Abstract
Parvalbumin-expressing, fast-spiking basket cells play key roles in the generation of synchronous, rhythmic population activities in the hippocampus. Here we show that GABAA receptor-mediated synaptic inputs from murine parvalbumin-expressing basket cells are selectively modulated by the membrane voltage- and intracellular chloride-dependent chloride channel ClC–2. These data demonstrate a novel cell type-specific regulation of intracellular chloride homeostasis in the perisomatic region of hippocampal pyramidal neurons.
Keywords: interneuron, GABAA receptor, excitability, microcircuit, intracellular chloride
There are two distinct basket cell classes specialized to provide GABAergic innervation to the perisomatic region of principal cells, the parvalbumin- or cholecystokinin- expressing basket cells (PVBCs or CCKBCs, respectively). The intrinsic and synaptic properties of PVBCs enable them to perform circuit functions related to precise time keeping and generation of network oscillations, whereas CCKBCs are thought to serve as modulators that adapt network activity to behavioral states1,2. Because synapses from PVBCs and CCKBCs co-exist on the perisomatic membrane, it has been assumed that the regulation of the intracellular concentration of Cl−, the major charge carrying anion for GABAA receptor-channels, is uniform at PVBC and CCKBC synapses. Here we demonstrate using paired recording techniques3 in slices (Supplementary Methods online) that the chloride channel ClC–2 robustly modulates synaptic inputs specifically from PVBCs, providing a molecular safety mechanism for the prevention of the accumulation of intracellular chloride at the highly active GABAergic synapses formed by the fast-spiking PVBCs. Our experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
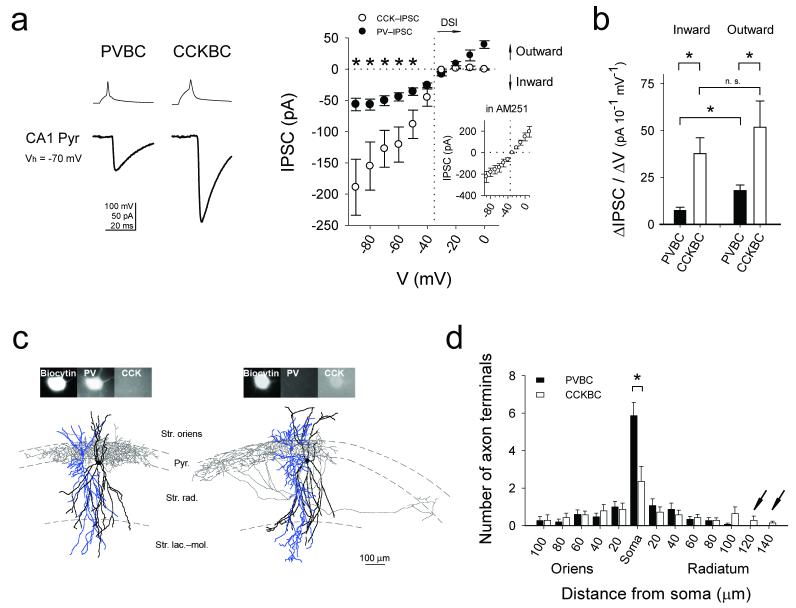
Paired interneuron-pyramidal cell whole-cell patch clamp recordings showed that, at membrane potentials more depolarized than −35 mV, the amplitudes of the unitary IPSCs evoked by CCKBCs (CCK-IPSCs) were smaller than the PVBC-evoked IPSCs (PV-IPSCs) (Fig. 1a). On the other hand, below the reversal potential for GABAA receptor-mediated events (EGABAA), it was the PV-IPSCs that were significantly smaller than the CCK-IPSCs (Fig. 1a). Furthermore, examination of the current-voltage relationships across a wide voltage range (Fig. 1a) indicated that CCK-IPSCs exhibited inward rectification (inward current flowed more easily than outward current), while PV-IPSCs showed apparent outward rectification.
Figure 1. Outward rectification of PV-IPSCs and distribution of PVBC axon terminals on the somata of pyramidal cells.
(a) Left: Averaged example traces; upper: presynaptic spikes; lower: postsynaptic responses ([Cl−]pip=48.7 mM). Right: current-voltage plots of IPSCs (failures included; PVBC: n=6 pairs; CCKBC: n=5 pairs; asterisks indicate P<0.05, errors are s.e.m; probability of release was similar between the two groups, see Supplementary Methods). Inset: CCK-IPSCs in AM251 (n=4 pairs). (b) Average ΔIPSC/ΔVs of the plot in (a) (CCK-IPSCs in AM251). (c) Examples of basket cells (axons: gray; dendrites: black) and pair-recorded postsynaptic pyramidal cells (blue). (d) Distribution of terminals. (P=0.03). Arrows indicate distal CCKBC terminals.
The origin of the inward rectification of CCK-IPSCs was readily identifiable, as it was due to depolarization-induced suppression of inhibition1 (DSI) that was sensitive to the CB1 receptor antagonist AM251 (10 μM) (Fig. 1a, inset). However, the difference in amplitude of the inward IPSCs was unexpected, because the number of GABAA receptor-channels is similar at PVBC and CCKBC synapses on CA1 pyramidal cells4. In order to characterize the apparent outward rectification of PV-IPSCs, we compared the inward and outward portions of the current-voltage relationships (ΔIPSC/ΔV, reflecting synaptic conductance; see Supplementary Methods). While the average ΔIPSC/ΔVs of inward and outward CCK-IPSCs were not different (in AM251; 37.7±8.5 and 51.8±14, n=4 pairs, P=0.424), the average ΔIPSC/ΔV for inward PV-IPSCs was significantly smaller than for outward PV-IPSCs (Fig. 1b; 7.6±1.5 and 18±2.9, n=8 pairs, P=0.007, Fig. 1b). Consequently, the ratio of the average ΔIPSC/ΔV of inward versus outward currents (reflecting rectification) was also significantly smaller for the PV-IPSCs (0.43±0.04 vs. 0.75±0.06, n=8 vs. n=4 pairs, P=0.001).
An explanation for the smaller inward PV-IPSCs compared to the CCK-IPSCs is that the driving force for Cl− is lower at PVBC compared to CCKBC synapses. Indeed, paired recordings with low (4 mM) intracellular Cl− concentration close to physiological values5 revealed that the difference in inward IPSC amplitude between the PVBC and CCKBC inputs was accompanied by differences between the EGABAA values (PVBC: −70.8±0.9 mV, n=8 pairs; CCKBC: −67.8±0.9 mV, n=13 pairs, P=0.04; Supplementary Fig. 1a1), indicating lower intracellular [Cl−]i at PVBC synapses. Such differential regulation of [Cl−]i could conceivably occur at the level of individual synapses and/or sub-cellular domains5-8. Since synapses from the two types of basket cells intermingle and are assumed to distribute similarly on perisomatic membranes, we performed a morphological analysis of our recorded pre- and postsynaptic cell pairs (Fig. 1c). The results revealed that PVBC axons formed more putative synaptic terminals on the postsynaptic pyramidal cells compared to CCKBCs (11±0.6, n=15 pairs vs. 8.3±0.8, n=14, P=0.02; note, however, that the number of release sites per terminal may differ between PVBCs and CCKBCs; for a review, see Ref. 1). In addition to differences in the total number of terminals, the distribution of the terminals within the perisomatic compartment was also different. Namely, PVBCs formed approximately twice as many axon terminals on the soma (5.8±0.7, n=15 pairs vs. 2.3±0.8, n=14 pairs, P=0.02), while the CCKBC terminals extended farther out onto the apical dendrites (Fig. 1d).
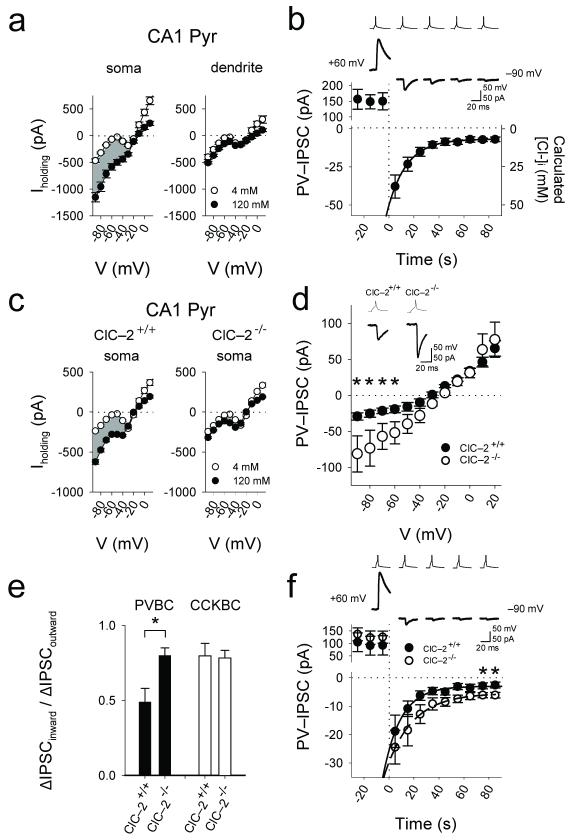
The preferential cell type-dependent innervation of sub-cellular compartments may provide anatomical basis for a hypothetical mechanism conveying domain-specific regulation of [Cl−]i. Indeed, whole-cell recordings with either low (4 mM) or high (120 mM) intra-pipette Cl− ([Cl−]pip) from the soma or apical dendrite (60–80 μm from soma, close to the middle of the basket cell synapse distribution in the stratum radiatum, see Fig. 1d) of single CA1 pyramidal cells revealed presence of a hyperpolarization-gated, sustained Cl−-conductance preferentially at the pyramidal cell soma (Fig. 2a; note that these data do not exclude the presence of such a Cl−-conductance elsewhere in the dendritic tree).
Figure 2. Somatic hyperpolarization-gated, sustained Cl−-conductance mediated by ClC–2.
(a) Whole-cell recordings from the somata and proximal apical dendrites of pyramidal cells with different [Cl−]pip (4mM: nsoma=14, ndendrite=4; 120mM: nsoma=11, ndendrite=4; shading indicates the difference current reflecting whole-cell Cl− current). (b) Time-dependent decrease of PV-IPSCs after stepping the membrane voltage of the postsynaptic cell from +60 mV to −90 mV in rat. (c) Large sustained somatic Cl−-conductance in somata of CA1 pyramidal cells in the wild-type (ClC–2+/+) but not the ClC–2−/− mice (4mM: nsoma,+/+ =13, nsoma,−/− =20; 120mM: nsoma,+/+ =24, nsoma,−/− =24). (d) Current-voltage plots of PV-IPSCs from ClC–2+/+ (n=6 pairs) and ClC–2−/− (n=3 pairs). Inset: PV-IPSCs from ClC–2−/− mice compared to ClC–2+/+ (example traces at −70 mV; [Cl−]pip=40 mM). (e) Significantly decreased outward rectification of the PV-IPSCs in the ClC–2−/− mice, and lack of change in rectification in the case of CCK-IPSCs. (f) Slower time-dependent decrease of PV-IPSCs after stepping the membrane voltage of the postsynaptic cell from +60 mV to −90 mV in the ClC–2−/− mice compared to ClC–2+/+. Asterisks indicate significant difference (note that the larger IPSCs indicated by asterisks in these Cl− extrusion experiments are in general agreement with the presence of larger IPSCs at hyperpolarized holding potentials in the ClC–2−/− animals in Fig. 2d).
Next, paired recording experiments between PVBCs and pyramidal cells were conducted by first evoking large outward PV-IPSCs at +60 mV (and presumably loading the postsynaptic cell body with Cl−; [Cl−]pip=4 mM), and then stepping the membrane potential to −90 mV. Inward PV-IPSCs immediately after the step to −90 mV were large (−37.8±7.5 pA, n=5 pairs), but then the amplitude decreased over tens of seconds (τ=14.4±1.8 sec, n=5 pairs; Fig. 2b), consistent with the presence of a mechanism that lets Cl− ions exit from the inside to the outside according to the Cl− electrochemical gradient (note that similar Cl− extrusion experiments with CCK-IPSCs resulted in a significantly slower decrease in the event amplitude after the step to −90 mV; τ=24.7±3.8 sec, n=5 pairs; P=0.04).
A mechanism that could potentially underlie the above-described effects is the hyperpolarization-activated, inwardly rectifying plasma membrane Cl−-channel ClC–2 whose gating also depends on [Cl−]i (a rise in intraneuronal Cl−-concentration opens ClC–2 and results in an efflux of Cl−)9-11. Both mRNA and protein for the ClC–2 channel are known to be expressed in CA1 pyramidal cells, but not in granule cells of the dentate gyrus (GCs)12,13. Consistent with the lack of ClC–2 expression in GCs, paired recordings from PVBCs and postsynaptic GCs revealed no marked outward rectification of the PV-IPSCs, and the somatic Cl−-conductance was also lacking in GCs (Supplementary Fig. 1b). Furthermore, the somatic Cl−-conductance was not present in CA1 pyramidal cells from mice lacking the ClC–2 channel14 (ClC–2−/−; Fig. 2c). In addition, paired recordings from PVBCs and postsynaptic CA1 pyramidal cells in mice showed increased inward currents (Fig. 2d) and, consequently, significantly reduced outward rectification of PV-IPSCs in ClC–2−/− mice (ClC–2+/+: 0.42±0.05, n=8 pairs and ClC–2−/−: 0.89±0.06, n=7 pairs, P=0.0005; Fig. 2e). Note that the rectification of the CCK-IPSCs did not change in the ClC–2−/− mice (ClC–2+/+: 0.79±0.08, n=3 pairs and ClC–2−/−: 0.78±0.05, n=4 pairs, P=0.8; Fig. 2e). Finally, Cl− extrusion experiments (similar to those in rat in Fig. 2b) showed a significantly slower decrease in the PV-IPSC amplitude after the step to −90 mV in the ClC–2−/− (ClC–2+/+: τ=14.9±1.1 sec, n=4 pairs; ClC–2−/−: τ=22.6±2.5 sec, n=4 pairs; P=0.03). Additional experiments showed a significantly longer time to reversal of the inward (depolarizing) IPSCs to outward (hyperpolarizing) IPSCs in CA1 pyramidal cells from ClC–2−/− mice compared to ClC–2+/+ after a brief period of intense presynaptic GABAergic fiber activity evoked by multi-fiber extracellular stimulation resulting in increased intracellular [Cl−] (Supplementary Fig. 1d).
Data in this paper reveal a novel regulation of PVBC synapses by ClC–2. The ClC–2-mediated selective modulation of PVBC inputs appear to be ideally suited to prevent potentially dangerous rises in [Cl−]i (and thus depolarizing GABAA responses) during episodes of intense synchronized firing during hippocampal network oscillations by populations of fast-spiking PVBCs that form convergent inputs on single pyramidal cells15 (in contrast, CCKBCs fire at lower frequencies in vivo2). Unlike several other [Cl−]i-regulating mechanisms5, ClC–2 does not influence the resting [Cl−]i under normal circumstances when EGABAA is more hyperpolarized than the resting membrane potential. Activation of ClC–2 may also be aided by K+-conductances (e.g., postsynaptic GABAB receptors) that can hyperpolarize the membrane potential below EGABAA, or by extracellular acidification11. Future studies will be required to demonstrate whether differential [Cl−]i regulation exist even at adjacent synapses from PVBCs and CCKBCs, and whether the differential activity of ClC–2 at PVBC inputs is due to differences in the levels of ClC–2 expression (i.e., ClC–2 may exist at some CCK synapses13) and/or channel modulation11.
Supplementary Material
Acknowledgements
We thank Ms R.Zhu for excellent technical assistance, Mr K.Ding and Ms D.Szabadics for camera lucida reconstructions, Ms M.Case for genotyping, Dr J.E.Melvin for the ClC–2 knockout mouse, and Drs L.Hilgenberg, M.A.Smith, M.Cahalan, M.V.Jones, Z.Nusser and R.L.McDonald for discussions. This work was supported by the US National Institutes of Health grant NS38580 (to I.S.) and the Epilepsy Foundation through the generous support of the Eric W. Lothman Training Fellowship (to S.-H.L.).
References
- 1.Freund TF, Katona I. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Klausberger T, Somogyi P. Science. 2008;321(5885):53–7. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Földy C, Lee SY, Szabadics J, Neu A, Soltesz I. Nat. Neurosci. 2007;10(9):1128–30. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- 4.Klausberger T, Roberts JD, Somogyi P. J.Neurosci. 2002;22(7):2513–21. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Szabadics J, Varga C, Molnár G, et al. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 7.Duebel J, Haverkamp S, Schleich W, et al. Neuron. 2006;49(1):81–94. doi: 10.1016/j.neuron.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 8.Inglefield JR, Schwartz-Bloom RD. J.Neurosci.Methods. 1997;75(2):127–35. doi: 10.1016/s0165-0270(97)00054-x. [DOI] [PubMed] [Google Scholar]
- 9.Madison DV, Malenka RC, Nicoll RA. Nature. 1986;321(6071):695–7. doi: 10.1038/321695a0. [DOI] [PubMed] [Google Scholar]
- 10.Staley KJ. J.Neurophysiol. 1994;72:273–284. doi: 10.1152/jn.1994.72.1.273. [DOI] [PubMed] [Google Scholar]
- 11.Jentsch TJ. Crit.Rev.Biochem.Mol.Biol. 2008;43:3–36. doi: 10.1080/10409230701829110. [DOI] [PubMed] [Google Scholar]
- 12.Smith RL, Clayton GH, Wilcox CL, Escudero KW, Staley KJ. J.Neurosci. 1995;15:4057–4067. doi: 10.1523/JNEUROSCI.15-05-04057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sík A, Smith RL, Freund TF. Neuroscience. 2000;101:51–65. doi: 10.1016/s0306-4522(00)00360-2. [DOI] [PubMed] [Google Scholar]
- 14.Nehrke K, Arreola J, Nguyen HV, et al. J.Biol.Chem. 2002;277(26):23604–11. doi: 10.1074/jbc.M202900200. [DOI] [PubMed] [Google Scholar]
- 15.Bartos M, Vida I, Jonas P. Nat.Rev.Neurosci. 2007;8(1):45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.