Abstract
The purpose of this study was to investigate the effect of directional fluid flow on periosteal chondrogenesis. Periosteal explants were harvested from two-month-old rabbits and sutured onto poly-ε-caprolactone (PCL) scaffolds with the cambium layer facing away from the scaffolds. The periosteum/PCL composites were cultured in suspension in spinner flask bioreactors and exposed to various fluid flow velocities: 0, 20, 60, 150 rpm for 4 hours each day for 6 weeks. The application of fluid flow significantly increased percent cartilage yield in periosteal explants from 17% in the static controls to 65-75% under fluid flow (there was no significant difference between 20, 60, or 150 rpm). The size of the neocartilage was also significantly greater in explants exposed to fluid flow compared to static culture. The development of zonal organization within the engineered cartilage was observed predominantly in the tissue exposed to flow conditions. The Young's modulus of the engineered cartilage exposed to 60 rpm was significantly greater than the samples exposed to 150 rpm and 20 rpm. These results demonstrate that application of directional fluid flow to periosteal explants secured onto PCL scaffolds enhances cell proliferation, chondrogenic differentiation, cell organization, and alters the biomechanical properties of the engineered cartilage.
Keywords: periosteum, cartilage, tissue engineering, polycaprolactone, bioreactors, Youngs's modulus
Introduction
Damaged articular cartilage, either traumatic or degenerative, predisposes patients to osteoarthritic changes in the joint, which leads to debilitating joint pain and is a major problem in orthopedics today1,2. Since articular cartilage lacks a suitable intrinsic repair mechanism, repair of the damaged articular cartilage becomes a complex undertaking. Tissue engineering has emerged as a viable option to induce repair and ultimately restore pain free joint function. Functional tissue engineering, in particular, is focused on the biomechanical properties of tissues and in vitro mechanical stimulation to produce a tissue, which meets the functional and mechanical needs of the damaged area3,4. Scientists have used this approach to improve cell adherence, distribution and nutrient diffusion in scaffolds through applying mechanical forces5-9. However, an engineered construct that consistently meets all of the properties of healthy articular cartilage has yet to be found10.
Periosteum, the connective tissue that surrounds bones, has been established as a viable autologous tissue for cartilage repair and cartilage tissue engineering11,12. In addition, although age is a limitation13, recent studies demonstrate that periosteum remains a viable source for musculoskeletal tissue engineering throughout adult life and has the potential to be rejuvenated by local injection of growth factors14-16. Importantly, the quality of neocartilage produced by transplanted periosteum is enhanced by joint motion, especially continuous passive motion17-19. Periosteal explants also respond to mechanical stimulation in tissue culture. Dynamic fluid pressure can enhance periosteal cell proliferation and chondrogenesis in vitro when cultured in agarose suspension20,21. However, other forces such as fluid flow and shear may also be important aspects of mechanical stimulation through joint motion. Therefore, we hypothesized that the application of directional fluid flow on periosteal explants would enhance periosteal cell proliferation and chondrogenesis.
A simple way to produce media flow when culturing chondrogenic cells is by using a spinner flask bioreactor5,22,23. Previous studies demonstrated that the application of mixing in a spinner flask influences the nature of tissue-engineered cartilage produced from chondrocyte-seeded scaffolds5,22,24,25. However, the effects of directional fluid flow on cultured periosteal explants in vitro have not been reported.
Previously, we demonstrated that periosteal tissue grafts sutured to porous poly-ε-caprolactone (PCL), or porous tantalum scaffolds, with the cambium layer facing away from the scaffold, supports the regeneration of osteochondral tissue in vivo26. PCL scaffolds are attractive for tissue regeneration because they are biocompatible and combine slow degradation kinetics with initial mechanical stability27.
In this study, in order to study the effects of directional fluid flow on periosteal explants, we immobilized the explants by suturing them onto porous PCL scaffolds with the cambium facing away from the scaffolds26. The composites were cultured in spinner flasks at various flow velocities and the effects on periosteal tissue growth, chondrogenesis and the biomechanical properties of the resulting neocartilage were analyzed.
Materials and Methods
Scaffold synthesis
Porous PCL scaffolds (10×10×5 mm) were fabricated using a custom-designed rapid prototyping system as previously reported28,29. Briefly, molten PCL polymer (Mw=65,000, MN=42,500; Sigma, MO, USA) was extruded onto a collection platform by air pressure (630 kPa) through the system's nozzle (Ø 200 μm), which is attached to a temperature-controlled reservoir (60°C). This reservoir is mounted onto a 3-axis, computer-controlled 3D motion system. Positioning of the system is controlled via a user-defined computer DMC Smart Terminal program (Galil Motion Control, CA, USA) and the motion controller for the servomotors. X-axis and y-axis servomotors control the system's planar positioning. The PCL was deposited onto a platform connected to the z-axis-servomotor that lowered the platform at the start of each new scaffold layer. The architectural geometry of the scaffolds produced was 0/45/135° with a porosity and pore size ranging from 60-65% and 100-150 μm respectively. The architecture of the scaffolds was selected to be the same as our previous in vivo study in which periosteal grafts were sutured to the PCL scaffolds and implanted into osteochondral defects in rabbits26. The scaffolds were sterilized in 70% ethanol.
Periosteal tissue harvesting and culture
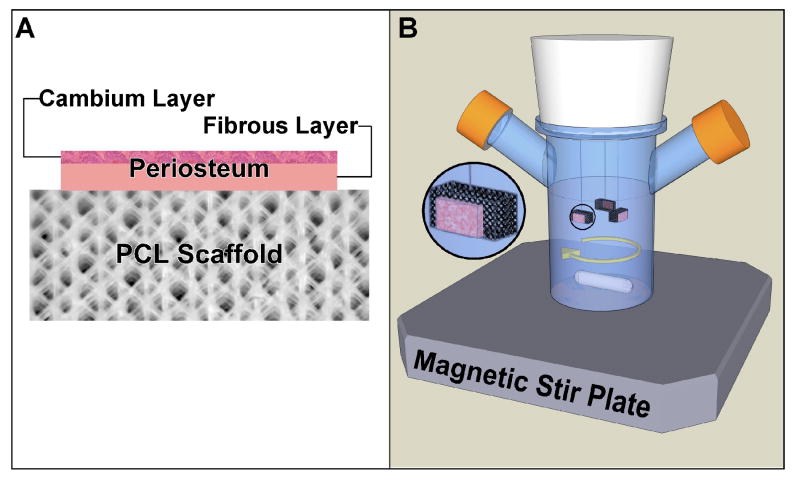
The Institutional Animal Care and Use Committee (IACUC) at Mayo Clinic approved the methods used in this study. Periosteal explants (8×4 mm) were harvested by sharp subperiosteal dissection from the proximal medial tibia of 12 two-month-old New Zealand white rabbits, four from each rabbit30. Explants were obtained within 30 minutes after euthanasia to minimize post-mortem effects on chondrogenic potential31 and sutured onto PCL scaffolds using prolene 7-0 with the cambium layer facing up (Fig. 1). All periosteal explants were placed in Dulbecco's modified Eagle medium (DMEM) with penicillin/streptomycin (50 U/ml and 50 μg/ml) and 1 mM L-proline at 4°C for no longer than 1.5 hours prior to placement into incubator. The scaffolds were threaded onto Kirschner wires that pointed out from the silicone stopper of 100 ml spinner flasks (Bellco Glass, Vineland, NJ). The periosteum/PCL composites were positioned with the periosteum facing the perimeter of the spinner flask (Fig. 1). Each Kirschner wire carried two composites and each flask contained three Kirschner wires. Magnetic stir plates and stir bars (38 mm) generated fluid flow. The composites were divided into four groups defined by the stir rate: 0, 20, 60 and 150 rpm for four hours of spinning each day for the 6-week culture period. Each flask contained 100 ml of DMEM supplemented with 0.1% BSA plus ITS+ (2.08 μg/ml each of insulin, transferring, and selenious acid, plus 1.78 μg/ml linoleic acid and 0.42 mg/ml BSA), 1 mM L-proline, Pen/Strp (50 U/ml and 50 μg/ml), and 50 μg/ml ascorbic acid. The medium was replaced once every week, and cultures were maintained at 37°C, 5% CO2. After six weeks, the length, width, and thickness of the engineered cartilage were measured.
Figure 1.
Illustration of the experimental set up for culturing periosteal explants in spinner flask bioreactors after suturing to PCL scaffolds. A) Illustration of periosteum/PCL scaffold composite with periosteum sutured to the PCL scaffold with the cambium layer facing away from the scaffold. B) Illustration of the spinner flask bioreactor showing the positioning of periosteum/PCL composites with periosteum facing the perimeter of the spinner flask.
Histological Analysis and Scoring
Specimens were fixed in 10% neutral formalin buffer, embedded in paraffin, and 3-μm thick sections were cut from the central portion of the parallel long axis and stained with Safranin-O/fast green for histological analyses32. An automated histomorphometry method was used to determine the percentage red staining in our samples (i.e. cartilage yield) as previously described and validated33.
A blinded observer used a simple histology score (O'Driscoll score), modified from a previously published and validated method34, to assess the cartilage cell and matrix organization. A score of 0 indicated either no cartilage tissue to evaluate or little to no organization of the cartilage cells present (almost homogeneous mixture of cell and cell matrix organization). A score of 3 was given if there were clearly defined layers and the organization of the cartilage cells closely resembled the layering of normal articular cartilage. A score of 1 or 2 was given to those tissue samples that displayed some organization and heterogeneity but were not a 3. A score of 1 was assigned to tissue samples that were closer to being a homogeneous cell mixture and had little organization. While a score of 2 were assigned to those tissue samples that more closely resembled articular cartilage but did not have the clear organization.
Biomechanical testing
The experimental design was repeated with four additional rabbits, in order to produce specimens for biomechanical analysis. After harvesting the samples, we obtained a 3 mm plug for biomechanical tests, from the same area on each scaffold, using a sample corer (FST 18035-03, Ø3 mm). The thickness of each sample was measured with a caliper prior to mechanical testing and the surface of each sample tested was reasonably flat for valid use in biomechanical testing. The mechanical tests were performed on a dynamic testing device (EnduraTec ELF 3200) with a displacement resolution of 1.0 μm. A 50N load cell (Transducer techniques, MDB-10) was used (0.01N resolution). Throughout the tests, the samples were immersed in PBS at room temperature. To determine the equilibrium Young's modulus, we performed unconfined compression using a stress relaxation protocol35. A preload (0.2N) was applied to ensure contact at the cartilage-indenter interface. Following the preload, a stepwise stress-relaxation compression was performed starting at 10% of the sample thickness followed by four incremental steps of 5% up to 30% thickness at a displacement rate of 0.01 mm/s. The relaxation time was estimated at 3, 5, 5, 10 and 10 min for 10%, 15%, 20%, 25% and 30% thickness, respectively. Equilibrium Young's modulus was determined as a linear fit to the equilibrium stress strain curve35.
Statistics
Data were analyzed statistically by Kruskal-Wallis and Wilcoxon signed-rank non-parametric tests and one-way analysis of variance (ANOVA) where appropriate. Statistical differences between treatment groups were evaluated with post hoc testing using the Least Squares Means Differences Student's t-Test (p = 0.05).
Results
Cartilage yield in the engineered tissue
The use of fluid flow for four hours per day resulted in approximately a four-fold increase in cartilage yield compared to static culture. This increase was significant (p < 0.0001) with a cartilage yield of 17 % in the control static culture group compared to 65-75% in the groups subjected to flow (Fig. 2 & 5). The application of fluid flow rather than the velocity of flow was the major determinant of cartilage production from the periosteal explants cultured on PCL scaffolds (Fig. 2 & 5).
Figure 2.
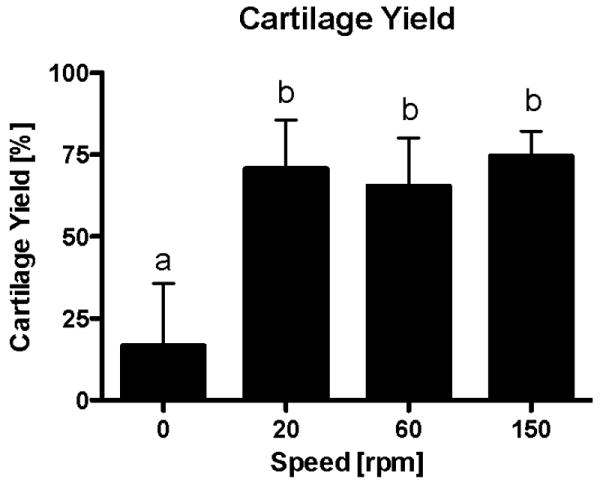
Mean percent cartilage yield from periosteum/PCL composites cultured for six weeks in spinner flask bioreactors. Groups exposed to fluid flow produced significantly more cartilage than static controls (p<0.0001). There was no significant difference due to the velocity of fluid flow applied. The data are presented as mean ± S.E.M., n=10. Lowercase letters indicate the results of post-hoc testing using the Least Squares Means Differences Student's t test (p<0.05). Columns with letters in common are not statistically different.
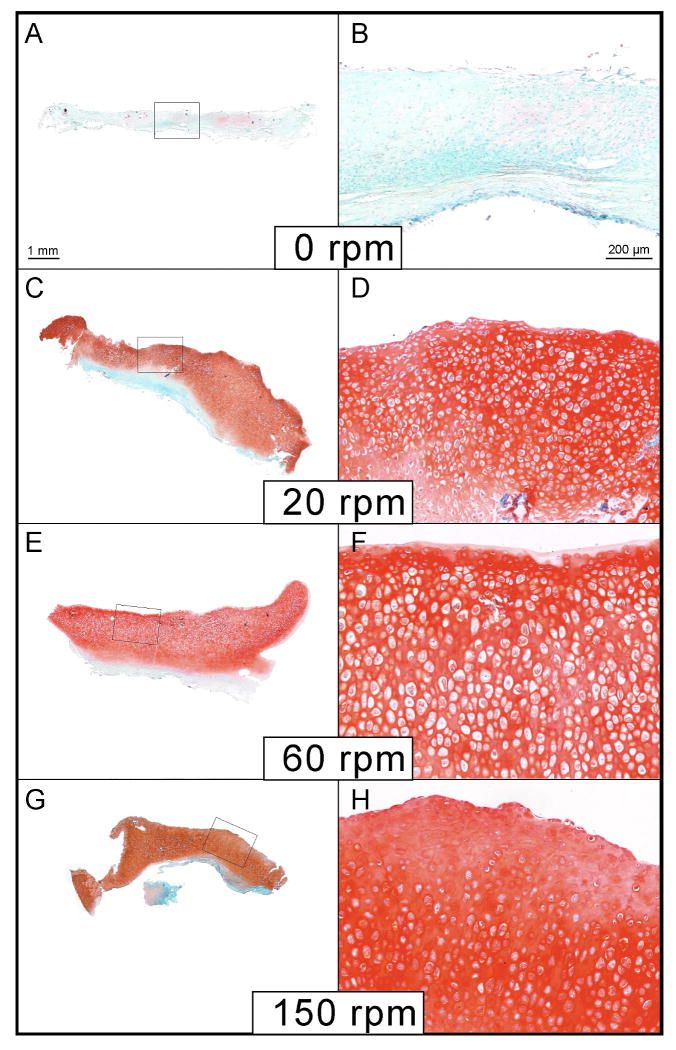
Figure 5.
Histological examples of periosteum/PCL composites cultured in spinner flask bioreactors for six weeks. Sections of the engineered cartilage were stained with safranin O/fast green. (A & B) Minimal cartilage was produced from periosteum/PCL composites cultured under static conditions (0 rpm), as indicated by safranin O/fast green stain (red). Significantly more cartilage was produced under fluid flow, 20 rpm (C &D), 60 rpm (E & F), and 150 rpm (G&H).
Periosteal tissue growth
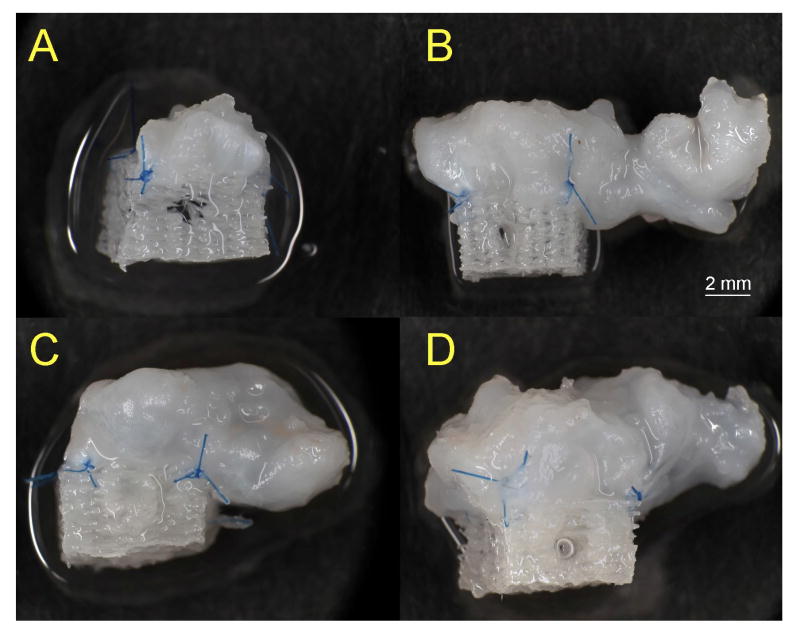
As evident in the gross images in Figure 3, the application of fluid flow for four hours per day to periosteal explants secured to PCL scaffolds resulted in a significant increase in the size of the resulting cartilage after six weeks of culture. In fact, the tissue grew well beyond the dimensions of the scaffold. Notably, the majority of the longitudinal growth of the tissue was in the direction of flow. Similar to the cartilage yield, the primary determinant for tissue growth was the presence or absence of fluid flow producing cartilage approximately 3-fold larger than those produced under static culture condition (p < 0.001).
Figure 3.
Gross view of the resulting engineered cartilage after culturing periosteum/PCL composites in spinner flasks under various velocities of fluid flow for six weeks. A significant increase in tissue growth was observed in the samples exposed to fluid flow compared to those cultured statically. Examples of the engineered cartilage from the A) 0 rpm, B) 20 rpm, C) 60 rpm and D) 150 rpm groups. The greatest tissue outgrowth from the scaffolds was in the direction of the fluid flow (from left to right in these images).
Cellular organization
There was a significant difference in the cell morphology and organization between the tissue samples exposed to fluid flow and those in the static culture group (p < 0.05) (Fig. 5 & 6). There was more heterogeneity and organization of the cells towards the resemblance of articular cartilage in the tissue exposed to flow. The only group that received scores of zero was the static culture group. There was no statistically significant difference in the organization and heterogeneity among the different velocities of fluid flow.
Figure 6.
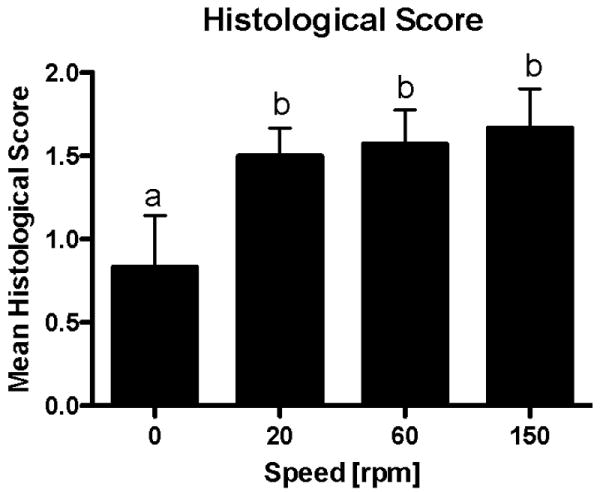
Mean histological score. Scores of 0, 1, 2, or 3 were assigned to each sample of engineered cartilage produced from periosteum/PCL composites cultured in spinner flask bioreactors for six weeks. The scores reflect cell morphology, matrix orientation and organization, and layer development with a score of 3 resembling articular cartilage. The groups experiencing fluid flow during the six-week culture period had a significantly higher mean histological score than those groups cultured under static conditions (p<0.05). The data are presented as mean ± S.E.M., n=6-10. Lowercase letters indicate the results of post-hoc testing using the Least Squares Means Differences Student's t test (p<0.05). Columns with letters in common are not statistically different.
Biomechanical analysis
The experimental design was repeated with four additional rabbits (16 periosteal explants), in order to produce specimens for biomechanical analysis. None of the static culture group produced sufficient tissue for biomechanical tests. The equilibrium Young's modulus for the 20 rpm samples had a mean value of 0.10 ± 0.01 MPa, for the 60 rpm group 0.18 ± 0.04 MPa, and for the 150 rpm group 0.06 ± 0.01 MPa (Fig. 7). We found statistically significant differences across all groups: between 60 and 20 rpm groups (p = 0.0008); 60 and 150 rpm groups (p < 0.0001); and 20 and 150 rpm groups (p = 0.032).
Figure 7.
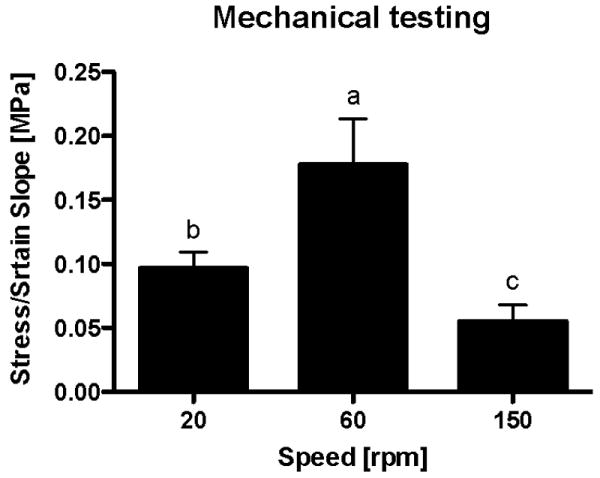
Biomechanical properties of the engineered cartilage produced from periosteum/PCL composites cultured in spinner flask bioreactors for six weeks. None of the samples from the 0 rpm group produced sufficient tissue for biomechanical tests. We found statistically significant differences across all fluid flow groups: between 60 and 20 rpm groups (p = 0.0008); 60 and 150 rpm groups (p < 0.0001); and 20 and 150 rpm groups (p = 0.032). The data are presented as mean ± S.D., n=4. Lowercase letters indicate the results of post-hoc testing using the Least Squares Means Differences Student's t test (p<0.05). Columns with letters in common are not statistically different.
Discussion
In this study, the use of a porous PCL scaffold as a solid support for explanted periosteum enabled us to determine the effects of directional fluid flow on this chondrogenic tissue using a spinner flask bioreactor. Consistent with previous reports23, the application of mechanical stimuli produced dramatic effects on the engineered cartilage compared to static culture. Specifically, similar to reported results with chondrocyte-seeded polyglycolic acid scaffolds cultured in spinner flasks25, the primary determinant of periosteal tissue growth and cartilage production in this experiment was the presence or absence of fluid flow as opposed to the velocity of the flow. Exposure of periosteal explants to directional fluid flow resulted in a significant increase in tissue growth and cartilage yield. Notably, the amount of tissue growth observed under directional fluid flow in this study exceeded what we typically observed previously using other culture protocols on over twenty thousand periosteal explants during the last fifteen years11,20,21,36-38. In addition, much like previous reports, fluid flow altered cell morphology, enhanced the zonal organization of cells within the engineered cartilage and altered the biomechanical properties of the tissue22,39. The zonal cell organization within the engineered cartilage documented in this study is also something that we have not observed previously from cultured periosteal explants. Interestingly, in the present study, the biomechanical properties of the engineered cartilage were dependent on the velocity of fluid flow with the highest Young's modulus observed in the 60 rpm group.
The main roles of the PCL scaffolds used in this experiment were to immobilize the periosteum in order to provide directional fluid flow, and provide sufficient porosity for nutrient exchange and tissue attachment. It is likely that similar results could be achieved with porous scaffolds other than those selected for this study. For example, we previously demonstrated that periosteal explants could be induced to grow cartilage on porous tantalum scaffolds under static culture conditions40 and in vivo26.
Based on what is known about periosteum, and previous studies with engineering cartilage in bioreactors, it might seem obvious that culturing periosteal explants under fluid flow conditions would improve tissue growth and chondrogenesis. However, other bioreactor systems such as roller bottles, agitators, and rotating vessels produced a negative effect on periosteal chondrogenesis in our laboratory (unpublished findings). In addition, while we have been able to increase periosteal cell proliferation and cartilage production using dynamic fluid pressure20,21, over the course of many experiments, this device proved to be inconsistent41. It is important to note that the periosteum was not secured to a solid support in the other bioreactors. Therefore, an important aspect for applying mechanical stimulation to periosteal explants may be to establish and maintain the orientation of the tissue in order to apply directional stimulation.
One of the main advantages of periosteal transplantation is the fact that a tissue culture step is not required for joint resurfacing. However, it is possible that a prior optimized culture step with growth factor and/or mechanical stimulation would enhance the quality and durability of the regenerated tissue produced by periosteum. Future studies are needed to address this issue. In addition, because the cartilage produced from periosteum in the spinner flask was non-uniform, modification of the bioreactor system to minimize turbulent flow will be needed to produce optimized engineered cartilage of appropriate dimensions and shape with a smooth and even surface. Nevertheless, the results from this experiment may help to explain the beneficial effects of continuous passive motion on periosteal chondrogenesis in vivo17-19, and provide a means to further decipher the mechanisms of periosteal tissue response to fluid flow.
Figure 4.
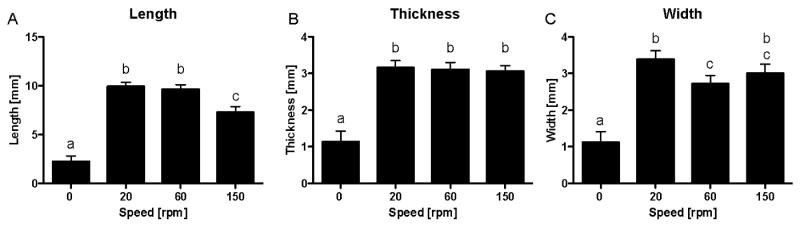
Measurements of tissue engineered cartilage after culturing periosteum/PCL composites in spinner flasks under various velocities of fluid flow for six weeks. For all parameters measured, the mean tissue size in groups exposed to fluid flow was significantly larger than the static control group (p<0.001). (A) As the fluid flow increased, the mean length of the tissue decreased. This difference was significant when increasing the fluid flow from 60 rpm to 150 rpm. For the measurements of thickness (B) and width (C), no such pattern emerged although in mean width there was a significant difference between the 20 rpm and the 60 rpm groups (p<0.05). The data are presented as mean ± S.E.M., n=10. Lowercase letters indicate the results of post-hoc testing using the Least Squares Means Differences Student's t test (p<0.05). Columns with letters in common are not statistically different.
Acknowledgments
This work was supported by the Mayo Clinic Rochester and NIH/NIAMS grant AR45755. This manuscript is dedicated to the memory of our dear friend and colleague Dr. James J. Stone.
References
- 1.CDC. Prevalence of Disabilities and Associated Health Conditions Among Adults---United States, 1999. MMWR. 2001;50(7):120–125. [PubMed] [Google Scholar]
- 2.Buckwalter JA, Martin JA. Osteoarthritis. Adv Drug Deliv Rev. 2006;58(2):150–67. doi: 10.1016/j.addr.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Guilak F, Butler DL, Goldstein SA. Functional tissue engineering: the role of biomechanics in articular cartilage repair. Clin Orthop. 2001;(391 Suppl):S295–305. [PubMed] [Google Scholar]
- 4.Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122(6):570–5. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 5.Freed LE, Vunjak-Novakovic G, Langer R. Cultivation of cell-polymer cartilage implants in bioreactors. J Cellr Biochem. 1993;51(3):257–64. doi: 10.1002/jcb.240510304. Review. [DOI] [PubMed] [Google Scholar]
- 6.Freed LE, Langer R, Martin I, Pellis NR, Vunjaknovakovic G. Tissue Engineering of Cartilage in Space. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):13885–13890. doi: 10.1073/pnas.94.25.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bancroft GN, Sikavitsas VI, Mikos AG. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 2003;9(3):549–54. doi: 10.1089/107632703322066723. [DOI] [PubMed] [Google Scholar]
- 8.Vunjak-Novakovic G, Obradovic B, Martin I, Bursac PM, Langer R, Freed LE. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998;14(2):193–202. doi: 10.1021/bp970120j. [DOI] [PubMed] [Google Scholar]
- 9.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17(1):130–8. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 10.McNickle AG, Provencher MT, Cole BJ. Overview of existing cartilage repair technology. Sports Med Arthrosc. 2008;16(4):196–201. doi: 10.1097/JSA.0b013e31818cdb82. [DOI] [PubMed] [Google Scholar]
- 11.O'Driscoll SW, Recklies AD, Poole AR. Chondrogenesis in periosteal explants. An organ culture model for in vitro study. J Bone Joint Surg Am. 1994;76(7):1042–51. doi: 10.2106/00004623-199407000-00013. [DOI] [PubMed] [Google Scholar]
- 12.O'Driscoll SW. Articular cartilage regeneration using periosteum. Clin Orthop. 1999;367(Suppl):186–203. doi: 10.1097/00003086-199910001-00020. [DOI] [PubMed] [Google Scholar]
- 13.O'Driscoll SW, Saris DB, Ito Y, Fitzimmons JS. The chondrogenic potential of periosteum decreases with age. J Orthop Res. 2001;19(1):95–103. doi: 10.1016/S0736-0266(00)00014-0. [DOI] [PubMed] [Google Scholar]
- 14.De Bari C, Dell'Accio F, Vanlauwe J, Eyckmans J, Khan IM, Archer CW, Jones EA, McGonagle D, Mitsiadis TA, Pitzalis C, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54(4):1209–21. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- 15.Jansen EJ, Emans PJ, Guldemond NA, van Rhijn LW, Welting TJ, Bulstra SK, Kuijer R. Human periosteum-derived cells from elderly patients as a source for cartilage tissue engineering? J Tissue Eng Regen Med. 2008;2(6):331–9. doi: 10.1002/term.100. [DOI] [PubMed] [Google Scholar]
- 16.Reinholz GG, Fitzsimmons JS, Casper ME, Ruesink TJ, Chung HW, Schagemann JC, O'Driscoll SW. Rejuvenation of periosteal chondrogenesis using local growth factor injection. Osteoarthritis Cartilage. 2009;17(6):723–34. doi: 10.1016/j.joca.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubak JM, Poussa M, Ritsilä V. Effects of joint motion on the repair of articular cartilage with free periosteal grafts. Acta Orthop Scand. 1982;53:187–191. doi: 10.3109/17453678208992199. [DOI] [PubMed] [Google Scholar]
- 18.O'Driscoll SW, Salter RB. The repair of major osteochondral defects in joint surfaces by neochondrogenesis with autogenous osteoperiosteal grafts stimulated by continuous passive motion. An experimental investigation in the rabbit. Clin Orthop Relat Res. 1986;(208):131–40. [PubMed] [Google Scholar]
- 19.O'Driscoll SW, Keeley FW, Salter RB. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J Bone Joint Surg. 1988;70A:595–606. [PubMed] [Google Scholar]
- 20.Saris DB, Sanyal A, An KN, Fitzsimmons JS, O'Driscoll SW. Periosteum responds to dynamic fluid pressure by proliferating in vitro. J Orthop Res. 1999;17(5):668–77. doi: 10.1002/jor.1100170508. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee N, Saris DBF, Schultz FM, Berglund LJ, An KN, O'Driscoll SW. The enhancement of Periosteal chondrogenesis by Dynamic Fluid Pressure. J Orthop Res. 2001;19(4):524–530. doi: 10.1016/S0736-0266(00)00045-0. [DOI] [PubMed] [Google Scholar]
- 22.Freed LE, Vunjak-Novakovic G. Tissue Culture Bioreactors: Chondrogenesis as a Model System. In: Lanza R, Langer R, Chick W, editors. Principles of Tissue Engineering. Austin, TX: R.G. Landes Company; 1997. pp. 151–165. [Google Scholar]
- 23.Darling EM, Athanasiou KA. Articular cartilage bioreactors and bioprocesses. Tissue Eng. 2003;9(1):9–26. doi: 10.1089/107632703762687492. [DOI] [PubMed] [Google Scholar]
- 24.Gooch KJ, Blunk T, Courter DL, Sieminski AL, Bursac PM, Vunjak-Novakovic G, Freed LE. IGF-I and mechanical environment interact to modulate engineered cartilage development. Biochem Biophys Res Commun. 2001;286(5):909–15. doi: 10.1006/bbrc.2001.5486. [DOI] [PubMed] [Google Scholar]
- 25.Gooch KJ, Kwon JH, Blunk T, Langer R, Freed LE, Vunjak-Novakovic G. Effects of mixing intensity on tissue-engineered cartilage. Biotechnol Bioeng. 2001;72(4):402–7. doi: 10.1002/1097-0290(20000220)72:4<402::aid-bit1002>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Mrosek EH, Schagemann JC, Chung HW, Fitzsimmons JS, Yaszemski MJ, Mardones RM, O'Driscoll SW, Reinholz GG. Porous tantalum and poly-epsilon-caprolactone biocomposites for osteochondral defect repair: preliminary studies in rabbits. J Orthop Res. 2010;28(2):141–8. doi: 10.1002/jor.20983. [DOI] [PubMed] [Google Scholar]
- 27.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21(24):2529–43. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 28.Stone J, Thoreson A, Langner K, Norton J, Stone D, Wang F, O'Driscoll S, An K. Computer-Aided Design, Manufacturing, and Modeling of Polymer Scaffolds for Tissue Engineering. IMECE 2005-81621; ASME International Mechanical Engineering Conference 2005. [Google Scholar]
- 29.Schagemann JC, Chung HW, Mrosek EH, Stone JJ, Fitzsimmons JS, O'Driscoll SW, Reinholz GG. Poly-epsilon-caprolactone/gel hybrid scaffolds for cartilage tissue engineering. J Biomed Mater Res A. 2009 Jul 6; doi: 10.1002/jbm.a.32521. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.O'Driscoll SW, Fitzsimmons JS. The importance of procedure specific training in harvesting periosteum for chondrogenesis. Clin Orthop. 2000;380:269–278. doi: 10.1097/00003086-200011000-00036. [DOI] [PubMed] [Google Scholar]
- 31.O'Driscoll SW, Meisami B, Miura Y, Fitzsimmons JS. Viability of periosteal tissue obtained postmortem. Cell Transplant. 1999;8(6):611–6. doi: 10.1177/096368979900800607. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg L. Chemical basis for the histological rise of safranin 0 in the study of articular cartilage. J Bone Joint Surg. 1971;53A:69–82. [PubMed] [Google Scholar]
- 33.O'Driscoll SW, Marx RG, Fitzsimmons JS, Beaton DE. Method for automated cartilage histomorphometry. Tissue Eng. 1999;5:13–23. doi: 10.1089/ten.1999.5.13. [DOI] [PubMed] [Google Scholar]
- 34.O'Driscoll SW, Marx RG, Beaton DE, Miura Y, Gallay SH, Fitzsimmons JS. Validation of a simple histological-histochemical cartilage scoring system. Tissue Eng. 2001;7(3):313–320. doi: 10.1089/10763270152044170. [DOI] [PubMed] [Google Scholar]
- 35.Boschetti F, Peretti GM. Tensile and compressive properties of healthy and osteoarthritic human articular cartilage. Biorheology. 2008;45(3-4):337–44. [PubMed] [Google Scholar]
- 36.O'Driscoll SW, Fitzsimmons JS. The role of periosteum in cartilage repair. Clin Orthop. 2001;(391 Suppl):S190–207. doi: 10.1097/00003086-200110001-00019. [DOI] [PubMed] [Google Scholar]
- 37.Miura Y, Parvizi J, Fitzsimmons JS, O'Driscoll SW. Brief exposure to high-dose transforming growth factor-beta1 enhances periosteal chondrogenesis in vitro: a preliminary report. J Bone Joint Surg Am. 2002;84-A(5):793–9. doi: 10.2106/00004623-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA, O'Driscoll SW. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage. 2003;11(1):55–64. doi: 10.1053/joca.2002.0869. [DOI] [PubMed] [Google Scholar]
- 39.Vunjak-Novakovic G, Freed LE, Biron RJ, Langer R. Effects of mixing on the composition and morphology of tissue-engineered cartilage. AIChE Journal. 1996;42:850–860. [Google Scholar]
- 40.Mardones RM, Reinholz GG, Fitzsimmons JS, Zobitz ME, An KN, Lewallen DG, Yaszemski MJ, O'Driscoll SW. Development of a biologic prosthetic composite for cartilage repair. Tissue Eng. 2005;11(9/10):1368–78. doi: 10.1089/ten.2005.11.1368. [DOI] [PubMed] [Google Scholar]
- 41.Fitzsimmons JS, O'Driscoll SW, Reinholz GG. Serum-soluble factor(s) regulate the mechanical stimulation of chondrogenesis. Orthopaedic Research Society 51st Annual Meeting; Washington, D.C.. 2005. [Google Scholar]