Abstract
Resistance to thyroid hormone (RTH), a syndrome of reduced end-organ responsiveness to thyroid hormone (TH), is mostly caused by mutations in the TH receptor (TR) β gene. Diagnosis is based on persistent elevations of serum free T4 and often T3 levels in the absence of TSH suppression, and confirmation in most cases is by way of genetic testing. The mainstay in the management of RTH patients who are asymptomatic is to recognize the correct diagnosis and avoid antithyroid treatment. Deciding whether to manage these patients with TH replacement is made even more challenging when an affected individual is pregnant. How one approaches such a patient with pregnancy and RTH would depend on the genotype of the fetus. This requires obtaining prenatal information on the genotype of the fetus and a thorough history of the outcome of previous pregnancies as well as a history of the course and outcome of other family members with RTH. If the TRβ mutation is known in the mother, the fetus can be rapidly genotyped from DNA from amniocentesis for the same mutation, and then management decisions could be made regarding thyroid or antithyroid hormone treatment.
The pregnant patient with resistance to thyroid hormone requires the consideration of prenatal diagnosis; on the basis of these findings additional modifications of RTH treatment are warranted.
Accreditation and Credit Designation Statements
The Endocrine Society is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The Endocrine Society has achieved Accreditation with Commendation.
The Endocrine Society designates this educational activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Learning Objectives
Upon completion of this educational activity, participants should be able to
Recognize the importance of prenatal diagnosis of RTH by amniocentesis.
Identify the symptoms and signs that warrant therapy in a gravida with RTH.
Understand that RTH mothers harboring an unaffected fetus should be given propylthiouracil to maintain a serum free T4 not higher than 20% above the upper limit of normal.
Target Audience
This continuing medical education activity should be of substantial interest to endocrinologists.
Disclosure Policy
Authors, editors, and Endocrine Society staff involved in planning this CME activity are required to disclose to learners any relevant financial relationship(s) that have occurred within the last 12 months with any commercial interest(s) whose products or services are discussed in the CME content. The Endocrine Society has reviewed all disclosures and resolved or managed all identified conflicts of interest, as applicable.
The following individuals reported relevant financial relationships:
Samuel Refetoff, M.D., has received an academic associate stipend from Quest Diagnostics.
Leonard Wartofsky, M.D., has received speaker honorarium from Genzyme Corp.
The following individuals reported NO relevant financial relationships:
Roy E. Weiss, M.D., and Alexandra Dumitrescu, M.D., reported no relevant financial relationships.
Endocrine Society staff associated with the development of content for this activity reported no relevant financial relationships.
Acknowledgement of Commercial Support
This activity is not supported by grants, other funds, or in-kind contributions from commercial supporters.
Privacy and Confidentiality Statement
The Endocrine Society will record learner’s personal information as provided on CME evaluations to allow for issuance and tracking of CME certificates. No individual performance data or any other personal information collected from evaluations will be shared with third parties.
Method of Participation
This Journal CME activity is available in print and online as full text HTML and as a PDF that can be viewed and/or printed using Adobe Acrobat Reader. To receive CME credit, participants should review the learning objectives and disclosure information; read the article and reflect on its content; then go to http://jcem.endojournals.org and find the article, click on CME for Readers, and follow the instructions to access and complete the post-activity test questions and evaluation. The estimated time to complete this activity, including review of material, is 1 hour. If you have questions about this CME activity, please direct them to education@endo-society.org.
Activity release date: July 2010
Activity expiration date: July 2011
Introduction
The proposita was 14 10/12 yr old when referred for management of resistance to thyroid hormone (RTH). She was the first child born (at 36 wk by spontaneous vaginal delivery) to a healthy 30-yr-old mother and 35-yr-old father of Austrian/German ethnic background. Her weight was 2.8 kg, and length was 59.7 cm. Soon after birth, the child appeared “colicky,” with frequent crying episodes. From age 6–14 months, she had monthly ear infections treated with antibiotics. At the age of 6 yr, the parents noticed increased energy that was later diagnosed as attention deficit hyperactivity disorder and treated with methylphenidate. Behavioral aberrations continued and worsened at age 11 yr, resulting in violent episodes and insomnia, necessitating psychiatric admission for 27 d. She was diagnosed with bipolar disorder. Although the possibility of thyroid disease was entertained at ages 4, 5, 10, and 11 yr, serum TSH level was the only thyroid test obtained and was always normal. Her growth was consistently in the 25th–50th percentile for height and the 50th percentile for weight. When the patient entered ninth grade, she had academic difficulty, anxiety, panic attacks, and hostility. Treatment with Risperdal and Depakote helped the behavioral abnormality. Her energy was always good. In September 2001, she was first noted to have a goiter. An ultrasound confirmed a diffusely enlarged thyroid gland. Thyroid function tests 1 wk before her visit to our clinic revealed a normal TSH of 2.5 mU/liter (normal range, 0.3–4.6); an elevated free T4 by analog method of 3.0 ng/dl (normal range, 0.7–1.5); and free T3 of 1070 pg/dl (normal range, 200–440). Magnetic resonance imaging of the pituitary was normal. Her medications at that time were Risperdal, 0.5 mg every morning and 1 mg every evening; Aderall XR, 30 mg every evening; Depakote ER, 500 mg, three tablets daily; and a multivitamin. There was no family history of thyroid disease or mental illness. Her past medical history was remarkable for a tonsillectomy and adenoidectomy at age 3 yr. Menarche occurred at age 13 yr with regular menses.
On physical examination she had a blood pressure of 134/70 mm Hg; a pulse of 109 beats per minute; a height of 158 cm (25–50th percentile), and a weight of 52.6 kg (25th percentile). Her thyroid gland was approximately two times enlarged (20 g), and she appeared hyperkinetic. The remainder of her examination was unremarkable, and she was Tanner stage 4.
The proposita was found to be heterozygous for a TRβ gene mutation (A317T). Both parents had normal thyroid function tests and had no TRβ gene mutation (Table 1). It is of note that the mutation is located in a CG dinucleotide hot spot, prone to de novo mutations (1). The patient was treated with atenolol while the Risperdal was titrated down and discontinued. Yearly thyroid function tests remained consistent with RTH.
Table 1.
Thyroid function tests
| ID | Age (yr, months) | Comment | Total T4 (μg/dl) | Total T3 (ng/dl) | rT3 (ng/dl) | Free T4I (units) | TSH (μU/ml) | TG (ng/dl) | TG/TPO antibodies |
|---|---|---|---|---|---|---|---|---|---|
| Proposita | 14, 10 | 18.0 | 268 | 66.9 | 23.0 | 3.0 | 34 | Neg/neg | |
| 16, 1 | 14.6 | 204 | 19.3 | 1.2 | |||||
| 17, 6 | 16 wk pregnant | 19.6 | 367 | 61.5 | 13.8 | 0.9 | 44 | Neg/neg | |
| 17, 8 | 20 wk pregnant | 23.7 | 314 | 19.2 | 1.5 | 54 | |||
| Father of proposita | 41 | 7.2 | 76 | 23.8 | 10.0 | 1.2 | 9 | Neg/neg | |
| Mother of proposita | 35 | 8.0 | 99 | 21.7 | 9.3 | 1.3 | 22 | 40/320 | |
| Normal range | 5–12 | 90–185 | 14.5–30 | 6–10.5 | 0.4–3.6 | 1–20 | Neg/neg |
TPO, Thyroid peroxidase; Neg, negative; Free T4I, free T4 index; rT3, reverse T3.
Background
RTH, a syndrome of reduced end-organ responsiveness to thyroid hormone (TH), was described in 1967 (2). The cardinal features of RTH are: 1) elevated serum levels of free T4 and often free T3; 2) normal or slightly increased serum TSH; and 3) absence of typical symptoms and metabolic consequences of TH excess (3,4).
Although a defect at the intracellular level was postulated in 1972 (5), a definitive proof had to await the identification in 1989 of TH receptor (TR) β gene mutations (6,7). The term RTH, thus become synonymous with defects of the TRβ (3). The recent discoveries of genetic defects that reduce the effectiveness of TH through altered cell membrane transport (8) and metabolism (9) have broadened the definition of TH insensitivity to encompass all defects that can interfere with the biological activity of a chemically intact hormone secreted in normal amounts. Use of the acronym RTH is limited to the syndrome produced by reduced intracellular action of the active TH, T3.
Expression of TH effects requires the presence of a sufficient amount of the active hormone T3 within the cell. Rapid, nongenomic action is exerted at the level of the plasma membrane and cytoplasm (10). However, the principal, best-studied and -characterized effect requires the translocation of the hormone into the nucleus where it interacts with TRs to activate or repress transcription of specific target genes.
A limited neonatal screen for high T4 values found a prevalence of 1:40,000 live births (11). Equal numbers of males and females are affected, although the prevalence of RTH without TR mutations (see Genetic testing) is more common in females (12). In the majority of cases, RTH is caused by mutations in the TRβ gene, located on chromosome 3. Mutations have been found in the carboxyl terminus of the TRβ covering the ligand-binding domain and adjacent hinge domain of the TRβ protein (1,13,14). They are contained within three clusters rich in CG “hot spots,” separated by areas devoid of mutations (cold regions). The latter are located between codons 282 and 310 and (with the exception of 383) codons 353 and 429. No mutation has been reported upstream of codon 234. Because cold regions are not devoid of “hot spots,” the lack of mutations reflects the observation that mutations in the second cold region do not impair TR function and therefore are not expected to produce a phenotype (15). The mutant TRβ molecules have either reduced affinity for T3 (13,14) or impaired interaction with one of the cofactors involved in the mediation of TH action (14,16,17,18).
Presenting complaints
RTH lacks specific clinical manifestations. When symptomatic, patients have variable symptoms with stigmata of TH deficiency and excess that often coexist (3,19). Investigation may be initiated when hypothyroidism is suspected in a child with short stature, learning disability, or mental retardation. In contrast, thyrotoxicosis may be the reason for investigation in a hyperactive youngster or an adult with tachycardia. The detection of a goiter has also been a reason for thyroid testing in children as well as adults. Formerly, the diagnosis was often missed due to failure to recognize the normal or elevated TSH, leading to treatment aimed at normalizing the elevated TH levels. In such patients, symptoms of fatigue, somnolence, depression, and weight gain associated with bradycardia have ensued. More dramatic is the growth retardation resulting from treatment of children with antithyroid drugs. This has been less common in the last decade with the wider recognition of RTH. Nevertheless, unrelated symptoms suggestive of thyrotoxicosis still lead to inappropriate ablative treatments.
Although most patients with RTH are clinically euthyroid, their presentation can range from a stare suggestive of exophthalmos to hypotonia, umbilical hernia, and cretinoid facies. Symptoms and signs and their frequency are shown in Table 2.
Table 2.
Clinical features: frequency of symptoms and signs
| Findings | Frequency (%) | |
|---|---|---|
| Thyroid gland | Goiter | 66–95 |
| Heart | Tachycardia | 33–75 |
| Nervous system | Emotional disturbances | 60 |
| Hyperkinetic behavior | 33–68 | |
| Attention deficit hyperactivity disorder | 40–60 | |
| Learning disability | 30 | |
| Mental retardation (IQ <70) | 4–16 | |
| Hearing loss (sensorineural) | 10–22 | |
| Growth and development | Short stature (<5%) | 18–25 |
| Delayed bone age >2 sd | 29–47 | |
| Low body mass index (in children) | 33 | |
| Recurrent ear and throat infections | 55 |
IQ, Intellectual quotient.
Fertility and outcome of pregnancy
An accurate evaluation of fertility and pregnancy outcome in RTH had been difficult to obtain until the discovery of a large Azorean kindred harboring the TRβ gene mutation R343Q (20,21). A 3- to 4-fold increase in the rate of miscarriages was observed in affected women compared with that in spouses of affected fathers or unaffected first-degree relatives.
Fertility was not impaired in affected couples regardless of whether women or men harbored the mutant TRβ gene. The difference in genotype frequency in the progeny of affected mothers (20 affected vs. 11 unaffected offspring), combined with a significantly higher miscarriage rate, suggests that these women tend to lose more normal than affected fetuses. This was not found in the progeny of affected fathers, whose spouses had almost equal numbers of affected and unaffected offspring (15 and 12, respectively). Because the mothers with RTH were not thyrotoxic and had no thyroid autoantibodies, it may be concluded that miscarriages were the consequence of the fetal exposure to the high levels of maternal TH. This is supported by the improved survival of the affected fetuses for whom high TH levels were appropriate, as in their affected mothers. Contrary to findings in uncontrolled maternal hyperthyroidism, women with RTH have no increased frequency of premature labor, preeclampsia, stillbirths, and perinatal loss.
Unaffected infants born to affected mothers have a significantly lower weight at birth than their affected siblings. This suggests that the high maternal TH levels were able to induce a catabolic state during fetal life, similar to what happens in children and adults with uncontrolled hyperthyroidism. That these infants were thyrotoxic is supported by their suppressed blood TSH at birth (20,21). In contrast, affected mothers with lower TH levels due to prior ablative treatment gave birth to unaffected infants of normal weight and nonsuppressed TSH.
Diagnostic Strategies
Baseline thyroid function tests
RTH should be considered in subjects with elevated TH levels and nonsuppressed TSH values. The differential diagnosis of “euthyroid hyperthyroxinemia” includes transport defects as well as T4 to T3 conversion defects. The presence of a goiter with elevation of free T4 and, in most cases, free T3 concentration measured by equilibrium dialysis with normal or elevated TSH levels is strongly suggestive of RTH. Although RTH and autoimmune thyroid disease can coexist, the presence of autoantibodies raises the suspicion that circulating substances may interfere with the measurement of either TH or, more rarely, TSH. Exclusion of such antibodies by direct testing or confirmation using different assays is advisable. rT3 concentrations are also high, and the levels of thyroglobulin (TG) reflect the degree of serum TSH elevation. Thyroidal radioiodide uptake is increased, and ultrasound of the thyroid gland demonstrates the presence of gland enlargement, diffuse or multinodular. The finding of goiter in the presence of normal serum TSH levels is explained by the increase of TSH bioactivity in RTH (22).
Distinction between RTH and a TSH-secreting pituitary adenoma can be challenging. No single test is conclusive, and diagnosis of RTH must rest on a combination of test and observations: 1) the absence of an elevated serum concentration of the α pituitary glycoprotein subunit; 2) stimulation of TSH after the administration of TRH; 3) the presence of thyroid test abnormalities compatible with RTH in other family members; 4) absence of elevated serum SHBG concentration, reflecting a euthyroid state; and 5) ability to suppress serum TSH with supraphysiological doses of l-T3.
Genetic testing
The ability to identify mutations in the TRβ gene provides a means to confirm the diagnosis and to obtain prenatal diagnosis. In addition to providing a definitive diagnosis, the demonstration of a TRβ gene mutation precludes the necessity for more complex and expensive testing. Gene sequencing is provided by several commercial laboratories at a reasonable cost. The reader may refer to GeneTests (http://www.genetests.org) for an up-to-date list of available facilities.
It is of note that 15% of subjects with RTH have no detectable mutations in the TRβ gene. RTH in the absence of a TRβ gene mutation is clinically and biochemically indistinguishable from RTH with TRβ gene mutations (12). Therefore, the absence of a mutation does not necessarily indicate a diagnosis other than RTH, and a solid endocrine diagnosis should be made by the in vivo demonstration of reduced sensitivity to the acute administration of TH.
l-T3 Suppression test
The measurement of responses to the administration of incremental doses of TH is the best means to assess the presence and magnitude of the hormonal resistance and obtain a clinical diagnosis of RTH when a TRβ gene mutation is not detected. The rationale for the use of l-T3 rather than l-T4 is its direct effect on tissues, independent of variations in T4 metabolism. The rapid onset of l-T3 action reduces the period of hormone administration, and the shorter half-life of this hormone decreases the duration of symptoms that may arise in hormonally responsive subjects. The test described in detail previously (3) involves the administration of three incremental doses of l-T3, each for the duration of 3 d. With each incremental dose, measurements reflecting responses of central and peripheral tissues to TH are obtained and compared with those observed in normal individuals.
Management Strategies
There is no available treatment to correct the specific defect in RTH. Current treatments are aimed to alleviate symptoms when present. Most important is not to treat asymptomatic, fully compensated individuals with the sole purpose of correcting the laboratory test abnormalities. Prior ablative treatment, resulting from misdiagnosis, requires the administration of TH often in supraphysiological doses.
When not to treat
There is no reason to treat subjects with elevated levels of TH appropriate for the degree of both thyrotroph and peripheral tissue resistance. Although the theoretical probability of developing thyrotroph adenomas due to long-standing increase in thyrotroph activity has been suggested, only one case of a pituitary adenoma in a subject with RTH has been reported (23). In mouse models of RTH, pituitary pathology consists of thyrotroph hyperplasia only, particularly in homozygotes, which rarely occurs in humans. Using the same logic, the thyroid gland, which is under increased stimulation by TSH, may also be prone to tumor development, but there is no increased incidence of thyroid cancer in RTH and goiters are rarely obstructive. A mouse model homozygous for a mutation in the TRβ gene did develop papillary thyroid cancer (24), but the relevance to humans is unknown. Therefore, the mainstay in the management of RTH patients who are asymptomatic is to recognize the correct diagnosis and avoid antithyroid treatment.
When to treat real and apparent TH deficiency
Intervention is recommended in patients who present with objective findings of TH deprivation usually because of treatment aimed to decrease the circulating TH level. If the consequence is reversible, such as with antithyroid drugs, treatment should be discontinued. In the case of prior ablative treatment (surgery or radioiodide), judicious administration of supraphysiological doses of TH is usually required. The dose of TH needs to be titrated in an incremental manner to normalize the serum TSH concentration. Doses of l-T4 as high as 500 to 1000 μg/d may be necessary to obtain the desired TH effect. Tachycardia should not be a contraindication for T4 treatment because it can be managed with atenolol, as noted in When to treat apparent TH excess. Most difficult is the treatment of children with apparent hypothyroidism manifesting as growth retardation with delayed bone age and failure to thrive. A guide for TH dosage in such children is growth, bone maturation, and mental development. In addition, it is suggested that basal metabolic rate, nitrogen balance, and serum SHBG be monitored at each dose increment.
When to treat apparent TH excess
The most common symptom suggestive of hyperthyroidism is sinus tachycardia, present in about half of patients with RTH. When symptomatic, or limiting exercise tolerance, treatment with a β-adrenergic blocking agent is very effective. Some β-blockers, such as Inderal, have an added effect of inhibiting conversion of T4 to T3, which is not desirable in RTH. We prefer to use atenolol, which does not have this added effect of depriving the TH-resistant cells of TH.
More generalized symptoms of hyperthyroidism, including tremor, heat intolerance, sweating, and agitation, may also benefit from treatment with atenolol. However, this may not be effective in the extreme cases. Two other approaches have been used, but experience is limited. These are reduction of TSH secretion and blocking the action of TH.
Agents reducing TSH include glucocorticoids, somatostatin, and dopaminergic drugs. Although effective at reducing the TSH concentration, glucocorticoids have unacceptable side effects and therefore are not clinically useful. Dopaminergic agents such as bromocriptine or pergolide can reduce the TSH concentration but lose their effectiveness when used for a prolonged period of time. In addition, patients may not tolerate the gastrointestinal side effects (25,26,27,28). The somatostatin analog, SMS201-995, was studied in three patients with RTH and found to have a weaker and a more transient effect when compared with that in patients with TSH-secreting pituitary adenomas (29).
Treatment of goiter
Although thyroid gland enlargement is usually modest, large goiters occasionally occur. Surgical treatment is not effective in the long term because goiters are notorious for their recurrence because contrary to autoimmune thyroid disease, there is no underlying destructive process. Thus, it is more effective to inhibit thyroid gland growth by suppression of TSH. The latter has been achieved by treating with a single large dose of l-T3, given every other day (30). The high levels of serum T3 occurring a few hours after ingestion of the hormone are effective in suppressing TSH but do not persist due to the short half-life of T3. Thus, symptoms of TH excess do not develop (30).
Treatments that reduce TH action using analogs
Triiodothyroacetic acid (TRIAC) is a TH analog with low hormonal potency but high affinity for the TR (31) and very rapid turnover, requiring the use of doses more that 1,000-fold those of l-T3. Long-term studies on the effect of TRIAC have been reported in several subjects with RTH (25,27,29,32,33,34,35,36,37,38,39). Although there was a significant reduction in the basal and TRH-stimulated TSH observed in the majority of cases, there was no appreciable change in parameters that measure TH action. Most investigators that have used TRIAC report receiving the drug from Laboratories ANA (Neuilly sur-Seine, France). This drug is not available in the United States.
Dextrothyroxine (d-T4) had been thought to be useful in reducing plasma cholesterol without producing adverse thyromimetic effects (40) in some subjects, but not in others (41,42). Investigators have tried to treat RTH with d-T4 in an effort to decrease TSH (43,44,45). Several patients with RTH, of various severities, have received 2 to 8 mg of d-T4 daily (44,45,46,47). Clinical changes have been minimal and often were not supported by objective findings. Given that most preparations contain small amounts of l-T4, 2 to 3% of the levo stereoisomer could fully account for the thyromimetic effect (43). In most cases, d-T4 (Dynothel, 2 mg tablet) was obtained from Henning (Berlin, Germany).
Treatment of children and infants
Infants may be found to have RTH by early testing because of a known affected sibling or parent or, more rarely, because routine neonatal testing revealed an elevated T4 and a nonsuppressed TSH. Treatment of these infants is controversial, especially when asymptomatic, because there have been no long-term outcome studies. In general, we tend to treat infants and children with l-T4 only if any of the following are present: 1) marked elevation of TSH; 2) history of adverse symptoms in other affected family members such as mental retardation; 3) evidence of failure to thrive; 4) growth retardation; or 5) developmental delays.
Strategy for Management of Pregnancy
Given the outcome of pregnancy in mothers with RTH carrying unaffected fetuses, it is obviously important to ascertain the fetal genotype when the mother harbors a TRβ gene mutation. Such information may lead to a rationale therapy of the mother to accommodate the thyroid needs of the fetus. Although there are no guidelines for the treatment of fetuses, based on the study described above (20), it seems reasonable to reduce the TH level of a mother with RTH that carries a normal fetus to prevent suppression of fetal TSH and low birth weight. Unfortunately, miscarriages occur early in pregnancy when prenatal diagnosis is not possible. Although subjects with RTH born to normal mothers, compared with RTH mothers, showed childhood short stature (48), it is unclear whether treatment with TH during pregnancy would be beneficial in such circumstances.
The first report of treatment in a pregnant RTH (TRβ T337A) patient was a person with symptoms of severe hyperthyroidism, who was managed symptomatically with TRIAC before pregnancy (49). TRIAC was discontinued until the genotype of the fetus could be confirmed. Fetal DNA obtained by chorionic villus sampling confirmed that the fetus carried the mutant maternal TRβ gene mutation. TRIAC was restarted at 20 wk gestation. Blood samples for the determination of fetal TH levels were obtained by cordocentesis. Fetal goiter was noted by ultrasound, and the dose of TRIAC was increased. This resulted in a 50% reduction of TSH levels and a reduction in fetal goiter. However, acute complications occurred from umbilical blood sampling, prompting delivery by cesarean section. The female neonate was critically ill, with multiple organ failure and respiratory distress syndrome. In addition, a small goiter and biochemical features of hypothyroidism were noted transiently and were felt to be related to the prematurity of the infant.
The second study reports three mothers with RTH (R383H, R438C, and I431M) who had presumably unaffected fetuses because thyroid tests were normal at birth. However, fetal genotyping was not reported antenatal or postpartum (50). Asymptomatic at the time of diagnosis before pregnancy, two mothers did well throughout the pregnancy, whereas one mother (R383H) complained of palpitations, anxiety, and excessive sweating. She was treated with propylthiouracil (PTU) during the pregnancy. Her baby, a male weighing 2.14 kg, was born at 34 wk.
Prenatal diagnosis
The first step in developing a plan for a pregnant woman with RTH is to determine the genotype of the fetus. Although prenatal diagnosis of RTH based on genetic testing of amniotic fluid has not been reported, the authors have successfully identified the genotype of fetuses carried by women with RTH and known to have TRβ gene mutations. Once the mutation in the TRβ gene has been identified in the mother, amniotic fluid is obtained under standard conditions. DNA is extracted and subjected to PCR amplification of the region harboring the mutation. Direct sequencing of the TRβ gene region harboring the mutation and/or restriction enzyme digestion of the PCR fragment to confirm the presence or absence of the mutation is performed. To rule out contamination by maternal DNA in the amniotic fluid, relative abundance of the mutant allele is assessed by dilution of template.
Back to the Case
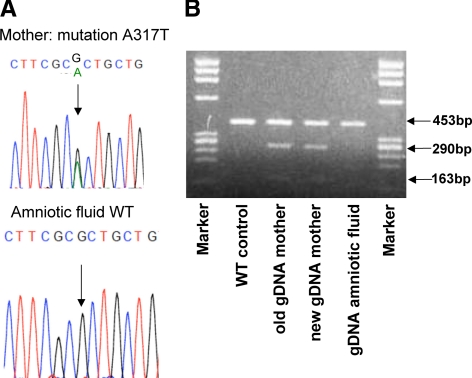
The proposita did well, and when she was 18 yr old, we were notified by her obstetrician that she was 3 months pregnant. Amniotic fluid was obtained at the request of the proposita, demonstrating that the fetus did not carry the mutant maternal TRβ gene (A317T) (Fig. 1). The options to continue monitoring the pregnancy without intervention or decrease the proposita’s TH levels were presented to the mother, her parents, and her treating physicians. It was decided to decrease her serum free T4 to a level 25% above the upper limit of normal. PTU was administered at 150 mg three times a day. Unfortunately, the patient did not return for hormone testing until delivery (Tables 1 and 3).
Figure 1.
Prenatal screening for the presence of TRβ gene mutation in a patient with RTH. A, Results of sequencing exon 9 of the TRβ gene on genomic DNA (gDNA) isolated from amniotic fluid and maternal blood. gDNA from maternal blood confirmed that she was heterozygous for the TRβ gene mutation, A317T. gDNA from the amniotic fluid was homozygous for the wild-type (WT) A317. B, Presence of the mutation in the mother but not the fetus (amniotic DNA) was confirmed by restriction enzyme digestion with MsI.
Table 3.
Thyroid function tests at birth and infancy
| Age (d) | Comment | Total T4 (μg/dl) | Total T3 (ng/dl) | rT3 (ng/dl) | Free T4I (units) | Free T4 (ng/dl) | TSH (μU/ml) | TG (ng/dl) |
|---|---|---|---|---|---|---|---|---|
| 0 | Cord blood | 10.7 | 73 | 47.6 | 8.9 | 7.5 | 89 | |
| 0 | Venous | 10.5 | 169 | 23.7 | 10.5 | 33.0 | >100 | |
| 4 | 1.53 | 30.8 | ||||||
| 13 | 25 μg T4 | 258 | 2.33 | 3.7 | ||||
| 24 | 12.5 μg T4 | 218 | 1.77 | 2.7 | ||||
| 56 | No Rx | 240 | 1.55 | 4.2 | ||||
| 86 | No Rx | 176 | 1.21 | 2.1 | ||||
| Normal range | ||||||||
| 0–2 d | 6–13 | 50–80 | 30–60 | 7–12 | 0.7–1.9 | <30 | up to 200 | |
| 3–30 d | 1–5 | up to 60 | ||||||
| >30 d | 5–12 | 90–215 | 16–36 | 6–10.5 | 0.5–4.0 | 1–40 |
Free T4I, Free T4 index; Rx, treatment; rT3, reverse T3.
A female baby was born to the proposita after a full-term, vacuum-assisted normal vaginal delivery. Birth weight was 3.1 kg, and cord blood TH and TSH concentrations were within the expected range of normal. The neonate did well without jaundice until d 3 of life when she was noted to be hypothermic (96 F). Sepsis workup was negative. She was started on 25 μg of l-T4 because of a TSH of 30.8 mU/liter on the fourth day, although free T4 was 1.53 ng/dl, well within the normal range, and there was no goiter (Table 3). Anterior fontanelle was 7.5 cm, and the posterior fontanelle was not palpable. Neurological exam was normal. At the age of 2 months, and after withdrawal of l-T4 treatment, TSH was 4.2 μ/liter and free T4 1.55 ng/dl. The impression was mild transient hyperthyrotropinemia, possibly due to transplacental transfer of PTU. The suppressed TSH and low birth weight seen in unaffected infants born to mothers with RTH did not occur.
Controversies and Areas of Uncertainty
In the absence of prospective data on a large number of pregnant subjects, hard and fast approaches cannot be recommended. The concept to treat maternal hyperthyrotropinemia to avoid maternal thyrotroph hyperplasia and to reduce fetal goiter remains unproven in pregnant RTH mothers. Although we still believe that aggressive management of pregnant RTH mothers is not warranted (51), data from pregnancy and outcome from the Azorean population with RTH (20) and data in a mouse model of RTH (52) raised concern for the health of the fetus. We believe that investigation into the efficacy of treatment of affected gravid subjects with RTH is in order. Given the low frequency of this condition, it would be helpful for the scientific community to cooperate in the sharing of information regarding pregnancy and outcomes.
In the following paragraphs, three scenarios are being considered as indications for special testing and consideration for intervention. This requires obtaining prenatal information on the genotype of the fetus and a thorough history of the outcome of previous pregnancies as well as a history of the course and outcome of other family members with RTH.
Mothers carrying an unaffected fetus
Limited, although solid, data indicate that the high levels of TH in pregnant mothers with RTH adversely affect normal fetuses, suppressing their TSH and producing infants with low weight for gestational age (20). In such mothers, the free T4 should be maintained not higher than 20% of the upper limit of normal. This can be achieved by the judicious use of PTU. Careful follow-up is mandatory to avoid overtreatment and induction of fetal hypothyroidism. An elevation in maternal TSH is expected but is, probably, of no consequence to the fetus. Current data indicate that affected fetuses carried by affected mothers have normal birth weights and TSH concentration. In such circumstances, no therapeutic intervention is recommended.
Normal mothers carrying an affected fetus
The detrimental effect of maternal hypothyroidism on the fetus is well established (detailed in Chapter 14 of the online “Thyroid disease manager” by DeGroot L, “Thyroid regulation and dysfunction in the pregnant patient,” http://www.thyroidmanager.org/Chapter14/14-framehtm). It is, therefore, surprising that affected fetuses carried by normal mothers do not show the effects of hormone deprivation during gestation. No increased rate of miscarriage, no birth complications, or elevated serum TSH at birth have been observed (20). Therefore, there is no basis for treatment unless fetal goiter or distress occurs. In such instances, intraamniotic instillation of l-T4 is in order. Alternatively, treatment of the mother with TRIAC (in countries where this compound is available) may be appropriate.
Family history of abnormal pregnancies or fetal outcome among subjects with RTH
Although, with exception to the problems mentioned above, the great majority of pregnancies in subjects with RTH have no major consequences, in some families RTH has been associated with serious maternal and fetal problems. In addition to frequent miscarriages, these include preeclampsia, hydramnios, fetal goiter, and fetal distress. Newborns can have large goiters and very abnormal thyroid function tests with TSH values well above 400 mU/liter. Frame shift mutations in the amino terminus of the TRβ and homozygotes for missense mutations in the TRβ gene defects are particularly prone to such problems (53,54). The obstetrician and pediatrician should be prepared to provide the necessary supportive care. Sometimes treatments to increase the TH level or decrease TSH are necessary.
Supplementary Material
Footnotes
This work was supported in part by grants from the National Institutes of Health (DK17050, DK07011, DK20595, and RR04999). Sections describing mechanisms and testing have appeared in various publications by the authors.
Disclosure Summary: R.E.W. and A.D. have nothing to declare. S.R. is an Academic Associate for Quest Diagnostics.
Abbreviations: d-T4, Dextrothyroxine; PTU, propylthiouracil; RTH, resistance to TH; TG, thyroglobulin; TH, thyroid hormone; TR, TH receptor; TRIAC, triiodothyroacetic acid.
References
- Weiss RE, Weinberg M, Refetoff S 1993 Identical mutations in unrelated families with generalized resistance to thyroid hormone occur in cytosine-guanine-rich areas of the thyroid hormone receptor β gene. Analysis of 15 families. J Clin Invest 91:2408–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refetoff S, DeWind LT, DeGroot LJ 1967 Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab 27:279–294 [DOI] [PubMed] [Google Scholar]
- Refetoff S, Weiss RE, Usala SJ 1993 The syndromes of resistance to thyroid hormone. Endocr Rev 14:348–399 [DOI] [PubMed] [Google Scholar]
- Refetoff S, Weiss RE, Usala SJ, Hayashi Y 1994 The syndromes of resistance to thyroid hormone: update 1994. In: Braverman LE, Refetoff S, eds. Endocrine Reviews Monographs. Bethesda, MD: The Endocrine Society; 336–343 [Google Scholar]
- Refetoff S, DeGroot LJ, Benard B, DeWind LT 1972 Studies of a sibship with apparent hereditary resistance to the intracellular action of thyroid hormone. Metabolism 21:723–756 [DOI] [PubMed] [Google Scholar]
- Sakurai A, Takeda K, Ain K, Ceccarelli P, Nakai A, Seino S, Bell GI, Refetoff S, DeGroot LJ 1989 Generalized resistance to thyroid hormone associated with a mutation in the ligand-binding domain of the human thyroid hormone receptor β. Proc Natl Acad Sci USA 86:8977–8981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usala SJ, Tennyson GE, Bale AE, Lash RW, Gesundheit N, Wondisford FE, Accili D, Hauser P, Weintraub BD 1990 A base mutation of the C-erbA β thyroid hormone receptor in a kindred with generalized thyroid hormone resistance. Molecular heterogeneity in two other kindreds. J Clin Invest 85:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S 2004 A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Abdullah MS, Lado-Abeal J, Majed FA, Moeller LC, Boran G, Schomburg L, Weiss RE, Refetoff S 2005 Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet 37:1247–1252 [DOI] [PubMed] [Google Scholar]
- Bassett JH, Harvey CB, Williams GR 2003 Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Mol Cell Endocrinol 213:1–11 [DOI] [PubMed] [Google Scholar]
- Lafranchi SH, Snyder DB, Sesser DE, Skeels MR, Singh N, Brent GA, Nelson JC 2003 Follow-up of newborns with elevated screening T4 concentrations. J Pediatr 143:296–301 [DOI] [PubMed] [Google Scholar]
- Sadow PM, Reutrakul S, Weiss RE, Refetoff S 2000 Resistance to thyroid hormone in the absence of mutations in the thyroid hormone receptor genes. Curr Opin Endocrinol Diabetes 7:253–259 [Google Scholar]
- Adams M, Matthews C, Collingwood TN, Tone Y, Beck-Peccoz P, Chatterjee KK 1994 Genetic analysis of 29 kindreds with generalized and pituitary resistance to thyroid hormone: identification of thirteen novel mutations in the thyroid hormone receptor β gene. J Clin Invest 94:506–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingwood TN, Wagner R, Matthews CH, Clifton-Bligh RJ, Gurnell M, Rajanayagam O, Agostini M, Fletterick RJ, Beck-Peccoz P, Reinhardt W, Binder G, Ranke MB, Hermus A, Hesch RD, Lazarus J, Newrick P, Parfitt V, Raggatt P, de Zegher F, Chatterjee VK 1998 A role for helix 3 of the TRβ ligand-binding domain in coactivator recruitment identified by characterization of a third cluster of mutations in resistance to thyroid hormone. EMBO J 17:4760–4770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Sunthornthepvarakul T, Refetoff S 1994 Mutations of CpG dinucleotides located in the triiodothyronine (T3)-binding domain of the thyroid hormone receptor (TR) β gene that appears to be devoid of natural mutations may not be detected because they are unlikely to produce the clinical phenotype of resistance to thyroid hormone. J Clin Invest 94:607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Takeshita A, Misiti S, Chin WW, Yen PM 1998 Lack of coactivator interaction can be a mechanism for dominant negative activity by mutant thyroid hormone receptors. Endocrinology 139:4197–4204 [DOI] [PubMed] [Google Scholar]
- Safer JD, Cohen RN, Hollenberg AN, Wondisford FE 1998 Defective release of corepressor by hinge mutants of the thyroid hormone receptor found in patients with resistance to thyroid hormone. J Biol Chem 273:30175–30182 [DOI] [PubMed] [Google Scholar]
- Yoh SM, Chatterjee VK, Privalsky ML 1997 Thyroid hormone resistance syndrome manifests as an aberrant interaction between mutant T3 receptor and transcriptional corepressor. Mol Endocrinol 11:470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck-Peccoz P, Chatterjee VK 1994 The variable clinical phenotype in thyroid hormone resistance syndrome. Thyroid 4:225–232 [DOI] [PubMed] [Google Scholar]
- Anselmo J, Cao D, Karrison T, Weiss RE, Refetoff S 2004 Fetal loss associated with excess thyroid hormone exposure. JAMA 292:691–695 [DOI] [PubMed] [Google Scholar]
- Anselmo J, César R 1998 Resistance to thyroid hormone: report of 2 kindreds with 35 patients. Endocr Pract 4:368–374 [DOI] [PubMed] [Google Scholar]
- Persani L, Asteria C, Tonacchera M, Vitti P, Krishna V, Chatterjee K, Beck-Peccoz P 1994 Evidence for the secretion of thyrotropin with enhanced bioactivity in syndromes of thyroid hormone resistance. J Clin Endocrinol Metab 78:1034–1039 [DOI] [PubMed] [Google Scholar]
- Safer JD, Colan SD, Fraser LM, Wondisford FE 2001 A pituitary tumor in a patient with thyroid hormone resistance: a diagnostic dilemma. Thyroid 11:281–291 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Willingham MC, Cheng SY 2002 Mice with a mutation in the thyroid hormone receptor β gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid 12:963–969 [DOI] [PubMed] [Google Scholar]
- Beck-Peccoz P, Piscitelli G, Cattaneo MG, Faglia G 1983 Successful treatment of hyperthyroidism due to nonneoplastic pituitary TSH hypersecretion with 3,5,3′-triiodothyroacetic acid (TRIAC). J Endocrinol Invest 6:217–223 [DOI] [PubMed] [Google Scholar]
- Dorey F, Strauch G, Gayno JP 1990 Thyrotoxicosis due to pituitary resistance to thyroid hormones. Successful control with D thyroxine: a study in three patients. Clin Endocrinol (Oxf) 32:221–228 [DOI] [PubMed] [Google Scholar]
- Dulgeroff AJ, Geffner ME, Koyal SN, Wong M, Hershman JM 1992 Bromocriptine and Triac therapy for hyperthyroidism due to pituitary resistance to thyroid hormone. J Clin Endocrinol Metab 75:1071–1075 [DOI] [PubMed] [Google Scholar]
- Sasaki J, Tada T, Saito K, Kurihara H 1989 [A case report of Refetoff’s syndrome]. Nippon Naibunpi Gakkai Zasshi 65:1286–1293 [DOI] [PubMed] [Google Scholar]
- Beck-Peccoz P, Mariotti S, Guillausseau PJ, Medri G, Piscitelli G, Bertoli A, Barbarino A, Rondena M, Chanson P, Pinchera A, Faglia G 1989 Treatment of hyperthyroidism due to inappropriate secretion of thyrotropin with the somatostatin analog SMS 201–995. J Clin Endocrinol Metab 68:208–214 [DOI] [PubMed] [Google Scholar]
- Anselmo J, Refetoff S 2004 Regression of a large goiter in a patient with resistance to thyroid hormone by every other day treatment with triiodothyronine. Thyroid 14:71–74 [DOI] [PubMed] [Google Scholar]
- Koerner D, Surks MI, Oppenheimer JH 1974 In vitro demonstration of specific triiodothyronine binding sites in rat liver nuclei. J Clin Endocrinol Metab 38:706–709 [DOI] [PubMed] [Google Scholar]
- Salmela PI, Wide L, Juustila H, Ruokonen A 1988 Effects of thyroid hormones (T4, T3), bromocriptine and Triac on inappropriate TSH hypersecretion. Clin Endocrinol (Oxf) 28:497–507 [DOI] [PubMed] [Google Scholar]
- Lind P, Eber O 1986 [Treatment of inappropriate TSH secretion with Triac]. Acta Med Austriaca 13:13–16 [PubMed] [Google Scholar]
- Medeiros-Neto G, Kallas WG, Knobel M, Cavaliere H, Mattar E 1980 Triac (3,5,3′-triiodothyroacetic acid) partially inhibits the thyrotropin response to synthetic thyrotropin-releasing hormone in normal and thyroidectomized hypothyroid patients. J Clin Endocrinol Metab 50:223–225 [DOI] [PubMed] [Google Scholar]
- Darendeliler F, Ba° F 1997 Successful therapy with 3,5,3′-triiodothyroacetic acid (TRIAC) in pituitary resistance to thyroid hormone. J Pediatr Endocrinol Metab 10:535–538 [DOI] [PubMed] [Google Scholar]
- Kunitake JM, Hartman N, Henson LC, Lieberman J, Williams DE, Wong M, Hershman JM 1989 3,5,3′-triiodothyroacetic acid therapy for thyroid hormone resistance. J Clin Endocrinol Metab 69:461–466 [DOI] [PubMed] [Google Scholar]
- Radetti G, Persani L, Molinaro G, Mannavola D, Cortelazzi D, Chatterjee VK, Beck-Peccoz P 1997 Clinical and hormonal outcome after two years of triiodothyroacetic acid treatment in a child with thyroid hormone resistance. Thyroid 7:775–778 [DOI] [PubMed] [Google Scholar]
- Rivolta CM, Mallea Gil MS, Ballarino C, Ridruejo MC, Miguel CM, Gimenez SB, Bernacchi SS, Targovnik HM 2004 A novel 1297–1304delGCCTGCCA mutation in the exon 10 of the thyroid hormone receptor β gene causes resistance to thyroid hormone. Mol Diagn 8:163–169 [PubMed] [Google Scholar]
- Torre P, Bertoli M, Di Giovanni S, Scommegna S, Conte C, Novelli G, Cianfarani S 2005 Endocrine and neuropsychological assessment in a child with a novel mutation of thyroid hormone receptor: response to 12-month triiodothyroacetic acid (TRIAC) therapy. J Endocrinol Invest 28:657–662 [DOI] [PubMed] [Google Scholar]
- Schneeberg NG, Herman E, Menduke H, Altschuler NK 1962 Reduction of serum cholesterol by sodium dextrothyroxine in euthyroid subjects. Ann Intern Med 56:265–275 [DOI] [PubMed] [Google Scholar]
- Bantle JP, Hunninghake DB, Frantz ID, Kuba K, Mariash CN, Oppenheimer JH 1984 Comparison of effectiveness of thyrotropin-suppressive doses of D- and L-thyroxine in treatment of hypercholesterolemia. Am J Med 77:475–481 [DOI] [PubMed] [Google Scholar]
- Gorman CA, Jiang NS, Ellefson RD, Elveback LR 1979 Comparative effectiveness of dextrothyroxine and levothyroxine in correcting hypothyroidism and lowering blood lipid levels in hypothyroid patients. J Clin Endocrinol Metab 49:1–7 [DOI] [PubMed] [Google Scholar]
- Hamon P, Bovier-Lapierre M, Robert M, Peynaud D, Pugeat M, Orgiazzi J 1988 Hyperthyroidism due to selective pituitary resistance to thyroid hormones in a 15-month-old boy: efficacy of D-thyroxine therapy. J Clin Endocrinol Metab 67:1089–1093 [DOI] [PubMed] [Google Scholar]
- Pohlenz J, Knöbl D 1996 Treatment of pituitary resistance to thyroid hormone (PRTH) in an 8-year-old boy. Acta Pædiatr 85:387–390 [DOI] [PubMed] [Google Scholar]
- Schwartz ID, Bercu BB 1992 Dextrothyroxine in the treatment of generalized thyroid hormone resistance in a boy homozygous for a defect in the T3 receptor. Thyroid 2:15–19 [DOI] [PubMed] [Google Scholar]
- Sarkissian G, Dace A, Mesmacque A, Bony-Trifunovic H, Malezet-Desmoulins C, Torresani J, Margotat A 1999 A novel resistance to thyroid hormone associated with a new mutation (T329N) in the thyroid hormone receptor β gene. Thyroid 9:165–171 [DOI] [PubMed] [Google Scholar]
- Usala SJ, Menke JB, Watson TL, Wondisford FE, Weintraub BD, Bérard J, Bradley WE, Ono S, Mueller OT, Bercu BB 1991 A homozygous deletion in the c-erbAβ thyroid hormone receptor gene in a patient with generalized thyroid hormone resistance: isolation and characterization of the mutant receptor. Mol Endocrinol 5:327–335 [DOI] [PubMed] [Google Scholar]
- Brucker-Davis F, Skarulis MC, Grace MB, Benichou J, Hauser P, Wiggs E, Weintraub BD 1995 Genetic and clinical features of 42 kindreds with resistance to thyroid hormone. The National Institutes of Health Prospective Study. Ann Intern Med 123:572–583 [DOI] [PubMed] [Google Scholar]
- Asteria C, Rajanayagam O, Collingwood TN, Persani L, Romoli R, Mannavola D, Zamperini P, Buzi F, Ciralli F, Chatterjee VK, Beck-Peccoz P 1999 Prenatal diagnosis of thyroid hormone resistance. J Clin Endocrinol Metab 84:405–410 [DOI] [PubMed] [Google Scholar]
- Dhingra S, Owen PJ, Lazarus JH, Amin P 2008 Resistance to thyroid hormone in pregnancy. Obstet Gynecol 112:501–503 [DOI] [PubMed] [Google Scholar]
- Weiss RE, Refetoff S 1999 Treatment of resistance to thyroid hormone—primum non nocere. J Clin Endocrinol Metab 84:401–404 [DOI] [PubMed] [Google Scholar]
- Alonso M, Goodwin C, Liao X, Page D, Refetoff S, Weiss RE 2007 Effects of maternal levels of thyroid hormone (TH) on the hypothalamus-pituitary-thyroid set point: studies in TH receptor β knockout mice. Endocrinology 148:5305–5312 [DOI] [PubMed] [Google Scholar]
- Ono S, Schwartz ID, Mueller OT, Root AW, Usala SJ, Bercu BB 1991 Homozygosity for a dominant negative thyroid hormone receptor gene responsible for generalized resistance to thyroid hormone. J Clin Endocrinol Metab 73:990–994 [DOI] [PubMed] [Google Scholar]
- Wu SY, Cohen RN, Simsek E, Senses DA, Yar NE, Grasberger H, Noel J, Refetoff S, Weiss RE 2006 A novel thyroid hormone receptor-β mutation that fails to bind nuclear receptor corepressor in a patient as an apparent cause of severe, predominantly pituitary resistance to thyroid hormone. J Clin Endocrinol Metab 91:1887–1895 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.