Abstract
Serotonin receptors of the 5-HT2A subtype are robustly expressed in the cerebral cortex where they have been implicated in the pathophysiology and therapeutics of mental disorders and the actions of hallucinogens. Much less is known, however, about the specific cell types expressing 5-HT2A receptors in cortex. In the current study we use immunohistochemical and electrophysiological approaches in genetically modified mice to address the expression of the Htr2a gene and 5-HT2A receptors in cortex. We first use an EGFP-expressing BAC transgenic mice and identify three main Htr2A gene expressing neuronal populations in cortex. The largest of these cell populations corresponds to layer V pyramidal cells of the anterior cortex, followed by GABAergic interneurons of the middle layers, and non-pyramidal cells of the subplate/Layer VIb. We then use 5-HT2A receptor knockout mice to identify an antibody capable of localizing 5-HT2A receptors in brain and use it to map these receptors. We find strong laminar expression of 5-HT2A receptors in cortex, especially along a diffuse band overlaying layer Va. This band exhibits a strong anteroposterior gradient that closely matches the localization of Htr2A expressing pyramidal cells of layer V. Finally we use electrophysiological and immunohistochemical approaches to show that most, but not all, GABAergic interneurons of the middle layers are parvalbumin expressing Fast-spiking interneurons and that these cells are depolarized and excited by serotonin, most likely through the activation of 5-HT2A receptors. These results clarify and extend our understanding of the cellular distribution of 5-HT2A receptors in the cerebral cortex.
Keywords: serotonin, cortex, serotonin receptors, 5-HT2A, prefrontal, pyramidal cell, interneuron
Introduction
5-HT2A receptors are class A heptahelical receptors activated by serotonin (5-Hydroxytryptamine, 5-HT) that signal, at least in part, by activating heterotrimeric G proteins of the Gαq-11 subtype (Hoyer et al., 1994). In the brain, 5-HT2A receptors are abundantly expressed in the anterior forebrain and especially in the anterior cerebral cortex (Pazos et al., 1985; Hoyer et al., 1986) where they are thought to mediate at least some of the effects of serotonin (Hoyer et al., 1994; Barnes and Sharp, 1999; Zhang, 2003; Béïque et al., 2007). Converging lines of evidence indicate that 5-HT2A receptors, especially those located in the prefrontal cortex, play an important role in the pathophysiology and therapeutics of neuropsychiatric disorders including schizophrenia and depression and the mechanism of action of hallucinogens (Vollenweider et al., 1998; Dean, 2003; Weisstaub et al., 2006; Gray and Roth, 2007; Serretti et al., 2007).
The distribution of 5-HT2A receptors in the brain was originally outlined by studies using receptor autoradiographic approaches (Pazos et al., 1985; Blue et al., 1988; Lopez-Gimenez et al., 1997, 2001) and was subsequently confirmed by studies using in situ hybridization (Mengod et al., 1990; Pompeiano et al., 1994; Wright et al., 1995). These complementary approaches outlined the distribution of 5-HT2A binding sites and 5-HT2A receptor mRNA in the rodent brain and demonstrated that 5-HT2A receptors are expressed in the rodent cerebral cortex along a strong anteroposterior gradient that exhibits robust laminar specificity. The limited resolution of these techniques, however, precluded a cellular level analysis of 5-HT2A receptor distribution.
Subsequent attempts to expand on this early work and localize 5-HT2A receptors at the cellular and subcellular levels have relied mostly on the use of anti-5-HT2A receptor antibodies. Unfortunately, although several different antibodies directed against the N and the C terminus of the receptor have been used (Morilak et al., 1993; Willins et al., 1997; Hamada et al., 1998; Jakab and Goldman-Rakic, 1998; Wu et al., 1998; Jansson et al., 2001; Martin-Ruiz et al., 2001; Miner et al., 2003; Doly et al., 2004; McDonald and Mascagni, 2007), this work has yielded conflicting results in terms of the areal, cellular and subcellular distribution of 5-HT2A receptors (Morilak et al., 1993; Backstrom and Sanders-Bush, 1997; Willins et al., 1997; Hamada et al., 1998; Cornea-Hebert et al., 1999; Miner et al., 2003). In broad terms, there has been only limited congruency between the distributions of 5-HT2A receptor immunoreactivity reported by these different antibodies. Furthermore, the distribution of 5-HT2A receptor immunoreactivity reported in most of these studies has also differed, in some cases quite substantially, from the distribution of 5-HT2A binding sites and 5-HT2A receptor mRNA identified using receptor autoradiographic and in situ hybridization approaches. This has resulted in uncertainty regarding the distribution of 5-HT2A receptors in the brain.
In the present work we have taken advantage of genetically modified mice to re-examine the expression of Htr2A, the gene coding for the 5-HT2A receptor, and the 5-HT2A receptor protein itself in the cerebral cortex. We use three genetically modified mice strains in this study. First, we use BAC transgenic mice engineered to express EGFP under the control of the 5-HT2A receptor promoter [5-HT2AR-EGFP mice, strain Tg(Htr2a-EGFP)118Gsat/Mmcd, GENSAT]. Previous studies have shown that reporter gene expression cassettes within BACs experience limited positional effects from their insertion into the genome and thus expression of the reporter can be used to map the native expression of the gene of interest (Heintz, 2001; Gong et al., 2003). Therefore EGFP expression in these mice should identify Htr2A gene expressing neurons both in vivo and in vitro. Second, we used mice in which the 5-HT2A receptor has been genetically deleted (5-HT2A receptor knockout mice, Villalobos et al., 2005; Béïque et al., 2007). This knockout mouse makes it possible to unambiguously determine antibody specificity and labeling conditions for the immunolocalization of 5-HT2A receptors in native tissues, and also greatly facilitates the identification of 5-HT2A receptor-mediated responses in vivo. Third and finally, we used B6/Cg-Tg(Thy1-YFPH)2Jrs/J mice (Thy1-YFP mice), which express YFP under the control of the Thy-1 promoter in pyramidal cell of layer Vb (Feng et al., 2000; Shepherd, 2009). This mouse provide an unambiguous laminar marker for the study of 5-HT2A receptor distribution in cortex. Together, these mice have allowed us to re-examine the distribution of 5-HT2A receptors in the cerebral cortex.
Materials and Methods
Experimental animals
All experiments described in the current report were carried out in accordance with the U.S. Public Health Service's Policy on Humane Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Wayne State University. Breeding colonies for each of the mice strains were maintained within the animal facilities of the Wayne State University School of Medicine. The Tg(Htr2a-EGFP)118Gsat/Mmcd mouse strain was originally obtained from the NIH's Mutant Mouse Regional Resource Center at the University of California, Davis. The 5-HT2A receptor knockout mouse strain was originally obtained from Dr. Jay A. Gingrich (Villalobos et al., 2005) and has been crossed through several generations into C57Black and Swiss Webster backgrounds. The B6/Cg-Tg(Thy1-YFPH)2Jrs/J mouse strain was obtained from the Jackson labs. The 5-HT2A receptor knockout and Tg(Htr2a-EGFP)118Gsat/Mmcd strains were maintained as heterozygote × heterozygote mating pairs and the offspring were genotyped to select mice of the needed genotype. The B6/Cg-Tg(Thy1-YFPH)2Jrs/J strain was maintained as homozygote × wild type mating pairs and thus all offspring expressed YFP.
Immunohistochemistry and imaging
Adult mice were anaesthetized with a lethal dose of pentobarbital and perfused transcardially with PBS followed by ice cold 4% paraformaldehyde. Brains were postfixed for 24–72 h in 4% paraformaldehyde at 4°C, washed in PBS and sectioned using a vibratome to produce horizontal or coronal sections nominally 50 μm thick. Standard immunohistochemical procedures were used to localize antigens of interest. The primary antibodies used in the current study were (1) rabbit anti-GFP (1:1000, Invitrogen), (2) chicken anti-GFP (1:1000, Aves Labs), (3) affinity purified rabbit anti-5-HT2A receptor (1:100–1:1000, ImmunoStar), (4) Rabbit anti-5-HT2A receptor (1:1000, Ab51, a kind gift of Dr. Bryan L. Roth, University of North Carolina), (5) Rabbit anti-5-HT2A receptor (1:300, Ab16028, Abcam), (6) rabbit anti-parvalbumin (1:2000, Swant), (7) Rabbit anti-calretinin (1:2000, Swant), (8) mouse anti-GABA (1:1000, Swant). The affinity purified rabbit anti-5-HT2A receptor antibody was originally developed by Dr. Mark S. Brownfield, University of Wisconsin, Madison (Brownfield et al., 1998) and is currently distributed by ImmunoStar. This same antibody also appears to be distributed by other companies (Dr. M.S. Brownfield, personal communication) and may be the same antibody used by McDonald and Mascagni (2007) and by Doly et al. (2004). Secondary antibodies conjugated to Alexafluor 488 or Alexafluor 568 were obtained from Molecular Probes/Invitrogen. Secondary antibodies conjugated to Dylight 488 or Cy3 were obtained from Jackson Immunoresearch. Biotinylated secondary antibodies were obtained from Vector labs. Immunoperoxidase staining was performed using the Vectastain ABC kit (Vector Labs) using DAB as a substrate and nickel intensification. Sections stained for immunofluorescence were mounted using Vectashield (Vector labs) while sections reacted using the HRP/DAB procedure were mounted, dehydrated in alcohol/xylene and coverslipped using Krystalon. Brain sections were imaged using an Olympus Fluoview laser scanning confocal microscope equipped with Argon and Xenon lasers, or a Zeiss Axioskop microscope equipped with a Spot digital camera (Diagnostic Instruments). For experiments aimed at comparing the immunostaining in wild type and 5-HT2A receptor knockout mice, the slices were notched with a scalpel for identification purposes, mixed, and processed as a single batch to avoid any potential differences in the immunolabeling procedure.
Electrophysiology
Whole cell electrophysiological recordings were obtained from neurons in cortical brain slices as previously described (Béïque et al., 2007; Yan et al., 2009). Briefly, mice aged between postnatal day 14 and 24 were anaesthetized with isofluorane and sacrificed by decapitation. The brain was cooled in ice cold Ringer (composition in mM: 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1 NaH2PO4, 26.2 NaHCO3 and 22 glucose, bubbled to saturation with 95% O2–5% CO2) and cortical brain slices were cut at a nominal thickness of 300 μM using a vibratome. Slices were allowed to recover in bubbled Ringer supplemented with 10 mM HEPES and then were transferred, one at a time, to a recording chamber on the stage of an upright microscope (Nikon E600). In this chamber, slices were continuously perfused with Ringer at 30° ± 1° C. In general EGFP expression in cortical interneurons was reasonably robust through the third and fourth postnatal week thus allowing for the targeting of EGFP expressing interneurons of the prefrontal, primary motor and primary sensory cortices using fluorescence and DIC imaging. In most experiments, whole-cell recordings were obtained using a potassium based intracellular recording solution (composition in mM: 125 KMeSO4, 5 KCl, 5 NaCl, 0.02 EGTA, 10 Hepes, 1 MgCl2, 10 NaH2PO4, 4, ATP Mg salt, 0.3 GTP Na salt, pH 7.3). Recordings examining sIPSCs were conducted in the presence of 10 μM CNQX and used a cesium based intracellular solution containing high chloride to allow for the detection of isolated IPSCs as large inward currents (composition in mM: 130 CsCl, 10 EGTA, 15 Hepes, 10 Phosphocreatine, 4 ATP Mg salt, 0.5 GTP Na salt, pH 7.3). We confirmed the effectiveness of this pharmacological isolation of sIPSCs using bicuculline (30 μM, n = 6 cells tested), which completely blocked all synaptic activity in the slice. Patch pipettes filled with these intracellular solutions exhibited resistances ranging from 2.5 to 3.5 MΩ and series resistances ranging from 8 to 15 MΩ upon gaining whole cell access. Drugs were administered in the bath dissolved at known concentrations. Most drugs were obtained from Sigma. MDL100,907 was a kind gift of Dr. Kenner Rice (Laboratory of Medicinal Chemistry, NIDDK).
Results
Htr2A gene expression in the cerebral cortex
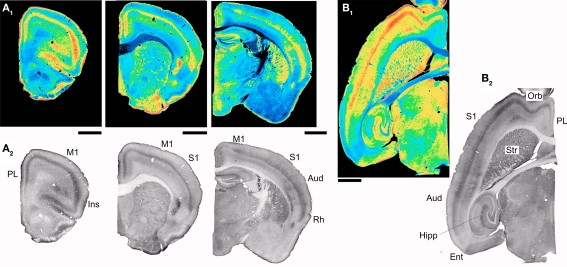
To address the cellular distribution of 5-HT2A receptors in the cerebral cortex we first examined Htr2A gene expression, as reported by EGFP, in 5-HT2AR-EGFP transgenic mice. The distribution of EGFP immunoreactivity in the forebrain of this mouse is illustrated in Figure 1. In broad terms, numerous EGFP expressing cells were observed in iso- and proisocortex, with much lower levels of expression in allocortex, including the pyriform, olfactory cortices and hippocampus. As previously noted in the preliminary GENSAT characterization of this mouse, this areal expression pattern is in general agreement with the results of in situ hybridization studies localizing the distribution of 5-HT2A receptor mRNA (Pompeiano et al., 1994; Wright et al., 1995). The main discrepancies in the anterior forebrain are the very low levels of EGFP expression in pyriform cortex, olfactory tubercle and striatum, where previous in situ hybridization studies have reported reasonably robust levels of 5-HT2A receptor mRNA expression.
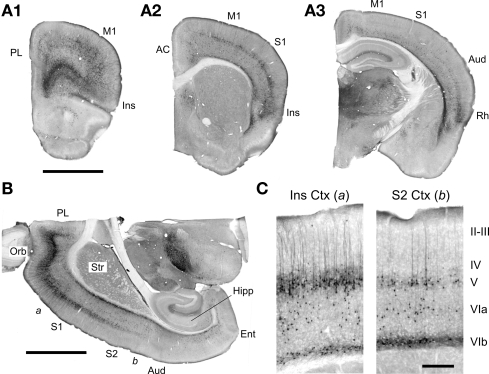
Figure 1.
EGFP expression in the forebrain of the 5-HT2AR-EGFP mouse. (A,B) Low magnification images illustrating the distribution of EGFP expressing cells in coronal and horizontal sections. Calibration bar = 2 mm. AC, anterior cingulate cortex; Aud, auditory cortex; Ent, Entorhinal cortex; Hipp, hippocampus; Ins, insular cortex; M1, primary motor cortex; Orb, orbital cortex; PL, prelimbic cortex; Rh, rhinal cortex; S1, primary somatosensory cortex; S2, secondary somatosensory cortex, Str, striatum. Low magnification images of parasagital brain sections for this mouse are available at the GENSAT web site. (C) Higher magnification images depicting the EGFP immunoreactivity in the insular [a in (B)] and secondary somatosensory cortex [b in (B)]. Calibration bar = 250 μm. In this figure EGFP was immunolocalized using a rabbit anti-GFP antibody and detected using immunoperoxidase histochemistry.
The expression of EGFP in cortex exhibits a very strong laminar pattern which reflects the labeling of three main populations of cells. First, EGFP expression defines a strong band of pyramidal neurons in layer V. This band extends, in anterior coronal sections, from the medial prefrontal cortex to the insular cortex overlaying the rhinal fissure (Figure 1A) and exhibits a very strong anteroposterior gradient. As illustrated in Figure 1, the number of labeled pyramidal cells in this band is highest in the orbital cortex and decreases at posterior levels such that only scattered pyramidal cells are labeled at the level of the secondary somatosensory cortex (Figures 1B,C). Second, EGFP expression defines a band of scattered non-pyramidal cells in Layer V and VIa (Figure 1C). In contrast to the band of pyramidal cells of layer V, this second band of cells does not exhibit an obvious anteroposterior gradation but rather appears relatively homogeneous through the visual cortex and laterally through the entorhinal cortex (Figures 1A3,C). Third, EGFP expression defines a narrow band of non-pyramidal cells immediately above the white matter (Figures 1 and 2), in what has been called the subplate, layer VIb or layer VII (Reep, 2000). These cells appear continuous with a group of EGFP expressing cells in the claustrum/endopiriform nucleus and form a thin sheet of EGFP expressing cells overlaying the entire corpus callosum. In addition to these three main cell populations, EGFP expression can also be detected in scattered pyramidal cells of layer III in the medial prefrontal, orbital and insular cortices (Figures 2B,C) as well as in sporadic non-pyramidal cells in layer I–III scattered through all cortical areas (e.g. Figures 1C and 2B).
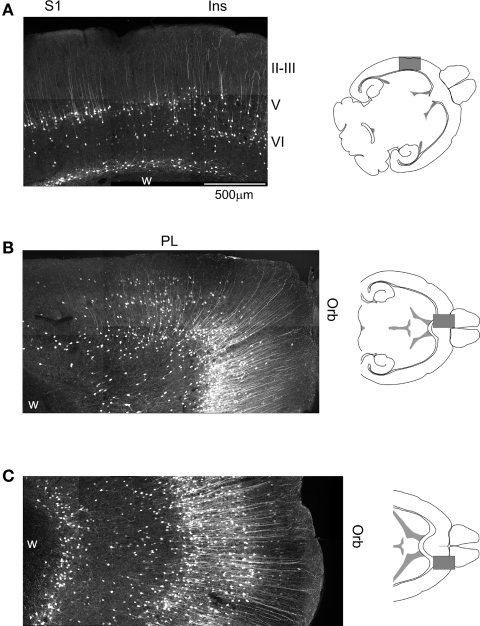
Figure 2.
Cellular expression of EGFP in the somatosensory, prefrontal and orbital cortices of 5-HT2AR-EGFP mice. (A) Confocal image illustrating the distribution of EGFP immunoreactive cells in the primary sensory and adjacent insular cortex. Calibration bar = 500 μm. (B) Distribution of EGFP in the medial prefrontal and medial orbital cortices. (C) Distribution of EGFP in the lateral orbital cortex. Ins, insular cortex; Orb, orbital cortex; PL, prelimbic cortex; S1, primary somatosensory cortex. EGFP was immunolocalized in horizontal sections using a rabbit anti-GFP antibody and detected using an Alexa 488 conjugated secondary goat anti-rabbit antibody. Images depict flattened confocal stacks containing 15–20 optical sections spanning 20–30 μm along the z axis.
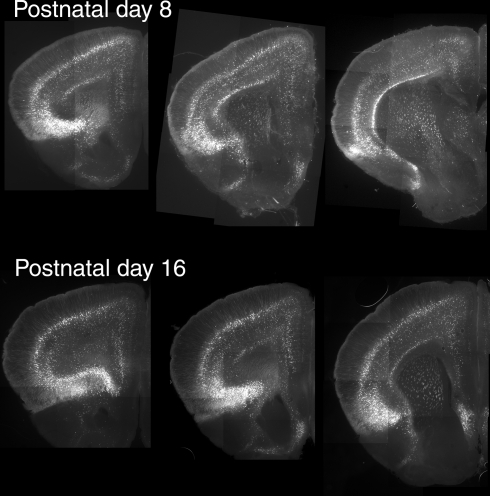
Previous studies have reported developmental changes in the ability of 5-HT2A receptors to regulate neuronal excitability in the cerebral (prefrontal) cortex (Zhang, 2003; Béïque et al., 2004). In particular, these studies have shown that 5-HT2A receptors robustly depolarize pyramidal cells of layer V during the early postnatal period and that this effect progressively diminishes with age in spite of relatively constant levels of 5-HT2A receptor mRNA. Therefore we also examined the distribution of EGFP at postnatal day 8, when 5-HT2A robustly depolarize pyramidal cells of layer V, and at postnatal day 16 when the 5-HT2A receptor-mediated depolarizing responses approach those seen in adults (Béïque et al., 2004). As illustrated in Figure 3, EGFP expression in the anterior cerebral cortex at postnatal day 8 and 16 was qualitatively similar to that seen in adult mice. The main difference observed was stronger native EGFP fluorescence during the early postnatal period (not shown).
Figure 3.
Expression of EGFP in the anterior cerebral cortex during the early postnatal period. For these experiments EGFP was immunolocalized in horizontal sections using a rabbit anti-GFP antibody and detected using an Alexa 488 conjugated secondary goat anti-rabbit antibody. Mosaic composite images for each section were assembled from 9–12 subfields acquired with a digital camera.
Validation of an anti-5-HT2A receptor antibody
While GFP expression identifies cells capable of expressing the Htr2A gene, it provides only indirect information regarding expression of the 5-HT2A receptor itself. Therefore we next sought to directly localize these receptors. We first used a commercially available affinity purified antibody directed against residues 22–41 on the extracellular N terminus of the 5-HT2A receptor (ImmunoStar cat# 24288). As illustrated in Figure 4A, using this antibody we observed a strong, diffuse laminar staining of the cerebral cortex that was accompanied by variable labeling of the pyramidal cells apical dendritic trunk and secondary branches (Figure 4C). The diffuse laminar staining pattern observed with this antibody closely matched the laminar distribution of 5-HT2A binding sites suggesting specific labeling of 5-HT2A receptors (Pazos et al., 1985; Blue et al., 1988; Mengod et al., 1990; Lopez-Gimenez et al., 1997). However, because anti-5-HT2A receptor antibodies have been plagued by specificity issues, we sought to validate the immunostaining. As a first control, we immunostained hippocampus, an area that is rich in pyramidal cells but where 5-HT2A receptor expression is very low (Pazos et al., 1985; Mengod et al., 1990; Pompeiano et al., 1994; Wright et al., 1995) and is restricted to a small subset of non-pyramidal cells (Wright et al., 1995; Shen and Andrade, 1998). As illustrated in Figure 4B, we observed only faint labeling in this region. To further test the specificity of this antibody we turned to 5-HT2A receptor knockout mice. As illustrated in Figure 4C, the laminar staining observed in cortex was completely absent in 5-HT2A receptor knockout mice. In contrast, the fainter pyramidal cell apical dendritic staining was still detectable in these mice. Dilution of the primary antibody as well as immunoabsorption through re-use of the antibody solution could reduce this non-specific dendritic staining, but could not completely eliminate it. This limitation notwithstanding, these results indicate that the ImmunoStar antibody is suitable for the detection of 5-HT2A receptors in fixed brain sections.
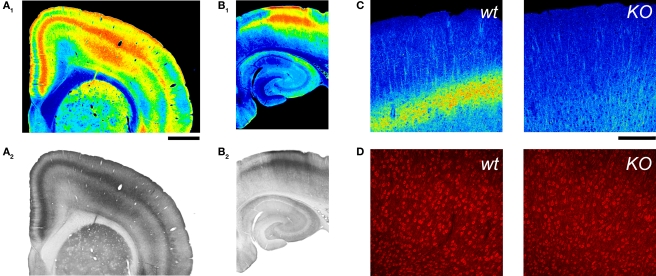
Figure 4.
5-HT2A receptor detection in the cerebral cortex using an anti-5HT2A receptor antibody. (A,B) 5-HT2A receptor immunoreactivity in the anterior cerebral cortex (A) and hippocampus (B) in a wild type mouse. 5-HT2A receptor immunodetection for these images used the ImmunoStar antibody at a 1:100 dilution and HRP/DAB detection (A2,B2). (A1) and (B1) depict pseudocoloring of images in (A2) and (B2).Calibration bar: 1 mm. (C) 5-HT2A receptor immunoreactivity in the cerebral cortex of wild type and 5-HT2A receptor knockout (KO) mice. Notice the disappearance of the layer V band in the KO mouse. 5-HT2A receptor immunodetection for these images used the ImmunoStar antibody at a 1:500 dilution and an Alexa 488 conjugated secondary antibody. Images are pseudocolored for emphasis and depict comparable flattened confocal stacks through motor-frontal cortex. (D) Immunoreactive staining obtained with the Ab51 antibody. Images correspond to single confocal sections through layers V and VI of the M1 region in a wild type and a 5-HT2A receptor knockout mice. (C,D) Calibration bar: 200 μm.
In addition to the ImmunoStar anti-5-HT2A antibody reported above, we tested two additional anti-5-HT2A receptor antibodies. First we tested a commercially available rabbit polyclonal antibody against an unspecified epitope within the first 100 residues of the rat 5-HT2A receptor (AbCam; Cat # ab16028). We could not obtain staining above background in any region of cortex with this antibody. Second, we tested a different rabbit polyclonal antibody again directed against residues 22–41 of the 5-HT2A receptor (antibody Ab51, Willins et al., 1997). Consistent with previous observations (Willins et al., 1997; Martin-Ruiz et al., 2001; Nocjar et al., 2002), this antibody stained both pyramidal and non-pyramidal cells in cortex with a strong punctuate pattern (Figure 4D). However this staining showed little regional or laminar specificity, with the soma and proximal apical dendrite of pyramidal cells of layers II–VI exhibiting comparable labeling. Most importantly, Ab51 produced comparable staining in wild type and 5-HT2A receptor knockout mice indicating that, at least under our experimental conditions, this antibody does not primarily recognize 5-HT2A receptors. We did not pursue any further studies with either of these antibodies. Unfortunately the widely used G186-1117 anti-5-HT2A receptor monoclonal antibody (Wu et al., 1998) originally distributed by Pharmingen is no longer available and could not be tested.
Immunohistochemical localization of 5-HT2A receptors in the cerebral cortex
Figure 5 illustrates the distribution of 5-HT2A receptor immunoreactivity in the cerebral cortex. In general, there is a very strong areal correspondence between anti-5-HT2A receptor immunoreactivity and Htr2A expression as reported by EGFP expression in the 5-HT2AR-EGFP mouse. This is most dramatically illustrated by the very close correspondence between the distribution of EGFP expressing pyramidal cells of layer V and the 5-HT2A receptor immunoreactive band in this same layer (Figures 1B and 4B). These results support the idea that EGFP expression in the 5-HT2AR-EGFP mouse labels 5-HT2A receptor expressing cells in the anterior forebrain. The main discrepancies observed in the anterior forebrain concerned the pyriform cortex, olfactory tubercle, and to a lesser extent the striatum. In these three areas we observed only very few and widely scattered EGFP expressing cells but robust 5-HT2A receptor immunoreactivity. In situ hybridization (Pazos et al., 1985; Blue et al., 1988; Lopez-Gimenez et al., 1997, 2001) and receptor autoradiographic studies (Mengod et al., 1990; Pompeiano et al., 1994; Wright et al., 1995) have consistently detected expression of 5-HT2A receptors in these areas. Furthermore the anti 5-HT2A receptor antibody staining in these areas was likely specific, since only background staining was detected in these areas in the 5-HT2A receptor knockout mouse (not shown). Therefore it seems likely that these discrepancies reflect silencing of the EGFP expression cassette in these areas in the 5-HT2AR-EGFP mouse rather than non-specific antibody staining. The 5-HT2A receptor immunoreactivity in iso- and proisocortex exhibits a strong laminar staining pattern (Figure 5) with the most conspicuous staining located to the middle layers of cortex. This laminar pattern is most evident in sensory and motor cortices where a dense superficial and a second deeper and less dense band are clearly recognizable (Figures 4A and 5). In addition, in strongly stained material, it is possible to also recognize an additional weak band of immunoreactivity over layer I and a second weak band over layer VIb (Figures 4A,B). Previous receptor autoradiographic studies have assigned the densest 5-HT2A receptor band to either layer IV or to layer V. To identify the specific cortical layer(s) stained by the anti-5-HT2A receptor antibody in midcortex we counterstained the brain sections using a fluorescent Nissl stain. This stain defines the densely packed layer IV in granular cortex and thus provides an unambiguous laminar landmark. As illustrated in Figure 6A, in primary somatosensory cortex the denser, more superficial 5-HT2A receptor immunoreactive band localized to layer Va, immediately below layer IV, while the second, less dense band localized to deep layer V and upper layer VI. This laminar distribution in somatosensory cortex matches the careful mapping of 5-HT2A binding sites in this same area by Blue and colleagues in rats (Blue et al., 1988).
Figure 5.
5-HT2A receptor distribution in mouse cerebral cortex. (A1-A2) Distribution of 5-HT2A receptor immunoreactivity in coronal sections through the mouse brain. (B1-B2) Distribution of 5-HT2A receptor immunoreactivity in a horizontal section through the mouse brain. The images in (A1-A2) and (B1-B2) were obtained using the ImmunoStar antibody. (A2) and (B2) depict pseudocolor renditions of the images illustrated in (A1) and (B1) respectively.Calibration bar: 1 mm. Aud, auditory cortex; Ent, Entorhinal cortex; Hipp, hippocampus; Ins, insular cortex; M1, primary motor cortex; Orb, orbital cortex; PL, prelimbic cortex; Rh, rhinal cortex; S1, primary somatosensory cortex; Str, striatum.
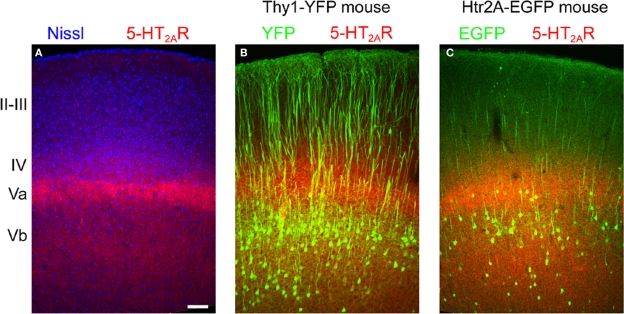
Figure 6.
Laminar distribution of 5-HT2A receptor immunoreactivity in motor-frontal cortex. (A) Laminar distribution of 5-HT2A receptors (red) in mouse cortex counterstained using a fluorescent Nissl dye (blue, Neurotrace, Invitrogen). (B) 5-HT2A receptors (red) distribution in the motor (agranular) cortex of the Thy1-YFP mouse. In this mouse YFP (green) is expressed in the large pyramidal cells of layer Vb. Notice the double banded distribution of YFP expressing cells that characterizes agranular cortex in this mouse (Shepherd, 2009). (C) 5-HT2A receptor distribution in the cortex of the 5-HT2AR-EGFP mouse. Calibration bar: 100 μm.
To extend this laminar analysis to agranular areas, which constitute the largest portion of the anterior cerebral cortex in rodents, we next took advantage of a transgenic mouse [B6/Cg-Tg(Thy1-YFPH)2Jrs/J] in which the large pyramidal cells of layer Vb are unambiguously labelled by the expression of YFP (Bartos et al., 2007; Shepherd, 2009). As illustrated for primary motor cortex in Figure 6B, the more superficial 5-HT2A receptor immunoreactive band was clearly localized immediately above the YFP immunoreactive somata, that is to say in layer Va, while the deeper band overlapped the YFP labelled cell layer (layer Vb). Similar results were observed in other agranular areas.
The diffuse 5-HT2A receptor immunoreactivity, while unambiguous, offered few clues as to the cellular localization of 5-HT2A receptors. Unfortunately, in the current material, we could not unambiguously resolve the 5-HT2A receptor immunoreactive cellular elements responsible for the banded pattern. To try to gain insight into this issue we colocalized EGFP and 5-HT2A receptors in the 5-HT2A R-EGFP mouse. As illustrated in Figure 6C, EGFP immunoreactive somata were located mostly between, but also overlaping, the two 5-HT2A receptor immunoreactive bands (Figure 6C). However, again, the weak dendritic EGFP and diffuse 5-HT2A receptor staining did not allow us to unambiguously assess colocalization. In the Thy1-YFP mouse, where pyramidal cell dendritic arborizations can be resolved in superb detail, the 5-HT2A receptor immunoreactive band located in layer Va was observed to overlap with a dense YFP immunoreactive neuropil composed predominantly of fine, horizontal and oblique oriented spiny dendrites (not shown).
Htr2A gene and 5-HT2A receptor expression in GABAergic interneurons
As outlined above, many EGFP expressing cells in the 5-HT2AR-EGFP mouse lack a clear apical dendrite and thus represent non-pyramidal cells. Such cells are most commonly located in layers V and VI and are distributed along two bands, a broad band of scattered cells located in layers V and VIa, and a second narrow band of cells located in the subplate/layer VIb. Here we investigated the cells located in the more superficial of these bands. The cells located in deeper cell band will be the subject of a separate report.
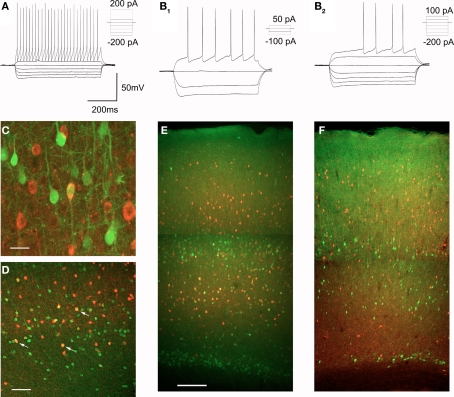
The vast majority of non-pyramidal cells in layers V and VIa can be expected to be GABAergic interneurons. In agreement with this idea, many EGFP expressing non-pyramidal cells of midcortex in the 5-HT2AR-EGFP mouse are immunoreactive for GABA (Figure 7C). Cortical GABAergic interneurons are highly heterogeneous and differ in terms of their morphology and projections, intrinsic electrophysiological properties, and the expression of specific marker genes (Kawaguchi and Kubota, 1997; Markram et al., 2004; Miyoshi et al., 2007; Ascoli et al., 2008). It is generally well accepted that GABAergic interneurons can be divided into two main types, fast-spiking (FS) and non fast-spiking (nFS) interneurons (Kawaguchi and Kubota, 1997; Markram et al., 2004; Miyoshi et al., 2007). This distinction, while based upon electrophysiological properties, is thought to reflect a fundamental division in interneuronal subtypes. Therefore we recorded from GFP expressing non-pyramidal cells and examined their spiking characteristics in response to constant current depolarizing stimuli (Kawaguchi and Kubota, 1997; Markram et al., 2004; Miyoshi et al., 2007). Most EGFP expressing interneurons could be classified as FS interneurons of the continuous firing or stuttering types (16 out of 25 cells recorded, Figure 7A). The remaining cells represented nFS interneurons (9 out of 25 cells recorded, Figure 7B). A fraction of these cells displayed a characteristic delayed spiking (Figure 7B2, n = 5 cells) and thus resembled the late spiking interneuron subtype previously described by Kawaguchi and Kubota (Kawaguchi and Kubota, 1997).
Figure 7.
Characterization of EGFP expressing GABAergic interneurons. (A,B) EGFP expressing interneurons fall into two broad classes based upon their spiking characteristics, FS interneurons (A) and nFS interneurons (B), a subset of which exhibit delayed spiking (B2). (C) Immunocolocalization of EGFP (green) and GABA (red) in layer V of motor-frontal cortex. Calibration bar: 20 μm. (D) Immunocolocalization of EGFP (green) and parvalbumin (red) in the deep layers of motor-frontal cortex. Arrows point to a subset of double labeled cells. Calibration bar 200 μm. (E) Immunocolocalization of EGFP (green) and parvalbumin (red) in motor-frontal cortex. Calibration bar: 500 μm. (F) Immunocolocalization of EGFP (green) and calretinin (red) in the same area.
Fast-spiking interneurons are characterized neurochemically by the expression of the calcium binding protein parvalbumin (Markram et al., 2004; Ascoli et al., 2008) and many EGFP expressing non-pyramidal cells were found to express this protein (Figure 7D). Parvalbumin expressing interneurons are distributed throughout cortex but only a very modest fraction of the parvalbumin expressing cells in layers II–III were found to coexpress EGFP (4/274 or 1.4% of all PV+ cells examined). This observation is consistent with the low number of EGFP positive cells in the superficial layers of cortex. In contrast, in layer V and especially in layer VIa, a much larger fraction of the parvalbumin-immunoractive cells was found to coexpress EGFP (74/483 or 15.3% of PV+ cells examined, Figure 7E). Interneurons of the nFS type represent a highly heterogeneous group (Kawaguchi and Kubota, 1997; Miyoshi et al., 2007) and we did not conduct a systematic effort to identify neurochemical markers for other Htr2A expressing interneurons. However, we observed little if any coexpression of EGFP and calretinin, a marker for a subset of nFS interneurons, in the cortex of the 5-HT2AR-EGFP mouse (Figure 7F).
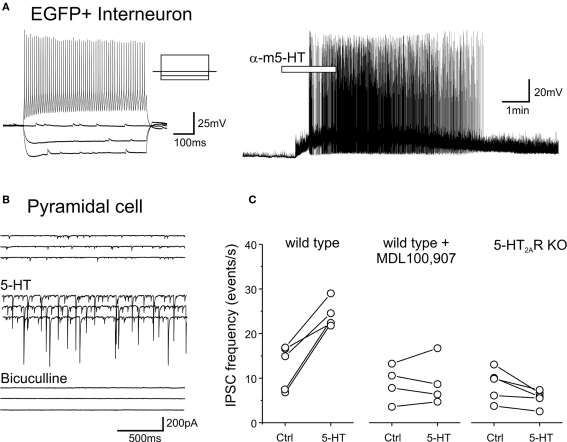
While the effects signaled by 5-HT2A receptors in pyramidal cells have been extensively investigated (e.g. Araneda and Andrade, 1991; Aghajanian and Marek, 1997; Zhang, 2003; Béïque et al., 2007), little is currently known about the effects signaled by 5-HT2A receptors in GABAergic interneurons. Therefore we examined the effects of serotonin or of the preferential 5-HT2 receptor agonist α-methyl-serotonin on EGFP expressing interneurons of layers V and VIa. As illustrated in Figure 8A, the most common effect observed in these experiments was a membrane depolarization (9 of 15 cells tested, 7 FS and 2 nFS cells). Overall the depolarizations induced by serotonin (30 μM) averaged 7.6 ± 2 mV (mean ± SEM, n = 9 cells) and ranged from barely detectable (1–2 mV) to capable of initiating sustained spiking activity (Figure 8A). No obvious effect of serotonin on membrane potential was seen in the remaining six cells.
Figure 8.
5-HT depolarizes EGFP expressing interneurons and increase sIPSCs recorded in pyramidal cells through the activation of 5-HT2A receptors. (A) Administration of α-methyl-5-HT (30 μM) depolarizes and excites an EGFP expressing FS interneuron (left panel). (B) Administration of 5-HT (30 μM) in the presence of tropisetron (1 μM, to block 5-HT3 receptors) and CNQX (10 μM to block AMPA/kainite receptors) results in a large increase in sIPSCs. Subsequent administration of bicuculline (30 μM) blocks all spontaneous synaptic activity in the slice, thus confirming that the recorded synaptic currents represented sIPSCs. (C) Plots illustrating the effect of 5-HT (30 μM) on sIPSC frequency recorded from pyramidal cells of layer V in slices derived from wild type mice, in slices derived from wild type mice but pretreated (>10 min) with the 5-HT2A receptor antagonist MDL100,907 (1 μM) and in slices derived from 5-HT2A receptor knockout mice.
The serotonin-induced depolarization observed in EGFP expressing GABAergic interneurons could be mediated by any number of serotonin receptors. The pharmacological dissection of this effect however was made difficult by the presence of considerable desensitization to repeated applications of serotonin (not shown). Interneurons, including FS interneurons provide a highly convergent synaptic input onto pyramidal cells (Bartos et al., 2007) and therefore the sIPSC frequency detected within pyramidal cells can be used to assess the integrated activity of interneurons presynaptic to the recorded cell. This provided an alternative for examining the possible role of 5-HT2A receptors in the serotonin-induced depolarization. As predicted from the direct recordings from EGFP expressing interneurons, administration of serotonin (30 μM) greatly increased the sIPSC frequency recorded from pyramidal cells of layer V, and this effect persisted even in slices pretreated with tropisetron (1 μM) to block 5-HT3 receptors (n = 5 cells, Figure 8B). These observations are consistent with similar observations in rat by Zhou and Hablitz (1999). The serotonin-induced increase in sIPSC frequency was completely blocked by pretreatment with the selective 5-HT2A receptor antagonist MDL100,907 (1 μM, Figure 8C) while 5-HT administration had no detectable effect on sIPSC frequency in slices derived from 5-HT2A receptor knockout mice (Figure 8C). These results indicate that a population of GABAergic interneurons in the cerebral cortex is excited by 5-HT2A receptor activation.
Discussion
While the distribution of 5-HT2A receptors in the brain has been mapped at a macroscopic level using receptor autoradiographic and in situ hybridization approaches, the precise cellular elements expressing 5-HT2A receptors in cortex have remained much less well understood. In the current work we have readdressed the distribution of 5-HT2A receptors in the cerebral cortex by taking advantage of genetically modified mice. The results of these studies lead us to conclude that 5-HT2A receptors in cortex are expressed predominantly by three discrete populations of cells in the cerebral cortex: layer V pyramidal cells of the anterior cerebral cortex, a subpopulation of GABAergic interneurons restricted to the middle layers of cortex, and non-pyramidal cells of the subplate/layer VIb.
Previous studies have suggested that, because of their large size, BACs experience limited positional effect from their insertion into the mouse genome, and therefore that expression of EGFP from the engineered BAC should recapitulate expression of the gene of interest (Heintz, 2001; Gong et al., 2003). In the current study we observed very good correspondence between the distribution of EGFP and 5-HT2A receptors in the cerebral cortex of the 5-HT2AR-EGFP mouse. Furthermore, we also noted a very good correspondence between the distribution of EGFP in mouse cortex and the distribution of 5-HT2A receptor mRNA and 5-HT2A binding sites previously reported in rat cortex. This strong correspondence supports the idea that EGFP expression in the 5-HT2AR-EGFP mouse identifies specific cell populations expressing 5-HT2A receptors in cortex.
While this approach identifies Htr2A gene expressing cell populations, it is important to note that the extent and completeness of the labeling of Htr2A expressing cells is less certain. For example in a series of six Drd1A (Dopamine receptor 1A) BAC mice generated by the GENSAT [g(Drd1a-cre)150 Gsat, Tg(Drd1a-cre)262 Gsat, Tg(Drd1a-cre)164 Gsat, Tg(Drd1a-cre)266 Gsat, Tg(Drd1a-cre)217 Gsat and Tg(Drd1a-cre)242 Gsat], four expressed the transgene only in a subset of the cells natively expressing the Drd1a gene (http://www.gensat.org/cre.jsp). Furthermore, a recent study has also suggested that even within a single cell type, transgenes may not be universally expressed (Shuen et al., 2008). These anomalies presumably reflect some positional silencing and/or the loss of regulatory regions not included in the BAC. Therefore it is possible that EGFP may label only a subset of Htr2A expressing cells in the 5-HT2AR-GFP mouse. In addition, Htr2A gene expression does not guarantee expression of 5HT2A receptor protein. These concerns notwithstanding, the close correspondence between EGFP and 5-HT2A receptor mRNA expression observed at the macroscopic level suggests that most cell populations expressing the Htr2A gene are likely labeled in this mouse. However it remains possible that EGFP may label only a fraction of the cells natively expressing the Htr2A gene and 5-HT2A receptors, and thus that EGFP may function more like a trace label for specific cell populations rather than a universal tag for Htr2A gene expressing cells.
Given the well documented expression of 5-HT2A receptor in cortex, the most striking finding from the present study is the very selective expression of the Htr2A gene in specific neuronal populations of the cerebral cortex. Numerically the largest labeled population corresponded to layer V pyramidal neurons of the anterior cerebral cortex. These cells show a remarkable graded anteroposterior distribution that likely accounts for the strong anteroposterior gradient in 5-HT2A receptor expression in cortex (Pazos et al., 1985; Blue et al., 1988; Lopez-Gimenez et al., 1997, and current work). This strongly localized expression contrasts with the much more widespread expression of 5-HT2A receptors suggested by previous studies using anti-5-HT2A receptor antibodies (Morilak et al., 1993; Willins et al., 1997; Hamada et al., 1998; Jakab and Goldman-Rakic, 1998; Wu et al., 1998; Cornea-Hebert et al., 1999). Discrepancies in the apparent distribution of 5-HT2A receptors when localized using anti-5-HT2A receptor antibodies and receptor autoradiography have been noted previously, and have been attributed to the ability of the antibodies to recognize immature forms of the receptor (Nocjar et al., 2002; McDonald and Mascagni, 2007) or to intracellular segmentation (Jakab and Goldman-Rakic, 1998). These discrepancies, however, generally persist even when considering 5-HT2A receptor mRNA distribution, as for example in the observation of strong staining of pyramidal cells of the CA1 region of the hippocampus (e.g. Cornea-Hebert et al., 1999), a group of cells that expresses few if any 5-HT2A receptors (Pazos et al., 1985; Mengod et al., 1990; Ciaranello et al., 1993; Wright et al., 1995; Lopez-Gimenez et al., 1997). Therefore we believe antibody cross-reactivity with non 5-HT2A receptor epitopes offers a more likely explanation for this discrepancy. As such, these considerations suggest a need to revisit previous work relying on immunodetection of 5-HT2A receptors in central neurons.
Htr2A gene expression in the cortex of the 5-HT2AR-EGFP mouse is not restricted to pyramidal cell but also extends to non-pyramidal cells, and specially GABAergic interneurons. A large fraction of these cells are parvalbumin-expressing FS interneurons, an observation that is consistent with previous in situ hybridization studies reporting coexpression of 5-HT2A receptor and parvalbumin mRNA in cortex (de Almeida and Mengod, 2007; Puig et al., 2010). These Htr2A expressing GABAergic interneurons form an extensive band of cells scattered through the middle layers and extending throughout iso- and proisocortex. Administration of serotonin depolarizes and excites these cells and also increases spontaneous GABAergic synaptic transmission onto pyramidal cells of layer V through the activation of 5-HT2A receptors. These results suggest that GABAergic interneurons, especially FS interneurons, of the cerebral cortex express functional 5-HT2A receptors and are regulated by serotonin in the mouse cortex. Admittedly, these functional studies were, by necessity, conducted on young animals. However the relatively conserved Htr2A gene expression pattern observed during postnatal development in the 5-HT2AR-EGFP mouse suggests these findings should be extensible to adult animals. Overall these observations are consistent with, and extend previous work in rat cortex (Zhou and Hablitz, 1999; Foehring et al., 2002) suggesting a role for serotonin receptors of the 5-HT2 family (subtypes 2A, 2B and 2C) in the control of GABAergic interneurons.
While the results above identify specific cell populations expressing 5-HT2A receptors in the cerebral cortex, they do not address the subcellular localization of these receptors. The strong correspondence between 5-HT2A binding sites and 5-HT2A receptor mRNA has been interpreted to indicate that these receptors are localized to the somatodendritic region, although the precise localization in this compartment is less certain. Previous observations using mostly the G186-1117 antibody (Jakab and Goldman-Rakic, 1998; Wu et al., 1998; Cornea-Hebert et al., 1999), but also other antibodies (Willins et al., 1997; Hamada et al., 1998; McDonald and Mascagni, 2007), have led to the widely accepted idea that 5-HT2A receptors are targeted to the apical dendritic trunk of pyramidal cells. In the current study we detected faint but clearly detectable staining of the pyramidal cell apical trunk and primary dendrites, in addition to the stronger, diffuse laminar staining. A similar staining pattern has also been observed in three additional studies (Hamada et al., 1998; Doly et al., 2004; McDonald and Mascagni, 2007). In our hands the apical dendritic trunk staining was still detectable in the 5-HT2A receptor knockout mice while the laminar staining was completely eliminated (but see Doly et al., 2004), suggesting it does not represent 5-HT2A receptor labeling. These results lead us to conclude that 5-HT2A receptors are neither targeted to the apical dendritic shaft nor uniformly distributed on the apical dendrite of pyramidal cells but rather cluster primarily within a narrow band overlapping layer Va. In the current experiments, we could not resolve the specific cellular elements labeled within this immunoreactive band. Our current hypothesis is that this diffuse labeling may reflect low density expression of 5-HT2A receptors in fine dendritic processes, but other possibilities cannot be ruled out. Ultrastructural studies examining the distribution of 5-HT2A receptors in the middle layers of cortex have reported the labeling of different subcellular compartments depending on the antibody used. Hamada et al. (1998) and Miner et al. (2003) have reported 5-HT2A expression in postsynaptic structures, including dendritic spines. Such a synaptic localization would be consistent with the recent report that 5-HT2A receptor expression is reduced in PSD-95 knockout mice (Abbas et al., 2009) but needs to be confirmed using better characterized antibodies. In contrast, Doly et al. (2004) reported a non-synaptic dendritic localization for these receptors in preliminary studies using an antibody validated using 5-HT2A knockout mice. Clearly further studies are needed to resolve this important issue.
At a functional level, the present results suggest that, far from being widespread regulators of cortical pyramidal neurons, 5-HT2A receptors are much more likely to play a circumscribed role mediating the effects of serotonin in the cerebral cortex. Specifically, the dense and selective localization of these receptors to layer Va strongly suggests a role regulating thalamic inputs to the anterior cerebral cortex. This idea is based upon recent work showing that thalamic input to rodent primary sensory (granular) cortex is divided into two anatomically segregated streams (Brecht, 2007), the lemniscal pathway that originates in the ventral posterior medial nucleus, terminates in layer IV and carries information regarding whisker deflections and the paralemniscal pathways that originates in the posterior medial nucleus, terminates in layer Va and carries information regarding “whisking”, the exploration-related movement of the whiskers. Thalamic inputs to agranular regions in the frontal cortex similarly receive overlapping input from multiple thalamic nuclei and may be similarly organized. Therefore, 5-HT2A receptors, by virtue of their robust and selective expression in layer Va, would appear to be ideally located to mediate the serotonergic modulation of thalamocortical information along the paralemniscal (or comparable) pathway. In addition, the expression of 5-HT2A receptors on FS GABAergic interneurons suggest a role in the regulation of gamma oscillations (Bartos et al., 2007), a possibility that has received independent support from the recent work by Puig et al. (2010) showing that raphe stimulation modulates gamma oscillation in cortex by activating 5-HT1A and also possibly 5-HT2A receptors. Gamma oscillations are thought to provide temporal structure for cortical processing including the possible “binding” of sensory streams into coherent percepts (Bartos et al., 2007 and references therein). Combined, these considerations indicate that 5-HT2A receptors are expressed at loci critical for controlling information processing in cerebral cortex. It is tempting to speculate that the location of 5-HT2A receptors at such sites, if preserved in humans, could contribute to the hallucinogenic activity that is the hallmark of 5-HT2A receptor agonists.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by NIH grant R01MH43985. We would like to thank Drs S. Beck, B.L. Roth, and Z.H. Pan for kindly providing to us anti-5-HT2A antibody samples and Dr. J.-C. Beique for reading the manuscript.
References
- Abbas A. I., Yadav P. N., Yao W. D., Arbuckle M. I., Grant S. G., Caron M. G., Roth B. L. (2009). PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J. Neurosci. 29, 7124–7136 10.1523/JNEUROSCI.1090-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian G. K., Marek G. J. (1997). Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology 36, 589–599 10.1016/S0028-3908(97)00051-8 [DOI] [PubMed] [Google Scholar]
- Araneda R., Andrade R. (1991). 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 40, 399–412 10.1016/0306-4522(91)90128-B [DOI] [PubMed] [Google Scholar]
- Ascoli G. A., Alonso-Nanclares L., Anderson S. A., Barrionuevo G., Benavides-Piccione R., Burkhalter A., Buzsaki G., Cauli B., Defelipe J., Fairen A., Feldmayer D., Fishell G., Fregnac Y., Freund T. F., Gardner D., Gardner E. P., Goldberg J. H., Helmstaedter M., Hestrin S., Karube F., Kisvarday Z. F., Lambolez B., Lewis D. A., Marin O., Markram H., Munoz A., Packer A., Petersen C. C., Rockland K. S., Rossier J., Rudy B., Somogyi P., Staiger J. F., Tamas G., Thomson A. M., Toledo-Rodriguez M., Wang Y., West D. C., Yuste R. (2008). Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 9, 557–568 10.1038/nrn2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom J. R., Sanders-Bush E. (1997). Generation of anti-peptide antibodies against serotonin 5-HT2A and 5-HT2C receptors. J. Neurosci. Methods 77, 109–117 10.1016/S0165-0270(97)00102-7 [DOI] [PubMed] [Google Scholar]
- Barnes N. M., Sharp T. (1999). A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152 10.1016/S0028-3908(99)00010-6 [DOI] [PubMed] [Google Scholar]
- Bartos M., Vida I., Jonas P. (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 8, 45–56 10.1038/nrn2044 [DOI] [PubMed] [Google Scholar]
- Béïque J. C., Campbell B., Perring P., Hamblin M. W., Walker P., Mladenovic L., Andrade R. (2004). Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J. Neurosci. 24, 4807–4817 10.1523/JNEUROSCI.5113-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béïque J. C., Imad M., Mladenovic L., Gingrich J. A., Andrade R. (2007). Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc. Natl. Acad. Sci. U.S.A. 104, 9870–9875 10.1073/pnas.0700436104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue M. E., Yagaloff K. A., Mamounas L. A., Hartig P. R., Molliver M. E. (1988). Correspondence between 5-HT2 receptors and serotonergic axons in rat neocortex. Brain Res. 453, 315–328 10.1016/0006-8993(88)90172-2 [DOI] [PubMed] [Google Scholar]
- Brecht M. (2007). Barrel cortex and whisker-mediated behaviors. Curr. Opin. Neurobiol. 17, 408–416 10.1016/j.conb.2007.07.008 [DOI] [PubMed] [Google Scholar]
- Brownfield M. S., Yracheta J., Chu F., Lorenz D., Diaz A. (1998). Functional chemical neuroanatomy of serotonergic neurons and their targets: antibody production and immunohistochemistry (IHC) for 5-HT, its precursor (5-HTP) and metabolite (5-HIAA), biosynthetic enzyme (TPH), transporter (SERT), and three receptors (5-HT2A, 5-ht5a, 5-HT7). Ann. N. Y. Acad. Sci. 861, 232–233 10.1111/j.1749-6632.1998.tb10195.x [DOI] [PubMed] [Google Scholar]
- Ciaranello R. D., Aimi J., Dean R., Desai R., Garlow S., Heller M. R., Morilak D., Roth B. L. (1993). Developmental regulation of 5-HT2 and 5-HT1c receptor gene expression in rat brain. Psychopharmacol. Ser. 10, 26–37 [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V., Riad M., Wu C., Singh S. K., Descarries L. (1999). Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J. Comp. Neurol. 409, 187–209 [DOI] [PubMed] [Google Scholar]
- de Almeida A. J., Mengod G. (2007). Quantitative analysis of glutamatergic and GABAergic neurons expressing 5-HT(2A) receptors in human and monkey prefrontal cortex. J. Neurochem. 103, 475–486 10.1111/j.1471-4159.2007.04768.x [DOI] [PubMed] [Google Scholar]
- Dean B. (2003). The cortical serotonin2A receptor and the pathology of schizophrenia: a likely accomplice. J. Neurochem. 85, 1–13 10.1046/j.1471-4159.2003.01693.x [DOI] [PubMed] [Google Scholar]
- Doly S., Madeira A., Fischer J., Brisorgueil M. J., Daval G., Bernard R., Verge D., Conrath M. (2004). The 5-HT2A receptor is widely distributed in the rat spinal cord and mainly localized at the plasma membrane of postsynaptic neurons. J. Comp. Neurol. 472, 496–511 10.1002/cne.20082 [DOI] [PubMed] [Google Scholar]
- Feng G., Mellor R. H., Bernstein M., Keller-Peck C., Nguyen Q. T., Wallace M., Nerbonne J. M., Lichtman J. W., Sanes J. R. (2000). Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 10.1016/S0896-6273(00)00084-2 [DOI] [PubMed] [Google Scholar]
- Foehring R. C., van Brederode J. F., Kinney G. A., Spain W. J. (2002). Serotonergic modulation of supragranular neurons in rat sensorimotor cortex. J. Neurosci. 22, 8238–8250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S., Zheng C., Doughty M. L., Losos K., Didkovsky N., Schambra U. B., Nowak N. J., Joyner A., Leblanc G., Hatten M. E., Heintz N. (2003). A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 10.1038/nature02033 [DOI] [PubMed] [Google Scholar]
- Gray J. A., Roth B. L. (2007). The pipeline and future of drug development in schizophrenia. Mol. Psychiatry 12, 904–922 10.1038/sj.mp.4002062 [DOI] [PubMed] [Google Scholar]
- Hamada S., Senzaki K., Hamaguchi-Hamada K., Tabuchi K., Yamamoto H., Yamamoto T., Yoshikawa S., Okano H., Okado N. (1998). Localization of 5-HT2A receptor in rat cerebral cortex and olfactory system revealed by immunohistochemistry using two antibodies raised in rabbit and chicken. Brain Res. Mol. Brain Res. 54, 199–211 10.1016/S0169-328X(97)00322-7 [DOI] [PubMed] [Google Scholar]
- Heintz N. (2001). BAC to the future: the use of bac transgenic mice for neuroscience research. Nat. Rev. Neurosci. 2, 861–870 10.1038/35104049 [DOI] [PubMed] [Google Scholar]
- Hoyer D., Clarke D. E., Fozard J. R., Hartig P. R., Martin G. R., Mylecharane E. J., Saxena P. R., Humphrey P. P. (1994). International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 46, 157–203 [PubMed] [Google Scholar]
- Hoyer D., Pazos A., Probst A., Palacios J. M. (1986). Serotonin receptors in the human brain. II. Characterization and autoradiographic localization of 5-HT1C and 5-HT2 recognition sites. Brain Res. 376, 97–107 10.1016/0006-8993(86)90903-0 [DOI] [PubMed] [Google Scholar]
- Jakab R. L., Goldman-Rakic P. S. (1998). 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc. Natl. Acad. Sci. U.S.A. 95, 735–740 10.1073/pnas.95.2.735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson A., Tinner B., Bancila M., Verge D., Steinbusch H. W., Agnati L. F., Fuxe K. (2001). Relationships of 5-hydroxytryptamine immunoreactive terminal-like varicosities to 5-hydroxytryptamine-2A receptor-immunoreactive neuronal processes in the rat forebrain. J. Chem. Neuroanat. 22, 185–203 10.1016/S0891-0618(01)00133-8 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Kubota Y. (1997). GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex 7, 476–486 10.1093/cercor/7.6.476 [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez J. F., Mengod G., Palacios J. M., Vilaro M. T. (1997). Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Arch. Pharmacol. 356, 446–454 10.1007/PL00005075 [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez J. F., Vilaro M. T., Palacios J. M., Mengod G. (2001). Mapping of 5-HT2A receptors and their mRNA in monkey brain: [3H]MDL100,907 autoradiography and in situ hybridization studies. J. Comp. Neurol. 429, 571–589 [DOI] [PubMed] [Google Scholar]
- Markram H., Toledo-Rodriguez M., Wang Y., Gupta A., Silberberg G., Wu C. (2004). Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807 10.1038/nrn1519 [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz R., Puig M. V., Celada P., Shapiro D. A., Roth B. L., Mengod G., Artigas F. (2001). Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J. Neurosci. 21, 9856–9866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A. J., Mascagni F. (2007). Neuronal localization of 5-HT type 2A receptor immunoreactivity in the rat basolateral amygdala. Neuroscience 146, 306–320 10.1016/j.neuroscience.2007.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengod G., Pompeiano M., Martinez-Mir M. I., Palacios J. M. (1990). Localization of the mRNA for the 5-HT2 receptor by in situ hybridization histochemistry. Correlation with the distribution of receptor sites. Brain Res. 524, 139–143 10.1016/0006-8993(90)90502-3 [DOI] [PubMed] [Google Scholar]
- Miner L. A., Backstrom J. R., Sanders-Bush E., Sesack S. R. (2003). Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience 116, 107–117 10.1016/S0306-4522(02)00580-8 [DOI] [PubMed] [Google Scholar]
- Miyoshi G., Butt S. J., Takebayashi H., Fishell G. (2007). Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J. Neurosci. 27, 7786–7798 10.1523/JNEUROSCI.1807-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak D. A., Garlow S. J., Ciaranello R. D. (1993). Immunocytochemical localization and description of neurons expressing serotonin2 receptors in the rat brain. Neuroscience 54, 701–717 10.1016/0306-4522(93)90241-7 [DOI] [PubMed] [Google Scholar]
- Nocjar C., Roth B. L., Pehek E. A. (2002). Localization of 5-HT(2A) receptors on dopamine cells in subnuclei of the midbrain A10 cell group. Neuroscience 111, 163–176 10.1016/S0306-4522(01)00593-0 [DOI] [PubMed] [Google Scholar]
- Pazos A., Cortes R., Palacios J. M. (1985). Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 346, 231–249 10.1016/0006-8993(85)90857-1 [DOI] [PubMed] [Google Scholar]
- Pompeiano M., Palacios J. M., Mengod G. (1994). Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res. Mol. Brain Res. 23, 163–178 10.1016/0169-328X(94)90223-2 [DOI] [PubMed] [Google Scholar]
- Puig M. V., Watakabe A., Ushimaru M., Yamamori T., Kawaguchi Y. (2010). Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J. Neurosci. 30, 2211–2222 10.1523/JNEUROSCI.3335-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep R. L. (2000). Cortical layer VII and persistent subplate cells in mammalian brains. Brain Behav. Evol. 56, 212–234 10.1159/000047206 [DOI] [PubMed] [Google Scholar]
- Serretti A., Drago A., De R. D. (2007). HTR2A gene variants and psychiatric disorders: a review of current literature and selection of SNPs for future studies. Curr. Med. Chem. 14, 2053–2069 10.2174/092986707781368450 [DOI] [PubMed] [Google Scholar]
- Shen R. Y., Andrade R. (1998). 5-Hydroxytryptamine2 receptor facilitates GABAergic neurotransmission in rat hippocampus. J. Pharmacol. Exp. Ther. 285, 805–812 [PubMed] [Google Scholar]
- Shepherd G. M. G. (2009). Intracortical cartography in an agranular area. Front. Neurosci. 3:30. doi: 10.3389/neuro.01.030.2009. 10.3389/neuro.01.030.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuen J. A., Chen M., Gloss B., Calakos N. (2008). Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J. Neurosci. 28, 2681–2685 10.1523/JNEUROSCI.5492-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos C., Beique J. C., Gingrich J. A., Andrade R. (2005). Serotonergic regulation of calcium-activated potassium currents in rodent prefrontal cortex. Eur. J. Neurosci. 22, 1120–1126 10.1111/j.1460-9568.2005.04307.x [DOI] [PubMed] [Google Scholar]
- Vollenweider F. X., Vollenweider-Scherpenhuyzen M. F. I., Bäbler A., Vogel H., Hell D. (1998). Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9, 3897–3902 10.1097/00001756-199812010-00024 [DOI] [PubMed] [Google Scholar]
- Weisstaub N. V., Zhou M., Lira A., Lambe E., Gonzalez-Maeso J., Hornung J. P., Sibille E., Underwood M., Itohara S., Dauer W. T., Ansorge M. S., Morelli E., Mann J. J., Toth M., Aghajanian G., Sealfon S. C., Hen R., Gingrich J. A. (2006). Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313, 536–540 10.1126/science.1123432 [DOI] [PubMed] [Google Scholar]
- Willins D. L., Deutch A. Y., Roth B. L. (1997). Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse 27, 79–82 [DOI] [PubMed] [Google Scholar]
- Wright D. E., Seroogy K. B., Lundgren K. H., Davis B. M., Jennes L. (1995). Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J. Comp. Neurol. 351, 357–373 10.1002/cne.903510304 [DOI] [PubMed] [Google Scholar]
- Wu C., Yoder E. J., Shih J., Chen K., Dias P., Shi L., Ji X. D., Wei J., Conner J. M., Kumar S., Ellisman M. H., Singh S. K. (1998). Development and characterization of monoclonal antibodies specific to the serotonin 5-HT2A receptor. J. Histochem. Cytochem. 46, 811–824 [DOI] [PubMed] [Google Scholar]
- Yan H. D., Villalobos C., Andrade R. (2009). TRPC channels mediate a muscarinic receptor-induced afterdepolarization in cerebral cortex. J. Neurosci. 29, 10038–10046 10.1523/JNEUROSCI.1042-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. W. (2003). Serotonin induces tonic firing in layer V pyramidal neurons of rat prefrontal cortex during postnatal development. J. Neurosci. 23, 3373–3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F. M., Hablitz J. J. (1999). Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J. Neurophysiol. 82, 2989–2999 [DOI] [PubMed] [Google Scholar]