Abstract
In preclinical studies, human adipose stem cells (ASCs) have been shown to have therapeutic applicability, but standard expansion methods for clinical applications remain yet to be established. ASCs are typically expanded in the medium containing fetal bovine serum (FBS). However, sera and other animal-derived culture reagents stage safety issues in clinical therapy, including possible infections and severe immune reactions. By expanding ASCs in the medium containing human serum (HS), the problem can be eliminated. To define how allogeneic HS (alloHS) performs in ASC expansion compared to FBS, a comparative in vitro study in both serum supplements was performed. The choice of serum had a significant effect on ASCs. First, to reach cell proliferation levels comparable with 10% FBS, at least 15% alloHS was required. Second, while genes of the cell cycle pathway were overexpressed in alloHS, genes of the bone morphogenetic protein receptor–mediated signaling on the transforming growth factor beta signaling pathway regulating, for example, osteoblast differentiation, were overexpressed in FBS. The result was further supported by differentiation analysis, where early osteogenic differentiation was significantly enhanced in FBS. The data presented here underscore the importance of thorough investigation of ASCs for utilization in cell therapies. This study is a step forward in the understanding of these potential cells.
Introduction
Human adipose stem cells (ASCs) are an attractive and abundant cell source that have been shown based on preclinical studies to have therapeutic applicability in diverse fields,1 but standard expansion methods have not yet been established. ASCs are an attractive option for multiple reasons. ASCs can easily be retrieved in high number from either liposuction aspirates or subcutaneous adipose tissue fragments and can readily be expanded in vitro. ASCs are multipotent cells that have multilineage differentiation capacity in vitro.2–11 Further, ASCs have been shown to be immunoprivileged,12–16 to prevent severe graft-versus-host disease in vitro and in vivo17–19 and to be more genetically stable in long-term culture20 than bone marrow stromal/stem cells (BMSCs).21
In vitro expansion of ASCs is necessary before performing clinical studies. Currently, standard cell expansion techniques utilize fetal bovine serum (FBS) and other culture reagents such as trypsin, serum albumin, and growth factors of animal origin as culture medium supplement. However, using animal serum that is by and large uncharacterized22,23 is not an option in clinical cell therapy applications due to several safety issues, such as immune reactions and infections.24–29
Replacing FBS and other animal-derived cell culture reagents with allogeneic human serum (alloHS) and xeno-free culture reagents may significantly enhance the safety and quality of transplanted in-vitro-expanded stem cells.30,31 Replacement of FBS with pooled alloHS, thrombin-activated platelet-rich plasma, or human platelet lysate (PL) is able to support equal or higher proliferation rates and multilineage differentiation capacity of BMSCs32–35 or ASCs.35,36 Moreover, no allogeneic antibodies against HS are found in patients that have received cell transplantation with BMSCs expanded in alloHS, whereas patients who have received cell transplantation with cells expanded in bovine serum exhibit antibodies against bovine antigens.30,37 Autologous HS (autoHS), which is a safe option for clinical applications, has been reported to make BMSCs proliferate faster and differentiate less rapidly than in FBS,21,33,38,39 but the results are conflicting.40 To date, no reports on the influence of autoHS on ASCs have thus far been published. However, the limited availability and high variability hinder the clinical applicability of autologous serum for large-scale stem cell production.41
BMSCs have been studied extensively and several reports have been published on the characteristics of BMSCs cultured in HS as compared to FBS.32–35,39,41,42 During recent years ASCs have gained appeal due to the ease of harvest and shorter expansion times,43–46 but there are yet many characteristics that remain to be investigated in ASCs. Here we report extensive characterization data on proliferation, surface markers, multipotentiality, and gene expression of ASCs expanded in alloHS versus FBS. Clinical stem cell therapy studies using ASCs are under way,1,47 a fact that calls for focus on safety, reproducibility, and quality of transplanted in-vitro-expanded stem cells. Thorough characterization is momentous for the utilization of ASCs in cell therapy applications. The characterization data presented here are an important step toward this goal.
Materials and Methods
The study was conducted in accordance with the Ethics Committee of the Pirkanmaa Hospital District, Tampere, Finland. The ASCs were isolated from adipose tissue samples collected from women (age 48.5 ± 6.6 years) undergoing elective surgical procedures in Tampere University Hospital.
Isolation and culture of ASCs
Isolation was carried out using mechanical and enzymatic isolation as described previously.1,10,44,48 The isolated cells were expanded in the medium containing Dulbecco's modified Eagle's medium/F-12 1:1 (Invitrogen) supplemented with 1% GlutaMAX I (Invitrogen), 1% antibiotics/antimycotic (100 U/mL penicillin, 0.1 mg/mL streptomycin, and 0.25 μg/mL amphotericin B; Invitrogen), and serum from either of these sources: 10% FBS (cat# 10108-165; Invitrogen) or 15% heat-inactivated HS off the clot type of blood groups A and B (AB) (alloHS; PAA Laboratories, cat# C15-021, lot# C02106-1878). ASCs cultured in the medium supplemented with FBS were detached using 1% trypsin (Lonza/Biowhittaker) and ASCs cultured in the medium supplemented with alloHS were detached using TrypLE Select (Invitrogen) to avoid introducing animal-derived substances into the HS cultures. In all analyses, ASCs expanded in both FBS and alloHS were used.
Cell proliferation analysis
The optimal alloHS concentration in the culture medium was assessed by cell viability and proliferation analysis using 10% FBS as reference. The proliferation of ASCs seeded on 48-well plates at a density of 4500 cells/cm2 in 10% FBS, 5% alloHS, 10% alloHS, 15% alloHS, or 20% alloHS (n = 4, donor cell samples/analysis passage 2–4) was assessed at 1, 4, and 7 days using PreMix WST-1 (tetrazolium salt) Cell Proliferation Assay System as described previously.44 Briefly, the cell culture medium was removed and Dulbecco's phosphate buffered saline (DPBS) and PreMix WST-1 (10:1) were added. The well plate was incubated for 4 h at +37°C and the cell proliferation activity was measured in a microplate reader (Victor 1429 Multilabel Counter) at 450 nm. A background control containing DPBS and PreMix WST-1 10:1 was used to evaluate spontaneous absorbance of the reagents in the absence of cells.
Flow cytometric cell cycle analysis
The cell cycle phase distribution was determined in cells cultured in 10% FBS or 15% alloHS (n = 4, passage 3) by staining DNA with propidium iodide as described previously.44 The percentage of cells in G0/G1, S, and G2/M phase was analyzed by flow cytometry data analysis software (ModFit LT 3.0; Verity Software House).
Flow cytometric surface marker expression analysis
The surface marker expression of ASCs expanded in either 10% FBS (n = 15, passages 1–5) or 15% alloHS (n = 15, passages 1–4) was analyzed by flow cytometry (FACSAria®; BDBiosciences) as described previously.1,44,49 The monoclonal antibodies used are listed in Supplemental online data S1 (available online at www.liebertonline.com/ten).
Differentiation analyses
The osteogenic, adipogenic, and chondrogenic induction experiments were performed as previously described.1,44 All differentiation and control cell cultures (n = 7, passages 2–3) were maintained for 14 days in the medium containing either 10% FBS or 15% alloHS. The osteogenic differentiation was detected by quantification of alkaline phosphatase (qALP) and staining of ALP1,49–51 and by quantitative real-time polymerase chain reaction (qRT-PCR) for bone-associated markers runt-related transcription factor 2 (Human Gene Nomenclature Committee [HGNC] name: RUNX2), collagen type I (COL1; HGNC: COL1A1), osteocalcin (OC; HGNC: BGLAP), and osteopontin (OPN; HGNC: SPP1).49,52 Adipogenic induction was detected by Oil red O staining1,44,53 and by qRT-PCR of adipogenic markers fatty acid binding protein 4, adipocyte (aP2, HGNC: FABP4) and peroxisome proliferator-activated receptor gamma (PPARγ, HGNC: PPARG). The names RUNX2, COL1, OC, OPN, aP2 and PPARγ will be used hereafter when referring to these genes. For the qRT-PCR, RNA was extracted from ASCs from osteogenic and adipogenic differentiation and control cultures using the RNeasy Plus Mini Kit (Qiagen) as per the manufacturer's instructions. First-strand cDNA syntheses and qRT-PCR were conducted as described previously49 with the primers designated with a D in Table 1. The mRNA values were normalized to that of the housekeeping gene RPLP0.54,55
Table 1.
Primers for Real-Time Quantitative Polymerase Chain Reaction
| Name | 5′-Sequence-3′ | Product size | Accessionnumber | Analysis | |
|---|---|---|---|---|---|
| hCOL1 | F | CCAGAAGAACTGGTACATCAGCAA | 94 | NM_00088 | D |
| R | CGCCATACTCGAACTGGAATC | ||||
| hOC | F | AGCAAAGGTGCAGCCTTTGT | 63 | NM 000711 | D |
| R | GCGCCTGGGTCTCTTCAC T | ||||
| hOP | F | GCCGACCAAGGAAAACTCACT | 71 | J04765 | D |
| R | GGCACAGGTGATGCCTAGGA | ||||
| hRUNX2 | F | CCCGTGGCCTTCAAGGT | 76 | NM_004348 | D |
| R | CGTTACCCGCCATGACAGTA | ||||
| haP2 | F | GGTGGTGGAATGCGTCATG | 71 | NM_001442 | D |
| R | CAACGTCCCTTGGCTTATGC | ||||
| hPPARγ | F | CAGTGTGAATTACAGCAAACC | 100 | NM_015869 | D |
| R | ACAGTGTATCAGTGAAGGAAT | ||||
| hRPLP0 | F | AATCTCCAGGGGCACCATT | 70 | NM_001002 | D, M |
| R | CGTTGGCTCCCACTTTGT | ||||
| hANGPT1 | F | TGCAAATGTGCCCTCATGTTA | 86 | NM_001146 | M |
| R | TCCCGCAGTATAGAACATTCCA | ||||
| hBMPR1A | F | CACTGCCCCCTGTTGTCATAG | 61 | NM_004329 | M |
| R | GAGCAAAACCAGCCATCGAA | ||||
| hBMPR2 | F | CAGCACTGCGGCTGCTT | 64 | NM_001204 | M |
| R | CTTGCTGATACGGATCTTTAAACG | ||||
| hFABP3 | F | CGGGAGCTAATTGATGGAAAAC | 71 | NM_004102 | M |
| R | CTCATAAGTGCGAGTGCAAACTG | ||||
| hFGF2 | F | CCGACGGCCGAGTTGAC | 89 | NM_002006 | M |
| R | TGATAGACACAACTCCTCTCTCTTCTG | ||||
| hHGF | F | TGCAAGGCTTTTGTTTTTGATAAAG | 102 | NM_000601 | M |
| R | GGTCAAATTCATGGCCAAATTC | ||||
| hIGF1 | F | CCATGTCCTCCTCGCATCTC | 64 | NM_000618 | M |
| R | CGTGGCAGAGCTGGTGAAG | ||||
| hLEP | F | ACAATTGTCACCAGGATCAATGAC | 73 | NM_000230 | M |
| R | TCCAAACCGGTGACTTTCTGT | ||||
| hLTBP1 | F | TGGTCCTGGATGCGTCAGA | 103 | NM_206943 | M |
| R | ACTCAGATGTTCCCAGCACAAA | ||||
| hRARB | F | CAAATCATCAGGGTACCACTATGG | 63 | NM_000965 | M |
| R | CTGAATACTTCTGCGGAAAAAGC | ||||
| hTNFAIP3 | F | AAGCTTGTGGCGCTGAAAA | 78 | NM_006290 | M |
| R | CCTGAACGCCCCACATGTAC |
Primers designated D were used in the differentiation analyses, primers designated M were used in the validation of microarray results.
M, microarray, D, differentiation.
The chondrogenic ASC pellets were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at 5 μm thickness. The sections were stained with Alcian blue (pH 1.0) for detection of sulfated glycosaminoglycans and counterstained with Nuclear Fast Red solution (Biocare Medical).
DNA microarray sample preparation
ASCs from donors (n = 5, passage 3) were cultured until 80% confluent in both 10% FBS and 15% alloHS and collected for the analysis. RNA was extracted using the RNeasy Plus Mini Kit according to the manufacturer's instructions. Two micrograms total RNA was used as starting material and the sample preparation was performed according to the One-Cycle Target Labeling protocol of the chip manufacturer (Affymetrix).56 The total RNA concentrations were checked with Nanodrop ND-1000 both before and after the amplifications, and cRNA quality was controlled by BioRad's Experion electrophoresis station. Each of the 10 samples was hybridized to GeneChip® Human Genome U133 Plus 2.0 Array and the arrays were scanned with the GeneChip Scanner 3000 with AutoLoader. The array data were deposited in NCBI's Gene Expression Omnibus57 at www.ncbi.nlm.nih.gov/geo/ (Series GSE17090).
Microarray data analysis
The microarray data were analyzed in R using Bioconductor (www.bioconductor.org) packages. The raw data were preprocessed to produce robust multiarray average summary measures.58,59 Good data quality and adequate normalization were ensured using several approaches60 (Supplemental online data S2, available online at www.liebertonline.com/ten).
Hierarchical clustering of the robust multiarray average normalized samples was performed using 1 − Pearson correlation as distance measure and the average linkage method. The differentially expressed genes between ASCs expanded in alloHS and FBS were identified using the Linear Models for Microarray Data package61 as instructed for paired samples.62 The p-values were adjusted for multiple testing using the Benjamini & Hochberg's method to control the false discovery rate.63 A threshold of adjusted p (false discovery rate) < 0.05 was used to identify genes with statistically significant differential expression. The genes were further divided into two groups, genes overexpressed and underexpressed in alloHS compared to FBS, according to the average log2 ratio calculated across all the samples.
The differentially expressed genes were analyzed using the database for annotation, visualization, and integrated discovery (DAVID) Functional Annotation Tool (http://david.abcc.ncifcrf.gov) to find enriched associations to Gene Ontology (GO) Biological Processes and to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways.64,65 The tool implements a modified Fisher's exact test [Expression Analysis Systematic Explorer Score (EASE score)].66 All the genes measured by the microarray were used as background population. Biological processes and KEGG pathways with a modified Fisher exact p-value <0.05 were considered significantly enriched. At least 10 genes in the analyzed gene group were required to be associated with each enriched biological process and at least 5 genes with each enriched KEGG pathway.
Validation of microarray genes by qRT-PCR
On the basis of the microarray results, 11 genes were selected for validation with qRT-PCR (Table 1). RNA from the same samples as in the microarray analysis was used. Further, to confirm that the microarray results were not serum lot specific, the same cell lines were expanded in another lot of HS (10% heat-inactivated alloHS; PAA Laboratories, cat# C15-021, lot# C02108-1021). qRT-PCR analysis was performed as described in the Differentiation Analyses section.
Statistics
Mann–Whitney U-test was applied to compare cell cycle phase from the fluorescence-activated cell sorting (FACS) analysis, alkaline phosphatase activity in the qALP assay, and sphere size in the chondrogenic differentiation assay. The analysis was performed using SPSS 13 (SPSS Inc.) and GraphPad Prism 5.01 (GraphPad Software Inc.). Analysis of variance by the methodology of mixed models with Bonferroni post hoc test was used for the proliferation and qRT-PCR data analysis utilizing SPSS. The surface marker expression levels from the FACS analysis were analyzed pairwise with Wilcoxon signed rank test using SPSS. All analyses were performed at the significance level p < 0.05.
Results
Isolation, proliferation, and morphology of ASCs expanded in alloHS versus FBS
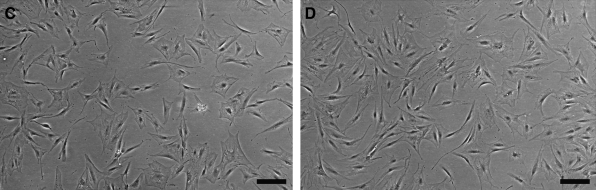
The isolation and expansion of ASCs from adipose tissue samples was successful for all donors irrespective of culture conditions. The HS concentration in the culture medium was optimized with 10% FBS supplemented culture medium as reference. WST-1 viability and proliferation analysis was performed at several time points to assess the proliferation rate of ASCs in different serum supplementation (Fig. 1A). After cell seeding, the proliferation rate (day 1) was similar in all culture conditions (p > 0.05). The ASCs cultured in the medium supplemented with 15% and 20% alloHS exhibited a significant rise in proliferation rate compared to FBS at day 4 (p < 0.001). At day 7, however, ASCs cultured in FBS surpassed ASCs cultured in 5%, 10%, and 15% alloHS in proliferation rate. Only 20% alloHS showed proliferation rates comparable to that of FBS (p > 0.05) (Fig. 1A). According to the proliferation analysis, supplementation with 20% alloHS results in a comparable cell proliferation rate as medium supplementation with 10% FBS. However, due to adequate proliferation rates and to reduce cell culture expenditures, 15% alloHS was used as medium supplementation in all subsequent analyses. Further, cell cycle phase distribution analysis by flow cytometry (Fig. 1B), light microscopic examination (Fig. 1C, D), and time-lapse imaging of the cell cultures (Supplemental videos S3A, B, available online at www.liebertonline.com/ten) supported the use of 15% alloHS, since no significant differences were detected between ASCs cultured in 10% FBS and 15% alloHS in these analyses.
FIG. 1.
Cell proliferation (A) of adipose stem cells (ASCs) in different culture conditions. Cell cycle distribution (B) analyzed by flow cytometry. Light micrographs of ASCs in 10% fetal bovine serum (FBS) (C) and 15% allogeneic human serum (alloHS) (D). Scale bar = 200 μm. The data in all diagrams are presented as mean ± standard deviation (SD). HS, human serum.
Surface marker expression in ASCs expanded in alloHS versus FBS
Flow cytometric analysis was used to compare the surface marker expression characteristics of ASCs cultured in 10% FBS versus 15% alloHS (Supplemental online data S1). Statistical analysis revealed no significant differences in surface marker expression of ASCs cultured in FBS versus alloHS. However, the FACS analysis revealed strong expression (>75%) in both FBS and alloHS of surface molecules CD10, CD13, CD29, CD44, CD90, and CD105. In both FBS and alloHS, moderate expression (<50% but >2%) was observed for CD9, CD34, CD49d, CD166, HLA-ABC, hFSP, and STRO-1. Conversely, in both FBS and alloHS, ASCs lacked (<2%) expression of CD31, CD45, and CD106, suggesting lack of hematopoietic and angiogenic cell lineages.
Multipotentiality of ASCs expanded in alloHS versus FBS
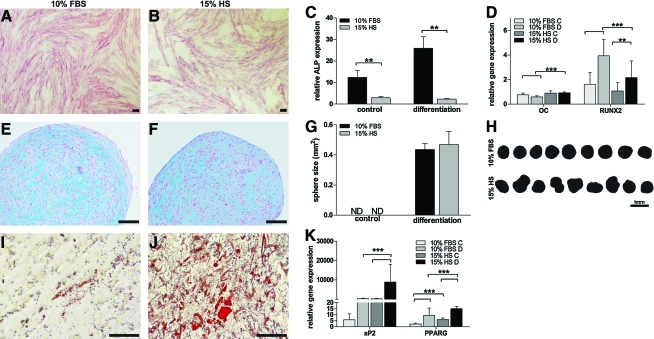
The multilineage differentiation capability of ASCs expanded in FBS and alloHS was examined by culturing cells under conditions that support osteogenic, adipogenic, and chondrogenic differentiation. Histological stainings were performed after 14 days of differentiation induction. In the osteogenic induction cultures, ASCs cultured in FBS exhibited a greater capacity for early osteogenic differentiation than ASCs cultured in alloHS based on ALP staining, qALP (p < 0.01), and qRT-PCR (Fig. 2A–D). ASCs cultured in the presence of FBS exhibited increased expression of the early osteogenic marker RUNX2 compared to ASCs cultured in alloHS after 14 days of differentiation (p < 0.001). ASCs cultured in the presence of alloHS exhibited higher expression levels of OC, although at very low levels (Fig. 2D). Markers of later stages of osteogenic commitment, such as OPN and COL1, were not expressed at detectable levels in either condition (data not shown). In the chondrogenic induction cultures, chondrogenic differentiation was evident at equal levels in both FBS and alloHS as verified by the intensity of the Alcian-blue-stained glycosaminoglycans (Fig. 2E, F). The size of the pellets appeared similar, whereas the morphology of the pellets varied between the conditions (Fig. 2G, H). In the adipogenic induction cultures, oil droplets were visible by light microscopic inspection in both FBS and alloHS cultured ASCs, but considerably more in alloHS conditions (Fig. 2I, J). On the basis of qRT-PCR, ASCs cultured in adipogenic differentiation conditions in the presence of alloHS showed significant upregulation of the adipogenic markers aP2 (p < 0.001) and PPARγ (p < 0.001) compared to ASCs cultured in FBS (Fig. 2K). Further, the adipogenic markers were also significantly upregulated in the undifferentiated control cultures supplemented with alloHS compared to FBS (p < 0.001), which is in line with the microarray results, where PPARγ was overexpressed in alloHS (Supplemental data S4, available online at www.liebertonline.com/ten). Spontaneous adipogenic differentiation was also seen in the osteogenic induction cultures in the presence of alloHS (data not shown).
FIG. 2.
Multipotentiality of ASCs cultured in 10% FBS and 15% alloHS. Alkaline phosphatase staining (A, B), quantitative alkaline phosphatase analysis (C), and quantitative real-time polymerase chain reaction (qRT-PCR) (D) confirming osteogenesis. Alcian blue staining verifying chondrogenesis (E, F), size (G), and sphere morphology (H) of chondrogenic pellet cultures. Oil red O staining (I, J) and qRT-PCR (K) substantiating adipogenesis. Scale bar = 100 μm. C, control; D, differentiation; ND, not detected; **p < 0.01, ***p < 0.001. Brackets without asterisks show similar statistical differences as in the brackets with asterisks above. The data in all diagrams are presented as mean ± SD. Color images available online at www.liebertonline.com/ten.
Gene expression of ASCs expanded in alloHS versus FBS
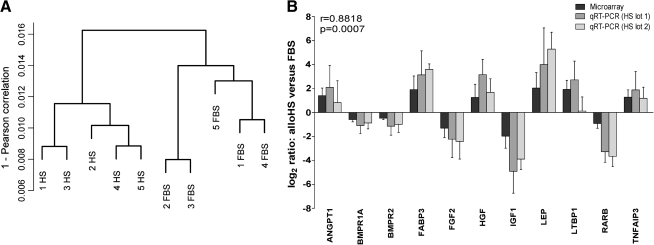
Whole-genome DNA microarray analysis was performed to assess how the serum supplementation of the culture medium affects the gene expression at large in ASCs. Hierarchical clustering performed on the normalized microarray data revealed that the samples clustered according to the serum supplement rather than according to the donor (Fig. 3A), which indicates that the choice of serum has a systematic effect on the ASCs.
FIG. 3.
Hierarchical clustering analysis of microarray data from donor samples (A). Donors are designated with numbers 1–5. FBS = 10% FBS, HS = 15% alloHS. Confirmation of differential gene expression by qRT-PCR (B). Microarray and qRT-PCR measurements were well correlated. The data in the qRT-PCR diagram are presented as mean ± SD.
Out of the total number of approximately 20,100 genes represented on the microarray, 1281 genes (6.4%) were differentially expressed between the conditions (p < 0.05). Of these, 844 genes (4.2%; 1184 probe sets) were overexpressed and 437 genes (2.2%; 652 probe sets) were underexpressed in alloHS compared to FBS (Supplemental online data S4).
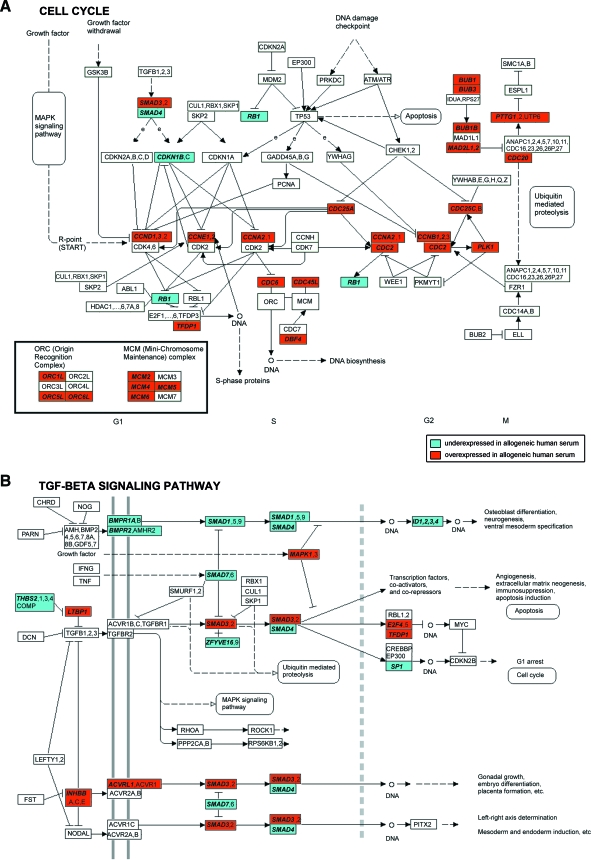
To explore the biological processes regulated through the differentially expressed genes, a GO enrichment analysis was carried out. Table 2 presents the most significantly enriched GO terms, of which many were involved with cell cycle. Further, the most significantly enriched GO term was “Cell cycle”, associated with 160 differentially expressed genes. The full list of enriched GO terms is presented in Supplemental online data S5 (available online at www.liebertonline.com/ten). To further explore the signaling pathways potentially affected by the differences in gene expression, a KEGG pathway enrichment analysis was also performed. The analysis revealed significantly enriched associations to altogether nine human KEGG pathways (Table 3, Supplemental online data S5). The cell cycle pathway and the transforming growth factor beta (TGF-β) signaling pathway were the most significantly enriched. Out of the 33 differentially expressed genes on the cell cycle pathway, 30 genes were overexpressed in alloHS compared to FBS and only 3 were underexpressed (Table 3, Fig. 4A, Supplemental online data S6, available online at www.liebertonline.com/ten). The overexpressed genes included cell division cycle (CDC) genes and cyclins (CCNs), which play important regulatory roles in cell cycle control and many of which are central for cell cycle progression.67,68 Moreover, minichromosome maintenance complex components and origin recognition complex genes, all essential for the genomic DNA replication,69,70 were also overexpressed in alloHS. Some of the differentially expressed genes from the cell cycle pathway are also present on other pathways. For example, the CCN genes CCND1, CCNE1, and CCNE2 are also present on some pathways associated with certain forms of cancer as defined by KEGG. Also the differentially expressed genes IGF1R, MAPK1, PDGFB, PTEN, RB1, and TCF7L2 associated with Cell cycle as defined by GO are associated with certain forms of cancer, such as the melanoma, colorectal or prostate cancer, or acute myeloid leukemia pathways, as defined by KEGG (Table 3; Supplemental online data S5). The consequences of differential expression of these genes were not investigated in this study, but point toward a significant finding that must receive more attention.
Table 2.
Twenty Most Enriched Gene Ontology Biological Process Terms for Genes Expressed Differentially Between Adipose Stem Cells Expanded in Allogeneic Human Serum and Fetal Bovine Serum
| GO term | Genes | p-Value | Genes overexpressed in HS | Genes underexpressed in HS | |
|---|---|---|---|---|---|
| 1 | GO:0007049 Cell cycle | 160 | 1.45E-30 | 130 | 30 |
| 2 | GO:0022403 Cell cycle phase | 89 | 5.90E-27 | 77 | 12 |
| 3 | GO:0022402 Cell cycle process | 137 | 1.73E-26 | 111 | 26 |
| 4 | GO:0000278 Mitotic cell cycle | 83 | 2.23E-26 | 73 | 10 |
| 5 | GO:0000279 M phase | 78 | 4.49E-26 | 70 | 8 |
| 6 | GO:0000087 M phase of mitotic cell cycle | 69 | 8.10E-26 | 63 | 6 |
| 7 | GO:0007067 Mitosis | 68 | 2.74E-25 | 62 | 6 |
| 8 | GO:0051301 Cell division | 68 | 5.65E-24 | 63 | 5 |
| 9 | GO:0000074 Regulation of progression through cell cycle | 85 | 5.70E-13 | 66 | 19 |
| 10 | GO:0051726 Regulation of cell cycle | 85 | 7.78E-13 | 66 | 19 |
| 11 | GO:0006260 DNA replication | 45 | 4.30E-11 | 38 | 7 |
| 12 | GO:0006996 Organelle organization and biogenesis | 140 | 4.96E-11 | 107 | 33 |
| 13 | GO:0000075 Cell cycle checkpoint | 23 | 9.17E-11 | 20 | 3 |
| 14 | GO:0007051 Spindle organization and biogenesis | 14 | 1.27E-10 | 14 | 0 |
| 15 | GO:0007059 Chromosome segregation | 23 | 1.32E-10 | 22 | 1 |
| 16 | GO:0007088 Regulation of mitosis | 23 | 1.80E-09 | 22 | 1 |
| 17 | GO:0007017 Microtubule-based process | 41 | 3.01E-09 | 34 | 7 |
| 18 | GO:0000226 Microtubule cytoskeleton organization and biogenesis | 24 | 3.94E-09 | 18 | 6 |
| 19 | GO:0006259 DNA metabolic process | 96 | 4.93E-09 | 74 | 22 |
| 20 | GO:0000070 Mitotic sister chromatid segregation | 14 | 6.57E-08 | 14 | 0 |
All significantly enriched GO terms and more extensive data are presented in Supplemental online data S5.
GO, Gene Ontology; HS, human serum.
Table 3.
Significantly Enriched Human Kyoto Encyclopedia of Genes and Genomes Pathways for Genes Expressed Differentially Between Adipose Stem Cells Expanded in Allogeneic Human Serum and Fetal Bovine Serum
| KEGG pathway | Genes | p-Value | Genes overexpressed in HS | Genes underexpressed in HS |
|---|---|---|---|---|
| 1 hsa04110 Cell cycle | 33 | 7.62E-11 | 30 | 3 |
| 2 hsa04350 TGF-β signaling pathway | 19 | 1.58E-04 | 7 | 12 |
| 3 hsa04115 p53 signaling pathway | 16 | 2.33E-04 | 12 | 4 |
| 4 hsa05218 Melanoma | 15 | 1.07E-03 | 6 | 9 |
| 5 hsa04540 Gap junction | 16 | 6.48E-03 | 10 | 6 |
| 6 hsa04520 Adherens junction | 13 | 1.36E-02 | 6 | 7 |
| 7 hsa05210 Colorectal cancer | 14 | 1.53E-02 | 6 | 8 |
| 8 hsa05215 Prostate cancer | 14 | 2.19E-02 | 4 | 10 |
| 9 hsa05221 Acute myeloid leukemia | 10 | 2.91E-02 | 4 | 6 |
More extensive data are presented in Supplemental online data S5.
KEGG, Kyoto Encyclopedia of Genes and Genomes; TGF-β, transforming growth factor beta.
FIG. 4.
Observation of the differentially expressed genes on the human cell cycle (A) and transforming growth factor beta (TGF-β) signaling pathways (B) modified from Kyoto Encyclopedia of Genes and Genomes (KEGG) (www.genome.jp/kegg/kegg1.html). The gene names were mapped to the pathway based on information provided by KEGG. Differentially expressed genes are denoted in bold italics. Color images available online at www.liebertonline.com/ten.
The TGF-β signaling pathway affects proliferation and differentiation of human mesenchymal stem cells.71 Altogether 19 genes associated with this pathway were differentially expressed between the culture conditions (Table 3; Fig. 4B; Supplemental online data S6). Genes of the bone morphogenetic protein (BMP) receptor–mediated signaling pathway, including the receptors BMPR1A and 2, the signal transducers SMAD1 and 4, and the transcriptional regulators ID1, 2, 3, and 4 had lower expression in ASCs cultured in alloHS than in FBS. Conversely, some genes of the TGF-β-receptor-mediated signaling pathway were overexpressed in alloHS compared to FBS. These included LTBP1, a gene whose protein product transfers TGF-β to its activation site,72 the signal transducer SMAD3, and the transcription factors E2F4 and TFDP1.
Since various fold changes in gene expression are required for different biological effects, we primarily considered all the genes with a statistically significant change (p < 0.05) as differentially expressed regardless of their fold change. To validate our approach, we performed the enrichment analyses on such significantly differentially expressed genes (p < 0.05) that had a greater than twofold change in expression. In this analysis, the most significantly enriched GO terms were similarly involved with cell cycle. Moreover, the cell cycle pathway and the TGF-β signaling pathway from KEGG were found significantly enriched (p < 0.05). The similarity of the enrichment results with both approaches implies that also the genes with smaller fold changes are relevant since they are associated with the same processes and pathways as the genes with greater fold changes.
Confirmation by qRT-PCR
Selected differentially expressed genes were confirmed by qRT-PCR based on their role in ASC metabolism and differentiation. Microarray and qRT-PCR demonstrated good correlation (r = 0.8818, p = 0.0007) in differential gene expression of ASCs cultured in HS (lot1) versus FBS. Further, the results were confirmed with another lot of HS (lot2) with strong correlation to the lot1 qRT-PCR results (r = 0.9545, p < 0.0001) (Fig. 3B); thus, it could be excluded that the alloHS serum lot affects the gene expression results.
Discussion
Safety, efficacy, and quality are key criteria for clinical applications using adult stem cells. Cells aimed for clinical therapy cultured in FBS pose a risk of infection and severe immune reactions in the recipient, a fact that makes FBS an unsuitable option with respect to patient safety.22 Replacement of FBS with HS derivatives, such as allogeneic AB serum, platelet-rich plasma, and human platelet lysate (PL), have been reported to support equal or higher proliferation rates and multilineage differentiation capacity of BMSCs21,32–35,39,41,42,73 or ASCs.31,35,74 AutoHS, which a priori is a reasonable option for clinical applications, has, nevertheless, been shown to have conflicting effects on the proliferation and differentiation capacity of BMSCs.40 Moreover, autoHS may not always be available in large enough quantities for clinical cell expansion. To date, no reports to verify its effect on ASCs have been published.
To define how alloHS performs in expansion of ASCs compared to FBS, thorough characterization of cells expanded in the presence of these serum supplements was carried out. The viability and proliferation analyses demonstrated differences between the culture conditions, requiring at least 15% alloHS supplementation as compared to 10% FBS to reach comparable proliferation rate. The 15% alloHS level was selected based on the satisfactory proliferation rates, cell morphology, and cell cycle analysis by flow cytometry, but also to not unduly raise cell culture expenditures.
Thorough characterization of stem cells is essential before clinical use, because different culture conditions change the characteristics of stem cells. The flow cytometric analysis revealed no significant differences in surface markers between 15% alloHS as compared to 10% FBS culture conditions, which also conforms to results reported both for BMSCs33,41 and ASCs.35,36 However, only one report has been published on surface marker expression of ASCs expanded in alloHS versus FBS.36 Nevertheless, the flow cytometric data comply with previous results for ASCs expanded in FBS10,43 with expression of markers verifying the mesenchymal origin of cells and the lack of hematopoietic and angiogenic markers in both culture conditions.
Nevertheless, the ASCs expanded in alloHS and FBS exhibited differences in gene expression. In the microarray data, genes present among other on the TGF-β signaling pathway were differentially expressed. Genes on this pathway control many biological processes such as embryogenesis, morphogenesis of tissues like bone and cartilage, and angiogenesis.75,76 Signaling of the TGF-β superfamily is mediated by related serine/threonine kinase receptors BMP, TGF-β, Activin, and Nodal receptors.77,78 Interestingly, in our data, genes related to the BMP-receptor-mediated signaling pathway affecting osteoblast differentiation79 were underexpressed in alloHS compared to FBS, whereas many genes related to the TGF-β-receptor-mediated pathway, affecting, for example, apoptosis, and cell cycle,80 were overexpressed in alloHS. Although the differentiation potential toward the chondrogenic lineages appeared similar in both culture conditions, early osteogenic differentiation was enhanced in ASCs expanded in FBS. This result conforms to the gene expression differences on the BMP-receptor-mediated signaling pathway detected in the microarray analysis. The differentiation analysis and microarray analysis also supported one another in pointing toward an enhanced adipogenic differentiation capacity of ASCs cultured in alloHS.
FBS is known to be rich in growth factors and stimulates protein accretion in cell cultures81,82; thus, the growth factors in the serum may have supported expression of genes related to the BMP-receptor-mediated pathway in the ASCs, leading to the activation of extracellular matrix genes, which in turn influence the matrix deposition.71,79,83,84 Recent reports also indicate that ID genes may be downstream targets in the TGF-β and BMP receptor pathways and that ID1, −2, and −3 expression promote the proliferation of early osteoblast progenitor cells.85,86 In our data, all known ID genes and genes of the BMP signaling cascade inducing these genes were underexpressed in alloHS-expanded ASCs. Further, genes of the TGF-β signaling cascade repressing ID genes, in particular SMAD3, had higher expression in alloHS. This result may be coupled to the enhanced osteogenic differentiation in FBS, although the precise molecular function of the ID proteins in BMP-induced osteogenic differentiation in ASCs remains to be defined. Overall, based on these findings, and the fact that FBS contains higher levels of BMP-4, −6, and −9 than alloHS,82 we find it reasonable to speculate that ASCs expanded in HS aimed for clinical bone tissue engineering may require supplementation with growth factors of the TGF-β family such as BMP-2, −6, −7, and −9, to enhance the osteogenic differentiation. Reports with similar observations have been published,1,87,88 but further studies are still needed. Further, supplementation with BMP-2 may also reduce the level of spontaneous adipogenic differentiation seen in alloHS supplemented cultures and may thus simultaneously enhance the osteogenic differentiation. Studies on BMSCs have shown that exposure to BMP-2 inhibits adipogenic differentiation by enhancing osteogenesis through elevated expression levels of ALP, RUNX2, type I procollagen, and OC and by increased mineralized nodule formation.89,90 To date, no reports have been published confirming whether BMP-2 has a similar effect on ASCs, and this remains to be examined.
Although the differentiation capacity of ASCs has been studied,10,91,93 little attention has been addressed on the cell-cycle-related events controlling proliferation and differentiation of these cells.94 Altogether, 160 genes associated with the GO term Cell cycle were found to be differentially expressed between the culture conditions. A considerable amount of these, 130 genes, were overexpressed in ASCs expanded in alloHS, among which several key regulators of the cell cycle, such as the CCNs and CDCs, were detected. Similarly, most of the differentially expressed genes on the KEGG cell cycle pathway were overexpressed in alloHS, including several genes central for DNA replication and cell cycle progression. Nevertheless, no distinct differences between 10% FBS and 15% alloHS culture conditions could be detected in the cell cycle analysis by flow cytometry. Since alloHS contains lower levels of growth factors than FBS,82 increasing the alloHS concentration in the culture medium speeds up the cell proliferation and consequently cell cycle progression. This may give an indication as to why no differences could be detected in the cell cycle phase analysis by flow cytometry between the two culture conditions. Alternatively, the higher levels of alloHS added to the culture medium may have affected the cell cycle by activating genes related to DNA synthesis. The serum concentration in the expansion medium may modify cell behavior and gene expression as shown in a study on murine BMSCs53; however, so far no reports have been published establishing how the cell behavior and gene expression of human ASCs is affected by the concentration of HS in the culture medium. The effects of serum concentration should be investigated on human ASCs, preferably by illuminating the progression of the cell cycle pathway. The cell cycle is a complex and strictly regulated process, where several factors act in concert for the timely progression of each cell cycle step. Such data may therefore prove useful when selecting cell culture conditions for ASCs in clinical applications.
Several of the differentially expressed genes involved in the process of cell cycle were also associated to various so-called cancer pathways as defined by KEGG. For example, melanoma, colorectal, prostate, or acute myeloid leukemia cancer pathways also contain the differentially expressed genes IGF1R, MAPK1, PDGFB, PTEN, RB1, and TCF7L2. Also, the CCN genes CCND1, CCNE1, and CCNE2 appear on cancer pathways as delimited in KEGG. These genes are all involved in normal cellular processes; however, the expression levels of the genes determine the fate of the cell, whether the cells will become normal or become cancerous. Rather, the cell behavior and gene expression seen in ASCs cultured in alloHS more likely corresponds to normal physiological conditions than FBS. The pleiotropic effect of these genes is interesting and requires to be explored further.
ASCs have been shown to be good candidates for cell-based therapies due to their capacity to survive in a low oxygen environment95–97 by secreting angiogenic cytokines such as vascular endothelial growth factor and hematopoietic growth factor,11,98,99 perhaps to a higher degree than BMSCs.100 Nevertheless, despite having apparently optimal characteristics for clinical applications, that is, showing immunomodulatory properties and protective effects in immunological disorders16 in addition to the angiogenic properties, these properties may have the opposite effect. Reports have shown that ASCs may in some cases favor the growth of tumor cells,34,101 but contradictory results exist102,103 and the question is a matter of intense debate. These contradictory results indicate that the investigation of the molecular characteristics of ASCs is in early stages, and further studies and consensual protocols are necessary to fully clarify the true effect of ASCs on tumor formation.
Conclusions
The data presented here show differences in expression of genes central for cell cycle progression and cell differentiation in ASCs expanded in alloHS versus FBS. ASCs cultured in alloHS and FBS have comparable proliferation characteristics and a similar cell surface marker phenotype, but some fundamental differences in the gene expression profile exist. Clinical stem cell therapy studies using ASCs are under way,1,47 which calls for focus on safety, efficacy, and quality of in-vitro-expanded cells. Thorough characterization of the cells to be used is important for successful stem cell therapy. This study is an important step toward this goal.
Supplementary Material
Acknowledgments
The authors thank Ms. Miia Juntunen, Ms. Anna-Maija Honkala, Ms. Minna Salomäki, and Ms. Sari Kalliokoski for technical assistance and Mr. Henrik Mannerström for graphic processing consultation. We also thank Christophe Roos, Ph.D., Jarno Tuimala, Ph.D., Reija Autio, Dr. Tech, and Jari Niemi, M.Sc., for valuable advice in the microarray analysis. The work was supported by TEKES, the Finnish Funding Agency for Technology and Innovation, the competitive research funding of the Pirkanmaa Hospital District, the Finnish Cultural Foundation Pirkanmaa Provincial Foundation, Tampere Graduate School in Information Science and Engineering, and the Academy of Finland (application number 129657, Finnish Programme for Centres of Excellence in Research 2006–2011).
Disclosure Statement
No competing financial interests exist.
References
- 1.Mesimäki K. Lindroos B. Törnwall J. Mauno J. Lindqvist C. Kontio R. Miettinen S. Suuronen R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Zuk P.A. Zhu M. Ashjian P. De Ugarte D.A. Huang J.I. Mizuno H. Alfonso Z.C. Fraser J.K. Benhaim P. Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuk P.A. Zhu M. Mizuno H. Huang J. Futrell J.W. Katz A.J. Benhaim P. Lorenz H.P. Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 4.Winter A. Breit S. Parsch D. Benz K. Steck E. Hauner H. Weber R.M. Ewerbeck V. Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 5.Timper K. Seboek D. Eberhardt M. Linscheid P. Christ-Crain M. Keller U. Muller B. Zulewski H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006;341:1135. doi: 10.1016/j.bbrc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 6.Seo M.J. Suh S.Y. Bae Y.C. Jung J.S. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- 7.Safford K.M. Hicok K.C. Safford S.D. Halvorsen Y.D. Wilkison W.O. Gimble J.M. Rice H.E. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;294:371. doi: 10.1016/S0006-291X(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 8.Planat-Benard V. Silvestre J.S. Cousin B. Andre M. Nibbelink M. Tamarat R. Clergue M. Manneville C. Saillan-Barreau C. Duriez M. Tedgui A. Levy B. Penicaud L. Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 9.Huang J.I. Zuk P.A. Jones N.F. Zhu M. Lorenz H.P. Hedrick M.H. Benhaim P. Chondrogenic potential of multipotential cells from human adipose tissue. Plast Reconstr Surg. 2004;113:585. doi: 10.1097/01.PRS.0000101063.27008.E1. [DOI] [PubMed] [Google Scholar]
- 10.Gimble J. Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 11.Gimble J.M. Katz A.J. Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui L. Yin S. Liu W. Li N. Zhang W. Cao Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007;13:1185. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 13.Niemeyer P. Vohrer J. Schmal H. Kasten P. Fellenberg J. Suedkamp N.P. Mehlhorn A.T. Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy. 2008;10:784. doi: 10.1080/14653240802419302. [DOI] [PubMed] [Google Scholar]
- 14.McIntosh K. Zvonic S. Garrett S. Mitchell J.B. Floyd Z.E. Hammill L. Kloster A. Di Halvorsen Y. Ting J.P. Storms R.W. Goh B. Kilroy G. Wu X. Gimble J.M. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Rey E. Gonzalez M.A. Rico L. Buscher D. Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;98:929. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Rey E. Gonzalez M.A. Varela N. O'Valle F. Hernandez-Cortes P. Rico L. Buscher D. Delgado M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T-cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69:241. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- 17.Yanez R. Lamana M.L. Garcia-Castro J. Colmenero I. Ramirez M. Bueren J.A. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 18.Puissant B. Barreau C. Bourin P. Clavel C. Corre J. Bousquet C. Taureau C. Cousin B. Abbal M. Laharrague P. Penicaud L. Casteilla L. Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus H. Curtin P. Devine S. McCarthy P. Holland K. Moseley A. Bacigalupo A. Role of mesenchymal stem cells (MSC) in allogeneic transplantation: early phase I clinical results. Blood. 2000;96:392A. (meeting abstract) [Google Scholar]
- 20.Meza-Zepeda L.A. Noer A. Dahl J.A. Micci F. Myklebost O. Collas P. High-resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence. J Cell Mol Med. 2008;12:553. doi: 10.1111/j.1582-4934.2007.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahl J.A. Duggal S. Coulston N. Millar D. Melki J. Shahdadfar A. Brinchmann J.E. Collas P. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. Int J Dev Biol. 2008;52:1033. doi: 10.1387/ijdb.082663jd. [DOI] [PubMed] [Google Scholar]
- 22.Gstraunthaler G. Alternatives to the use of fetal bovine serum: serum-free cell culture. Altex. 2003;20:275. [PubMed] [Google Scholar]
- 23.Price P.J. Gregory E.A. Relationship between in vitro growth promotion and biophysical and biochemical properties of the serum supplement. In Vitro. 1982;18:576. doi: 10.1007/BF02810081. [DOI] [PubMed] [Google Scholar]
- 24.Spees J.L. Gregory C.A. Singh H. Tucker H.A. Peister A. Lynch P.J. Hsu S.C. Smith J. Prockop D.J. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9:747. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Heiskanen A. Satomaa T. Tiitinen S. Laitinen A. Mannelin S. Impola U. Mikkola M. Olsson C. Miller-Podraza H. Blomqvist M. Olonen A. Salo H. Lehenkari P. Tuuri T. Otonkoski T. Natunen J. Saarinen J. Laine J. N-glycolylneuraminic acid xenoantigen contamination of human embryonic and mesenchymal stem cells is substantially reversible. Stem Cells. 2007;25:197. doi: 10.1634/stemcells.2006-0444. [DOI] [PubMed] [Google Scholar]
- 26.Martin M.J. Muotri A. Gage F. Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 27.Mackensen A. Drager R. Schlesier M. Mertelsmann R. Lindemann A. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol Immunother. 2000;49:152. doi: 10.1007/s002620050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selvaggi T.A. Walker R.E. Fleisher T.A. Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood. 1997;89:776. [PubMed] [Google Scholar]
- 29.Will R.G. Ironside J.W. Zeidler M. Cousens S.N. Estibeiro K. Alperovitch A. Poser S. Pocchiari M. Hofman A. Smith P.G. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347:921. doi: 10.1016/s0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- 30.Le Blanc K. Samuelsson H. Lonnies L. Sundin M. Ringden O. Generation of immunosuppressive mesenchymal stem cells in allogeneic human serum. Transplantation. 2007;84:1055. doi: 10.1097/01.tp.0000285088.44901.ea. [DOI] [PubMed] [Google Scholar]
- 31.Kim S.J. Cho H.H. Kim Y.J. Seo S.Y. Kim H.N. Lee J.B. Kim J.H. Chung J.S. Jung J.S. Human adipose stromal cells expanded in human serum promote engraftment of human peripheral blood hematopoietic stem cells in NOD/SCID mice. Biochem Biophys Res Commun. 2005;329:25. doi: 10.1016/j.bbrc.2005.01.092. [DOI] [PubMed] [Google Scholar]
- 32.Schallmoser K. Bartmann C. Rohde E. Reinisch A. Kashofer K. Stadelmeyer E. Drexler C. Lanzer G. Linkesch W. Strunk D. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 33.Shahdadfar A. Fronsdal K. Haug T. Reinholt F.P. Brinchmann J.E. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357. doi: 10.1634/stemcells.2005-0094. [DOI] [PubMed] [Google Scholar]
- 34.Yu J.M. Jun E.S. Bae Y.C. Jung J.S. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008;17:463. doi: 10.1089/scd.2007.0181. [DOI] [PubMed] [Google Scholar]
- 35.Mirabet V. Solves P. Minana M.D. Encabo A. Carbonell-Uberos F. Blanquer A. Roig R. Human platelet lysate enhances the proliferative activity of cultured human fibroblast-like cells from different tissues. Cell Tissue Bank. 2008;9:1. doi: 10.1007/s10561-007-9048-x. [DOI] [PubMed] [Google Scholar]
- 36.Kocaoemer A. Kern S. Kluter H. Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 2007;25:1270. doi: 10.1634/stemcells.2006-0627. [DOI] [PubMed] [Google Scholar]
- 37.Sundin M. Ringden O. Sundberg B. Nava S. Gotherstrom C. Le Blanc K. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007;92:1208. doi: 10.3324/haematol.11446. [DOI] [PubMed] [Google Scholar]
- 38.Parker A.M. Shang H. Khurgel M. Katz A.J. Low serum and serum-free culture of multipotential human adipose stem cells. Cytotherapy. 2007;9:637. doi: 10.1080/14653240701508452. [DOI] [PubMed] [Google Scholar]
- 39.Nimura A. Muneta T. Koga H. Mochizuki T. Suzuki K. Makino H. Umezawa A. Sekiya I. Increased proliferation of human synovial mesenchymal stem cells with autologous human serum: comparisons with bone marrow mesenchymal stem cells and with fetal bovine serum. Arthritis Rheum. 2008;58:501. doi: 10.1002/art.23219. [DOI] [PubMed] [Google Scholar]
- 40.Stute N. Holtz K. Bubenheim M. Lange C. Blake F. Zander A.R. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp Hematol. 2004;32:1212. doi: 10.1016/j.exphem.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Bieback K. Hecker A. Kocaomer A. Lannert H. Schallmoser K. Strunk D. Kluter H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27:2331. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- 42.Muller I. Kordowich S. Holzwarth C. Spano C. Isensee G. Staiber A. Viebahn S. Gieseke F. Langer H. Gawaz M.P. Horwitz E.M. Conte P. Handgretinger R. Dominici M. Animal serum-free culture conditions for isolation and expansion of multipotent mesenchymal stromal cells from human BM. Cytotherapy. 2006;8:437. doi: 10.1080/14653240600920782. [DOI] [PubMed] [Google Scholar]
- 43.Strem B.M. Hicok K.C. Zhu M. Wulur I. Alfonso Z. Schreiber R.E. Fraser J.K. Hedrick M.H. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 44.Lindroos B. Boucher S. Chase L. Kuokkanen H. Huhtala H. Haataja R. Vemuri M. Suuronen R. Miettinen S. Serum-free, xeno-free culture media maintain the proliferation rate and multipotentiality of adipose stem cells in vitro. Cytotherapy. 2009;11:958. doi: 10.3109/14653240903233081. [DOI] [PubMed] [Google Scholar]
- 45.Zuk P.A. Tissue engineering craniofacial defects with adult stem cells? Are we ready yet? Pediatr Res. 2008;63:478. doi: 10.1203/PDR.0b013e31816bdf36. [DOI] [PubMed] [Google Scholar]
- 46.Strem B.M. Hedrick M.H. The growing importance of fat in regenerative medicine. Trends Biotechnol. 2005;23:64. doi: 10.1016/j.tibtech.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Lendeckel S. Jodicke A. Christophis P. Heidinger K. Wolff J. Fraser J.K. Hedrick M.H. Berthold L. Howaldt H.P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Niemelä S.M. Miettinen S. Konttinen Y. Waris T. Kellomäki M. Ashammakhi N.A. Ylikomi T. Fat tissue: views on reconstruction and exploitation. J Craniofac Surg. 2007;18:325. doi: 10.1097/scs.0b013e3180333b6a. [DOI] [PubMed] [Google Scholar]
- 49.Lindroos B. Mäenpää K. Ylikomi T. Oja H. Suuronen R. Miettinen S. Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem Biophys Res Commun. 2008;368:329. doi: 10.1016/j.bbrc.2008.01.081. [DOI] [PubMed] [Google Scholar]
- 50.Haimi S. Suuriniemi N. Haaparanta A.M. Ellä V. Lindroos B. Huhtala H. Räty S. Kuokkanen H. Sandor G.K. Kellomäki M. Miettinen S. Suuronen R. Growth and osteogenic differentiation of adipose stem cells on PLA/bioactive glass and PLA/beta-TCP scaffolds. Tissue Eng Part A. 2009;15:1473. doi: 10.1089/ten.tea.2008.0241. [DOI] [PubMed] [Google Scholar]
- 51.Shi Y.Y. Nacamuli R.P. Salim A. Longaker M.T. The osteogenic potential of adipose-derived mesenchymal cells is maintained with aging. Plast Reconstr Surg. 2005;116:1686. doi: 10.1097/01.prs.0000185606.03222.a9. [DOI] [PubMed] [Google Scholar]
- 52.Madras N. Gibbs A.L. Zhou Y. Zandstra P.W. Aubin J.E. Modeling stem cell development by retrospective analysis of gene expression profiles in single progenitor-derived colonies. Stem Cells. 2002;20:230. doi: 10.1634/stemcells.20-3-230. [DOI] [PubMed] [Google Scholar]
- 53.Wu L. Cai X. Dong H. Jing W. Huang Y. Yang X. Wu Y. Lin Y. Serum regulates adipogenesis of mesenchymal stem cells via MEK/ERK dependent PPARgamma expression and phosphorylation. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00709.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fink T. Lund P. Pilgaard L. Rasmussen J.G. Duroux M. Zachar V. Instability of standard PCR reference genes in adipose-derived stem cells during propagation, differentiation and hypoxic exposure. BMC Mol Biol. 2008;9:98. doi: 10.1186/1471-2199-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steinau M. Rajeevan M.S. Unger E.R. DNA and RNA references for qRT-PCR assays in exfoliated cervical cells. J Mol Diagn. 2006;8:113. doi: 10.2353/jmoldx.2006.050088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Affymetrix Technical Manual. GeneChip Expression Analysis Technical Manual. Santa Clara, CA: Affymetrix Inc; 2009. [Google Scholar]
- 57.Edgar R. Domrachev M. Lash A.E. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Irizarry R.A. Bolstad B.M. Collin F. Cope L.M. Hobbs B. Speed T.P. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Irizarry R.A. Hobbs B. Collin F. Beazer-Barclay Y.D. Antonellis K.J. Scherf U. Speed T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 60.Parman C. Halling C. affyQCReport: A Package to Generate QC Reports for Affymetrix Array Data. Bioconductor Package Help Documentation. 2008.
- 61.Smyth G.K. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 62.Smyth G.K. Ritchie M. Thorne N. Wettenhall J. Bioconductor Package Help Documentation. Melbourne: 2007. LIMMA: Linear Models for Microarray Data User's Guide. [Google Scholar]
- 63.Benjamini Y. Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165. [Google Scholar]
- 64.Dennis G., Jr. Sherman B.T. Hosack D.A. Yang J. Gao W. Lane H.C. Lempicki R.A. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 65.Huang da W. Sherman B.T. Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 66.Hosack D.A. Dennis G., Jr. Sherman B.T. Lane H.C. Lempicki R.A. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murray A.W. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221. doi: 10.1016/s0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- 68.Malumbres M. Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 69.Bryant J.A. Moore K. Aves S.J. Origins and complexes: the initiation of DNA replication. J Exp Bot. 2001;52:193. [PubMed] [Google Scholar]
- 70.Prasanth S.G. Mendez J. Prasanth K.V. Stillman B. Dynamics of pre-replication complex proteins during the cell division cycle. Philos Trans R Soc Lond B Biol Sci. 2004;359:7. doi: 10.1098/rstb.2003.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou S. Eid K. Glowacki J. Cooperation between TGF-beta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res. 2004;19:463. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]
- 72.Oklu R. Hesketh R. The latent transforming growth factor beta binding protein (LTBP) family. Biochem J. 2000;352(Pt 3):601. [PMC free article] [PubMed] [Google Scholar]
- 73.Caterson E.J. Nesti L.J. Danielson K.G. Tuan R.S. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol. 2002;20:245. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- 74.Katz A.J. Parker A.M. Methods and compositions for growing adipose stem cells. 2006:69. WO 2007/030652 A2. [Google Scholar]
- 75.Barbara N.P. Wrana J.L. Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem. 1999;274:584. doi: 10.1074/jbc.274.2.584. [DOI] [PubMed] [Google Scholar]
- 76.Massague J. Chen Y.G. Controlling TGF-beta signaling. Genes Dev. 2000;14:627. [PubMed] [Google Scholar]
- 77.Massague J. Receptors for the TGF-beta family. Cell. 1992;69:1067. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- 78.Massague J. Cheifetz S. Boyd F.T. Andres J.L. TGF-beta receptors and TGF-beta binding proteoglycans: recent progress in identifying their functional properties. Ann N Y Acad Sci. 1990;593:59. doi: 10.1111/j.1749-6632.1990.tb16100.x. [DOI] [PubMed] [Google Scholar]
- 79.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 80.Heldin C.-H. Landström M. Moustakas A. Mechanism of TGF-[beta] signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr Opin Cell Biol. 2009;21:166. doi: 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 81.Muramatsu T. Pinontoan R. Okumura J. Biopotency of fetal bovine serum, and insulin and insulin-like growth factors I and II in enhancing whole-body protein synthesis of chicken embryos cultured in vitro. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1995;111:281. doi: 10.1016/0742-8413(95)00049-t. [DOI] [PubMed] [Google Scholar]
- 82.Herrera B. Inman G.J. A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins. Identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC Cell Biol. 2009;10:20. doi: 10.1186/1471-2121-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim H.-J. Im G.-I. Combination of transforming growth factor-beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A. 2009;15:1943. doi: 10.1089/ten.tea.2008.0368. [DOI] [PubMed] [Google Scholar]
- 84.Toom A. Arend A. Gunnarsson D. Ulfsparre R. Suutre S. Haviko T. Selstam G. Bone formation zones in heterotopic ossifications: histologic findings and increased expression of bone morphogenetic protein 2 and transforming growth factors beta2 and beta3. Calcif Tissue Int. 2007;80:259. doi: 10.1007/s00223-007-9000-x. [DOI] [PubMed] [Google Scholar]
- 85.Kang Y. Chen C.R. Massague J. A self-enabling TGFbeta response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol Cell. 2003;11:915. doi: 10.1016/s1097-2765(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 86.Peng Y. Kang Q. Luo Q. Jiang W. Si W. Liu B.A. Luu H.H. Park J.K. Li X. Luo J. Montag A.G. Haydon R.C. He T.C. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:32941. doi: 10.1074/jbc.M403344200. [DOI] [PubMed] [Google Scholar]
- 87.Dragoo J.L. Lieberman J.R. Lee R.S. Deugarte D.A. Lee Y. Zuk P.A. Hedrick M.H. Benhaim P. Tissue-engineered bone from BMP-2-transduced stem cells derived from human fat. Plast Reconstr Surg. 2005;115:1665. doi: 10.1097/01.prs.0000161459.90856.ab. [DOI] [PubMed] [Google Scholar]
- 88.Jeon O. Rhie J.W. Kwon I.K. Kim J.H. Kim B.S. Lee S.H. In vivo bone formation following transplantation of human adipose-derived stromal cells that are not differentiated osteogenically. Tissue Eng Part A. 2008;14:1285. doi: 10.1089/ten.tea.2007.0253. [DOI] [PubMed] [Google Scholar]
- 89.Gimble J.M. Morgan C. Kelly K. Wu X. Dandapani V. Wang C.S. Rosen V. Bone morphogenetic proteins inhibit adipocyte differentiation by bone marrow stromal cells. J Cell Biochem. 1995;58:393. doi: 10.1002/jcb.240580312. [DOI] [PubMed] [Google Scholar]
- 90.Gori F. Thomas T. Hicok K.C. Spelsberg T.C. Riggs B.L. Differentiation of human marrow stromal precursor cells: bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J Bone Miner Res. 1999;14:1522. doi: 10.1359/jbmr.1999.14.9.1522. [DOI] [PubMed] [Google Scholar]
- 91.Gronthos S. Franklin D.M. Leddy H.A. Robey P.G. Storms R.W. Gimble J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 92.Katz A.J. Tholpady A. Tholpady S.S. Shang H. Ogle R.C. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 93.Liu T.M. Martina M. Hutmacher D.W. Hui J.H. Lee E.H. Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 94.Ugland H. Boquest A.C. Naderi S. Collas P. Blomhoff H.K. cAMP-mediated induction of cyclin E sensitizes growth-arrested adipose stem cells to DNA damage-induced apoptosis. Mol Biol Cell. 2008;19:5082. doi: 10.1091/mbc.E08-01-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chung H.M. Won C.H. Sung J.H. Responses of adipose-derived stem cells during hypoxia: enhanced skin-regenerative potential. Expert Opin Biol Ther. 2009;9:1499. doi: 10.1517/14712590903307362. [DOI] [PubMed] [Google Scholar]
- 96.Lee E.Y. Xia Y. Kim W.S. Kim M.H. Kim T.H. Kim K.J. Park B.S. Sung J.H. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 97.Merceron C. Vinatier C. Portron S. Masson M. Amiaud J. Guigand L. Cherel Y. Weiss P. Guicheux J. The differential effects of hypoxia on the osteo-chondrogenic potential of human adipose-derived stem cells. Am J Physiol Cell Physiol. 2010;298:315. doi: 10.1152/ajpcell.00398.2009. [DOI] [PubMed] [Google Scholar]
- 98.Rehman J. Traktuev D. Li J. Merfeld-Clauss S. Temm-Grove C.J. Bovenkerk J.E. Pell C.L. Johnstone B.H. Considine R.V. March K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 375;298 doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 99.Moon M.H. Kim S.Y. Kim Y.J. Kim S.J. Lee J.B. Bae Y.C. Sung S.M. Jung J.S. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17:279. doi: 10.1159/000094140. [DOI] [PubMed] [Google Scholar]
- 100.Kilroy G.E. Foster S.J. Wu X. Ruiz J. Sherwood S. Heifetz A. Ludlow J.W. Stricker D.M. Potiny S. Green P. Halvorsen Y.D. Cheatham B. Storms R.W. Gimble J.M. Cytokine profile of human adipose-derived stem cells: expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 101.Muehlberg F.L. Song Y.H. Krohn A. Pinilla S.P. Droll L.H. Leng X. Seidensticker M. Ricke J. Altman A.M. Devarajan E. Liu W. Arlinghaus R.B. Alt E.U. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30:589. doi: 10.1093/carcin/bgp036. [DOI] [PubMed] [Google Scholar]
- 102.Kucerova L. Altanerova V. Matuskova M. Tyciakova S. Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;67:6304. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 103.Cousin B. Ravet E. Poglio S. De Toni F. Bertuzzi M. Lulka H. Touil I. Andre M. Grolleau J.L. Peron J.M. Chavoin J.P. Bourin P. Penicaud L. Casteilla L. Buscail L. Cordelier P. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS ONE. 2009;4:e6278. doi: 10.1371/journal.pone.0006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Narkilahti S. Rajala K. Pihlajamaki H. Suuronen R. Hovatta O. Skottman H. Monitoring and analysis of dynamic growth of human embryonic stem cells: Comparison of automated instrumentation and conventional culturing methods. Biomed Eng Online. 2007;6:11. doi: 10.1186/1475-925X-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sundberg M. Jansson L. Ketolainen J. Pihlajamäki H. Suuronen R. Skottman H. Inzunza J. Hovatta O. Narkilahti S. CD marker expression profiles of human embryonic stem cells and their neural derivatives, determined using flow-cytometric analysis, reveal a novel CD marker for exclusion of pluripotent stem cells. Stem Cell Res. 2009;2:113. doi: 10.1016/j.scr.2008.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.