Abstract
We found that rat central auditory neurons fire action potentials in a precise sequence of mini-bursts prior to hearing onset. This stereotyped pattern is initiated by hair cells within the cochlea, which trigger brief bursts of action potentials in auditory neurons each time they fire a Ca2+ spike. By generating theta-like activity, hair cells may limit the influence of synaptic depression in developing auditory circuits and promote consolidation of synapses.
Developing sensory systems rely on intrinsically-generated electrical activity to guide the maturation of circuits required for processing sensory information1. In all sensory modalities examined, this spontaneous activity occurs in the form of discrete bursts of action potentials separated by long periods of quiescence1,2, yet the mechanisms by which burst firing influences diverse aspects of development are largely unknown. In mature circuits, plasticity is enabled by distinct forms of activity3,4, raising the possibility that developing circuits also initiate stereotyped patterns of activity to promote efficient induction of certain signal transduction cascades.
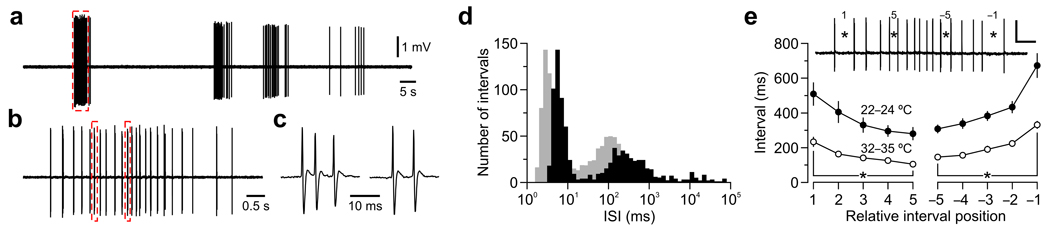
Atricial mammals are born deaf and do not respond to sound until the second postnatal week. Nevertheless, auditory neurons fire bursts of action potentials during the prehearing period5,6 that are likely initiated within the developing cochlea. Indeed, recent studies indicate that ATP is released spontaneously from supporting cells in the developing cochlea, which depolarizes inner hair cells (IHCs) and eventually induces trains of action potentials in spiral ganglion neurons (SGNs)7,8. To define the patterns of activity exhibited by SGNs during this period, we made extracellular recordings from SGNs in cochleae isolated from prehearing rats. Spontaneous activity in SGNs was clustered into discrete bursts that lasted 2.6 ± 0.3 s, contained 15.8 ± 1.8 action potentials and occurred at a frequency of 2.6 ± 0.4 min−1 (n = 27) (Fig. 1a). Action potentials within bursts did not occur randomly, but were grouped into discrete “minibursts” of one to six action potentials (average: 1.6 ± 0.1) that repeated every 100–300 ms (Fig. 1b,c), suggesting that SGNs are under the influence of a pacemaker. This firing pattern was consistent over time and similar between SGNs; inter-spike interval (ISI) histograms had three peaks: one near 10 ms, one between 100 and 300 ms and one broad peak near 10 s (n = 31) (Fig. 1d and Supplementary Fig. 1), representing the intervals separating action potentials within mini-bursts, intervals separating mini-bursts and long intervals separating bursts, respectively. Another conspicuous feature of this activity was that intervals between mini-bursts consistently decreased and then increased during each burst (Fig. 1e). Similar burst patterns were observed at near physiological temperature (n = 8) and in cochleae acutely-isolated from prehearing rats (n = 10) (Fig. 1d,e and Supplementary Fig. 2). Notably, efferent input was not required to initiate rhythmic activity in SGNs, as this discharge pattern was not altered upon blocking cholinergic transmission at efferent synapses (Supplementary Fig. 3).
Figure 1.
SGNs fire patterned action potential bursts during the prehearing period. (a) In vitro extracellular recording from a SGN at room temperature. (b) Detail of dashed red box in a. (c) Detail of mini-bursts highlighted in b. (d) Overlaid log-binned ISI histograms for the neuron in a (black) and a representative cell at near-physiological temperature (32–35 °C, gray). (e) Mean duration (± s.e.m.) of intervals separating mini-bursts as a function of their relative position within a burst, for recordings performed at 22–24 °C (n = 302 bursts in 23 cochleae) and 32–35 °C (n = 191 bursts in 8 cochleae). Inset: Example of relative mini-burst interval position at the beginning (1 and 5) and end (−1 and −5) of a spontaneous burst. Scale: 0.5 mV, 0.4 s. * P < 0.001, paired t-test. All experimental procedures used in this study were approved by the Animal Care and Use Committees at Johns Hopkins University and Erasmus MC.
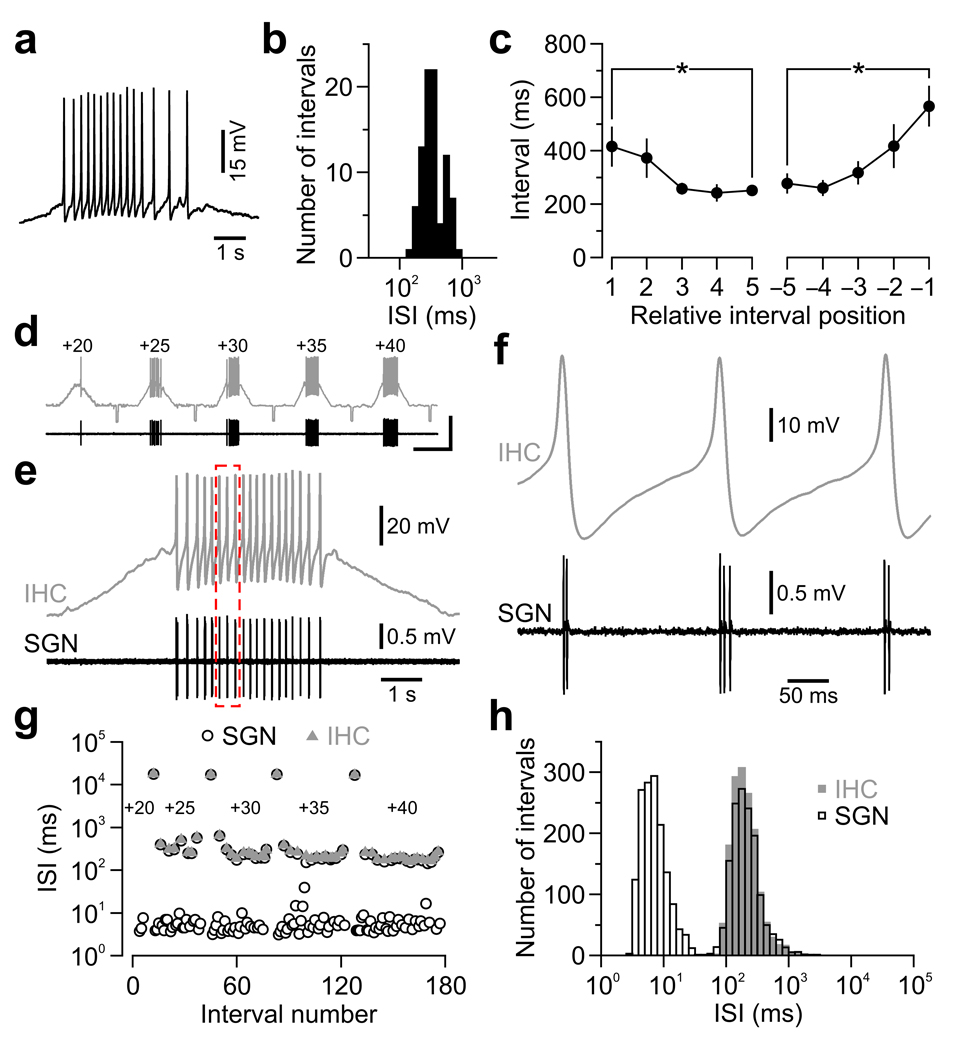
To determine the mechanisms responsible for these patterns, we made whole-cell current-clamp recordings from IHCs. Release of ATP from supporting cells periodically depolarized IHCs, which often triggered trains of Ca2+ spikes with ISIs of 361 ± 26 ms (n = 7) (Fig. 2a,b), similar to the delay between action potentials within mini-bursts in SGNs (356 ± 39 ms, n = 27; P = 0.9, two-sample t-test). Notably, intervals between Ca2+ spikes progressively decreased and then increased during these events (Fig. 2a,c). To determine if IHC Ca2+ spikes were sufficient to induce SGN mini-bursts, we recorded simultaneously from IHCs and their synaptically-connected SGNs (Supplementary Fig. 4). Slow depolarization of IHCs triggered trains of Ca2+ spikes in IHCs and discrete bursts of action potentials in SGNs (n = 11 pairs) (Fig. 2d,e). The vast majority of Ca2+ spikes (92 %; n = 1,634 spikes) elicited postsynaptic mini-bursts of 2.1 ± 0.1 action potentials (Fig. 2f) separated by 8.0 ± 0.7 ms (n = 11), similar to the first peak in ISI histograms (Fig. 1d). Furthermore, intervals separating IHC Ca2+ spikes and SGN action potential mini-bursts were indistinguishable (Fig. 2g,h). Thus, IHC Ca2+ spikes act as pacemakers to set the timing of action potentials in peripheral auditory neurons before hearing.
Figure 2.
IHC Ca2+ spikes initiate action potential mini-bursts in SGNs before hearing onset. (a) Spontaneous burst of Ca2+ spikes recorded from an IHC (22–24 °C). (b) Log-binned histogram of intervals separating Ca2+ spikes within spontaneous bursts (n = 7 IHCs). (c) Mean duration (± s.e.m.) of intervals separating Ca2+ spikes vs. relative position within a burst. * P < 0.05. (d–g) Simultaneous recording from an IHC (whole-cell; gray) and a synaptically-connected SGN (extracellular; black). (d) Continuous paired recording upon 5 consecutive depolarizing current injections of increasing amplitude (20–40 pA). Small hyperpolarizing current steps (−10 pA) were injected every 20 s. Scale bars: 50 mV (top trace), 2 mV (bottom trace); 10 s. (e) IHC membrane potential (Vrest = −80 mV) upon 40 pA injection and corresponding postsynaptic SGN firing. (f) Detail of dashed red box in e. (g) Plot of consecutive intervals (log scale) separating IHC Ca2+ spikes and SGN action potentials from the recording in d. (h) Superimposed log-binned ISI histograms for all IHC Ca2+ spikes and SGN action potentials pooled from 11 paired recordings. Long intervals separating current injections were excluded for clarity.
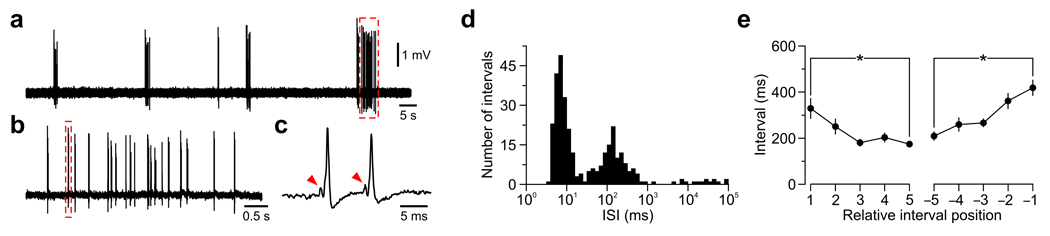
To determine whether activity patterns initiated within the cochlea propagate through central auditory nuclei, we made extracellular recordings in vivo from principal neurons in the medial nucleus of the trapezoid body (MNTB) (Supplementary Fig. 5), a relay nucleus in the brainstem involved in sound localization9. Spontaneous activity in most MNTB neurons (n = 31/34, 91 %) in prehearing postnatal day (P) 4–8 rats, consisted of discrete bursts of action potentials similar to those recorded from SGNs: bursts lasted for 2.6 ± 0.4 s, occurred at a frequency of 3.2 ± 0.3 min−1 and contained 16.5 ± 2.7 action potentials (Fig. 3a). Action potentials within bursts were typically clustered into discrete mini-bursts containing 1.5 ± 0.1 action potentials (Fig. 3b,c), and ISI histograms had distinct peaks near 10 ms, 100–300 ms and 10 s (Fig. 3d and Supplementary Fig. 1). In addition, the intervals between mini-bursts progressively decreased and then increased during each burst (Fig. 3e), a characteristic feature of SGN activity. This firing pattern was observed as early as P1 and was prevalent for most of the postnatal prehearing period (Supplementary Fig. 1). In recordings where pre-spikes were visible (n = 16/31), all action potentials were preceded by a pre-spike in 14 cells (Fig. 3c), and in the remaining two cells, 97 % of action potentials were preceded by a pre-spike, indicating that this activity was induced by synaptic input from the cochlear nucleus10. Moreover, spontaneously active MNTB neurons were not observed when the contralateral cochlea was removed (n = 6) (Supplementary Fig. 6), indicating that the patterns of activity exhibited by MNTB neurons in vivo are initiated within the cochlea.
Figure 3.
MNTB neurons fire patterned action potential bursts during the prehearing period. (a) In vivo extracellular recording from a P5 MNTB neuron. (b) Detail of dashed red box in a. (c) Detail of the mini-burst highlighted in b. Arrowheads indicate pre-spikes. (d) Log-binned ISI histograms for the cell in a. (e) Mean duration (± s.e.m.) of mini-burst intervals within bursts (n = 216 bursts in 19 units). * P < 0.003, paired t-test.
To explore whether neurons in other auditory centers exhibit similar activity, we made in vivo extracellular recordings from the central nucleus of the inferior colliculus (CIC), a major midbrain nucleus that integrates ascending auditory information. Most recordings from CIC were composed of multiple units that exhibited highly correlated activity (n = 92/120 recordings, 77 %), consisting of bouts of action potentials lasting several seconds (Supplementary Fig. 7); the remaining recordings contained either a few isolated spikes or regularly discharging units. The high degree of synchrony among CIC neurons in vivo is consistent with ATP-mediated events in the cochlea, which initiate synchronous activity in groups of IHCs in the same region of the organ of Corti7,8. In recordings where action potentials from individual bursting units could be discriminated (n = 21), CIC neurons displayed firing patterns similar to those recorded from SGNs and MNTB neurons: action potentials were clustered in discrete bursts of 8.5 ± 1.1 action potentials, which lasted 1.7 ± 0.2 s and occurred at 2.9 ± 0.4 min−1. Although extended mini-bursts were not as frequent, ISI histograms had distinct peaks near 100–300 ms and 10 s, and intervals between mini-bursts progressively shortened and then lengthened during each burst (Supplementary Figs. 1 and 7). Moreover, burst activity in CIC neurons was abolished following bilateral cochlear ablation (Supplementary Fig. 8), providing further evidence that the characteristic activity patterns initiated by Ca2+ spikes in cochlear hair cells before hearing propagate though central auditory circuits (see Supplementary Discussion).
Pioneering studies in newborn cats revealed that auditory neurons fire rhythmically in small groups of one to several action potentials every 100–300 ms upon exposure to loud sound12. Unexpectedly, the periodicity of this activity was influenced by the intensity but not the frequency of the stimulus, in marked contrast to sharply-tuned, sustained responses observed in adults. Our results suggest that the rhythmic nature of this activity arises from generation of Ca2+ spikes in IHCs, which promote transmitter release from immature ribbon synapses13, but impose strict limitations on the timing of action potentials in auditory neurons.
Calyceal synapses in the auditory pathway of prehearing animals undergo pronounced synaptic depression in response to repetitive, high frequency stimulation, which eventually prevents EPSPs from inducing action potentials9,14. Thus, clustering activity in mini-bursts is a more efficient means of propagating activity through these developing circuits. Notably, the patterns of activity that occur in the developing auditory system are similar to exogenous stimulation protocols, such as “theta burst” that reliably induce long-term potentiation of excitatory synapses15. Repeated initiation of this patterned activity by subsets of IHCs at similar locations within the cochlea could therefore promote the formation and maintenance of tonotopically-arranged connections in auditory centers of the brain.
Supplementary Material
Acknowledgements
This work was supported by grants from FP6, European Union (EUSynapse, LSHM-CT-2005–019055) and SenterNovem, The Netherlands (Neuro-Bsik, BSIK 03053) to J.G.G.B., and the NIH (DC008860 and DC009464) to D.E.B.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
References
- 1.Blankenship AG, Feller MB. Nat Rev Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Ari Y. Trends Neurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- 3.Malenka RC, Bear MF. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Linden DJ. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- 5.Jones TA, et al. J Neurophysiol. 2007;98:1898–1908. doi: 10.1152/jn.00472.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonntag M, et al. J Neurosci. 2009;29:9510–9520. doi: 10.1523/JNEUROSCI.1377-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tritsch NX, et al. Nature. 2007;450:50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- 8.Tritsch NX, Bergles DE. J Neurosci. 2010;30:1539–1550. doi: 10.1523/JNEUROSCI.3875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Gersdorff H, Borst JGG. Nat Rev Neurosci. 2002;3:53–64. doi: 10.1038/nrn705. [DOI] [PubMed] [Google Scholar]
- 10.Guinan JJ, Jr, Li RY. Hear Res. 1990;49:321–334. doi: 10.1016/0378-5955(90)90111-2. [DOI] [PubMed] [Google Scholar]
- 11.Oertel D. Annu Rev Physiol. 1999;61:497–519. doi: 10.1146/annurev.physiol.61.1.497. [DOI] [PubMed] [Google Scholar]
- 12.Walsh EJ, Romand R. In: Development of auditory and vestibular systems 2. Romand R, editor. Amsterdam: Elsevier; 1992. pp. 161–210. [Google Scholar]
- 13.Johnson SL, Marcotti W, Kros CJ. J Physiol. 2005;563:177–191. doi: 10.1113/jphysiol.2004.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenowitz S, Trussell LO. J Neurosci. 2001;21:9487–9498. doi: 10.1523/JNEUROSCI.21-23-09487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson J, Wong D, Lynch G. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.