Abstract
Background: Serum 25-hydroxyvitamin D (25OHD) is a key factor in determining monocyte induction of the antimicrobial protein cathelicidin, which requires intracrine conversion of 25OHD to 1,25-dihydroxyvitamin D [1,25(OH)2D]. Both vitamin D metabolites circulate bound to vitamin D-binding protein (DBP), but the effect of this on induction of monocyte cathelicidin remains unclear.
Methods: Human monocytes were cultured in medium containing 1) serum from DBP knockout (DBP−/−) or DBP+/− mice, 2) serum-free defined supplement reconstituted with DBP or albumin (control), and 3) human serum with different DBP [group-specific component [Gc]] genotypes with varying affinities for vitamin D metabolites. In each case, response to added 1,25(OH)2D3 or 25OHD3 was determined by measuring expression of mRNA for cathelicidin and 24-hydroxylase. Monocyte internalization of DBP was assessed by fluorescent tagging followed by microscopic and flow cytometric analysis of tagged DBP.
Results: Monocytes cultured in DBP−/− serum showed more potent induction of cathelicidin by 25OHD3 or 1,25(OH)2D3 when compared with DBP+/− serum. Likewise, DBP added to serum-free medium attenuated 25OHD3/1,25(OH)2D3 responses. Fluorescently tagged DBP showed low-level uptake by monocytes, but this did not appear to involve a megalin-mediated mechanism. Human serum containing low-affinity Gc2-1S or Gc2-2, respectively, supported 2.75-fold (P = 0.003) and 2.43-fold (P = 0.016) higher induction of cathelicidin by 25OHD relative to cells cultured with high affinity Gc1F-1F.
Conclusion: These data indicate that DBP plays a pivotal role in regulating the bioavailablity of 25OHD to monocytes. Vitamin D-dependent antimicrobial responses are therefore likely to be strongly influenced by DBP polymorphisms.
Innate immune responses to vitamin D are dependent on vitamin D binding protein genotype.
Recent studies have highlighted a role for vitamin D as a potent modulator of human immune responses (1). In monocytes, Toll-like receptor (TLR)-mediated up-regulation of CYP27b1 catalyzes the activation of precursor 25-hydroxyvitamin D (25OHD) to hormonal 25-dihydroxyvitamin D [1,25(OH)2D]. Coincidental TLR induction of the nuclear receptor for 1,25(OH)2D [vitamin D receptor (VDR)] then enables the induction of key vitamin D target genes, notably the antimicrobial protein cathelicidin (2,3,4,5). At a molecular level, this mechanism ultimately depends on the interaction between the liganded VDR and a specific vitamin D response element within the proximal promoter of the cathelicidin gene (6,7,8), but, in vivo, other factors are likely to be involved. In particular, we have shown that induction of monocyte cathelicidin is highly dependent on the initial component of the intracrine vitamin D pathway, namely the availability of substrate for CYP27b1, 25OHD (2,3). Serum levels of 25OHD vary considerably within humans and provide the best circulating marker for vitamin D status (9). As such, serum 25OHD is likely to be a key determinant of vitamin D-mediated immune activity, particularly for individuals who are vitamin D (25OHD) insufficient or deficient (10,11).
A key factor in regulating the availability of 25OHD to cells is the serum vitamin D-binding protein (DBP), also known as group-specific component (Gc). DBP is an abundant multifunctional protein structurally related to albumin and α-fetoprotein (12,13). Almost all vitamin D metabolites circulate bound to either DBP (high affinity for vitamin D ligands) or serum albumin (high abundance but low affinity for vitamin D ligands). In the proximal tubule of the kidney (14) and in mammary cells (15), delivery of substrate 25OHD to CYP27b1 occurs through megalin/cubilin-mediated endocytosis of this metabolite bound to DBP (16). However, as yet, it is unclear whether such a mechanism is crucial for synthesis of 1,25(OH)2D in other cells expressing CYP27b1. We have therefore characterized the effects of DBP on vitamin D-induced cathelicidin in monocytes and the extent to which this may be influenced by DBP gene variants.
Materials and Methods
Cell culture
Ficoll-isolated peripheral blood mononuclear cells (PBMCs) derived from anonymous healthy donors (screened in accordance with standard transfusion medicine protocols) were obtained from the Center for AIDS Research Virology Core/BSL3 Facility (supported by National Institutes of Health award AI-28697 and by the UCLA AIDS Institute and the UCLA Council of Bioscience Resources). Briefly, monocytes were enriched by adherence by incubating 5 × 106 PBMCs per well in 12-well plates for 2 h in RPMI (Invitrogen, Carlsbad, CA) with 1% fetal bovine serum (FBS) for DBP knockout mouse serum studies or macrophage serum-free (SF) medium (Invitrogen) for DBP add-back and Gc genotype studies. Adherent monocytes were then washed in SF RPMI and cultured overnight in RPMI with 10% FBS (Omega, Tarzana, CA) or in macrophage SF medium depending on experiment type as outlined below. After overnight incubation, cells were washed with SF RPMI and then 1) recultured in RPMI plus 5% DBP+/− mouse serum or 5% DBP−/− mouse serum for 6 h; 2) recultured in RPMI plus BSA (Calbiochem, San Diego, CA) plus human DBP (Calbiochem) for 6 h, with varying amounts of DBP and BSA used for treatments to normalize all samples to 10 μm of added protein; or 3) recultured in RPMI plus 5% human donor serum (Innovative Research, Novi, MI; or provided by Drs. Martineau and Wilkinson in accordance with local ethical approval) for 6 h. All culture media were supplemented with 10 U/ml granulocyte-macrophage colony-stimulating factor (Immunex, Seattle, WA). Serum was collected from C57BL/6 mice heterozygous (+/−) or homozygous (−/−) for the DBP/Gc gene. Monocytes were treated with the vitamin D3 form of 25OHD (25OHD3; 2–200 nm) or 1,25(OH)2D [1,25(OH)2D3; 0.02–2 nm] (Biomol, Plymouth Meeting, PA) or with vehicle (0.2% ethanol) for 6 h. Megalin-expressing BN16 cells (rat yolk sac carcinoma) were propagated in DMEM plus 10% FBS (kind gift of Dr. T. Willnow, Max-Delbrueck-Center for Molecular Medicine, Berlin, Germany) (17). In some cases, monocytes were treated with IL-15 (R&D Systems, Minneapolis, MN).
Quantitative real-time PCR
RNA was isolated by Trizol (Invitrogen) extraction and cDNA synthesized by SuperScript reverse transcriptase III (Invitrogen) according to the manufacturer’s protocol using random hexamers as previously described (2). Quantitative PCR analysis was performed on a Stratagene MX-3005P instrument using TaqMan reagents from Applied Biosystems (Foster City, CA). Specifically, we used FAM-labeled TaqMan gene expression assay probe/primer sets Hs00167999_m1 (CYP24A1) and Hs00189038_m1 (cathelicidin) in conjunction with VIC/MGB probe/primer for 18S rRNA (endogenous control) (part 4319413E). All cDNAs were amplified under the following conditions: 50 C for 2 min and then 95 C for 10 min followed by 45 cycles of 95 C for 15 sec and 60 C for 1 min. All reactions were performed in triplicate and reported as difference in cycle threshold (ΔΔCt) values (ΔCt value for vehicle-treated control − ΔCt for treated sample).
Fluorescent tagging of DBP
One milligram of human Gc-globulin (Calbiochem) was fluorescently tagged using Alexa-Fluor 488 protein labeling kit (catalog item A-10235; Molecular Probes/Invitrogen, Carlsbad, CA) reagents for 30 min at room temperature and separated from free dye through size exclusion resin provided with the kit according to manufacturer’s instructions. The resulting fluorescently labeled DBP is referred to as Alexa-DBP.
25OHD3-DBP-binding assays
Aliquots (0.2 μg) of Alexa-DBP were incubated with 40,000 cpm [3H]25OHD3 (NEN Life Science Products/PerkinElmer, Waltham, MA) in 200 μl PBS plus 0.1% gelatin for 30 min at room temperature. Dextran-coated charcoal buffer (200 μl) was then added and incubated for 30 additional minutes on ice. Nonspecific binding was determined using the same assay conditions but with 100 nm unlabeled 25OHD3 as competitor. DBP-bound [3H]25OHD3 was then obtained as a supernatant by centrifugation (Sorval H6000A) at 3500 rpm for 30 min at 4 C and measured by scintillation counting.
Flow cytometric analysis of DBP cellular uptake
PBMCs (2.5 × 106) were added to 24-well plates in RPMI containing 1% FBS and incubated for 2 h, and nonadherent cells were aspirated to enrich for monocytes. Cells were cultured with RPMI plus 10% FBS plus granulocyte-macrophage colony-stimulating factor (10 U/ml) and then washed with SF RPMI. Parallel cultures of megalin-positive BN16 cells were also used. Both types of cell were pretreated with 0.4 μm receptor-associated protein (RAP) (Calbiochem), a megalin-cubilin antagonist for 15 min. Aliquots of Alexa-DBP (0.4 or 0.1 μm in RAP antagonist studies) were added to cells in SF RPMI and incubated for 15 min at 37 C. Cells were then placed on ice for 5 min, IgG-R-phycoerythrin isotype control or CD14-R-phycoerythrin (Caltag/ Invitrogen, Carlsbad, CA) was added and incubated for 10 min on ice. The cells were then washed with ice-cold PBS, removed from culture wells by scraping in ice-cold PBS, collected by centrifugation, washed again in ice-cold PBS, and then fixed with 4% paraformaldehyde in PBS (pH 7.4). Flow cytometric analysis was carried out using a Beckman Coulter (Brea, CA) Cyan ADP with Summit 4.3 software.
DBP genotype (Gc) analysis
Twenty age-matched (30–39 yr) and sex-matched (male) human serum samples from Black and white donors were purchased from a commercial source (Innovative Research), and 16 donor serum samples from uninfected patients of Gujarati ethnicity were obtained from Dr. Martineau’s United Kingdom clinic, the latter in accordance with local ethical approval. Allelic forms of DBP were determined by DNA sequencing (18) or by serum isoelectric focusing as described previously (19).
Statistical analyses
Data are expressed as mean ± sd unless otherwise stated. Statistical analysis of dose response and single treatment was determined by two-tailed unpaired equal-variance Student’s t test. Statistical analysis of variations between sera with different DBP genotypes treatment studies was carried out by Kruskal-Wallis one-way ANOVA with the Dunn method as a post hoc multiple-comparison procedure applied to raw ΔCt values from RT-PCR assays (Sigmaplot 9.0 software; Systat Inc., San Jose, CA).
Results
DBP attenuates 25OHD3- and 1,25(OH)2D3-induced cathelicidin expression in monocytes
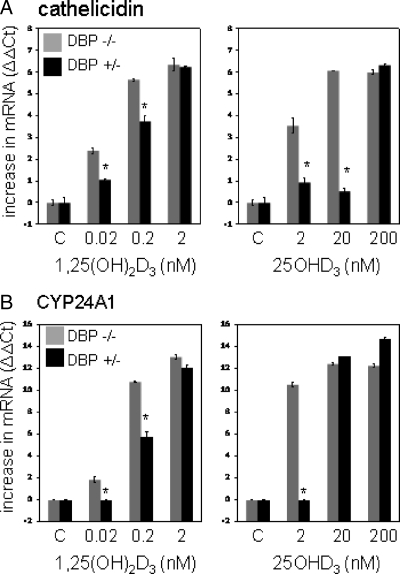
Initial experiments were carried out to determine whether monocyte responses to vitamin D metabolites are influenced by DBP. Data in Fig. 1 show that for monocytes cultured in medium containing mouse serum lacking DBP (DBP−/−), expression of cathelicidin and the classical vitamin D target gene 24-hydroxylase (CYP24A1) was induced when cells were treated for 6 h with 25OHD3 (2–200 nm) or 1,25(OH)2D3 (0.02–2 nm). However, this response was attenuated in cells cultured in medium containing serum from DBP+/− mice. At higher doses of 25OHD3 or 1,25(OH)2D3, the modulatory action of DBP was negated, suggesting a threshold level beyond which there is sufficient unbound vitamin D metabolite to elicit a response. In the case of 25OHD3, DBP suppression of CYP24A1 induction could be overcome with 20 nm 25OHD3, whereas cathelicidin required 200 nm.
Figure 1.
DBP knockout serum increases monocyte responses to vitamin D metabolites. Human monocytes cultured in medium supplemented with 5% serum from DBP+/− and DBP−/− mice were treated with 25OHD3 (2–200 nm), 1,25(OH)2D3 (0.02–2 nm), or vehicle control (C, 0.2% ethanol) for 6 h. RNA from the resulting cells were then analyzed by RT-PCR for cathelicidin (panel A) and 24-hydroxylase (CYP24A1) (panel B). Data are shown as mean (n = 3) changes in RT-PCR ΔΔCt values relative to vehicle-treated cells. *, Statistically different from DBP+/− serum cells at P < 0.001.
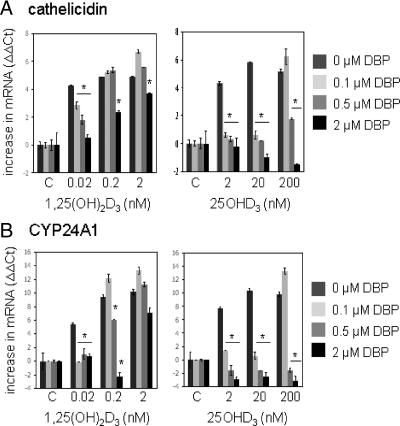
Additional experiments were carried out using monocytes cultured in SF medium containing only 10 μm BSA. Under these conditions, both 25OHD3 and 1,25(OH)2D3 potently induced expression of monocyte cathelicidin (Fig. 2). As with the DBP+/− mouse serum in Fig. 1, response to 25OHD3 and 1,25(OH)2D3 was attenuated by the addition of DBP to SF cultures. The DBP was added with BSA in different combinations to provide a final concentration of 10 μm protein per well to prevent any bias from variable total protein levels. Data indicated that doses of DBP as low as 0.1 μm were sufficient to completely suppress induction of cathelicidin or CYP24A1 by 25OHD3. This squelching response to DBP was much less pronounced in cells treated with 1,25(OH)2D3: 2 μm DBP moderately suppressed responses to a physiologically normal level of 1,25(OH)2D3 (0.2 nm), whereas 0.5 μm DBP was sufficient to completely inhibit responses to a physiologically high level of 25OHD3 (200 nm).
Figure 2.
DBP add-back decreases monocyte responses to vitamin D metabolites. Human monocytes were cultured in SF medium supplemented with DBP (0.1–2 μm) or 10 μm BSA control (0 μm DBP) with all treatment groups receiving 10 μm total protein (DBP and BSA). Cells were treated with 25OHD3 (2–200 nm), 1,25(OH)2D3 (0.02–2 nm), or vehicle (C, 0.2% ethanol) for 6 h. RNA from the resulting cells was then analyzed by RT-PCR for cathelicidin (panel A) and 24-hydroxylase (CYP24A1) (panel B). Data are shown as mean (n = 3) changes in RT-PCR ΔΔCt values relative to vehicle-treated cells. *, Statistically different from cells in medium containing no DBP at P < 0.001.
We have reported previously that even in the absence of treatments known to induce CYP27b1 such as TLR ligands (2,3) or IL-15 (4), monocytes at day 1 of culture exhibit baseline conversion of 25OHD3 to 1,25(OH)2D3 which can act in an intracrine fashion to induce expression of genes such as cathelicidin (2). Thus, in the current study, cells were cultured without added immune activators to minimize confounding effects that might influence responses to DBP and vitamin D metabolites. However, in a series of parallel experiments we showed that suppression of 25OHD3 action by DBP was also observed in monocytes cultured for up to ten days to generate a more macrophage-like phenotype (Supplemental Fig. 1, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org), and in monocytes treated with IL-15 to stimulate CYP27b1 expression (Supplemental Fig. 2).
Internalization of DBP by human monocytes
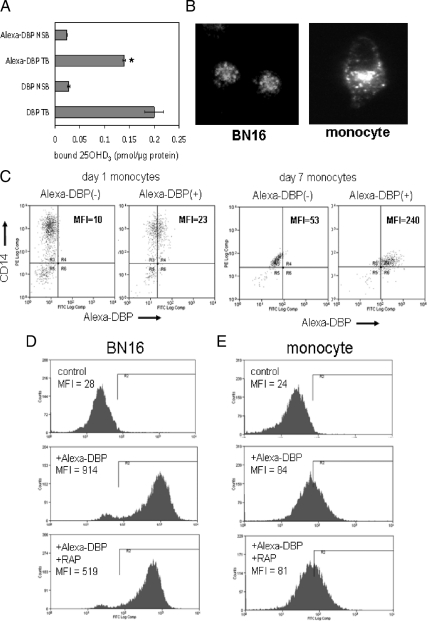
Previous reports have shown that DBP can be internalized by cells via a megalin- mediated endocytic pathway in kidney (14) and mammary (15) tissue. In both cases, receptor-mediated uptake of DBP facilitated local conversion of 25OHD3 to 1,25(OH)2D3 and downstream VDR responses. To determine whether the effects of DBP on monocyte responses to 25OHD3 or 1,25(OH)2D3 also involve an internalization pathway, further studies were carried out using fluorescently tagged DBP. Initial ligand-binding analyses confirmed that Alexa-DBP was able to specifically bind [3H]25OHD3, albeit at a slightly lower level than unlabeled DBP (Fig. 3A). Alexa-DBP was then incubated with monocytes and uptake assessed by fluorescent microscopy (Fig. 3B) and flow cytometry (Fig. 3, C–E). Microscopy indicated that Alexa-DBP was internalized by monocytes in a similar fashion to megalin-positive BN16 cells, (Fig. 3B). To demonstrate similar cytosolic patterns of DBP expression in the two cell lines, longer exposure times were required for monocytes, indicating higher levels of DBP uptake in BN16 cells. This was further underlined by flow cytometric analyses that showed higher mean fluorescence intensity for BN16 cells (Fig. 3D, middle panel) compared with monocytes (Fig. 3E, middle panel). Nevertheless, measurable uptake of Alexa-DBP was detectable in CD14-positive monocytes at both d 1 and 7 of culture (Fig. 3C). To determine whether this uptake was mediated via megalin, cells were preincubated with RAP, a megalin antagonist. RAP reduced Alexa-DBP uptake in BN16 cells (Fig. 3D) but had no effect on Alexa-DBP uptake in monocytes (Fig. 3E). In additional studies, RT-PCR analysis revealed no significant mRNA expression for either megalin or its coreceptor cubulin in monocytic cells (data not shown).
Figure 3.
DBP is internalized by monocytes via a non-megalin-mediated mechanism. A, Binding of [3H]25OHD3 to unlabeled DBP and fluorescently tagged DBP (Alexa-DBP). Nonspecifically bound (NSB) [3H]25OHD3 was determined in the presence of a molar excess of nontritiated 25OHD3. Total binding (TB) data are shown as picomoles 25OHD3 bound per microgram DBP. B, Fluorescence microscopy of megalin-positive BN16 cells and monocytes showing that DBP is internalized by human monocytes. Longer exposure time was used to demonstrate DBP uptake by monocytes. C, Flow cytometric analysis of DBP uptake by monocytes at different stages of differentiation. Aliquots (0.4 μm) of Alexa-DBP were incubated with d-1 or -7 cultures of human monocytes. Flow cytometry was then carried out to identify cells with coexpression of CD14 and Alexa-DBP; dual-label data are shown as mean fluorescence intensity (MFI). D and E, Effect of the megalin inhibitor RAP on BN16 cells (D) and monocytes (E) (d 7). Cells were pretreated with 0.4 μm RAP, a megalin antagonist and then incubated with Alexa-DBP (0.1 μm). Data show flow cytometric data for Alexa-DBP uptake in untreated (control) cells and cells treated with Alexa-DBP in the absence or presence of RAP. Data in C–E show representative flow cytometry plots for each cell treatment incorporating 40,000 cells per plot. Flow cytometry assays were repeated at least twice with similar results. *, Statistically different from unlabeled DBP at P < 0.05. FITC, Fluorescein isothiocyanate.
Induction of monocyte cathelicidin by 25OHD3 varies with DBP genotype
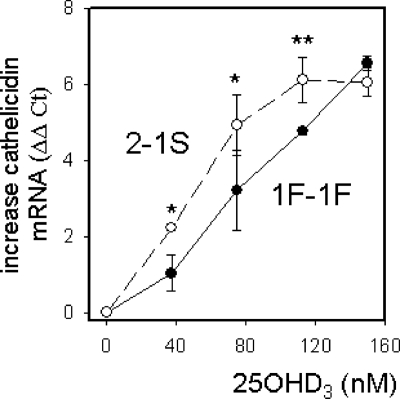
Having demonstrated the importance of DBP as a determinant of vitamin D-induced responses in monocytes, we then sought to determine whether established genetic differences in DBP provide an additional level of variation to this effect. To assess the impact of DBP gene variants on 25OHD3-induced cathelicidin, monocytes were cultured in medium containing 5% human serum from donors with the six different combinations of the three major allelic variants of DBP: Gc1F, Gc1S, and Gc2. Initial dose-response studies showed more sensitive responses to 25OHD3 using serum from Gc2-1S donors compared with those with the Gc1F-1F genotype (Fig. 4).
Figure 4.
Dose-responsive induction of cathelicidin by 25OHD3 in monocytes cultured in medium supplemented with serum from donors with different DBP genotypes. Human monocytes cultured in medium supplemented with 5% serum from sex-matched (male) and age-matched (30–39 yr) donors were treated with 25OHD3 (0–150 nm) for 6 h. RNA from the resulting cells was then analyzed by RT-PCR for cathelicidin mRNA expression. Data are shown as mean increase in cathelicidin mRNA (ΔΔCt value) relative to vehicle-treated (0.2% ethanol) controls for donor serum with a Gc2-1S genotype (n = 3) vs. a Gc1F-1F genotype (n = 3). *, Statistically different from Gc1F-1F at P < 0.05; **, P < 0.01.
Further analysis by ANOVA of combinations of Gc alleles using 36 serum samples from donors with different ethnic backgrounds confirmed a statistically significant (P = 0.01) increase in cathelicidin induction after treatment with a single dose of 25OHD3 in monocytes cultured with Gc2-1S (P = 0.003) or Gc2-2 (P = 0.016) serum compared with Gc1F-1F serum (Table 1). Indeed, a straightforward comparison between donors with least one allele of Gc1F (Gc1F-1F, Gc1F-1S, and Gc1F-2) and those without Gc1F (Gc1S-1S and Gc2-1S, and Gc2-2) showed that monocytes cultured in the latter were 1.97-fold more sensitive to 25OHD3 (P = 0.002). Likewise, cells cultured in serum from donors with at least one Gc2 allele showed 1.73-fold higher sensitivity to 25OHD3 than cells cultured in serum from donors without a Gc2 allele (P = 0.011). Analysis of a subset of the sera revealed no statistical difference in levels of 25OHD or 1,25(OH)2D between donors with different Gc types (Supplemental Table 1).
Table 1.
Serum from donors with DBP genotype Gc2–1S and Gc2–2 supports increased monocyte responses to 25OHD3
| DBP phenotype | Black | White | Asian | Mean ΔΔCt | sd | Fold change relative to Gc1F-1F | P value relative to Gc1F-1F |
|---|---|---|---|---|---|---|---|
| Gc1F-1F | 4 | 0 | 0 | −0.70 | 0.52 | 1.00 | |
| Gc1F-1S | 4 | 1 | 1 | −0.66 | 1.53 | 1.03 | 0.951 |
| Gc1F-2 | 0 | 3 | 1 | −0.35 | 0.88 | 1.27 | 0.407 |
| Gc1S-1S | 1 | 3 | 3 | 0.00 | 0.85 | 1.62 | 0.063 |
| Gc2-1S | 1 | 3 | 5 | 0.76 | 1.08 | 2.75 | 0.003 |
| Gc2-2 | 0 | 0 | 6 | 0.43 | 0.86 | 2.19 | 0.016 |
Human monocytes were cultured in medium supplemented with 5% serum from 36 donors with different DBP allelic combinations and different ethnic backgrounds [African-American (Black), Caucasian-American (white), United Kingdom Gujarati (Asian)]. Each monocyte culture was then treated with either vehicle (0.1% ethanol) or a single dose of 25OHD3 (112.5 nm) for 6 h. RNA from the resulting cells was then analyzed by RT-PCR for cathelicidin. Data are shown as mean increase in cathelicidin mRNA (ΔΔCt) for donor DBP genotype relative to the Gc1S-1S genotype (equal to zero) and the fold change in expression relative to Gc1F-1F.
Discussion
Serum levels of 25OHD vary considerably within humans and provide the best circulating marker for vitamin D status (9). As such, serum 25OHD is likely to be a key determinant of many vitamin D functions, notably its antibacterial effects, which are known to be mediated via intracrine/autocrine mechanisms (2,3). However, we hypothesized that biological responses to 25OHD will also be strongly influenced by serum proteins that bind vitamin D metabolites, in particular DBP. In data presented here, we confirm that DBP plays a pivotal role in modulating monocyte responses to 25OHD3 and that these effects vary according to DBP genotype. Analysis of DBP function using in vivo and in vitro models is complex. Animals lacking DBP are viable and physiologically normal under conditions of vitamin D sufficiency but have greatly reduced circulating levels of 25OHD and 1,25(OH)2D (20). More recent studies have shown that DBP−/− mice also have normal accumulation of 1,25(OH)2D in peripheral tissues (21), but to date, there has been no similar analysis of 25OHD. Studies of DBP in vitro are complicated by the fact that supplementation of culture media with 5–10% FBS is unrepresentative of conditions in vivo. However, in vitro comparisons using the same cells cultured with similar levels of serum from DBP+/− and DBP−/− mice has shed light on some key features of DBP. Notably, osteoblastic MC3T3-E1 cells cultured in medium lacking DBP were shown to be more sensitive to 1,25(OH)2D3 when compared with DBP-positive equivalents (21). Data presented here using CYP27b1-positive monocytes (Figs. 1 and 2) confirm these previous observations while also showing that the regulatory effects of DBP are more pronounced for responses to 25OHD, the major circulating form of vitamin D. These observations, coupled with the fact that 25OHD has a higher binding affinity for DBP than 1,25(OH)2D (22), suggest that it is free rather than DBP-bound vitamin D that is biologically active in monocytes, at least in vitro.
In serum, vitamin D metabolites bind primarily to DBP but may also associate with other abundant circulating proteins such as albumin. The affinity of albumin for 25OHD3 (dissociation constant, Kd = 1.7 μm) or 1,25(OH)2D3 (Kd = 19 μm) is substantially lower than that observed for DBP and 25OHD3 (Kd = 1.4 nm) or 1,25(OH)2D3 (Kd = 25 nm) (23,24). However, because of the relative abundance of albumin in serum (650 μm) compared with DBP (5 μm), the potential remains for some vitamin D metabolites to be transported in the circulation by albumin. Despite this, it is generally assumed that vitamin D metabolites are biologically active when unbound, even though this fraction is likely to be very small (24,25). Indeed, the free-hormone hypothesis has been proposed as a general mechanism for the cellular uptake of steroid-like molecules because they are highly lipophilic and therefore have the potential to passively diffuse across cell membranes (26,27). The concentrations of 25OHD3 and 1,25(OH)2D3 used in the current study were chosen to represent physiological to supraphysiological levels of these metabolites (9). Under such conditions, one would predict that the proportion of free (relative to DBP-bound) will be greater for 1,25(OH)2D3 than 25OHD3. This is endorsed by data in Figs. 1 and 2 showing that DBP is less effective at attenuating monocyte responses to 1,25(OH)2D3 and provides further support for the free-hormone hypothesis.
The free-hormone hypothesis is not universal. For some cell types such as renal proximal tubule cells (14,16) and breast epithelial cells (15,28), uptake of 25OHD, and subsequent conversion to 1,25(OH)2D, has been shown to involve endocytosis of DBP mediated via the megalin and cubilin receptors. Flow cytometry suggests that monocytes are also able to internalize DBP (see Fig. 3). However, the magnitude of this uptake was relatively low when compared with cells known to express megalin and cubilin. Moreover, we were unable to detect transcripts for megalin/cubilin in monocytes (data not shown), and inhibition of these receptors using RAP had no effect on monocyte uptake of DBP. The functional significance of non-megalin-mediated DBP uptake by monocytes is unclear but may be related to the alternative function of DBP as an actin-binding protein (29). This facet of DBP has been shown to be a highly effective mechanism for disrupting the intracellular translocation of infectious agents such as Listeria monocytogenes, which occurs via hijack of host actin filaments (30). DBP can also act as a macrophage-activating factor (Maf) precursor after posttranslational modification involving glycosidase enzymes found in T and B cells (31). Although DBP-Maf has been reported to act as a pluripotent cytokine affecting a variety of biological systems (32,33,34), these effects do not appear to be influenced by binding of vitamin D metabolites.
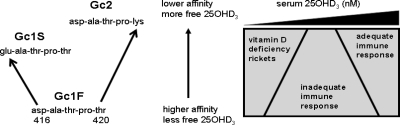
A key feature of DBP is that polymorphic variants of this protein were first described as long ago as 1959 (35). The ancestral DBP allele, Gc1F, has undergone two amino acid changes: a D416E change to form Gc1S and a T420K change to form Gc2 (see Fig. 5). These alterations in amino acid sequence have been shown to affect the affinity of DBP for vitamin D ligands. The reported Kd values for 25OHD3 with Gc1F = 0.9 nm, with Gc1S = 1.7 nm, and with Gc2 = 2.8 nm (22). Similar Kd variations have also been reported for 1,25(OH)2D3: Gc1F = 56 nm, Gc1S = 160 nm, and Gc2 = 240 nm (22). Gc alleles have also been linked to varying concentrations of DBP in serum, with Gc2 being the least abundant and Gc1F exhibiting the highest levels (19). It was therefore interesting to observe that the DBP genotype with the highest affinity/abundance (Gc1F-1F allelic combination) exhibited a significantly weaker cathelicidin response to 25OHD3 than the low-affinity/abundance Gc2-1S genotype (Table 1 and Fig. 4). This contrasts the endocrine function of the DBP variants, where Gc1F-1F is better able to maintain serum levels of vitamin D metabolites as a consequence of more efficient retention of 25OHD3 and 1,25(OH)2D3 after megalin-mediated uptake of DBP by kidney cells (36).
Figure 5.
Evolution of low-affinity forms of DBP enhances monocyte responses to vitamin D. Schematic representation of the impact of DBP genotype (Gc type) on monocyte responses to 25OHD3, the major circulating form of vitamin D is shown. The left part of the panel shows primary DBP peptide sequence variations corresponding to the three major GC forms. The right part of the panel shows proposed variations in sensitivity to the immunomodulatory effects of 25OHD3 for low- or high-affinity Gc forms.
Data presented here suggest that in humans, the ancestral form of DBP (Gc1F) has undergone loss-of-function amino acid changes that nevertheless confer important biological advantages with respect to the antibacterial actions of vitamin D. As outlined in Fig. 5, alterations in DBP would have had little or no effect on vitamin D physiology under conditions of high serum 25OHD status. However, with lower levels of 25OHD, loss of the Gc1F allele would be advantageous with respect to improved induction of antimicrobial agents such as cathelicidin. Maintenance of vitamin D function is considered to have been a pivotal factor in the adaptive changes in skin pigmentation that occurred as early humans migrated toward Northern latitudes (37). Under these conditions, individuals with darker skin pigmentation would have been less able to generate vitamin D via the epidermal action of UV light. As a consequence, early humans may have experienced a decline in serum 25OHD levels sufficient to compromise biological functions such as innate immune antibacterial activity. Kappelman and colleagues (38) have postulated that this may have been the underlying basis for their detection of tuberculosis infection in 500,000-yr-old hominid fossils discovered in Western Turkey. With this in mind, it is tempting to speculate that evolutionary changes in DBP may have occurred against a backdrop of increased bacterial infection and decreased vitamin D status as early humans migrated out of Africa.
Insufficient serum levels of 25OHD have been correlated with a wide range of detrimental health outcomes including infectious diseases (11,39,40,41). However, data presented here suggest that the DBP genotype of individuals is also an important determinant of the bioavailability of vitamin D metabolites to key target cells such as monocytes. It is therefore interesting to note studies showing association between the Gc1F allele and increased risk of chronic obstructive pulmonary disease (42) and syphilis infection (43). Paradoxically, a more recent study has linked the Gc2 allele with susceptibility to active tuberculosis (18). There are several potential explanations for this. Notably, the Gc2 form of DBP lacks the threonine at amino acid 420 required for posttranslational modification of DBP to DBP-Maf. Individuals with a Gc2-2 genotype will thus be unable to exhibit this facet of DBP action, although the impact of this with respect to innate immune response to infection is unclear. It should also be noted that association between the Gc2-2 genotype and active tuberculosis was observed only for a specific cohort of Gujarati origin living in London, in which the mean serum levels of 25OHD were extremely low (approximately 20 nm). Moreover, the association was lost for those patients with 25OHD levels greater than 20 nm. With this in mind, it is interesting to note that in patients with chronic obstructive pulmonary disease, the Gc2-2 allelic combination has been linked to low serum 25OHD levels (44). Thus, it is important to recognize that variations in DBP will affect both local (intracrine) and systemic (endocrine) actions of vitamin D. We therefore postulate that future studies of vitamin D and human disease will need to incorporate analysis of both serum vitamin D status and DBP genotype.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grants AR050626 to M.H. and AI073539 to R.L.M. and Wellcome Trust (072070) and Medical Research Council support to R.J.W.
Disclosure Summary: The authors have no conflict of interest to declare.
First Published Online April 28, 2010
Abbreviations: ΔΔCt, Difference in cycle threshold; DBP, vitamin D-binding protein; FBS, fetal bovine serum; Gc, group-specific component; Maf, macrophage-activating factor; 25OHD, 25-hydroxyvitamin D; 1,25(OH)2D, 25-dihydroxyvitamin D; PBMC, peripheral blood mononuclear cell; RAP, receptor-associated protein; SF, serum-free; TLR, Toll-like receptor; VDR, vitamin D receptor.
References
- Adams JS, Hewison M 2008 Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 4:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M 2009 Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol 182:4289–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL 2006 Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773 [DOI] [PubMed] [Google Scholar]
- Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL 2008 IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol 181:7115–7120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, Davidson RN, Sørensen OE, Kampmann B, Griffiths CJ, Wilkinson RJ 2007 IFN-γ- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol 178:7190–7198 [DOI] [PubMed] [Google Scholar]
- Gombart AF, Borregaard N, Koeffler HP 2005 Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 19:1067–1077 [DOI] [PubMed] [Google Scholar]
- Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH, Hanrahan JH 2004 Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173:2909–2912 [DOI] [PubMed] [Google Scholar]
- Gombart AF, Saito T, Koeffler HP 2009 Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genomics 10:321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF 2009 Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 19:73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF, Chen TC 2008 Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87:1080S–1086S [DOI] [PubMed] [Google Scholar]
- Holick MF 2007 Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- White P, Cooke N 2000 The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol Metab 11:320–327 [DOI] [PubMed] [Google Scholar]
- Laing JCN 2005 Vitamin D-binding protein. In: Feldman D, Pike JW, Glorieux FH, eds. Vitamin D. Amersterdam: Elsevier; 117–152 [Google Scholar]
- Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE 1999 An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96:507–515 [DOI] [PubMed] [Google Scholar]
- Rowling MJ, Kemmis CM, Taffany DA, Welsh J 2006 Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr 136:2754–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI 2001 Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D3. Proc Natl Acad Sci USA 98:13895–13900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Panse S, Galceran M, Pontillon F, Lelongt B, van de Putte M, Ronco PM, Verroust PJ 1995 Immunofunctional properties of a yolk sac epithelial cell line expressing two proteins gp280 and gp330 of the intermicrovillar area of proximal tubule cells: inhibition of endocytosis by the specific antibodies. Eur J Cell Biol 67:120–129 [PubMed] [Google Scholar]
- Martineau AR, Leandro AC, Anderson ST, Newton SM, Wilkinson KA, Nicol MP, Pienaar SM, Skolimowska KH, Rocha MA, Rolla VC, Levin M, Davidson RN, Bremner SA, Griffiths CJ, Eley BS, Bonecini-Almeida MG, Wilkinson RJ 24 September 2009 Association between Gc genotype and susceptibility to TB is dependent on vitamin D status. Eur Respir J 0:09031936.00087009v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauridsen AL, Vestergaard P, Nexo E 2001 Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin Chem 47:753–756 [PubMed] [Google Scholar]
- Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, Liebhaber SA, Cooke NE 1999 Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest 103:239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zella LA, Shevde NK, Hollis BW, Cooke NE, Pike JW 2008 Vitamin D-binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinology 149:3656–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud J, Constans J 1993 Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Hum Genet 92:183–188 [DOI] [PubMed] [Google Scholar]
- Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG 1986 Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab 63:954–959 [DOI] [PubMed] [Google Scholar]
- Bikle DD, Gee E, Halloran B, Haddad JG 1984 Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J Clin Invest 74:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon R, Van Assche FA, Van Baelen H, Heyns W, De Moor P 1981 Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. J Clin Invest 67:589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel CM 1989 The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev 10:232–274 [DOI] [PubMed] [Google Scholar]
- Hammond GL 2002 Access of reproductive steroids to target tissues. Obstet Gynecol Clin North Am 29:411–423 [DOI] [PubMed] [Google Scholar]
- Chlon TM, Taffany DA, Welsh J, Rowling MJ 2008 Retinoids modulate expression of the endocytic partners megalin, cubilin, and disabled-2 and uptake of vitamin D-binding protein in human mammary cells. J Nutr 138:1323–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JM, Dabiri G, Mittal B, Kowalski MA, Haddad JG, Sanger JW 1990 Disruption of microfilament organization in living nonmuscle cells by microinjection of plasma vitamin D-binding protein or DNase I. Proc Natl Acad Sci USA 87:5474–5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger JM, Mittal B, Southwick FS, Sanger JW 1995 Listeria monocytogenes intracellular migration: inhibition by profilin, vitamin D-binding protein and DNase I. Cell Motil Cytoskeleton 30:38–49 [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Homma S, Millman I 1991 Identification of the serum factor required for in vitro activation of macrophages. Role of vitamin D3-binding protein (group specific component, Gc) in lysophospholipid activation of mouse peritoneal macrophages. J Immunol 147:273–280 [PubMed] [Google Scholar]
- Yamamoto N, Naraparaju VR 1996 Role of vitamin D3-binding protein in activation of mouse macrophages. J Immunol 157:1744–1749 [PubMed] [Google Scholar]
- Kanda S, Mochizuki Y, Miyata Y, Kanetake H, Yamamoto N 2002 Effects of vitamin D3-binding protein-derived macrophage activating factor (GcMAF) on angiogenesis. J Natl Cancer Inst 94:1311–1319 [DOI] [PubMed] [Google Scholar]
- Swamy N, Ghosh S, Schneider GB, Ray R 2001 Baculovirus-expressed vitamin D-binding protein-macrophage activating factor (DBP-maf) activates osteoclasts and binding of 25-hydroxyvitamin D3 does not influence this activity. J Cell Biochem 81:535–546 [DOI] [PubMed] [Google Scholar]
- Hirschfled J, Jonsson B, M R 1959 Immune electrophoretic demonstration of qualitative differences in human sera and their relation to the haptoglobins. Acta Pathol Microbiol 47:160–168 [DOI] [PubMed] [Google Scholar]
- Lauridsen AL, Vestergaard P, Hermann AP, Brot C, Heickendorff L, Mosekilde L, Nexo E 2005 Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): a cross-sectional study on 595 early postmenopausal women. Calcif Tissue Int 77:15–22 [DOI] [PubMed] [Google Scholar]
- Jablonski NG, Chaplin G 2000 The evolution of human skin coloration. J Hum Evol 39:57–106 [DOI] [PubMed] [Google Scholar]
- Kappelman J, Alçiçek MC, Kazanci N, Schultz M, Ozkul M, Sen S 2008 First Homo erectus from Turkey and implications for migrations into temperate Eurasia. Am J Phys Anthropol 135:110–116 [DOI] [PubMed] [Google Scholar]
- Shoenfeld N, Amital H, Shoenfeld Y 2009 The effect of melanism and vitamin D synthesis on the incidence of autoimmune disease. Nat Clin Pract Rheumatol 5:99–105 [DOI] [PubMed] [Google Scholar]
- Grant WB, Mohr SB 2009 Ecological studies of ultraviolet B, vitamin D and cancer since 2000. Ann Epidemiol 19:446–454 [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM 2007 Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab 92:3517–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Keicho N, Teramoto S, Azuma A, Kudoh S, Fukuchi Y, Ouchi Y, Matsuse T 2001 Association of Gc-globulin variation with susceptibility to COPD and diffuse panbronchiolitis. Eur Respir J 18:753–757 [DOI] [PubMed] [Google Scholar]
- Pollard DR, Gill P, Day A 1988 The group-specific protein marker: a possible indicator of syphilis, not human immunodeficiency virus infection. CMAJ 138:1013–1015 [PMC free article] [PubMed] [Google Scholar]
- Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, Coolen J, Mathieu C, Decramer M, Lambrechts D 2009 Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 65:215–220 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.