Abstract
In mammals, synchronization of the circadian pacemaker in the hypothalamus is achieved through direct input from the eyes conveyed by intrinsically photosensitive retinal ganglion cells (ipRGCs). Circadian photoentrainment can be maintained by rod and cone photoreceptors, but their functional contributions and their retinal circuits that impinge on ipRGCs are not well understood. We demonstrate in genetic mouse models lacking functional rods, or where rods are the only functional photoreceptors, that rods are solely responsible for photoentrainment at scotopic light intensities. Surprisingly, rods were also capable of driving circadian photoentrainment at photopic intensities where they were incapable of supporting a visually–guided behavior. Using animals in which cone photoreceptors were ablated, we demonstrate that rods signal through cones at high light intensities, but not low light intensities. Thus two distinct retinal circuits drive ipRGC function to support circadian photoentrainment across a wide range of light intensities.
Keywords: melanopsin, outer retinal photoreceptors, ipRGCs, retinal ganglion cells, cones, non–image forming vision
Introduction
Daily variations in sleep, feeding, and hormone regulation are synchronized to the solar day via circadian photoentrainment allowing organisms to anticipate the availability of food or predator activity for optimal survival. In mammals, circadian photoentrainment is dependent on the light–evoked output from intrinsically photosensitive retinal ganglion cells (ipRGCs)1–4 to the master clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus. These ipRGCs express the photopigment melanopsin3, 5, 6, and their phototransduction cascade generates depolarizing light responses that evoke action potentials5. However, phototransduction in ipRGCs is relatively insensitive, and cannot drive physiological responses at low light intensities7–9. Instead, the outer retinal photoreceptors, the rods and cones, drive ipRGC activity through the retinal circuitry8, 10 and along with phototransduction in ipRGCs can account fully for non–image forming visual functions including phase shifting of the circadian oscillator, pupil constriction, and masking11, 12.
A goal of recent studies has been to identify the relative contributions of rods and cones to circadian light responses. However, a limitation of these studies are that mouse models used to delineate the contributions of rods from cones either alter the development of the retina13, or cause retinal degeneration14–16. The broad spectral tuning of the photoreceptors and electrical coupling between rods and cones further complicate the determination of the sufficiency of rods and cones for driving non–image forming visual functions17, 18. Ultimately strategies for determining the functional contribution of rods versus cones should silence individual photoreceptor classes without influencing the remaining retinal cells or circuits.
To determine the contribution of individual photoreceptors to circadian photoentrainment, especially the sufficiency of rods and cones to drive photoentrainment, we used several lines of transgenic mice that eliminate selectively rod or cone phototransduction pathways without the induction of non–specific retinal degeneration. We show that rod photoreceptors are capable of driving non–image forming visual functions across a surprisingly wide range of light intensities. We also demonstrate that for rod photoreceptors to mediate this wide–ranging function, two distinct retinal circuits are used. Rod input through the rod bipolar pathway is necessary for circadian photoentrainment at low light intensity, but rod signaling through cone photoreceptors is required for photoentrainment at high light intensities.
Results
Rods drive circadian photoentrainment
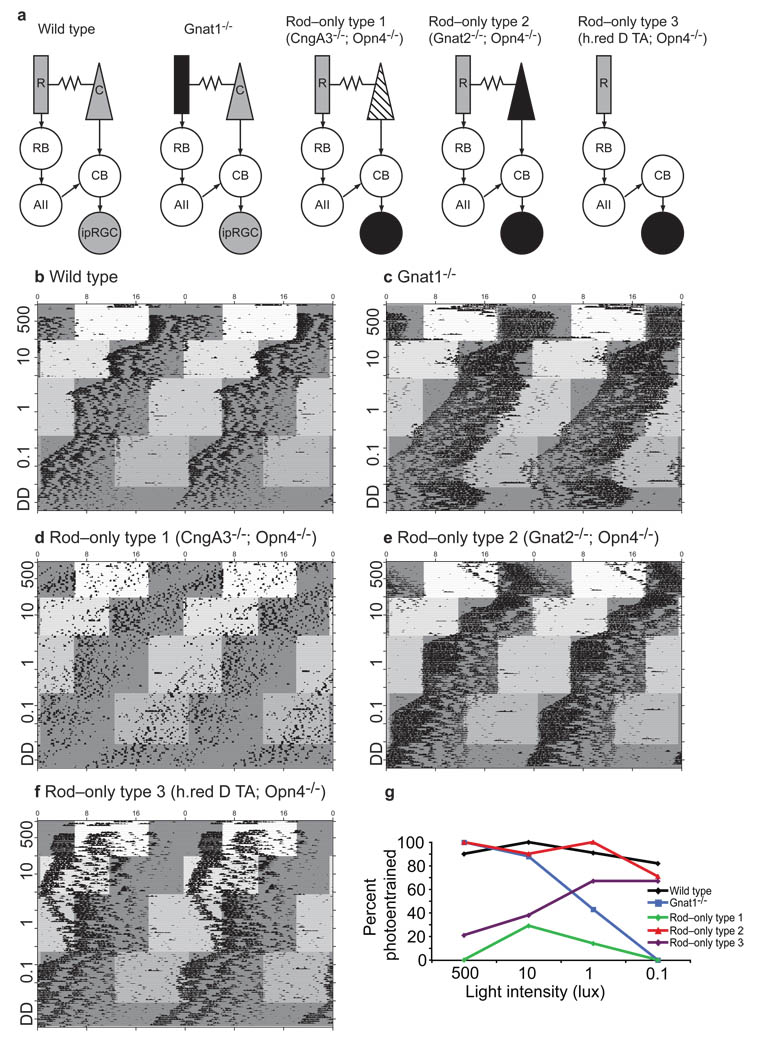
To determine whether rod phototransduction is necessary for circadian photoentrainment, we used mice homozygous for an inactivating mutation at the rod transducin locus19 (Gnat1–/–, thereby leaving cones and ipRGCs as the only remaining retinal photoreceptors; Fig. 1a). This mutation renders rods incapable of transducing light information without complications from retinal degeneration, which is a common outcome of rod dysfunction14. We tested the ability of Gnat1–/– mice to entrain over a 5000–fold range of irradiances (500 lux, 1.7 W/m2, or 365,000 photoisomerizations rod–1 sec–1 through 0.1 lux, 0.34 mW/m2, or 73 photoisomerizations rod–1 sec–1) by decreasing the light intensity concurrently with a 6–hour phase advance in the light cycle (Fig. 1). As expected, the Gnat1–/– mice photoentrained at high light intensities (Figs. 1c, S1, and Table 1), in agreement with previous studies showing that ipRGCs alone are sufficient for circadian light responses using mice with retinal photoreceptor degeneration15, 20–22. However, Gnat1–/– mice fail to photoentrain with a stable phase onset at low light intensities, with some animals showing a complete free running rhythm (Figs. 1c, S1, and Table 1). It is important to note that while some Gnat1–/– mice show photic responses at low light intensities (Fig. S1), not a single Gnat1–/– mouse maintained a 24–hour period or a stable phase relation to the light–dark cycle, criteria important for defining an animal as photoentrained. These results show that cone and ipRGC phototransduction pathways do not have sufficient sensitivity to photoentrain animals at low light intensity, indicating that rod photoreceptors are necessary under these conditions.
Figure 1. Rods drive circadian photoentrainment across a wide range of light intensities.
(A) Retinal schematics for all transgenic mouse lines used; gray is functional photoreceptor, black is non–functional resembling dark state, and striped is non–functional resembling saturating light state. (B–F) Representative double–plotted wheel running activity records for (B) WT, (C) Gnat1–/–, (D) Rod–only type 1, (E) Rod–only type 2, and (F) Rod–only type 3 mice assaying for photoentrainment to a 12: 12 hour light: dark cycle which advances six hours concurrently with each intensity decrease. Local time is indicated at the top of each graph and light intensity (lux) is indicated along y–axis of each actogram. Mice were exposed to a 6 hour advanced cycle before the start of this experiment. Note the re–entrainment time course at the 500 lux intensity in all mice which were able to photoentrain (panels B, C, and E, but not D and F). (G) Summary of percentage of photoentrained animals for all genotypes. Refer to Table 1 for number of animals and statistics for each genotype at each light intensity.
Table 1. Summary of photoentrainment of each genotype at all light intensities.
Percent photoentrained, length of circadian period, mean phase angle and variance of phase angle are shown at each light intensity for WT, Gnat1−/−, Rod–only type 1, Rod–only type 2 and Rod–only type 3 mice. Phase angle and average phase angle variance are only calculated for the animals which photoentrained. 500 lux statistics were taken from analysis of photoentrainment shown in Figure S2. Free–running animals were not included in photoentrainment analysis.
| 500 lux | 10 lux | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample size |
Percent photoentrained |
Phase angle |
Variance | Sample size |
Percent photoentrained |
Phase angle |
Variance | |
| Wild type | 12 | 100 | −0.09 | 0.17 | 10 | 100 | 0.73 | 0.54 |
| Gnat1−/− | 12 | 100 | 1.81 | 1.01 | 9 | 88 | 0.18 | 0.61 |
| Rod–only type 1 |
11 | 0 | –– | –– | 7 | 29 | 0.19 | 0.32 |
| Rod–only type 2 |
10 | 100 | −0.3 | 0.26 | 10 | 90 | 0.37 | 0.3 |
| Rod–only type 3 |
13 | 7 | –– | –– | 13 | 31 | 2.07 | 0.32 |
| 1 lux | 0.1 lux | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample size |
Percent photoentrained |
Phase angle | Variance | Sample size |
Percent photoentrained |
Phase angle |
Variance | |
| Wild type | 11 | 91 | 0.33 | 0.31 | 11 | 82 | −0.27 | 0.41 |
| Gnat1−/− | 9 | 43 | 0.36 | 0.44 | 6 | 0 | –– | –– |
| Rod–only type 1 |
7 | 14 | –– | –– | 3 | 0 | –– | –– |
| Rod–only type 2 |
9 | 100 | 0.32 | 0.28 | 7 | 71 | −1.18 | 0.36 |
| Rod–only type 3 |
13 | 62 | 0.35 | 0.52 | 10 | 50 | 0.31 | 0.42 |
| Period in constant dark | ||
|---|---|---|
| Mean | SEM | |
| Wild type | 23.71 | 0.06 |
| Gnat1−/− | 23.88 | 0.06 |
| Rod–only type 1 |
23.29 | 0.20 |
| Rod–only type 2 |
23.83 | 0.06 |
| Rod–only type 3 |
23.47 | 0.10 |
We studied two lines of mice where rods are the only functional photoreceptors to investigate whether rod phototransduction is sufficient for circadian photoentrainment (Fig. 1a). First, we studied mice lacking cone and ipRGC phototransduction pathways through the deletion of both the cone cyclic nucleotide gated (CNG) channel and melanopsin protein3, 23 (Cnga3–/–; Opn4–/–, referred to as rod–only type 1). Second, we studied mice lacking cone and ipRGC phototransduction pathways through the loss of the alpha subunit of cone transducin and melanopsin protein3, 24 (Gnat2cpfl3/cpfl3; Opn4–/–, referred to as rod–only type 2). Rod and cone photoreceptors remain depolarized in the dark and respond to absorbed light with graded hyperpolarizations in membrane potential that saturate when all photoreceptor outer segment CNG channels are closed. Therefore, rod–only type 1 and rod–only type 2 mice both lack light–driven signals from the cone phototransduction pathway, but have important differences. In the rod–only type 1 mice, the loss of the CNG channels in cones should relegate cones to persistent hyperpolarization, mimicking saturating light. Alternatively, in rod–only type 2 mice the loss of cone transducin leaves cones persistently depolarized, mimicking the dark state. We sought to determine how each of these manipulations would influence non–image forming visual functions.
To determine rod contributions to circadian photoentrainment, we measured the ability of rod–only type 1 and rod–only type 2 mice to photoentrain. We found surprisingly that rod–only type 1 animals were unable to photoentrain to either low or high light intensities, in apparent contradiction to the Gnat1–/– results showing that rods are necessary for entrainment at low light intensities (compare Figs. 1c, d and Table 1). In contrast, the rod–only type 2 animals photoentrained at both low and high light intensities (Figs. 1e, S1, and S2). To confirm these findings, we delayed the light onset by 6 hours and assayed for re–entrainment (Fig. 1). Rod–only type 2 animals were able to re–entrain (Figs. 1b,c,e) demonstrating that rods signal light information from the outer retina to the ipRGCs for photoentrainment even under conditions where a significant fraction of the visual pigment in rods is bleached (Fig. 1e and S1). However, rod–only type 1 animals remained unable to photoentrain. Thus it appears that the ‘saturating light’ state of rod–only type 1 cones interferes with the ability of rods to drive photoentrainment, while rod–only type 2 cones in the ‘dark’ state do not.
Rods use two retinal circuits to signal to ipRGCs
In the mammalian retina, rods exploit cone circuitry to signal the presence of light to ganglion cells25. At scotopic light intensities signals pass through the rod bipolar pathway, a highly convergent pathway where rod signals pass to rod bipolar cells and onto AII amacrine cells before feeding to ON and OFF cone bipolar terminals and their respective ganglion cells25, 26. At mesopic light intensities, gap junctions between rods and cones are believed to allow rods to signal light–evoked activity to ganglion cells via cone pathways27.
To distinguish the retinal pathway for circadian photoentrainment, we used a mouse line where cone photoreceptors have been ablated (thus eliminating the rod–cone pathway while keeping the remaining rod pathways intact). These mice were generated by expressing an attenuated diphtheria toxin A subunit under the control of a promoter selective for cones in a melanopsin knockout background3, 28 (h.red DT–A; Opn4–/–, referred to as rod–only type 3, Fig 1a). We found that while the rod–only type 3 mice were unable to stably photoentrain at high light intensities (Figs. 1f, S2, and Table 1), most animals were able to photoentrain at lower light intensities (Figs. 1f,g, S1 and Table 1). These results reveal that for circadian photoentrainment, rods signal through the rod bipolar pathway at low light intensities. In addition, since rod–only type 2 but not rod–only type 3 animals show normal photoentrainment at high light intensities, these results indicate that rods use the rod–cone pathway for photoentrainment at these light intensities.
Visual functions are normal in all rod–only types
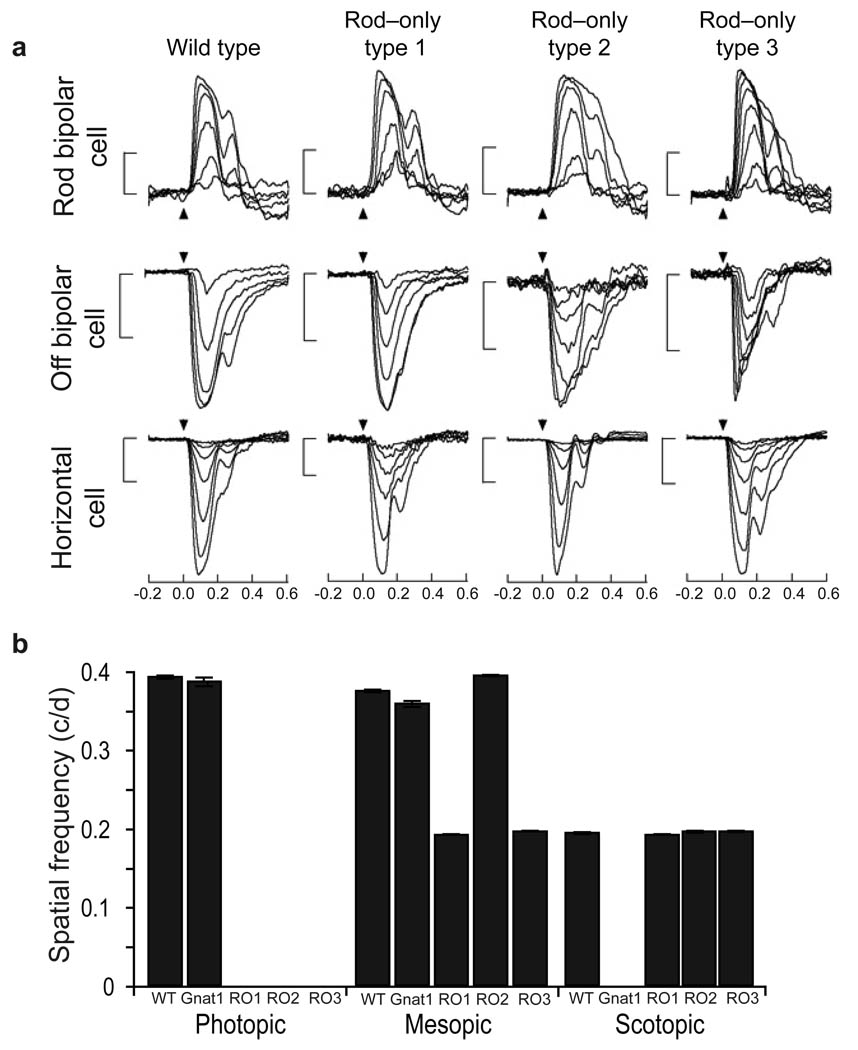
The differences in circadian photoentrainment between the rod–only animals prompted us to investigate whether spatial vision and the retinal circuitry for rod signaling are compromised in these models. We investigated how the sensitivity of light–evoked signals in the identified rod retinal circuits was influenced in all rod–only animals. Current clamp recordings were used to measure light–evoked changes in membrane potential for rod bipolar, OFF bipolar, and horizontal cells in response to brief flashes of light of increasing strength. We found that all rod–only type mice exhibit responses of similar sensitivity to WT mice (Fig. 2a). Combined, these results show that the sensitivity of rod signaling within the retina of rod–only type 1, rod–only type 2, and rod–only type 3 animals is indistinguishable from WT animals.
Figure 2. Rod–cone pathway is important for mesopic light signaling.
(A) Representative traces from current clamp recordings of membrane potential as a function of time from rod bipolar, off bipolar and horizontal cells of WT, rod–only type 1, rod–only type 2, and rod–only type 3 mice show that rod input to each cell type is intact. Arrow represents timing of a 10 ms flash whose strength was increased by a factor of two from generating a just–detectable response to response saturation. Flash strengths for all cells ranged from 0.2 to 30 R* per rod. All scale bars are 10mV. (B) The acuity (cycles/ degree) of mice lacking rod function (Gnat1–/–) is comparable to WT mice at photopic light intensities, while the acuity of rod–only type 1, rod–only type 2, and rod–only type 3 animals is equivalent to that seen in WT animals in scotopic light. Rod–only type 2, but not rod–only types 1 and rod–only type 3, showed similar visual acuity to WT at mesopic light levels. Note that all rod–only mice failed to track at photopic light intensities, while Gnat1–/– animals did not track at scotopic light intensities. Error bars represent SEM.
Furthermore, to determine if the rod signals contribute to vision in the rod–only mouse models, we measured the spatial frequency threshold utilizing an optokinetic tracking task where mice track reflexively a virtual cylinder patterned with a moving sine wave grating29. At scotopic light levels30, all rod–only mice performed similarly to WT animals, whereas Gnat1–/– animals were unable to track the grating at any spatial frequency (Fig. 2b). As expected, all rod–only animals failed to perform the tracking at photopic light intensities, likely due to the bleaching and background desensitization of rods, whereas Gnat1–/– animals tracked normally using cone phototransduction (Fig. 2b). Together, these observations indicate that although rod–only type 1 animals show strong defects in circadian photoentrainment, they retain similar sensitivity as rod–only type 2 animals (and WT) for visual function. Thus the mechanisms underlying rod signaling for circadian photoentrainment may be distinct from those for spatial vision.
Previous studies have established that rods signal through a cone–cell dependent pathway at mesopic light intensities25, 27. We sought to understand how well the rod–only mice performed the optokinetic tracking at mesopic light levels. We found that the rod–cone pathway is utilized to signal light information from rods, since rod–only type 3 mice had impaired tracking at mesopic intensities (Fig. 2b). We also show that rod–only type 1 and rod–only type 3 mice have lower acuity than rod–only type 2 mice, which show WT–like tracking at mesopic light intensities. The higher acuity of rod–only type 2 animals indicates that rods confer greater acuity at mid–light levels when they signal via the rod–cone pathway (Fig. 2b). Finally, rod–only type 1 animals track with the same acuity as rod–only type 3 animals suggesting that when cones are in a saturating light state or are absent, rods cannot utilize the rod–cone pathway for signaling light to the brain (Fig. 2b), in agreement with circadian photoentrainment results (Fig. 1).
Rods influence several light dependent behaviors
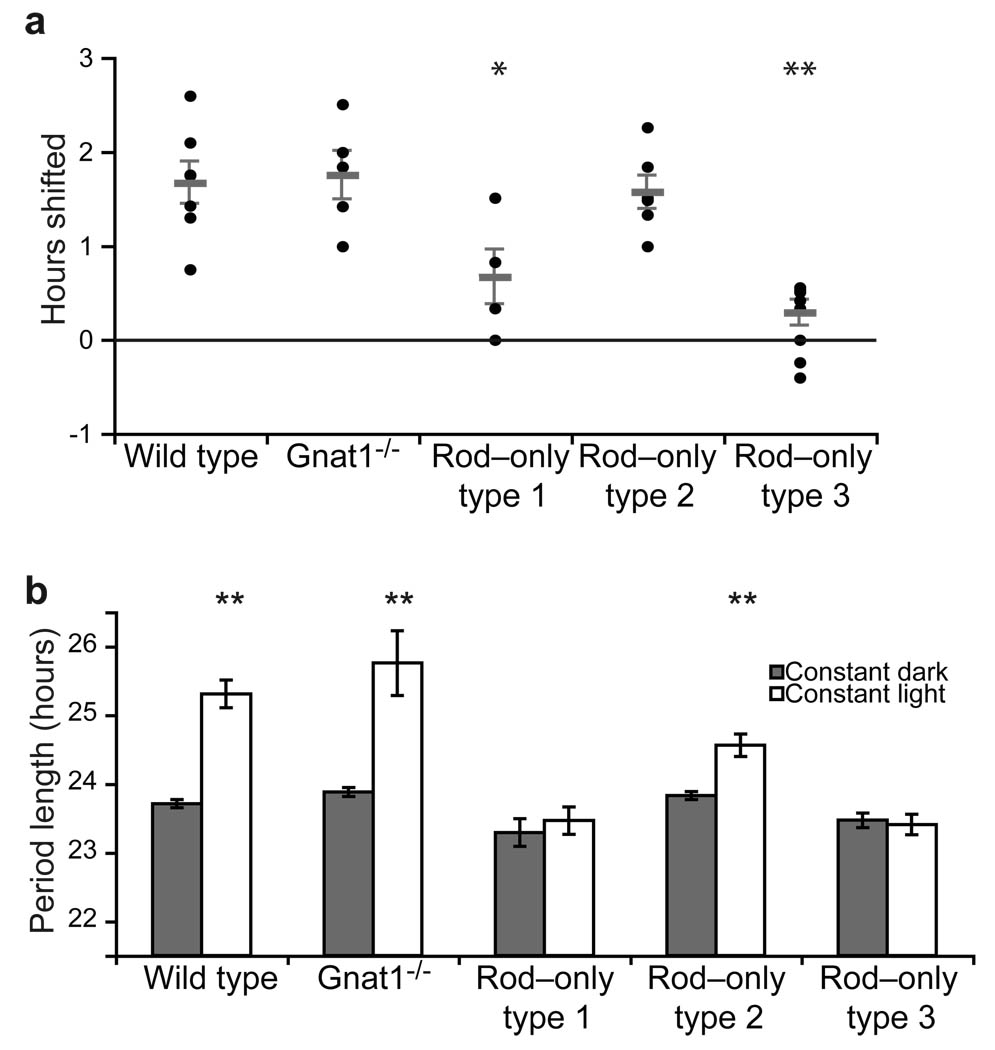
To determine whether the strength of light–evoked signals in rods is sufficient to influence the phase of the circadian system, we measured phase shifts in free running rhythms in response to a 15–minute light pulse (1000 lux, 3.41 W/m2, or 730,000 photoisomerizations rod–1 sec–1) in Gnat1–/–, and rod–only animals. We found that WT, Gnat1–/–, and rod–only type 2 mice produced similar phase delays in response to light (WT: 1.7±0.2, Gnat1–/–: 1.7±0.3, and rod–only type 2: 1.6±0.2 hours; Fig. 3a). However, this high intensity light pulse failed to produce a significant change in the phase of the rhythm for rod–only type 1 or rod–only type 3 animals (Fig. 3a). These results are consistent with the observation that rod–only type 3 mice fail to photoentrain at high light intensity (Figs 1f, and S2) and highlight that the rod–cone pathway is necessary for the circadian phase shifting effects of light.
Figure 3. Rods contribution to phase shifts and period lengthening in constant light is dependent on cone state.
(A) WT, Gnat1–/–, and rod–only type 2 mice respond similarly in response to 15 minute, 1000 lux white light pulse administered at CT16 while rod–only type 1 and rod–only type 3 animals show minimal shift in activity onset. (B) Period length in constant darkness is compared to period length in constant light in all animals. WT, Gnat1–/– and rod–only type 2 animals all show significant period lengthening in constant light however, rod–only type 2 period length in constant light in significantly shorter than that seen in WT animals. Rod–only type 1 and 3 animals show no significant period lengthening in constant light. p<0.05 is represented by * and p<0.01 is represented by **. Error bars represent SEM.
Previous reports in animals that lack melanopsin–based phototransduction (Opn4–/–) showed attenuated period lengthening in constant light as compared to WT animals31, 32. To investigate if animals with only functional rods show period lengthening in constant light conditions, we measured the period lengths of Gnat1–/–, and rod–only animals in both constant darkness and constant light (500 lux, 1.7 W/m2, or 365,000 photoisomerizations rod–1 sec–1). In constant darkness, mice free run with an endogenous period shorter than 24 hours (WT: 23.7±0.1, Gnat1–/–: 23.9±0.1, rod–only type 1: 23.3±0.2, rod–only type 2: 23.8±0.6 and rod–only type 3: 23.5±0.1). Gnat1–/– animals increased their period length in constant light to the same level as WT animals (Fig. 3b). Rod–only type 1 animals, which showed limited ability to photoentrain in all light intensities and rod–only type 3 animals, which failed to photoentrain at high light intensities, did not increase their period length in response to constant light (Fig. 3b; rod–only type 1: 23.5±0.2, rod–only type 3: 23.4±0.2). In contrast, rod–only type 2 animals increased their period length significantly in constant light (Fig. 3b; rod–only type 2: 24.6±0.2, p=0.0007). However, in rod–only type 2 mice the increases in period length were attenuated significantly compared to WT and Gnat1–/– animals (p=0.012), and matched that previously observed for Opn4–/– animals32. These results indicate that rods are capable of signaling light to the brain to influence the period length under constant light conditions.
Discussion
It has been appreciated for several decades that the circadian oscillator in the SCN can be entrained to the day–night cycle by virtue of light–evoked signaling originating in the eyes33, even in the absence of rod and cone photoreceptors. These observations led to the discovery of a specialized class of retinal ganglion cells, ipRGCs, that themselves are light sensitive and send projections to the SCN3, 5. Subsequent work has been focused on accounting for how the ipRGCs, and the rod and cone photoreceptors, contribute to various circadian functions34. While ipRGCs have been recognized as the only input to the SCN for relaying light–evoked signals for circadian photoentrainment1, 2, 4, melanopsin phototransduction is relatively insensitive. Instead the rods and cones must provide input to the ipRGCs to account for the robust operating range of non–image forming functions7, 8, 35. However, for circadian photoentrainment, the relative contribution of the three retinal photoreceptors has proven quite controversial12, 14–16, 20, 21, 36. Here we distinguished the role of rods and cones (and ipRGCs) to circadian functions and found: 1) Gnat1–/– mice lacking functional rods, but retaining both cone and melanopsin phototransduction pathways, do not photoentrain at scotopic light levels demonstrating that rods are necessary for circadian photoentrainment at low light intensities, 2) the ability of rod–only type 2 animals to photoentrain at all tested light levels indicating that light detection by rods is sufficient for photoentrainment across a surprisingly wide range of light intensities (ranging from approximately 102 to 106 photoisomerizations rod–1 sec–1), 3) rod–only type 3 animals entrain at low but not high light intensities suggesting that rod photoreceptors use two distinct pathways for photoentrainment; the primary rod bipolar pathway at low light intensities and electrical coupling to cones in the rod–cone pathway at high light intensities, 4) the relatively high threshold for phase shifting response is mediated by rods through the rod–cone pathway and the intrinsic photosensitivity of ipRGCs, whereas the more sensitive rod bipolar pathway can support photoentrainment with prolonged scotopic illumination.
Despite previous evidence that responses from both rods and cones are necessary for circadian light responses, we show that rods are the major contributor to photoentrainment, reconciling several published observations. First the peak of the action spectra for circadian responses in both humans37–39 and rodents40 is near 500 nm, closely resembling the spectral sensitivity of rods but not cones. Second, RPE65–/– animals, which lack a key component of the visual cycle leading to complete loss of cone function and attenuated rod function, still show photoentrainment in the background of the melanopsin knockout41. In fact, even with the highly attenuated rod function, these animals show better photoentrainment than animals with fully functional cones36, although their photoentrainment is impaired compared to WT animals41. Third, our results show that rods can continue to entrain the circadian oscillator into photopic light intensities even under conditions when the persistent activity of the rods renders them incapable of supporting spatial vision.
Of interest is the fact that rod–only type 1 animals failed to photoentrain at low light intensities, despite demonstrating normal vision at scotopic light intensities. This apparent contradiction may result from the ‘continuous light’ condition in rod–only type 1 mice resulting from the deletion of CNG channels in the cone outer segments, which we propose adapts the retinal circuit that signals to ipRGCs. Thus, the adapted state in rod–only type 1 animals may not be sufficient to influence image formation, which will largely depend on encoding contrast, but it might influence circadian photoentrainment whereby rod signals feed through cone circuits to signal absolute irradiance levels in the environment.
In rod–only type 3 animals, we surprisingly observed responses in OFF cone bipolar cells despite the absence of cone photoreceptors; signals that we suspect originate from the rod spherules themselves. In the rodent retina a third rod pathway has been identified whereby OFF cone bipolar cells synapse onto the rod spherule28, 42, and the sensitivity of this pathway is comparable to the rod–cone pathway43. Under conditions where the cones are absent we postulate that the cone bipolar cells are reflecting signals through this third rod pathway. Interestingly, this pathway on its own appears insufficient for circadian photoentrainment at high light intensity.
Several studies have inferred a contribution of cone phototransduction to circadian light responses based on the action spectrum for photoentrainment16, 44. One study, using mice that lack M–cones (515nm), found attenuated phase shifts in response 530nm but not 480nm13. This data lead to a model whereby cones and ipRGCs account fully for light effects on the circadian oscillator13. However, since photoreceptors have a broad absorption spectrum, the light intensities used in this study would have activated rods. Furthermore the developmental loss of M–cones might have hindered rod signals from using the rod–cone pathway thereby attenuating phase shift responses at 530nm.
Two recent studies attempted to exploit the differences in the spectral sensitivity of rod and cone photopigments to drive one photoreceptor type in preference of the other to determine their relative roles in non–image forming functions. One of these studies was carried out in humans17, whereas the other utilized a mouse line that substituted transgenically the human L cone opsin in mouse M cones allowing them to increase spectral separation between the rod and cone light responses18. Despite using similar strategies to drive selectively rod and cone phototransdution, both groups reached opposing conclusions about the role of rods and cones in circadian functions. It should be appreciated that despite greater efficacy in activating one photoreceptor type versus another, these approaches also don’t fully separate the light–evoked activity of rods and cones17. Our strategy of selectively eliminating rod or cone phototransduction, or cone photoreceptors altogether, provides a stringent separation of these photoreceptors’ contributions to circadian functions.
Since rods use the cone circuits to drive photoentrainment, it seems paradoxical that that cone phototransduction alone fails to photoentrain animals. The tremendous capacity of cone phototransduction to adapt to increases in light intensity may ultimately be responsible for this phenomenon, which will prevent their photocurrent from saturating even under bright, bleaching light conditions. The recovery of dark current in cones allows both the resting membrane potential and thus glutamate release to recover toward basal levels in darkness. As such we propose that cone phototransduction would be much less able to signal steady light intensity. The persistent hyperpolarization of rods during bright, bleaching light exposures45, 46 may thus be better suited to signaling irradiance through the cone pedicle to ipRGCs which influence circadian photoentrainment. Consistent with this notion, cone adaptation impairs their ability to signal light for non–image functions, especially under prolonged light treatments17, 18.
These results provide a simple model (Fig. S3) for how the outer retinal photoreceptors and ipRGCs account for photoentrainment. At low light intensity, ipRGCs lack sensitivity while rods are known to respond to increasing light levels and thus reliably relay this information to higher centers. Rods will continue to signal persistent light exposure through the rod–cone pathway even under conditions where their photocurrent is saturated. Finally, at high light intensities and for prolonged light exposures, melanopsin phototransduction in ipRGCs will extend the range of light intensities that allow circadian photoentrainment. Ultimately the properties of rod and melanopsin phototransduction, as well as the rod pathways that impinge on ipRGCs, can account fully for the ability of mammals to photoentrain throughout physiologically relevant light conditions5, 10.
Methods
Mice
All animals were between the ages of 3 and 12 months. For photoentrainment, phase shift, and pupillary light reflex, all animals were males. All experiments were conducted in accordance with National Institutes of Health guidelines and were approved by Institutional Animal Care and Use Committees of Johns Hopkins University, the Weill Cornell Medical College, and the University of Southern California Keck School of Medicine.
Wheel running activity
Mice were placed in cages with a 4.5–inch running wheel, and their activity was monitored with VitalView software (Mini Mitter, OR). Photoentrainment experiments were done under a 12 hours light: 12 hours dark cycle. Initial light intensity was provided by Philips Daylight deluxe fluorescent lamps, and intensity decreases were accomplished with neutral density filters (Rosco, CT). Light intensity decreases were accompanied by 6–hour phase advances to measure the re–entrainment ability of animals. The periods in constant darkness and constant light were calculated with ClockLab (Actimetrics, IL). For phase–shifting experiments, each animal was exposed to a light pulse (1,000 lux; CT16) for 15 min.
Measuring the spatial frequency threshold for optokinetic tracking
A virtual cylinder comprised of a vertical sine wave grating was projected in 3D coordinate space on computer monitors arranged in a quadrangle (square) around a testing arena made of black Plexiglas box. A platform was situated at the epicenter of the arena by securing a white Plexiglas disk to a threaded bolt secured to the base of the apparatus. A vented and hinged lid enclosed the top of the apparatus. A video camera was secured to the lid directly above the platform. Four– 20” LCD computer monitors were attached one to each outside wall of the apparatus such that they projected through the rectangular openings into the arena. Whisper fans were used to cool the monitors and vent the testing arena.
A computer program (OptoMotry; CerebralMechanics, Lethbride, Alberta), running on a dual–processor G4 or G5 Power Macintosh (Apple Computer Corporation, Mountain View, CA), was used to drive video cards and project on the monitors a virtual cylinder in 3D coordinate space. The gamma response of the monitors was used to linearize the output to the screens, and the screen luminance was adjusted to equalize the screen intensity.
Individual mice were placed on the platform, and the lid was closed. As the animal moved freely about the platform, the experimenter followed a position the head between its eyes with a crosshair superimposed on the video image. The X–Y positional coordinates of the crosshair were used to center the rotation of the cylinder at the mouse’s viewing position, thereby maintaining the virtual walls of the cylinder at a constant ‘distance’ from the animal. When a grating detectable to the mouse was projected on the cylinder wall and the cylinder was rotated (12 deg/sec), the mouse normally stopped moving its body and would begin to track the grating with reflexive head movements in concert with the rotation. An experimenter assessed whether the animals tracked the cylinder by monitoring in the video window the image of the cylinder, the animal, and the cross–hair simultaneously. If the mouse’s head tracked the cylinder rotation a minimum of 3 times, which was evident as a sweeping movement against the stationary arms of the crosshair, it was judged that the animal's visual system could detect the grating. The highest spatial frequency capable of driving a response was adopted as the threshold.
Scotopic thresholds were obtained with the aid of neutral density film placed over the screens. Scotopic intensity was 1.4 × 10−4 cd/m2, which is between 0.9 and 2.1 log units above rod threshold, with mouse rod threshold estimated at 1×10−6 to 1.8 ×10−5 cd/m2 of light intensity30. Photopic intensity, 142 cd/m2, was more than 3 log units above rod saturation47.
Single cell recordings
Whole–cell current clamp recordings from rod bipolar cells, cone Off bipolar cells, and horizontal cells in 200 µm thick dark–adapted retinal slices from WT, rod–only type 1 and rod–only type 2 mice as described previously48, 49. Retinal slices were superfused with Ames media heated to 35 – 37 °C, and the internal solution for these experiments consisted of: 125 K–Aspartate, 10 KCl, 10 HEPES, 5 NMG–HEDTA, 0.5 CaCl2, 1 ATP–Mg, 0.2 GTP–Mg; pH was adjusted to 7.2 with NMG–OH. Flash families were measured in response to a 10 ms flash from a blue LED (λmax ~ 470 nm) whose strength varied from generating a just–measurable response and increased by factors of 2.
Light Calibration
Light intensities both for physiological and photoentrainment experiments were converted to the number of rhodopsin molecules activated as described previously49. Briefly, light intensities were measured using a calibrated photodiode (United Detector Technologies, San Diego, CA) and were converted to equivalent 501 nm photons by convolving the power–scaled spectral output of the LED with the normalized spectral sensitivity curve for mouse rhodopsin (Rh). The flash strengths and background light intensities were then converted to activated Rh per rod using the estimated collecting area of rod photoreceptors in the experimental setup, or a collecting area of 0.2 µm2 for photoentrainment experiments50.
Supplementary Material
Acknowledgements
We would like to thank Dr. Jeremy Nathans for the h.red DT–A mice. We also want to thank Drs. Rejji Kuruvilla, Haiqing Zhao and Marnie Halpern for their careful reading of the manuscript and helpful suggestions, and the Johns Hopkins University Mouse Tri–Lab for support. This work was supported by the National Institutes of Health grants GM076430 (SH), EY017606 (APS), the David and Lucile Packard Foundation (SH), the Alfred P. Sloan Foundation (SH), and the McKnight Endowment Fund for Neurosciences (APS).
Footnotes
Contributions:
Experiments were conceived and designed by CMA, ADG, APS, and SH. Wheel running experiments were carried out by CMA. ACA performed current clamp recordings of retinal cells. NMA, CMA and GTP carried out virtual optomoter experiments. The paper was written by CMA, ADG, APS and SH and edited by all.
References
- 1.Guler AD, et al. Melanopsin cells are the principal conduits for rod-cone input to nonimage-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatori M, et al. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goz D, et al. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE. 2008;3:e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 6.Provencio I, et al. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas RJ, et al. Diminished pupillary light reflex at high irradiances in melanopsinknockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 8.Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do MT, et al. Photon capture and signalling by melanopsin retinal ganglion cells. Nature. 2009;457:281–287. doi: 10.1038/nature07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dacey DM, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 11.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panda S, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 13.Dkhissi-Benyahya O, Gronfier C, De Vanssay W, Flamant F, Cooper HM. Modeling the role of mid-wavelength cones in circadian responses to light. Neuron. 2007;53:677–687. doi: 10.1016/j.neuron.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster RG, et al. Circadian photoreception in the retinally degenerate mouse (rd/rd) J Comp Physiol A. 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- 15.Freedman MS, et al. Regulation of mammalian circadian behavior by non-rod, noncone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- 16.Provencio I, Foster RG. Circadian rhythms in mice can be regulated by photoreceptors with cone-like characteristics. Brain Res. 1995;694:183–190. doi: 10.1016/0006-8993(95)00694-l. [DOI] [PubMed] [Google Scholar]
- 17.Gooley JJ, et al. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lall GS, et al. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvert PD, et al. Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit. Proc Natl Acad Sci U S A. 2000;97:13913–13918. doi: 10.1073/pnas.250478897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- 21.Mrosovsky N. Contribution of classic photoreceptors to entrainment. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2003;189:69–73. doi: 10.1007/s00359-002-0378-7. [DOI] [PubMed] [Google Scholar]
- 22.Ebihara S, Tsuji K. Entrainment of the circadian activity rhythm to the light cycle: effective light intensity for a Zeitgeber in the retinal degenerate C3H mouse and the normal C57BL mouse. Physiol Behav. 1980;24:523–527. doi: 10.1016/0031-9384(80)90246-2. [DOI] [PubMed] [Google Scholar]
- 23.Biel M, et al. Selective loss of cone function in mice lacking the cyclic nucleotide-gated channel CNG3. Proc Natl Acad Sci U S A. 1999;96:7553–7557. doi: 10.1073/pnas.96.13.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang B, et al. Cone photoreceptor function loss-3, a novel mouse model of achromatopsia due to a mutation in Gnat2. Invest Ophthalmol Vis Sci. 2006;47:5017–5021. doi: 10.1167/iovs.05-1468. [DOI] [PubMed] [Google Scholar]
- 25.Sharpe LT, Stockman A. Rod pathways: the importance of seeing nothing. Trends Neurosci. 1999;22:497–504. doi: 10.1016/s0166-2236(99)01458-7. [DOI] [PubMed] [Google Scholar]
- 26.Dacheux RF, Raviola E. The rod pathway in the rabbit retina: a depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith RG, Freed MA, Sterling P. Microcircuitry of the dark-adapted cat retina: functional architecture of the rod-cone network. J Neurosci. 1986;6:3505–3517. doi: 10.1523/JNEUROSCI.06-12-03505.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493. doi: 10.1016/s0896-6273(00)80560-7. [DOI] [PubMed] [Google Scholar]
- 29.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- 30.Umino Y, Solessio E, Barlow RB. Speed, spatial, and temporal tuning of rod and cone vision in mouse. J Neurosci. 2008;28:189–198. doi: 10.1523/JNEUROSCI.3551-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- 32.Panda S, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 33.Ibuka N, Inouye SI, Kawamura H. Analysis of sleep-wakefulness rhythms in male rats after suprachiasmatic nucleus lesions and ocular enucleation. Brain Res. 1977;122:33–47. doi: 10.1016/0006-8993(77)90660-6. [DOI] [PubMed] [Google Scholar]
- 34.Guler AD, Altimus CM, Ecker JL, Hattar S. Multiple photoreceptors contribute to nonimage-forming visual functions predominantly through melanopsin-containing retinal ganglion cells. Cold Spring Harb Symp Quant Biol. 2007;72:509–515. doi: 10.1101/sqb.2007.72.074. [DOI] [PubMed] [Google Scholar]
- 35.Lucas RJ, et al. Identifying the photoreceptive inputs to the mammalian circadian system using transgenic and retinally degenerate mice. Behav Brain Res. 2001;125:97–102. doi: 10.1016/s0166-4328(01)00274-1. [DOI] [PubMed] [Google Scholar]
- 36.Mrosovsky N, Hattar S. Diurnal mice (Mus musculus) and other examples of temporal niche switching. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:1011–1024. doi: 10.1007/s00359-005-0017-1. [DOI] [PubMed] [Google Scholar]
- 37.Wright HR, Lack LC, Kennaway DJ. Differential effects of light wavelength in phase advancing the melatonin rhythm. J Pineal Res. 2004;36:140–144. doi: 10.1046/j.1600-079x.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 38.Brainard GC, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- 41.Doyle SE, Castrucci AM, McCall M, Provencio I, Menaker M. Nonvisual light responses in the Rpe65 knockout mouse: rod loss restores sensitivity to the melanopsin system. Proc Natl Acad Sci U S A. 2006;103:10432–10437. doi: 10.1073/pnas.0600934103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hack I, Peichl L, Brandstatter JH. An alternative pathway for rod signals in the rodent retina: rod photoreceptors, cone bipolar cells, and the localization of glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:14130–14135. doi: 10.1073/pnas.96.24.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Protti DA, Flores-Herr N, Li W, Massey SC, Wassle H. Light signaling in scotopic conditions in the rabbit, mouse and rat retina: a physiological and anatomical study. J Neurophysiol. 2005;93:3479–3488. doi: 10.1152/jn.00839.2004. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimura T, Ebihara S. Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd) and normal CBA/N (+/+)mice. J Comp Physiol A. 1996;178:797–802. doi: 10.1007/BF00225828. [DOI] [PubMed] [Google Scholar]
- 45.Cornwall MC, Fain GL. Bleached pigment activates transduction in isolated rods of the salamander retina. J Physiol. 1994;480(Pt 2):261–279. doi: 10.1113/jphysiol.1994.sp020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cornwall MC, Fein A, MacNichol EF., Jr Cellular mechanisms that underlie bleaching and background adaptation. J Gen Physiol. 1990;96:345–372. doi: 10.1085/jgp.96.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xin D, Bloomfield SA. Comparison of the responses of AII amacrine cells in the dark- and light-adapted rabbit retina. Vis Neurosci. 1999;16:653–665. doi: 10.1017/s0952523899164058. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong-Gold CE, Rieke F. Bandpass filtering at the rod to second-order cell synapse in salamander (Ambystoma tigrinum) retina. J Neurosci. 2003;23:3796–3806. doi: 10.1523/JNEUROSCI.23-09-03796.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sampath AP, et al. Recoverin improves rod-mediated vision by enhancing signal transmission in the mouse retina. Neuron. 2005;46:413–420. doi: 10.1016/j.neuron.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Lyubarsky AL, Pugh EN., Jr Recovery phase of the murine rod photoresponse reconstructed from electroretinographic recordings. J Neurosci. 1996;16:563–571. doi: 10.1523/JNEUROSCI.16-02-00563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.