Abstract
Objectives
Hot flashes are a common adverse effect of hormonal therapy for prostate cancer. We sought to determine the effect of acupuncture on hot flash frequency and intensity, quality of life, and sleep quality.
Methods
Men who had a hot flash score (HFS) > 4 while on androgen deprivation therapy for prostate cancer received acupuncture with electrostimulation biweekly for 4 weeks, then weekly for 6 weeks using a predefined treatment plan. The primary endpoint was a 50% reduction in HFS after 4 weeks of therapy, calculated from the patient daily hot flash diary. Hot flash related quality of life and sleep quality, and biomarkers potentially related to hot flashes, including serotonin, calcitonin gene-related peptide (CGRP), and urinary 5-HIAA were examined.
Results
Twenty-five men were enrolled between 9/2003 and 4/2007; 22 were eligible and evaluable. After four weeks, 9 of 22 patients (41%, 95%CI 21 to 64%) had a > 50% reduction in HFS. Twelve of 22 patients (55%, 95%CI 32 to 76%) met this response definition at any time during the course of therapy. No patients had a significant increase in HFS on therapy. Reduced HFS was associated with improvement in hot flash related quality of life and sleep quality.
Conclusions
Multiple placebo-controlled trials have demonstrated a 25% response rate to placebo treatment for hot flashes. 41% of patients responded by week 4 and 55% overall in this pilot study providing evidence of a potentially meaningful benefit. Further studies of acupuncture for hot flashes in this population are warranted.
Keywords: prostate cancer, hot flashes, acupuncture, androgen deprivation therapy
INTRODUCTION
Androgen deprivation therapy (ADT) is the principal medical treatment for advanced prostate cancer and is increasingly used in earlier stages of the disease. Up to 80% of men treated with androgen deprivation for prostate cancer suffer from hot flashes.1 Insomnia, fatigue, and irritability are often associated with hot flashes and adversely affect the quality of life for these patients.2–4 Twenty-seven percent indicated that hot flashes were greatly distressing.5,6
Pharmacologic therapies for hot flashes in androgen-deprived men with prostate cancer have focused on both hormonal and non-hormonal approaches. Non-androgen hormones including estrogens1,7,8 and progestins9 reduce hot flashes. Non-hormonal therapies with activity against hot flashes include clonidine10 and phenobarbital plus ergotamine.10 Recently the serotonin reuptake inhibitor (SSRI) class antidepressants have shown promising activity. While most of the data for these agents has been gathered in women,11,12 preliminary data suggest activity in androgen-deprived men with prostate cancer.13 Adverse effects of these treatments have been extensively reviewed in the literature.14 Notably, estrogen-based treatments have been associated with thromboembolic complications and gynecomastia. Progestins have been reported to stimulate prostate cancer progression in very rare instances15 and may also increase the risk of thrombosis. Adverse effects of clonidine include mouth dryness, constipation, and drowsiness, while SSRIs have been associated with mouth dryness, decreased appetite, constipation, and nausea.14
A Swedish pilot study of seven men treated by acupuncture for hot flashes due to androgen deprivation therapy for prostate cancer show promising results. Six of 7 men completed a 10-week course of acupuncture. Frequency of hot flashes was reduced between 50 and 70% at various time points. Hot flash intensity was not reported. No adverse effects were reported.16 In a larger, uncontrolled study, auricular acupuncture was shown to be active in prostate cancer patients treated with hormonal therapy.17
Similar benefits were observed in a pilot study in postmenopausal women.18 The examination of acupuncture has moved forward more rapidly in breast cancer survivors than in men living with prostate cancer. Acupuncture was shown to be superior to sham acupuncture in one randomized trial19 while a second study did not show a statistically significant improvement over sham acupuncture.20 Acupuncture yielded similar results to venlafaxine in a randomized comparison.21 In a fourth randomized comparison, acupuncture was shown to be active, but less effective than hormonal treatment in breast cancer survivors.22
Two systemic reviews recently reported on the state of the art of acupuncture for the treatment of hot flashes in breast cancer23 and prostate cancer patients.24 Both analyses called for additional research to further define the potential of acupuncture to ameliorate this common symptom.
The biologic basis of hot flashes remains incompletely understood, but several potential biomarkers are suggested by the literature. Women with hot flashes have higher mean plasma levels of the main metabolite of brain norepinephrine, 3-methoxy-4-hydroxyphenylglycol (MHPG), than do asymptomatic women. Furthermore, MHPG levels rise significantly in symptomatic women just after they experience a hot flash.25 The alpha-2 receptor agonists and antagonists alter both plasma MHPG levels and hot flashes.26,27
Multiple alterations in the serotonin system have also been associated with hot flashes. Circulating serotonin (5-HT) and urinary 5-HIAA (principal 5-HT metabolite) levels are decreased in menopause and restored with estrogen replacement therapy.28,29 Upregulation of the 5-HT2A receptors, also associated with estrogen withdrawal, has been postulated to be an important contributor to the disordered thermoregulation in post menopausal women.30 The clinical effects of mirtazipine31 and by the serotonin/norepinephrine reuptake inhibitor venlafaxine32,33 provide supportive evidence serotonin’s role in hot flashes.
Plasma calcitonin gene-related peptide (CGRP), an endothelium-dependent vasodilator, increases during a hot flash in both postmenopausal women and castrate men.34–37 Intravenous administration of exogenous CGRP increases cutaneous blood flow and causes flushing in the upper body in healthy male volunteers.38 Evidence that acupuncture can reduce CGRP levels in women with hot flashes18 provides an intriguing hypothesis for one of the mechanisms by which acupuncture may reduce vasomotor symptoms.
We sought to prospectively examine acupuncture’s effects on hot flashes using a validated hot flash instrument.39 The impact of treatment on hot flash related quality of life was also determined. To extend current understanding of the physiologic changes associated with hot flashes and with acupuncture, several relevant biologic markers were evaluated before and after treatment.
METHODS
Study Design
This was an open label single-arm phase II study. The primary endpoint was a 50% reduction in the hot flash score after 4 weeks of therapy. The hot flash score summed the number of hot flashes multiplied by the intensity of each hot flash to estimate the overall hot flash burden.39 Change in hot flash scores over time, and change in hot flash related quality of life assessed by the HFRDIS were also examined. The effect of treatment on sleep quality and the patient’s overall vitality were assessed by the PSQI and SF-36. Blood and urine were collected for analyses of serum serotonin, 5-HIAA, and plasma CGRP.
A sample of size of 25 was chosen as recommended by Sloan et al based on our interest in detecting a minimum HFS reduction of 50%.39 Based on findings in 375 patients treated in 7 clinical trials, it was expected that placebo would reduce the hot flash score by 25% and the standard deviation of hot flash score percentage reduction is 25%.
Patients
Men treated for prostate cancer either by bilateral orchiectomy, GnRH agonist or antagonists with or without antiandrogens who reported a minimum hot flash score of 4/day were eligible. Concurrent chemotherapy, estrogens (prescribed or herbal including PC-SPES), progestational agents, and gabapentin were prohibited. A two week washout (2 months for fluoxitine) was required for SSRI class antidepressants, however the SSRI prohibition was removed after 16 patients were enrolled to speed accrual. Patients with pace makers or other implantable electrical devices were excluded.
Treatment
Acupuncture treatment was administered by a qualified practitioner twice each week for the first four weeks and once per week for an additional 6 weeks. Every effort was made for each patient to receive treatment from the same practitioner throughout the entire treatment period. Sterile, disposable Seirin needles were placed bilaterally at the point Gallbladder 34, and along the spine at Bladder 15, Bladder 23, and Bladder 32. Low intensity electrostimulation at 2 Hz was used for both Bladder 23 and Bladder 32. Points were also placed unilaterally at Governing vessel 20, Heart 7, Pericardium 6, Liver 2, and Spleen 6. Needles were retained for 30 minutes with manual stimulation at 10 minute intervals. Basic dietary suggestions were made to limit the intake of caffeine, spicy foods, and alcohol as is common practice by Chinese medical providers when treating hot flashes. No herbal treatments, vitamins, or supplements were recommended. The treatment plan follows that of the Swedish pilot study16 with slight modifications based on classic text of acupuncture.40 Specifically, Gall Bladder 34 was added due to its ability to strongly reduce heat and relax muscles and tendons, Spleen 9 was omitted to keep the total number of points unchanged, and Liver 3 was substituted for Liver 2, due to its classical function of heat reduction.
Assessments
The hot flash score was calculated from the Hot Flash Diary (HFD) as described by Sloan at al.39 Patients were asked to fill out the HFD daily for 7 days prior to treatment and daily throughout the study. Each hot flash was multiplied by its severity (1 – mild, 2 – moderate, 3 – severe, 4 – very severe) to yield a severity-adjusted hot flash. All severity-adjusted hot flashes were summed over a 7 day period and divided by 7 for a daily hot flash score expressed as points/day.
Hot flash related quality of life measured by the Hot Flash Related Daily Interference Scale (HFRDIS)41 was administered immediately before starting therapy and then after 2, 4, 6 and 10 weeks of treatment.
To determine the effect of treatment on sleep quality the Pittsburgh Sleep Quality Index (PSQI) was administered immediately before starting therapy and then after 4 to 6 weeks of treatment and again after 10 weeks of treatment.
To assess the effect of treatment on a patient’s overall vitality the Short Form Health Survey (SF-36) was administered immediately before starting therapy and ten after 4 and 10 weeks of treatment.
Biomarkers
Baseline assessment included morning serum serotonin, 24-hour urinary 5-HIAA, as well as a detailed blood collection for CGRP. Morning serum serotonin and 24-hour urinary 5-HIAA were collected after 2, 4, 6 and 10 weeks of treatment and again 6 weeks after completion of treatment. Detailed blood collection of plasma for CGRP was obtained once after 4 weeks of therapy. The schedule for sample collection is detailed in the results section. The start time for drawing blood and plasma markers was no sooner than 1 hour after a hot flash to avoid interference from acute changes associated with a hot flash.
5-HIAA was assayed in duplicate from a 24-hour urine aliquot preserved with 6N HCL by Enzyme immunoassay, EIA (Rocky Mountain Diagnostics Inc, Colorado Springs, CO 80903). The manufactures reported sensitivity was 0.17 mg/l and the average interassay CV 10.3%. For CGRP analysis blood was collected in 5 ml EDTA vacutainers and immediately placed on ice prior to centrifugation at 5°C. CGRP was then assayed in duplicate by enzyme immunoassay, EIA (Cayman Chemical Company, Ann Arbor, Michigan, 48108). The analytical sensitivity as reported by the manufacturer was 2 pg/mL and the average interassay CV 6.7%. The standard curve range was 7.81 to 500 pg/mL. Serotonin was assayed in duplicate from serum by radioimmunoassay, RIA (ALPCO, American laboratory Products Company, Windham, NH 03087). The manufactures reported sensitivity was 6.7 ng/mL and the average interassay CV 5.1 %. The standard curve range was 15 to 2,500 ng/mL.
RESULTS
Patients
Twenty-five patients were registered to the study between September 2003 and April 2007. Two registered patients were ineligible and 23 began treatment. One patient withdrew during the first week of treatment without significant toxicity, therefore 22 patients were evaluable for efficacy. Toxicity assessment included all 23 patients who started therapy. Characteristics of the 22 eligible and evaluable patients are summarized in Table 1.
Table 1.
Patient Characteristics at Study Entry
| Characteristic | |
|---|---|
| Number of patients | 22 |
| Age, years | |
| Median (Range) | 71 (56–88) |
| Type of Hormonal Therapy | |
| LHRH agonist alone | 15 |
| LHRH agonist + antiandrogen | 4 |
| LHRH agonist + Ketoconazole | 1 |
| Orchiectomy | 1 |
| Orchiectomy + Ketoconazole | 1 |
| Baseline hot flash score | |
| Mean (Standard Deviation) | 16.2 (12.3) |
| Median (Range) | 10.8 (7.3– 51.6) |
Hot flash reduction
The mean ± 95% CI in the hot flash score is shown in figure 1.
Figure 1.
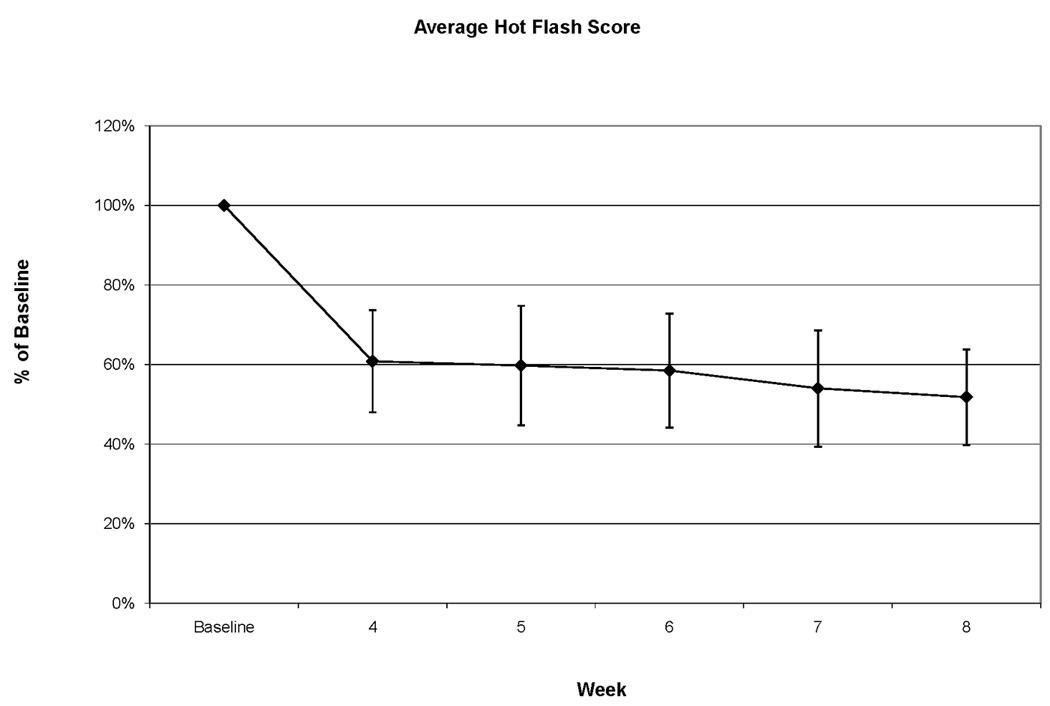
Mean + 95% CI of Hot Flash Score at baseline and after 4, 5, 6, 7, and 8 weeks of therapy.
When examined in all patients, the mean hot flash score was reduced to 60% of baseline after 4 weeks of therapy and 52% of baseline after 8 weeks of therapy. Many patients found keeping daily hot flash diaries quite taxing. As a result, hot flash frequency and intensity ascertainment beyond week 8 of therapy was incomplete. Hot flash scores were therefore not calculated for week 10 and 16.
After four weeks of therapy, 9 of 22 patients (41%, 95%CI 21 to 64%) had a greater than 50% reduction in hot flashes. The number of patients who reached this criterion varied between 9 and 12 at their weekly assessments after 4, 5, 6, 7, and 8 of therapy. The largest fraction of responders was seen after 7 weeks of therapy: 12 of 22 patients (55%, 95%CI 32 to 76%). While reductions in the hot flash score were common, increases were not. Only 1 patient, and only on a single assessment, reported an increase in excess of 50%. By week 8, only 2 of 22 patients had a hot flash score that was not lower than baseline. In those patients, hot flash scores were essentially unchanged with therapy and stood at 102% and 108% of baseline.
Hot flash related quality of life
Baseline HFDIS scores were available for 21 of the 22 patients. At week 4 and at week 10, HFDIS data were available for 16 and 17 patients respectively. To determine the effect of treatment on hot flash related quality of life, we compared HFDIS scores at week 4 and week 10 to baseline in those patients for whom data were available at both of the relevant time points. After 4 weeks of treatment, mean HFDIS scores improved from 35.9 (SD 25.2) to 18.4 (SD 23.8) (p=0.002, n=16). After 10 weeks of treatment, mean HFDIS scores improved from 34.3 (SD 22.1) to 22.6 (SD 22.8) (p=0.0003, n=17).
Pittsburgh Sleep Quality Index
Baseline PSQI scores were available for 19 of the 22 patients. On treatment analyses after 4 to 6 weeks of treatment and at week 10 PSQI data was available for 17 patients. For each comparison, patients with a measurement at each time point were included. When all patients were analyzed, we saw no statistically significant difference between PSQI scores at baseline and 6 and 10 weeks although a favorable trend was seen at the initial on treatment evaluation (PSQI 7.4 (SD 3.5) at baseline and 5.8 (SD 2.7) on treatment, p=0.08).
A global PSQI score of ≥ 5 is consistent with severe sleep difficulties in at least 2 domains or moderate difficulties in more than 3 areas and is therefore considered to represent significant impairment in sleep quality. At baseline, 16 patients had a global PSQI score ≥ 5. Of those 20% and 9% saw their PSQI global score drop below 5 at 6 and 10 week evaluations respectively.
Interestingly, responders (defined as those patients who had at least a 50% reduction in their hot flash score) experienced a mean global scores improvement from 8.1 (SD 3.8) to 5.4 (SD 2.8) (p=0.007), while no improvement was seen in non-responders (6.2 (SD 2.9) and 6.4 (SD 2.7), p=0.9).
Thus the PSQI data suggest that successful treatment of hot flashes with acupuncture has the potential to meaningfully improve sleep quality.
The Short Form Health Survey-36
We did not see improvements in overall vitality as measured by the SF-36.
Toxicity
All adverse events reported by patients at any visit were tabulated and are reported in Table 2 regardless of deemed relationship to study treatment. There were no clinically significant adverse events and no treatment delays due to an adverse event. All events were considered mild (equivalent of grade 1 in the CTCAE).
Table 2.
Adverse Events
| Event | Number of patients reporting |
|---|---|
| Fatigue | 6 |
| Mood Changes | 6 |
| Sweating | 6 |
| Somnolence | 5 |
| Dizziness | 4 |
| Insomnia | 4 |
| Dry Mouth | 4 |
| Appetite Loss | 1 |
| Nausea | 1 |
| Nervousness | 1 |
| Chills | 1 |
| Leg Cramps | 1 |
| Diarrhea | 1 |
| Rapid Heartbeat | 1 |
Biomarkers
Morning Serum Serotonin
We saw no changes in mean serum serotonin on therapy in all patients or when patients were stratified by responder status.
Urinary 5-HIAA
Results of urinary 5-HIAA analyses are shown in Figure 2. In all subjects, there was a non-significant trend towards reduced 5-HIAA concentrations. The average 5-HIAA concentration on therapy was 84.1% of baseline. Interestingly, once therapy was discontinued, the average 5-HIAA concentration rose to 104.3% of baseline. Different patterns of 5-HIAA concentration changes emerged when the analysis was stratified by response status. 5-HIAA concentrations increased over time in non-responders. While statistical power was limited by the sample size, this observation approached significance in weeks 2 and 6 and was statistically significant in week 10. The average 5-HIAA concentration in non-responders during treatment was 129% of baseline. Conversely, 5-HIAA concentrations decreased during treatment in responders and this decrease was statistically significant in week 10. The average 5-HIAA concentration during treatment in responders was 73.3% of baseline (Figure 2).
Figure 2.
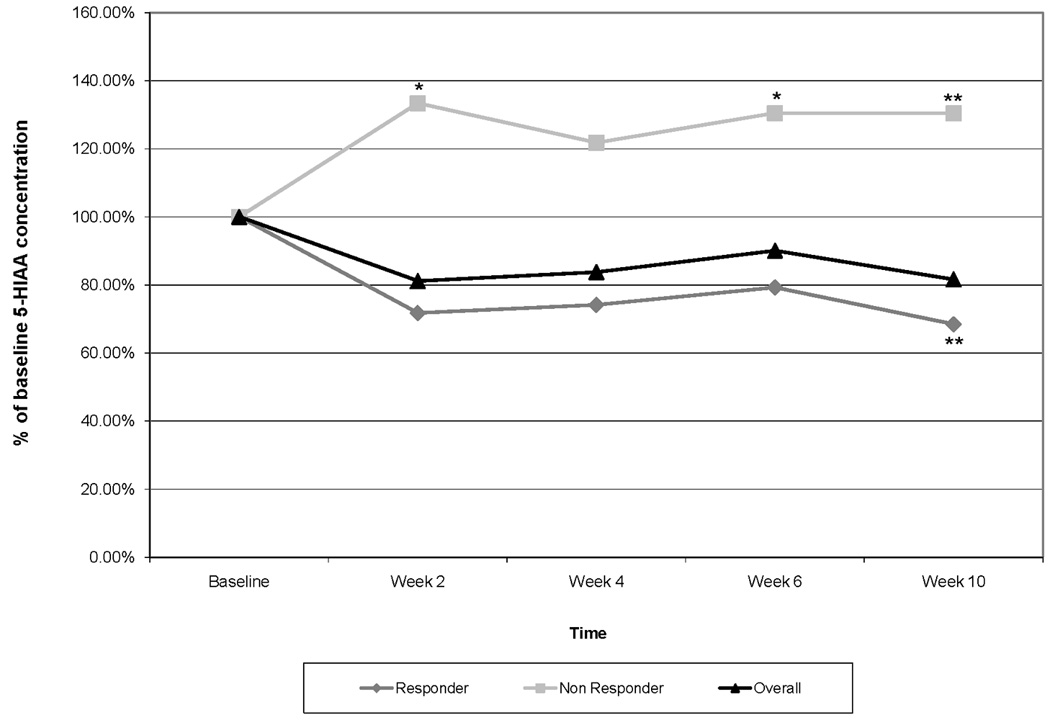
Urinary 5-HIAA concentrations shown as a % of baseline for all subjects and for responding and non-responding subjects. For this analysis, responders were defined as those subjects whose hot flash score was reduced by ≥ 50% at any time on therapy.
* indicates p<0.1, ** indicates p <0.05.
CGRP
To determine the extent to which relief of hot flashes with acupuncture is associated with changes in CGRP, CGRP was collected at baseline and after 4 weeks of therapy using timed pre-hot flash measurements (30, 60, 120, 180, 240, 300, 360) and hot flash measurements (immediately after a hot flash was noted, and 1 and 5 minutes after a hot flash was noted).
We found considerable heterogeneity in CGRP levels at baseline. Mean CGRP was 187.7 and median CGRP was 94.06 with a range of 7.8 to 500 and a standard deviation of 203.2. The variability on CGRP reduced the statistical power of this study to detect differences. When the entire population was examined, we found no clinically significant trends.
When the analysis was stratified by hot flash response status, a non-significant trend toward reduced mean timed pre-hot flash measurements of CGRP concentrations in responders and increased mean timed pre-hot flash measurements of CGRP concentrations in non-responders emerged. In responders there was a 9.9% decrease in mean timed pre-hot flash measurement on therapy compared to baseline, as compared to a 14.8% increase in non-responders (p=0.065).
There were no significant trends in mean timed hot flash measurement CGRP concentrations on therapy drawn immediately, and 1 and 5 minutes after a hot flash.
DISCUSSION
Multiple placebo-controlled trials have demonstrated that 25% of patients respond to placebo treatment for hot flashes. Forty-one percent of patients responded by week 4 and 55% by week 7 in this pilot study providing evidence of a potentially meaningful benefit with acupuncture therapy. Hot flash-related quality of life was also significantly improved with acupuncture treatment and treatment was well tolerated. Consistent with our hypothesis, we observed a trend towards improvement in sleep quality when all patients were analyzed. When stratified by hot flash response status, there was improvement in sleep quality in hot flash responders but not non-responders. Both of these results suggest that the improvement in hot flashes was clinically meaningful to our participants. The absence of improvements on SF-36 may reflect both limited power and the broader nature of this instrument that does not focus tightly on the impact of hot flashes.
These results suggest a clinically meaningful benefit of acupuncture in the treatment of hot flashes. The hot flash related quality of life and sleep quality data strengthen these results and illustrate the potential of successful hot flash therapy to improve cancer survivorship. The clinical activity of acupuncture, as observed in this study, is comparable to SSRI antidepressants but less than hormonal agents like megestrol acetate and estradiol. The clinical activity of acupuncture seen in this single arm study should be confirmed in a randomized comparative trial.
Correlative biomarker analyses showed a trend towards a decrease in urinary 5-HIAA concentrations in all subjects who received acupuncture treatment. Patients who experienced a ≥ 50% reduction in hot flash scores had a statistically significant decrease in 5-HIAA concentrations while urinary 5-HIAA concentrations increased in non-responders. We did not detect significant changes in serum serotonin or CGRP. These exploratory analyses represent the first analyses of these potential biomarkers of acupuncture activity in the treatment of hot flashes. Our inability to detect an overall difference is not surprising. While these neurotransmitters have been implicated in hot flashes, it is not clear that their assessment in the blood rather than in the central nervous system, is sufficiently sensitive. Changes in neurotransmitters associated with hot flashes would be expected to be present in the brain. Systemic levels reflect not only the central nervous system, but also peripheral nervous system and other sources. For example platelets are a major reservoir of serotonin. Our results suggest that measurement of circulating neurotransmitters and their metabolites may not be sufficiently sensitive to monitor either the effects of acupuncture or other treatments for hot flashes.
CONCLUSIONS
In summary, this single arm study non-comparative study suggests that acupuncture may be an active non-pharmacologic treatment option for prostate cancer patients with hot flashes. Further studies of the efficacy of acupuncture for hot flashes in men undergoing hormonal treatment for prostate cancer that include a control group as well as the biologic basis of the observed treatment effect are warranted.
Acknowledgments
Sources of support: Supported in part by grant M01 RR000334 from the OHSU General Clinical Research Center (GCRC), grant UL1RR024140 from the Oregon Clinical and Translational Research Institute (OCTRI) and grant R21 CA098406 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gerber GS, Zagaja GP, Ray PS, et al. Transdermal estrogen in the treatment of hot flushes in men with prostate cancer. Urology. 2000;55:97–101. doi: 10.1016/s0090-4295(99)00370-2. [DOI] [PubMed] [Google Scholar]
- 2.Rebar RW, Spitzer IB. The physiology and measurement of hot flushes. Am J Obstet Gynecol. 1987;156:1284–1288. doi: 10.1016/0002-9378(87)90165-7. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg F. Hot flashes: epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. [DOI] [PubMed] [Google Scholar]
- 4.Oldenhave A, Jaszmann LJ, Haspels AA, et al. Impact of climacteric on well-being. A survey based on 5213 women 39 to 60 years old. Am J Obstet Gynecol. 1993;168:772–780. doi: 10.1016/s0002-9378(12)90817-0. [DOI] [PubMed] [Google Scholar]
- 5.Frodin T, Alund G, Varenhorst E. Measurement of skin blood-flow and water evaporation as a means of objectively assessing hot flushes after orchidectomy in patients with prostatic cancer. Prostate. 1985;7:203–208. doi: 10.1002/pros.2990070210. [DOI] [PubMed] [Google Scholar]
- 6.Charig CR, Rundle JS. Flushing. Long-term side effect of orchiectomy in treatment of prostatic carcinoma. Urology. 1989;33:175–178. doi: 10.1016/0090-4295(89)90385-3. [DOI] [PubMed] [Google Scholar]
- 7.Atala A, Amin M, Harty JI. Diethylstilbestrol in treatment of postorchiectomy vasomotor symptoms and its relationship with serum follicle-stimulating hormone, luteinizing hormone, and testosterone. Urology. 1992;39:108–110. doi: 10.1016/0090-4295(92)90264-w. [DOI] [PubMed] [Google Scholar]
- 8.Miller JI, Ahmann FR. Treatment of castration-induced menopausal symptoms with low dose diethylstilbestrol in men with advanced prostate cancer. Urology. 1992;40:499–502. doi: 10.1016/0090-4295(92)90401-h. [DOI] [PubMed] [Google Scholar]
- 9.Loprinzi CL, Michalak JC, Quella SK, et al. Megestrol acetate for the prevention of hot flashes. N Engl J Med. 1994;331:347–352. doi: 10.1056/NEJM199408113310602. [DOI] [PubMed] [Google Scholar]
- 10.Smith JA., Jr A prospective comparison of treatments for symptomatic hot flushes following endocrine therapy for carcinoma of the prostate. J Urol. 1994;152:132–134. doi: 10.1016/s0022-5347(17)32835-5. [DOI] [PubMed] [Google Scholar]
- 11.Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356:2059–2063. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 12.Stearns V, Isaacs C, Rowland J, et al. A pilot trial assessing the efficacy of paroxetine hydrochloride (Paxil) in controlling hot flashes in breast cancer survivors. Ann Oncol. 2000;11:17–22. doi: 10.1023/a:1008382706068. [DOI] [PubMed] [Google Scholar]
- 13.Roth AJ, Scher HI. Sertraline relieves hot flashes secondary to medical castration as treatment of advanced prostate cancer. Psychooncology. 1998;7:129–132. doi: 10.1002/(SICI)1099-1611(199803/04)7:2<129::AID-PON294>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 14.Barton D, Loprinzi C, Wahner-Roedler D. Hot flashes: aetiology and management. Drugs Aging. 2001;18:597–606. doi: 10.2165/00002512-200118080-00004. [DOI] [PubMed] [Google Scholar]
- 15.Sartor O, Eastham JA. Progressive prostate cancer associated with use of megestrol acetate administered for control of hot flashes. South Med J. 1999;92:415–416. doi: 10.1097/00007611-199904000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Hammar M, Frisk J, Grimas O, et al. Acupuncture treatment of vasomotor symptoms in men with prostatic carcinoma: a pilot study. J Urol. 1999;161:853–856. [PubMed] [Google Scholar]
- 17.Harding C, Harris A, Chadwick D. Auricular acupuncture: a novel treatment for vasomotor symptoms associated with luteinizing-hormone releasing hormone agonist treatment for prostate cancer. BJU Int. 2009;103:186–190. doi: 10.1111/j.1464-410X.2008.07884.x. [DOI] [PubMed] [Google Scholar]
- 18.Wyon Y, Lindgren R, Lundberg T, et al. Effects of acupuncture on climacteric vasomotor symptoms, quality of life, and urinary excretion of neuropeptides among postmenopausal women. Monopause. 1995;2:3–12. [Google Scholar]
- 19.Hervik J, Mjaland O. Acupuncture for the treatment of hot flashes in breast cancer patients, a randomized, controlled trial. Breast Cancer Res Treat. 2009;116:311–316. doi: 10.1007/s10549-008-0210-3. [DOI] [PubMed] [Google Scholar]
- 20.Deng G, Vickers A, Yeung S, et al. Randomized, controlled trial of acupuncture for the treatment of hot flashes in breast cancer patients. J Clin Oncol. 2007;25:5584–5590. doi: 10.1200/JCO.2007.12.0774. [DOI] [PubMed] [Google Scholar]
- 21.Walker EM, Rodriguez AI, Kohn B, et al. Acupuncture versus venlafaxine for the management of vasomotor symptoms in patients with hormone receptor-positive breast cancer: a randomized controlled trial. J Clin Oncol. 2010;28:634–640. doi: 10.1200/JCO.2009.23.5150. [DOI] [PubMed] [Google Scholar]
- 22.Frisk J, Carlhall S, Kallstrom AC, et al. Long-term follow-up of acupuncture and hormone therapy on hot flushes in women with breast cancer: a prospective, randomized, controlled multicenter trial. Climacteric. 2008;11:166–174. doi: 10.1080/13697130801958709. [DOI] [PubMed] [Google Scholar]
- 23.Lee MS, Kim KH, Choi SM, et al. Acupuncture for treating hot flashes in breast cancer patients: a systematic review. Breast Cancer Res Treat. 2009;115:497–503. doi: 10.1007/s10549-008-0230-z. [DOI] [PubMed] [Google Scholar]
- 24.Lee MS, Kim KH, Shin BC, et al. Acupuncture for treating hot flushes in men with prostate cancer: a systematic review. Support Care Cancer. 2009;17:763–770. doi: 10.1007/s00520-009-0589-3. [DOI] [PubMed] [Google Scholar]
- 25.Freedman RR. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil Steril. 1998;70:332–337. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 26.Freedman RR, Woodward S, Sabharwal SC. Alpha 2-adrenergic mechanism in menopausal hot flushes. Obstet Gynecol. 1990;76:573–578. [PubMed] [Google Scholar]
- 27.Charney DS, Heninger GR, Sternberg DE. Assessment of alpha 2 adrenergic autoreceptor function in humans: effects of oral yohimbine. Life Sci. 1982;30:2033–2041. doi: 10.1016/0024-3205(82)90444-1. [DOI] [PubMed] [Google Scholar]
- 28.Gonzales GF, Carrillo C. Blood serotonin levels in postmenopausal women: effects of age and serum oestradiol levels. Maturitas. 1993;17:23–29. doi: 10.1016/0378-5122(93)90120-7. [DOI] [PubMed] [Google Scholar]
- 29.Lippert TH, Filshie M, Muck AO, et al. Serotonin metabolite excretion after postmenopausal estradiol therapy. Maturitas. 1996;24:37–41. doi: 10.1016/0378-5122(95)00998-1. [DOI] [PubMed] [Google Scholar]
- 30.Berendsen HH. The role of serotonin in hot flushes. Maturitas. 2000;36:155–164. doi: 10.1016/s0378-5122(00)00151-1. [DOI] [PubMed] [Google Scholar]
- 31.Waldinger MD, Berendsen HH, Schweitzer DH. Treatment of hot flushes with mirtazapine: four case reports. Maturitas. 2000;36:165–168. doi: 10.1016/s0378-5122(00)00152-3. [DOI] [PubMed] [Google Scholar]
- 32.Loprinzi CL, Pisansky TM, Fonseca R, et al. Pilot evaluation of venlafaxine hydrochloride for the therapy of hot flashes in cancer survivors. J Clin Oncol. 1998;16:2377–2381. doi: 10.1200/JCO.1998.16.7.2377. [DOI] [PubMed] [Google Scholar]
- 33.Quella SK, Loprinzi CL, Sloan J, et al. Pilot evaluation of venlafaxine for the treatment of hot flashes in men undergoing androgen ablation therapy for prostate cancer. J Urol. 1999;162:98–102. doi: 10.1097/00005392-199907000-00024. [DOI] [PubMed] [Google Scholar]
- 34.Spetz AC, Pettersson B, Varenhorst E, et al. Momentary increase in plasma calcitonin gene-related peptide is involved in hot flashes in men treated with castration for carcinoma of the prostate. J Urol. 2001;166:1720–1723. [PubMed] [Google Scholar]
- 35.Wyon YA, Spetz AC, Theodorsson GE, et al. Concentrations of calcitonin gene-related peptide and neuropeptide Y in plasma increase during flushes in postmenopausal women. Menopause. 2000;7:25–30. doi: 10.1097/00042192-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 36.Valentini A, Petraglia F, De Vita D, et al. Changes of plasma calcitonin gene-related peptide levels in postmenopausal women. Am J Obstet Gynecol. 1996;175:638–642. doi: 10.1053/ob.1996.v175.a74287. [DOI] [PubMed] [Google Scholar]
- 37.Chen JT, Hirai Y, Seimiya Y, et al. Menopausal flushes and calcitonin-gene-related peptide. Lancet. 1993;342:49. doi: 10.1016/0140-6736(93)91911-5. [DOI] [PubMed] [Google Scholar]
- 38.Jernbeck J, Edner M, Dalsgaard CJ, et al. The effect of calcitonin gene-related peptide (CGRP) on human forearm blood flow. Clin Physiol. 1990;10:335–343. doi: 10.1111/j.1475-097x.1990.tb00795.x. [DOI] [PubMed] [Google Scholar]
- 39.Sloan JA, Loprinzi CL, Novotny PJ, et al. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 40.Ellis A, Wiseman N, Boss K. Fundamentals of Chinese Acupuncture. Brookline, MA: Paradigm Publications; 1991. pp. 1–479. [Google Scholar]
- 41.Carpenter JS. The Hot Flash Related Daily Interference Scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage. 2001;22:979–989. doi: 10.1016/s0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]


