF, 1 mM DTT, 1 mM NaVO4, 1 mM PMSF and 1.5% aprotinin. Cell extracts were clarified by centrifugation, and the supernatants were immunoprecipitated with antibodies to Flag, MEKK2, CHIP and control rabbit IgG using protein A-sepharose (Zymed). The beads were washed twice with the washing buffer A containing 500 mM NaCl, 20 mM Tris–HCl pH7.5, 5 mM EGTA and 1% Triton X-100 and twice with the washing buffer B containing 150 mM NaCl, 20 mM Tris–HCl pH7.5 and 5 mM EGTA. After boiling with SDS sample buffer, immunoprecipitated samples or aliquots of cell lysates were subjected to SDS–PAGE followed by electroblotting onto PVDF membranes. After blocking with 5% skim milk in TBS-T containing 150 mM NaCl, 50 mM Tris–HCl pH8.0 and 0.05% Tween20 for 1 h, the membranes were probed with antibodies. The antibody–antigen complexes were detected using enhanced chemiluminescence system.
IVK assay
The IVK assay has been described (Nishitoh et al, 2008). The plasmid of GST-mouse SEK1 kinase negative mutant (GST-SEK1KN) for bacterial fusion protein was constructed in pGEX-4T-1 (GE Healthcare). HEK293 cells were lysed and immunoprecipitated with anti-MEKK2 antibody as described above. The beads were incubated with 0.5 μg of GST-SEK1KN as a substrate for MEKK2 for 15 min at 30°C in a final volume of 50 μl of kinase buffer containing 20 mM Tris–HCl pH8.0, 20 mM MgCl2 and 0.3 μCi [γ-32P]ATP. Kinase reactions were quenched with SDS sample buffer. Immunoprecipitated samples were subjected to SDS–PAGE. Phosphorylation of endogenous MEKK2 and GST-SEK1KN were analysed by STORM imaging analyser (GE Healthcare). Immunoprecipitated MEKK2 was subjected to immunoblotting analysis to confirm appropriate expression of MEKK2.
In vitro-binding assay
The plasmid of 6xHis-tagged HA-CHIP (His-HA-CHIP) for bacterial fusion protein was constructed in pTrcHisA (Invitrogen). Recombinant His-HA-CHIP proteins were purified with Ni-NTA (Qiagen) according to the manufacturer's instructions. Flag-MEKK2WT or Flag-MEKK2KN were transfected into HEK293 cells and purified by immunoprecipitated with anti-Flag antibody. Then, after washing with washing buffer A and B, immunoprecipitated samples were incubated with λPPase (New England Biolab). After washing with washing buffer A and B, samples were incubated with 40 ng of His-HA-CHIP for 1 h at 4°C. Finally, after washing with washing buffer A and B, samples were analysed by SDS–PAGE.
Ubiquitination assay
In vivo ubiquitination assay. HEK293 cells were transfected with expression vectors. After 24 h, cells were incubated with 0.5 μM MG132 (Sigma-Aldrich). After 16 h, cell lysates were immunoprecipitated with antibodies to Flag or MEKK2. After washed with washing buffer A and B, beads were boiled with the denaturing buffer containing 1% SDS, 150 mM NaCl, 20 mM Tris–HCl pH7.5 and 5 mM EGTA. Supernatants were diluted by 0.02% SDS and re-immunoprecipitated with antibodies (2ndIP) and analysed by SDS–PAGE.
In vitro ubiquitination assay. Flag-MEKK2WT or Flag-MEKK2KN purified from HEK293 cells were incubated with recombinant E1 (Sigma-Aldrich), recombinant E2 (Sigma-Aldrich), recombinant Hsp70 (Sigma-Aldrich) and recombinant His-HA-CHIP in the buffer containing 4 mM ATP, 1 mM DTT, 2 mM MgCl2 and 50 mM HEPES-HCl pH7.6. Samples were analysed as described above.
CHX-chase assay
MEFs infected with lentivirus encoding CHIP or mock virus. After 48 h, MEFs pre-cultured in DMEM containing 0.1% FBS for 24 h were treated with or without 300 mM sorbitol in the presence of 100 μg/ml CHX. Cell lysates were analysed by immunoblotting with antibodies to MEKK2 and actin.
Reporter assay
HEK293 cells transfected with siRNA for 24 h were transfected with pFA-Elk1 (Stratagene), pFR-Luc (Stratagene) and β-galactosidase vector (pSV7d-βGal). After 24 h, cells were treated with 500 mM sorbitol for 1 h and medium were changed to mew medium without sorbitol. The Elk-promoter activity determined by luciferase activity was normalized to β-galactosidase activity. Relative activities were shown as fold activation relative to the activities in non-treated cells.
siRNA knockdown of MEKK2, MEKK3 and CHIP
HEK293 cells were transfected with MEKK2, CHIP and control siRNA oligo (Invitrogen) using Lipofectamine RNAiMAX reagent (Invitrogen). After 24 h, cells were incubated in new medium for 24 h. Knockdown was analysed by immunoblotting with antibody to MEKK2 or CHIP. Sequences were as follows: MAP3K2-HSS116573 Stealth Select RNAi (MEKK2#1; UUGGAUAGCUCUCUCCAUCCAAAGG and CCUUUGGAUGGAGAGAGCUAUCCAA), MAP3K2-HSS116574 Stealth Select RNAi (MEKK2#2; AAUUCCUGAUGAUUAUCUGGGUAGC and GCUACCCAGAUAAUCAUCAGGAAUU), MAP3K3-HSS1106451 Stealth Select RNAi (MEKK3#1; ACAUCUUCAUAUUUCACAGGCCGGC and GCCGGCCUGUGAAAUAUGAAGAUGU), MAP3K3-HSS106452 Stealth Select RNAi (MEKK3#2; AGGAUUGGCAGCUUCCAGACAAGGA and UCCUUGUCUGGAAGCUGCCAAUCCU), STUB1-HSS145537 Stealth Select RNAi (CHIP#1; UUCGGCCCACGAACAGACGAUUGCC and GGCAAUCGUCUGUUCGUGGGCCGAA), STUB1-HSS145539 Stealth Select RNAi (CHIP#2; UUCUUCGCGAUUCGAAGAGCGCUGG and CCAGCGCUCUUCGAAUCGCGAAGAA) and Stealth RNAi Negative Control Medium GC Duplex.
Real-time PCR
Complementary DNA corresponding to 1 μg of total RNA was used per reaction in quantitative PCR performed on ABI PRISM 7000 (Applied Biosystems), using Power SYBER Green Master Mix (Applied Biosystems) and following primers: human TonEBP (TCAGACAAGCGGTGGTGA and AGGGAGCTGAAGAAGCATCA), human AQP1 (GCCATCCTCTCAGGCATC and CCCGAGTTCACACCATCAG), human AQP4 (GGAAATTGGGAAAACCATTG and GACATACTCATAAAGGCCACCAG), human AQP5 (CTACTTCACTGGCTGCTCCAT and ATGGGCCCTACCCAGAAA), human AQP9 (GCAACCGTCTTTGGCATTTA and TTTTCTCCCACGATCAGCA), human 18S-ribosomal RNA (GGAGAGGGAGCCTGAGAAAC and TCGGGAGTGGGTAATTTGC) and human Na+K+-ATPaseα1 (TCTCTGATTCTCCAGCGACA and GGCTCATACTTATCACGTCCAA).
Supplementary Material
Acknowledgments
We thank all the members of Cell Signaling Laboratory for their critical comments. This work was supported by grants-in aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation. TM is a Research Fellow of the Japan Society of the Promotion of Science.
Footnotes
The authors declare that they have no conflict of interest.
References
- Arima H, Yamamoto N, Sobue K, Umenishi F, Tada T, Katsuya H, Asai K (2003) Hyperosmolar mannitol simulates expression of aquaporins 4 and 9 through a p38 mitogen-activated protein kinase-dependent pathway in rat astrocytes. J Biol Chem 278: 44525–44534 [DOI] [PubMed] [Google Scholar]
- Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C (1999) Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol 19: 4535–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank JL, Gerwins P, Elliott EM, Sather S, Johnson GL (1996) Molecular cloning of mitogen-activated protein/ERK kinase kinases (MEKK) 2 and 3. Regulation of sequential phosphorylation pathways involving mitogen-activated protein kinase and c-Jun kinase. J Biol Chem 271: 5361–5368 [DOI] [PubMed] [Google Scholar]
- Blatch GL, Lassle M (1999) The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays 21: 932–939 [DOI] [PubMed] [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC (1993) An osmosensing signal transduction pathway in yeast. Science 259: 1760–1763 [DOI] [PubMed] [Google Scholar]
- Burg MB, Ferraris JD, Dmitrieva NI (2007) Cellular response to hyperosmotic stresses. Physiol Rev 87: 1441–1474 [DOI] [PubMed] [Google Scholar]
- Cardozo CP, Michaud C, Ost MC, Fliss AE, Yang E, Patterson C, Hall SJ, Caplan AJ (2003) C-terminal Hsp-interacting protein slows androgen receptor synthesis and reduces its rate of degradation. Arch Biochem Biophys 410: 134–140 [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M (2001) Mammalian MAP kinase signalling cascades. Nature 410: 37–40 [DOI] [PubMed] [Google Scholar]
- Cheng J, Yu L, Zhang D, Huang Q, Spencer D, Su B (2005a) Dimerization through the catalytic domain is essential for MEKK2 activation. J Biol Chem 280: 13477–13482 [DOI] [PubMed] [Google Scholar]
- Cheng J, Zhang D, Kim K, Zhao Y, Su B (2005b) Mip1, an MEKK2-interacting protein, controls MEKK2 dimerization and activation. Mol Cell Biol 25: 5955–5964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C (2001) The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol 3: 93–96 [DOI] [PubMed] [Google Scholar]
- Guo Z, Clydesdale G, Cheng J, Kim K, Gan L, McConkey DJ, Ullrich SE, Zhuang Y, Su B (2002) Disruption of Mekk2 in mice reveals an unexpected role for MEKK2 in modulating T-cell receptor signal transduction. Mol Cell Biol 22: 5761–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammaker DR, Boyle DL, Chabaud-Riou M, Firestein GS (2004) Regulation of c-Jun N-terminal kinase by MEKK-2 and mitogen-activated protein kinase kinase kinases in rheumatoid arthritis. J Immunol 172: 1612–1618 [DOI] [PubMed] [Google Scholar]
- Hoffert JD, Leitch V, Agre P, King LS (2000) Hypertonic induction of aquaporin-5 expression through an ERK-dependent pathway. J Biol Chem 275: 9070–9077 [DOI] [PubMed] [Google Scholar]
- Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66: 300–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A (2006) Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol Cell 21: 737–748 [DOI] [PubMed] [Google Scholar]
- Hunter T (2007) The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol Cell 28: 730–738 [DOI] [PubMed] [Google Scholar]
- Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Hohfeld J, Patterson C (2001) CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J Biol Chem 276: 42938–42944 [DOI] [PubMed] [Google Scholar]
- Kesavan K, Lobel-Rice K, Sun W, Lapadat R, Webb S, Johnson GL, Garrington TP (2004) MEKK2 regulates the coordinate activation of ERK5 and JNK in response to FGF-2 in fibroblasts. J Cell Physiol 199: 140–148 [DOI] [PubMed] [Google Scholar]
- Kuivinen E, Hoffman BL, Hoffman PA, Carlin CR (1993) Structurally related class I and class II receptor protein tyrosine kinases are down-regulated by the same E3 protein coded for by human group C adenoviruses. J Cell Biol 120: 1271–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Xu S, Joazeiro C, Cobb MH, Hunter T (2002) The PHD domain of MEKK1 acts as an E3 ubiquitin ligase and mediates ubiquitination and degradation of ERK1/2. Mol Cell 9: 945–956 [DOI] [PubMed] [Google Scholar]
- Marshall CJ (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80: 179–185 [DOI] [PubMed] [Google Scholar]
- Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM (2001) The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol 3: 100–105 [DOI] [PubMed] [Google Scholar]
- Murata S, Chiba T, Tanaka K (2003) CHIP: a quality-control E3 ligase collaborating with molecular chaperones. Int J Biochem Cell Biol 35: 572–578 [DOI] [PubMed] [Google Scholar]
- Murata S, Minami Y, Minami M, Chiba T, Tanaka K (2001) CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep 2: 1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J (2002) Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol 4: 556–564 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Johnson GL (2003) PB1 domains of MEKK2 and MEKK3 interact with the MEK5 PB1 domain for activation of the ERK5 pathway. J Biol Chem 278: 36989–36992 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Johnson GL (2007) Noncanonical function of MEKK2 and MEK5 PB1 domains for coordinated extracellular signal-regulated kinase 5 and c-Jun N-terminal kinase signaling. Mol Cell Biol 27: 4566–4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Uhlik MT, Johnson NL, Hahn KM, Johnson GL (2006) PB1 domain-dependent signaling complex is required for extracellular signal-regulated kinase 5 activation. Mol Cell Biol 26: 2065–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitoh H, Kadowaki H, Nagai A, Maruyama T, Yokota T, Fukutomi H, Noguchi T, Matsuzawa A, Takeda K, Ichijo H (2008) ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev 22: 1451–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui MS, Green SH (1992) PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron 9: 705–717 [DOI] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17: 2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasagawa S, Ozaki Y, Fujita K, Kuroda S (2005) Prediction and validation of the distinct dynamics of transient and sustained ERK activation. Nat Cell Biol 7: 365–373 [DOI] [PubMed] [Google Scholar]
- Schaefer BC, Ware MF, Marrack P, Fanger GR, Kappler JW, Johnson GL, Monks CR (1999) Live cell fluorescence imaging of T cell MEKK2: redistribution and activation in response to antigen stimulation of the T cell receptor. Immunity 11: 411–421 [DOI] [PubMed] [Google Scholar]
- Shimura H, Schwartz D, Gygi SP, Kosik KS (2004) CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J Biol Chem 279: 4869–4876 [DOI] [PubMed] [Google Scholar]
- Su B, Cheng J, Yang J, Guo Z (2001) MEKK2 is required for T-cell receptor signals in JNK activation and interleukin-2 gene expression. J Biol Chem 276: 14784–14790 [DOI] [PubMed] [Google Scholar]
- Sun W, Wei X, Kesavan K, Garrington TP, Fan R, Mei J, Anderson SM, Gelfand EW, Johnson GL (2003) MEK kinase 2 and the adaptor protein Lad regulate extracellular signal-regulated kinase 5 activation by epidermal growth factor via Src. Mol Cell Biol 23: 2298–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TT, Guttapalli A, Agrawal A, Albert TJ, Shapiro IM, Risbud MV (2007) MEK/ERK signaling controls osmoregulation of nucleus pulposus cells of the intervertebral disc by transactivation of TonEBP/OREBP. J Bone Miner Res 22: 965–974 [DOI] [PubMed] [Google Scholar]
- Uhlik MT, Abell AN, Johnson NL, Sun W, Cuevas BD, Lobel-Rice KE, Horne EA, Dell'Acqua ML, Johnson GL (2003) Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat Cell Biol 5: 1104–1110 [DOI] [PubMed] [Google Scholar]
- Umenishi F, Verkman AS, Gropper MA (1996) Quantitative analysis of aquaporin mRNA expression in rat tissues by RNase protection assay. DNA Cell Biol 15: 475–480 [DOI] [PubMed] [Google Scholar]
- Urushitani M, Kurisu J, Tateno M, Hatakeyama S, Nakayama K, Kato S, Takahashi R (2004) CHIP promotes proteasomal degradation of familial ALS-linked mutant SOD1 by ubiquitinating Hsp/Hsc70. J Neurochem 90: 231–244 [DOI] [PubMed] [Google Scholar]
- Werlen G, Hausmann B, Palmer E (2000) A motif in the alphabeta T-cell receptor controls positive selection by modulating ERK activity. Nature 406: 422–426 [DOI] [PubMed] [Google Scholar]
- Wiederkehr T, Bukau B, Buchberger A (2002) Protein turnover: a CHIP programmed for proteolysis. Curr Biol 12: R26–R28 [DOI] [PubMed] [Google Scholar]
- Wu SJ, Liu FH, Hu SM, Wang C (2001) Different combinations of the heat-shock cognate protein 70 (hsc70) C-terminal functional groups are utilized to interact with distinct tetratricopeptide repeat-containing proteins. Biochem J 359(Part 2): 419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Marcu M, Yuan X, Mimnaugh E, Patterson C, Neckers L (2002) Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc Natl Acad Sci USA 99: 12847–12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Ying SX, Zhang GM, Li C, Cheng SY, Deng CX, Zhang YE (2005) Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell 121: 101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Facchinetti V, Wang X, Huang Q, Qin J, Su B (2006) Identification of MEKK2/3 serine phosphorylation site targeted by the Toll-like receptor and stress pathways. EMBO J 25: 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Windheim M, Roe SM, Peggie M, Cohen P, Prodromou C, Pearl LH (2005) Chaperoned ubiquitylation—crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP-Ubc13-Uev1a complex. Mol Cell 20: 525–538 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.