1 Current Knowledge about the Pathogen
Malaria occurs world-wide in more than 100 countries in the tropical and subtropical regions and is the most significant parasitic disease in humans. Each year, it causes disease in approximately 500 million people and kills between 1 and 3 million people, above all children under 5 years. Four different types of protozoae are responsible for the disease. They belong to the genus Plasmodium (class haematozoea, order haemosporida). These include:
-
–
Plasmodium falciparum – the agent of tropical malaria
-
–
Plasmodium vivax und Plasmodium ovale – the agent of tertian malaria.
-
–
Plasmodium malariae – the agent of quartan malaria.
P. falciparum consists of genetically different types and, based on the analyses of core and mitochondria nucleic acid sequences, is the agent most closely related to Plasmodium galli-naceum. It probably originates from Africa [1]. As can be derived from the mutations found, the age of P. falciparum is estimated to be 10,000–100,000 years which corresponds to the evolution of the hominids since in Central and North Europe no markers can be found for selection pressure by malaria [2]. Humans are the only hosts for these four human pathogenic Plasmodium species. In rare cases, infections are caused by malaria parasites of other primates (e.g. Plasmodium knowlesi, Plasmodium cynomolgie and Plasmodium simium).
1.1 Characteristics of Malaria
Plasmodium is a parasite that grows intracellularly. The life cycle of plasmodium develops in two phases: an asexual phase in the human host and a sexual phase in the carrier, the Anopheles mosquito. The sporozoites transmitted during a blood meal rapidly penetrate from the blood stream into the liver parenchymal cells in which they replicate asexually. Depending on the plasmodium species, this so-called schizogony phase lasts between 5–7 days in P. falciparum and between 6–18 days in the other species. Schizogony, formerly referred to as merogony, is the asexual replication of the protozoae. A schizont contains several cell nuclei; the daughter nuclei are surrounded by cytoplasm and organise themselves into single individuals, the merozoites. One single sporozoite can produce between 10,000 and more than 30,000 merozoites. For P. vivax and P. ovale, part of the schizonts remain in a kind of inactive phase (hypnozoites); they can remain in the liver cell for months or years, and may then lead to the relapses characteristic of tertian malaria.
After schizogony is completed, the swollen liver cell ruptures and releases the mobile merozoites into the blood stream. These adhere to the red blood cells via specific surface receptors (receptor in the case of P. vivax e.g. Duffy blood group antigen, Fya or Fyb, or in case of P. falciparum glycophorin A). Then, they enter the red blood cells and turn into trophozoites. At the end of the 48- to 72-hour erythrocytic phase, the schizonts will have formed in the red blood cells. During this phase, so-called seal-ring shapes (vacuoles with parietal nuclei) may form (cf. fig. 1). From decayed red blood cells, new merozoites may be released which can infect further red blood cells. A part of the merozoites differentiates within erythrocytes into sexual stages, forming macro- and microgametocytes. In the intra-ery-throcytic vacuoles, haemozoin is formed as an insoluble metabolite of haemoglobin, called malaria pigment.
Fig. 1.
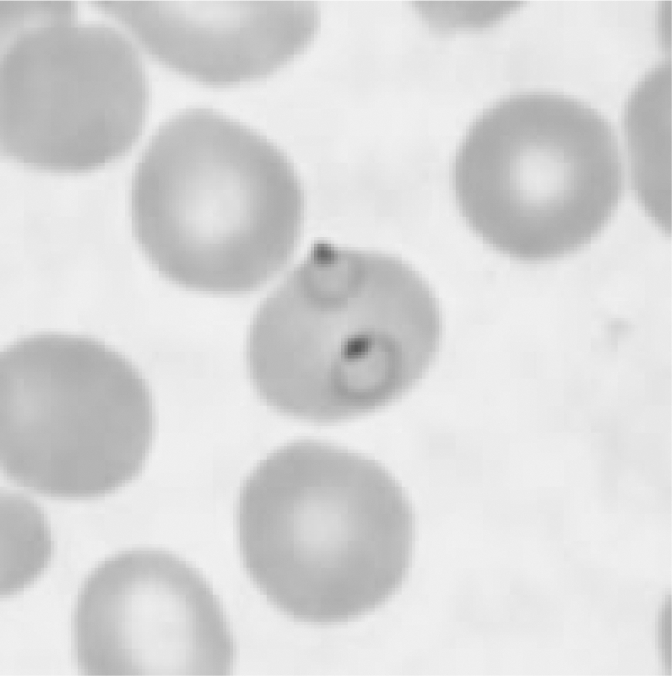
Plasmodium falciparum ring shapes in thin blood smear.
After ingestion of male and female gametocytes during a blood meal, a motile flagellated zygote is formed in the midgut of the Anopheles mosquito. This zygote moves into the salivary gland. An oocyst is formed releasing sporozoites which can infect a new human host via the saliva of the mosquito.
1.2 lnfection and Infectious Disease [3,4,5]
Malaria infection in humans is caused by a sting of the female Anopheles mosquito during which sporozoites are released from the salivary gland of the mosquito into the organism of the host during a blood meal. The sporozoites then go through the cycle described in 1.1 ‘Characteristics of Malaria’. The symptoms in humans are caused by the invasion and destruction of the red blood cells by the asexual parasites and the immune response of the host. The red blood cells are affected by strong consumption and degradation of intracellular proteins, especially of haemoglobin, caused by the growing parasites. Changes occur in the membrane of the red blood cell, and their deformability is reduced. Above all, the adhesion protein PfEMP-1 (P. falciparum erythrocyte membrane protein-1) plays an important part in the pathogenesis of P. falciparum. This protein mediates the adherence to the receptors of the venous and capillary endothelium (cell adherence). Around 60 different var-genes encode for different variants of PfEMP-1, each with individual antigenic and adhesive properties.
It is assumed that one particular PfEMP-1 each is prevalent on the surface of one individual infected red blood cell. Receptor molecules for PfEMP-1 above all include the intercellular adhesion molecule 1 (ICAM-1) in the brain, chondroitin sulphate B in the placenta, and CD36 in other organs. Infected red blood cell can also adhere to other non-affected red blood cells (rosette formation) and other infected red blood cells (agglutination). Cell adhesion and rosette formation will lead to sequestration of the red blood cells which contain mature forms of the parasite in the capillaries of different organs (especially the brain). The impairment of microcirculation, caused by the cell adherence of the red blood cells amongst each other and the cell adherence to the endothelium, the resulting reduction of blood flow in the capillaries, the possible intravasal coagulation activation and the changes in the metabolism will lead to reduced oxygen supply in the brain, kidneys, liver and lungs, which are responsible for the severe course and deadly complications of tropical malaria.
The symptoms of acute malaria are non-specific and begin no sooner than 6 days after a sting by an infected mosquito during the intraerythocytic phase of the development cycle. The symptoms which first occur include malaise, headache, abdominal pain, limb and muscle pain as well as fever. Frequent symptoms are nausea, vomiting, diarrhoea, and orthostatic hypotension. In case of tropical malaria caused by P. falciparum, the fever occurs irregularly while symptomatic phases with chills and fever in 48- or 72-hour cycles occur in case of infections with P. vivax, P. ovale or P. malariae (tertian or quartan malaria). Individuals who are not immune may develop body temperatures of 40 °C and higher. In many cases, thrombope-nia is present, and in addition, splenomegaly and hepatomegaly as well as diarrhoea in 30% of the cases.
If tropical malaria is left untreated or is inappropriately treated, complicated malaria can develop in different organs due to sequestration of infected red blood cells. Characteristic phenomena for such complicated courses of tropical malaria include impairments of consciousness up to coma, often in connection with seizures (cerebral malaria), hypoglycaemia, lactic acidosis, impairment of the kidney and liver functions, ARDS (adult respiratory distress syndrome) and haematological changes, especially pronounced haemolytic anaemia. In addition, coagulation disorders (only very seldom as disseminated intravasal coagulation), thrombocytopenia and haemoglobinuria may occur. The pathogenetic mechanisms underlying the symptoms of tropical malaria are only partly known and include disorders of the microcirculation (cerebral malaria, impairment of the kidney function), anaerobic glycolysis (lactic acidosis), haemolysis (anaemia) and disorders of the metabolism such as reduced gluconeogenesis in the liver, hyperinsuli-naemia and increased glucose consumption by parasites (hypoglycaemia).
The most severe course of tropical malaria is cerebral malaria. This is the course which is in most cases responsible for the high lethality, despite appropriate treatment, and for neurological deficits, especially in children. During pregnancy, tropical malaria is related to increased maternal and foetal morbidity and lethality. In semi-immune primigravid and secundi-gravid women, cerebral malaria results in low birth weight and increased infant and child mortality due to reduced oxygen supply. An HIV infection of the mother predisposes for increased parasitaemia and increases the risk of connatal malaria. In non-immune pregnant women, the risk of infection with severe outcome and high parasitaemia and anaemia is high. Severe tropical malaria, as a rule, leads to a miscarriage. Connatal malaria occurs in less than 5% of the newborns of infected mothers in endemic areas.
The clinical course of tropical malaria in children resembles that of adults. Above all in infants more than 6 months of age, who no longer have a certain protection from malaria by maternal antibodies, and in small children, the course is often serious with a high complication rate in contrast to adults, with serious anaemia causing cerebral malaria. According to WHO estimates, more than half the estimated 1–3 million annual deaths in African children is caused by tropical malaria.
Clinical symptoms of tertian malaria caused by P. vivax resembles tertian malaria caused by P. vivax. Both parasites infect only young red blood cells (reticulocytes) so that parasitaemia in the blood is detectable in only 1-2% of the red blood cells. Symptoms that may occur include anaemia due to haemolysis, thrombocytopenia and in rare cases rupture of the spleen. Mi-crocirculatory disorders due to sequestration of the red blood cells may also occur very seldom.
Because of the occurrence of dormant parasite forms in the liver (hypnozoites), relapses can occur after remitting tertian malaria after up to 4 years. In extreme cases, even longer relapse periods were observed.
Quartan malaria which is caused by P. malariae as a rule has a benign course with only mild symptoms. The parasites infect predominantly old red blood cells; the result is low parasitaemia (1–2%) in the blood, as in tertian malaria. Glomeru-lonephritis due to chronic formation of immune complexes with deposits in the kidney may occur. Unlike tertian malaria, quartan malaria displays no hypnozoites in the liver and therefore no latent hepatic courses. Late quartan malaria attacks (recrudescences) may result from persisting forms of P. Malariae in blood vessel endothelia [5]. Quartan malaria can persist for up to 40 years.
Chronic complications of malaria include the tropical splenomegaly syndrome, the nephrotic syndrome in case of quartan malaria and possibly an increased occurrence of Epstein-Barr virus (EBV) associated Burkitt lymphoma during childhood.
1.3 Epidemiology
According to the WHO, there were possible transmissions of malaria in a total of 107 countries in 2004 [6]. Although this figure has declined since the 1950s (140 countries with endemic malaria), roughly 3.2 billion people currently live at a permanent risk of malaria. It is estimated that every year between 350 and 600 million clinical cases of malaria occur world-wide, out of which 60% occur in Africa south of the Sahara alone. Out of the more than 1 million deaths in Africa caused by malaria, roughly half are children under 5 years.
Almost all of these deaths are due to tropical malaria. P. falciparum mainly occurs in Africa and in certain regions of SouthEast Asia, the Caribbean and South America. The second most frequent malaria species, P. vivax, is found in wider parts of Asia, America, and North Africa. Altogether, more than 40 different Anopheles mosquito species are able to transmit malaria. The most important malaria vector, Anopheles gambi-ae, occurs exclusively in Africa.
The extent of endemic occurrence of malaria is calculated from the degree of parasitaemia or the rate in children between 2 and 9 years with an enlargement of the spleen. If the rate is below 10%, we talk about hypoendemic, between 11 and 50% about mesoendemic, between 51 and 75% of hyperendemic and with a portion of spleen enlargements of over 75% of holoendemic regions. In certain holo- and hyperendemic regions of tropical Africa or New Guinea with a very high transmission rate of P. falciparum, the inhabitants are repeatedly infected with plasmodia throughout their lifetime. In these regions, there is a high morbidity and mortality during childhood. Whereas health impairments are serious in children, malaria infections in adults frequently show few symptoms because of the adult's partial immunity. Such a situation with frequent re-infections throughout the entire year is described as ‘stable malaria’ and occurs in holo- and hyperendemic regions. In hypoendemic regions, the transmission rate is low, and infections only occasionally occur seasonally or in certain districts. Depending on external circumstances (e.g. rainy season), the incidence of malaria can be markedly increased, and epidemics can occur. In general, the epidemiology of malaria is influenced by the number of infectious stings, the mosquito density, the number of infected mosquitoes, the number of chronically infected individuals, the degree of an-thropophilia of the vector, the interval between the blood meals, the lifetime of the mosquitoes and the sporozoite infection dose. The ambient temperature also plays an important role, since development of human pathogenic plasmodia persists below 16 °C. Thus, an endemic spread of P. vivax as occurred about 100 years ago in Central Europe is no longer possible since the human host is missing thanks to a successful treatment of malaria.
In Germany, altogether 628 cases of malaria were reported in 2005 pursuant to the § 7 IfSG (Infektionsschutzgesetz; German Infection Protection Act). This corresponds to an incidence of 0.8 cases of infection per 100,000 inhabitants. In the years before 2005, the reported figures were 707 cases (2004), 820 cases (2003), 859 cases (2002), 1,045 cases (2001), 836 cases (2000), 931 cases (1999), and 1,008 cases (1998) [7]. The largest portion of malaria infections in 2005 was imported from African countries (88%). 7% of the reported cases originated from Asia, 3% from America, and 2% from Australia/Oceania. Among those countries from which malaria was imported, Ghana, Nigeria, Cameroon and Kenya were at the top of the list. In 78% of the malaria cases reported in 2005, P. falciparum was identified as the agent of tropical malaria while P. vivax ranked second with 12%, and P. ovale and P. malariae were registered with only 4 and 3%. Among the total of 6 deaths by tropical malaria in 2005, three cases had infection with P. falciparum, one case had a mixed infection, and in 2 cases no pathogen species was identified. One of the individuals originated from Cameroon, and 5 were German. Evidence showed that 2 of the individuals who died had not performed chemoprophylaxis. The countries of origin indicated were Cameroon, Gambia, Senegal and Ghana.
Out of the cases of malaria reported to the Robert Koch Institute for 2001 to 2006 (as of 12 July 2007), altogether 4,639 cases fulfilled the reference definition. For part of the cases reported, both the time of travel and the onset of symptoms of the disease were available. Although no exact incubation period can be derived from these data, the time of the onset of clinical symptoms after returning from an endemic area is important for blood safety concerns, i.e. the determination of deferral periods. This especially applies to infection with P. vivax and P. ovale as well as P. malariae, since these may have longer incubation periods. An analysis of the data on individuals who did not stay in an endemic area for malaria for more than 8 weeks (individuals who do not have their temporary centre of activity there), shows that in 14.6% of the returnees reported on tertian malaria in accordance with the IfSG symptoms occurred only after more than 6 months (table 1). Similar information was also obtained from Switzerland [8].
Table 1.
Time from end of travel up to the occurrence of symptoms in cases of malaria reported in accordance with the IfSG (Infection Protection Act) who did not stay in the country of travel for longer than 8 weeks
| Form of malaria | Number of cases from 2001-2006 | Number of cases with information on end of travel and beginning of symptoms | Months after end of travel | Portion of cases with symptoms, % |
|---|---|---|---|---|
| Tropical | 3,334 | 1,446 | 2 | 98.8 |
| 6 | 99.8 | |||
| 12 | 99.9 | |||
| 13 | 100 | |||
| Tertian | 796 | 261 | 2 | 55.6 |
| 6 | 85.4 | |||
| 12 | 97.7 | |||
| 60 | 100 | |||
| Quartan | 103 | 33 | 2 | 81.8 |
| 6 | 100 | |||
The rare cases of malaria infections acquired in non-endemic areas are either transfusion associated malaria cases (cf. 3 ‘Recipients’) or so-called airport malaria and/or nosocomial transmissions. Airport malaria is transmitted during the flight or a stop-over and/or by mosquitoes e.g. transported in the luggage [9]. Nosocomial malaria transmissions can be caused e.g. by needle injuries, contaminated fluids and contaminated medical devices [10,11,12,13].
Besides, autochthonous malaria transmissions (i.e. transmissions which developed on site) can occur in regions in which malaria does not usually exist or has been eradicated if the respective vector is present (Anopheles spp.). Thus, 7 locally restricted transmissions of P. vivax were reported in Palm State County, FL, USA, in 2003 [14].
Anopheles spp. native to Germany, too, can transmit plasmodia; depending on ambient temperature, infectious chains are therefore well possible in Germany and have occurred before. P. vivax and tertian malaria were wide-spread in Southern Germany up to the middle of the 19th century. At the Upper Rhine, malaria did not recede before the straightening of the Rhine which reduced the breeding grounds for Anopheles. Sporogeny of P. vivax in Anopheles occurs up to a summer isotherm of 16 °C. The main areas of distribution are within the 25 °C summer isotherm which runs through the middle of Germany [15]. In the past few decades, no case of endemic malaria has been reported in Germany. The last autochthonic cases of malaria were observed up to around 1950 in Berlin and its surroundings [16].
Malaria caused by P. falciparum has recently been reported in 2 German children, who had no history of international travels but stayed at a hospital at the same time as a child from Angola infected with tropical malaria. Since breeding grounds of Anopheles plumbeus (a potential plasmodium carrier) were found in the vicinity of the hospital and, in addition, temperatures were between 21 and 27 °C on average during the day time, transmission by A. plumbeus was assumed as the possible cause [17].
1.4 Detection Methods and Their Significance
1.4.1 Thick Drop and Blood Smear
The gold standard for malaria diagnostics is still the microscopic examination of the so-called thick drop or thin blood smears stained in accordance with May-Grünwald or Wright-Giemsa, respectively [18], or by means of fluorescence colouring (figs 1, 2). In the thick drop method, plasmodia are enriched to the 6- to 10-fold concentration compared with the blood smear. The degree of parasitaemia/μl can be determined by means of the parasite and leucocyte count via a correlation with the total leucocyte count. A negative test result does not reliably exclude a malaria infection since at the beginning of clinical manifestation the parasite density might be low in the peripheral blood. In case of continued clinical suspicion and negative results, the test must be repeated several times, e.g. at 12-hour intervals. The experience of the investigator plays an important part in malaria diagnostics. The detection limit is 5–10 parasites/μl In the case of less experienced investigators, it is increased to the power of 10.
Fig. 2.
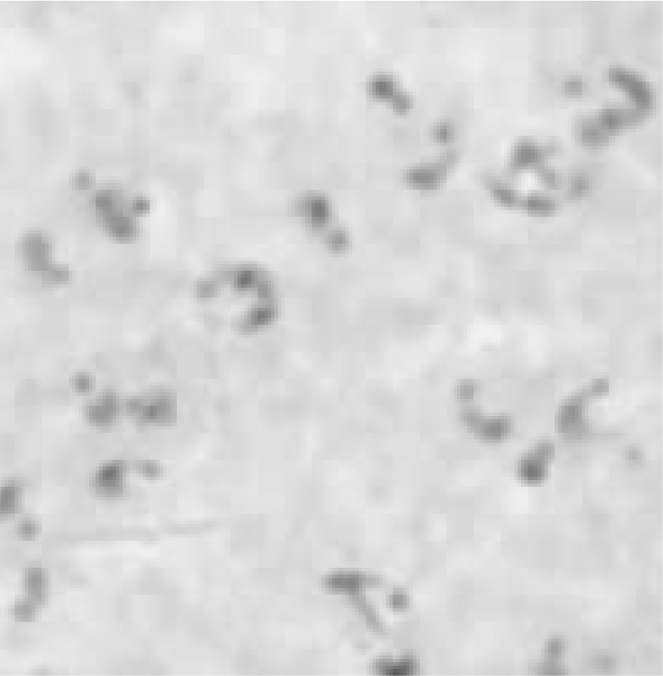
Plasmodium falciparum ring shapes in thick drop.
1.4.2 NAT Method
Various investigators have compared in particular the sensitivity of NAT tests with conventional microscopy after staining in accordance with Giemsa or Wright-Giemsa. All studies showed a higher sensitivity after DNA extraction by means of NAT. Around 5–10 μl blood are used for blood smear or thick drop; for DNA extraction, the quantity is 200 μl
For the conventional nested PCR, different primers are used to include all plasmodia species. These primers bind in the 18s RNA [19, 20]. Sensitivity of microscopy is approximately 93% if 100% are set in the PCR. Other methods included real-time PCR [21] and real-time PCR with probes as molecular beacon [22]. An arithmetic sensitivity of 0.004 parasites/μl blood was obtained for P. falciparum, and for genus-specific PCR this figure was 0.16/μl [22]. Compared with microscopy, a sensitivity of 97.4% was obtained with genus-specific sensitivity [23]. According to another report, no cross-reaction with Toxoplasma gondii and Leishmania infantum was found at a sensitivity of 0.7 parasites/μl for P. falciparum, 4 parasites/μl for P. vivax, and 1.5 parasites/μl for P. ovale [24].
1.4.3 Plasmodium Antigen Detection
The first few tests for the determination of plasmodium components showed low sensitivity and specificity [25,26,27], even though parasitic LDH was used as antigen. Rapid tests were developed to support travellers in malaria diagnostics in the event of fever or exposure. Direct detection tests for plasmodium have been improved in the meantime so that a sensitivity of 85% and a specificity of 96% can be reached for P. falciparum infections [28]. Low parasite density could be a reason for low sensitivity. The antibody cross-reacts with P. vivax, but at a low sensitivity. When antibodies were used against histi-dine-rich protein II of P. falciparum, a sensitivity of 97% and a specificity of 96% were obtained in travel returnees [29].
Depending on the exposure, a sensitivity of 88% and a specificity of 99% can be reached for P. falciparum. For P. vivax, the respective figures are 76% and 100%[30]. Monoclonal antibody based ELISA has shown a sensitivity of 90% for P. falciparum in Thailand and Nepal [31].
The quality of the plasmodium indicated in each study quoted depends on the comparative tests used in these studies, i.e. PCR or microscopy, and on the investigator's expertise in interpreting the test result.
1.4.4 Diagnostics UsingAnti-Plasmodium Antibodies
Most of the studies published in this field deal with the determination of antibodies which are formed after a vaccination or which can be used for the appropriate antigens for a putative vaccine. The cytoadherence-linked asexual gene 9 (clag 9) [32], merozoite surface proteins 6 and 7 (MSP6, MSP7) [33] and the variant surface antigens (VSA) are immunogenic and can still be detected 10 years after infection [34].
Exposure to malaria parasites which was measured via IgM and IgG responses using a test containing 5 different proeryrthocyte antigens was published by some working groups from France in 2006. The test antigens were circuMSporozoite protein, sporozoite threonine- and asparagine-rich protein, sporozoite and liver stage antigen, liver stage antigen 1 and SR11.1. The immune response was measured in 106 individuals 3 months after exposure and seemed plausible [35]. If leucin-rich protein is used, cross-reactions occur between Schistosoma mansoni and P. falciparum [36].
Since the infection with P. falciparum in the blood causes serious clinical symptoms, the testing of returnees from tropical regions after a delay of several months in the case of an asymptomatic state of health is immaterial from an epidemiological point of view. Testing for anti-plasmodium antibodies has more informational value as regards the exposure and possible circulation if no clinical abnormality has shown.
2 Blood and Plasma Donors
2.1 Prevalence and Incidence in Donor Populations
Little is known about the prevalence and incidence of malaria in blood donors in Germany. In an up-to-date study by Okocha et al. [37], a prevalence of antibodies of 30.2% was determined in donors in Nigeria. In Venezuela, 890 blood donors were studied by Nunez et al. [38] by means of ELISA. The total antibody prevalence found was 1.7%.
2.2 Definition of Exclusion Criteria
Conforming to the guidelines of the Bundesärztekammer (German Medical Association) on haemotherapy, individuals in Germany are excluded from donating blood for a period of 4 years following medically documented recovery from malaria [39]. In addition, persons who were born or raised in a malaria endemic area or temporarily had their centre of life in such an area are deferred from donating blood for a total of 4 years after their last stay in the endemic area. Before donating blood, infectiveness has to be excluded by means of a validated immunological or nucleic acid test. Persons visiting a malaria endemic area for a short period are not allowed to donate blood for a period of at least 6 months following their stay. The decision of a possible deferral is independent from the occurrence of febrile episodes. For persons exclusively donating plasma for fractionation, exclusion from the donation due to a malaria risk need not be considered.
In the UK, potential donors who had a malaria infection or fever of unclear origin within 6 months after returning from an endemic area, or after having stayed in a malaria endemic area during the past 12 months or for an uninterrupted period of more than 6 months, are excluded from the donation. A negative test result for anti-plasmodium antibodies is required for re-admittance to the donor panel 3 years after the end of treatment or after 6 months free of symptoms after returning from the endemic area [40].
In France, antibody tests (indirect immunofluorescence antibody test; IFAT) are also performed within a period of between 4 months and up to 3 years after returning from an endemic area. The test algorithm depends on the duration of the stay in the malaria endemic area.
2.3 Donor Testing and Significance
Because of existing exclusion rules, no donor screening for plasmodium is carried out in Germany.
2.4 Donor Interviews
In countries with endemic occurrence of malaria in which a high portion of the donors is infected, the kind of donor exclusion which is common practice in non-endemic countries cannot be performed because a lack of donated blood supply would result. Besides, a great number of recipients have partial immunity. In some malaria endemic countries, recipients received administrations of chloroquine or, in the event of chloroquine resistance, sulfadoxin-pyrimethmin in order to prevent transfusion associated malaria. However, this type of preventative measure is not reliable in the very regions of Central and West Africa as well as South-East Asia due to increased resistance against these two substances.
When potential donors in Germany are interviewed, they are asked whether they originated or grew up in a malaria endemic region or whether they stayed in such a region in the past 6 months. A possible malaria prophylaxis is not taken into account here. In addition, the donor is obliged to indicate whether he/she has an acute malaria infection or ever had a malaria infection.
2.5 Donor Information and Counselling
Information or advice for a donor concerning malaria is not provided. If the donor is suspected to be infected with malaria, further clarification by a physician experienced in tropical medicine is required.
3 Recipients
3.1 Prevalence and Incidence of Blood-Associated Infections and Infectious Diseases in Recipient Populations
The first transmission of malaria by blood transfusion was described in 1911 in a patient with pernicious anaemia [41]. Although the donor indicated that he had never suffered from malaria, the recipient developed a febrile reaction 11 days following the donation, and P. vivax could be detected both in the donor and in the recipient. More than roughly 350 malaria infections following blood transfusions were reported for the years between 1910 and 1950 [42]. An analysis of the cases that became known between 1911 and 1979 [43] showed an increase in the incidence of malaria from 6 to 145 cases per year, parallel to the increasing number of transfusions. At first, P. vivax was the most frequently observed species in case of transfusion associated malaria while P. malariae prevailed in the 1950s. In the 1970s, P. vivax was again the prevalent species in cases of transfusion associated malaria followed by P. malariae and P. falciparum. Since the 1980s, however, P. falciparum has been the most frequently registered species of malaria following blood transfusions in countries in which malaria does not occur endemically (e.g. USA, Canada and UK). Thus, the portion of P. falciparum in the UK has risen from 37% in 1984 to 55% in 1993 [44].
Altogether 93 cases of transfusion associated malaria have been documented in the USA during the period from 1963 to 1999 [45]. 35% could be ascribed to P. falciparum, 27% to P. vivax, 27% to P. malariae, 5% to P. ovale and 3% to mixed infections. 11% of the patients died. Of 91 identified donors altogether, 67 could be associated with the transmission. 59% of the donors whose country of origin was known came from endemic areas. The estimated incidence of transfusion associated malaria in the USA is one case per 1 million donors. Around 1–3 cases per year are reported in the US Center for Disease Control and Prevention (CDC). It is assumed that the frequency of transfusion associated malaria in malaria endemic countries is more than 50 cases per 1 million donors. In Canada, the frequency of transfusion associated malaria is estimated to be 1 case per 4 million transfused red blood cell concentrates [46]. The three cases of tropical malaria published in 2001 were all transmitted by donors originating from malaria endemic areas but had lived in Canada symptom free for several years. Based on these 3 cases, the Canadian donor deferral criteria were amended to exclude persons who had recovered from malaria (2 of the 3 cases) permanently from donation. Up to 1965, a total of 12 cases of transfusion associated malaria were reported from Germany [47,48,49,50,51,52,53]. The cases in question were 2 infections with P. falciparum and 7 infections with P. vivax, the 3 remaining infections could not be reliable assigned [overview in 42].
In the past few years, 3 reports were published on transfusion associated cases of malaria [54,55,56]. In 1998, Witt et al. [56] reported on an 18-month-old boy who developed antibiotic refractory fever 14 days after a heart operation. On day 23, postoperative, intraerythrocytic ring shapes and gametocytes of P. falciparum were found in the peripheral blood smear. The child recovered rapidly after a 3-day administration of quinine and halofantrine. The retrospective analysis of the deferred samples of 7 donors from which the child had received red blood cell concentrates showed antibodies against P. falciparum in one of the donors.
Malaria caused by P. falciparum with lethal outcome was recently reported from a 70-year-old patient after preceding operation due to coronary heart disease and aortic aneurysm which was caused by transfusion of red blood cell concentrates. One of the blood donors was a 30-year-old man born in Cameroon who had left the country 10 years before and had ever since lived in Paris (near the International Airport) and later on in Switzerland. His last visit to his home country had been 6 years back at the time of the medical investigations. A possible cause of the infection of the donor was assumed to be airport malaria or persistence with partial immunity [57].
3.2 Immune Status (Resistance, Existing Immunity, Immune Response, Age, Exogenous Factors) [3, 4]
The course of malaria strongly depends on the degree of immunity of the infected individual. Complete sterile immunity is never reached, but merely partial immunity (semi-immunity). Immunity is proportional to the age, the cumulative number of malaria episodes and time spent continuously in a malaria endemic region.
Based on transplacentally transmitted maternal antibodies, newborns have a certain degree of immunity to malaria up to an age of around 4–6 months, whereby foetal haemoglobin inhibits maturing of the schizonts. Older children and young adults in regions with stable malaria and a high transmission rate (holoendemic or hyperendemic regions) possess the highest degree of immunity. Here, asymptomatic parasitaemia can often be found. In semi-immune individuals – polyclonal increase in IgM, IgG and IgA antibodies as well as specific T-lymphocytes can act cytotoxically against parasites or infected cells in the liver. The most important antigen in infections with P. falciparum is probably variable protein PfEMP-1. The acquisition of antibodies against a number of variant PfEMP-1 antigens seems to be of special significance for the immunity situation. Specific immunity is directed both against the species and against the respective subpopulation of the parasites.
Non-immune individuals only have the non-specific defence mechanisms, and the course of tropical malaria in such individuals is by far more severe than in semi-immune persons. Congenital red blood cell anomalies also play an important role in immunity to malaria. In malaria endemic areas, hereditary diseases such as sickle cell anaemia, thalassaemia or glucose-6-phosphate dehydrogenase deficiency of the red blood cells can be found. In individuals with heterozygoty of HbAS (sickle cell gene carriers), there is a certain immunity against tropical malaria. This seems to be due to the inhibited growth of the parasites caused by strongly reduced oxygen content. Higher resistance can also be found in heterozygote a-thalassaemia. Glucose-6-phosphate dehydrogenase deficiency is also associated with reduced susceptibility to malaria in children and pregnant women and is caused by inhibition of growth of the parasites in red blood cells in which the latter enzyme is missing. Ovalocytosis, which occurs in South-East Asia, leads to lower parasitaemia in heterozygote carriers.
Red blood cells where the Duffy blood group antigen Fya is missing are partly resistant to P. vivax. This is how the very low incidence of malaria caused by P. vivax in West Africa can explained, where a high percentage of the population does not possess the Duffy blood group antigen Fya [58].
3.3 Severity and Course of the Disease
Untreated tropical malaria always has a fatal outcome in nonimmune individuals who do not originate from an endemic area.
In a US study performed on transfusion associated malaria from the years 1963–1998 with a total of 93 cases, a lethality rate of 11% (10 cases) was observed [45]. Six of these patients had an infection with P. falciparum, 2 were infected with P. vivax, and 2 with P. malariae. The two patients with P. vivax died of their underlying disease.
Out of 5 patients with post-transfusion malaria in the UK [59], 1 died of cerebral tropical malaria and another P. falciparum infected patient died of multiple organ failure. In another study [60], lethality of transfusion associated malaria is indicated as 15%. No figures are available from Germany. The high lethality rate of transfusion associated malaria can be explained by the fact that most of the cases documented are cases of immunologically naïve recipients with a more or less serious underlying disease, or this rare route of infection was diagnosed too late.
The incubation period of transfusion associated malaria depends on the species and the number of transmitted parasites, and is between 10 and 60 days [43]. For P. falciparum, it is 10 days on average, for P. vivax 16 days, and for P. malariae 40 days [42]. Symptoms of transfusion associated malaria are many and varied. They include dizziness, vomiting, muscle pain, slight icterus, abdominal pain and diarrhoea. Usually, no fever periodicity can be found. In patients with serious underlying disease, above all immune suppression, transfusion associated malaria often displays a severe course with early cerebral involvement.
3.4 Therapy and Prophylaxis
3.4.1 Vaccine Development
Although a variety of attempts have been made to develop effective malaria vaccines, practically no immunisation is commercially available yet [61]. In the past few years, malaria has been recognised as a global social problem, and intensive activities are underway to develop new vaccines, which is to a considerable extent the merit of the generous funding from the European Union and the Bill and Melinda Gates Foundation. The number of clinical trials is on the increase so that new vaccines can be expected in the next few years. The distribution of roles between humoral and cell-bound factors in effective immunity against plasmodia, however, is not understood [58].
The development work focuses on vaccines against P. falciparum and/or tropical malaria as the most dangerous form of malaria. Four stages of the life cycle of P. falciparum are eligible as targets of immune prophylaxis in humans:
-
–
sporozoites after a blood meal or before infection of the hepatocytes,
-
–
the merozoites after release from the liver or before invasion of the red blood cells,
-
–
the merozoites during invasion of the red blood cells or replication in the red blood cells, and
-
–
the gametocytes released from the red blood cells in the anopheles mosquito after the next blood meal [61].
The overwhelming majority of the development studies focus on potential vaccines which are intended to prevent the invasion in the red blood cells, and thus the massive replication of the parasites. It is expected that this would inhibit the clinical symptoms of malaria, and above all, the complications relating to these symptoms. If the red blood cell stage could be completely blocked, this would automatically break the infection chain which would also be significant from an epidemiological point of view. Strictly speaking, only vaccines against sporozoites preventing the invasion of the liver cell and leading to the killing of parasites should be considered as causal immune prophylaxis. Such variants, however, will probably not be a subject of discussion in the near future, due to the complexity of the parasites and the number of unresolved scientific problems. From the transfusion medicine point of view, we must bear in mind that, even if a donor is protected by a vaccination, it should not automatically be assumed that no transfusion related malaria will occur. What is more relevant here is to calculate this risk taking into account the epidemiological and infectiological background and the type of immune protection.
3.4.2 Prophylaxis and Therapy
Primary prevention of malaria is brought about by mosquito bite prevention. Other preventative measures include the reduction of the parasite reservoir in the population in malaria endemic regions, measures to eradicate vectors (removal of breeding grounds, use of larvicides, and insecticides), as well as measures to reduce contact with the vector.
Attempts have been made to immunise the Anopheles mosquito against the parasite in order to break the infection chain. Travellers to malaria endemic areas are still strongly recommended to undergo exposure prophylaxis, e.g. the use of repellents, wearing clothes that provide protection, sleeping under mosquito netting and staying in mosquito proof rooms. In addition, chemoprophylaxis is recommended depending on the destination and the type of travel. Depending on the resistance situation, the Deutsche Gesellschaft für Tropenmedizin und Internationale Gesundheit (DTG; German Society for Tropical Medicine and International Health) recommends chloroquine, mefloquine, atovaquone-proguanil, or, in some cases, doxycyclin for malaria prophylaxis (table 2) [62]. In this context, the distinction is made between continued chemoprophylaxis (which as a rule begins one week or day before travelling to the malaria area and continues up to 4 weeks or 1 week after leaving the malaria area, depending on the situation), and the stand-by treatment with the appropriate medicine (mefloquine, atovaquone-proguanil, artemeter-lume-fantrine). The use of halofantrin for malaria prophylaxis was stopped in Germany many years ago because of its risk of cardiac adverse effects.
Table 2.
Overview of products for malaria prophylaxis and stand-by treatment for malaria (modified in accordance with the recommendations from the Deutsche Gesellschaft für Tropenmedizin und Internationale Gesundheit (DTG, German Society for Tropical Medicine and International Health)
| Product | Prophylaxis | Stand-by treatment |
|---|---|---|
| Chloroquine | 300 mg chloroquine base weekly – for persons > 75 kg body weight: 450 mg weekly – 1 week before up to 4 weeks after a stay in a malaria area | 600 mg base, 6 h after the beginning of treatment and 300 mg at a time 24 and 48 h after the beginning of treatment |
| Mefloquine | 250 mg weekly, 1–3 weeks before up to 4 weeks after a stay in a malaria area | Initial dose 750 mg, after 6–8 h further 500 mg; if body weight >60 kg after another 6–8 h further 250 mg |
| Atovaquone-proguanil | 250 mg/100 mg daily, 1–2 days before up to 7 day after a stay in a malaria area (adults with a body weight > 40 kg; maximal duration of stay 28 days) | 1,000 mg / 400 mg as a single dose on 3 subsequent days if the body weight is > 40kg |
| Doxycyclin | 100 mg daily 1–2 days before and up to 4 weeks after a stay in a malaria area | not suitable |
| Artemether-lumefantrine | not suitable | 80 mg / 480 mg initial dose after 8 h further 80 mg / 480 mg, then 2× daily 80 mg / 480 mg on days 2 and 3 |
| Proguanil | 200 mg daily | not suitable |
In pregnant women, the use of a combination of chloroquine and proguanil is possible as from the second trimester. For children primarily consistent exposure prophylaxis is recommended. Prophylaxis with atovaquone-proguanil is possible for children with a body weight >11 kg while mefloquine can be used in children with a body weight over 5 kg. Doxycyclin must not be used in children under 8 years of age. However, chemoprophylaxis does not guarantee that the patient will be free from malaria, but will reduce the risk significantly.
Because of the world-wide occurrence of e.g. chloroquine and sulfadoxin-pyrimethamine resistent isolates of P. falciparum, meloqine, atovaquone-proguanil, or arthemether-lumefanine are now the treatments of choice in non-complicated tropical malaria. Treatment of serious tropical malaria (with CNS involvement, acute kidney failure or other organ complications) should be performed under intensive-ward conditions at all means. Treatment with medicinal products usually consists of parenteral administration of quinine in combination with doxycyclin.
Due to the still relatively favourable resistance situation, chloroquine is still the treatment of choice in tertian malaria. After completion of the chloroquine course of treatment, completion treatment with primaquine which is efficacious against hypnozoites of P. vivax and P. ovale should follow. Quartan malaria should also be treated with chloroquine. Subsequent treatment with primaquine is not required (table 3). The same principles as for general malaria treatment apply for treatment of transfusion associated malaria, except that in infections caused by P. vivax and P. ovale, no subsequent treatment with primaquine is required because a liver cycle, i.e. hypnozoites, is not involved in this type of infection (cf. 1.2. ‘Infection and Infectious Disease’). In this type of infection, too, it is early start of treatment which is decisive for the outcome. Prophylaxis is currently possible only by strict exclusion criteria for donors, in combination with serological and/or molecular biology screening (NAT) if required. A vaccination which confers protection is so far not available.
Table 3.
Treatment of malaria, modified in accordance with [63]
| Product | Dosage | |
|---|---|---|
| Malaria tertiana | chloroquine | 10 mg base/kg body weight initial dose, followed by |
| Malaria quartana | 5 mg base/kg body weight after 6, 24, and 48 h | |
| plus primaquine (only in case of tertian malaria) | 0.5 mg base/kg body weight 1 × daily over a period of 14 days | |
| Malaria tropica | ||
| Non-complicated malaria | mefloquine | initially 750 mg base, followed by 500 mg base 6 h after the beginning of treatment and 250 mg base 12 h after the beginning of treatment (at >60 kg body weight) |
| or atovaquone-proguanil | 1,000 mg/day atovaquone, 400 mg/day proguanil 1 × daily over a period of 3 days | |
| or arthemether-lumefantrine | 80 mg/480 mg arthemether-lumefantrine initial dose, followed by 80 mg/480 mg arthemether-lumefantrine after 8 h, 2 × 80 mg/480 mg arthemether-lumefantrine on day 2, 2 × 80 mg/480 mg arthemether-lumefantrine on day 3 | |
| Complicated forms | quinine salt (quinine-dihydrochloride) | 3 × 10 mg p.inf./kg body weight/day for 7–10 days |
| plus doxycyclin | 3 mg/kg body weight/day for 7 days | |
3.5 Transmissibility
A risk of transmissibility of malaria by transfusion exists because of the primary presence of parasites in red blood cells, above all in transfusion of whole blood or red blood cells. However, there is also a risk of transmission with platelet concentrates [64], leucocyte concentrates [65], and even fresh frozen plasma; the latter has only been observed with the administration of fresh frozen plasma within one day following collection [66]. Three infections with malaria were described due to non-observance of the basic principles of hygiene [67] or needle perforation injury [10, 68], and three other transfusion associated cases of malaria recently were described in Canada [46]. These give rise to the assumption that even low numbers of infected red blood cells can cause malaria infection.
Due to the long survival times in the human organism (e.g. P. malariae), a donor can still have malaria parasites in the blood after years and can cause an infection in the recipient. In general, P. vivax and P. ovale rarely persist longer than 3 years while P. falciparum persists about 1–2 years. The longest intervals between blood donation and malaria exposure of the donor in the past were 13 years in the case of a P. falciparum infection [69], 27 years for a P. vivax infection [69] and 7 years in a P. ovale infection [70]. For P. malariae, considerably longer time intervals have been described – in an extreme case – up to 50 years or even more.
3.6 Frequency of Administration, Type and Amount of Blood Products
For plasma derivatives, contamination with plasmodia can be excluded thanks to the manufacturing procedure.
In principle, transmission is possible with only one single product of a non-pathogen-inactivated blood component since all products show residual red blood cell contents.
4 Blood Products
4.1 Infectious Load of the Starting Material and Test Methods
Most donors involved in the cases of transfusion associated malaria are semi-immune individuals with low parasitaemia in the blood. As is known from previous studies, plasmodia can survive in blood reserves for at least 10–12 days, possibly longer [43, 71]. Apparently, the minimum infective dose in humans is 10 parasites (for P. vivax) [65]. In the case of assumed (low) parasitaemia in the donor of 1–2 parasites per μl blood, however, as many as 250,000–500,000 parasites would be transmitted if 250 ml red blood cell concentrate was donated. Methods for detecting a potentially infected donation which would have to contain as few as 10 parasites, would have to be able to detect as little as 0.00004 parasites per μl blood [43, 72]. This degree of sensitivity is not even achieved with the NAT method.
The test methods usually applied for malaria such as microscopic examination of ‘thick drop’ or blood smear in accordance with Giemsa (sensitivity for both methods between 5–500 parasites/μl blood) are not suitable for donor screening because of the usually very low parasitaemia of the donor. Antigen tests based on the detection of HRP/2 or pLDH, too, only detect between 100 and 1,000 parasites/μl blood. Compared with that, NAT methods display higher sensitivity [73, 74]. In a study [75], as little as 0.004 parasites/μl blood could be detected in potentially malaria exposed donors. However, NAT is not able to detect all potentially infectious donors, even at this high sensitivity.
Targeted serological examination of donors returning from malaria endemic areas has been successfully performed in France since 1983. After a deferral period of 4 months, donors are tested using the IFAT. If the result is negative, the donor can be re-admitted. No transfusion associated case of malaria became known in France between 1984 and 2002 [76]. Disadvantages of the IFAT however, include its limitation to antibodies against P. falciparum with low cross-reactivity against the other plasmodia species, high workload in the laboratory and its poor reproducibility due to the subjective assessibility of the test method. Enzyme immunoassays have recently been developed, partly also with recombinant antigens [44, 72]. Thus, a combination of donor interviewing and testing with enzyme immunoassays with recombinant antigen is currently recommended in England for reducing the malaria risk after blood transfusion.
4.2 Methods for Removal and Inactivation of the Infectious Agent
For plasma derivatives, transmission of malaria can be excluded thanks to the manufacturing procedure. Therefore, no cases of malaria due transmission of fractionated plasma products have so far been described.
4.3 Feasibility and Validation of Procedures for Removal/ Inactivation of the Infectious Agent
Some papers have recently been published on the inactivation of plasmodia in blood components by means of different methods (gamma irradiation, photochemical and photodynamic inactivation) [77,78,79,80,81], but without being able to establish whether these methods can be considered as sufficiently reliable and without being considered as routine procedures up to now. In contrast to that, the INTERCEPT system for pathogen inactivation in platelet concentrates and plasma for transfusion is currently beginning to become established in the blood bank routine of some countries. This photochemical method in which the photoactive compound amotosalen HCI is added to the blood components which are at the same time irradiated with long-wavelength UV light (UVA) has proven to be effective for P. falciparum by showing a pathogen reduction of >6 log levels [82].
5 Assessment
With by far more than 1 billion people affected, malaria, which occurs in the world's tropical and subtropical areas, is one of the most significant parasitary infections in humans. The protozoae, which are transmitted by the sting of the female Anopheles mosquito, (P. falciparum, P. vivax, P. ovale and P. malariae) cause different forms of malaria in humans. Tropical malaria which is caused by P. falciparum is the infection mainly responsible for the 1–3 million deaths occurring per year world-wide, and out of which more than half are African children.
In Germany, the number of malaria infections reported in the past few years varies between around 600 and 1,000 cases per year. These are caused by travelling, very rarely by importing infected Anopheles (airport malaria) and in very isolated cases by autochthonous infections.
Depending on the resistance situation, medicinal products such as chloroquine, mefloquine, atovaquone-proquanil, or doxycyclin are used for chemical malaria prophylaxis. Donors are deferred independently from such a malaria prophylaxis. No vaccination is currently commercially available which confers protection against malaria.
During the period up to 1965, altogether 12 cases of transfusion associated malaria became known in Germany. Three reports on malaria following blood transfusions in Germany are available in the literature from a more recent time. Since the Paul Ehrlich Institute became responsible for blood products (1994), one single case of transfusion associated malaria was reported from 1997.
To prevent transfusion associated malaria, persons are excluded from donating blood after medically documented cure from malaria for a period of 4 years. In addition, persons who were born or grew up or temporarily had there centre of life in a malaria endemic area are excluded from donating blood for 4 years following their stay in the endemic area. Returnees from a malaria endemic area are currently deferred from donating blood for a minimum of 6 months following their last stay in a malaria endemic area. If only plasma for fractionation is donated, no exclusion from the donation based on a malaria risk is required.
Altogether, this risk of transfusion associated malaria is considered as low in Germany. A high standard of safety is guaranteed thanks to the thorough investigation of the history of the patient and donor selection as well as the exclusion criteria used for returnees from tropical areas. New epidemiological data for Germany show that with the current referral period of 6 months after travels to endemic areas not all plasmodia infections are detected, especially infections with P. vivax or P. ovale, since in 15% of the cases reported, symptoms occur only after that period. An extension of the deferral period to 12 months would include nearly 99% of the malaria infections. An argument against an extension of the deferral period and the loss of donors relating to this would be the fact that there has so far been no case of transmission since the introduction of the 6-month regulation. If the epidemiological situation changes, however, the deferral period will have to be reviewed and adjusted.
Even though testing of blood donors for malaria antibodies or the presence of plasmodium antigens or for plasmodium genome by means of the NAT method is laid down in the ‘Richtlinien zur Gewinnung von Blut und Blutbestandteilen und zur Anwendung von Blutprodukten (Hämotherapie)’ (Guidelines for the collection of blood and components from blood, as well as for the use of blood products (haemotherapy)) [39] for donors before re-admittance as donor after the 4-year deferral, no tests are commercially available guaranteeing the detection of antibodies against all four human pathogenic Plasmodium spp., which would, however, be imperative for diagnostics with informational value. The inactivation of plasmodia in blood components, which is feasible in principle, should not be required as a general method for Germany because of the low malaria prevalence. Concerning this issue as well as concerning the development of a suitable vaccine, extensive research is certainly required in order to optimise the safety for recipients of blood and blood products regarding malaria.
Footnotes
This paper was completed on March 19, 2007 and approved by the German Advisory Committee Blood (Arbeitskreis Blut) on October 1, 2007. It was compiled by the members of the subgroup ‘Assessment of Pathogens Transmissible by Blood’ of the German Advisory Committee Blood (Arbeitskreis Blut):
Dr. Johannes Blümel
Prof. Dr. Reinhard Burger
Prof. Dr. Christian Drosten
Dr. Albrecht Gröner
Prof. Dr. Lutz Gürtler
Dr. Margarethe Heiden
Prof. Dr. Dr. Bernd Jansen
Dr. Horst Klamm
Prof. Dr. Wolf-Dieter Ludwig
Dr. Thomas Montag-Lessing
Dr. Ruth Offergeld
Prof. Dr. Georg Pauli
Prof. Dr. Rainer Seitz
Dr. Uwe Schlenkrich
Dr. Volkmar Schottstedt
Dr. Hannelore Willkommen
Prof. Dr. Karl-Heinz Wirsing von König with special support by Prof. Dr. Jürgen Knobloch (University of Tübingen)
References
- 1.Conway DJ. Molecular epidemiology of malaria. Clin Microbiol Rev. 2007;20:188–204. doi: 10.1128/CMR.00021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartl DL. The origin of malaria: mixed messages from genetic diversity. Nature Rev Microbiol. 2004;2:15–22. doi: 10.1038/nrmicro795. [DOI] [PubMed] [Google Scholar]
- 3.Fairhurst R, Wellems T. Plasmodium species (malaria) In: Mandell G, Bennett G, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia: Elsevier Churchill Livingstone; 2005. pp. 3121–3144. [Google Scholar]
- 4.Zoller T, Suttorp N. Malaria und Babesiose: Parasitäre Erkrankungen der Erythrozyten. In: Dietel M, Suttorp N, Zeitz M, editors. Harrisons Innere Medizin. Berlin: ABW Wissenschaftsverlag; 2005. pp. 1309–1323. [Google Scholar]
- 5.Kretschmer H, Bienzle U, Klauß V, Kremsner P, Leichsenrring M. Malaria. In: Knobloch J, editor. Tropen- und Reisemedizin. Jena: Gustav Fischer; 1996. pp. 134–163. [Google Scholar]
- 6.WHO: World Malaria Report 2005. http://rbm.who.int./wmr2005
- 7.Robert Koch-Institut Reiseassoziierte infektionsbedingte Erkrankungen im Jahr 2004. Epidemiol Bull. 2005;35:317–324. [Google Scholar]
- 8.Lehky Hagen MR, Haley TJ, Christoph Hatz FR. Factors influencing the pattern of imported malaria. J Travel Med. 2005;12:72–79. doi: 10.2310/7060.2005.12203. [DOI] [PubMed] [Google Scholar]
- 9.Mouchet J. Airport malaria: a rare disease still poorly understood. Eurosurveillance. 2000;5:75–80. doi: 10.2807/esm.05.07.00016-en. [DOI] [PubMed] [Google Scholar]
- 10.Alweis RL, DiRosario K, Conidi G, Kain KC, Olans R, Tully JL. Serial nosocomial transmission of Plasmodium falciparum malaria from patient to nurse to patient. Infect Control Hosp Epidemiol; 2004;25:55–59. doi: 10.1086/502293. [DOI] [PubMed] [Google Scholar]
- 11.Moro ML, Romi R, Severini C, Casadio GP, Sarta G, Tampieri G, Scardovi A, Pozzetti C, Malaria Outbreak Group Patient-to-patient transmission of nosocomial malaria in Italy. Infect Control Hosp Epidemiol. 2002;23:338–341. doi: 10.1086/502062. [DOI] [PubMed] [Google Scholar]
- 12.Piro S, Sammud M, Badi S, Al Ssabi L. Hospital-acquired malaria transmitted by contaminated gloves. J Hosp Infect. 2001;47:156–158. doi: 10.1053/jhin.2000.0907. [DOI] [PubMed] [Google Scholar]
- 13.Chen KT, Chen CJ, Chang PY, Morse DL. A nosocomial outbreak of malaria associated with contaminated catheters and contrast medium of a computed tomographic scanner. Infect Control Hosp Epidemiol. 1999;20:22–25. doi: 10.1086/501557. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Local transmission of Plasmodium vivax malaria. – Palm Beach County, Florida, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(38):908–911. [PubMed] [Google Scholar]
- 15.Lucius R, Frank B. Parasitologie. Heidelberg: Spektrum Akademischer Verlag; 1997. [Google Scholar]
- 16.Fischer L. Einheimische Malaria und Anophelis-mus in der Nachkriegszeit. Dtsch Med Wochenschr. 1948;73:515–518. [Google Scholar]
- 17.Krüger A, Rech A, Xin-Zhuan S, Tannich E. Two cases of autochthonous Plasmodium falciparum malaria in Germany with evidence for local transmission by indigenous Anopheles plumbeus. Trop Med Intern Health. 2001;6:983–985. doi: 10.1046/j.1365-3156.2001.00816.x. [DOI] [PubMed] [Google Scholar]
- 18.Warhurst DC, Williams JE. ACP Broadsheet no 148, July 1996. Laboratory diagnosis of malaria. J Clin Pathol. 1996;49:533–538. doi: 10.1136/jcp.49.7.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snounou GS, Viriyakosol S, Zhu XP, Jara W, Pin-heiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 20.Johnston SP, Pieniazek NJ, Xayavong MV, Slemenda SB, Wilkins PP, da Silva AJ. PCR as a confirmatory technique for laboratory diagnosis of malaria. J Clin Microbiol. 2006;44:1087–1089. doi: 10.1128/JCM.44.3.1087-1089.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malhotra I, Dent A, Mungai P, Muchiri E, King CL. Real-time quantitative PCR for determining the burden of Plasmodium falciparum parasites during pregnancy and infancy. J Clin Microbiol. 2005;43(8):3630–3635. doi: 10.1128/JCM.43.8.3630-3635.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsayed S, Plewes K, Church D, Chow B, Zhang K. Use of molecular beacon probes for real-time PCR detection of Plasmodium falciparum and other Plasmodium species in peripheral blood specimens. J Clin Microbiol. 2006;44:622–624. doi: 10.1128/JCM.44.2.622-624.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangold KA, Manson RU, Koay ESC, Stephens L, Regner MA, Thomson RB, Peterson LR, Kaul KL. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43:2435–2440. doi: 10.1128/JCM.43.5.2435-2440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perandin F, Manca N, Calderaro A, Piccolo G, Galati L, Ricci L, Medici MC, Arcangeletti MC, Snounou G, Dettori G, Chezzi C. Development of a real-time PCR assay for detection of Plasmodium falciparum, Plasmodium vivax, and Plasmodium ovale for routine clinical diagnosis. J Clin Microbiol. 2004;42:1214–1219. doi: 10.1128/JCM.42.3.1214-1219.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grobusch MP, Alpermann U, Schwenke S, Jelinek T, Warhurst DC. False-positive rapid tests for malaria in patients with rheumatoid factor. Lancet. 1999;353:297. doi: 10.1016/s0140-6736(05)74930-8. [DOI] [PubMed] [Google Scholar]
- 26.Hänscheid T, Valadas E. Poor accuracy of rapid diagnostic tests and misdiagnosis of imported malaria: are PCR-based reference laboratories the answer? J Clin Microbiol. 2002;40:736–737. doi: 10.1128/JCM.40.2.736-737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mankhambo L, Kanjala M, Rudman S, Lema VM, Rogerson SJ. Evaluation of the OptiMal rapid antigen test and species-specific PCR to detect placental Plasmodium falciparum infection at delivery. J Clin Microbiol. 2002;40:155–158. doi: 10.1128/JCM.40.1.155-158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Playford EG, Walker J. Evaluation of the ICT malaria P.f/P.v and the OptiMal rapid diagnostic tests for malaria in febrile returned travellers. J Clin Microbiol. 2002;40:4166–4171. doi: 10.1128/JCM.40.11.4166-4171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson DC, Ciach M, Zhong KJY, Crandall I, Kain KC. Evaluation of the Makromed dipstick assay versus PCR for diagnosis of Plasmodium falciparum malaria in returned travelers. J Clin Microbiol. 2002;40:4528–4530. doi: 10.1128/JCM.40.12.4528-4530.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jelinek T, Grobusch MP, Harms G. Evaluation of a dipstick test for the rapid diagnosis of imported malaria among patients presenting within the network TropNetEurop. Scand J Infect Dis. 2001;33:752–754. doi: 10.1080/003655401317074563. [DOI] [PubMed] [Google Scholar]
- 31.Joshi HH. Monoclonal antibody based ELISA: an effective diagnostic tool for the diagnosis of falciparum malaria. J Nep Med Assoc. 2005;44:79–83. [PubMed] [Google Scholar]
- 32.Trenholme KR, Boutlis CS, Kuns R, Lagog M, Bockarie MJ, Gatton M, Kemp DJ, Good MF, Anstey NM, Gardiner DL. Antibody reactivity to linear epitopes of Plasmodium falciparum cytoad-herence linked asexual gene 9 in asymptomatic children and adults from Papua New Guinea. Am J Trop Med Hyg. 2005;72:708–713. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Crouch L, Richie TL, Nhan DH, Coppel RL. Naturally acquired antibody responses to the components of the Plasmodium falciparum merozoite surface protein 1 complex. Parasite Immunol. 2003;25:403–412. doi: 10.1111/j.1365-3024.2003.00647.x. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen MA, Vestergaard LS, Lusingu J, Kurtzhals JAL, Giha HA, Grevstad B, Goka BQ, Lemnge MM, Jensen JB, Akanmori BD, Theander TG, Staalsoe Hviid L. Geographical and temporal conservation of antibody recognition of Plasmodium falciparum variant surface antigens. Infect Immun. 2004;72(6):3531–3535. doi: 10.1128/IAI.72.6.3531-3535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orlandi-Pradines E, Penhoat K, Durand C, Pons C, Bay C, Pradines B, Fusai T, Josse R, Dubrous P, Meynard JP, Durand JP, Migliani R, Boutin JP, Druilhe P, Rogier C. Antibody responses to several malaria pre-erythrocytic antigens as a marker of malaria exposure among travelers. Am J Trop Med Hyg. 2006;74:979–985. [PubMed] [Google Scholar]
- 36.Pierrot C, Wilson S, Lallet H, Lafitte S, Jones FM, Daher W, Capron M, Dunne DW, Khalife J. Identification of a novel antigen of Schistosoma mansoni shared with Plasmodium falciparum and evaluation of different cross-reactive antibody subclasses induced by human schistosmiasis and malaria. Infect Immun. 2006;74:3347–3354. doi: 10.1128/IAI.01724-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okocha EC, Ibeh CC, Ele PU, Ibeh NC. The prevalence of malaria parasitaemia in blood donors in a Nigerian teaching hospital. J Vector Borne Dis. 2005;42:21–24. [PubMed] [Google Scholar]
- 38.Nunez L, Linares J, Perez AH. Seroprevalence of antibodies against Plasmodium falciparum in volunteer donors from various cities in Venezuela. Sangre (Barc) 1992;37(2):141–143. [PubMed] [Google Scholar]
- 39.Bundesärztekammer: Richtlinien zur Gewinnung von Blut und Blutbestandteilen und zur Anwendung von Blutprodukten (Hämotherapie) gemäß ßß 12 und 18 des Transfusionsgesetzes (Novelle 2005). Bundesanzeiger 2005;57 (Nr.209a).
- 40.Joint UKBTS / NIBSC Professional Advisory Committee: Whole Blood & Components Donor Selection Guidelines. http://www.transfusion-guidelines.org.uk/index.asp?Publication=WB
- 41.Woolsey G. Transfusion for pernicious anemia: two cases. Ann Surg. 1911;53:132–134. [Google Scholar]
- 42.Bruce-Chwatt LJ. Blood transfusion and tropical disease. Trop Dis Bull. 1972;69:825–862. [PubMed] [Google Scholar]
- 43.Bruce-Chwatt LJ. Transfusion malaria revisited. Trop Dis Bull. 1982;79:827–840. [PubMed] [Google Scholar]
- 44.Chiodini PL, Hartley S, Hewitt PE, Barbara JA, Lalloo K, Bligh J, Voller A. Evaluation of a malaria antibody ELISA and its value in reducing potential wastage of red cell donations from blood donors exposed to malaria, with a note on a case of transfusion-transmitted malaria. Vox Sang. 1997;73:143–148. doi: 10.1046/j.1423-0410.1997.7330143.x. [DOI] [PubMed] [Google Scholar]
- 45.Mungai M, Tegtmeier G, Chamberland M, Parise M. Transfusion-transmitted malaria in the United States from 1963 through 1999. N Engl J Med. 2001;344:1973–1978. doi: 10.1056/NEJM200106283442603. [DOI] [PubMed] [Google Scholar]
- 46.Slinger R, Giulivi A, Bodie-Collins M, Hindieh F, John RS, Sher G, Goldman M, Ricketts M, Kain KC. Transfusion-transmitted malaria in Canada. CMAJ. 2001;164:377–379. [PMC free article] [PubMed] [Google Scholar]
- 47.Oehlecker F. Übertragung latenter Malaria bei direkter Bluttransfusion. Dtsch Med Wochenschr. 1920;46:1025–1026. [Google Scholar]
- 48.Schnitzler H. Übertragung von latenter Malaria tertiana durch Bluttransfusion. Zentralbl Chir. 1929;56:1438. [Google Scholar]
- 49.Majanz A. Beiträge zur Frage der Bluttransfusion. Dtsch Z Chir. 1930;224:171–185. [Google Scholar]
- 50.Stohlmann H. Malariaübertragung auf Blutspende. Münch Med Wochenschr. 1943;90:84–85. [Google Scholar]
- 51.Menk W. Bluttransfusion und Malaria. Münch Med Wochenschr. 1944;91:349–350. [Google Scholar]
- 52.Lampen H. Induzierte Malaria nach Bluttransfusion. Med Klin. 1947;42:371–372. [Google Scholar]
- 53.Mohr W. Malariaübertragung durch Bluttransfusionsgerät. Med Klin. 1950;45:94. [Google Scholar]
- 54.Nolte K, Stibbe W, Kuhlencord A, Bommer W, Gallasch E. Transfusionsbedingte Malaria tropica. Beitr Infusionsther Klin Ernähr. 1987;18:42–46. [PubMed] [Google Scholar]
- 55.Schunkert H, Handt S, de Wit M, Gladziwa U, Glöckner W, Sieberrth H. Transfusionsmalaria bei Promyelozytenleukämie. Dtsch Med Wochenschr. 1988;113:1841–1843. doi: 10.1055/s-2008-1067898. [DOI] [PubMed] [Google Scholar]
- 56.Witt O, Iglauer A RJ, Bommer W, Eber S. Transfusionsmalaria als Ursache von unklarem postoperativen Fieber. Monatsschr Kinderheilkd. 1998;146:1054–1056. [Google Scholar]
- 57.Frey-Wettstein M, Maier A, Markwalder K, Munch U. A case of transfusion transmitted malaria in Switzerland. Swiss Med Wkly. 2001;131:320. doi: 10.4414/smw.2001.09720. [DOI] [PubMed] [Google Scholar]
- 58.Knobloch, Jürgen., editor. Malaria – Grundlagen und klinische Praxis. Bremen: Unimed; 2003. [Google Scholar]
- 59.Kitchen AD, Barbara JA, Hewitt PE. Documented cases of post-transfusion malaria occurring in England: a review in relation to current and proposed donor-selection guidelines. Vox Sang. 2005;89:77–80. doi: 10.1111/j.1423-0410.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- 60.Guerrero IC, Weniger BG, Schultz MG. Transfusion malaria in the United States, 1972-1981. Ann Intern Med. 1983;99:221–226. doi: 10.7326/0003-4819-99-2-221. [DOI] [PubMed] [Google Scholar]
- 61.Graves P, Gelband H: Vaccines for preventing malaria. Cochrane Database Syst Rev 2006 (2 und 4): CD000129. [DOI] [PubMed]
- 62.Deutsche Gesellschaft für Tropenmedizin und Internationale Gesundheit (DTG): Empfehlungen zur Malariavorbeugung, 2006. www.dtg.org/Malar-ia.html.
- 63.Leitlinie der AWMF (Association of the Medical Societies in Germany): Diagnostik und Therapie der Malaria. Leitlinienregister Nr. 042/001.
- 64.Garfield MD, Ershler WB, Maki DG. Malaria transmission by platelet concentrate transfusion. JAMA. 1978;240:2285–2286. [PubMed] [Google Scholar]
- 65.Dover AS, Guinee VF. Malaria transmission by leukocyte component therapy. JAMA. 1971;217:1701–1702. [PubMed] [Google Scholar]
- 66.Lazner E, Newhouser E. Studies on the transmission of malaria by blood transfusions. Am J Med Sci. 1943;204:141–146. [Google Scholar]
- 67.Al-Saigul AM, Fontaine RE, Haddad Q. Nosocomial malaria from contamination of a multidose heparin container with blood. Infect Control Hosp Epidemiol. 2000;21:329–330. doi: 10.1086/501765. [DOI] [PubMed] [Google Scholar]
- 68.Winterberg DH, Wever PC, Rheenen-Veerberg CV, Kempers O, Durand R, Bos AP, Teeuw AH, Spanjaard L, Dunkert J. A boy with nosocoial malaria tropica contracted in a Dutch hospital. Ped Infect Dis J. 2005;24:89–91. doi: 10.1097/01.inf.0000148881.92329.f6. [DOI] [PubMed] [Google Scholar]
- 69.Besson P, Robert JF, Reviron J, Richard-Lenoble D, Gentilini M. 2 cases of transfusional malaria. Attempted prevention combining an indirect immunofluorescence test with clinical selection crit-era. Rev Fr Transfus Immunohematol. 1976;19:369–373. doi: 10.1016/s0338-4535(76)80076-1. [DOI] [PubMed] [Google Scholar]
- 70.Nahlen BL, Lobel HO, Cannon SE, Campbell CC. Reassessment of blood donor selection criteria for United States travelers to malarious areas. Transfusion. 1991;31:798–804. doi: 10.1046/j.1537-2995.1991.31992094665.x. [DOI] [PubMed] [Google Scholar]
- 71.Bruce-Chwatt LJ. Transfusion malaria. Bull World Health Organ. 1974;50:337–346. [PMC free article] [PubMed] [Google Scholar]
- 72.Seed CR, Kitchen A, Davis TM. The current status and potential role of laboratory testing to prevent transfusion-transmitted malaria. Transfus Med Rev. 2005;19:229–240. doi: 10.1016/j.tmrv.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15:66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vu TT, Tran VB, Phan NT, Le TT, Luong VH, O'Brien E, Morris GE. Screening donor blood for malaria by polymerase chain reaction. Trans R Soc Trop Med Hyg. 1995;89:44–47. doi: 10.1016/0035-9203(95)90652-5. [DOI] [PubMed] [Google Scholar]
- 75.Benito A, Rubio JM. Usefulness of seminested polymerase chain reaction for screening blood donors at risk for malaria in Spain. Emerg Infect Dis. 2001;7:1068. doi: 10.3201/eid0706.010632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Silvie O, Rubinstein E, Franetich JF, Prenant M, Belnoue E, Rénia L, Hannoun L, Eling W, Levy S, Boucheix C, Mazier D. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- 77.Smith TG, Kain KC. Inactivation of Plasmodium falciparum by photodynamic excitation of hemecycle intermediates derived from delta-aminolevulinic acid. J Infect Dis. 2004;190:184–191. doi: 10.1086/421503. [DOI] [PubMed] [Google Scholar]
- 78.Zavizion B, Serebryanik D, Chapman J, Alford B, Purmal A. Inactivation of Gram-negative and Gram-positive bacteria in red cell concentrates using INACTINE PEN110 chemistry. Vox Sang. 2004;87:143–149. doi: 10.1111/j.1423-0410.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- 79.Ferreira-da-Cruz MF, Teva A, Espindola-Mendes EC, dos Santos LG, Daniel-Ribeiro CT. Inactivation of Plasmodium falciparum parasites using gamma-irradiation. Mem Inst Oswaldo Cruz. 1997;92:137–138. doi: 10.1590/s0074-02761997000100029. [DOI] [PubMed] [Google Scholar]
- 80.Grellier P, Santus R, Mouray E, Agmon V, Maziere JC, Rigomier D, Dagan A, Gatt S, Schrével J. Photosensitized inactivation of Plasmodium falciparum and Babesia divergens-infected erythrocytes in whole blood by lipophilic pheophorbide derivatives. Vox Sang. 1997;72:211–220. doi: 10.1046/j.1423-0410.1997.7240211.x. [DOI] [PubMed] [Google Scholar]
- 81.Lustigman S, Ben Hur E. Photosensitized inactivation of Plasmodium falciparum in human red cells by phthalocyanines. Transfusion. 1996;36:543–546. doi: 10.1046/j.1537-2995.1996.36696269514.x. [DOI] [PubMed] [Google Scholar]
- 82.Singh Y, Sawyer LS, Pinkoski LS, Dupuis KW, Hsu JC, Lin L, Corash L. Photochemical treatment of plasma with amotosalen and long-wavelength ultraviolet light inactivates pathogens while retaining coagulation function. Transfusion. 2006;46:1168–1177. doi: 10.1111/j.1537-2995.2006.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


