Abstract
Background/Rationale: Autoantibodies to islet antigen-2 (IA-2A) and glutamic acid decarboxylase (GADA) are markers for diagnosis, screening, and measuring outcomes in National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) consortia studies. A harmonization program was established to increase comparability of results within and among these studies.
Methods: Large volumes of six working calibrators were prepared from pooled sera with GADA 4.8–493 World Health Organization (WHO) units/ml and IA-2A 2–235 WHO units/ml. Harmonized assay protocols for IA-2A and GADA using 35S-methionine-labelled in vitro transcribed and translated antigens were developed based on methods in use in three NIDDK laboratories. Antibody thresholds were defined using sera from patients with recent onset type 1 diabetes and healthy controls. To evaluate the impact of the harmonized assay protocol on concordance of IA-2A and GADA results, two laboratories retested stored TEDDY study sera using the harmonized assays.
Results: The harmonized assays gave comparable but not identical results in the three laboratories. For IA-2A, using a common threshold of 5 DK units/ml, 549 of 550 control and patient samples were concordantly scored as positive or negative, specificity was greater than 99% with sensitivity 64% in all laboratories. For GADA, using thresholds equivalent to the 97th percentile of 974 control samples in each laboratory, 1051 (97.9%) of 1074 samples were concordant. On the retested TEDDY samples, discordance decreased from 4 to 1.8% for IA-2A (n = 604 samples; P = 0.02) and from 15.4 to 2.7% for GADA (n = 515 samples; P < 0.0001).
Conclusion: Harmonization of GADA and IA-2A is feasible using large volume working calibrators and common protocols and is an effective approach to ensure consistency in autoantibody measurements.
Improved concordance of measurement of islet autoantibodies among international laboratories is achieved by the establishment of harmonized, sensitive, and specific reference immunoassays.
The measurement of islet autoantibodies is used extensively in diabetes research to identify individuals at risk of developing type 1 diabetes, in particular as selection criteria for clinical prevention trials. It is also increasingly used in the classification of diabetes (1). Such activities often require multicenter recruitment with islet autoantibody screening carried out in central laboratories. There has been substantial progress toward standardization of glutamic acid decarboxylase (GAD) and islet antigen-2 (IA-2) antibodies through the Diabetes Autoantibody Standardization Program (DASP), a collaboration between the Immunology of Diabetes Society and Centers for Disease Control, and reliable assays and laboratories can be identified and new assays evaluated (2). Previous comparisons have, however, demonstrated that, despite high sensitivity and specificity and general concordance in ranking samples, there were still differences in absolute levels of GAD and IA-2 antibodies expressed in standardized World Health Organization (WHO) units/ml (2).
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) has launched a number of multicenter studies that use central laboratories for the measurement of islet autoantibodies (3,4,5,6,7). For both historical and logistic reasons, several different central laboratories are used. To facilitate comparison of quantitative islet autoantibody results between studies, the NIDDK set up an Islet Autoantibody Harmonization Committee to align measurement and reporting of islet autoantibodies in all NIDDK-sponsored studies. This manuscript reports the process and results of the harmonization exercise. This has included the introduction of common working calibrators, units, and methods and has resulted in high concordance of GAD and IA-2 autoantibody (GADA and IA-2A) measurement among central laboratories of the NIDDK consortia.
Patients and Methods
Study plan
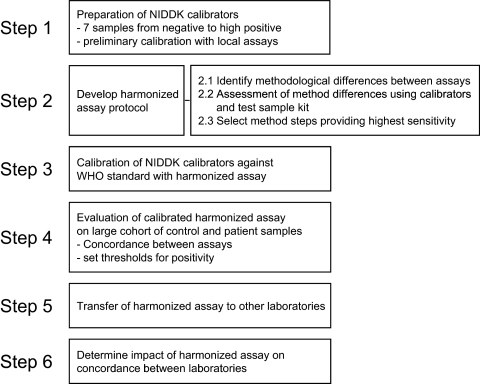
The steps in the harmonization exercise are summarized in Fig. 1.
Figure 1.
Sequential steps taken in harmonization process.
Laboratories
Four laboratories participated in the harmonization process: 1) the Barbara Davis Center (Denver, CO) (BDC), North American reference laboratory for The Environmental Determinants of Diabetes in the Young (TEDDY) (4), TrialNet (5), and T1DGC (3); 2) the University of Bristol (Bristol, UK), European reference laboratory for TEDDY and T1DGC (Bristol); 3) the Diabetes Research Institute (Munich, Germany), reference laboratory for SEARCH (Munich); and 4) the University of Washington (Seattle, WA), central islet autoantibody laboratory for SEARCH (6) and TODAY (7) studies (Seattle).
Sera
Calibrators
Large volumes of positive and negative calibrator samples were prepared from pooled sera. For the positive calibrator, 25–50 ml serum were collected from each of 21 patients with type 1 diabetes aged 18 to 30 yr with a median time since diagnosis of 1.1 yr (range, 0.2 to 2.2 yr). The presence of moderate/high levels of antibodies to GAD and IA-2 in individual sera was confirmed in the BDC, Bristol, and Munich laboratories before the sera were pooled. For the negative serum diluents and calibrator, a total of 13 frozen serum donations (median volume for each sample, 228 ml) were obtained from the blood bank of the Städt. Klinikum München GmbH, Munich. One sample was found to have low titer GAD antibodies at screening in the Munich laboratory and was excluded. The remaining 12 sera were negative in all three laboratories and were pooled. All 34 sera had negative hepatitis B, hepatitis C, and HIV serology.
Positive and negative calibrator pools were cleared through filter paper (4- to 7-μm pore size), high-speed centrifugation, and finally filtered through gauze to remove the resultant lipid layer. The final volumes obtained were 795 ml of the positive calibrator pool and 2600 ml of the negative calibrator. Six dilutions from 1/3.85 to 1/457 (1/2.6-fold dilution intervals) were selected to cover a range of titers from at or just below the expected threshold for positivity up to the upper limit of linearity in the binding characteristics of the assays (Table 1). Approximately 400 ml of each dilution was prepared, aliquoted, and stored at −20 C. An additional 629 ml of the positive pool calibrator remains and has been stored at −20 C for future preparations.
Table 1.
Details of NIDDK calibrators
| Calibrator | Volume prepared (ml) | Dilution of stock positive in negative pool | Assigned GADA (DK units/ml) | Assigned IA-2A (DK units/ml) |
|---|---|---|---|---|
| A | 394 | 1/3.85 | 493 | 235 |
| B | 397 | 1/10 | 228 | 106 |
| C | 392 | 1/26 | 92 | 42 |
| D | 393 | 1/67.6 | 38 | 16 |
| E | 394 | 1/176 | 13 | 5.8 |
| F | 395 | 1/457 | 4.8 | 2 |
Other sera
1) The initial test set comprised 18 samples from recent onset type 1 diabetes patients and two nondiabetic controls (test set 1).
2) A set of samples from 500 blood donors and 50 patients with recent onset type 1 diabetes was used to define thresholds (DASP set 2), which were validated in a second set of samples from cases comprising another 50 sera from patients with recent onset diabetes (DASP) and 500 adult controls from the Coronary Artery Calcification in Type 1 (CACTI) Diabetes study (8).
3) Concordance was evaluated in previously discordant samples from the TEDDY study (TEDDY discordant set). These were selected from samples previously reported as discordant for insulin autoantibodies, GADA, and/or IA-2A using local assays in the two TEDDY central laboratories (BDC and Bristol). Sufficient sample remained in the aliquots retained in both laboratories for testing GADA in 514 samples and IA-2A in 604 samples with both the local and harmonized assays.
All test samples and aliquots were individually coded to ensure masked testing throughout the harmonization exercise. For all sera, collection and use were obtained with informed consent.
Local IA-2A and GADA assays
At the start of the harmonization exercise, all laboratories were running radio-binding assays based on the use of 35S-methionine or 3H-lysine labeled in vitro transcribed and translated antigen with protein A Sepharose immunoprecipitation. Local assays for IA-2A and GADA were as previously reported for BDC (9), Bristol (10), and Munich (11). The local assays in BDC used filtration for washing protein A Sepharose beads, and the local assays in Bristol and Munich used centrifugation-based washing procedures. To allow comparison, local assays were run in parallel with the harmonized protocols as they were developed.
Harmonized IA-2A and GADA assays
The assay formats of the BDC, Bristol, and Munich laboratories were compared, and differences were noted. Steps of the assays that were brought into alignment in the harmonized protocol were: 1) the plasmid clones used for in vitro transcription/translation of labeled antigen [pSP64-PolyA-IA-2ic, kindly provided by Dr. Vito Lampasona (San Raffaele Institute, Milan, Italy) for IA-2, and pTh-GAD65, kindly provided by Prof. Åke Lernmark (Lund University, Malmö, Sweden) for GAD65]; 2) the radio-label (35S for all antigens) and the procedure for labeling antigen; 3) the counts per minute (cpm) of labeled antigen added to each assay well (approximately 20,000 cpm); 4) the assay wash buffer [50 mm Tris-buffered saline (pH 7.4) containing 0.15% Tween 20 (TBST)] and the assay sample buffer [TBST containing 0.1% BSA]; 5) the capture reagent—protein A Sepharose; and 6) the procedure for measuring bound radio-label and for calculating results of test samples. A full assay protocol can be obtained from the Diabetes Autoantibody Standardization Program (pwm2@cdc.gov).
Statistical analyses
The NIDDK calibrators were tested together with dilutions of the WHO reference serum using harmonized assays on five occasions in the BDC, Bristol, and Munich laboratories and reported as WHO units/ml by calibration as previously described (2). For each of the NIDDK calibrators, the median value of the WHO units/ml obtained for the 15 measurements was assigned as its calibrator unit. The assigned units were termed digestive and kidney units (DK units)/ml. In subsequent assays, calibration of signals into DK units/ml from the harmonized assays was performed by plotting the natural log of the assigned calibrator units against the natural log of cpm obtained for the calibrators minus cpm obtained for the negative calibrator and deriving a standard curve using linear regression. For each sample, the curve equation was used to convert the cpm of the test sample minus the cpm of the negative calibrator into DK units/ml. Distributions of results in control and patient samples were examined using QQ-plots. A comparison of the concordance obtained for the TEDDY samples measured by the local assays and the harmonized assays was made by a two-tailed χ2 analysis. All analyses were performed using the Statistical Package for Social Science (SPSS 17.0; SPSS, Chicago, IL).
Results
Harmonization steps
Introduction of NIDDK working calibrators
Test set 1 was assayed in the BDC, Bristol, and Munich laboratories using local assays to evaluate the working calibrators and the comparability of results between laboratories. This demonstrated linearity of measurement for the calibrators in each laboratory, but also showed that, despite the use of common working calibrators, some substantial differences remained for individual samples (data not shown).
Harmonization of IA-2A and GADA methods
The local methods in use in the BDC, Bristol, and Munich laboratories were compared, and the impact of differences between methods was addressed in individual participating laboratories. Using 35S-methionine improved assay performance compared with 3H-leucine in the Denver assay (data not shown). Antigen clones were harmonized to the pSP64-PolyA-IA-2ic clone from Dr. Vito Lampasona and the pTh-GAD65 clone from Prof. Åke Lernmark on the basis of improved signal-to-noise ratio and/or superior labeling efficiency compared with other clones tested. Higher protein A Sepharose concentration and smaller primary incubation volume increased IA-2A and GADA binding. The buffer concentration of Tris was shown to have little impact, but other factors in the assay buffers were shown to be critical for IA-2A assay performance. The inclusion of sodium azide or high Tween 20 concentrations (1%) affected results in selected sera (12), whereas BSA reduced background binding for some sera (data not shown). Both filtration and centrifugation washing procedures were in use in participating laboratories, and the harmonized assays include the option to perform either washing procedure. Overall, the resultant method produced a standard assay across the laboratories with relatively few changes in procedures.
Assignment of standard units to NIDDK working calibrators
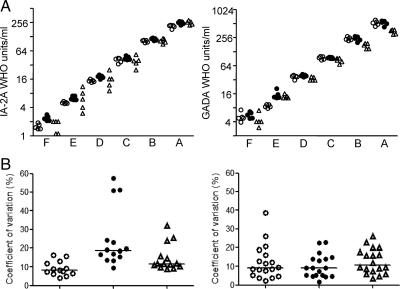
To assign units that were approximately equivalent to WHO units/ml for these autoantibodies, the NIDDK calibrators were tested together with the WHO reference standard in five separate assays in each of the BDC, Bristol, and Munich laboratories using the harmonized assay protocols, and results were reported as WHO units/ml (Fig. 2A). All laboratories achieved linearity across the calibrators. There were some between-laboratory differences in GADA levels reported for the highest calibrators and in IA-2A levels reported for the lowest calibrators. Units that were termed DK units/ml were assigned to each of the calibrators on the basis of the median WHO units/ml of the 15 results (Table 1 and Fig. 2A).
Figure 2.
Calibration of NIDDK calibrator samples. A, NIDDK calibrators A to F (abscissa) were tested together with the WHO reference preparation for IA-2A and GADA using the harmonized assays on five separate occasions in the Munich (open circles), Bristol (filled circles), and Denver (open triangles) laboratories to determine their WHO units/ml equivalents. The calibrated WHO units/ml for each of these measurements are shown. The median of the WHO units/ml was used to assign DK units/ml to the NIDDK calibrators. B, A set of coded samples that included 18 samples from patients with type 1 diabetes were also included in the calibration exercise. Each set was tested together with NIDDK calibrator samples on five occasions. Using calibration curves of the NIDDK calibrators, DK units/ml were assigned to each of these samples. For each laboratory, the coefficients of variation of the five replicate measurements for each of these samples are shown for IA-2A with values greater than 2.5 DK units/ml (left; n = 14 samples) and for GADA with values greater than 2.5 DK units/ml (right; n = 18 samples) for each of the laboratories. The median coefficient of variation for the sample is shown in each laboratory by the horizontal line.
Precision and concordance using the harmonized assays
Aliquots of test set 1 sera were measured together with the calibrators on five occasions by each laboratory. All samples from control subjects were reported as less than 2.5 DK units/ml for IA-2A and GADA in all laboratories in all assays. Of the patient samples, four were consistently reported as IA-2A less than 2.5 DK units/ml in all laboratories, and none was consistently reported as GADA less than 2.5 DK units/ml. The precision of measurement was determined in the patient samples with values above 2.5 U/ml (n = 14 for IA-2A, and n = 18 for GADA; Fig. 2B). The median (interquartile range) of the coefficients of variation for IA-2A measurements in the BDC, Bristol, and Munich laboratories were 11.6% (10.6–21.5); 18.8% (15.8–23.9); and 8.7% (6.8–12.9), respectively; and for GADA measurements were 10.8% (7.8–17.1), 9.1% (5.2–13.9), and 9% (6–15.3).
Sensitivity, specificity, concordance, and thresholds in a large sample set
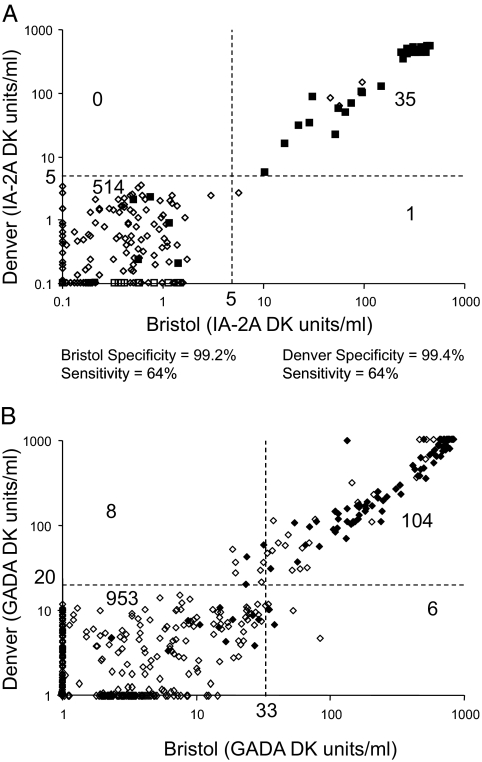
The results of IA-2A and GADA measurement of the DASP set (550 samples) using the harmonized assays are shown in Fig. 3. For IA-2A there was almost perfect concordance. QQ plots of the data showed clear separation of results of negative and positive with a threshold in the range of 3 to 5 DK units/ml in each of the laboratories. Using a common threshold of 5 DK units/ml, 496 of 500 control samples were reported as negative in all laboratories, three samples were reported as positive in all three laboratories, and one sample was scored positive in two of the three laboratories with results ranging between 2.6 and 8.1 DK units/ml. Of the 50 patient samples, the three laboratories identified 32 as positive, 18 as negative, and none as discordant.
Figure 3.
Concordance of measurement. A, IA-2A measured in Bristol (abscissa) and Denver (ordinate axis) using the harmonized assay in 500 samples from the DASP blood donor control set (open diamonds) and 50 samples from patients with recent onset type 1 diabetes (filled squares). Thresholds of 5 DK units/ml are indicated for both laboratories, and the number of samples falling in each quadrant are indicated. B, GADA measured in Bristol (abscissa) and Denver (ordinate axis) using the harmonized assay in 974 samples representing 500 controls from the DASP blood donor control set and a second set of adult control samples (open diamonds) and 100 samples from patients with recent onset type 1 diabetes (filled diamonds). Thresholds representing the 97th percentile of the control samples are indicated for both laboratories, and the number of samples falling in each quadrant are indicated.
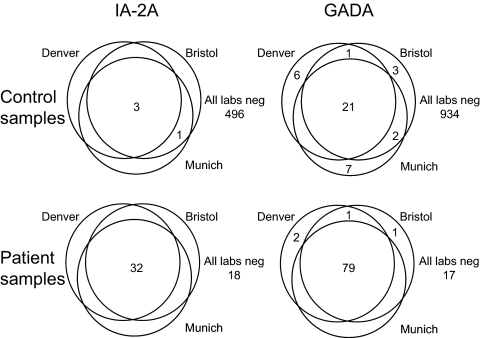
For GADA, there was more variation among the laboratories in the distribution of results, and it was not possible to identify a common threshold suitable for all laboratories. A second set of 524 samples was, therefore, tested by each laboratory. Results from this second set confirmed the distributions for the DASP sample set, and therefore GADA results were combined. The combined results are shown in Fig. 3B for the Bristol and Denver laboratories. The clearest distinction between positive and negative signals was obtained in the harmonized BDC assay with a potential threshold identified at around 20 DK units/ml, which corresponded to the 97th percentile of the adult control samples. A threshold corresponding to the 97th percentile was selected for each of the laboratories (20 DK units/ml for Denver, 33 DK units/ml for Bristol, 43 DK units/ml for Munich). Using these thresholds to identify positive and negative samples, 97.9% of the samples were concordant in all three laboratories (Fig. 4): 21 of 974 control samples and 79 of 100 patient samples were identified as positive in all three laboratories; 934 control samples and 17 patient samples were negative in all three laboratories; 19 control samples were discordant (16 positive in one laboratory, three positive in two laboratories); and four patient samples were discordant (three positive in one laboratory, one positive in two laboratories).
Figure 4.
Venn diagram showing concordance of IA-2A (left panels) and GADA (right panels) in the Munich, Bristol, and Denver laboratories on control samples (upper panels; n = 500 for IA-2A, n = 974 for GADA) and patient samples (lower panels; n = 50 for IA-2A, n = 100 for GADA). Numbers refer to the number of positive samples.
Transfer to a fourth laboratory
The efforts of the standardization exercise were then extended to include the central laboratory for autoantibody measurements in SEARCH (6). Training was performed in the Denver laboratory, and a series of validation exercises were undertaken to establish that the SEARCH laboratory achieved high-quality measurement. The laboratory was then requested to perform the measurement of the DASP sample set. For IA-2A, results from the SEARCH laboratory were very similar to those of the other three laboratories: a threshold of 5 DK units/ml provided identical sensitivity and specificity. For GADA, the distribution of results from the SEARCH laboratory was similar to those of the Bristol and Munich laboratories, and the 97th percentile of the adult control samples corresponded to 31 DK units/ml.
Improved concordance in the clinical setting
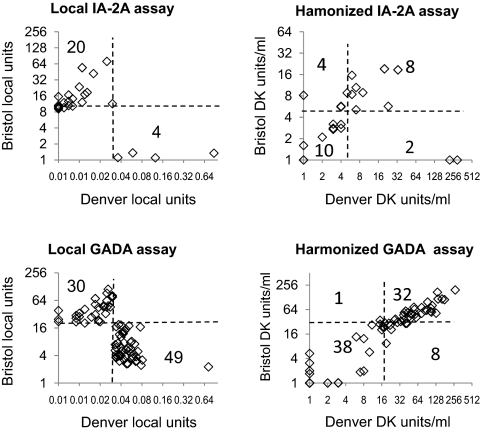
There was a substantial improvement in concordance when samples from the TEDDY study previously measured for IA-2A (n = 604) and/or GADA (n = 514) in the BDC and Bristol laboratories using local assays were remeasured by the two central laboratories using the harmonized assays (Fig. 5). For IA-2A, 18 of 24 (75%) samples that were previously discordant became concordant, and five of 580 previously concordant samples became discordant. The overall discordance rate in these samples improved from 4 to 1.8% (P = 0.02). The harmonized assays identified 28 of the samples as positive in both laboratories, compared with 25 using local assays. For GADA, 70 of 79 (89%) samples that were previously discordant became concordant, and five of 435 previously concordant samples became discordant. The overall discordance rate in these samples improved from 15.4 to 2.7% (P < 0.0001). The harmonized assay identified 143 of the samples as GADA positive in both laboratories, compared with 114 using local assays.
Figure 5.
Improved concordance of measurement of samples from the TEDDY study. Results are shown for children samples that were found to be discordant for IA-2A (upper panels, n = 24) and GADA (lower panels, n = 79) when measured in the Bristol and Denver laboratories using their local assays (left panels) and remeasured using the harmonized assays (right panels). Threshold for positivity in the respective assays are shown by the broken lines, and numbers represent the samples falling in each quadrant.
Discussion
We provide a process of harmonization of assays for multicenter studies. The assays that underwent harmonization were the measurement of islet autoantibodies, which unlike the majority of biochemical analytes are heterogeneous populations of molecules with multiple binding sites and potentially multiple affinities to target ligand. The harmonization process included the preparation and introduction of working calibrators, their calibration against reference calibrators, and the identification of common assay reagents and procedures that can be applied in different laboratory sites. Finally, using common samples, potential thresholds for identification of positive signals were established and the benefit of the harmonization was shown in the clinical research setting of the TEDDY study.
These efforts built on many years of standardization of these assays in international workshop programs (2,13,14). Previous standardization exercises had established that radio-binding assays had high sensitivity and specificity and introduced WHO reference units to the measurement of GADA and IA-2A (15). Despite these efforts, when large-scale testing was performed, it was apparent that there were persistent differences in results among laboratories, and in particular in the definition of positive and negative signals for samples. Hence, harmonization targeted to multicenter studies was required to supplement these previous standardization activities.
The NIDDK calibrators were prepared with a vision that they would be used by the NIDDK consortia autoantibody laboratories and serve as true working calibrators i.e. daily throughout the course of the autoantibody measurement for current and future NIDDK studies. The calibrators were made from a pool of 21 patient samples to avoid bias in measurements due to overrepresentation of atypical autoantibody epitopes in single sera. The negative serum used as diluent was also from a pool of screened negative blood donors. The calibrators covered a range of values from at or just below the expected thresholds of positivity, through to the upper limits of reliable discrimination. They were calibrated against the WHO reference preparation, and therefore the DK units/ml should be equivalent to WHO units/ml for these antibodies. We did, however, observe differences in WHO units/ml vs. DK units/ml reported for a minority of sera, which we attribute to the fact that the WHO standard is a single serum preparation and may over- or underrepresent antibodies to certain epitopes of IA-2 or GAD. The limitations of the NIDDK calibrators include the fact that they do not cover very high titers of antibodies, and results beyond the upper calibrator therefore require extrapolation without knowledge of the extended curve shape. It is also possible that there will be variable matrix effects related to the negative serum pool used as diluent when different assays are used. Nevertheless, they provide for the first time multiple point calibrators that can be used daily by participating laboratories, and as such should improve consistency of measurement over time and between laboratories.
With respect to methodology, the current exercise revealed unexpected and sometimes substantial differences in results related to the composition of assay buffers. Substantial improvement in the Denver GADA assay performance was achieved by substituting a 35S tracer for the 3H tracer previously used in the Denver local GADA assay. One important aspect of the assay that was not harmonized was the washing procedure. This decision was pragmatic because both procedures appeared to work well when used in the experienced laboratories, and it was anticipated that successful introduction of a different washing method to a laboratory would be expensive and likely to require a considerable learning phase.
The transferability of the harmonization exercise was shown in its application to a fourth laboratory, namely the SEARCH central autoantibody laboratory. After a period of assay validation that improved the specificity of measurement in the laboratory, we were able successfully to introduce the harmonized assay and working calibrators and achieve results that were remarkably similar to those obtained in the other three participating laboratories. Thus, it is envisaged that with the assistance of DASP it should also be possible to introduce the harmonized assays to other consortia and that this should improve the comparability of findings among different study cohorts. It should also be possible to perform a similar exercise with the other autoantibody measurements, in particular insulin autoantibodies and the recently introduced ZNT8 antibodies (16). Nevertheless, despite the use of common standards and methods, we still had differences with respect to thresholds of positivity in the GAD antibody assays, suggesting that relatively minor differences in assay procedure can affect signal strengths.
We formally evaluated whether the introduction of the harmonized assays would improve comparability across studies by retesting the TEDDY samples. Although discordance was not completely abolished, there was a dramatic improvement in the concordance in calling samples positive in the two laboratories; more samples were designated as confirmed positive for GADA, and the majority of these also contained other islet autoantibodies (data not shown).
In summary, on the basis of our efforts toward harmonization of islet autoantibody assays, we offer the following recommendations for large-scale multicenter autoantibody testing. First, a procedure that involves confirmation of positive results in a second laboratory is likely to be valuable to identify discrepancies. Second, where possible, laboratories should use common calibrators, which are available in sufficient volumes to last for the whole duration of the study and have been calibrated against reference standards. Third, the methods used in more than one center should be compared and, where possible, harmonized to eliminate factors likely to contribute to discrepancies. Fourth, laboratories should measure a common set of samples that include a large number of samples from controls and patients to provide knowledge of concordance, sensitivity, and specificity and to establish working thresholds of positivity that are similar between the participating laboratories. These steps should lead to more reproducible identification of individuals at risk of type 1 diabetes and improve monitoring in long-term prospective studies.
Acknowledgments
We thank those who provided samples, including Janet Snell-Bergeon and Marian Rewers who provided control samples from the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study.
Footnotes
The CACTI study was supported by the National Institutes of Health (NIH), National Heart, Lung and Blood Institute Grants R01 HL61753 and R01 HL079611, and Diabetes Endocrinology Research Center Clinical Investigation Core P30 DK57516. The CACTI study was performed at the Adult General Clinical Research Center at the University of Colorado Denver Anschutz Medical Center, supported by the NIH Grant M01 RR000051; at the Barbara Davis Center for Childhood Diabetes in Denver, Colorado; and at the Colorado Heart Imaging Center in Denver, Colorado.
This work was supported by Grants DK 63829, 63861, 63821, 63865, 63863, 63836, and 63790 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, National Institute of Environmental Health Sciences, Juvenile Diabetes Research Foundation, Centers for Disease Control and Prevention, and the Kompetenznetz Diabetes mellitus (Competence Network for Diabetes mellitus), funded by the Federal Ministry of Education and Research in Germany (FKZ 01GI0805-07).
Disclosure Summary: All authors have nothing to declare.
First Published Online May 5, 2010
Abbreviations: cpm, Counts per minute; DK units, digestive and kidney disease units; GAD, glutamic acid decarboxylase; GADA, GAD autoantibody; IA-2, islet antigen-2; IA-2A, IA-2 autoantibody.
References
- American Diabetes Association 2008 Diagnosis and classification of diabetes mellitus. Diabetes Care 31:S55–S60 [DOI] [PubMed] [Google Scholar]
- Törn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ; Participating Laboratories 2008 Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia 51:846–852 [DOI] [PubMed] [Google Scholar]
- Qu HQ, Polychronakos C 2009 The effect of the MHC locus on autoantibodies in type 1 diabetes. J Med Genet 46:469–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEDDY Study Group 2008 The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann NY Acad Sci 1150:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyler JS, Greenbaum CJ, Lachin JM, Leschek E, Rafkin-Mervis L, Savage P, Spain L; Type 1 Diabetes TrialNet Study Group 2008 Type 1 Diabetes TrialNet—an international collaborative clinical trials network. Ann NY Acad Sci 1150:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The SEARCH Study Group 2004 SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 25:458–471 [DOI] [PubMed] [Google Scholar]
- Zeitler P, Epstein L, Grey M, Hirst K, Kaufman F, Tamborlane W, Wilfley D 2007 Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 8:74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, Garg S, Hamman RF, Rewers M 2003 Coronary Artery Calcification in Type 1 Diabetes Study. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes 52:2833–2839 [DOI] [PubMed] [Google Scholar]
- Yu L, Rewers M, Gianani R, Kawasaki E, Zhang Y, Verge C, Chase P, Klingensmith G, Erlich H, Norris J, Eisenbarth GS 1996 Antiislet autoantibodies develop sequentially rather than simultaneously. J Clin Endocrinol Metab 81:4264–4267 [DOI] [PubMed] [Google Scholar]
- Bingley PJ, Williams AJ, Gale EA 1999 Optimized autoantibody-based risk assessment in family members. Implications for future intervention trials. Diabetes Care 22:1796–1801 [DOI] [PubMed] [Google Scholar]
- Ziegler AG, Hummel M, Schenker M, Bonifacio E 1999 Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes 48:460–468 [DOI] [PubMed] [Google Scholar]
- Williams AJ, Somerville M, Rokni S, Bonifacio E, Yu L, Eisenbarth G, Akolkar B, Steffes M, Bingley PJ 2009 Azide and Tween-20 reduce binding to autoantibody epitopes of islet antigen-2; implications for assay performance and reproducibility. J Immunol Methods 351:75–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidli RS, Colman PG, Bonifacio E, Bottazzo GF, Harrison LC 1994 High level of concordance between assays for glutamic acid decarboxylase antibodies. The First International Glutamic Acid Decarboxylase Antibody Workshop. Diabetes 43:1005–1009 [DOI] [PubMed] [Google Scholar]
- Bingley PJ, Bonifacio E, Mueller PW 2003 Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes 52:1128–1136 [DOI] [PubMed] [Google Scholar]
- Mire-Sluis AR, Gaines Das R, Lernmark A 2000 The World Health Organization International Collaborative Study for islet cell antibodies. Diabetologia 43:1282–1292 [DOI] [PubMed] [Google Scholar]
- Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC 2007 The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]