Abstract
We show here that the voltage-gated K+ channel Kv12.2 is a potent regulator of excitability in hippocampal pyramidal neurons. Genetic deletion and pharmacologic block of Kv12.2 significantly reduced firing threshold in these neurons. Kv12.2−/− mice displayed signs of persistent neuronal hyperexcitability including frequent interictal spiking, spontaneous seizures and increased sensitivity to the chemoconvulsant pentylenetetrazol.
Hippocampal and cortical hyperexcitability is a defining feature of most epilepsies1, 2. The molecular mechanisms regulating excitability in these brain regions are not fully understood, but genetic studies have revealed a critical role for sub-threshold voltage-gated K+ currents, which are in part encoded by Kv7 (KCNQ)–based M-channels3. We therefore reasoned that Kv12.2 (KCNH3, Elk2), which is highly expressed in hippocampal and cortical pyramidal neurons4, could also significantly contribute to the control firing because of its hyperpolarized voltage-activation range5, 6 (Supplementary Fig. 1). Kv12 channels have been highly conserved since the beginnings of the metazoan lineage, implying an important and non–redundant physiological function. They are present in the genomes of the earliest sequenced eumetazoans, the sea anemone Nematostella vectensis and the placozoan Trichoplax adherens7–9 (Supplementary Fig. 1). Genetic analysis suggests that Kv12.2 regulates spatial memory processing10, but its role in the control of excitability has not been defined. We produced an allele for conditional deletion of Kv12.2 with Cre–recombinase (Kv12.2f); expression of Kv12.2 was completely eliminated following germline excision (Supplementary Fig. 2).
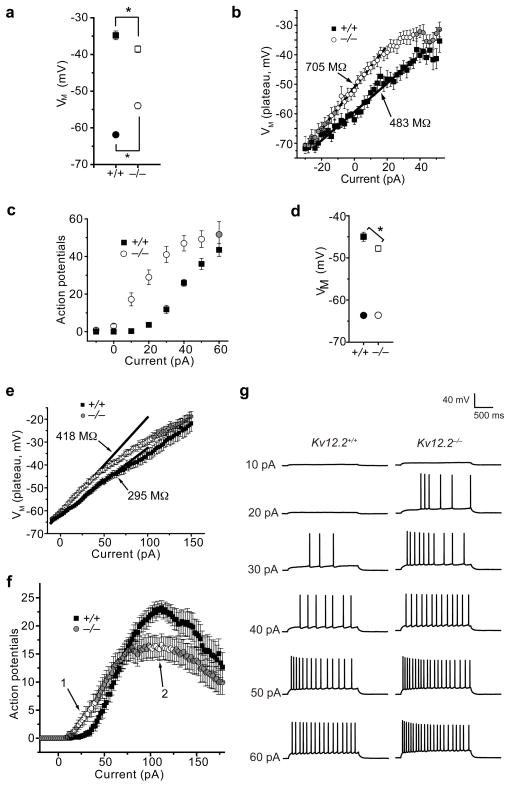
We examined the intrinsic firing properties of hippocampal pyramidal neurons cultured at P2 from Kv12.2+/+ and Kv12.2−/− mice. Kv12.2−/− neurons had significantly depolarized resting membrane potentials, a reduced firing threshold and increased input resistance (Fig.1 a,b). The interval between resting potential and firing threshold was also significantly compressed in Kv12.2−/− neurons (15.8 ± 2.2 mV, n = 16) relative to Kv12.2+/+ neurons (27.5 ± 2.1 mV, n = 17). Deletion of Kv12.2 from cultured Kv12.2f/f neurons with lentivirus expressing Cre–recombinase11 produced a similar phenotype (Supplementary Fig. 3). Steady state K+ current measured under voltage clamp at −20 mV was significantly reduced (p < 0.01) in Kv12.2−/− neurons (58.1 ± 6.1 pA, n = 29) compared to Kv12.2+/+ neurons (89 ± 6.9 pA, n = 30). Consistent with this finding, Kv12.2−/− neurons required ~30 pA less current injection than Kv12.2+/+ neurons to achieve a given spike frequency (Fig. 1c). This pronounced shift in the current input/spike output relationship in Kv12.2−/− neurons suggests that Kv12.2 plays a key role in limiting firing in response to small excitatory stimuli.
Figure 1. Kv12.2−/− neurons are hyperexcitable.
(a–c) Comparisons of excitability and K+ currents in hippocampal pyramidal neurons cultured at P2 from Kv12.2−/− and Kv12.2+/+ mice. (a) Resting membrane potential (circles) and action potential threshold (squares) for Kv12.2−/− and Kv12.2+/+ neurons; asterisks indicate significant difference. (b) Input resistance calculated from plateau voltages elicited by current injections for Kv12.2−− and Kv12.2+/+ neurons. Values are derived from linear fits (lines) and significant difference is indicated with open symbols. (c) Number of action potentials elicited during 2 s current injections for Kv12.2−/− and Kv12.2+/+ neurons; significant difference is indicated with open symbols. (d–g) Comparison of excitability in Kv12.2−/− and Kv12.2+/+ CA1 pyramidal neurons recorded in acute slices taken from 8–9 week old animals: (d) resting membrane potential (circles) and action potential threshold (squares), (e) input resistance calculated from plateau voltages observed during 2 s current injections as in (b), (f) number of action potentials recorded during 2 s current injections, and (g) example voltage traces elicited by current injection. Asterisks (d) or open symbols (e,f) indicate significant difference. Values given in (a–f) are mean ± s.e.m. (n = 16–73); we used p < 0.05 as the threshold for significance.
We also observed a significant reduction in firing threshold and increased input resistance in Kv12.2−/− CA1 pyramidal neurons recorded in acutely isolated slices from 8–9 week old animals (Fig. 1d–h). Resting potential was not altered in mature Kv12.2−/− CA1 neurons (−63.6 ± 0.5 mV for Kv12.2+/+; −63.2 ± 0.6 mV for Kv12.2−/−), possibly reflecting developmental or synaptic regulation of compensatory conductances. Nevertheless, the interval between resting potential and threshold remained significantly compressed in the Kv12.2−/− neurons (15.8 ± 0.9, n = 37) vs. Kv12.2+/+ neurons (18.7 ± 1.1, n = 27). These results demonstrate that the role of Kv12.2 in setting threshold excitability is preserved through development. Maximal firing rate was also reduced in Kv12.2−/− CA1 neurons in slices (Fig. 1g).
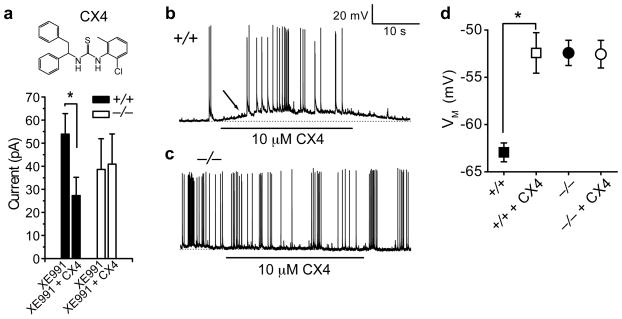
We identified a specific pharmacological inhibitor of Kv12.2, CX4 (1-(2-chloro-6-methylphenyl)-3-(1,2-diphenylethyl) thiourea) in a high throughput screen for Kv12.2 inhibitors (Supplementary Fig. 4). We applied CX4 to cultured P2 hippocampal pyramidal neurons in the presence of 10 μM XE991 to block M-current. CX4 blocked ~ half of the remaining steady-state K+ current at −20 mV in Kv12.2+/+ neurons, but had no effect on Kv12.2−/− neurons (Fig. 2a). CX4 depolarized the resting potential and increased the spontaneous firing rate in Kv12.2+/+ but not Kv12.2−/− neurons (Fig. 2a–d), corroborating genetic evidence of a role for Kv12.2 in determining firing threshold.
Figure 2. Pharmacological block of Kv12.2 increases neuronal excitability.
(a) The Kv12.2 inhibitor CX4 blocks steady state K+ current at −20 mV in Kv12.2+/+ but not Kv12.2−/− hippocampal pyramidal neurons cultured at P2. We used 10 μM XE991 to block the M-current. (b–d) Resting membrane potential of Kv12.2+/+ but not Kv12.2−/− neurons depolarized during CX4 application. The depolarization in Kv12.2+/+ neurons is accompanied by an increase in firing (b, arrow); Kv12.2−/− neurons (c) typically had high spontaneous firing rates that were not affected by CX4. Baseline resting potentials in (b) and (c) are indicated with dotted lines. Values in d are mean ± s.e.m.; asterisks indicate significance (p < 0.05); n = 8–20.
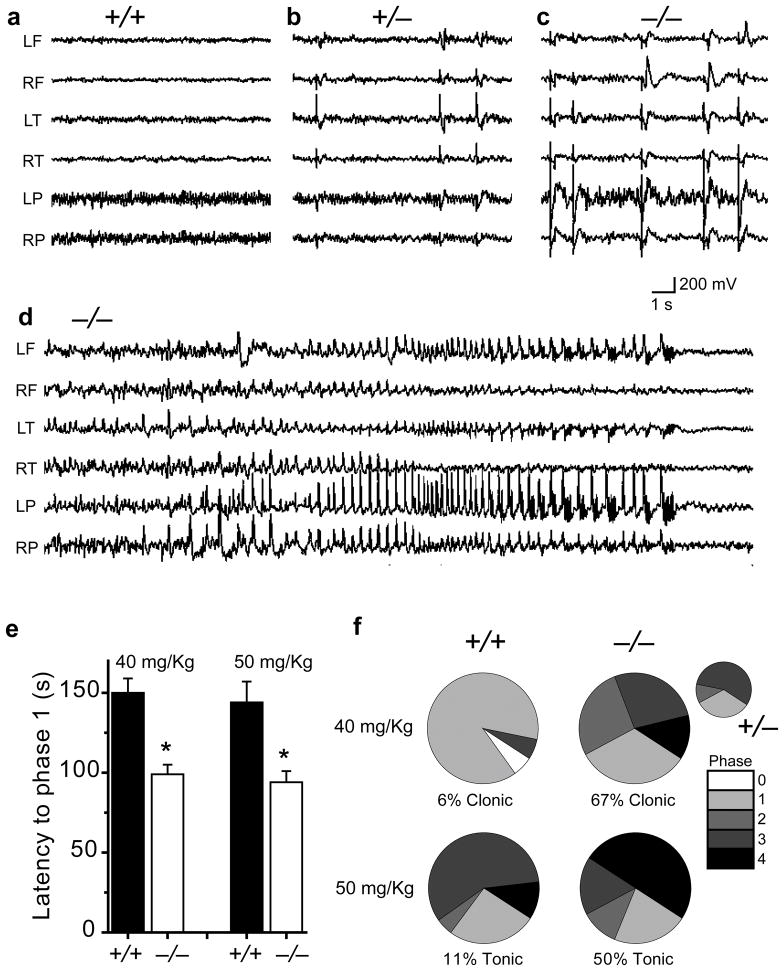
Simultaneous video/EEG monitoring revealed significant epileptic activity in Kv12.2−/− mice. Adult Kv12.2+/− and Kv12/2−/− mice showed a frequent (5–50/min) generalized pattern of sharp synchronous discharges in all cortical electrodes that were never seen in Kv12.2+/+ littermates (Fig. 3a–c). All Kv12.2−/− mice also had intermittent spontaneous non-convulsive seizures lasting from 30–60 seconds; four seizures were captured during 60 hours of monitoring. Seizures were characterized by a generalized onset of spike and slow wave discharges, increasing in frequency to a rapid spike discharge, then decelerating to slow (1–2/second) spike wave rhythms before abrupt termination (Fig. 3d). Seizures could occur while the mouse was asleep (Fig. 3d) or awake (Supplementary Video) and generally did not interrupt ongoing behaviour; brief myoclonic activity accompanied termination of seizures during sleep. Kv12.2−/− mice were hypersensitive to intraperitoneal injection of the chemoconvulsant pentylenetetrazol (PTZ)12. Genetic deletion of Kv12.2 reduced latency to seizure and increased seizure severity at threshold (40 mg/Kg) and convulsive (50 mg/Kg) PTZ doses (for Kv12.2+/+). Direct injection of CX4 into the hippocampus of Kv12.2+/+ mice also significantly reduced the latency to PTZ-induced seizure (Supplementary Fig. 5). Interestingly, Kv12.2−/− mice displayed no deficits in tests of motor coordination and learning (accelerating rotarod13), activity level and exploratory behaviour (open field test14) or contextual and cued memory (fear conditioning15) (Supplementary Fig. 6), in concordance with recent findings10.
Figure 3. Kv12.2−/− seizure phenotypes.
(a–c) Frequent generalized interictal spikes were detected in EEG recordings from Kv12.2−/− and Kv12.2+/− but not Kv12.2+/+ mice. (d) EEG recording of a seizure in a Kv12.2−/− mouse. Letters indicate recording electrode position: L (left), R (right), F (frontal), T (temporal) and P (parietal). (e) Latency to seizure (mean ± s.e.m., significance = *, p < 0.01, n = 15–18) and (f) seizure severity following intraperitoneal injection of 40 or 50 mg/Kg PTZ. Seizure phases were scored as reduced motility and prostrate position (1), partial clonus (2), generalized clonus involving the extremities (3), and tonic–clonic seizure with rigid paw extension (4). Heterozygotes had increased seizure severity at the 40 mg/Kg dose (inset, n = 9).
These results indicate that Kv12.2 significantly raises the firing threshold of hippocampal pyramidal neurons. It may therefore contribute to the ability of hippocampal pyramidal neurons to maintain “quiet” periods and respond selectively to strong or coincident stimuli. We propose that degradation of firing threshold in pyramidal neurons of the hippocampus and/or cortex contributes to persistent hyperexcitability and spontaneous seizures observed in EEG recordings from Kv12.2−/− mice.
Supplementary Material
Supplementary Figure 1. Evolution and biophysics of Kv12.2
Supplementary Figure 2. Conditional targeting strategy for genetic deletion of Kv12.2 in mice
Supplementary Figure 3. Conditional genetic deletion of Kv12.2 from cultured hippocampal pyramidal neurons results in hyperexcitability
Supplementary Figure 4. High throughput identification of a specific Kv12.2 inhibitor
Supplementary Figure 5. CX4 reduces latency to PTZ–induced seizure
Supplementary Figure 6. Normal Kv12.2 behavioural phenotypes
Supplementary Methods
Supplementary Video. Video and EEG recording of a seizure in an awake, behaving Kv12.2−/− mouse.
Sequences used in phylogenetic analysis in FASTA format
Acknowledgments
Lentivirus vectors were kindly provided by Dr. Anton Maximov. The project was funded by the Genomics Institute of the Novartis Research Foundation, NINDS grants awarded to T.J. and J.N., an AHA fellowship supporting X.Z. and INSERM funds supporting F.B.
Footnotes
Author Contributions V.L., C.C., C.W., J.A., T.J. and C.S. designed targeting constructs, handled ES cell work and produced germ line chimaeras; X.Z. and F.B. established the Kv12.2− mouse line, S.M.C. analyzed Kv12.2 expression and J.Y. and J.N. provided EEG analysis. X.Z. and F.B. conducted patch clamp experiments and contributed to manuscript preparation; X.Z., F.B. and K.B. conducted behavioral experiments. T.J. supervised the project, analyzed data and wrote the manuscript.
Supplementary Information will be linked to the online version of the paper.
References
- 1.Noebels JL. Annu Rev Neurosci. 2003;26:599–625. doi: 10.1146/annurev.neuro.26.010302.081210. [DOI] [PubMed] [Google Scholar]
- 2.McCormick DA, Contreras D. Annu Rev Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- 3.Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Nat Neurosci. 2005;8:51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- 4.Saganich MJ, Machado E, Rudy B. J Neurosci. 2001;21:4609–4624. doi: 10.1523/JNEUROSCI.21-13-04609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becchetti A, et al. Eur J Neurosci. 2002;16:415–428. doi: 10.1046/j.1460-9568.2002.02079.x. [DOI] [PubMed] [Google Scholar]
- 6.Trudeau MC, Titus SA, Branchaw JL, Ganetzky B, Robertson GA. J Neurosci. 1999;19:2906–2918. doi: 10.1523/JNEUROSCI.19-08-02906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jegla TJ, Zmasek CM, Batalov S, Nayak SK. Comb Chem High Throughput Screen. 2009;12:2–23. doi: 10.2174/138620709787047957. [DOI] [PubMed] [Google Scholar]
- 8.Putnam NH, et al. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava M, et al. Nature. 2008;454:955–960. doi: 10.1038/nature07191. [DOI] [PubMed] [Google Scholar]
- 10.Miyake A, et al. J Neurosci. 2009;29:14637–14645. doi: 10.1523/JNEUROSCI.0901-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho A, et al. J Neurosci. 2006;26:13089–13101. doi: 10.1523/JNEUROSCI.2855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes GL. Neurology. 2007;69:S28–32. doi: 10.1212/01.wnl.0000302369.24230.c6. [DOI] [PubMed] [Google Scholar]
- 13.Nolan MF, et al. Cell. 2003;115:551–564. doi: 10.1016/s0092-8674(03)00884-5. [DOI] [PubMed] [Google Scholar]
- 14.Streng J. Can J Psychol. 1971;25:62–68. doi: 10.1037/h0082368. [DOI] [PubMed] [Google Scholar]
- 15.Kim JJ, Fanselow MS. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Evolution and biophysics of Kv12.2
Supplementary Figure 2. Conditional targeting strategy for genetic deletion of Kv12.2 in mice
Supplementary Figure 3. Conditional genetic deletion of Kv12.2 from cultured hippocampal pyramidal neurons results in hyperexcitability
Supplementary Figure 4. High throughput identification of a specific Kv12.2 inhibitor
Supplementary Figure 5. CX4 reduces latency to PTZ–induced seizure
Supplementary Figure 6. Normal Kv12.2 behavioural phenotypes
Supplementary Methods
Supplementary Video. Video and EEG recording of a seizure in an awake, behaving Kv12.2−/− mouse.
Sequences used in phylogenetic analysis in FASTA format