This brief report by Negri and colleagues demonstrates that integration defective lentiviral vectors (IDLV) can infect human antigen-presenting cells and trigger the effective induction and expansion of antigen-specific human CD8+ T cells in vitro.
Abstract
Nonintegrating lentiviral vectors are being developed as a efficient and safe delivery system for both gene therapy and vaccine purposes. Several reports have demonstrated that a single immunization with integration-defective lentiviral vectors (IDLVs) delivering viral or tumor model antigens in mice was able to elicit broad and long-lasting specific immune responses in the absence of vector integration. At present, no evidence has been reported showing that IDLVs are able to expand preexisting immune responses in the human context. In the present study, we demonstrate that infection of human antigen-presenting cells (APCs), such as monocyte-derived dendritic cells (DCs) and macrophages with IDLVs expressing influenza matrix M1 protein resulted in effective induction of in vitro expansion of M1-primed CD8+ T cells, as evaluated by both pentamer staining and cytokine production. This is the first demonstration that IDLVs represent an efficient delivery system for gene transfer and expression in human APCs, useful for immunotherapeutic applications.
Introduction
There is substantial evidence suggesting that lentiviral vectors (LVs) offer considerable advantages over other delivery platforms for genetic immunization, including the induction of broad immune responses with high potency and durability after a single inoculum, with low interfering vector-specific responses (reviewed in He and Falo, 2007). Importantly, Beignon and colleagues provided the first evidence that an LV expressing simian immunodeficiency virus (SIV) Gag protein was able to induce control of viral replication in monkeys challenged with a high dose of SIV (Beignon et al., 2009). However, the use of integrating LVs in humans raises serious safety concerns, including the risk of insertional mutagenesis and vector mobilization. Integration-defective LVs (IDLVs) are being developed that improve the safety profile of these vectors. These IDLVs contain a mutated form of integrase in the viral particles that prevents the integration of the vector genome into host chromosomes, while allowing production of nonintegrated forms of vector DNA (Banasik and McCray, 2010). Several reports showed that the level of gene expression driven by the IDLVs remains long-lasting, as long as the transduced cells are not dividing (Cara and Klotman, 2006; Wanisch and Yáñez-Muñoz, 2009). In addition, we demonstrated that a single immunization with an IDLV expressing the HIV-1 envelope protein in the mouse immunogenicity model was able to elicit strong and long-lasting specific immune responses in the absence of vector integration, thus providing safe and efficient delivery for vaccine purposes (Negri et al., 2007). Importantly, several groups confirmed the use of IDLVs as an effective vaccine delivery strategy (Coutant et al., 2008; Hu et al., 2009; Karwacz et al., 2009), and we provided evidence that SIV-based IDLVs can be constructed and used as well (Michelini et al., 2009).
Development of effective vaccines for the immunotherapy of cancer or persistent infectious diseases will require the reactivation of a suboptimal immune response. Dendritic cells (DCs) are the most potent antigen-presenting cells (APCs) of the immune system, and macrophages, present in almost every tissue and in the lymphoid organs, have been demonstrated to be able to cross-present antigens in vitro and to prime in vivo naive CD8+ T cells to proliferate and mature into both effector and memory cells (Pozzi et al., 2005). Gene transfer to APCs has several advantages over pulsing with protein or peptide, such as the possibility of presentation of multiple epitopes for a functional cytotoxic T lymphocyte (CTL) response, a continuous supply of antigen for sustained presentation, and the coexpression of cytokine genes along with the antigen of interest. We and others have already demonstrated that IDLVs are able to transduce both murine and human APCs (Gillim-Ross et al., 2005; Coutant et al., 2008; Berger et al., 2009; Michelini et al., 2009), and that integrase (IN) mutants prevent virus integration in transduced macrophages (Cara et al., 1995; Kelly et al., 2008; Zheng et al., 2008). However, a proof-of-concept study showing that IDLV-transduced human APCs are able to induce the antigen-specific T cell expansion of primed T cells is still lacking. With this aim in mind, in the current study we examined the in vitro efficiency of IDLV-transduced human monocyte-derived DCs and macrophages to induce expansion of functional antigen-specific CD8+ T cell responses against the vector-encoded influenza matrix M1 model antigen.
Materials and Methods
Vector construction and production of recombinant vector particles
A schematic depiction of the vectors used in this study is provided in Fig. 1a. Briefly, the woodchuck posttranscriptional regulatory element (WPRE) was introduced downstream of the green fluorescent protein (GFP) sequence into the XhoI-restricted pTY2CMV-GFP plasmid (Bona et al., 2006), to produce the pTY2CMV-GFPW plasmid. The influenza matrix protein (M1) coding sequence was obtained from plasmid pET-15b-M1 and was inserted in place of the GFP sequence to obtain the Flu-M1-expressing pTY2CMV-M1W plasmid. The IN-competent packaging plasmid pCMVdR8.2, the IN-defective packaging plasmid pcHelp/IN-, and the vesicular stomatitis virus G glycoprotein (VSV.G) envelope-expressing pMD.G plasmids have been already described (Bona et al., 2006). The human kidney epithelial cell line 293T was maintained in Dulbecco's modified Eagle's medium (Euroclone, Life Sciences Division, Pero, Milan, Italy) supplemented with 10% fetal calf serum (Euroclone, Life Sciences Division) and penicillin–streptomycin–glutamine (100 units/ml; GIBCO Invitrogen, Paisley, UK). For production of recombinant LVs expressing GFP (LV-GFP) and M1 (LV-M1), 293T cells were transiently transfected with the calcium phosphate-based ProFection mammalian transfection system (Promega, Madison WI) as previously described (Negri et al., 2007). Integrase-defective and integrase-competent recombinant LVs were produced with pcHelp/IN- and pCMVdR8.2, respectively. To produce the control recombinant vector LV-GFP, the pTY2CMV-GFPW vector was used in place of the pTY2CMV-M1W plasmid. Vector-containing supernatants were clarified and concentrated by ultracentrifugation (Beckman Coulter, Fullerton, CA) on a 20% sucrose gradient (Sigma-Aldrich, St. Louis, MO). Viral pellets were resuspended in 1 × phosphate-buffered saline (PBS) and viral titers were normalized by reverse transcriptase (RT) (Weiss et al., 1992) and p24 ELISAs (Innotest; Innogenetics, Ghent, Belgium).
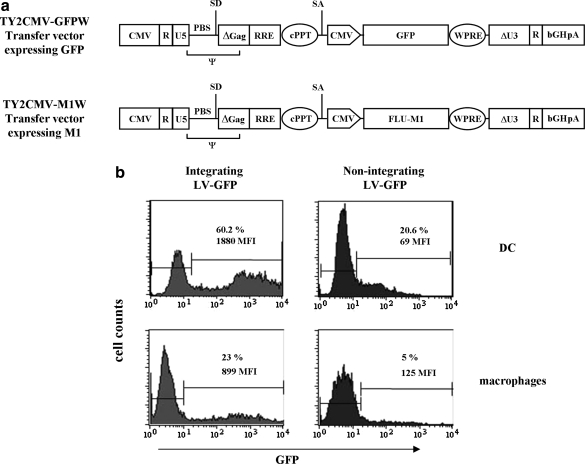
FIG. 1.
(a) Transfer vectors used in this study. The packaging signal (ψ), primer-binding site (PBS), major splice donor (SD) and splice acceptor (SA) sites, bovine growth hormone polyadenylation signal (bGHpA), central polypurine tract (cPPT), woodchuck posttranscriptional regulatory element (WPRE), and green fluorescent protein (GFP) and influenza matrix M1 protein (FLU-M1) coding sequences are indicated. ΔGag, deleted Gag region; ΔU3, deleted U3 region in the 3′ long terminal repeat; CMV, cytomegalovirus promoter; DC, dendritic cells; LV, lentiviral vector; R and U5, repeated and unique 5′ regions, respectively; RRE, Rev response element. (b) Transduction of DCs and macrophages with integrating or nonintegrating LVs expressing GFP was evaluated by flow cytometry. Percentages of GFP expression and mean fluorescence intensity (MFI) are indicated within the histograms. Results from one representative experiment are shown.
Selection of healthy donors by pentamer staining
Peripheral blood mononuclear cells (PBMCs) from HLA-A*0201-positive healthy donors were isolated by density gradient centrifugation, using Lympholyte (Cedarlane Laboratories, Burlington, NC). Phycoerythrin (PE)-labeled HLA-A*0201 pentamer presenting the influenza matrix M1 epitope (amino acids 58–66) (M1–pentamer) and control PE-labeled HLA-A*0201 pentamer presenting the HIV Gag peptide (SLY–pentamer) were provided by Proimmune (Magdalen Centre, Oxford Science, Oxford, UK). PBMCs (2 × 106) were washed in wash buffer (0.1% sodium azide, 0.1% bovine serum albumin [BSA] in PBS), spun down, and resuspended in residual liquid. Either M1– or SLY–pentamer (2 μl) was added to the cells, incubated at 4°C for 20 min, and further incubated for 15 min on ice with PE–Cy5-labeled anti-CD8 monoclonal antibody (Immunological Sciences, Rome, Italy). After washing, cells were resuspended in 1% formaldehyde in PBS and CD8+pentamer+ cells were analyzed with a FACSCalibur (BD Biosciences, San Jose, CA), using CellQuest software.
Generation of primary APCs and transduction with lentiviral vectors
PBMCs isolated from M1-positive donors were incubated with anti-CD14 MicroBeads (Miltenyi Biotec, Calderara di Reno, Bologna, Italy) in accordance with the manufacturer's instructions. Purified monocytes were seeded in a 24-well plate at 0.5 × 106 per well in 1 ml in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF, 50 ng/ml; Immunological Sciences) and interleukin (IL)-4 (35 ng/ml; Immunological Sciences) or GM-CSF (5 ng/ml) alone to differentiate monocytes to DCs or macrophages, respectively. DCs (on day 4) and macrophages (on day 8) were infected with 5 × 106 RT counts of concentrated viral supernatant per 106 cells. After 3 hr of incubation at 37°C, cells were washed once and seeded in complete medium supplied with cytokines. Maturation of DCs was induced by adding lipopolysaccharide (LPS, 0.5 μg/ml; Sigma-Aldrich) to the medium on day 7, and on day 9 mature DCs were used for stimulation of autologous PBMCs, as described subsequently. DC maturation was confirmed by evaluating upregulation of CD83, CD86, and HLA-DR expression, using fluorescein isothiocyanate (FITC)- or PE-labeled monoclonal antibodies from Immunological Sciences. Macrophages were used as APCs in coculture experiments 3 days after infection. DCs and macrophages infected with LV-M1 or LV-GFP were cocultured with autologous PBMCs depleted of CD14+ cells, at an effector (T cell)-to-stimulator (APC) ratio of 10:1 for 9 days in the presence of IL-2 (50 U/ml; BD Biosciences) and IL-7 (5 ng/ml; Thermo Scientific, Rockford, IL).
Analysis of M1-specific CD8+ T cells by pentamer staining, interferon-γ enzyme-linked immunospot analysis, and intracellular staining
The presence of M1-specific CD8+ T cells was evaluated with PE-labeled M1–pentamer, as described previously. An interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assay was performed with reagents from BD Biosciences. A 9-mer containing the HLA-A*0201-restricted M1 epitope (GILGFVFTL) (Primm, Milan, Italy) and the HLA-A*0201-restricted HIV-1 Gag 9-mer peptide (SLYNTVATL) (Primm) were used at 5 μg/ml as specific and unrelated stimuli, respectively. Medium alone and phytohemagglutinin (PHA; Sigma-Aldrich) were used as negative and positive controls, respectively. Samples were scored positive when they presented a minimum of 50 spots per 106 cells and a 2-fold or higher number compared with the unrelated peptide.
The presence of M1-specific CD8+ cytokine-producing T cells was evaluated by intracellular staining for IFN-γ, TNF-α, and IL-2 on M1–pentamer-positive cells. To analyze the effector function of antigen-specific T cells, the cells were first stained with either M1– or SLY–pentamer (2 μl) incubated at room temperature for 20 min, washed, resuspended in complete medium, and then incubated with monensin (GolgiStop, 0.7 μg/ml; BD Biosciences) plus costimulatory antibodies (anti-CD28 and anti-CD49d, 1 μg/ml; BD Biosciences) in the presence of M1-specific peptide, HIV-1 Gag-unrelated peptide, phorbol myristate acetate (PMA, 50 ng/ml; Sigma-Aldrich), and ionomycin (0.2 μM; Sigma-Aldrich) as positive control or medium as negative control, at 37°C for 6 hr. Cells were then washed and stained with anti-human CD8 monoclonal antibody before fixation, permeabilization, and intracellular staining for IFN-γ, TNF-α, and IL-2, using Fluorochrome-labeled monoclonal antibodies provided by BD Biosciences. Samples were analyzed with a FACSCanto equipped with Diva software (BD Biosciences).
Results and Discussion
Transduction of DCs and macrophages with IDLVs
Evaluation of the transduction efficiency of IDLVs on human APCs was performed with either integrating or nonintegrating LVs expressing GFP. A representative experiment is reported in Fig. 1b, showing GFP expression in transduced monocyte-derived DCs and macrophages evaluated in terms of percentage of GFP-positive cells and mean fluorescence intensity (MFI) in the transduced cells. On day 7 from infection, 60.2 and 20.6% of GFP-expressing DCs were detected after infection with integrating and nonintegrating LVs, respectively. As expected, the integrating LV was more efficient in transducing DCs, showing both a higher percentage of GFP-expressing cells and higher MFI (MFI, 1880 vs. 69). In the same experiment macrophages from the same donors were generated and infected with both vectors. As shown in Fig. 1b, 23% (MFI, 899) and 5% (MFI, 125) of GFP+ macrophages were detected 7 days after infection with integrating and nonintegrating LV-GFP, respectively. These data are in agreement with previously reported experiments (Schroers et al., 2000), and confirm that DCs are more efficiently transduced by LVs than are macrophages.
IDLV-transduced human DCs are able to induce expansion of M1-specific CD8+ T cells
To demonstrate the functionality of human APCs transduced with IDLV, integrating and nonintegrating LVs expressing influenza matrix (M1) protein were generated and used as described in Materials and Methods. HLA-A*0201-positive human healthy donors were selected and tested for the presence of CD8+ T cells specific for the M1 immunodominant epitope (Gotch et al., 1987). Selected M1-positive donors showed a percentage ranging from 0.2 to 0.3% of M1–pentamer-positive cells within gated peripheral CD8+ T lymphocytes (data not shown). Monocyte-derived DCs and macrophages infected with either integrating or nonintegrating LV-M1 or control vector (LV-GFP) were cocultured with CD14-depleted autologous PBMCs for 9 days. The presence of M1-specific CD8+ T cells was evaluated by pentamer staining and the functional activity of these cells was analyzed by cytokine production.
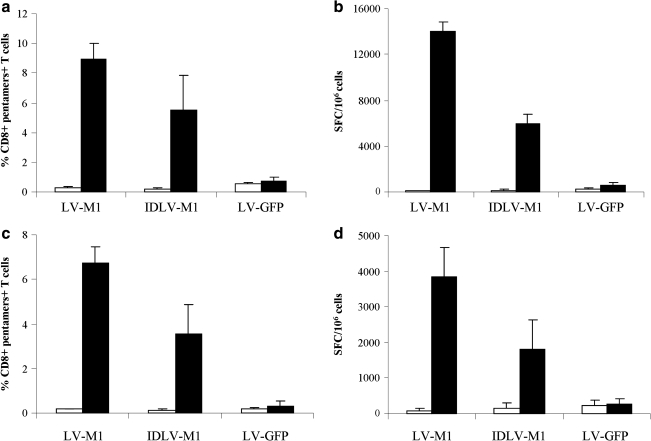
As shown in Fig. 2a, DCs transduced with either LV-M1 vector were able to expand M1-specific T cells. In particular, the IDLV-transduced DCs were able to induce an expansion of 5.52 ± 2.3% of M1-specific CD8+ T cells, representing an approximately 25-fold increase compared with day 0, whereas, as expected, integrase-competent LV-transduced DCs induced higher expansion of M1–pentamer-positive cells (8.94 ± 1.03%). DCs infected with LVs expressing GFP (unrelated antigen) did not induce significant expansion of M1-specific CD8+ T cells (0.76 ± 0.2%).
FIG. 2.
Evaluation of M1-specific CD8+ T cell expansion by using DCs (a and b) and macrophages (c and d) as APCs infected with either integrating or nonintegrating LVs expressing FLU-M1 or GFP as an unrelated antigen. (a and c) Cells were stained with PE–Cy5-labeled anti-CD8 antibody and PE-labeled HLA-A*0201 pentamer presenting the influenza matrix M1 epitope (solid columns) or control PE-labeled HLA-A*0201 pentamer presenting HIV Gag peptide (open columns). The percentage of pentamer+ cells was calculated within CD8+ T cells. (b and d) The functionality of CD8+ T cells was evaluated by ELISPOT for IFN-γ in the presence of M1-specific peptide (solid columns) or HIV Gag-unrelated peptide (open columns). Results are expressed as spot-forming cells (SFC) per 1 × 106 cells.
The functional analysis of M1-specific CD8+ T cells was performed by IFN-γ ELISPOT (Fig. 2b). Stimulation of CD14− PBMCs with IDLV-M1-transduced DCs was able to induce a high number of IFN-γ-producing cells in the presence of M1-specific peptide (average, 6000 ± 1000 SFC/106 cells). As expected, the number of IFN-γ-producing cells was higher in lymphocytes stimulated with the integrase-competent transduced DCs (13,900 ± 990 SFC/106 cells). Neither IFN-γ production in the presence of the unrelated peptide, or M1-specific IFN-γ-producing cells in the case of DCs infected with LV-GFP, were detected (Fig. 2b).
Overall these results indicate that IDLV-transduced DCs efficiently enable the in vitro expansion of functional primed antigen-specific CD8+ T cells.
IDLV-transduced macrophages induce polyfunctional antigen-specific CD8+ T cells
Macrophages are important APCs, present in several tissues and able to efficiently present antigen to T cells. LV-M1-transduced macrophages were used as APCs in coculture experiments, as described previously in the case of DCs. IDLV-M1-transduced macrophages were able to expand M1-specific CD8+ T cells as evaluated by pentamer staining (Fig. 2c) and to induce IFN-γ-producing M1-specific CD8+ T cells in the ELISPOT assay (Fig. 2d). IDLV-M1-transduced macrophages were able to induce expansion of M1-specific T cells (3.56 ± 1.33% pentamer+ cells) and IFN-γ-producing T cells in the presence of the M1-specific peptide (1806 ± 814 SFC/106 cells). As expected, the higher efficiency of integrase-competent LVs, compared with IDLVs, in transducing APCs resulted in more efficient expansion of functional M1-specific T cells (6.75 ± 0.72% pentamer+ cells and 3822 ± 823 SFC/106 cells) (Fig. 2c and d).
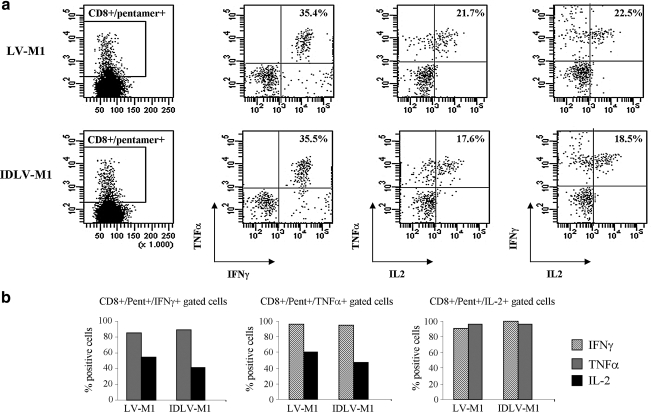
Finally, to appreciate whether the M1-specific CD8+ T cells induced by IDLV-transduced macrophages were qualitatively similar to those induced by integrase-competent LV-transduced macrophages, analysis of the production of various cytokines was evaluated by intracellular staining for IFN-γ, TNF-α, and IL-2. As shown in Fig. 3a, in both conditions, CD8+pentamer+ T cells were able to produce all the cytokines analyzed. Indeed, even if the numbers of M1–pentamer T cells were different between integrating and nonintegrating LV-transduced APC cultures, the percentage of cytokine-producing cells within the M1–pentamer-positive population was similar, as indicated by the percentage number within the dot plots (Fig. 3a). By gating the population producing a single cytokine, we were able to appreciate the polyfunctionality of these cells (Fig. 3b). In particular, almost all the IFN-γ-producing cells were able to produce TNF-α and vice versa (Fig. 3b, left and middle). All the IL-2-producing cells produced both IFN-γ and TNF-α (Fig. 3b, right) and about 50% of IFN-γ- and TNF-α-producing cells expressed IL-2. Overall, these results indicated that human macrophages transduced with IDLVs effectively induce expansion of functional antigen-specific CD8+ T cells at levels nearing those observed when using the integration-competent LV counterpart.
FIG. 3.
Polyfunctionality of M1-specific CD8+ T cells. Macrophages transduced with either integrating (LV-M1) or nonintegrating LVs expressing M1 (IDLV-M1) were used as APCs in coculture experiments with autologous lymphocytes. After 9 days cells were recovered and analyzed by intracellular staining for IFN-γ, TNF-α, and IL-2, after stimulation with M1 peptide for 6 hr. Results from one representative experiment are shown. (a) The percentages of double cytokine-producing cells were calculated on CD8+pentamer+ gated T cells and are indicated within the dot plots. (b) By gating on single cytokine-producing CD8+pentamer+ T cells, the percentages of the other cytokine-producing cells were calculated and indicated in the histograms (cells producing IFN-γ, TNF-α, and IL-2 are indicated as lined, gray, and black columns, respectively).
In the present work, we have demonstrated that IDLVs efficiently transduce human APCs, such as monocyte-derived DCs and macrophages, while allowing maintenance of their biological properties. In particular, the transduction efficiency of both integrating and nonintegrating LVs was different in the two cell populations, being higher in DCs than in macrophages. These results are in agreement with previously published data regarding the efficiency of transduction of DCs and macrophages by using integrase-competent LVs (Schroers et al., 2000). Indeed, the possibility that endogenous factors in various cell types may influence transduction efficiency and/or transgene expression should be considered and improved on.
In this study, we quantified the number (by pentamer staining) and functionality (by cytokine production) of DCs and macrophages, and report that both DCs and macrophages, transduced with a relatively low vector dose, effectively induced in vitro expansion of functional antigen-specific primed CD8+ T cells. Overall, transduced DCs showed greater ability than transduced macrophages as APCs, in terms of number and functionality of expanded antigen-specific CD8+ T cells. These data could be explained by considering both the higher efficiency of DC transduction and their well-known capacity for antigen presentation. Results indicated that IDLV-transduced macrophages induced polyfunctional CD8+ T cells qualitatively similar to those induced by the integrase-competent counterpart. Further studies aimed at improving the transduction of DCs and macrophages with IDLVs, using lower multiplicities of infection, might be considered. In this context, one report showed that noninfectious SIVmac virus-like particles carrying vpx efficiently improved transduction of human APCs with IDLVs (Berger et al., 2009). Alternatively, other approaches, including the insertion of a specific APC activator into the IDLV genome, could be considered to improve the efficiency of APC activity (Escors et al., 2008; Yang et al., 2008).
Reports and reviews have been published suggesting that IDLVs represent a class of novel viral vectors with several established and emerging applications (Wanisch and Yáñez-Muñoz, 2009; Banasik and McCray, 2010). Results here described support the use of IDLVs as an appealing strategy for antigen delivery to APCs. Indeed, there is exciting potential for the effective use of IDLVs in immunotherapy applications, as they would appear to have significant safety benefits compared with their integrating counterparts.
Acknowledgments
The authors thank Patrizia Cocco and Ferdinando Costa for technical support, and Stefania Donnini for secretarial assistance. This study was carried out with financial support from NIH grant 1R21AI073926-01A2 (to M.S.) and by grants from the Italian AIDS National Program (to A.C.).
Author Disclosure Statement
The authors declare no conflict of interest.
References
- Banasik M.B. McCray P.B., Jr. Integrase-defective lentiviral vectors: Progress and applications. Gene Ther. 2010;17:150–157. doi: 10.1038/gt.2009.135. [DOI] [PubMed] [Google Scholar]
- Beignon A.S. Mollier K. Liard C. Coutant F. Munier S. Rivière J. Souque P. Charneau P. A lentiviral vector-based prime/boost vaccination against AIDS: A pilot study shows protection against SIVmac251 challenge in macaques. J. Virol. 2009;83:10963–10974. doi: 10.1128/JVI.01284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger G. Goujon C. Darlix J.L. Cimarelli A. SIVMAC Vpx improves the transduction of dendritic cells with nonintegrative HIV-1-derived vectors. Gene Ther. 2009;16:159–163. doi: 10.1038/gt.2008.128. [DOI] [PubMed] [Google Scholar]
- Bona R. Andreotti M. Buffa V. Leone P. Galluzzo C.M. Amici R. Palmisano L. Mancini M.G. Michelini Z. Di Santo R. Costi R. Roux A. Pommier Y. Marchand C. Vella S. Cara A. Development of a human immunodeficiency virus vector-based, single-cycle assay for evaluation of anti-integrase compounds. Antimicrob. Agents Chemother. 2006;50:3407–3417. doi: 10.1128/AAC.00517-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cara A. Klotman M.E. Retroviral E-DNA: persistence and gene expression in nondividing immune cells. J. Leukoc. Biol. 2006;80:1013–1017. doi: 10.1189/jlb.0306151. [DOI] [PubMed] [Google Scholar]
- Cara A. Guarnaccia F. Reitz M.S. Gallo R.C. Lori F. Self-limiting, cell type-dependent replication of an integrase-defective human immunodeficiency virus type 1 in human primary macrophages but not T lymphocytes. Virology. 1995;208:242–248. doi: 10.1006/viro.1995.1148. [DOI] [PubMed] [Google Scholar]
- Coutant F. Frenkiel M.P. Despres P. Charneau P. Protective antiviral immunity conferred by a nonintegrative lentiviral vector-based vaccine. PLoS One. 2008;3:e3973. doi: 10.1371/journal.pone.0003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escors D. Lopes L. Lin R. Hiscott J. Akira S. Davis R.J. Collins M.K. Targeting dendritic cell signaling to regulate the response to immunization. Blood. 2008;111:3050–3061. doi: 10.1182/blood-2007-11-122408. [DOI] [PubMed] [Google Scholar]
- Gillim-Ross L. Cara A. Klotman M.E. HIV-1 extrachromosomal 2-LTR circular DNA is long-lived in human macrophages. Viral Immunol. 2005;18:190–196. doi: 10.1089/vim.2005.18.190. [DOI] [PubMed] [Google Scholar]
- Gotch F. Rothbard J. Howland K. Townsend A. McMichael A. Cytotoxic T lymphocytes recognize a fragment of influenza virus matrix protein in association with HLA-A2. Nature. 1987;326:881–882. doi: 10.1038/326881a0. [DOI] [PubMed] [Google Scholar]
- He Y. Falo L.D., Jr. Lentivirus as a potent and mechanistically distinct vector for genetic immunization. Curr. Opin. Mol. Ther. 2007;9:439–446. [PMC free article] [PubMed] [Google Scholar]
- Hu B. Yang H. Dai B. Tai A. Wang P. Nonintegrating lentiviral vectors can effectively deliver ovalbumin antigen for induction of antitumor immunity. Hum. Gene Ther. 2009;20:1652–1664. doi: 10.1089/hum.2009.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwacz K. Mukherjee S. Apolonia L. Blundell M.P. Bouma G. Escors D. Collins M.K. Thrasher A.J. Nonintegrating lentivector vaccines stimulate prolonged T-cell and antibody responses and are effective in tumor therapy. J. Virol. 2009;83:3094–3103. doi: 10.1128/JVI.02519-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. Beddall M.H. Yu D. Iyer S.R. Marsh J.W. Wu Y. Human macrophages support persistent transcription from unintegrated HIV-1 DNA. Virology. 2008;372:300–312. doi: 10.1016/j.virol.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini Z. Negri D.R. Baroncelli S. Spada M. Leone P. Bona R. Klotman M.E. Cara A. Development and use of SIV-based Integrase defective lentiviral vector for immunization. Vaccine. 2009;27:4622–4629. doi: 10.1016/j.vaccine.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri D.R. Michelini Z. Baroncelli S. Spada M. Vendetti S. Buffa V. Bona R. Leone P. Klotman M.E. Cara A. Successful immunization with a single injection of non-integrating lentiviral vector. Mol. Ther. 2007;15:1716–1723. doi: 10.1038/sj.mt.6300241. [DOI] [PubMed] [Google Scholar]
- Pozzi L.A. Maciaszek J.W. Rock K.L. Both dendritic cells and macrophages can stimulate naive CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells. J. Immunol. 2005;175:2071–2081. doi: 10.4049/jimmunol.175.4.2071. [DOI] [PubMed] [Google Scholar]
- Schroers R. Sinha I. Segall H. Schmidt-Wolf I.G. Rooney C.M. Brenner M.K. Sutton R.E. Chen S.Y. Transduction of human PBMC-derived dendritic cells and macrophages by an HIV-1-based lentiviral vector system. Mol. Ther. 2000;1:171–179. doi: 10.1006/mthe.2000.0027. [DOI] [PubMed] [Google Scholar]
- Wanisch K. Yáñez-Muñoz R.J. Integration-deficient lentiviral vectors: A slow coming of age. Mol. Ther. 2009;17:1316–1332. doi: 10.1038/mt.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. Teich N. Varmus H. Coffin J. RNA Tumor Viruses. 2nd. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1992. pp. 1205–1218. [Google Scholar]
- Yang L. Yang H. Rideout K. Cho T. Joo K.I. Ziegler L. Elliot A. Walls A. Yu D. Baltimore D. Wang P. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat. Biotechnol. 2008;26:326–334. doi: 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. Ourmanov I. Hirsch V.M. Persistent transcription of a nonintegrating mutant of simian immunodeficiency virus in rhesus macrophages. Virology. 2008;372:291–299. doi: 10.1016/j.virol.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]