Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) can induce severe reproductive failure in sows, and is involved in the porcine respiratory disease complex. The glycoprotein GP4 of the European prototype PRRSV strain Lelystad virus (LV) contains a linear neutralizing epitope that is located in a highly variable region. The current study aimed to evaluate the antibody response against this and other epitopes on GP4 to infection of pigs with European-type PRRSV. It was shown that three virus strains, differing in the region that corresponds to the neutralizing epitope on GP4 of LV, strongly induce antibodies against this area. Antibodies against the epitopes of the different virus strains were purified from polyclonal swine sera, and used in virus-neutralization tests on primary alveolar macrophages. This revealed that antibodies against the variable region in GP4 of different virus strains are able to neutralize infection with homologous but not heterologous virus strains.
Introduction
Porcine reproductive and respiratory syndrome virus (PRRSV) is an enveloped positive single-stranded RNA virus that belongs to the family Arteriviridae in the order Nidovirales (18,49). PRRSV can cause severe reproductive failure in sows, characterized by late-term abortion, early farrowing, stillbirth, and the birth of weak piglets (5,6,41,49). Furthermore, the virus is associated with the porcine respiratory disease complex, causing respiratory disease in combination with secondary infections (42,44). Alveolar macrophages are considered the primary in vivo target cells for PRRSV, and it has been shown that the virus requires the cell-specific entry-mediators sialoadhesin and CD163 to establish efficient infection in those cells (2,14,43,46). In vitro, the virus can be grown in primary alveolar macrophages (PAM), and African green monkey kidney cell lines such as Marc-145, in which it uses an alternative receptor mechanism (13,19,20). The PRRSV virion consists of a nucleocapsid core, composed by the N protein that encapsulates the viral genome. The nucleocapsid is surrounded by a lipid envelope, in which six structural proteins are embedded. The membrane protein M and glycoprotein GP5 represent the majority of envelope proteins and are present as disulfide-linked heterodimers, while glycoproteins GP2, GP3, and GP4, together with the non-glycosylated protein E, are minor envelope proteins (8,26–28,52). PRRSV shows extensive genetic variability, and virus strains are classified into European (EU) type strains and North American (NA) type strains (1,39). In addition, a high variability exists within each genotype, and EU-type strains can be further subdivided into three subtypes (12,15,30,40). Upon infection with PRRSV, virus-specific antibodies appear within 1–2 wk, but virus-neutralizing antibodies are generally not detected earlier than 3–5 wk post-infection in small amounts, and by that time an extensive viremia has already taken place (11,22,47,55). Nevertheless, virus-neutralizing antibodies are able to inhibit replication of virus in PAM, and the presence of sufficiently high titers in serum before infection offers in vivo protection against viremia, virus replication in lungs, and transplacental spread of the virus (10,24,25). The identification of viral proteins and epitopes that are able to induce virus-neutralizing antibodies is thus a main topic of interest regarding the development of novel PRRSV vaccines. A neutralizing epitope on GP5 of NA-type PRRSV has been identified by the use of mouse monoclonal antibodies (mAbs), and the appearance of serum antibodies in pigs against GP5, and against this epitope in particular, correlates with virus neutralization. This has led to the assumption that GP5 of NA-type PRRSV is the main target for virus-neutralizing antibodies (17,35,37). A neutralizing epitope has also been identified on GP5 of an EU-type PRRSV strain. This epitope is situated upstream of the neutralizing epitope on GP5 of NA-type strains; however, only a very narrow range of virus strains that contain a rare mutation in the putative N-terminal signal peptide of GP5 are susceptible to neutralization by mAbs against this epitope, questioning the relevance of this epitope in vivo (48,50,51). On GP4 of the prototype EU strain Lelystad virus (LV), an epitope has been identified that is a target for virus-neutralizing mAbs in continuous cell lines as well as in PAM (7,28,45). This epitope is immunogenic in pigs, but shows a huge genetic variability, and antibodies against this epitope show little or no reactivity with other EU-type PRRSV strains (12,28,34). Although it is known that pigs produce antibodies against this epitope on GP4 upon infection with different EU-type PRRSV strains, no detailed information is available concerning the kinetics of the antibody response against this or other epitopes on GP4. Moreover, it remains unclear whether the hypervariable region corresponding to the neutralizing epitope on GP4 of LV also serves as a target for virus-neutralizing antibodies on PRRSV isolates other than LV. The aim of the current study was to investigate the antibody response against GP4 upon infection of pigs with different EU-type PRRSV strains. The kinetics of the GP4-specific antibody response after initial infection with LV in naïve piglets was determined. Subsequently, linear epitopes on GP4 that are targeted by porcine serum antibodies were identified, and it was determined whether antibodies against these epitopes were able to reduce PRRSV-replication in macrophages. Finally, the influence of genetic variability on induction of antibodies and recognition of epitopes was determined by the use of two recent EU-type field virus strains that differ from LV and from each other in the neutralizing epitope on GP4.
Materials and Methods
Cell cultures
Primary porcine alveolar macrophages (PAM) were obtained from 4-wk-old conventional Belgian Landrace pigs from a PRRSV-negative herd as previously described, and cultivated in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 2 mM L-glutamine, 1% non-essential amino acids, and 1 mM sodium pyruvate (49). Hek-293T cells were cultivated in Dulbecco's modified Eagle's medium (DMEM), with 5% FCS, 2 mM L-glutamine, and 1 mM sodium pyruvate. Marc-145 cells were cultivated in minimum essential medium (MEM), with 5% FCS, and 2 mM L-glutamine. All cells were cultivated in their respective media, supplemented with a mixture of antibiotics at 37°C in a humidified atmosphere with 5% CO2.
Viruses
PRRSV strain 07V063 was isolated from an aborted fetus, derived from a Belgian farm during an outbreak of PRRSV-associated reproductive disorders. PRRSV strain 08V204 was isolated from a Belgian farm from serum of 8-wk old healthy piglets that showed PRRSV-specific antibodies. For inoculation of piglets, the prototype EU-type PRRSV strain LV and field virus strains 07V063 and 08V204 were propagated in PAM, derived from gnotobiotic piglets (LV: fifth passage, 07V063: second passage, 08V204: third passage) (49). For a single-replication virus-neutralization test on PAM, virus was propagated in PAM from conventional pigs (LV: 13th passage, 07V063: second passage, 08V204: third passage). For a classical seroneutralization test on Marc-145 cells, virus was propagated in Marc-145 cells (LV: fourth passage, 07V063: second passage, 08V204: third passage).
ORF4 sequencing
The ORF4 sequences of all virus stocks used in this study were determined. RNA was extracted using an RNeasy Protect Mini Kit (Qiagen Inc., Valencia, CA) according to the manufacturer's protocol. Total RNA was reverse transcribed using a multiprime cDNA synthesis kit (Applied Biosystems, Foster City, CA). A region containing the complete ORF4 sequence was amplified using Taq Polymerase (Invitrogen Corp., Carlsbad, CA), and the following primers: forward primer 5′-cggcccaittccatccigag-3′, recognizing the complement sequence of nucleotides 12756–12775 upstream of ORF4 in LV (5′-cggcccaattccatccggag-3′), and reverse primer 5′-cattcagctcgcataicgtcaag-3′, recognizing nucleotides 13631–13653 downstream of ORF4 in LV (5′-cttgacgatatgcgagctgaatg-3′). This resulted in an 898-bp fragment for LV and 07V063, and a 886-bp fragment for 08V204. PCR products were treated with Exonuclease I and Antarctic Phosphatase (New England BioLabs, Ipswich, MA), and used directly for cycle sequencing with a Big Dye Terminator Cycle sequencing kit V1.1 (Applied Biosystems), and the aforementioned primers. Cycle sequencing reaction products were purified by ethanol precipitation and separated on an ABI Genetic 310 (Applied Biosystems). The ORF4 sequences of 07V063 and 08V204 were submitted to Genbank (accession numbers GU737264 and GU737266). The ORF4 sequence of LV was 100% identical to the one that was described earlier (Genbank accession number M96262.2). No differences in ORF4 sequence were observed between the viral passages on PAM, as well as Marc-145 cells, for any of the virus strains.
Experimental inoculation of animals, serum collection and detection of viremia and virus-specific and virus-neutralizing antibodies
Nineteen piglets were derived from a PRRSV-negative farm and their seronegative status was confirmed by immunoperoxidase monolayer assay (IPMA). Two groups of six and one group of seven piglets were inoculated intranasally at the age of 14 wk with 106 TCID50 of the PRRSV strain LV, 07V063, or 08V204, respectively. Sera were collected at different time points between 3 and 44 d post-infection (p.i.) for LV-infected animals, and just at 44 days pi for 07V063- and 08V204-infected animals. Virus titers were determined by titration on PAM, followed by immunoperoxidase staining with mAb P3/27 against the nucleocapsid protein (22,50). Sera for antibody detection were heat inactivated at 56°C for 30 min. PRRSV-specific antibodies were detected by IPMA, and virus-neutralizing antibodies by classical seroneutralization testing on Marc-145 cells as previously described, with the respective virus strain as antigen (22,47).
Detection of GP4-specific serum antibodies
Synthesis of an ORF4 DNA construct
RNA was extracted from LV and reverse transcribed using Superscript RT (Invitrogen) with oligodT primers. The cDNA was used for PCR with Platinum Pfx DNA polymerase (Invitrogen) and the following primers: forward primer 5′-gctctagacccacaatggctgcggccactcttttc-3′, recognizing the complement sequence of nucleotides 12946–12966 at the start of LV ORF4 (5′-atggctgcggccactcttttc-3′), and reverse primer 5′-atcatcggcgcctattgccaagagaatggcgaa-3′, recognizing nucleotides 13474–13494 at the end of LV ORF4 (5′-ttcgccattctcttggcaata-3′). The forward primer contained an XbaI site, while the reverse primer contained a KasI site (the underlined sequences). The XbaI-KasI PCR fragment was cloned in expression vector pEXPRIBA-3 (IBA BioTAGnology; IBA GmbH, Göttingen, Germany). The 549-bp nucleotide sequence of the insert was determined as described above, and was 100% identical to LV ORF4 (Genbank accession number M96262.2).
Transient transfection of Hek-293T cells
Hek-293T cells were seeded in a 96-well plate at a concentration of 25,000 cells per well, and incubated for 24 h before transfection. The cells were transfected with 1.5 μg per well of the LV ORF4 plasmid DNA, using calcium phosphate precipitation (4). At 48 h post-transfection, the cells were fixed by air drying and stored at −20°C. Expression of GP4 was verified by immunoperoxidase staining with mAb XVI11C/5F10 against GP4 of LV (7).
IPMA on GP4-expressing cells
GP4-specific serum antibody titers were determined by IPMA on GP4-expressing Hek-293T cells. The cells were thawed, fixed with 4% paraformaldehyde for 10 min at room temperature, washed with PBS, and treated with 1% H2O2 in methanol for 5 min. A 1:4 dilution series of heat-inactivated serum was prepared in PBS with 10% negative goat serum and 1% Tween-80, and incubated for 1 h at 37°C on the cells. The cells were washed with PBS with 1% Tween-80, and incubated for 1 h at 37°C with an optimal dilution of peroxidase-conjugated goat-anti-swine polyclonal antibodies (Jackson ImmunoResearch West Grove, PA). The cells were washed and incubated with a substrate solution of 3-amino-9-ethylcarbazole (AEC) in 0.05 M acetate buffer (pH 5) with 0.05% H2O2. The reaction was blocked by replacing the substrate with acetate buffer. The GP4-specific IPMA titer was defined as the reciprocal value of the highest serum dilution that showed staining of GP4-expressing cells.
Pepscan analysis
Epitope mapping was performed by pepscan analysis, using sets of overlapping peptides in a peptide ELISA (16). Sets of 44 overlapping dodecapeptides with an offset of 4 and an overlap of 8 were synthesized by solid-phase synthesis, based on the amino acid (aa) sequences of GP4 of virus strains LV, 07V063, and 08V204. All peptides were provided by JPT Peptide Technologies, GmbH (Berlin, Germany), as a set of BioTides that contained a hydrophilic spacer with a biotin molecule at the C-terminal end. Streptavidin-coated 96-well plates (VWR International, LLC) were coated for 1 h with 0.1 μg of biotinylated peptide in PBS with 0.5% Tween-20, washed, and blocked overnight with blocking buffer containing 1% bovine serum albumin (R&D Systems, Inc., Minneapolis, MN), and 0.5% Tween-20. Serum (1:100) or epitope-specific serum antibodies (100 μg/mL) were diluted in blocking buffer and incubated for 1 h on the peptide-coated plates, followed by washing and incubation with an optimal dilution of peroxidase-conjugated goat-anti-swine polyclonal antibodies. The plates were washed and developed with a substrate solution of tetramethylbenzidine and H2O2 (R&D Systems). The reaction was stopped after 10 min with 1 M H2SO4, and the optical density (OD) at 450 nm was measured. All wash steps were performed with PBS with 0.5% Tween-20, and all incubation steps were carried out at room temperature. Background OD levels were determined by including serum or protein A purified serum antibodies from a serologically PRRSV-negative pig. Specific signals within a pepscan analysis were determined as follows. For each separate peptide, the OD value obtained with the test sample was expressed relative to the OD value obtained with the negative control sample (% sample/negative). Next, the mean percentage sample/negative over all 44 peptides was calculated. If the percentage sample/negative at a certain peptide was more than two times the mean percentage sample/negative over all peptides, the signal was considered to be specific.
Purification of peptide-specific serum antibodies
We had 12-mer peptides with purity >80% synthesized by JPT Peptide Technologies. For each epitope, 1 mg peptide was covalently coupled to an N-hydroxysuccinimide-activated sepharose column (HiTrap NHS-activated HP; GE Healthcare, Piscataway, NJ), following the manufacturer's instructions, and a blank purification procedure was performed prior to use. The serum was clarified by centrifugation and filtration, inactivated for 30 min at 56°C, and the pH was adjusted with 200 mM Na2HPO4 (pH 7.0). After equilibration of the column with 20 mM Na2HPO4 (pH 7.0), serum was run over it and the column was subsequently washed with 20 mM Na2HPO4 buffer. When no more protein could be detected by spectrophotometry at 278 nm in the wash fractions, peptide-specific antibodies were eluted from the column with 0.1 M glycine (pH 2.7), and collected in 1 M Tris (pH 8.0). Elution fractions that contained protein, as measured by photospectrometry at 278 nm, were pooled, dialyzed against PBS, and stored at −70°C. The specificity of the obtained antibodies was evaluated by peptide ELISA as described above.
Single replication virus-neutralization test on PAM
Twofold serial dilutions of epitope-specific serum antibodies in PBS (1000–32 μg/mL) were mixed with equal volumes of PAM-grown virus with a titer of 2 × 105 TCID50/mL, resulting in 105 TCID50/mL virus, and an antibody concentration range of 500–16 μg/mL. Protein A purified antibodies from a serologically PRRSV-negative pig were included as mock antibody condition. Virus-antibody mixtures were incubated for 1 h at 37°C, and then added to a 96-well plate (100 μL/well), containing PAM at a concentration of 105 cells/well, that were cultivated for 48 h prior to use. After 1 h of incubation, the inoculum was removed and replaced by medium, after which the cells were further incubated for another 10 h, fixed by drying, and stored at −20°C. The cells were stained for PRRSV infection with mAb P3/27 against the nucleocapsid protein of PRRSV and peroxidase conjugated goat-anti-mouse polyclonal antibodies (Dako Denmark A/S, Glostrup, Denmark), followed by development with AEC. The number of infected cells in each well was counted in three fields at 200× magnification, and expressed relative (%) to the mean number of infected cells for all mock conditions within the same experiment. The experiments were performed in triplicate. The relative percentage of infected cells was analyzed by two-way analysis of variance, followed by Bonferoni post-tests to determine statistically significant differences between treatment and mock antibody conditions for a given antibody concentration. Statistical analysis was performed using GraphPad Prism version 5.0a software (GraphPad Software, San Diego, CA).
Results
Viremia, virus-specific, and virus-neutralizing antibody response upon infection with LV
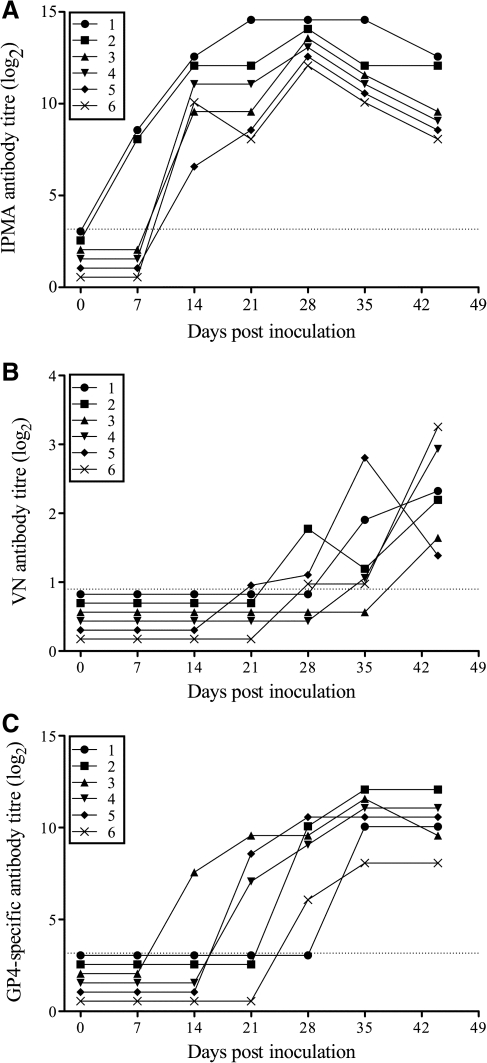
Six piglets were inoculated with the European prototype PRRSV strain LV, and serum virus titers, virus-specific antibody titers, and virus-neutralizing antibody titers were determined. All pigs showed viremia, starting from 3 d p.i. until 3–4 wk p.i. (Table 1). Virus-specific antibodies, as measured by IPMA, appeared at 1 (n = 2) or 2 (n = 4) weeks p.i., and maximum antibody titers varied between 11.3 and 13.3 log2 (Fig. 1A). Virus-neutralizing antibodies, as measured by classical seroneutralization testing on Marc-145 cells, were not detected earlier than 3 (n = 1), 4 (n = 2), 5 (n = 2), or 6 (n = 1) weeks p.i., with maximum titers no higher than 3.6 log2 (Fig. 1B).
Table 1.
Serum Virus Titers Upon Infection with Lelystad Virus
| |
Days post-inoculation |
|||||||
|---|---|---|---|---|---|---|---|---|
| Pig no. | 0 | 3 | 5 | 7 | 10 | 14 | 21 | 28 |
| 1 | <0.96 | 1.97 | 3.80 | 1.97 | 2.80 | <0.96 | <0.96 | <0.96 |
| 2 | <0.96 | 3.80 | 3.63 | 2.07 | 2.80 | <0.96 | 2.30 | <0.96 |
| 3 | <0.96 | 2.05 | 3.30 | <0.96 | 3.30 | 1.80 | <0.96 | <0.96 |
| 4 | <0.96 | 1.97 | 3.80 | 0.96 | 2.80 | 2.53 | <0.96 | <0.96 |
| 5 | <0.96 | 1.97 | 3.67 | <0.96 | 3.63 | 4.30 | 2.86 | <0.96 |
| 6 | <0.96 | 1.97 | 2.63 | 1.97 | 3.30 | <0.96 | <0.96 | <0.96 |
Six pigs were inoculated intranasally with 106 TCID50 of Lelystad virus and bled at different time points post-infection. Serum virus titers (log10 TCID50/mL) were determined by titration on primary alveolar macrophages.
FIG. 1.
Virus-specific (A), virus-neutralizing (B), and GP4-specific (C) antibody titers upon infection with LV. Virus-specific antibody titers (log2) were determined by IPMA, virus-neutralizing antibody titers (log2) by classical seroneutralization testing on Marc-145 cells, and GP4-specific antibody titers (log2) by IPMA on GP4-expressing Hek-293T cells for six LV-infected pigs at different time points post-inoculation. The dotted lines represent detection limits (1–6 = pig no. 1–6).
GP4-specific antibody response upon infection with LV
Antibodies against GP4 were detected by IPMA on GP4-expressing Hek-293T cells in serum of all LV-infected pigs. GP4-specific antibodies appeared at two (n = 1), three (n = 3), or four (n = 2) weeks p.i., and reached maximum titers between 7.3 and 11.3 log2 (Fig. 1C).
Epitope-specificity of GP4-specific serum antibodies in LV-infected pigs
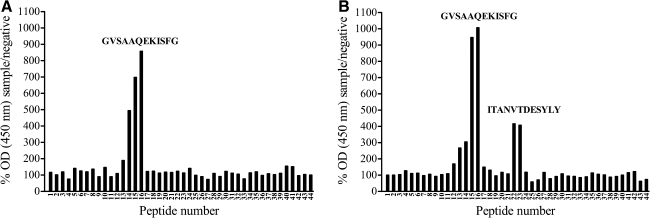
To discover linear epitopes against which the GP4-specific serum antibodies of LV-infected pigs were directed, a pepscan analysis was performed with sera collected between 7 and 44 d p.i. Sera of all six pigs contained antibodies directed against overlapping peptides 14, 15, and 16. The central peptide 15, with sequence GVSAAQEKISFG at aa position 57–68, was considered the core of this epitope. Antibodies against this epitope were detected starting from 2 wk p.i. for pig 3, and from 3 wk p.i. for the other pigs. An additional epitope, defined by peptides 22 and 23, was recognized by serum from pigs 2 and 3, starting from 4 wk p.i. Peptide 22, with sequence ITANVTDESYLY at aa position 85–96, was considered the core of this epitope, because signals were systematically higher at peptide 22 than at peptide 23. Fig. 2 represents an example of a pepscan profile of sera that recognize only peptides 14–16 (A), or both peptides 14–16 and 22–23 (B).
FIG. 2.
GP4 pepscan profiles of sera derived from LV-infected pigs. Sera collected at 44 d p.i. with LV were diluted 1:100 and used in a pepscan analysis with overlapping peptides covering GP4 of LV. OD (450 nm) values at each peptide were determined for both the test sample and a negative serum sample, and the OD for the test sample was expressed as the relative percentage of the negative control value for each peptide separately (percentage sample/negative). Signals were considered positive when the percentage sample/negative at a certain peptide was more than two times the mean percentage sample/negative over all peptides. Graph A represents a serum sample in which antibodies against peptides 14–16 were detected (core sequence GVSAAQEKISFG). Graph B represents a serum sample in which antibodies against peptides 14–16 as well as 22–23 (core sequence ITANVTDESYLY) were detected.
Virus-neutralization by antibodies against epitopes on GP4 of LV
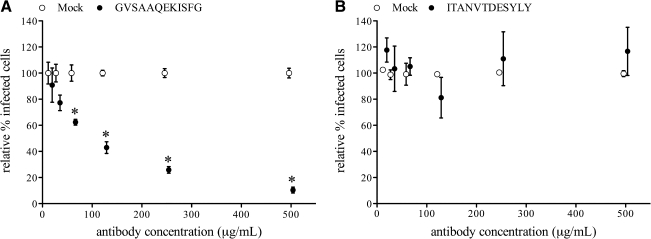
To evaluate the ability of serum antibodies against the identified epitopes to inhibit virus replication in vitro, antibodies against peptide 15 (GVSAAQEKISFG) or peptide 22 (ITANVTDESYLY) were purified from polyclonal serum by peptide affinity chromatography. Pepscan analysis revealed that antibodies purified against GVSAAQEKISFG recognized peptides 14, 15, and 16, while antibodies purified against ITANVTDESYLY recognized peptides 22 and 23. Subsequently, the epitope-specific antibodies were used in a single replication virus-neutralization test with LV on PAM. Antibodies against GVSAAQEKISFG were able to reduce infection of LV in PAM in a concentration-dependent manner (Fig. 3A). The number of infected cells was significantly reduced (p < 0.01), by more than 50% by antibody concentrations of 125 μg/mL or higher, compared to infection in the presence of similar concentrations of mock antibodies. Antibodies against ITANVTDESYLY did not reduce replication of LV in PAM in any concentration up to 500 μg/mL (Fig. 3B).
FIG. 3.
Reduction of LV replication in PAM by serum antibodies against peptides GVSAAQEKISFG (A) and ITANVTDESYLY (B). A single replication virus-neutralization test was performed with a 1:2 dilution series (500–16 μg/mL) of epitope-specific serum antibodies, purified from polyclonal serum by peptide affinity chromatography. Protein A purified immunoglobulins of a PRRSV-negative pig were included as mock antibodies (open symbols). The number of infected cells for each antibody dilution was set relative to the mean number of infected cells for all mock conditions within one experiment. The means and standard deviations of three experiments were calculated and are given in the graphs. Statistically significant differences (*p < 0.01) were observed with GVSAAQEKISFG-specific antibodies, compared to mock antibodies for antibody concentrations between 500 and 63 μg/mL.
ORF4 sequence analysis of PRRSV strains 07V063 and 08V204
To determine whether the GP4-specific antibody response is similar for EU-type virus strains other than LV, two Belgian PRRSV field isolates were selected: 07V063 and 08V204. ORF4 sequence analysis of these strains revealed that 07V063 shows 87% aa homology with LV in GP4, while 08V204 shows 85% aa homology with LV in GP4. Alignment of the GP4 aa sequences of LV, 07V063, and 08V204 revealed that the majority of the variation is located between aa positions 46 and 70 (Fig. 4). Out of 12 aa that determine the neutralizing epitope of LV (aa 57–68), only five are conserved between LV and 07V063, and no more than two between LV and 08V204, and a deletion of 4 aa is present in the 08V204 sequence. Moreover, 07V063 and 08V204 only share three common aa in this region, and except for the glutamine at position 62, no aa are conserved over the three virus strains. In the non-neutralizing epitope at position 85–96, all but one or two aa are conserved between LV and 07V063 or 08V204, respectively.
FIG. 4.
Alignment of the GP4 aa sequences of PRRSV strains LV, 07V063, and 08V204. The ORF4 sequences of PRRSV strain 07V063 and 08V204 were determined by PCR and cycle sequencing, and the deduced aa sequences were aligned to the GP4 sequence of LV. Dots represent residues that are identical to LV, and hyphens indicate gaps. Boxes indicate regions that are recognized by sera in pepscan analyses.
Linear serum antibody epitopes on GP4 of 07V063 and 08V204
Six and seven pigs were infected with 07V063 or 08V204, respectively, and all pigs showed viremia and a virus-specific antibody response (data not shown). To determine the linear epitopes on GP4 against which serum antibodies were directed, a homologous pepscan analysis was performed with sera collected at 44 days p.i. All but one pig infected with virus strain 07V063 possessed antibodies against peptides 15 and 16, with the highest signals at peptide 15 (RVTAAQGRIYTR, aa position 57–68). Two pigs had antibodies against peptides 22 and 23, with the highest signals at peptide 22 (VTANVTDESYLY, aa position 85–96). Similarly, all seven pigs infected with virus strain 08V204 had antibodies against peptides 14 and 15, with the highest signals at peptide 15 (RTNTTQGKVPSQ, aa position 57–68), and three of them had antibodies against peptides 21 and 22, with the highest signals at peptide 21 (MTANVTDKSYLY, aa position 81–92) (Fig. 4).
Virus-neutralization by antibodies against epitopes 57–68 on GP4 of 07V063 and 08V204
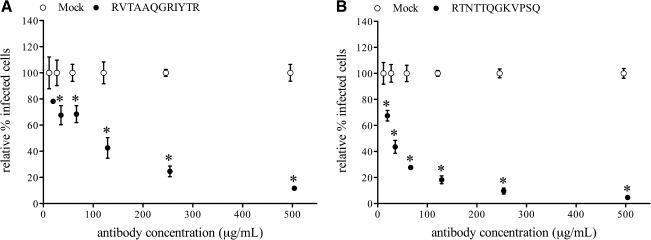
Antibodies against aa sequences RVTAAQGRIYTR or RTNTTQGKVPSQ were purified from polyclonal serum of pigs infected with PRRSV strain 07V063 or 08V204, respectively, and their specificity was verified by pepscan analysis on homologous GP4. Antibodies against epitope RVTAAQGRIYTR on GP4 of 07V063 reduced replication of the homologous virus strain in PAM in a dose-dependent manner. The number of infected cells was significantly reduced (p < 0.01), by more than 50%, with antibody concentrations of 125 μg/mL or higher, compared to infection in the presence of mock antibodies (Fig. 5A). Similarly, antibodies against epitope RTNTTQGKVPSQ of 08V204 reduced the number of cells infected with the homologous virus in a dose-dependent matter, and here the lowest antibody concentration that gave a significant reduction (p < 0.01) of more than 50% was 31 μg/mL (p < 0.01) (Fig. 5B).
FIG. 5.
Reduction of replication of 07V063 (A), or 08V204 (B), in PAM by serum antibodies against peptides RVTAAQGRIYTR and RTNTTQGKVPSQ, respectively. A single replication virus-neutralization test with homologous virus was performed with a 1:2 dilution series (500–16 μg/mL) of epitope-specific serum antibodies, purified from polyclonal serum by peptide affinity chromatography. Protein A purified immunoglobulins of a PRRSV-negative pig were included as mock antibodies (open symbols). The number of infected cells for each antibody dilution was set relative to the mean number of infected cells for all mock conditions within one experiment. The means and standard deviations of three experiments were calculated and are given in the graphs. Statistically significant differences (*p < 0.01) were observed with RVTAAQGRIYTR-specific antibodies, compared to mock antibodies, for antibody concentrations between 500 and 31 μg/mL in the test with virus strain 07V063. In the test with virus strain 08V204, statistically significant differences (*p < 0.01) were observed with RTNTTQGKVPSQ-specific antibodies, compared to mock antibodies, for antibody concentrations between 500 and 16 μg/mL.
Cross-recognition and cross-neutralization by antibodies against the neutralizing epitope on GP4 of LV, 07V063, and 08V204
Sera derived from LV-, 07V063-, or 08V204-infected pigs at 44 d p.i. were tested in a pepscan analysis on GP4 of the heterologous virus strains (data not shown). None of the sera that reacted with aa position 57–68 in the homologous pepscan recognized this region in the heterologous pepscans for each virus strain. Sera of LV-infected pigs that reacted with aa position 87–98 in the homologous pepscan also recognized the corresponding region of 07V063, but not the one of 08V204. Similarly, sera of 07V063-infected pigs that recognized position 87–98 of the homologous virus strain still reacted with the corresponding region of LV, but not with the one of 08V204. Sera derived from 08V204-infected pigs did not recognize any regions in GP4 of LV or 07V063 at all.
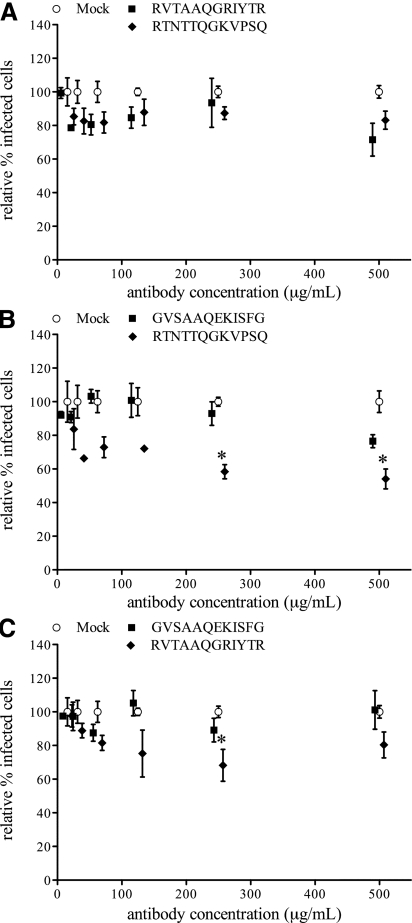
Antibodies against aa position 57–68 on GP4 of LV (GVSAAQEKISFG), 07V063 (RVTAAQGRIYTR), and 08V204 (RTNTTQGKVPSQ), were used in single-replication virus-neutralization tests on PAM with each of the respective heterologous virus strains. LV-replication in PAM was not reduced by antibodies directed against aa 57–68 of either 07V063 or 08V204 in any of the concentrations tested (Fig. 6A). Similarly, replication of 07V063 (Fig. 6B) or 08V204 (Fig. 6C) in PAM was not reduced by antibodies against aa 57–68 of LV in any concentration. Infection with 07V063 and 08V204 was slightly but significantly reduced with high concentrations of antibodies against aa 57–68 of the respective heterologous virus strain (Fig. 6B and C). However, this reduction never reached 50%, and no dose-dependent reduction of infection was observed for these conditions.
FIG. 6.
Reduction of replication of LV (A), 07V063 (B) and 08V204 (C) in PAM by antibodies against the neutralizing epitopes on GP4 of the respective heterologous virus strains. Single-replication virus-neutralization tests were performed with LV, 07V063, and 08V204, with a 1:2 dilution series (500–16 μg/mL) of serum antibodies against the neutralizing epitope of the two respective heterologous virus strains. Epitope-specific antibodies were purified from polyclonal serum by peptide affinity chromatography. Protein A purified immunoglobulins of a PRRSV-negative pig were included as mock antibodies (open symbols). The number of infected cells for each antibody dilution was set relative to the mean number of infected cells for all mock conditions within one experiment. The means and standard deviations of three experiments were calculated and are given in the graphs. Statistically significant differences (*p < 0.01) were observed with RTNTTQGKVPSQ-specific antibodies, compared to mock antibodies, for antibody concentrations of 500 and 250 μg/mL in the test with virus strain 07V063 (B). In the test with virus strain 08V204 (C), statistically significant differences (*p < 0.01) were observed with RVTAAQGRIYTR-specific antibodies, compared to mock antibodies, for an antibody concentration of 250 μg/mL.
Discussion
Two neutralizing epitopes have been identified on EU-type PRRSV by the use of mAbs, one on GP5 and one on GP4 (28,51). The neutralizing epitope on GP4 of the EU prototype LV strain has been shown to be immunogenic in pigs, but the kinetics of the antibody response against this or other epitopes on GP4 was never explored (34). In the current study, it was shown that naïve pigs produced high titers of GP4-specific antibodies upon infection with LV. Between 2 and 3 wk post-infection GP4-specific antibodies were directed against a linear region covering aa position 57–68, determining the epitope that was described earlier as a target for virus-neutralizing mAbs (7,28). Purified serum antibodies against peptides 57–68 clearly reduced single-virus replication in PAM in a concentration-dependent manner, showing that this epitope is a target for porcine virus-neutralizing antibodies as well. At the time antibodies against this epitope appeared, the sera, however, did not yet show neutralizing activity in a classical seroneutralization test on Marc-145 cells. This can be explained by the lower sensitivity of this test, compared to the single-replication virus-neutralization test on PAM (10,54). However, no clear correlation was observed between the levels of GP4-specific antibodies and the levels of virus-neutralizing antibodies in sera, and it remains to be determined to what extent antibodies against GP4 contribute to the global neutralizing activity of polyclonal serum upon PRRSV-infection. Nevertheless, this study clearly shows that pigs are able to produce antibodies against a linear epitope on GP4 that reduce PRRSV replication in its natural host cell, the alveolar macrophage. Therefore, it is reasonable to expect that such antibodies, if present in sufficient amounts, will contribute to a significant reduction in virus replication in the host.
In addition to the neutralizing epitope, a second linear epitope at aa position 85–96 on GP4 of LV was recognized by some sera that were collected at later time points. However, antibodies against this less immunogenic epitope were not neutralizing at all.
The neutralizing epitope on GP4 of LV is located in a highly variable region, and it is not known thus far whether the corresponding regions of PRRSV strains other than LV are also targets for neutralizing antibodies (12,28). In the current study it was shown that similarly to LV, two Belgian PRRSV field isolates of EU genotype (07V063 and 08V204) strongly induce antibodies towards aa 57–68 of GP4, even though the aa sequences at this position are different. Serum antibodies against the corresponding peptides of each virus strain were able to reduce replication of the homologous virus in PAM. These data indicate that aa 57–68 on GP4 of PRRSV determines a highly immunogenic epitope that induces virus-neutralizing antibodies. In addition to LV, 07V063 and 08V204, also an LV-like vaccine strain, an EU-type field isolate, and even a NA-type PRRSV strain, have been shown to induce antibodies against the corresponding region on GP4 (9,33,34). The fact that this variable epitope on GP4 is highly immunogenic for a series of different PRRSV strains suggests that its immunogenicity is determined by the location rather than by the aa sequence.
It was previously shown that mAbs against the neutralizing epitope on GP4 of LV do not recognize or neutralize a series of European field isolates that differ in the corresponding region (28). Likewise, in the current study no dose-dependent cross-neutralization by serum antibodies against aa 57–68 was observed between LV, 07V063, and 08V204. Considering the fact that different virus strains strongly induce neutralizing antibodies against aa 57–68 on GP4, it is thus very likely that hypervariability in this region is the outcome of a high immunological selective pressure in vivo. In agreement with this, Costers et al. recently showed that cultivation of LV in the continuous presence of mAbs against the neutralizing epitope on GP4 results in the selection of PRRSV variants that show aa changes in this epitope, making them insensitive to neutralization by the mAbs (7). ORF4 of PRRSV partially overlaps with ORF3, and the coding sequence for the neutralizing epitope on GP4 also determines a variable region in the C-terminal end of GP3 (39). The data presented here indicate that variability in this region is probably an additional effect of selective pressure exerted by antibodies against GP4 (32).
Neutralizing antibodies can interfere with viral infectivity in different ways (21,38). The antibodies may directly bind to the viral ligand for a certain cellular receptor or entry mediator, inhibiting attachment, internalization, membrane fusion, or another post-entry step in infection. However, the variability in the neutralizing epitope on GP4, including non-conservative substitutions and even deletions, makes it improbable that this particular part of the protein is somehow involved in a crucial step in the virus replication cycle. More likely, neutralizing antibodies against aa 57–68 of GP4 block functional interactions of other, more conserved regions on the virus with their cellular counterparts by sterical hindrance or by inducing conformational changes. If this is the case, the infectivity of virus quasispecies that show mutations in the neutralizing epitope will not be compromised, and viruses that escape neutralization by antibodies against this epitope will have a better chance of survival.
Several PRRSV proteins and epitopes have been associated with virus neutralization in the past on both EU- and NA-type strains. mAbs with neutralizing activity towards the virus have been characterized for their protein specificity and in some cases for their epitope specificity (3,28,35,36,48,51,53,56). This approach, however, does not offer any information about the way in which the natural host produces antibodies against these viral proteins and epitopes. Western blot analysis, phage display libraries, and ELISA with recombinant proteins and peptides, have been used to evaluate the specificity of the antibody response upon PRRSV infection in pigs (10,17,23,29,31,33,35,37,55). In some studies, statistical correlations were observed between serum reactivity against certain proteins or epitopes and the presence of virus-neutralizing antibodies, giving an indication about which viral domains may be the main inducers of virus-neutralizing antibodies (17,35,37). However, this approach does not directly show to what extent serum antibodies against particular epitopes are actually able to neutralize the virus. In the present study, the kinetics of the antibody response against linear epitopes on GP4 was determined, and antibodies against distinct epitopes were purified from polyclonal pig serum and directly used in virus-neutralization tests to determine their neutralizing capacity. This approach circumvents the limited availability of specific mAbs, allows the identification of in vivo relevant antibody targets, and makes it possible to directly examine to what extent porcine serum antibodies against each of these targets separately are able to neutralize the virus. In the current study, this has led to the discovery that a highly variable region in GP4 of EU-type PRRSV induces antibodies that neutralize homologous, but not heterologous, virus strains. The use of a similar approach for the study of the antibody response against other structural PRRSV proteins may lead to identification and characterization of more in vivo relevant antibody targets.
Acknowledgments
The authors want to acknowledge Chantal Vanmaercke, Carine Boone, Dries Helderweirt, and Geert Opsomer for excellent technical assistance. Merijn Vanhee was funded by the Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT-SB 071316). This work was funded by the EU (Seventh Framework Programme, Project Number 245141). All animal experiments were approved by the local ethical committee of the Faculty of Veterinary Medicine, Ghent University.
Author Disclosure Statement
No competing financial interests exist for any of the authors of this manuscript.
References
- 1.Allende R. Lewis TL. Lu Z, et al. North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions. J Gen Virol. 1999;80(Pt 2):307–315. doi: 10.1099/0022-1317-80-2-307. [DOI] [PubMed] [Google Scholar]
- 2.Calvert JG. Slade DE. Shields SL. Jolie R. Mannan RM. Ankenbauer RG. Welch SK. CD163 expression confers susceptibility to porcine reproductive and respiratory syndrome viruses. J Virol. 2007;81:7371–7379. doi: 10.1128/JVI.00513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancel-Tirado SM. Evans RB. Yoon KJ. Monoclonal antibody analysis of porcine reproductive and respiratory syndrome virus epitopes associated with antibody-dependent enhancement and neutralization of virus infection. Vet Immunol Immunopathol. 2004;102:249–262. doi: 10.1016/j.vetimm.2004.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C. Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christianson WT. Collins JE. Benfield DA, et al. Experimental reproduction of swine infertility and respiratory syndrome in pregnant sows. Am J Vet Res. 1992;53:485–488. [PubMed] [Google Scholar]
- 6.Collins JE. Benfield DA. Christianson WT, et al. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J Vet Diagn Invest. 1992;4:117–126. doi: 10.1177/104063879200400201. [DOI] [PubMed] [Google Scholar]
- 7.Costers S. Lefebvre DJ. Van Doorsselaere J. Vanhee M. Delputte PL. Nauwynck HJ. GP4 of porcine reproductive and respiratory syndrome virus contains a neutralizing epitope that is susceptible to immunoselection in vitro. Arch Virol. 2010;155:371–378. doi: 10.1007/s00705-009-0582-7. [DOI] [PubMed] [Google Scholar]
- 8.de Lima M. Ansari IH. Das PB. Ku BJ. Martinez-Lobo FJ. Pattnaik AK. Osorio FA. GP3 is a structural component of the PRRSV type II (US) virion. Virology. 2009;390:31–36. doi: 10.1016/j.virol.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 9.de Lima M. Pattnaik AK. Flores EF. Osorio FA. Serologic marker candidates identified among B-cell linear epitopes of Nsp2 and structural proteins of a North American strain of porcine reproductive and respiratory syndrome virus. Virology. 2006;353:410–421. doi: 10.1016/j.virol.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 10.Delputte PL. Meerts P. Costers S. Nauwynck HJ. Effect of virus-specific antibodies on attachment, internalization and infection of porcine reproductive and respiratory syndrome virus in primary macrophages. Vet Immunol Immunopathol. 2004;102:179–188. doi: 10.1016/j.vetimm.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Diaz I. Darwich L. Pappaterra G. Pujols J. Mateu E. Immune responses of pigs after experimental infection with a European strain of porcine reproductive and respiratory syndrome virus. J Gen Virol. 2005;86:1943–1951. doi: 10.1099/vir.0.80959-0. [DOI] [PubMed] [Google Scholar]
- 12.Drew TW. Lowings JP. Yapp F. Variation in open reading frames 3, 4 and 7 among porcine reproductive and respiratory syndrome virus isolates in the UK. Vet Microbiol. 1997;55:209–221. doi: 10.1016/s0378-1135(96)01328-4. [DOI] [PubMed] [Google Scholar]
- 13.Duan X. Nauwynck HJ. Pensaert MB. Effects of origin and state of differentiation and activation of monocytes/macrophages on their susceptibility to porcine reproductive and respiratory syndrome virus (PRRSV) Arch Virol. 1997;142:2483–2497. doi: 10.1007/s007050050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan X. Nauwynck HJ. Pensaert MB. Virus quantification and identification of cellular targets in the lungs and lymphoid tissues of pigs at different time intervals after inoculation with porcine reproductive and respiratory syndrome virus (PRRSV) Vet Microbiol. 1997;56:9–19. doi: 10.1016/S0378-1135(96)01347-8. [DOI] [PubMed] [Google Scholar]
- 15.Forsberg R. Storgaard T. Nielsen HS, et al. The genetic diversity of European type PRRSV is similar to that of the North American type but is geographically skewed within Europe. Virology. 2002;299:38–47. doi: 10.1006/viro.2002.1450. [DOI] [PubMed] [Google Scholar]
- 16.Geysen MH. Meloen RH. Barteling SJ. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci USA. 1984;81:3398–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonin P. Pirzadeh B. Gagnon CA. Dea S. Seroneutralization of porcine reproductive and respiratory syndrome virus correlates with antibody response to the GP5 major envelope glycoprotein. J Vet Diagn Invest. 1999;11:20–26. doi: 10.1177/104063879901100103. [DOI] [PubMed] [Google Scholar]
- 18.Gorbalenya AE. Enjuanes L. Ziebuhr J. Snijder EJ. Nidovirales: evolving the largest RNA virus genome. Virus Res. 2006;117:17–37. doi: 10.1016/j.virusres.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HS. Kwang J. Yoon IJ. Joo HS. Frey ML. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch Virol. 1993;133:477–483. doi: 10.1007/BF01313785. [DOI] [PubMed] [Google Scholar]
- 20.Kim JK. Fahad AM. Shanmukhappa K. Kapil S. Defining the cellular target(s) of porcine reproductive and respiratory syndrome virus blocking monoclonal antibody 7G10. J Virol. 2006;80:689–696. doi: 10.1128/JVI.80.2.689-696.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klasse PJ. Sattentau QJ. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J Gen Virol. 2002;83:2091–2108. doi: 10.1099/0022-1317-83-9-2091. [DOI] [PubMed] [Google Scholar]
- 22.Labarque GG. Nauwynck HJ. Van Reeth K. Pensaert MB. Effect of cellular changes and onset of humoral immunity on the replication of porcine reproductive and respiratory syndrome virus in the lungs of pigs. J Gen Virol. 2000;81:1327–1334. doi: 10.1099/0022-1317-81-5-1327. [DOI] [PubMed] [Google Scholar]
- 23.Loemba HD. Mounir S. Mardassi H. Archambault D. Dea S. Kinetics of humoral immune response to the major structural proteins of the porcine reproductive and respiratory syndrome virus. Arch Virol. 1996;141:751–761. doi: 10.1007/BF01718333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez OJ. Oliveira MF. Garcia EA. Kwon BJ. Doster A. Osorio FA. Protection against porcine reproductive and respiratory syndrome virus (PRRSV) infection through passive transfer of PRRSV-neutralizing antibodies is dose dependent. Clin Vaccine Immunol. 2007;14:269–275. doi: 10.1128/CVI.00304-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez OJ. Osorio FA. Role of neutralizing antibodies in PRRSV protective immunity. Vet Immunol Immunopathol. 2004;102:155–163. doi: 10.1016/j.vetimm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Mardassi H. Mounir S. Dea S. Molecular analysis of the ORFs 3 to 7 of porcine reproductive and respiratory syndrome virus, Quebec reference strain. Arch Virol. 1995;140:1405–1418. doi: 10.1007/BF01322667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meulenberg JJ. Petersen-den Besten A. De Kluyver EP. Moormann RJ. Schaaper WM. Wensvoort G. Characterization of proteins encoded by ORFs 2 to 7 of Lelystad virus. Virology. 1995;206:155–163. doi: 10.1016/S0042-6822(95)80030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meulenberg JJ. van Nieuwstadt AP. van Essen-Zandbergen A. Langeveld JP. Posttranslational processing and identification of a neutralization domain of the GP4 protein encoded by ORF4 of Lelystad virus. J Virol. 1997;71:6061–6067. doi: 10.1128/jvi.71.8.6061-6067.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulupuri P. Zimmerman JJ. Hermann J, et al. Antigen-specific B-cell responses to porcine reproductive and respiratory syndrome virus infection. J Virol. 2008;82:358–370. doi: 10.1128/JVI.01023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelsen CJ. Murtaugh MP. Faaberg KS. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J Virol. 1999;73:270–280. doi: 10.1128/jvi.73.1.270-280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson EA. Christopher-Hennings J. Benfield DA. Serum immune responses to the proteins of porcine reproductive and respiratory syndrome (PRRS) virus. J Vet Diagn Invest. 1994;6:410–415. doi: 10.1177/104063879400600402. [DOI] [PubMed] [Google Scholar]
- 32.Oleksiewicz MB. Botner A. Toft P. Grubbe T. Nielsen J. Kamstrup S. Storgaard T. Emergence of porcine reproductive and respiratory syndrome virus deletion mutants: correlation with the porcine antibody response to a hypervariable site in the ORF 3 structural glycoprotein. Virology. 2000;267:135–140. doi: 10.1006/viro.1999.0103. [DOI] [PubMed] [Google Scholar]
- 33.Oleksiewicz MB. Botner A. Toft P. Normann P. Storgaard T. Epitope mapping porcine reproductive and respiratory syndrome virus by phage display: the nsp2 fragment of the replicase polyprotein contains a cluster of B-cell epitopes. J Virol. 2001;75:3277–3290. doi: 10.1128/JVI.75.7.3277-3290.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oleksiewicz MB. Stadejek T. Mackiewicz Z. Porowski M. Pejsak Z. Discriminating between serological responses to European-genotype live vaccine and European-genotype field strains of porcine reproductive and respiratory syndrome virus (PRRSV) by peptide ELISA. J Virol Methods. 2005;129:134–144. doi: 10.1016/j.jviromet.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Ostrowski M. Galeota JA. Jar AM. Platt KB. Osorio FA. Lopez OJ. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J Virol. 2002;76:4241–4250. doi: 10.1128/JVI.76.9.4241-4250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pirzadeh B. Dea S. Monoclonal antibodies to the ORF5 product of porcine reproductive and respiratory syndrome virus define linear neutralizing determinants. J Gen Virol. 1997;78(Pt 8):1867–1873. doi: 10.1099/0022-1317-78-8-1867. [DOI] [PubMed] [Google Scholar]
- 37.Plagemann PG. Rowland RR. Faaberg KS. The primary neutralization epitope of porcine respiratory and reproductive syndrome virus strain VR-2332 is located in the middle of the GP5 ectodomain. Arch Virol. 2002;147:2327–2347. doi: 10.1007/s00705-002-0887-2. [DOI] [PubMed] [Google Scholar]
- 38.Reading SA. Dimmock NJ. Neutralization of animal virus infectivity by antibody. Arch Virol. 2007;152:1047–1059. doi: 10.1007/s00705-006-0923-8. [DOI] [PubMed] [Google Scholar]
- 39.Snijder EJ. Meulenberg JJ. The molecular biology of arteriviruses. J Gen Virol. 1998;79:961–979. doi: 10.1099/0022-1317-79-5-961. [DOI] [PubMed] [Google Scholar]
- 40.Stadejek T. Oleksiewicz MB. Scherbakov AV. Timina AM. Krabbe JS. Chabros K. Potapchuk D. Definition of subtypes in the European genotype of porcine reproductive and respiratory syndrome virus: nucleocapsid characteristics and geographical distribution in Europe. Arch Virol. 2008;153:1479–1488. doi: 10.1007/s00705-008-0146-2. [DOI] [PubMed] [Google Scholar]
- 41.Terpstra C. Wensvoort G. Pol JM. Experimental reproduction of porcine epidemic abortion and respiratory syndrome (mystery swine disease) by infection with Lelystad virus: Koch's postulates fulfilled. Vet Q. 1991;13:131–136. doi: 10.1080/01652176.1991.9694297. [DOI] [PubMed] [Google Scholar]
- 42.Thacker EL. Immunology of the porcine respiratory disease complex. Vet Clin North Am Food Anim Pract. 2001;17:551–565. doi: 10.1016/S0749-0720(15)30006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Gorp H. Van Breedam W. Delputte PL. Nauwynck HJ. Sialoadhesin and CD163 join forces during entry of the porcine reproductive and respiratory syndrome virus. J Gen Virol. 2008;89:2943–2953. doi: 10.1099/vir.0.2008/005009-0. [DOI] [PubMed] [Google Scholar]
- 44.Van Gucht S. Labarque G. Van Reeth K. The combination of PRRS virus and bacterial endotoxin as a model for multifactorial respiratory disease in pigs. Vet Immunol Immunopathol. 2004;102:165–178. doi: 10.1016/j.vetimm.2004.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Nieuwstadt AP. Meulenberg JJ. van Essen-Zanbergen A. Petersen-den Besten A. Bende RJ. Moormann RJ. Wensvoort G. Proteins encoded by open reading frames 3 and 4 of the genome of Lelystad virus (Arteriviridae) are structural proteins of the virion. J Virol. 1996;70:4767–4772. doi: 10.1128/jvi.70.7.4767-4772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanderheijden N. Delputte PL. Favoree HW. Vandekerckhove J. Van Damme J. van Woensel PA. Nauwynck HJ. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J Virol. 2003;77:8207–8215. doi: 10.1128/JVI.77.15.8207-8215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanhee M. Delputte PL. Delrue I. Geldhof MF. Nauwynck HJ. Development of an experimental inactivated PRRSV vaccine that induces virus-neutralizing antibodies. Vet Res. 2009;40:63. doi: 10.1051/vetres/2009046. [DOI] [PubMed] [Google Scholar]
- 48.Weiland E. Wieczorek-Krohmer M. Kohl D. Conzelmann KK. Weiland F. Monoclonal antibodies to the GP5 of porcine reproductive and respiratory syndrome virus are more effective in virus neutralization than monoclonal antibodies to the GP4. Vet Microbiol. 1999;66:171–186. doi: 10.1016/s0378-1135(99)00006-1. [DOI] [PubMed] [Google Scholar]
- 49.Wensvoort G. Terpstra C. Pol JM, et al. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet Q. 1991;13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- 50.Wieczorek-Krohmer M. Weiland F. Conzelmann K, et al. Porcine reproductive and respiratory syndrome virus (PRRSV): monoclonal antibodies detect common epitopes on two viral proteins of European and U.S. isolates. Vet Microbiol. 1996;51:257–266. doi: 10.1016/0378-1135(96)00047-8. [DOI] [PubMed] [Google Scholar]
- 51.Wissink EH. van Wijk HA. Kroese MV. Weiland E. Meulenberg JJ. Rottier PJ. van Rijn PA. The major envelope protein, GP5, of a European porcine reproductive and respiratory syndrome virus contains a neutralization epitope in its N-terminal ectodomain. J Gen Virol. 2003;84:1535–1543. doi: 10.1099/vir.0.18957-0. [DOI] [PubMed] [Google Scholar]
- 52.Wu WH. Fang Y. Farwell R. Steffen-Bien M. Rowland RR. Christopher-Hennings J. Nelson EA. A 10-kDa structural protein of porcine reproductive and respiratory syndrome virus encoded by ORF2b. Virology. 2001;287:183–191. doi: 10.1006/viro.2001.1034. [DOI] [PubMed] [Google Scholar]
- 53.Yang L. Frey ML. Yoon KJ. Zimmerman JJ. Platt KB. Categorization of North American porcine reproductive and respiratory syndrome viruses: epitopic profiles of the N, M, GP5 and GP3 proteins and susceptibility to neutralization. Arch Virol. 2000;145:1599–1619. doi: 10.1007/s007050070079. [DOI] [PubMed] [Google Scholar]
- 54.Yoon IJ. Joo HS. Goyal SM. Molitor TW. A modified serum neutralization test for the detection of antibody to porcine reproductive and respiratory syndrome virus in swine sera. J Vet Diagn Invest. 1994;6:289–292. doi: 10.1177/104063879400600326. [DOI] [PubMed] [Google Scholar]
- 55.Yoon KJ. Zimmerman JJ. Swenson SL, et al. Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J Vet Diagn Invest. 1995;7:305–312. doi: 10.1177/104063879500700302. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y. Sharma RD. Paul PS. Monoclonal antibodies against conformationally dependent epitopes on porcine reproductive and respiratory syndrome virus. Vet Microbiol. 1998;63:125–136. doi: 10.1016/s0378-1135(98)00231-4. [DOI] [PubMed] [Google Scholar]